Abstract
Objectives
Cytomegalovirus (CMV) congenital infection and in immunodeficiency can have deleterious effects on human cortex. In immunocompetent adults, the putative association between CMV infection and cortical measures has not been explored. We hypothesized that CMV exposure is associated with smaller cortical surface area or cortical thinning mainly in patients with schizophrenia spectrum disorders.
Study Design
We included 67 adult patients with schizophrenia spectrum disorders and 262 adult healthy controls. We measured circulating CMV IgG antibody concentrations with solid-phase immunoassay techniques. We measured the total cortical surface area, regional cortical surface areas and the overall mean cortical thickness based on T1-weighted MRI scans processed in FreeSurfer v6.0.
Study Results
In the whole sample analysis, we found a significant diagnostic group-by-CMV status interaction on the total surface area (P = .020). Among patients, CMV antibody positivity was significantly associated with smaller total surface area (P = .002, partial eta2 = 0.138) whereas no such association was found in healthy controls (P = .059). Post hoc analysis among patients showed that higher CMV antibody concentrations were also significantly associated with smaller total surface area (P = .038), and that CMV antibody positivity was significantly inversely associated with 14 left and 16 right regional surface areas mainly in the frontal and temporal lobes. CMV infection was not associated with the overall mean cortical thickness.
Conclusions
The results are indicative of a cortical surface area vulnerability to CMV infection in patients with schizophrenia spectrum disorders but not in healthy controls. CMV infection may contribute to the established cortical surface area aberrations in schizophrenia.
Keywords: CMV, MRI, cortex, psychosis
Introduction
Human cytomegalovirus (CMV) also known as Human Herpesvirus 5 (HHV-5) is a DNA virus of the Herpesviridae family. Antenatal CMV infection as well as postnatal infection of immunodeficient hosts of any age are both associated with considerable mortality and morbidity.1 In marked contrast, postnatal CMV infection of immunocompetent hosts is typically either asymptomatic or followed by flu-like symptoms, but cannot be cleared by the host and results in lifetime latency.1,2 This latent infection has a prominent impact on the adaptive immune system which adjusts its recourses such that a considerable fraction focuses on CMV immunosurveillance.3 The latent CMV infection can be complicated with periodic reactivations. In immunocompromised hosts, CMV reactivations characteristically lead to apparent illness, but there is evidence that frequent reactivations also occur in immunocompetent hosts, are typically asymptomatic and may be related to chronic disease exacerbation.4 It is established that hematopoietic cell lineages are main sites of latency.1 Neural stem cells are the main sites of CMV latency in the brain.5,6
Studies on congenital CMV infection as well as infection of immunodeficient individuals have revealed substantial cerebral cortex involvement. In particular, congenital CMV infection has been associated with MRI aberrations including cortical malformations and enlarged ventricles as well as white matter and hippocampal disturbances.7 In a neuropathological study of CMV infected human fetal brains, the majority had widespread cortical abnormalities, microcephaly, or hippocampal aberrations.8 A neuropathological study of AIDS patients with CMV brain infection showed that numerous brain regions had CMV-related abnormalities, and that cerebral cortex was involved in half of the cases.9 We here hypothesized that such cortical involvement is present even in the latent form of the CMV infection among immunocompetent adults. We base this hypothesis on (a) the apparent CMV neurotropism and the cortical CMV-related aberrations when the human brain is infected prenatally or in the context of immunodeficiency7–9 and (b) the nonsilent chronic viral latency in CMV-infected immunocompetent individuals with continuous expression of proteins and noncoding RNAs as well as recurrent reactivation events.1,2 The cortical volume is a product of two components, the cortical surface area (SA) and the cortical thickness (CT) with different developmental courses.10,11 We hypothesized that circulating CMV immunoglobulin G (IgG) positivity, showing previous CMV infection and current latency, is associated with smaller SA or cortical thinning. To the best of our knowledge, the putative associations between CMV IgG status and cortical measures have not been previously explored in schizophrenia (SCZ).
Immune system disturbances have been repeatedly reported in SCZ12,13 and may result in a less efficient CMV control. Blood–brain barrier (BBB) hyperpermeability and an inflammatory environment have also been implicated in SCZ.14,15 Of note, BBB deficiency facilitates CMV brain penetration while inflammation accelerates CMV reactivation rates.1,16 Furthermore, the female immune system may more efficiently control latent CMV in healthy adults,17,18 but to our knowledge such studies have not been conducted in SCZ. We therefore hypothesized that a putative association between CMV exposure and cortical measures is greater in patients with SCZ spectrum disorders relative to healthy controls (HC), and that such a putative association may be sex-dependent.
Methods
Participants
We included 67 patients with SCZ spectrum disorders and 262 HC (age range 18–53 years). Specifically, we included 45 patients with SCZ, 5 patients with schizophreniform disorder and 17 patients with schizoaffective disorder. Medical doctors and psychologists evaluated the patients with the Structured Clinical Interview for DSM-IV axis I disorder (SCID-I) module A–E19 and HC with the Primary Care Evaluation of Mental Disorders (Prime-MD).20 We recruited the patients from outpatient and inpatient psychiatric units in Oslo, as part of the Thematically Organized Psychosis (TOP) research study, and the HC from the same catchment areas using the national population register. We applied the following exclusion criteria for all participants: previous moderate or severe head injury, a neurological disorder or medical conditions that could affect brain function. We excluded HC with previous or current psychiatric disorders including substance use disorders (including alcohol use disorder) or with first-degree relatives with severe mental illness. The study was approved by the Regional Committee for Medical Research Ethics South East Norway (2009/2485), and was conducted in accordance with the Declaration of Helsinki as revised in 2008. We obtained written informed consent from all participating patients and HC.
Measures and Medication
Education level has been largely used as a socioeconomic status indicator capturing the important shift from parental to own socioeconomic status.21 We therefore used years of education as proxy indicator for socioeconomic status for all participants. Psychosis can impact patients’ education level, and in patient analysis, we also used a categorical maternal education variable (1. primary school; 2. upper secondary school; 3. college/university). In patients, we evaluated alcohol use with the alcohol use disorder identification test (AUDIT)22 and drug use with the drug use disorder identification test (DUDIT).23 We further assessed the patients with the Positive and Negative Syndrome Scale (PANSS).24 We defined the duration of illness (DOI) as the time passed since the first psychotic episode. We finally assessed the current use of antipsychotic medication (binary variable) and we calculated the current chlorpromazine equivalent doses (CPZ) in mg/day.25
MRI
We obtained 329 T1-weighted MRI scans with a General Electric 3T Signa HDxt scanner with an 8-channel head coil. A 3D fast spoiled gradient echo (FSPGR) sequence was obtained applying the following parameters: 170 sagittal slices, slice thickness = 1.2 mm, voxel size = 1 × 1 × 1.2 mm, inversion time (TI) = 450 ms, echo time (TE) = MinFull, repetition time (RT) = 7.8 ms, flip angle = 12°. MRI scans were processed using the FreeSurfer v6.0.26 The total cortical SA was calculated as the sum of the left and right SAs. To calculate the average CT across both hemispheres, we computed a weighted average which takes interhemispheric differences in SA into account, using the following formula: (Mean CT(left) * SA(left)) + (Mean CT(right) * SA(right)) divided by (Sum SA(right + left)). We obtained regional SAs based on the Desikan–Killiany (DK) FreeSurfer Atlas.27 Quality inspection and editing was performed by trained research assistants following standard FreeSurfer procedures.28
Serology Assessment
Blood samples were drawn from all participants. Serology assessment was performed at the Stanley Neurovirology Laboratory, Johns Hopkins University School of Medicine, Baltimore, MD, USA. CMV IgG antibody concentrations were measured by solid-phase immunoassay techniques and were expressed as continuous (antibody concentrations) and dichotomous measures (seropositivity vs. seronegativity), derived via comparisons of the reactivity generated by the samples in the immunoassay with the optical density generated by standard samples as previously described.29,30
Statistics
In the bivariate analysis among all participants (n = 329), we assessed group differences between CMV seropositive (CMV+) and CMV seronegative (CMV−) participants in patient-control status, sex, age, education years and handedness, as well as the correlations between each of these variables and the dependent variables (SA and CT) (table 1). In the bivariate analysis among patients with SCZ spectrum disorders (n = 67), we assessed group differences between CMV+ and CMV− patients in sex, age, education years, maternal education level, daily use of tobacco, handedness, DOI, PANSS total score, the percentage of patients on antipsychotics and the CPZ among patients on antipsychotics, as well as their correlations with SA (table 2). In the bivariate analysis among HC (n = 262), we assessed group differences between CMV+ and CMV− HC in sex, age, education years and handedness, as well as their correlations with SA (table 2).
Table 1.
Group differences between cytomegalovirus (CMV) immunoglobulin G (IgG) seronegative (CMV−) and seropositive (CMV+) participants in patient-control (PC) status, sex, age, education years and handedness. For the whole sample, correlations with the total cortical surface area (SA) and the overall mean cortical thickness (CT) are shown. P values < .05 shown in bold.
| CMV− | CMV+ | Correlation with SA | Correlation with CT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N a | Mean (SD) or % | N a | Mean (SD) or % | P valueb | Direction (+ or −) |
P Valuec | Direction (+ or −) |
P valuec | |
| PC status (% patients) | 146 | 22.6 | 183 | 18.6 | .368 | – | .062 | - | .928 |
| Sex (% females) | 146 | 34.9 | 183 | 42.6 | .156 | -d | <.001 | + | .665 |
| Age (years) | 146 | 30 (7.5) | 183 | 32.6 (7.9) | <.001 | − | .380 | − | <.001 |
| Education years (years) | 144 | 14 (2.6) | 183 | 14.1 (2.3) | .609 | + | .363 | − | <.001 |
| Handedness (% right-handedness) |
143 | 83.9 | 183 | 90.2 | .091 | + | .322 | + | .663 |
Note:
aNumber of participants with data in each variable.
bChi-square test or t-test.
cPoint-biserial correlations for PC status, sex and handedness; Spearman’s correlations for age and education years.
dWomen had smaller SA than men.
Table 2.
Group differences between cytomegalovirus (CMV) immunoglobulin G (IgG) seronegative (CMV−) and seropositive (CMV+) patients with schizophrenia (SCZ) spectrum disorders in sex, age, education years, maternal education level (primary school/upper secondary school/college or university), daily use of tobacco, handedness (right-handedness vs. left-handedness/ambidexterity), duration of illness (DOI), Positive and Negative Syndrome Scale (PANSS) total score, the percentage of patients on antipsychotics as well as the chlorpromazine equivalent doses (CPZ) among patients on antipsychotics. Group differences between CMV− and CMV+ healthy controls in sex, age, education years and handedness. Separately for all patients and all healthy controls, correlations with the total cortical surface area (SA) are shown. P values < .05 shown in bold.
| CMV− | CMV+ | Correlation with SA |
|||||
|---|---|---|---|---|---|---|---|
| N a | Mean (SD) or % | N a | Mean (SD) or % | P valueb | Direction (+ or -) |
P valuec | |
| Patients with SCZ spectrum disorders | |||||||
| Sex (% women) | 33 | 30.3 | 34 | 41.8 | .353 | −d | .003 |
| Age (years) | 33 | 26.8 (6.5) | 34 | 28.6 (8.9) | .347 | − | .074 |
| Education years | 31 | 11.7 (2) | 34 | 12.4 (2.6) | .246 | − | .058 |
| Maternal education | 30 | 5/7/18 | 27 | 5/6/16 | 1.000e | .396f | |
| Tobacco use (%) | 33 | 60.6 | 34 | 64.7 | .729 | + | .264 |
| Handedness (% right-handedness) |
30 | 83.3 | 34 | 97.1 | .090e | .629g | |
| DOI (years) | 33 | 6.5 (6) | 34 | 6.8 (7.3) | .860 | − | .019 |
| PANSS total score | 33 | 61.3 (19.2) | 34 | 57.1 (13.3) | .296 | + | .120 |
| On antipsychotics (%) | 33 | 90.9 | 34 | 97.1 | .356e | + | .815 |
| CPZ (mg/day) | 29 | 397.6 (274.2) | 33 | 326.5 (214) | .257 | + | .632 |
| Healthy Controls | |||||||
| Sex (% women) | 113 | 36.3 | 149 | 43 | .275 | −d | <.001 |
| Age (years) | 113 | 30.4 (7.6) | 149 | 33.5 (7.3) | .001 | − | .620 |
| Education years | 113 | 14.6 (2.4) | 149 | 14.5 (2) | .764 | + | .176 |
| Handedness (% right-handedness) |
113 | 84.1 | 149 | 86.6 | .287 | .568g | |
aNumber of participants with data in each variable.
bChi-square test or t-test.
cPoint-biserial correlations for binary variables; Spearman’s correlations for quantitative variables.
dWomen had smaller SA than men.
eFisher’s exact test.
fAnalysis of variance (ANOVA).
gMann–Whitney U test.
Among all participants, based on our main hypothesis, we ran two full factorial analyses of covariance (ANCOVAs) investigating main and interaction effects of CMV status (seropositivity/seronegativity), diagnostic group (SCZ spectrum/HC) and sex on (a) SA and (b) CT, whilst controlling for variables that differed between CMV+ and CMV− participants or were correlated with the dependent variables in the bivariate analysis among all participants. As we ran two full factorial ANCOVAs, we accepted statistical significance for main and interaction effects at a Bonferroni corrected alpha level of 0.025 (0.05/2). In the SA ANCOVA among all participants, there was a significant diagnostic group-by-CMV status interaction (as shown in the Results section) which we followed up stratifying by diagnostic group and investigating main CMV effects on SA whilst controlling for variables that differed between CMV+ and CMV− participants or were correlated with SA in the bivariate analysis for the diagnostic group analyzed.
We conducted all the analyses with IBM SPSS Statistics 28.
Results
Patient-control Analysis
To investigate putative patient-control differences in SA and CT, we ran the following ANCOVAs in the whole sample. In the SA ANCOVA (patient-control status, sex, and age on SA) patients had significantly smaller SA than HC, F(1,325) = 10.102, P = .002; women had smaller SA than men (P < .001) and age was inversely associated with SA (P = .008). In the CT ANCOVA (patient-control status, sex and age on CT), patients had significantly thinner cortex than HC, F(1,325) = 4.601, P = .033; sex was not associated with CT (P = .968) whereas age was inversely associated with CT (P < .001).
Surface Area Analysis
Whole Sample.
The bivariate analysis of the whole sample (n = 329) showed that CMV+ participants were older than CMV− participants (P < .001), while females had smaller SA than males (P < .001) (table 1). In the multivariate model of the whole sample (full factorial ANCOVA), we searched for main and interaction effects of diagnostic group (SCZ spectrum/HC), CMV status (seropositivity/seronegativity) and sex on SA whilst controlling for age. There was no three-way (CMV-by-diagnostic group-by-sex), CMV-by-sex or diagnostic group-by-sex interactions, whereas there was a significant CMV-by-diagnostic group interaction, F(1,320) = 5.482, P = .020 on SA. Furthermore, there were significant main effects of CMV status, diagnostic group and sex on SA while age was associated with SA. Specifically, CMV+ participants had smaller SA than CMV− participants (P < .001), patients had smaller SA than HC (P = .012), women had smaller SA than men (P < .001) while age was inversely associated with SA (P = .027) (table 3). We followed up the statistically significant two-way interaction investigating the CMV-SA association by diagnostic group.
Table 3.
The results of the full factorial analysis of covariance (ANCOVA) on the total cortical surface area (SA) in the whole sample of patients with schizophrenia (SCZ) spectrum disorders and healthy controls (HC) are presented
| F | P value | |
|---|---|---|
| Whole sample (n = 329) | ||
| CMV status (CMV−/CMV+) | 13.077 | <.001 |
| Diagnostic group (SCZ/HC) | 6.397 | .012 |
| Sex | 63.095 | <.001 |
| CMV status-by-diagnostic group-by-sex | 0.157 | .692 |
| CMV status-by-diagnostic group | 5.482 | .020 |
| CMV status-by-sex | 0.174 | .677 |
| Diagnostic group-by-sex | 3.646 | .057 |
| Age | 4.948 | .027 |
| SCZ spectrum (n = 67) | ||
| CMV status | 10.091 | .002 |
| Sex | 6.892 | .011 |
| Duration of illness | 5.248 | .025 |
| HC (n = 262) | ||
| CMV status | 3.600 | .059 |
| Sex | 131.667 | <.001 |
| Age | 2.035 | .155 |
Note: There was a significant cytomegalovirus (CMV) status-by-diagnostic group interaction which we followed up stratifying by diagnostic group (we ran an ANCOVA among patients and an ANCOVA among HC). CMV seropositive (CMV+) patients had smaller SA than CMV seronegative (CMV−) patients, whereas CMV+ and CMV- HC did not significantly differ in SA. The selection of covariates in the three ANCOVAs was based on the bivariate analyses.
Patients With Schizophrenia Spectrum Disorders.
The bivariate analysis of the patient group showed that CMV+ and CMV− patients did not significantly differ in any of the analyzed variables (table 2). Among all patients, women had smaller SA than men (P = .003), while DOI was inversely correlated with SA (P = .019). Stratifying by CMV status, DOI was significantly inversely correlated with SA among CMV+ (P = .016), but not among CMV− patients (P = .270).
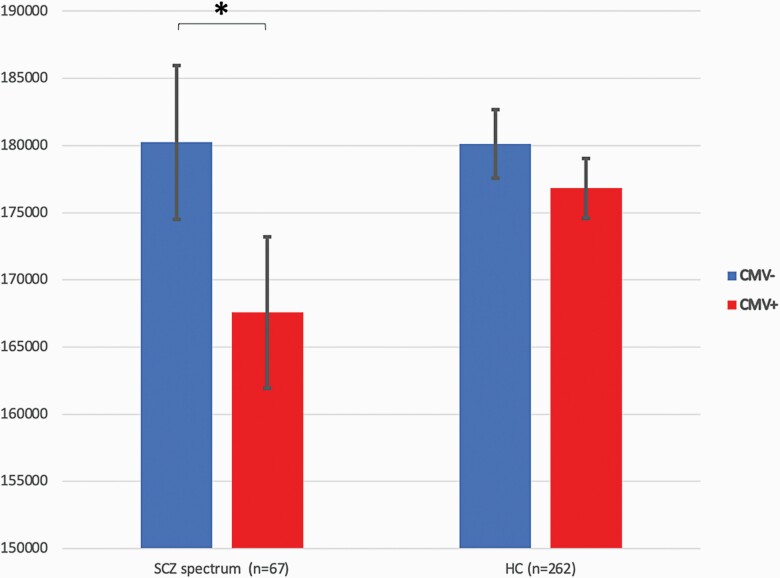
In the multivariate model (ANCOVA), we searched for main effects of CMV status on SA whilst controlling for sex and DOI. There was a significant main effect of CMV status on SA, F(1,63) = 10.091, P = .002, partial eta2 = 0.138 (figure 1); as in the bivariate analysis women had smaller SA than men (P = .011) while DOI was inversely associated with SA (P = .025) (table 3).
Fig. 1.
Total cortical surface area (SA) in mm2 in cytomegalovirus (CMV) immunoglobulin G (IgG) seronegative (CMV−) and seropositive (CMV+) patients with schizophrenia (SCZ) spectrum disorders and healthy controls (HC). CMV+ patients had significantly smaller total SA than CMV− patients. * P = .002.
Healthy Controls.
The bivariate analysis of the HC group showed that CMV+ HC were older than CMV− HC (P = .001) but did not significantly differ in sex, education year or handedness (table 2); women had smaller SA than men (P < .001) (table 2). In the multivariate analysis of the HC group (ANCOVA), we therefore searched for main effects of CMV status on SA whilst controlling for sex and age. In the multivariate model, there was no significant main effect of CMV status on SA, F(1,258) = 3.600, P = .059 (figure 1). Furthermore, there was a significant main effect of sex, with women having smaller SA than men (P < .001) and no significant association between age and SA (P = .155) (table 3).
Post-hoc Analysis Among Patients With Schizophrenia Spectrum Disorders
CMV Antibody Concentrations.
The mean (standard deviation) CMV IgG antibody concentration was 2.8 (1.9). As shown in table 2, among patients with SCZ spectrum disorders, sex and DOI were correlated with SA and were therefore included in the multivariate model. We ran a multiple regression to predict the total cortical SA from CMV antibody concentrations, sex and DOI. An increase of CMV IgG concentration by 1 unit was associated with a 1987 mm2 decrease in SA (P = .038), women had 8113 mm2 smaller SA than men (P = .035), and an increase in DOI by one year was associated with 764 mm2 decrease in SA (P = .006) (supplementary table S1).
Extended MRI Analysis.
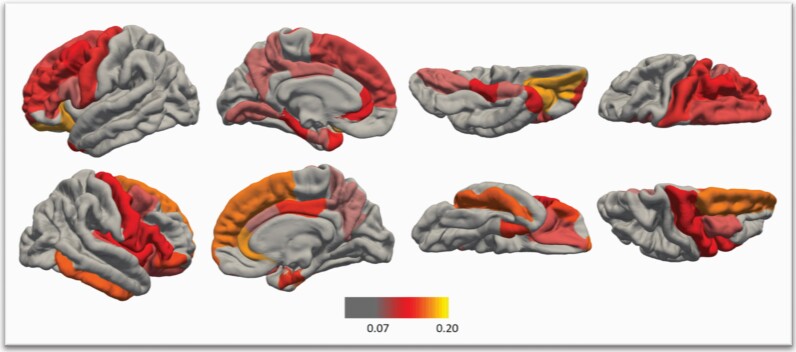
We aimed to investigate the putative associations between CMV status and all left and right regional SAs based on the DK FreeSurfer Atlas.27 The mean age of the CMV+ and the CMV− patients differed 1.8 years, and further, there were 11% more women among CMV+ patients compared to CMV− patients. Although these differences did not reach statistical significance (table 2), we considered reasonable to include both sex and age in the extended analysis. We ran 34 ANCOVAs by hemisphere investigating CMV IgG status main effect on regional SAs whilst controlling for sex and age. We applied a false discovery rate (FDR) of 5% by hemisphere to correct for multiple testing.31 CMV seropositivity was significantly inversely associated with 14 left and 16 right regional SAs (figure 2 and supplementary table S3).
Fig. 2.
Cytomegalovirus (CMV) immunoglobulin IgG seropositive (CMV+) patients with schizophrenia spectrum disorders compared to seronegative (CMV−) patients displayed significantly smaller (after false discovery rate correction of 5% by hemisphere) left (L) and right (R) surface areas whilst controlling for age and sex in the following regions: R caudal anterior cingulate, L &R caudal middle frontal, L & R entorhinal, R frontal pole, R inferior temporal, R Insula, L & R lateral orbitofrontal, L lingual, L parahippocampal, L & R pars opercularis, R pars orbitalis, L & R pars triangularis, L & R posterior cingulate, L & R precentral, L & R precuneus, L & R rostral anterior cingulate, L rostral middle frontal, L & R superior frontal, R transverse temporal and L temporal pole. Color bar represents effects sizes: the variation in SAs explained by CMV status (partial eta2 derived from the analyses of covariance).
Cortical Thickness Analysis
As shown in table 1, CMV+ participants were older than CMV− participants (P < .001), while age and education years were both inversely correlated with mean overall CT (P < .001 for both). We ran a full factorial ANCOVA searching for main and interaction effects of CMV status (seropositivity/seronegativity), diagnostic group (SCZ spectrum/HC) and sex on CT whilst controlling for age and education years (supplementary table S5). There were no three-way (P = .653) or two-way interactions (P-values for CMV-by-sex, diagnostic group-by-sex and CMV-by-diagnostic group interactions were 0.514, 0.967 and 0.621, respectively). CMV status (P = .268), sex (P = .928), diagnostic group (P = .062) and education years (P = .737) were not associated with CT; age was inversely associated with CT (P < .001).
Discussion
We found that among patients with SCZ spectrum disorders, CMV IgG seropositivity was significantly associated with smaller total cortical SA (figure 1). The effect size was large with CMV status explaining 14% of the variation in the total SA. CMV IgG seropositivity indicates previous CMV infection but does not indicate when a person was infected. As the virus is never cleared,2 CMV IgG seropositivity reflects previous infection and subsequent latency. Post-hoc analysis among patients with SCZ spectrum disorders showed that higher CMV IgG antibody concentrations were also associated with smaller total cortical SA. The biological explanation of the higher CMV antibody concentrations is not established, but they may indicate more frequent reactivations.30,32 Not only reactivation rate but also reactivation intensity and duration have been linked to elevated CMV antibody levels.32 Our results of both seropositivity and antibody concentrations being inversely associated with SA may indicate a SA vulnerability to CMV infection in SCZ especially among patients with more frequent, longer or intense reactivation events. Furthermore, there was no association between HSV1 seropositivity and the total SA indicating a CMV specificity (supplementary material). In addition, we failed to find any associations between CMV status and the mean overall CT. Finally, in the whole sample, patients had smaller SA and thinner cortex than HC, men had larger total SA than women while increasing age was associated with cortical thinning already from young adulthood and with smaller SA after the age of 35 (supplementary material).
CMV seropositivity was associated with significantly smaller regional SAs mainly in the frontal and the temporal lobe regions. Out of the 34 left and 34 right DK atlas SAs, CMV+ patients had significantly smaller SAs (pFDR < .05) in 16/34 right and 14/34 left SAs compared with CMV− patients: 10 right and 8 left frontal regions, three right and three left temporal regions, two right and two left parietal regions, one left occipital region and the right insula (figure 2 and supplementary table S3). Furthermore, CMV+ patients had nominally significantly (puncorrected < .05) smaller SAs in totally 19/34 right and 20/34 left regional SA. Interestingly, all 68 DK atlas SAs were smaller in CMV+ patients than CMV− patients (supplementary table S4). In the recent ENIGMA (Enhancing Neuro Imaging Genetics Through Meta Analysis) meta-analysis, patients with SCZ had smaller regional SAs than HC in all DK atlas regional SAs without regional specificity and the largest effect sizes in the frontal and temporal lobes (top 15 SAs were all frontal or temporal).33 The right superior frontal, left superior frontal and right precentral SA were the frontal SAs with the largest effect sizes in the ENIGMA study, and CMV seropositivity was significantly associated with all three in the present study. Concerning the temporal lobe, the SAs with the largest effect sizes in the ENIGMA were the right middle temporal, right fusiform and right inferior temporal SAs. In the present study, CMV seropositivity was significantly associated with smaller right inferior temporal SA (pFDR < .05, partial eta2 = 0.156), and nonsignificantly associated with the right middle temporal (puncorrected = .066) and the right fusiform (puncorrected = .166) SA. Interestingly, the top regional SA in the ENIGMA was the right superior frontal SA which was one of the top findings in the present study with a large effect size (partial eta2 = 0.165). Our results may indicate that CMV seropositivity contributes to the widespread smaller regional SAs in some patients with SCZ especially in the frontal lobe.
The observed association between CMV latent infection and cortical SA may be a mild equivalent of the established CMV impact on the human cortex observed in congenital CMV infections and in immunodeficiency.7,9 Congenital CMV infection is often devastating for the central nervous system, but its incidence is less than 2%,16 0.2% in Norway,34 whereas in our study, approximately 50% of the participating patients have been exposed to CMV. Thus, although we cannot determine from the present results when the primary CMV infection or its hypothesized impact on the cortical SA took place, we can conclude that for the vast majority of the CMV+ patients it occurred postnatally, and we can thereby rule out that the smaller SA in CMV+ patients is related to nonlethal congenital CMV infection. The inverse DOI-SA association also indicates that the smaller SA is distinct from congenital CMV and supports thereby a postnatal CMV involvement. Importantly, normal aging is associated with a declining brain volume which is linked to age-related reductions of both grey and white matter.35,36 The shrinkage of the cortical grey matter in healthy adults is more prominent in the frontal and parietal lobes,36 and is linked to cortical volume, cortical SA and CT reductions.37 Compared with HC patients with SCZ show larger age-related decrement in the whole brain, total and frontal grey matter, and frontal, parietal and temporal white matter38,39 with the frontal lobes being predominately affected.39 We are tempted to speculate that CMV is a key environmental factor playing a role in the excess grey matter loss in SCZ. We still cannot know if the smaller SA in CMV+ patients is a result of the primary CMV infection, the CMV reactivations and/or the chronic infection. DOI was linked to smaller SA in CMV+ but not CMV− patients suggestive of a progression of tissue loss with extended illness and CMV seropositivity.
The observed vulnerability of patients with SCZ to CMV infections may be due to genetic variations implicated in both SCZ and CMV infections. The majority of genes involved in CMV infections, including major histocompatibility complex-, interleukin- and tumor necrosis factor-related genes, has also been implicated in SCZ.40,41 Furthermore, maternal CMV infection interacts with CTNNA3, a gene encoding catenin alpha-3 which is a part of a complex that CMV during infection can disconnect disrupting thereby intercellular connections, to predict SCZ risk.42 Furthermore, CMV latent infection has a surprisingly profound impact on host immune system.43 It is mainly associated with a decrease of naïve and increase of late-differentiated T-cell subsets.44 One of the most abundant cytokines that CMV-infected cells secrete is interleukin-6 (Il-6),45,46 which in turn robustly boosts CMV reactivation rates.47 Interestingly, increased Il-6 levels have been reported in SCZ48 and in children at increased risk for psychosis.49 CMV infection may thereby have a greater impact on hosts with or at risk for psychosis.
It has been shown based on evidence from human and murine CMV that neural stem cells are the main sites of CMV latency in the brain,5,6 while neural stem cell differentiation to progenitor cells is linked to CMV reactivation.5 Neural stem and progenitor cells are present in the human subependymal zone (SEZ) in all ages indicating adult neurogenesis.50 In the SEZ of patients with SCZ, there is a decrease in neurogenesis markers with a parallel increase in inflammation markers.50 This may be consistent with a CMV infection resulting in inflammation and cell loss. An impaired neurogenesis might contribute to the observed cortical abnormalities not only found in patients with SCZ relative to HC but also in CMV+ patients with SCZ relative to CMV− patients. Interestingly, the hippocampal dentate gyrus is also a major neurogenic niche in the adult mammalian brain with neural stem and progenitor cells giving rise to new neurons.51–53 In a recent study, among male patients with SCZ spectrum disorders, CMV+ patients had smaller dentate gyrus than CMV− patients.54
Finally, even among HC, CMV+ participants had (nonsignificantly, P = .059) smaller SA than CMV− participants (figure 1). Considering our directional hypothesis that CMV seropositivity would be associated with smaller SA and dividing thereby the P-value by two (P = .03) the CMV-SA association in HC may also be considered significant. Still, the significant CMV-by-diagnosis interaction in the whole group indicates that the impact (if we assume causal relationship) of CMV on SA is significantly larger in patients possibly due to resilience in HC as a result of BBB integrity, a noninflammatory environment and an adequate host immune response.
The present study has certain limitations. First, we cannot know when the CMV primary infection and the subsequent CMV reactivations occurred. The study has a cross-sectional design, and long-term course or causal effects cannot be determined. Another limitation of the study is the relatively small number of patients (n = 67). However, the patient group was well-characterized and we could therefore take account of possible confounders including medication variables, education variables, DOI and substance use. Furthermore, the patient analysis was the follow-up analysis of the significant two-way (diagnostic group-by-CMV status) interaction from the whole sample analysis, where 329 individuals were included.
To conclude, we have shown that CMV seropositivity, reflecting previous CMV infection and current latency, is associated with smaller total cortical SA and smaller regional SAs mainly in the frontal and temporal lobe in patients with SCZ spectrum disorders. CMV may be an environmental factor with a key role in the established SA aberrations in SCZ.
Supplementary Material
Acknowledgments
The authors would like to thank the clinicians collaborating in patient recruitment, the research assistants at NORMENT and all participating patients and controls. OAA received speaker’s honorarium from Lundbeck. All other authors reported no potential conflicts of interest.
Contributor Information
Dimitrios Andreou, Norwegian Centre for Mental Disorders Research (NORMENT), Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Psychiatric Research, Diakonhjemmet Hospital, Oslo, Norway; Department of Clinical Neuroscience, Centre for Psychiatry Research, Karolinska Institutet & Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden.
Kjetil Nordbø Jørgensen, Norwegian Centre for Mental Disorders Research (NORMENT), Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Psychiatric Research, Diakonhjemmet Hospital, Oslo, Norway.
Stener Nerland, Norwegian Centre for Mental Disorders Research (NORMENT), Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Psychiatric Research, Diakonhjemmet Hospital, Oslo, Norway.
Robert H Yolken, Department of Pediatrics, Stanley Division of Developmental Neurovirology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Unn K Haukvik, Norwegian Centre for Mental Disorders Research (NORMENT), Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Norwegian Centre for Mental Disorders Research (NORMENT), Division of Mental Health and Addiction, Oslo University Hospital, Oslo, Norway; Department of Forensic Research and Education, Oslo University Hospital, Oslo, Norway.
Ole A Andreassen, Norwegian Centre for Mental Disorders Research (NORMENT), Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Norwegian Centre for Mental Disorders Research (NORMENT), Division of Mental Health and Addiction, Oslo University Hospital, Oslo, Norway.
Ingrid Agartz, Norwegian Centre for Mental Disorders Research (NORMENT), Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Psychiatric Research, Diakonhjemmet Hospital, Oslo, Norway; Department of Clinical Neuroscience, Centre for Psychiatry Research, Karolinska Institutet & Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden.
Funding
This work was supported by the Research Council of Norway (213700, 223273, 248778) and the South-Eastern Norway Regional Health Authority (2017-112, 2017-097).
References
- 1. Dupont L, Reeves MB. Cytomegalovirus latency and reactivation: recent insights into an age old problem. Rev Med Virol. 2016;26(2):75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wills MR, Poole E, Lau B, Krishna B, Sinclair JH. The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immunotherapeutic strategies? Cell Mol Immunol. 2015;12(2):128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol. 2009;21(4):440–445. [DOI] [PubMed] [Google Scholar]
- 4. Forte E, Zhang Z, Thorp EB, Hummel M. Cytomegalovirus latency and reactivation: an intricate interplay with the host immune response. Front Cell Infect Microbiol. 2020;10:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsutsui Y, Kosugi I, Kawasaki H, Arai Y, Han GP, Li L, Kaneta M. Roles of neural stem progenitor cells in cytomegalovirus infection of the brain in mouse models. Pathol Int. 2008;58(5):257–267. [DOI] [PubMed] [Google Scholar]
- 6. Belzile JP, Stark TJ, Yeo GW, Spector DH. Human cytomegalovirus infection of human embryonic stem cell-derived primitive neural stem cells is restricted at several steps but leads to the persistence of viral DNA. J Virol. 2014;88(8):4021–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diogo MC, Glatter S, Binder J, Kiss H, Prayer D. The MRI spectrum of congenital cytomegalovirus infection. Prenat Diagn. 2020;40(1):110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teissier N, Fallet-Bianco C, Delezoide AL, et al. Cytomegalovirus-induced brain malformations in fetuses. J Neuropathol Exp Neurol. 2014;73(2):143–158. [DOI] [PubMed] [Google Scholar]
- 9. Setinek U, Wondrusch E, Jellinger K, Steuer A, Drlicek M, Grisold W, Lintner F. Cytomegalovirus infection of the brain in AIDS: a clinicopathological study. Acta Neuropathol. 1995;90(5):511–515. [DOI] [PubMed] [Google Scholar]
- 10. Houston SM, Herting MM, Sowell ER. The neurobiology of childhood structural brain development: conception through adulthood. Curr Top Behav Neurosci. 2014;16:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tamnes CK, Herting MM, Goddings AL, et al. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 2017;37(12):3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, Kahn RS, Sommer IE. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7(3):e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muller N, Schwarz MJ. Immune system and schizophrenia. Curr Immunol Rev. 2010;6(3):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Najjar S, Pahlajani S, De Sanctis V, Stern JNH, Najjar A, Chong D. Neurovascular unit dysfunction and blood–brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawasaki H, Kosugi I, Meguro S, Iwashita T. Pathogenesis of developmental anomalies of the central nervous system induced by congenital cytomegalovirus infection. Pathol Int. 2017;67(2):72–82. [DOI] [PubMed] [Google Scholar]
- 17. van der Heiden M, van Zelm MC, Bartol SJW, de Rond LGH, Berbers GAM, Boots AMH, Buisman AM. Differential effects of cytomegalovirus carriage on the immune phenotype of middle-aged males and females. Sci Rep. 2016;6:26892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Benedetto S, Derhovanessian E, Steinhagen-Thiessen E, Goldeck D, Muller L, Pawelec G. Impact of age, sex and CMV-infection on peripheral T cell phenotypes: results from the Berlin BASE-II Study. Biogerontology. 2015;16(5):631–643. [DOI] [PubMed] [Google Scholar]
- 19. First MB, Spitzer RL, Gibbon M, Williams JB.. Structured clinical interview for DSM-IV axis I disorders (SCID-I), clinician version, administration booklet. American Psychiatric Association Publishing; 2012. [Google Scholar]
- 20. Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruyFV, 3rd, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 21. Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position (part 1). J Epidemiol Community Health. 2006;60(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423–432. [DOI] [PubMed] [Google Scholar]
- 23. Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res. 2005;11(1):22–31. [DOI] [PubMed] [Google Scholar]
- 24. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 25. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 28. McCarthy CS, Ramprashad A, Thompson C, Botti JA, Coman IL, Kates WR. A comparison of FreeSurfer-generated data with and without manual intervention. Front Neurosci. 2015;9:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60(5):466–472. [DOI] [PubMed] [Google Scholar]
- 30. Dickerson F, Stallings C, Origoni A, Katsafanas E, Schweinfurth LA, Savage CL, Yolken R. Association between cytomegalovirus antibody levels and cognitive functioning in non-elderly adults. PLoS One. 2014;9(5):e95510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 32. Iglesias-Escudero M, Moro-Garcia MA, Marcos-Fernandez R, et al. Levels of anti-CMV antibodies are modulated by the frequency and intensity of virus reactivations in kidney transplant patients. PLoS One. 2018;13(4):e0194789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Erp TGM, Walton E, Hibar DP, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol Psychiatry. 2018;84(9):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barlinn R, Dudman SG, Trogstad L, Gibory M, Muller F, Magnus P, Rollag H. Maternal and congenital cytomegalovirus infections in a population-based pregnancy cohort study. APMIS. 2018;126(12):899–906. [DOI] [PubMed] [Google Scholar]
- 35. Peters R. Ageing and the brain. Postgrad Med J. 2006;82(964):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bagarinao E, Watanabe H, Maesawa S, et al. Reserve and maintenance in the aging brain: a longitudinal study of healthy older adults. eNeuro. 2022;9(1):ENEURO.0455-21.2022 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33(3):617 e611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70(1):88–96. [DOI] [PubMed] [Google Scholar]
- 39. Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70(7):672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carter CJ. Schizophrenia susceptibility genes directly implicated in the life cycles of pathogens: cytomegalovirus, influenza, herpes simplex, rubella, and Toxoplasma gondii. Schizophr Bull. 2009;35(6):1163–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Torrey EF, Leweke MF, Schwarz MJ, Mueller N, Bachmann S, Schroeder J, Dickerson F, Yolken RH. Cytomegalovirus and schizophrenia. CNS Drugs. 2006;20(11):879–885. [DOI] [PubMed] [Google Scholar]
- 42. Borglum AD, Demontis D, Grove J, et al. Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol Psychiatry. 2014;19(3):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Picarda G, Benedict CA. Cytomegalovirus: shape-shifting the immune system. J Immunol. 2018;200(12):3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Heiden M, van Zelm MC, Bartol SJ, de Rond LG, Berbers GA, Boots AM, Buisman AM. Differential effects of cytomegalovirus carriage on the immune phenotype of middle-aged males and females. Sci Rep. 2016; 6:26892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dumortier J, Streblow DN, Moses AV, et al. Human cytomegalovirus secretome contains factors that induce angiogenesis and wound healing. J Virol. 2008;82(13):6524–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77(8):4588–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reeves MB, Compton T. Inhibition of inflammatory interleukin-6 activity via extracellular signal-regulated kinase-mitogen-activated protein kinase signaling antagonizes human cytomegalovirus reactivation from dendritic cells. J Virol. 2011;85(23):12750–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801–808. [DOI] [PubMed] [Google Scholar]
- 49. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71(10):1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weissleder C, North HF, Bitar M, et al. Reduced adult neurogenesis is associated with increased macrophages in the subependymal zone in schizophrenia. Mol Psychiatry. 2021;26(11):6880–6895. [DOI] [PubMed] [Google Scholar]
- 51. Drew LJ, Fusi S, Hen R. Adult neurogenesis in the mammalian hippocampus: why the dentate gyrus? Learn Mem. 2013;20(12):710–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, Avila J, Llorens-Martin M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25(4): 554–560. [DOI] [PubMed] [Google Scholar]
- 53. Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. [DOI] [PubMed] [Google Scholar]
- 54. Andreou D, Jorgensen KN, Nerland S, Engen K, Yolken RH, Andreassen OA, Agartz I. Cytomegalovirus infection associated with smaller dentate gyrus in men with severe mental illness. Brain Behav Immun. 2021;96:54–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.