Abstract
Background
To examine the effect of frailty on cognitive decline independent of cerebral small vessel disease (cSVD) and brain atrophy, and whether associations between neuropathology and cognition differed depending on frailty status.
Methods
The Tasmanian Study of Cognition and Gait was a population-based longitudinal cohort study with data collected at 3 phases from 2005 to 2012. Participants aged 60–85 were randomly selected from the electoral roll. Various data were used to operationalize a 36-item frailty index (FI) at baseline. Brain MRI was undertaken to obtain baseline measures of neuropathology. A neuropsychological battery was used to assess cognition at each time point. Generalized linear mixed models were used to examine the effect of frailty and MRI measures on cognition over time. The associations between MRI measures and cognition were explored after stratifying the sample by baseline frailty status. All analyses were adjusted for age, sex, and education.
Results
A total of 385 participants were included at baseline. The mean age was 72.5 years (standard deviation [SD] 7.0), 44% were female (n = 171). In fully adjusted linear mixed models, frailty (FI × time β −0.001, 95% confidence interval [CI] −0.003, −0.001, p = .03) was associated with decline in global cognition, independent of brain atrophy, and cSVD. The association between cSVD and global cognition was significant only in those with low levels of frailty (p = .03).
Conclusion
These findings suggest that frailty is an important factor in early cognitive dysfunction, and measuring frailty may prove useful to help identify future risk of cognitive decline.
Keywords: Brain atrophy, Cerebral small vessel disease, Cognitive decline, Frailty
The neuropathological features of dementia show considerable variability in their association with the clinical diagnosis of dementia (1,2). This discrepancy suggests the involvement of other factors, of which, frailty may be one. Frailty is conceptually defined as a vulnerability state with a reduced capacity to respond to stressors, which is mediated by dysfunction across multiple bodily homeostatic systems (3). Frailty has been shown to be associated with cognitive decline and incident dementia (4,5), as well as their associated neuropathology, including cerebral small vessel disease (cSVD) (6,7), beta-amyloid, tau accumulation (8), and brain atrophy (9). However, little is known as to whether frailty is associated with cognitive decline or dementia independent of these brain pathologies. A recent cross-sectional study showed that frailty was associated with the clinical expression of Alzheimer Disease (AD) independent of measures of neurofibrillary tangles and neuritic and diffuse plaques at autopsy (10). Furthermore, those with higher levels of frailty were more likely to express AD at a lower burden of pathology (10). However, it remains unknown whether these relationships exist earlier in the course of cognitive dysfunction, if they are maintained in longitudinal analyses, or are still applicable when considering other neuropathology such as cSVD and brain atrophy.
In a sample of community-dwelling older individuals, we aimed to (a) examine if baseline frailty is associated with cognition over time independent of cSVD and brain atrophy and (b) to assess whether relationships between these brain measures and cognitive decline varied by frailty status. We hypothesized that in a sample of community-dwelling older individuals without dementia that (a) frailty would be associated with decline in cognition over time independent of cSVD and brain atrophy and (b) the relationship for cSVD and brain atrophy with cognitive decline would differ when stratified by baseline frailty status.
Method
Study Population
The Tasmanian Study of Cognition and Gait (TASCOG) is a population-based longitudinal cohort study of community-dwelling older individuals aged between 60 and 85 years who were randomly recruited from the Southern Tasmanian Electoral Roll, Australia between 2005 and 2012. Phase 1 data were collected between January 2005 and December 2008, Phase 2 data collected between March 2008 and March 2010, and Phase 3 data collected between March 2010 and June 2012. Exclusion criteria were inability to walk unaided; a diagnosis of dementia; any contraindication to MRI; or residing in an aged-care facility. Written consent was obtained from all participants, and the study was approved by the Southern Tasmania Health and Medical human research ethics committee.
Cognitive Function (At Each Time Point)
All participants underwent a comprehensive neuropsychological battery to measure cognitive function in the following seven domains: (a) executive function: Victoria Stroop Test (color minus word subtests) (11); (b) attention-processing speed: Victoria Stroop (dot subtest), digit-symbol coding and symbol search tests (Wechsler Adult Intelligence Scale―Third Edition) (11,12); (c) visuospatial ability: Rey-Osterrieth Complex Figure test (copying task) (13); (d) visual memory: Rey-Osterrieth complex figure task (20-minute delayed reproduction) (13); (e) verbal fluency: Controlled Word Association Test (category fluency, animals; letter fluency with F, A, and S) (13); (f) working memory: Digit span subtest of the Wechsler Adult Intelligence Scale―Third Edition (12); (g) verbal memory: Hopkins Verbal Learning Test-Revised (immediate recall, delayed recall, and recognition memory) (13). To allow for comparison between domains, individual tests were standardized by creating Z scores with reference to the baseline visit mean and standard deviation (SD). The primary outcome measure was a global cognitive domain, derived using all neuropsychological tests. Global and other cognitive domains were created by adding the means of constituent cognitive tests. Domain scores with more than 1 constituent cognitive test were restandardized to an SD of 1 at baseline.
Frailty (Baseline)
The frailty index (FI) was used to measure frailty. The FI operationalizes frailty according to the accumulation of health deficits (14). The index describes a ratio of deficits, divided by the total number of potential deficits for the individual, having a theoretical range from 0 to 1.0, with higher values indicative of worse health. The predictive validity is preserved provided at least 30 items are included (15). The FI in this study comprised 36 items (Supplementary eTable 1). Candidate variables were selected, screened, and scored according to standard procedures (16).
MRI Acquisition (Baseline)
MRI data were obtained at baseline using a 1.5-Tesla machine (LX Horizon, General Electric, Milwaukee, WI) with the following sequences: high-resolution T1-weighted spoiled gradient echo (GRE; repetition time [TR] 35 ms, echo time [TE] 7 ms, flip angle 35°, field of view 24 mm; voxel size 1 mm3) comprising 120 contiguous slices; T2-weighted fast spin echo (TR 4 300 ms, TE 120 ms, 1 excitation, turbo factor 48; voxel size 0.90 × 0.90 × 3 mm); fluid-attenuated inversion recovery (FLAIR; TR 8 802 ms, TE 130 ms, time interval 2 200 ms; voxel size 0.50 × 0.50 × 3 mm); and GRE (TR 800 ms, TE 15 ms, flip angle 30°; voxel size 0.93 × 0.93 × 7 mm).
Brain Atrophy and cSVD Measures
Scans were registered to a standard 152-brain Montreal Neurological Institute template in stereotaxic space and baseline gray matter volume (GMV), and total intracranial volume (TIV) were classified using statistical parametric mapping software following previously described procedures (17). cSVD was rated at baseline only and defined in accordance with the Neuroimaging Standards for Research into Small Vessel Disease criteria (18). White matter hyperintensities (WMH) were manually assessed on T2-FLAIR sequences and rated by 2 trained reviewers according to the modified Fazekas scale (19). Small subcortical infarcts (SI) were evaluated by 2 experts in the field on T2-FLAIR sequences using a definition of a small (3–20 mm) region of signal hypointensity with a surrounding hyperintense rim located in subcortical regions. Cerebral microbleeds (CMB) were identified by a single expert reviewer on GRE images using a definition of small (2–10 mm) rounded hypointense lesions with clear margins in subcortical regions. Enlarged perivascular spaces (EPVS) were manually rated by 2 trained reviewers using a single, predefined slice in the basal ganglia (slice immediately superior to the anterior commissure) and on the most affected hemisphere only, on T2-weighted images. EPVS were phenotypically defined as small, sharply delineated structures of cerebrospinal fluid intensity measuring <3 mm following the course of perforating vessels and scored using a semiquantitative scale (<10, 10–20, 20–40, and >40). All reviewers of cSVD were blinded to the remaining data. These data were used to generate a total cSVD rating scale as previously described (20). Overall burden of cSVD is described on an ordinal scale of 0–4, inclusive, with points awarded for: WMH with a modified Fazekas score of 3 for the periventricular location or ≥2 for deep location; SI, the presence of one or more; CMB, the presence of one or more; EPVS, number >20.
Statistics
Descriptive statistics were used to show participant characteristics in the total sample, as well as by high and low frailty status. Prior to analyses, the FI was multiplied by 100 to aid with interpretation. First, in separate models, linear mixed models (maximum likelihood estimation, unstructured covariance) were used to examine the relationship between baseline total cSVD (exposure variable), GMV (exposure variable), and baseline FI (exposure variable) with global cognitive decline over time (primary outcome variable). Time since baseline was a fixed effect, the exposure variables and their interactions with time (eg, FI × time) were main effects. Estimated intercepts and slopes were permitted to vary between individuals, allowing participants to have different scores at baseline and rates of change in the dependent variable. All analyses were adjusted for age, sex, and level of education and when GMV was included, additionally adjusted for TIV (21). This analysis was then repeated for each cognitive domain. Where frailty and at least 1 of the brain variables were associated with decline in cognition Akaike and Bayesian information criterion were employed to assess whether the addition of frailty improved model fit.
To examine the effect of frailty on the association between brain variables and cognition we stratified analyses by high and low frailty (at the 66th percentile, a value of 0.26, similar to prior studies (22)). This analysis was then repeated for each cognitive domain.
Alpha-levels of p < .05 were considered significant for all statistical tests. All analyses were conducted using Stata version 16.1 (StataCorp., College Station, TX).
Results
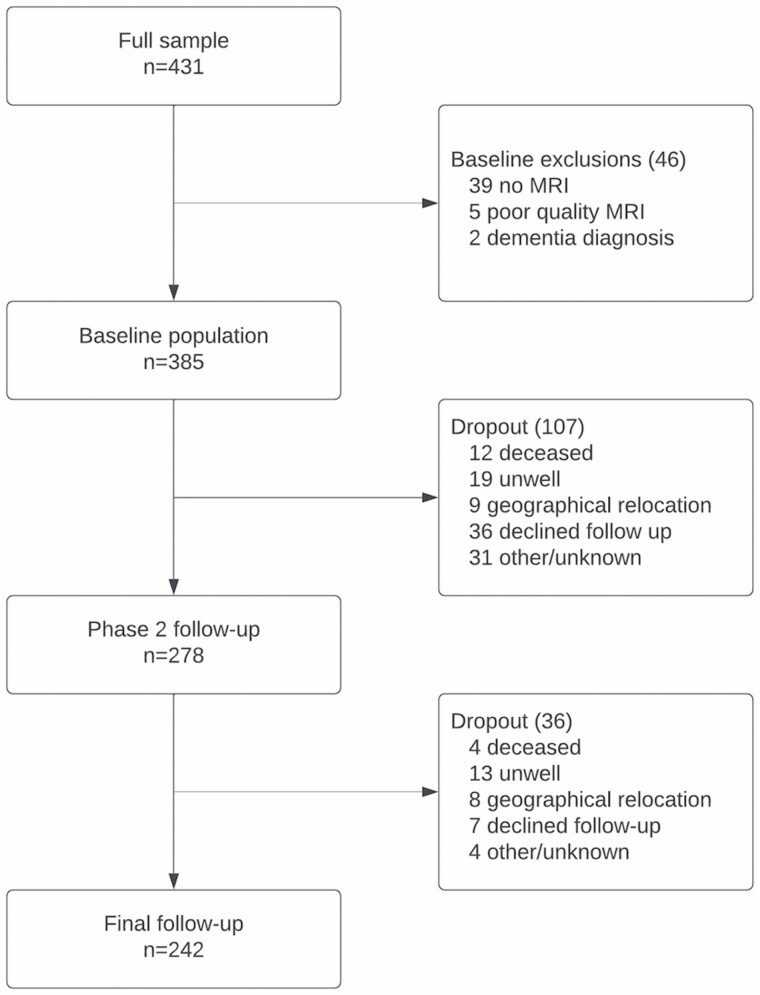
Median follow-up time was 4.4 years. The initial response rate was 54% (n = 431/804). Those included in the study tended to be younger (p < .05) but did not differ by sex (p > .05) when compared to those who declined participation. Figure 1 illustrates participant dropout over time. A total of 242 participants were retained at the final follow-up. When participants who were lost to follow-up (37%; n = 143) were compared to those retained, they tended to be older, frailer, have a higher burden of total cSVD and perform worse on all neuropsychological tests (p < .05 for all). At baseline, most participants (n = 330; 86%) had complete data for the FI, 42 participants (11%) had 1 missing variable, 7 participants (2%) had 2 missing variables, and 6 participants (2%) had 3 or more missing variables.
Figure 1.
Participant flowchart.
Table 1 presents baseline sample characteristics stratified by high (n = 126) and low (n = 259) frailty status.
Table 1.
Baseline Sociodemographic, MRI, and Cognitive Data by Baseline Frailty Status
| Entire Sample | Low Frailty (0.26) | High Frailty (0.26) | |
|---|---|---|---|
| n = 385 | n = 259 | n = 126 | |
| Age (mean in years, SD) | 72.5 (7.0) | 70.9 (6.4) | 75.8 (7.2) |
| Female sex (n, %) | 171 (44.4%) | 109 (42%) | 62 (49.2%) |
| Level of education (mean in years, SD) | 10.9 (3.6) | 11.2 (3.8) | 10.3 (3.2) |
| Frailty index (%, median, IQR) | 0.21 (0.14, 0.29) | 0.16 (0.11, 0.21) | 0.33 (0.29, 0.39) |
| MRI measures | |||
| Total cSVD (median, IQR) | 1 (0, 2) | 1 (0, 2) | 2 (1, 2) |
| cSVD by strata (n, %) | |||
| 0 | 126 (32.7%) | 103 (39.8%) | 23 (18.3%) |
| 1 | 130 (33.8%) | 91 (35.1%) | 39 (31.0%) |
| 2 | 84 (21.8%) | 48 (18.5%) | 36 (28.6%) |
| 3 | 29 (7.5%) | 13 (5.0%) | 16 (12.7%) |
| 4 | 16 (4.2%) | 4 (1.5%) | 12 (9.5%) |
| WMH (n, %) | |||
| Periventricular = 3 | 72 (18.7%) | 33 (12.7%) | 39 (31%) |
| Deep ≥ 2 | 96 (24.9%) | 48 (18.5%) | 48 (38.1%) |
| SI (n, %) | 71 (18.4%) | 35 (13.5%) | 36 (28.6%) |
| CMB (n, %) | 30 (7.8%) | 12 (4.6%) | 18 (14.3%) |
| EPVS >20 (n, %) | 229 (59.5%) | 136 (52.5%) | 93 (73.8%) |
| GMV (mL, median, IQR) | 540 (502, 575) | 550 (508, 585) | 519 (487, 557) |
| TIV (mL, median, IQR) | 1 435 (1 323, 1 520) | 1 441 (1 331, 1 535) | 1 421 (1 308, 1 497) |
| Cognitive function (z scores) | |||
| Global cognition (mean, SD) | 0.21 (0.88) | −0.47 (1.09) | |
| Processing speed (mean, SD) | 0.26 (0.85) | −0.54 (1.07) | |
| Visuospatial ability (mean, SD) | 0.20 (0.78) | −0.42 (1.25) | |
| Executive function (mean, SD) | 0.17 (0.59) | −0.36 (1.49) | |
| Verbal fluency (mean, SD) | 0.13 (0.92) | −0.27 (1.10) | |
| Working memory (mean, SD) | 0.13 (0.99) | −0.26 (0.98) | |
| Verbal memory (mean, SD) | 0.14 (0.94) | −0.28 (1.07) | |
| Visual memory (mean, SD) | 0.16 (0.99) | −0.33 (0.94) |
Notes: CMB = cerebral microbleeds; cSVD = cerebral small vessel disease; EPVS = enlarged perivascular spaces; GMV = gray matter volume; IQR = interquartile range; MRI = magnetic resonance imaging; SD = standard deviation; SI = subcortical infarcts; TIV = total intracranial volume; WMH = white matter hyperintensities, presented as n (%) above threshold as rated by Modified Fazekas Scale.
Associations Between Frailty, Brain Measures, and Cognitive Decline
Table 2 shows the associations between baseline FI and cognition over time. In the adjusted model (Model 2) there were associations between a higher FI and decline in both global cognition (FI × time β −0.002, 95% CI −0.003, −0.001, p = .002) and verbal fluency (FI × time β −0.002, 95% CI −0.004, −0.001, p = .001).
Table 2.
Frailty Index at Baseline and Cognitive Domains Over Time
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p Value | Beta | 95% CI | p Value | |
| Global cognition | ||||||
| Time | −0.029 | −0.053, −0.004 | .024 | 0.012 | −0.016, 0.040 | .40 |
| FI | −0.027 | −0.040, −0.019 | <.001 | −0.017 | −0.025, −0.009 | <.001 |
| FI × time | −0.002 | −0.003, −0.0006 | .003 | −0.002 | −0.003, −0.001 | .002 |
| Attention and processing | ||||||
| Time | −0.004 | −0.030, 0.022 | .76 | 0.038 | 0.009, 0.066 | .01 |
| FI | −0.034 | −0.041, −0.026 | <.001 | −0.022 | −0.030, −0.015 | <.001 |
| FI × time | −0.001 | −0.002, 0.001 | .13 | −0.001 | −0.002, 0.001 | .12 |
| Visuospatial | ||||||
| Time | −0.301 | −0.365, −0.236 | <.001 | −0.274 | −0.340, −0.208 | <.001 |
| FI | −0.024 | −0.033, −0.016 | <.001 | −0.015 | −0.024, −0.001 | .001 |
| FI × time | −0.002 | −0.005, 0.001 | .12 | −0.002 | −0.005, 0.001 | .12 |
| Executive function | ||||||
| Time | −0.002 | −0.051, 0.046 | .92 | 0.020 | −0.031, 0.070 | .44 |
| FI | −0.017 | −0.024, −0.009 | <.001 | −0.011 | −0.019, −0.003 | .005 |
| FI × time | −0.001 | −0.002, 0.002 | .94 | −0.001 | −0.003, 0.002 | .89 |
| Verbal fluency | ||||||
| Time | 0.055 | 0.023, 0.088 | .001 | 0.081 | 0.045, 0.116 | <.001 |
| FI | −0.016 | −0.024, −0.008 | <.001 | −0.009 | −0.017, −0.001 | .03 |
| FI × time | −0.002 | −0.004, −0.001 | .002 | −0.002 | −0.004, −0.001 | .001 |
| Working memory | ||||||
| Time | 0.032 | −0.005, 0.069 | .09 | 0.036 | −0.003, 0.076 | .07 |
| FI | −0.017 | −0.025, −0.010 | <.001 | −0.013 | −0.022, −0.005 | .002 |
| FI × time | −0.001 | −0.002, 0.001 | .48 | −0.001 | −0.002, 0.001 | .50 |
| Verbal memory | ||||||
| Time | 0.048 | 0.010, 0.086 | .014 | 0.083 | 0.043, 0.123 | <.001 |
| FI | −0.018 | −0.025, −0.010 | <.001 | −0.010 | −0.017, −0.002 | .01 |
| FI × time | −0.001 | −0.002, 0.002 | .87 | −0.001 | −0.002, 0.001 | .72 |
| Visual memory | ||||||
| Time | −0.154 | −0.194, −0.116 | <.001 | −0.127 | −0.168, −0.086 | <.001 |
| FI | −0.021 | −0.028, −0.013 | <.001 | −0.012 | −0.020, −0.004 | .002 |
| FI × time | −0.001 | −0.002, 0.002 | .93 | −0.001 | −0.002, 0.002 | .92 |
Notes: Model 1: unadjusted model. Model 2: adjusted for age, sex, and level of education. 95% CI = 95% confidence intervals; FI = frailty index.
Supplementary eTable 2 shows associations between baseline brain measures and cognition over time. In Model 2 a higher burden of cSVD was associated with greater decline in global cognition (cSVD × time β −0.019, 95% CI −0.031, −0.007, p = .003), attention and processing speed (cSVD × time β −0.017, 95% CI −0.030, −0.007, p = .007), and visuospatial ability (cSVD × time β −0.036, 95% CI −0.067, −0.006, p = .02). There was no association between GMV and global cognition (GMV × time β 0.0001, 95% CI −0.0001, 0.0003, p = .20) or the remaining cognitive domains.
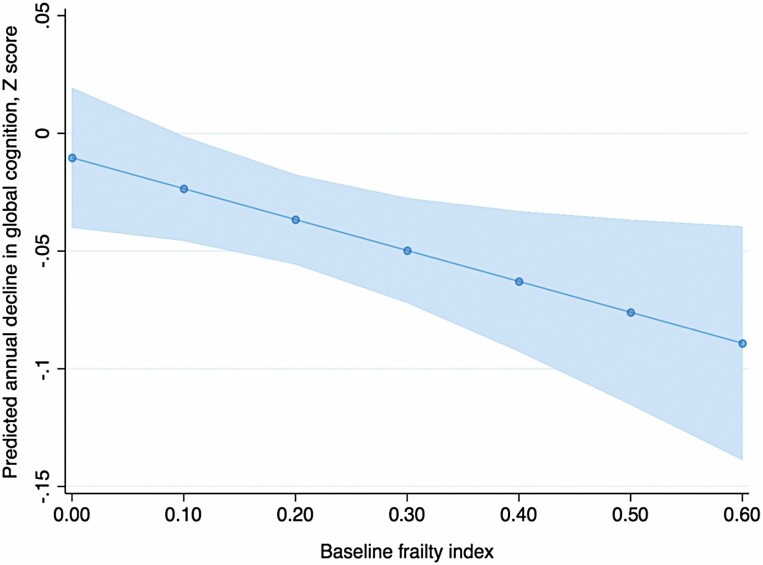
Where frailty and a brain measure were both associated with a cognitive variable (global cognition only) we examined a final model including all exposure variables. In the model, frailty (FI × time β −0.001, 95% CI −0.003, −0.001, p = .03) was associated with decline in global cognition independent of cSVD (cSVD × time β −0.014, 95% CI −0.027, −0.002, p = .03) and GMV (GMV × time β 0.00005, 95% CI −0.0002, 0.0002, p = .96), after adjusting for age, sex, education, and TIV. Additionally, adjusting for various vascular measures (history of hypertension, angina, myocardial infarction, stroke, diabetes, and dyslipidemia) did not significantly alter the results (results not shown). The addition of frailty to the model improved model fit according to a reduction in Akaike information criterion (from 1 490 to 1 473) and Bayesian information criterion (from 1 552 to 1 544). Figure 2 shows the predicted annual change of global cognition (Z score) by the baseline FI.
Figure 2.
Predicted annual change in global cognition by baseline frailty index.
Table 3 (global cognition only) and Supplementary eTable 3 (remaining cognitive domains) present the results for associations between brain measures and global cognition over time stratified by high and low frailty. Higher burden of baseline cSVD was associated with decline in global cognition (cSVD × time β −0.017, 95% CI −0.033, −0.002, p = .03) and attention and processing speed (cSVD × time β −0.022, 95% CI −0.037, −0.007, p = .004) only in those lower, but not higher frailty scores (cSVD × time β −0.014, 95% CI −0.037, 0.008, p = .21) and (cSVD × time β −0.008, 95% CI −0.033, 0.017, p = .53), respectively. There were no significant associations between GMV and global cognition in those with either lower (GMV × time β 0.0001, 95% CI −0.0001, 0.0004, p = .28) or higher (GMV × time β −0.0001, 95% CI −0.0004, 0.0004, p = .94) frailty.
Table 3.
Associations Between MRI Measures and Cognitive Domains Over Time Stratified by Baseline Frailty Status, Adjusting for Age, Sex, and Years of Formal Education and Additionally Adjusting for Total Intracranial Volume in Gray Matter Models
| Low Frailty (FI < 0.26) | High Frailty (FI > 0.26) | |||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p Value | Beta | 95% CI | p Value | |
| cSVD (n) | ||||||
| Global cognition | ||||||
| Time | 0.010 | −0.014, 0.034 | .41 | −0.041 | −0.090, 0.009 | .11 |
| cSVD | −0.038 | −0.136, 0.061 | .45 | −0.001 | −0.178, 0.177 | .99 |
| cSVD × time | −0.017 | −0.033, −0.002 | .03 | −0.014 | −0.037, 0.008 | .21 |
| GMV (mL) | ||||||
| Global cognition | ||||||
| Time | −0.100 | −0.248, 0.050 | .19 | −0.066 | −0.267, 0.135 | .52 |
| GMV | 0.005 | 0.001, 0.008 | .009 | 0.007 | 0.001, 0.013 | .03 |
| GMV × time | 0.0002 | −0.0001, 0.0004 | .28 | −0.0001 | −0.0004, 0.0004 | .94 |
Notes: cSVD = cerebral small vessel disease; 95% CI = 95% confidence intervals; FI = frailty index; GMV = gray matter volume; MRI = magnetic resonance imaging.
Discussion
We investigated the role of frailty in the relationship between neuropathology and cognitive decline. There were 2 main findings from our study. First, frailty was associated with greater global cognitive decline, independent of cSVD and brain atrophy, suggesting it has a role in cognitive decline over and above cerebrovascular and neurodegenerative disease. Second, cSVD burden was only associated with decline in global cognition in the group with low levels of frailty. Taken together these findings highlight the importance of frailty as a latent factor in the expression of cognitive decline and add to a growing body of literature conceptualizing cognitive impairment and dementia as a complex syndrome of aging with multiple potential causes that interact, rather than a discrete disease entity.
Our findings build on the previous literature by demonstrating the independent effect of frailty on cognitive decline over and above that of cSVD burden and brain atrophy. Prior studies have shown associations between both cSVD (6,7) and brain atrophy (17,23) and frailty, and between frailty (24–26), cSVD (27), brain atrophy (28) and cognitive decline and dementia. To the best of our knowledge no prior studies have evaluated the independent effect of frailty on cognitive decline over and above cSVD and brain atrophy. Our finding suggest frailty may represent other factors such as the effect of inflammation (29), vascular dysregulation (30), impaired white matter microstructure (7), or functional connectivity (31,32) that we were unable to measure. The FI may therefore be a good overall marker of multiple risk factors (eg, high blood pressure and diabetes) for dementia or of other factors such as slow gait speed that share common underlying neural networks (33). Furthermore, in-keeping with its conceptual framework, frailty may contribute indirectly to expression of cognitive decline by reducing the threshold in which brain pathology manifests as cognitive dysfunction. Possible contributors to this are reduced cognitive reserve, impaired sensory function, or lack of later-life social and cognitive engagement (34,35). Finally, frailty may contribute to cognitive decline in ways that are yet to be described.
We found that associations between cSVD and cognitive decline in domains of global cognition and attention and processing speed were only significant in those with low, but not high, levels of frailty. The associations of cSVD with attention and processing speed are consistent with previous research demonstrating an association between cSVD and domains most affected in vascular dementia (36). The weakened direct link in those with higher frailty is supportive of the latent role of frailty in cognitive function. Recent work by Wallace et al. (10) eloquently describes similar associations with frailty status, AD pathology and clinical expression of AD using a cross-sectional autopsy study design. They found that those with even a low burden of pathology may manifest clinical dementia in the face of a high degree of frailty. They controlled for vascular risk factors in analyses, which did not affect results, but were unable to consider cSVD or brain volumes directly, and causative inferences are limited by the cross-sectional design. Our work complements their findings and extends our understanding by demonstrating that the relationship between frailty and cognition dysfunction begins much earlier and is present in relatively healthy community dwellers with overall low levels of frailty. Furthermore, we show that the relationship persists longitudinally and is applicable to cerebrovascular and neurodegenerative diseases. Taken together our findings add to a growing body of literature elucidating cognitive decline and dementia as a complex syndrome with multiple potential causes, rather than a discrete entity with a singular cause. Refining our understanding of cognitive decline and dementia may assist in the development of more efficacious treatment paradigms in the future, an area that has been hampered by mostly negative results. Furthermore, frailty itself is emerging as a prognostic marker and therapeutic target to ameliorate the impact of cognitive decline, suggesting its importance in a comprehensive cognitive assessment. At this time, it remains unknown if treating frailty may mitigate cognitive decline in these populations and future research aiming to treat frailty may wish to consider cognition as an outcome measure.
Our study has several limitations. First, our sample demonstrated overall low levels of frailty, with a median FI at baseline of 0.20. We describe higher levels of frailty at > 66th percentile (FI > 0.26), a level that closely approximates the advocated 0.25 cut-point between “fit” and “frail” (22,37). Similarly, the burden of cSVD was low, with only 12% (n = 45) of the sample scoring >2 on the total cSVD score. Despite this we were able to detect associations between both frailty and cSVD and cognitive decline. Although the association between frailty and decline in global cognition was significant independent of brain atrophy and cSVD, the coefficient was small (ie, the coefficient represents a change in each unit of frailty per 1 SD of cognition per year.) We did not demonstrate an association between cSVD and cognition in those with higher levels of frailty (Table 3). Although this may reflect the latent role that frailty has in cognitive dysfunction, it may also be due to reduced numbers in the higher frailty group. Akin to other longitudinal studies, and in particular those with older participants, our population had a loss to follow-up over time. It is unlikely these data were missing at random, and these participants tended to be frailer, have a higher burden of cSVD and poorer cognitive performance at baseline compared to those who were retained until the end of the study. By using linear mixed models for longitudinal analyses we allowed for those with missing data to be retained in the models. However, it is possible this may have led to an underestimation of the true effect size. MRI data were limited to conventional sequences (T1, T2, FLAIR, and GRE), and we were unable to consider cSVD phenotypes detectable on novel sequences such as diffusion tensor imaging (DTI) or microinfarcts which require ultra-high field MRI. Previous studies have described associations between white matter microstructural changes on DTI and frailty (7,38). It is therefore possible that frailty could be associated with the preexistence of neuronal damage not seen by the conventional MRI sequences used in this study. The temporal associations between frailty, microstructural changes, and cognition warrants further investigation. Finally, we were unable to consider the potential for other neurodegenerative pathology, such as beta-amyloid and tau, and it is unlikely that decline in cognition was accounted for by cSVD alone when considering the high frequency of multiple pathology in autopsy studies (39) and the known association between frailty and AD pathology (8).
Our study has a number of strengths. The sample was randomly selected from the electoral roll and therefore more generalizable than samples from clinics or from people who volunteer for autopsy studies. However, we excluded those residing in nursing homes and this could have led to an underestimation of frailty in our population. Frailty was operationalized using the deficit accumulation approach, and although frailty can be operationalized in a myriad of ways the literature suggests that this approach may be best suited for research purposes, which is reflective of its robust predictive validity across diverse disease states and outcomes in different populations (40,41).
Conclusion
In a sample of community-dwelling older individuals higher levels of frailty were associated with decline in global cognition independent of overall burden of cSVD and brain atrophy. Our findings suggest that frailty is an important factor in early cognitive dysfunction and measuring frailty may prove useful to help identify future risk of cognitive decline.
Supplementary Material
Acknowledgments
T.P.S. and M.L.C. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Timothy P Siejka, Alfred Health, Melbourne, Victoria, Australia.
Velandai K Srikanth, Peninsula Clinical School, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Menzies Institute for Medical Research, University of Tasmania, Hobart, Tasmania, Australia.
Ruth E Hubbard, Faculty of Medicine, University of Queensland, Brisbane, Queensland, Australia.
Chris Moran, Alfred Health, Melbourne, Victoria, Australia; Peninsula Clinical School, Central Clinical School, Monash University, Melbourne, Victoria, Australia.
Richard Beare, Peninsula Clinical School, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Clinical Sciences, Murdoch Children’s Research Institute, Melbourne, Victoria, Australia.
Amanda G Wood, Clinical Sciences, Murdoch Children’s Research Institute, Melbourne, Victoria, Australia; School of Life and Health Sciences & Institute for Health and Neurodevelopment, Aston University, Birmingham, United Kingdom.
Taya A Collyer, Peninsula Clinical School, Central Clinical School, Monash University, Melbourne, Victoria, Australia.
Siddhanth Gujjari, Monash Health, Melbourne, Victoria, Australia.
Thanh G Phan, Peninsula Clinical School, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Monash Health, Melbourne, Victoria, Australia.
Michele L Callisaya, Peninsula Clinical School, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Menzies Institute for Medical Research, University of Tasmania, Hobart, Tasmania, Australia.
Funding
This work was supported by National Health and Medical Research Council (403000 and 491109), Perpetual Trustees, Brain Foundation, Royal Hobart Hospital Research Foundation (341M), Australian and New Zealand Charitable Trust, Masonic Centenary Medical Research Foundation. M.L.C. is supported by a Boosting Dementia Research Fellowship from the National Health and Medical Research Council of Australia (1135761). V.K.S. is supported by a Practitioner Fellowship from the National Health and Medical Research Council of Australia (1137837). The funding source(s) did not influence the conduction of the study in any way.
Conflict of Interest
T.G.P. has received speaker honorariums from Genzyme, Pfizer, Boehringer Ingelheim, Bayer and served on an advisory board for Genzyme. M.L.C. serves as an Associate Editor for Journal of Gerontology: Medical Sciences. T.P.S., V.K.S., R.E.H., C.M., R.B., A.G.W., T.A.C., and S.G. have no conflict to declare.
Author Contributions
T.P.S. designed and conceptualized the study, acquired, processed, and analyzed the data, wrote the manuscript. V.K.S. acquired the study funding, acquired the data, revised the manuscript, and provided expert opinion. R.E.H. reviewed the manuscript and provided expert opinion. C.M. acquired and analyzed the data, reviewed the manuscript, and provided expert opinion. R.B. acquired and analyzed the data, reviewed the manuscript, and provided expert opinion. A.G.W. acquired and analyzed the data, reviewed the manuscript, and provided expert opinion. T.A.C. provided statistical support, reviewed the manuscript, and provided expert opinion. S.G. acquired the data and reviewed the manuscript. T.G.P. acquired and analyzed the data, reviewed the manuscript, and provided expert opinion. M.L.C. designed and conceptualized the study, analyzed the data, wrote, revised and edited the manuscript, and provided expert opinion.
Data Availability
Requests for access to the data used in this study will be considered by the corresponding author.
References
- 1. Chetelat G, La Joie R, Villain N, et al. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin. 2013;2:356–365. doi: 10.1016/j.nicl.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Villemagne VL, Pike KE, Chetelat G, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–192. doi: 10.1002/ana.22248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–255. doi: 10.1111/j.1532-5415.2009.02671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kojima G, Taniguchi Y, Iliffe S, Walters K. Frailty as a predictor of Alzheimer disease, vascular dementia, and all dementia among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17(10):881–888. doi: 10.1016/j.jamda.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 6. Siejka TP, Srikanth VK, Hubbard RE, et al. White matter hyperintensities and the progression of frailty-The Tasmanian Study of Cognition and Gait. J Gerontol A Biol Sci Med Sci. 2020;75(8):1545–1550. doi: 10.1093/gerona/glaa024 [DOI] [PubMed] [Google Scholar]
- 7. Avila-Funes JA, Pelletier A, Meillon C, et al. Vascular cerebral damage in frail older adults: the AMImage study. J Gerontol A Biol Sci Med Sci. 2017;72(7):971–977. (In eng). doi: 10.1093/gerona/glw347 [DOI] [PubMed] [Google Scholar]
- 8. Wallace L, Theou O, Rockwood K, Andrew MK. Relationship between frailty and Alzheimer’s disease biomarkers: a scoping review. Alzheimers Dement (Amst). 2018;10:394–401. doi: 10.1016/j.dadm.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kant IMJ, de Bresser J, van Montfort SJT, et al. The association between brain volume, cortical brain infarcts, and physical frailty. Neurobiol Aging. 2018;70:247–253. doi: 10.1016/j.neurobiolaging.2018.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18(2):177–184. doi: 10.1016/S1474-4422(18)30371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spreen O, Strauss E.. A Compendium of Neuropsychological Test. Administration, Norms and Commentary. Oxford University Press; 1998. [Google Scholar]
- 12. Wechsler D. Wechsler Adult Intelligence Scale, 3rd edition. Psychological Corporation; 1997. [Google Scholar]
- 13. Lezak M. Neuropsychological Assessment. 3rd ed.Oxford University Press;1995. [Google Scholar]
- 14. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moorhouse P, Rockwood K. Frailty and its quantitative clinical evaluation. J R Coll Physicians Edinb. 2012;42(4):333–340. doi: 10.4997/JRCPE.2012.412 [DOI] [PubMed] [Google Scholar]
- 16. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Callisaya ML, Beare R, Phan TG, Chen J, Srikanth VK. Global and regional associations of smaller cerebral gray and white matter volumes with gait in older people. PLoS One. 2014;9(1):e84909. doi: 10.1371/journal.pone.0084909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 20. Huijts M, Duits A, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Staals J. Accumulation of MRI markers of cerebral small vessel disease is associated with decreased cognitive function. A study in first-ever lacunar stroke and hypertensive patients. Front Aging Neurosci. 2013;5:72. doi: 10.3389/fnagi.2013.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Westman E, Aguilar C, Muehlboeck JS, Simmons A. Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer’s disease and mild cognitive impairment. Brain Topogr. 2013;26(1):9–23. doi: 10.1007/s10548-012-0246-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–743. doi: 10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 23. Gallucci M, Piovesan C, Di Battista ME. Associations between the frailty index and brain atrophy: the Treviso Dementia (TREDEM) Registry. J Alzheimers Dis. 2018;62(4):1623–1634. doi: 10.3233/JAD-170938 [DOI] [PubMed] [Google Scholar]
- 24. Zheng L, Li G, Gao D, et al. Cognitive frailty as a predictor of dementia among older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2020;87:1–8. doi: 10.1016/j.archger.2019.103997 [DOI] [PubMed] [Google Scholar]
- 25. Avila-Funes JA, Carcaillon L, Helmer C, et al. Is frailty a prodromal stage of vascular dementia? Results from the Three-City Study. J Am Geriatr Soc. 2012;60(9):1708–1712. doi: 10.1111/j.1532-5415.2012.04142.x [DOI] [PubMed] [Google Scholar]
- 26. Solfrizzi V, Scafato E, Frisardi V, et al. Frailty syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Aging. Alzheimers Dement. 2013;9(2):113–122. doi: 10.1016/j.jalz.2011.09.223 [DOI] [PubMed] [Google Scholar]
- 27. Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164–173. doi: 10.1016/j.neubiorev.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kramer JH, Mungas D, Reed BR, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21(4):412–418. doi: 10.1037/0894-4105.21.4.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amarasekera AT, Chang D, Schwarz P, Tan TC. Vascular endothelial dysfunction may be an early predictor of physical frailty and sarcopenia: a meta-analysis of available data from observational studies. Exp Gerontol. 2021;148:111260. doi: 10.1016/j.exger.2021.111260 [DOI] [PubMed] [Google Scholar]
- 31. Suarez-Mendez I, Doval S, Walter S, et al. Functional connectivity disruption in frail older adults without global cognitive deficits. Front Med (Lausanne). 2020;7:322. doi: 10.3389/fmed.2020.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lammers F, Zacharias N, Borchers F, Morgeli R, Spies CD, Winterer G. Functional connectivity of the supplementary motor network is associated with Fried’s modified frailty score in older adults. J Gerontol A Biol Sci Med Sci. 2020;75(12):2239–2248. doi: 10.1093/gerona/glz297 [DOI] [PubMed] [Google Scholar]
- 33. Martin KL, Blizzard L, Wood AG, et al. Cognitive function, gait, and gait variability in older people: a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68(6):726–732. doi: 10.1093/gerona/gls224 [DOI] [PubMed] [Google Scholar]
- 34. Maltby J, Hunt SA, Ohinata A, Palmer E, Conroy S. Frailty and social isolation: comparing the relationship between frailty and unidimensional and multifactorial models of social isolation. J Aging Health. 2020;32(10):1297–1308. doi: 10.1177/0898264320923245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3 [DOI] [PubMed] [Google Scholar]
- 36. Ying H, Jianping C, Jianqing Y, Shanquan Z. Cognitive variations among vascular dementia subtypes caused by small-, large-, or mixed-vessel disease. Arch Med Sci. 2016;12(4):747–753. doi: 10.5114/aoms.2016.60962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tian Q, Williams OA, Landman BA, Resnick SM, Ferrucci L. Microstructural neuroimaging of frailty in cognitively normal older adults. Front Med (Lausanne). 2020;7:546344. doi: 10.3389/fmed.2020.546344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257(1–2):80–87. doi: 10.1016/j.jns.2007.01.045 [DOI] [PubMed] [Google Scholar]
- 40. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104–114. doi: 10.1016/j.arr.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 41. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to the data used in this study will be considered by the corresponding author.