Abstract
Background
Tofacitinib is an oral, small molecule JAK inhibitor for the treatment of ulcerative colitis. We evaluate baseline characteristics as predictors of sustained response and remission in patients with ulcerative colitis receiving tofacitinib maintenance therapy.
Methods
Patients with clinical response following OCTAVE Induction 1 and 2 entered OCTAVE Sustain and were rerandomized to receive tofacitinib 5 or 10 mg twice daily or placebo. Baseline characteristics were stratified by week 52 efficacy endpoints (remission, sustained remission, clinical response, sustained clinical response). Associations between baseline characteristics and efficacy endpoints were evaluated using logistic regression analyses.
Results
Overall, 170 of 487 (34.9%) patients were in remission at week 52. In multivariable modeling, endoscopic subscore at baseline of OCTAVE Induction 1 and 2 (2 vs 3; odds ratio [OR], 1.60; 95% confidence interval [CI], 1.06-2.44]), partial Mayo score (<2 vs ≥2; OR, 1.92; 95% CI, 1.27-2.90), and age (per 10-years; OR, 1.19; 95% CI, 1.02-1.39) at baseline of OCTAVE Sustain (following 8 weeks’ tofacitinib induction therapy) were associated with higher odds of remission at week 52. Oral corticosteroid use (OR, 0.63; 95% CI, 0.42-0.96) and C-reactive protein (per unit; OR, 0.94; 95% CI, 0.89-0.99) at baseline of OCTAVE Sustain were associated with reduced likelihood of remission at week 52. In general, opposite associations were observed for time to loss of response.
Conclusion
Patients with greater clinical improvement after 8 weeks of tofacitinib induction therapy are more likely to maintain response or remission with tofacitinib regardless of dose received during maintenance, highlighting the importance of a robust response to induction therapy.
Keywords: predictors of response, tofacitinib, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic disease of the colon, characterized by relapsing and remitting inflammation.1 Ulcerative colitis is a heterogenous disease in terms of severity, disease course, and response to therapy.2 Secondary loss of response can often occur with UC therapies, with randomized clinical trials of ustekinumab, vedolizumab, tofacitinib, and infliximab reporting loss of response rates of 30%-55% after maintenance therapy ranging from 44-54 weeks.3–6 Therefore, multiple treatments with different mechanisms of action are required.
In order to better characterize patient populations, optimize treatment, and minimize adverse events (AEs), there is a need to identify factors that are predictive of maintaining response to therapy.2 Finding a way to predict response to therapy will help clinicians better assess patients who are at risk of poor outcomes and who are most likely to benefit from specific therapies.
Prediction of response to UC therapy is complex and is influenced by multiple factors, including the environment and microbiome. Molecular studies have shown some evidence on predictors of response to vedolizumab, but these have been carried out in small numbers.7 Although there have been studies into predictors of primary response to induction therapy, there have been limited investigations into predictors of sustained response during maintenance therapy. Previous studies of sustained remission have mainly focused on tumor necrosis factor inhibitor (TNFi) therapies or been conducted in pediatric cohorts.8,9
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of UC. The efficacy and safety of tofacitinib have been evaluated in patients with UC in three phase 3 studies (2 identical 8-week induction studies [OCTAVE Induction 1 and 2] and a 52-week maintenance study [OCTAVE Sustain]).5
Here, we report on baseline demographics and clinical characteristics (from the baseline of either induction or maintenance) as predictors of sustained response and remission in patients with moderately to severely active UC receiving tofacitinib maintenance therapy.
Materials and Methods
Patients and Study Design
The OCTAVE Induction 1 and 2 studies (NCT01465763 and NCT01458951) were 2 identical, 8-week studies, in which patients with moderately to severely active UC were randomized to receive tofacitinib 10 mg twice daily (BID) or placebo. Patients who completed OCTAVE Induction 1 and 2 and achieved clinical response (defined as a decrease from induction study baseline total Mayo score of ≥3 points and ≥30%, plus a decrease in rectal bleeding subscore of ≥1 point or an absolute rectal bleeding subscore of 0 or 1) with either tofacitinib or placebo were eligible to enter the 52-week maintenance study, OCTAVE Sustain (NCT01458574), where they were rerandomized to receive tofacitinib 10 mg BID, tofacitinib 5 mg BID, or placebo. Details of concomitant medications are provided in the Supplementary Material. Full details of OCTAVE Induction 1 and 2 and OCTAVE Sustain have been reported previously.5
Efficacy Assessments
Efficacy endpoints were based on the Mayo score that includes measurements of stool frequency, rectal bleeding, endoscopic subscore, and Physician Global Assessment (PGA; each with a range of 0 to 3). Potential predictors were assessed based on the following efficacy endpoints in OCTAVE Sustain: remission at week 52, sustained remission at weeks 24 and 52, clinical response at week 52, sustained clinical response at weeks 24 and 52, and time to loss of response. Remission was defined as a total Mayo score of ≤2 with no individual subscore >1, and a rectal bleeding subscore of 0. Sustained remission was defined as remission at weeks 24 and 52 of OCTAVE Sustain. Sustained clinical response was defined as clinical response at weeks 24 and 52 of OCTAVE Sustain. Loss of response was defined by an increase in partial Mayo score (PMS) of ≥2 points from baseline in OCTAVE Sustain for 2 consecutive visits (at least 2 weeks apart), with an increase in rectal bleeding subscore of ≥1 from baseline of OCTAVE Sustain.
Safety Assessments
Safety outcomes and laboratory value changes were assessed in all patients with nonmissing efficacy responses in OCTAVE Sustain. The incidence and severity of AEs, classified by the Medical Dictionary for Regulatory Activities, including AEs of special interest, were reported. Gastrointestinal perforations, malignancies (excluding nonmelanoma skin cancer [NMSC]), NMSC, and major adverse cardiovascular events (MACE) were independently adjudicated. Safety outcomes and laboratory value changes were stratified by tofacitinib dose received during OCTAVE Sustain and remission status at week 52.
Statistical Analyses
Observed data were analyzed using the full analysis set, including all randomized patients who completed OCTAVE Induction 1 and 2, achieved clinical response, and were subsequently assigned to tofacitinib 10 mg BID, tofacitinib 5 mg BID, or placebo in OCTAVE Sustain. Baseline demographics and clinical characteristics were analyzed descriptively based on the achievement of efficacy endpoints at week 52 or at both weeks 24 and 52, and summarized by treatment group. The following characteristics were assessed at baseline of OCTAVE Induction 1 and 2: C-reactive protein (CRP; mg/L), albumin (g/dL), total Mayo score, extraintestinal manifestations (EIMs), prior TNFi exposure, and prior TNFi failure. The following characteristics were assessed at baseline of OCTAVE Sustain (following 8 weeks of tofacitinib induction therapy): age, gender, CRP (mg/L), total Mayo score, PMS, EIMs, and oral corticosteroid use.
Univariate regression analyses were performed to evaluate if any baseline demographics or clinical characteristics at baseline of OCTAVE Sustain and/or OCTAVE Induction 1 and 2 were associated with remission at week 52 or time to loss of response during OCTAVE Sustain. The full list of evaluated factors is provided in the Supplementary Material.
To assess the effect of various factors on the likelihood of achieving remission at week 52, multivariable logistic regression modeling was performed using a stepwise selection procedure using an entry criterion P value of .15 and a stay criterion P value of .05. To assess the effect of various factors on time to loss of response endpoint, a time to event analysis was carried out using multivariable Cox proportional hazards regression modeling. A stepwise selection process was implemented, with an entry criterion P value of .15 and a stay criterion P value of .05.
Based on the final model, odds ratios (ORs) and 95% confidence intervals (CIs) from the multivariable logistic regression analysis were reported for factors associated with remission at week 52 of OCTAVE Sustain; and hazard ratios (HRs; 95% CI) from the multivariable Cox proportional hazards regression analysis were reported for factors associated with time to loss of response during OCTAVE Sustain.
These analyses were post hoc for exploratory purposes only, and no multiplicity adjustment was performed. For the univariate and multivariable analyses, nominal P values of ≤0.05 were considered significant.
Ethical Considerations
All studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines and were approved by the Institutional Review Boards and/or Independent Ethics Committees at each of the investigational centers participating in the studies, or a central Institutional Review Board. All patients provided written informed consent.
Results
Patients
The OCTAVE Sustain study included 593 patients who had initially received placebo, tofacitinib 10 mg BID, or tofacitinib 15 mg BID (a dose that was subsequently discontinued following a protocol amendment) induction therapy and had clinical response after 8 weeks before being rerandomized to receive placebo, tofacitinib 5 mg BID, or tofacitinib 10 mg BID.
Of the patients with available data for the respective efficacy outcomes, 34.9% of patients were in remission at week 52; 20.6% of patients achieved sustained remission at weeks 24 and 52; 54.2% of patients had a clinical response at week 52; and 51.5% of patients achieved sustained clinical response at weeks 24 and 52. The majority of patients who achieved these efficacy outcomes received tofacitinib 5 or 10 mg BID. Baseline demographics and disease characteristics were generally similar between treatment groups within respective efficacy outcomes (Tables 1-4).
Table 1.
Baseline demographics and disease characteristics by week 52 remission status and treatment group.
| Tofacitinib 5 mg BID | Tofacitinib 10 mg BID | Placebo | ||||
|---|---|---|---|---|---|---|
| Remission at week 52 | Yes N = 68 | No N = 101 | Yes N = 80 | No N = 83 | Yes N = 22 | No N = 133 |
| Age (years), mean (SD) a | 43.5 (14.9) | 40.5 (12.5) | 45.5 (15.0) | 40.4 (13.0) | 43.4 (12.5) | 44.0 (14.0) |
| Age (<65 years), n (%)a | 63 (92.6) | 96 (95.0) | 72 (90.0) | 79 (95.2) | 21 (95.5) | 120 (90.2) |
| Male, n (%)a | 40 (58.8) | 49 (48.5) | 40 (50.0) | 54 (65.1) | 11 (50.0) | 84 (63.2) |
| CRP (mg/L) at baseline of OCTAVE Induction 1 and 2, mean (SD)b | 7.6 (12.6)c | 10.1 (19.4) | 8.6 (13.9)d | 8.5 (16.8) | 10.4 (20.5) | 10.5 (14.6) |
| CRP (mg/L) at baseline of OCTAVE Sustain, mean (SD)a | 1.5 (2.0) | 2.1 (3.5) | 1.8 (3.8) | 4.2 (7.3) | 2.7 (3.4) | 2.8 (5.0) |
| Albumin (g/dL), mean (SD)b | 4.3 (0.4) | 4.1 (0.4) | 4.2 (0.5) | 4.2 (0.4) | 4.3 (0.3) | 4.2 (0.4) |
| Total Mayo score, mean (SD)b | 8.6 (1.5) | 8.8 (1.4) | 8.6 (1.5) | 9.0 (1.5) | 8.9 (1.3) | 8.8 (1.3) |
| Total Mayo score <3, n (%)a | 34 (50.0) | 30 (29.7) | 35 (43.8) | 20 (24.1) | 9 (40.9) | 54 (40.6) |
| PMS <2, n (%)a | 41 (60.3) | 39 (38.6) | 48 (60.0) | 30 (36.1) | 10 (45.5) | 58 (43.6) |
| EIMs at baseline of OCTAVE Induction 1 and 2, n (%)b | 13 (19.1) | 31 (30.7) | 19 (23.8) | 19 (23.5)e | 5 (22.7) | 43 (32.3) |
| EIMs at baseline of OCTAVE Sustain, n (%)a | 3 (4.4) | 10 (9.9) | 5 (6.3) | 4 (4.9)f | 1 (4.5) | 18 (13.5) |
| Prior TNFi exposure, n (%)b | 24 (35.3) | 50 (49.5) | 37 (46.3) | 46 (55.4) | 11 (50.0) | 58 (43.6) |
| Prior TNFi failure, n (%)b | 20 (29.4) | 47 (46.5) | 34 (42.5) | 44 (53.0) | 10 (45.5) | 56 (42.1) |
| Oral corticosteroid use, n (%)a | 29 (42.6) | 60 (59.4) | 25 (31.3) | 39 (47.0) | 11 (50.0) | 60 (45.1) |
Remission was defined as a total Mayo score of ≤2 with no individual subscore >1, and a rectal bleeding subscore of 0. Abbreviations: BID, twice daily; CRP, C-reactive protein; EIM, extraintestinal manifestation; N, number of patients in the specified category with nonmissing data; n, number of patients with the specified characteristic within the given category; PMS, partial Mayo score; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
Data at baseline of OCTAVE Sustain (following 8 weeks’ tofacitinib induction therapy).
Data at baseline of OCTAVE Induction 1 and 2.
N = 67.
N = 78.
N = 81.
N = 82.
Table 2.
Baseline demographics and disease characteristics by sustained remission status and treatment group.
| Tofacitinib 5 mg BID | Tofacitinib 10 mg BID | Placebo | ||||
|---|---|---|---|---|---|---|
| Sustained remission at weeks 24 and 52 | Yes N = 44 | No N = 130 | Yes N = 50 | No N = 119 | Yes N = 10 | No N = 153 |
| Age (years), mean (SD)a | 41.9 (15.8) | 41.9 (12.6) | 44.2 (14.4) | 42.3 (14.3) | 38.0 (8.9) | 44.3 (14.0) |
| Age (<65 years), n (%)a | 40 (90.9) | 124 (95.4) | 46 (92.0) | 110 (92.4) | 10 (100.0) | 138 (90.2) |
| Male, n (%)a | 24 (54.5) | 69 (53.1) | 30 (60.0) | 69 (58.0) | 3 (30.0) | 96 (62.7) |
| CRP (mg/L) at baseline of OCTAVE Induction 1 and 2, mean (SD)b | 7.5 (13.0) | 9.4 (17.9)c | 7.7 (12.7) | 9.5 (16.8)d | 6.2 (8.1) | 10.6 (15.6) |
| CRP (mg/L) at baseline of OCTAVE Sustain, mean (SD)a | 1.7 (2.4) | 2.0 (3.2) | 1.4 (2.5) | 3.7 (6.8) | 3.2 (3.4) | 3.1 (6.0) |
| Albumin (g/dL), mean (SD)b | 4.3 (0.4) | 4.2 (0.4) | 4.2 (0.5) | 4.2 (0.4) | 4.3 (0.3) | 4.2 (0.4) |
| Total Mayo score, mean (SD)b | 8.6 (1.6) | 8.7 (1.3) | 8.5 (1.6) | 9.0 (1.4) | 8.9 (1.4) | 8.9 (1.3) |
| Total Mayo score of <3, n (%)a | 28 (63.6) | 35 (26.9) | 28 (56.0) | 27 (22.7) | 5 (50.0) | 59 (38.6) |
| PMS <2, n (%)a | 31 (70.5) | 48 (36.9) | 36 (72.0) | 42 (35.3) | 5 (50.0) | 65 (42.5) |
| EIMs at baseline of OCTAVE Induction 1 and 2, n (%)c | 9 (20.5) | 37 (28.5) | 10 (20.0) | 30 (25.6)e | 1 (10.0) | 50 (32.7) |
| EIMs at baseline of OCTAVE Sustain, n (%)a | 3 (6.8) | 11 (8.5) | 2 (4.0) | 7 (5.9)f | 0 (0.0) | 20 (13.1) |
| Prior TNFi exposure, n (%)b | 18 (40.9) | 59 (45.4) | 18 (36.0) | 68 (57.1) | 6 (60.0) | 65 (42.5) |
| Prior TNFi failure, n (%)b | 15 (34.1) | 55 (42.3) | 16 (32.0) | 64 (53.8) | 5 (50.0) | 63 (41.2) |
| Oral corticosteroid use, n (%)a | 16 (36.4) | 73 (56.2) | 14 (28.0) | 54 (45.4) | 5 (50.0) | 70 (45.8) |
Sustained remission was defined as remission at weeks 24 and 52 of OCTAVE Sustain. Remission was defined as a total Mayo score of ≤2 with no individual subscore >1, and a rectal bleeding subscore of 0. Abbreviations: BID, twice daily; CRP, C-reactive protein; EIM, extraintestinal manifestation; N, number of patients in the specified category with nonmissing data; n, number of patients with the specified characteristic within the given category; PMS, partial Mayo score; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
Data at baseline of OCTAVE Sustain (following 8 weeks’ tofacitinib induction therapy).
Data at baseline of OCTAVE Induction 1 and 2.
N = 129.
N = 114.
N = 117.
N = 118.
Table 3.
Baseline demographics and disease characteristics by week 52 clinical response status and treatment group.
| Tofacitinib 5 mg BID | Tofacitinib 10 mg BID | Placebo | ||||
|---|---|---|---|---|---|---|
| Clinical response at week 52 | Yes N = 102 | No N = 67 | Yes N = 122 | No N = 41 | Yes N = 40 | No N = 115 |
| Age (years), mean (SD)a | 42.8 (14.2) | 40.0 (12.4) | 44.8 (14.3) | 37.1 (12.4) | 44.3 (12.8) | 43.7 (14.2) |
| Age (<65 years), n (%)a | 95 (93.1) | 64 (95.5) | 111 (91.0) | 40 (97.6) | 37 (92.5) | 104 (90.4) |
| Male, n (%)a | 57 (55.9) | 32 (47.8) | 67 (54.9) | 27 (65.9) | 23 (57.5) | 72 (62.6) |
| CRP (mg/L) at baseline of OCTAVE Induction 1 and 2, mean (SD)b | 9.4 (19.0)c | 8.6 (13.6) | 8.6 (16.0)d | 8.2 (13.4)e | 10.9 (19.0) | 10.3 (14.2) |
| CRP (mg/L) at baseline of OCTAVE Sustain, mean (SD)a | 1.8 (2.6) | 1.9 (3.6) | 2.6 (5.5) | 4.2 (7.3) | 2.4 (3.1) | 2.9 (5.3) |
| Albumin (g/dL), mean (SD)b | 4.2 (0.4) | 4.1 (0.4) | 4.2 (0.4) | 4.1 (0.3) | 4.2 (0.4) | 4.2 (0.4) |
| Total Mayo score, mean (SD)b | 8.7 (1.4) | 8.8 (1.4) | 8.7 (1.5) | 9.1 (1.5) | 9.0 (1.3) | 8.8 (1.3) |
| Total Mayo score of <3, n (%)a | 44 (43.1) | 20 (29.9) | 44 (36.1) | 11 (26.8) | 14 (35.0) | 49 (42.6) |
| PMS <2, n (%)a | 55 (53.9) | 25 (37.3) | 64 (52.5) | 14 (34.1) | 19 (47.5) | 49 (42.6) |
| EIMs at baseline of OCTAVE Induction 1 and 2, n (%)b | 24 (23.5) | 20 (29.9) | 30 (24.6) | 8 (20.5)f | 9 (22.5) | 39 (33.9) |
| EIMs at baseline of OCTAVE Sustain, n (%)a | 6 (5.9) | 7 (10.4) | 7 (5.7) | 2 (5.0)g | 3 (7.5) | 16 (13.9) |
| Prior TNFi exposure, n (%)b | 42 (41.2) | 32 (47.8) | 58 (47.5) | 25 (61.0) | 14 (35.0) | 55 (47.8) |
| Prior TNFi failure, n (%)b | 37 (36.3) | 30 (44.8) | 55 (45.1) | 23 (56.1) | 13 (32.5) | 53 (46.1) |
| Oral corticosteroid use, n (%)a | 42 (41.2) | 47 (70.1) | 39 (32.0) | 25 (61.0) | 18 (45.0) | 53 (46.1) |
Clinical response was defined as a decrease from induction study baseline total Mayo score of ≥3 points and ≥30%, plus a decrease in rectal bleeding subscore of ≥1 point or an absolute rectal bleeding subscore of 0 or 1. Abbreviations: BID, twice daily; CRP, C-reactive protein; EIM, extraintestinal manifestation; N, number of patients in the specified category with nonmissing data; n, number of patients with the specified characteristic within the given category; PMS, partial Mayo score; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
Data at baseline of OCTAVE Sustain (following 8 weeks’ tofacitinib induction therapy).
Data at baseline of OCTAVE Induction 1 and 2.
N = 101.
N = 119.
N = 38.
N = 39.
N = 40.
Table 4.
Baseline demographics and disease characteristics by sustained clinical response status and treatment group.
| Tofacitinib 5 mg BID | Tofacitinib 10 mg BID | Placebo | ||||
|---|---|---|---|---|---|---|
| Sustained clinical response at weeks 24 and 52 | Yes N = 97 | No N = 72 | Yes N = 117 | No N = 45 | Yes N = 38 | No N = 120 |
| Age (years), mean (SD)a | 42.9 (14.2) | 40.0 (12.5) | 44.8 (14.4) | 37.3 (12.5) | 44.3 (13.1) | 43.9 (14.2) |
| Age (<65 years), n (%)a | 90 (92.8) | 69 (95.8) | 106 (90.6) | 44 (97.8) | 35 (92.1) | 108 (90.0) |
| Male, n (%)a | 53 (54.6) | 36 (50.0) | 65 (55.6) | 29 (64.4) | 22 (57.9) | 74 (61.7) |
| CRP (mg/L) at baseline of OCTAVE Induction 1 and 2, mean (SD)b | 9.2 (19.3)c | 8.9 (13.5) | 8.8 (16.2)d | 8.1 (12.9)e | 11.3 (19.4) | 10.0 (14.0) |
| CRP (mg/L) at baseline of OCTAVE Sustain, mean (SD)a | 1.8 (2.6) | 2.1 (3.5) | 2.6 (5.6) | 4.0 (7.0) | 2.5 (3.2) | 3.2 (6.5) |
| Albumin (g/dL), mean (SD)b | 4.3 (0.4) | 4.1 (0.4) | 4.2 (0.4) | 4.2 (0.3) | 4.2 (0.4) | 4.2 (0.4) |
| Total Mayo score, mean (SD)b | 8.6 (1.4) | 8.8 (1.4) | 8.8 (1.5) | 9.0 (1.5) | 9.0 (1.3) | 8.8 (1.3) |
| Total Mayo score of <3, n (%)a | 43 (44.3) | 20 (27.8) | 43 (36.8) | 11 (24.4) | 12 (31.6) | 50 (41.7) |
| PMS <2, n (%)a | 54 (55.7) | 25 (34.7) | 61 (52.1) | 15 (33.3) | 17 (44.7) | 51 (42.5) |
| EIMs at baseline of OCTAVE Induction 1 and 2, n (%)b | 22 (22.7) | 23 (31.9) | 27 (23.1) | 9 (20.9)f | 8 (21.1) | 42 (35.0) |
| EIMs at baseline of OCTAVE Sustain, n (%)a | 4 (4.1) | 9 (12.5) | 6 (5.1) | 3 (6.8)g | 3 (7.9) | 17 (14.2) |
| Prior TNFi exposure, n (%)b | 40 (41.2) | 34 (47.2) | 57 (48.7) | 27 (60.0) | 13 (34.2) | 57 (47.5) |
| Prior TNFi failure, n (%)b | 35 (36.1) | 32 (44.4) | 54 (46.2) | 25 (55.6) | 12 (31.6) | 55 (45.8) |
| Oral corticosteroid use, n (%)a | 40 (41.2) | 48 (66.7) | 37 (31.6) | 27 (60.0) | 16 (42.1) | 56 (46.7) |
Sustained clinical response was defined as clinical response at weeks 24 and 52 of OCTAVE Sustain. Clinical response was defined as a decrease from induction study baseline total Mayo score of ≥3 points and ≥30%, plus a decrease in rectal bleeding subscore of ≥1 point or an absolute rectal bleeding subscore of 0 or 1. Abbreviations: BID, twice daily; CRP, C-reactive protein; EIM, extraintestinal manifestation; N, number of patients in the specified category with nonmissing data; n, number of patients with the specified characteristic within the given category; PMS, partial Mayo score; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
Data at baseline of OCTAVE Sustain (following 8 weeks’ tofacitinib induction therapy).
Data at baseline of OCTAVE Induction 1 and 2.
N = 96.
N = 115.
N = 42.
N = 43.
N = 44.
Efficacy
Clinical characteristics at baseline of OCTAVE Induction 1 and 2 that may predict efficacy outcomes with maintenance therapy
In the group receiving tofacitinib 5 mg BID, prior TNFi failure was observed in numerically lower proportions of patients who achieved remission (29.4%), sustained remission (34.1%), clinical response (36.3%), or sustained clinical response (36.1%) at week 52 compared with those who failed to achieve these endpoints (46.5%, 42.3%, 44.8%, and 44.4% of patients, respectively; Tables 1-4). Similar trends were observed in patients who received tofacitinib 10 mg BID (Tables 1-4).
Clinical characteristics at baseline of OCTAVE Sustain that may predict efficacy outcomes with maintenance therapy
In the group receiving tofacitinib 5 mg BID, PMS <2 at baseline of OCTAVE Sustain (following 8 weeks of tofacitinib induction therapy) was observed in numerically higher proportions of patients who achieved remission (60.3%), sustained remission (70.5%), clinical response (53.9%), or sustained clinical response (55.7%) at week 52 compared with those who did not achieve these endpoints (38.6%, 36.9%, 37.3%, and 34.7% of patients, respectively; Tables 1-4).
Similarly, total Mayo score of <3 at baseline of OCTAVE Sustain was observed in numerically higher proportions of patients in the group receiving tofacitinib 5 mg BID who achieved remission (50.0%), sustained remission (63.6%), clinical response (43.1%), or sustained clinical response (44.3%) at week 52 compared with those who did not achieve these endpoints (29.7%, 26.9%, 29.9%, and 27.8% of patients, respectively; Tables 1-4).
In the group receiving tofacitinib 5 mg BID, oral corticosteroid use at baseline of OCTAVE Sustain was observed in numerically lower proportions of patients who achieved remission (42.6%), sustained remission (36.4%), clinical response (41.2%), or sustained clinical response (41.2%) at week 52 compared with those who failed to achieve these endpoints (59.4%, 56.2%, 70.1%, and 66.7% of patients, respectively; Tables 1-4).
Similar trends were observed in patients who received tofacitinib 10 mg BID (Tables 1-4). Clinical characteristics among patients who received placebo during OCTAVE Sustain are shown in Tables 1-4.
Association between baseline factors and achievement of remission at week 52
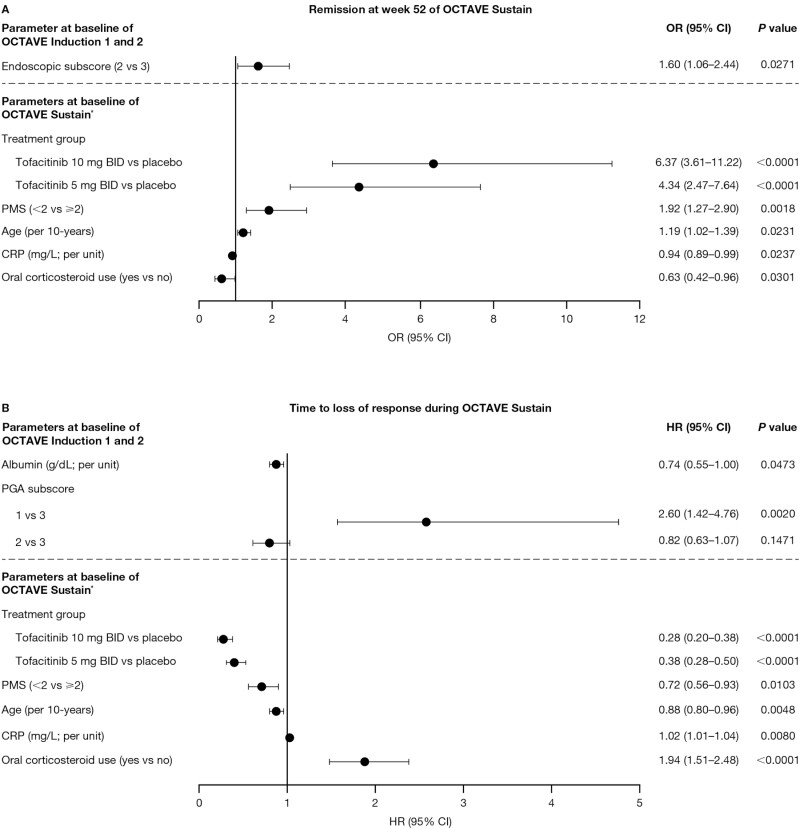
Univariate logistic regression analysis for factors associated with remission at week 52 are shown in Supplementary Tables 1-3. In the multivariable model, lower endoscopic subscore at baseline of OCTAVE Induction 1 and 2 was associated with higher odds of remission at week 52 (2 vs 3; OR, 1.60; 95% CI, 1.06-2.44; Figure 1A). Both tofacitinib 10 mg BID vs placebo and tofacitinib 5 mg BID vs placebo received during OCTAVE Sustain were significantly associated with higher odds of remission at week 52, with a higher likelihood of patients achieving remission in the group receiving 10 mg BID vs placebo compared with patients in the group receiving 5 mg BID vs placebo (OR, 6.37; 95% CI, 3.61-11.22 and OR, 4.34; 95% CI, 2.47-7.64, respectively; Figure 1A). Lower PMS (<2 vs ≥2; OR, 1.92; 95% CI, 1.27-2.90) and older age (continuous; per 10-years; OR, 1.19; 95% CI, 1.02-1.39) at baseline of OCTAVE Sustain were also associated with higher odds of remission at week 52 (Figure 1A).
Figure 1.
Multivariable analysis for factors associated with (A) remission at week 52 of OCTAVE Sustain and (B) time to loss of response during OCTAVE sustain. Stepwise selection (stay criterion at 0.05) was used to analyze the significant factors (P < .10) from the univariate analysis associated with (A) remission at week 52 of OCTAVE Sustain and (B) time to loss of response during OCTAVE Sustain. ∗Following 8 weeks’ tofacitinib induction therapy. Abbreviations: BID, twice daily; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; OR, odds ratio; PGA, Physician Global Assessment; PMS, partial Mayo score.
Conversely, factors associated with a reduced likelihood of remission at week 52 were oral corticosteroid use (OR, 0.63; 95% CI, 0.42-0.96) and higher CRP (per unit; OR, 0.94; 95% CI, 0.89-0.99) at baseline of OCTAVE Sustain (Figure 1A).
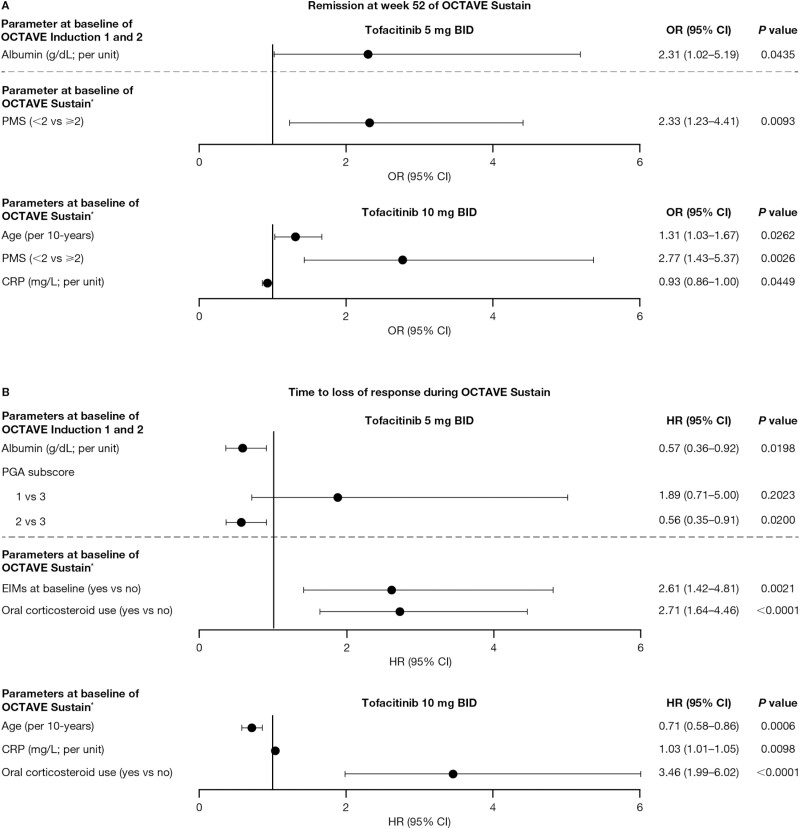
When analyzed by dose group, higher albumin (continuous; per unit) at baseline of OCTAVE Induction 1 and 2 was associated with higher odds of remission at week 52 among patients receiving 5 mg of tofacitinib BID—but not 10 mg of tofacitinib BID. Older age (continuous; per 10-years) was associated with higher odds, and higher CRP (continuous; per unit) at baseline of OCTAVE Sustain was associated with lower odds of remission at week 52 among patients receiving tofacitinib 10 mg BID, but not tofacitinib 5 mg BID (Figure 2A). Lower PMS (<2 vs ≥2) at baseline of OCTAVE Sustain was associated with higher odds of remission at week 52 among patients receiving tofacitinib 5 mg BID and tofacitinib 10 mg BID (Figure 2A).
Figure 2.
Multivariable analysis by tofacitinib dose group for factors associated with (A) remission at week 52 of OCTAVE Sustain and (B) time to loss of response during OCTAVE Sustain. Stepwise selection (stay criterion at 0.05) was used to analyze the significant factors (P < .10) from the univariate analysis associated with (A) remission at week 52 of OCTAVE Sustain and (B) time to loss of response during OCTAVE Sustain. ∗Following 8 weeks’ tofacitinib induction therapy. Abbreviations: BID, twice daily; CI, confidence interval; CRP, C-reactive protein; EIM, extraintestinal manifestation; HR, hazard ratio; OR, odds ratio; PGA, Physician Global Assessment; PMS, partial Mayo score.
A total of 462 (94.9%) patients had nonmissing data for all the factors included in the analysis of remission at week 52. Of these, 161 (34.8%) patients achieved remission at week 52, and 301 (65.2%) did not achieve remission.
Association between baseline factors and loss of response during OCTAVE Sustain
Univariate Cox proportional hazards regression analysis showing factors associated with time to loss of response during OCTAVE Sustain are shown in Supplementary Tables 4-6.
In the multivariable model, lower PGA subscore at baseline of OCTAVE Induction 1 and 2 was associated with an increased risk of loss of response during OCTAVE Sustain (1 vs 3; HR, 2.60; 95% CI, 1.42-4.76; Figure 1B).
Factors at baseline of OCTAVE Sustain that were significantly associated with increased risk of loss of response during OCTAVE Sustain were oral corticosteroid use (HR, 1.94; 95% CI, 1.51-2.48) and higher CRP (continuous; per unit; HR, 1.02; 95% CI, 1.01-1.04).
Conversely, factors associated with a reduced risk of loss of response were higher albumin at baseline of OCTAVE Induction 1 and 2 (continuous; per unit; HR, 0.74; 95% CI, 0.55-1.00), treatment with tofacitinib 5 or 10 mg BID maintenance therapy (vs placebo; HR, 0.38; 95% CI, 0.28-0.50 and HR, 0.28; 95% CI, 0.20-0.38, respectively), older age (continuous; per 10-years; HR, 0.88; 95% CI, 0.80-0.96), and lower PMS at baseline of OCTAVE Sustain (<2 vs ≥2; HR, 0.72; 95% CI, 0.56-0.93; Figure 1B).
When analyzed by dose group, higher albumin (continuous; per unit) and lower PGA subscore (2 vs 3) at baseline of OCTAVE Induction 1 and 2 were associated with a reduced risk, and the presence of EIMs at baseline of OCTAVE Sustain was associated with an increased risk of loss of response among patients receiving tofacitinib 5 mg BID, but not tofacitinib 10 mg BID. Older age (continuous; per 10-years) at baseline of OCTAVE Sustain was associated with a reduced risk, and higher CRP (continuous; per unit) at baseline of OCTAVE Sustain was associated with an increased risk of loss of response among patients receiving tofacitinib 10 mg BID, but not tofacitinib 5 mg BID (Figure 2B). Oral corticosteroid use at baseline of OCTAVE Sustain was associated with an increased risk of loss of response among patients receiving tofacitinib 5 mg BID and tofacitinib 10 mg BID.
Safety
A summary of safety, AEs of special interest, and laboratory parameters stratified by remission status at week 52 and treatment group is presented in Supplementary Table 7.
The proportion of patients with AEs was generally similar, regardless of remission status (Supplementary Table 7). A numerically lower proportion of patients with remission discontinued due to AEs compared with patients without remission, regardless of treatment group (Supplementary Table 7).
Among patients receiving tofacitinib 10 mg BID or placebo, a numerically higher proportion of patients with remission at week 52 had infection AEs compared with patients without remission (Supplementary Table 7). This was not observed in patients receiving tofacitinib 5 mg BID, where a comparable number of infection AEs were observed between those with remission at week 52 and those without remission. No serious infections, gastrointestinal perforations, or malignancies (excluding NMSC) occurred in any treatment group in OCTAVE Sustain (among patients with nonmissing efficacy responses). There were 2 cases of NMSC in the group of patients receiving tofacitinib 10 mg BID (1 of which occurred in a patient with remission at week 52) and 1 case in the placebo group in a patient without remission at week 52. There was 1 case of MACE in the group receiving tofacitinib 5 mg BID; the patient achieved remission at week 52. There was 1 case each of pulmonary embolism and deep vein thrombosis, both occurring in the placebo group in patients without remission at week 52. The proportions of patients with herpes zoster (nonserious and serious) were similar between patients, regardless of remission status, across treatment groups (Supplementary Table 7).
Among patients receiving tofacitinib 5 mg BID or tofacitinib 10 mg BID, a generally numerically higher proportion of patients with remission at week 52 had total cholesterol >1.3× upper limit of normal (ULN), triglycerides >1.3× ULN, low-density lipoprotein >1.2× ULN, and creatine kinase >2× ULN compared with patients without remission (Supplementary Table 7). The proportion of patients with high-density lipoprotein <0.8× lower limit of normal was low across treatment groups, regardless of remission status (Supplementary Table 7). Laboratory parameters were generally similar among patients receiving placebo, regardless of remission status (Supplementary Table 7).
Discussion
This report presents post hoc analyses evaluating baseline demographics and clinical characteristics as potential predictors of achieving efficacy outcomes following 52 weeks of tofacitinib maintenance therapy. A deeper response to tofacitinib induction therapy and no oral corticosteroid use at baseline of OCTAVE Sustain, following 8 weeks of tofacitinib induction therapy, were key characteristics associated with remission and maintaining clinical response at week 52 compared with more severe disease and oral corticosteroid use. Partial Mayo score <2 at baseline of OCTAVE Sustain was significantly associated with an increased likelihood of achieving remission and a reduced risk of loss of response during OCTAVE Sustain vs PMS ≥2. Lower endoscopic subscore (moderate vs severe disease) at baseline of OCTAVE Induction 1 and 2 was also associated with remission at week 52. Higher albumin at baseline of OCTAVE Induction 1 and 2 was associated with reduced risk of loss of response during OCTAVE Sustain. Conversely, higher CRP and corticosteroid use at baseline of OCTAVE Sustain were significantly associated with an increased risk of loss of response and a reduced likelihood of remission at week 52. Furthermore, lower PGA subscore (1 vs 3) at baseline of OCTAVE Induction 1 and 2 was associated with loss of response during OCTAVE Sustain. Physician Global Assessment subscore is a subjective measure of efficacy and a recognized Mayo score limitation.10
When analyzed by dose group, higher albumin at baseline of OCTAVE Induction 1 and 2 was associated with an increased likelihood of achieving remission and a reduced risk of loss of response among patients receiving tofacitinib 5 mg BID, but not tofacitinib 10 mg BID. The albumin level at baseline of OCTAVE Induction 1 and 2 was relatively high across treatment groups, being at the upper end of the normal range for the general population. A previous analysis of data from the tofacitinib UC clinical program showed that tofacitinib clearance was not related to albumin concentration, and after accounting for the significant effect of baseline Mayo score in the multivariable analysis, baseline albumin had no effect on efficacy endpoints in OCTAVE Induction 1 and 2 and OCTAVE Sustain. Therefore, baseline albumin may not be useful in determining optimal tofacitinib dose in patients with UC, beyond its association with disease severity as determined by the total Mayo score.11 The presence of EIMs at baseline of OCTAVE Sustain was associated with increased risk of loss of response among patients receiving tofacitinib 5 mg BID, but not tofacitinib 10 mg BID. Although sample sizes were small, when analyzed by dose group, tofacitinib 10 mg BID may be more efficacious in maintaining response in more complex UC (eg, UC with EIMs) compared with tofacitinib 5 mg BID. This is supported by recent findings showing that, in patients with EIMs at baseline, the proportion of patients achieving remission at week 52 of OCTAVE Sustain was higher among patients receiving tofacitinib 10 mg BID vs 5 mg BID (41.3% vs 24.1%, respectively).12 Higher CRP at baseline of OCTAVE Sustain was associated with a reduced likelihood of achieving remission and increased risk of loss of response among patients receiving tofacitinib 10 mg BID, but not tofacitinib 5 mg BID. This result could be influenced by small sample size in both dose groups and a large standard deviation in the group receiving tofacitinib 10 mg BID not in remission (mean CRP [mg/L] 4.2, standard deviation 7.3). The opposite association was observed with increasing age; however, this finding could also have been influenced by small sample size in both dose groups.
Partial Mayo score <2 at baseline of OCTAVE Sustain was significantly associated with an increased likelihood of achieving remission and a reduced risk of loss of response during OCTAVE Sustain vs PMS ≥2. It is noted that the extent and severity of UC is best assessed by endoscopy, with the endoscopic subscore as a core component of the Mayo score.1 In a comparison between total Mayo score and the noninvasive PMS, PMS has been found to perform as well as the total Mayo score in identifying patient-perceived clinical response.13 Therefore, PMS can be considered a practical measure of predicting which patients are likely to maintain response and achieve remission following 52 weeks of tofacitinib maintenance therapy.
Here, higher CRP at baseline of OCTAVE Sustain was associated with an increased risk of loss of response and a lower likelihood of achieving remission at week 52. Because CRP is an objective marker of inflammation, CRP levels have been observed to correlate with the extent of the disease.14 Consistent with this, higher CRP at baseline was independently associated with primary nonresponse to 8-week tofacitinib induction therapy in a recent real-world cohort study of patients with moderate to severe UC.15 Furthermore, in an observational study of patients with steroid-dependent UC treated with infliximab maintenance therapy, an endoscopic subscore of 3 (indicative of severe disease) at baseline and high CRP after infliximab induction therapy were independent predictors of colectomy.16
Oral corticosteroid use at baseline of OCTAVE Sustain was significantly associated with loss of response and lower odds of achieving remission at week 52 of tofacitinib treatment vs no corticosteroid use. Corticosteroid requirement is generally associated with poor UC prognosis; a meta-analysis looking at predictors of colectomy in patients with UC showed that the need for corticosteroids was associated with a higher risk of colectomy.17
Because active disease is a potential risk factor for AEs, it is important to find predictors of response that may reduce the risk of relapsing disease activity during maintenance therapy. The safety profile of tofacitinib 5 mg BID or tofacitinib 10 mg BID as maintenance therapy demonstrated no safety risks associated with remission status at week 52 of OCTAVE Sustain. Discontinuations due to AEs were generally less frequent among patients who achieved remission compared with those who did not. The safety data in these studies are consistent with the known safety profile of tofacitinib.5
A limitation of the current study is that these are post hoc analyses and the pooled studies were not designed, nor powered, to identify or evaluate predictors of response to maintenance treatment. Additional data interpretation is limited by small numbers. Although fecal calprotectin level has shown evidence of predicting sustained efficacy,18 this biomarker was not measured in the OCTAVE program and thus could not be included in this analysis. Furthermore, patients enrolled in OCTAVE Sustain were prior responders to 8-week treatment with tofacitinib 10 mg BID or placebo in OCTAVE Induction 1 and 2.
Conclusions
In conclusion, the results of these analyses suggest that PMS, CRP, and corticosteroid use are all characteristics that could be evaluated after 8 weeks of tofacitinib induction therapy to provide some prediction as to whether patients are likely to achieve remission or lose response to tofacitinib maintenance therapy. Furthermore, a lower endoscopic subscore at baseline of induction therapy may be predictive of remission at week 52 of tofacitinib maintenance therapy. Overall, these results suggest that patients with a deeper response to tofacitinib induction therapy are more likely to maintain response or remission compared with patients with more severe UC, regardless of tofacitinib dose received during maintenance. Therefore, this highlights the importance of a robust response to induction therapy in the treatment of UC.
Supplementary Material
Acknowledgments
The authors would like to thank the patients, investigators, and study teams who were involved in the tofacitinib ulcerative colitis clinical program. These studies were sponsored by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Sarah Mancini, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163:461–4).
Contributor Information
William J Sandborn, Division of Gastroenterology, University of California San Diego, La Jolla, CA, USA.
Alessandro Armuzzi, IBD Unit, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy.
Giuseppina Liguori, Pfizer Srl, Rome, Italy.
Peter M Irving, IBD Unit, Guy’s and St Thomas’ Hospital, London, UK.
Ala I Sharara, Division of Gastroenterology, American University of Beirut Medical Center, Beirut, Lebanon.
Rajiv Mundayat, Pfizer Inc., New York, NY, USA.
Nervin Lawendy, Pfizer Inc., Collegeville, PA, USA.
John C Woolcott, Pfizer Inc., Collegeville, PA, USA.
Silvio Danese, Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Italy.
Author Contributions
All authors have had full access to and have verified the underlying data. All authors contributed to the drafting of the article and critically reviewed/revised the article for important intellectual content. All authors approved the final version of the article before submission. W.J.S. and S.D. were investigators on the trials included in this analysis and made substantial contributions to the interpretation of the data. A.A., P.M.I., and A.I.S. made substantial contributions to the interpretation of the data. G.L., N.L., and J.C.W. contributed to the study design and the analysis and interpretation of the data. R.M. contributed to the statistical analysis and interpretation of the data. All authors reviewed and approved the final article, had access to the study data, and accept responsibility to submit for publication.
Funding
These studies were sponsored by Pfizer Inc. Pfizer Inc. were involved in study design, collection, analysis, and interpretation of the data. Medical writing support was funded by Pfizer Inc.
Conflicts of Interest
W.J.S. reports grants, personal fees, and nonfinancial support from Pfizer Inc. during the conduct of the study; research grants from AbbVie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Eli Lilly, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen, Pfizer Inc., Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, and Theravance Biopharma; consulting fees from AbbVie, Abivax, Admirx, Alfasigma, Alimentiv (Robarts Clinical Trials, owned by Health Academic Research Trust [HART]), Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health (Salix), BeiGene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Eli Lilly, Equillium, Escalier Biosciences, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Oppilan Pharma, Otsuka, Pandion Therapeutics, Pfizer Inc., Progenity, Prometheus Biosciences, Protagonist Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vedanta Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, and Zealand Pharma; and stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences, Progenity, Shoreline Biosciences, Ventyx Biosciences, and Vimalan Biosciences. The spouse of W.J.S. reports consultancy fees from Iveric Bio and Oppilan Pharma; stock options from Oppilan Pharma, Progenity, Prometheus Biosciences, Ventyx Biosciences, and Vimalan Biosciences; and is an employee of Prometheus Biosciences. A.A. reports consultancy fees from AbbVie, Allergan, Amgen, Biogen Idec, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring Pharmaceuticals, Hospira, Janssen, MSD, Mundipharma, Mylan, Pfizer Inc., Roche, Samsung Bioepis, Sandoz, Sofar, and Takeda; research grants from MSD, Pfizer Inc., and Takeda; lecture fees from AbbVie, Amgen, AstraZeneca, Biogen Idec, Bristol-Myers Squibb, Chiesi, Ferring Pharmaceuticals, Hospira, Janssen, Mitsubishi-Tanabe, MSD, Mundipharma, Nikkiso, Otsuka, Pfizer Inc., Samsung Bioepis, Sandoz, Takeda, TiGenix, and Zambon. P.M.I. reports grants and personal fees from Janssen, MSD, and Takeda; and personal fees from AbbVie, Arena Pharmaceuticals, Dr Falk Pharma GmbH, Eli Lilly, Ferring Pharmaceuticals, Genentech, Hospira, Pfizer Inc., Pharmacosmos, Samsung Bioepis, Sandoz, Shire, Tillotts Pharma, TopiVert, VHsquared, Vifor Pharma, and Warner Chilcott. A.I.S. reports consultancy fees from AbbVie, Amgen, Arena Pharmaceuticals, Gilead Sciences, Janssen, Pfizer Inc., and Takeda; research grants from Ferring Pharmaceuticals, Gilead Sciences, Janssen, Pfizer Inc., and Takeda; and lecture fees from AbbVie, Amgen, Ferring Pharmaceuticals, Gilead Sciences, Janssen, Pfizer Inc., Sandoz, Takeda, and Tillotts Pharma. G.L., R.M., N.L., and J.C.W. are employees and stockholders of Pfizer Srl and Pfizer Inc.. S.D. reports consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring Pharmaceuticals, Gilead Sciences, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Pfizer Inc., Roche, Sandoz, Takeda, TiGenix, UCB, and Vifor.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1. Ungaro R, Mehandru S, Allen PB, et al. . Ulcerative colitis. Lancet. 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee H-S, Cleynen I.. Molecular profiling of inflammatory bowel disease: is it ready for use in clinical decision-making? Cells. 2019;8:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sands BE, Sandborn WJ, Panaccione R, et al. ; UNIFI Study Group. . Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 4. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group. . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 5. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. . Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 6. Rutgeerts P, Sandborn WJ, Feagan BG, et al. . Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 7. Breidert M, Eftekhari P, Louis F, et al. Functional molecular network analysis enables prediction of response to vedolizumab therapy in anti-TNF refractory IBD patients. Crohns Colitis 360. 2020;2:otaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zampeli E, Gizis M, Siakavellas SI, Bamias G.. Predictors of response to anti-tumor necrosis factor therapy in ulcerative colitis. World J Gastrointest Pathophysiol. 2014;5:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner D, Mack D, Leleiko N, et al. . Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010;138:2282–2291. [DOI] [PubMed] [Google Scholar]
- 10. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Ulcerative colitis: clinical trial endpoints. Guidance for industry. Accessed July 8, 2021. http://www.fda.gov/downloads/Drugs/Guidances/UCM515143.pdf
- 11. Lichtenstein GR, Tinsley A, Roblin X, et al. . Baseline albumin level is not a significant predictor of tofacitinib efficacy in patients with ulcerative colitis: results of multivariate exposure-response analysis. Am J Gastroenterol. 2018;113:S354–S355. [Google Scholar]
- 12. Rubin DT, Reinisch W, Greuter T, et al. . Extraintestinal manifestations at baseline, and the effect of tofacitinib, in patients with moderate to severe ulcerative colitis. Therap Adv Gastroenterol. 2021;14:17562848211005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis JD, Chuai S, Nessel L, et al. . Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henriksen M, Jahnsen J, Lygren I, et al. ; IBSEN Study Group. . C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523. [DOI] [PubMed] [Google Scholar]
- 15. Honap S, Chee D, Chapman TP, et al. ; LEO [London, Exeter, Oxford] IBD Research Consortium. . Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 2020;14:1385–1393. [DOI] [PubMed] [Google Scholar]
- 16. Armuzzi A, Pugliese D, Danese S, et al. . Long-term combination therapy with infliximab plus azathioprine predicts sustained steroid-free clinical benefit in steroid-dependent ulcerative colitis. Inflamm Bowel Dis. 2014;20:1368–1374. [DOI] [PubMed] [Google Scholar]
- 17. Dias CC, Rodrigues PP, da Costa-Pereira A, Magro F.. Clinical predictors of colectomy in patients with ulcerative colitis: systematic review and meta-analysis of cohort studies. J Crohns Colitis. 2015;9:156–163. [DOI] [PubMed] [Google Scholar]
- 18. Bertani L, Blandizzi C, Mumolo MG, et al. . Fecal calprotectin predicts mucosal healing in patients with ulcerative colitis treated with biological therapies: a prospective study. Clin Transl Gastroenterol. 2020;11:e00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.