Abstract
Background
Total knee replacement (TKR) is a common procedure in older adults. Physical resilience may be a useful construct to explain variable outcomes. We sought to define a simple measure of physical resilience and identify risk factors for nonresilient patient outcomes.
Methods
Secondary analysis of Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR) cohort study, a prospective registry of total joint replacement. The analysis included 7 239 adults aged 60 or older who underwent TKR between 2011 and 2015. Measures included sociodemographic and health factors. Outcomes were categorized as physically resilient versus nonresilient based on the change from baseline to 1-year follow-up for 3 patient-reported outcomes: the physical component summary (PCS), bodily pain (BP), and vitality (VT) from the Short Form-36 subcomponent scores, at preop and 1-year postprocedure. Associations were expressed as relative risk (RR) of physically nonresilient outcomes using generalized linear regression models, with Poisson distribution and log link.
Results
Age, body mass index, and Charlson Comorbidity Index (CCI) were associated with increased risk of physically nonresilient outcomes across PCS, BP, and VT: age, per 5 years for PCS (RR = 1.18 [1.12–1.23]), BP (RR = 1.06 [1.01–1.11), and VT (RR = 1.09 [1.06–1.12]); body mass index, per 5 kg/m2, for PCS (RR = 1.13 [1.07–1.19]), BP (RR = 1.06 [1.00–1.11]), and VT (RR = 1.08 [1.04–1.11]); and CCI for PCS CCI = 1 (RR = 1.38 [1.20–1.59]), CCI = 2–5 (RR = 1.59 [1.35–1.88]), CCI ≥6 (RR = 1.55 [1.31–1.83]. Household income >$45 000 associated with lower risk for PCS (RR = 0.81 [0.70–0.93]), BP (RR = 0.80 [0.69–0.91]), and VT (RR = 0.86 [0.78–0.93]).
Conclusions
We operationalized physical resilience and identified factors predicting resilience after TKR. This approach may aid clinical risk stratification, guide further investigation of causes, and ultimately aid patients through the design of interventions to enhance physical resilience.
Keywords: Knee arthroplasty, Resilience, SF-36, Surgical risk
Background
Total knee replacement (TKR) is a common surgical procedure for older adults with knee osteoarthritis and is generally associated with excellent clinical results and a low rate of complications. TKR has proven benefits including restoration of physical function, pain relief, and preserved independence (1,2). More than 700 000 TKRs were performed in the United States in 2005, and, with an aging population, this number is projected to increase to between 935 000 and 1.26 million by 2030 (3,4). While most patients derive benefit, it is reported that about 20% of patients do not achieve satisfactory pain relief or functional improvement after TKR (5,6). These diverse trajectories highlight the need to understand who is most likely to demonstrate physically resilient outcomes and benefit from TKR and who is vulnerable to adverse outcomes.
How has physical resilience been operationalized to date in the context of surgical interventions in older adults? Physical resilience addresses physical stressors (eg, surgical interventions) and physiologic response to these stressors (7–9). Within the context of aging and geriatric medicine, physical resilience has been defined to include (a) preservation of health status in response to an acute stressor (more commonly termed “robustness” in other fields) or (b) recovery of health status after a perturbation to health induced by a stressor (8). The definition of physical resilience is still evolving and is typically built on a stressor, perturbation, and recovery model (10). The stressor perturbs the system and (often) induces an acute/transient decline in health status. This is typically followed by a recovery phase where the health status improves. The recovery phase may be divided into 2 parts: first an acute or subacute phase of recovery characterized by rate of improvement “recovery slope,” and second a “plateau” phase where the system has reached a steady state. Resilience is usually characterized by the overall change between prestressor and poststressor at the time of maximum recovery. The rate of recovery is seldom quantified in resilience studies due to sparse temporal sampling of phenotypic data following the stressor.
Prior studies have constructed measures of physical resilience and evaluated the clinical significance of such resilience. Few if any measures of resilience have been developed to evaluate older adults undergoing TKR (11). In the orthopedic setting, Colón-Emeric et al. (12) investigated recovery from hip fracture in adults 65 years of age and older using 10 outcome variables with the first measurement at 2 months after hip fracture. They identified 3 trajectories of recovery based on the relative degree to which all measured variables favored the more optimal recovery pattern: low 2-month values and a slow rate of recovery, defined as low resilience; intermediate 2-month values and intermediate rate of recovery, or intermediate resilience; and high 2-month values and higher rate of recovery, or high resilience. Interestingly, self-reported functioning prior to fall was the single variable with the greatest predictive value for a person’s recovery trajectory.
We have found no similar studies evaluating resilient outcomes in TKR. A variety of measures have been developed to characterize successful outcomes after TKR, and these measures can serve as a basis for defining resilient outcomes of TKR. Existing measures to evaluate TKR can be divided into broad categories of those that measure objective outcomes, those that measure subjective outcomes, and those that use a combination of these 2. Objective assessments of TKR success include falls, knee range of motion, gait speed, 6-minute walk, timed up and go, hospital length of stay, and aggregates of procedural complications (13–17). Subjective assessments include the Knee Injury and Osteoarthritis Outcome Score, the Oxford Knee Score, Lower Extremity Activity Scale, Western Ontario and McMaster Universities Arthritis Index, Fear of Falling, and the 36-item Short Form Survey (SF-36) (15,18–21). The revised Knee Society Clinical Rating system combines both objective and patient-reported parameters (15). The aims of our study are twofold: (a) to propose a novel metric of physical resilience in patients undergoing TKR and (b) to identify risk factors associated with lack of physical resilience in older adults undergoing TKR. These aims are a first step toward identifying older adults at risk of adverse outcomes, so that we can design interventions to improve outcomes following TKR.
We chose the SF-36 and its subdomains as outcome measures to characterize resilience because it is a highly validated patient-reported measure both in TKR and in other clinical contexts (22–24). SF-36 evaluates physical function/well-being within a broader scope than measures designed to focus on knee-specific outcomes (knee joint pain and function). We have proposed a measure of physical resilience for TKR based on SF-36 and have applied this measure to TKR data from the Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR) cohort study. We then examined predictors of resilient and nonresilient phenotypes. We specifically focused on changes to the physical component summary (PCS) between a presurgical baseline visit and 1-year follow-up after surgery. We further investigated how 2 subcomponents of the PCS, bodily pain (BP) and vitality (VT), compare in their trajectories and relevant predictive sociodemographic and health variables. Importantly, because of the relatively coarse time points available in this large cohort study—resilience is described using the difference in the outcome measure between prestressor and 1-year poststressor alone (without intervening time points). This means that resilience is characterized only by the “plateau” phase of recovery (at a time point expected to represent maximal clinical recovery), rather than by the rate of recovery (“recovery slope”).
Method
The FORCE-TJR cohort is a prospective, longitudinal study of adults who underwent either total hip replacement (THR) or TKR. The research cohort enrolled more than 30 000 participants drawing from 230 physicians in 28 states in the United States, including 25% academic and 75% community practices (National Clinical Trial number: NCT02566473). At surgical centers with an internal review board (IRB), participating surgeons obtained IRB approval from their local board; for those surgeons who did not have an IRB at their local institution (including community providers), study participation was covered by the University of Massachusetts Medical School IRB. Participating surgeons invited each THR or TKR patient who they saw to participate (25).
Inclusion Criteria
Eligible participants included persons who underwent TKR, had data available for scoring the SF-36 for both the preoperative visit and 1-year postoperative visit, and were aged 60 years or older at baseline. At each participating study setting, approval was obtained from the site’s associated institutional review board. All participants consented prior to joining the FORCE-TJR study.
Data Collection
For each participant, data were gathered by FORCE-TJR research staff both preoperatively and at postoperative follow-up, and a majority of participants had postoperative SF-36 collected at 1-year follow-up. Patient-reported data were gathered using a multimodal approach directed by participating-patient preference, with options including telephone interviews, mailed paper forms, and online surveys. We used the 1-year SF-36 values to characterize resilience, consistent with prior studies demonstrating that 1-year post-TKR is the time point at which mean recovery is maximal (26).
Measures
36-Item Short Form survey
The primary outcomes of interest in the present study were elements derived from the SF-36 Health survey (22), as follows:
PCS is a composite score of multiple measures and scored from 0 (worst function) to 100 (highest function).
BP is a subcomponent of PCS, is itself a composite of other questionnaire responses, and ranges from 0 (worst pain) to 100 (no pain).
VT is also a subcomponent of PCS and is a composite of questionnaire responses, ranging from 0 (least VT) to 100 (most VT).
These 3 scores were used to define phenotypes of physical resilience in our secondary analyses (see the Statistical Analysis section).
Demographics and Health Characteristics
Multiple covariates were available in the FORCE-TJR. Our analysis included the following variables: gender (female or male), age (years, continuous), body mass index (BMI, continuous), marital status (married or living with someone as married vs widowed, separated, or divorced vs never married), education (high school completed or less vs post-high school or more vs other), household income (≤$45 000 annually vs ≥$45 001 annually vs not reported), race (White, Black, or other), and smoking history (never smoker vs other). We also included the Charlson Comorbidity Index (CCI) to determine comorbidity count weighted by severity (0, 1, 2–5, or 6 or more) of the following diseases with associated scoring: 1 point—myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, and mild liver disease; 2 points—hemiplegia, moderate or severe renal disease, diabetes mellitus with end organ damage, any tumor, leukemia, and lymphoma; 3 points—moderate or severe liver disease; 6 points—metastatic solid tumor and AIDS (27).
Statistical Analysis
Outcomes were categorized as physically resilient versus nonresilient based on the change from baseline to 1-year follow-up for PCS and its subcomponents, BP and VT. For each of these measures, PCS, BP, and VT, a participant’s outcome was defined as resilient when one of the 2 conditions was met:
(1) the outcome variable (PCS, BP, or VT) “improved”—increasing by more than a clinically relevant threshold from preoperative to 1-year postoperative measurement, or
(2) the outcome variable (PCS, BP, or VT) “remained high,” defined as being in the upper quartile of the study population at preoperative baseline and not decreasing by more than a clinically relevant threshold at 1-year follow-up.
A clinically relevant threshold was defined as half of the clinically meaningful difference (CMD) as established in prior literature, or, if the CMD was not well established, as half of the median change in the sample population. For both PCS and BP, established values for CMD are approximately a 10-point change on the 100-point scale (18,23,24). For our study, this corresponded to a clinically relevant threshold of 5 points for both PCS and BP. For VT, we did not find literature to support an established definition of CMD; we instead estimated CMD by first calculating the median change from preoperative to postoperative measure, approximately 6 points, and then taking half of this value, leading to a clinically relevant threshold defined as a 3-point change.
We calculated the impact of risk factors on the binary outcome of physically resilient versus nonresilient response (defined above) as relative risks (RRs) in order to facilitate ease of clinical interpretation. We used a generalized linear regression model, with Poisson distribution and log link. We utilized the sandwich estimator for the calculation of robust standard errors (28). For each outcome variable, we adjusted for preoperative baseline value. All the analyses were performed using the R software environment (29).
We also analyzed the difference between pre- and postresponses to TKR, that is, postfunction minus prefunction scores by fitting a linear regression model with the pre–post difference as the outcome and the potential risk factors as covariates. The pre–post difference can be viewed as a continuous measure of resilience, with larger values indicating better recovery. In these models, we also adjusted for prefunction score as a covariate.
Results
Characteristics of Resilience in the FORCE-TJR Study
The mean age of participants was 70.3 years (SD 6.8), and the majority were female (63%), and identified as White (92%; Table 1). The mean BMI was 31.0 (SD 5.8). Using the binary classification of resilient/nonresilient defined in the Statistical Analysis section, based on the PCS, n = 5 648 (84%) participants were categorized as physically resilient, while n = 1 094 (16%) were categorized as nonresilient. For the subdomain BP, n = 5 973 (84%) had outcomes categorized as physically resilient and 1 120 (16%) participants had physically nonresilient outcomes. For physical resilience defined by VT, n = 4 817 (69%) participants were categorized as physically resilient and 2 199 (31%) as nonresilient. The VT domain had nearly double the probability of physically nonresilient response compared to PCS and BP responses.
Table 1.
Description of the FORCE-TJR Study Population’s Demographics and Health Characteristics
| Variable | Overall (N = 6 742) | Resilient (N = 5 648) | Nonresilient (N = 1 094) | |
|---|---|---|---|---|
| Age | Mean (SD) | 70.29 (6.75) | 70.03 (6.63) | 71.63 (7.16) |
| Gender | Female | 4 245 (63%) | 3 548 (63%) | 697 (64%) |
| Male | 2 497 (37%) | 2 100 (37%) | 397 (36%) | |
| Race | White | 6 169 (92%) | 5 185 (92%) | 984 (90%) |
| Black | 356 (5%) | 284(5%) | 72 (7%) | |
| Asian or Pacific Islander | 43 (1%) | 32 (1%) | 11 (1%) | |
| Native American or Alaska Native | 43 (1%) | 35 (1%) | 8 (1%) | |
| Other/do not know/refused | 43 (1%) | 41 (1%) | 2 (0%) | |
| Education | High school or less | 2 051 (32%) | 1 671 (31%) | 380 (36%) |
| Post high school or more | 4 297 (66%) | 3 644 (67%) | 653 (62%) | |
| Other | 135 (2%) | 115 (2%) | 20 (2%) | |
| Income | ≤$45 000 | 2 274 (34%) | 1 830 (32%) | 444 (41%) |
| ≥$45 001 | 3 208 (47%) | 2 764 (49%) | 444 (41%) | |
| Not reported | 1 260 (19%) | 1 054 (19%) | 206 (18%) | |
| Marital status | Married or living with someone as married | 4 583 (70%) | 3 897 (71%) | 686 (65%) |
| Widowed, separated or divorced | 1 673 (26%) | 1 351 (25%) | 322 (30%) | |
| Never married | 254 (4%) | 205 (4%) | 49 (5%) | |
| BMI | Mean (SD) | 30.98 (5.76) | 30.89 (5.72) | 31.42 (5.92) |
| Comorbidity | 0 | 3 555 (53%) | 3 089 (55%) | 466 (43%) |
| 1 | 1 470 (22%) | 1 194 (21%) | 276 (26%) | |
| 2–5 | 839 (12%) | 662 (12%) | 177 (16%) | |
| ≥6 | 811 (12%) | 647 (12%) | 164 (15%) | |
| Smoking history | Never smoked | 3 533 (54%) | 2 975 (54%) | 558 (53%) |
| Other | 2 992 (46%) | 2 490 (46%) | 502 (47%) | |
| Baseline PCS | Mean (SD) | 33.49 (8.40) | 33.24 (8.37) | 34.77 (8.47) |
| 1 year PCS | Mean (SD) | 43.63 (9.66) | 46.01 (8.09) | 31.34 (7.57) |
| Baseline vitality | Mean (SD) | 47.28 (9.99) | 46.01 (10.08) | 50.06 (9.17) |
| 1 year vitality | Mean (SD) | 52.02 (9.69) | 45.26 (9.04) | 55.11 (8.30) |
| Baseline pain | Mean (SD) | 35.79(7.76) | 35.35 (7.59) | 38.05 (8.22) |
| 1 year pain | Mean (SD) | 46.53 (9.78) | 48.89 (8.49) | 34.42 (6.50) |
Notes: BMI = body mass index; FORCE-TJR = Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement cohort study; PCS = physical component summary. (a) Ns are numbers of cases with both baseline and 1-year PCS values. (b) Percentages are calculated by dividing the related N. (c) Vitality and bodily pain are summarized by the corresponding resilience groups.
Protective Factors and Risk Factors for Resilient/Nonresilient Outcomes
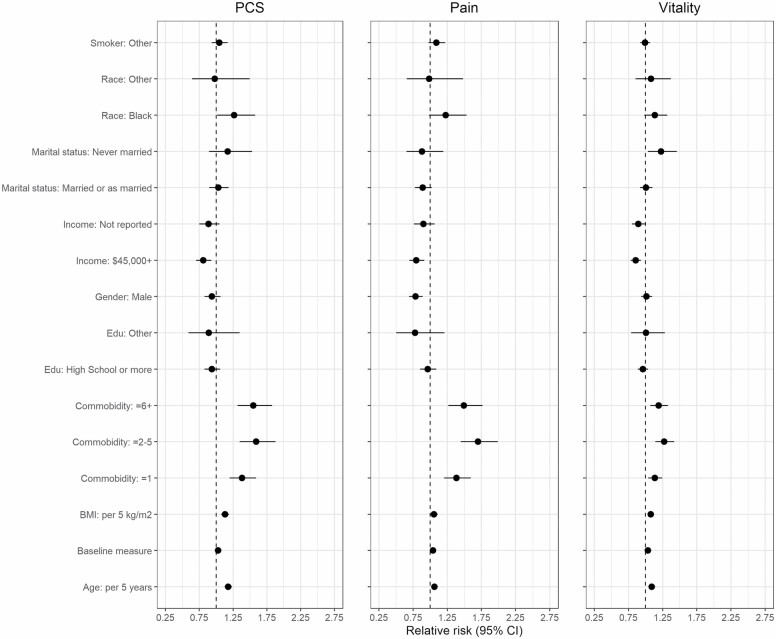
The results of logistic regression analyses including multiple (potential) risk factors for nonresilient outcomes are presented in Table 2. The coefficients of the models are also shown in a forest plot in Figure 1 to facilitate visualization.
Table 2.
Results of Logistic Regression Analyses of Risk Factors for Nonresilient Outcomes
| PCS | Bodily Pain | Vitality | ||
|---|---|---|---|---|
| Variable | Relative Risk (95% CI) | Relative Risk (95% CI) | Relative Risk (95% CI) | |
| Intercept | 0.090*** (0.07–0.11) | 0.030*** (0.02–0.04) | 0.046*** (0.04–0.06) | |
| Baseline value | 1.03*** (1.02–1.03) | 1.04*** (1.03–1.05) | 1.03*** (1.03–1.04) | |
| Age | Per 5 years | 1.18*** (1.12–1.23) | 1.06* (1.01–1.11) | 1.09*** (1.06–1.12) |
| Gender (ref = Female) | Male | 0.94 (0.83–1.06) | 0.78* (0.69–0.89) | 1.01 (0.94–1.10) |
| Race (ref = White) | Black | 1.26* (1.01–1.58) | 1.23 (0.98–1.53) | 1.14 (0.98–1.32) |
| Other | 0.98 (0.64–1.50) | 0.99 (0.66–1.48) | 1.08 (0.85–1.37) | |
| Education (ref = High school or less) | Post high school or more | 0.94 (0.83–1.06) | 0.96 (0.85–1.09) | 0.96 (0.89–1.04) |
| Other | 0.89 (0.59–1.35) | 0.78 (0.50–1.21) | 1.01 (0.79–1.28) | |
| Income (ref = ≤$45 000) | ≥$45 001 | 0.81** (0.70–0.93) | 0.80** (0.69–0.91) | 0.86*** (0.78–0.93) |
| Not reported | 0.89 (0.75–1.05) | 0.90 (0.76–1.06) | 0.89* (0.80–1.00) | |
| Marital status (ref = Married or as married) | Widowed, separated or divorced | 1.03 (0.90–1.18) | 0.89 (0.77–1.02) | 1.01 (0.92–1.10) |
| Never married | 1.17 (0.89–1.53) | 0.88 (0.65–1.19) | 1.23* (1.04–1.46) | |
| BMI | Per 5 unit | 1.13*** (1.07–1.19) | 1.06* (1.00–1.11) | 1.08*** (1.04,1.11) |
| Comorbidity (ref = 0) | 1 | 1.38*** (1.20–1.59) | 1.39*** (1.20–1.59) | 1.14** (1.04–1.24) |
| 2–5 | 1.59*** (1.35–1.88) | 1.70*** (1.45–1.99) | 1.27*** (1.14–1.42) | |
| ≥6 | 1.55*** (1.31–1.83) | 1.49*** (1.26–1.76) | 1.19** (1.07–1.33) | |
| Smoking history (ref = Never smoked) | Other | 1.05 (0.93–1.17) | 1.09 (0.98–1.22) | 0.99 (0.92–1.06) |
Notes: BMI = body mass index; PCS = physical component summary. (a) Age = (age-60)/5, BMI = (bmi-25)/5, estimates are relative risk. (b) Significance codes: ***p < .001, **p < .01, *p < .05.
Figure 1.
Forest plot, relative risk of physically nonresilient outcomes for each demographic factor. Relative risk associated with sociodemographic factors compared to reference value as a note. PCS = physical component summary; Edu = education; BMI = body mass index; comorbidity = Charlson Comorbidity Index.
For each outcome variable (PCS, BP, and VT), higher preoperative baseline value was associated with higher risk for nonresilient outcomes, PCS (RR = 1.03 [1.02–1.03], p < .001), BP (RR = 1.04 [1.03–1.05], p < .001), and VT (RR = 1.03 [1.03–1.04], p < .001).
Age (per 5-year increase) was associated with an increased risk for nonresilient outcomes for PCS (RR = 1.18 [1.12–1.23], p < .001), BP (RR = 1.06 [1.01–1.11], p = .011), and VT (RR = 1.09 [1.06–1.12], p < .001). Male gender did not have a significant association with resilient outcomes for PCS or VT. Male gender was associated with resilient outcomes for the BP phenotype (RR = 0.78 [0.69–0.89], p = .026). Race identified as Black was associated with increased risk for nonresilient outcomes for PCS (RR = 1.26 [1.01–1.58], p = .038), while race identified as other was not significantly associated with the PCS resilience phenotype. For BP and VT, race was not significantly associated with resilient outcomes. Household income greater than $45 000 was associated with resilient outcomes for PCS (RR = 0.81 [0.70–0.93], p = .003), BP (RR = 0.80 [0.69–0.91], p = .001), and VT (RR = 0.86 [0.78–0.93], p < .001). Marital status of never married was associated with increased risk for nonresilient outcomes for VT (RR = 1.23 [1.04–1.46], p = .018) when compared to those married or living as married. For PCS and BP, marital status was not significantly associated with resilient outcomes.
BMI was a risk factor for nonresilient outcomes for PCS per 5 kg/m2 (RR = 1.13 [1.07–1.19], p < .001), BP (RR = 1.06 [1.00–1.11] p = .041), and for VT (RR = 1.08 [1.04–1.11] p < .001). All categories of the CCI above zero were associated with an increased risk for nonresilient outcomes for PCS, BP, and VT when compared to no comorbidities (Table 2). Educational attainment and smoking history were not significantly associated with resilient outcomes for PCS, BP, or VT.
Predictors of Pre- to Post-TKR Mean Change in Function
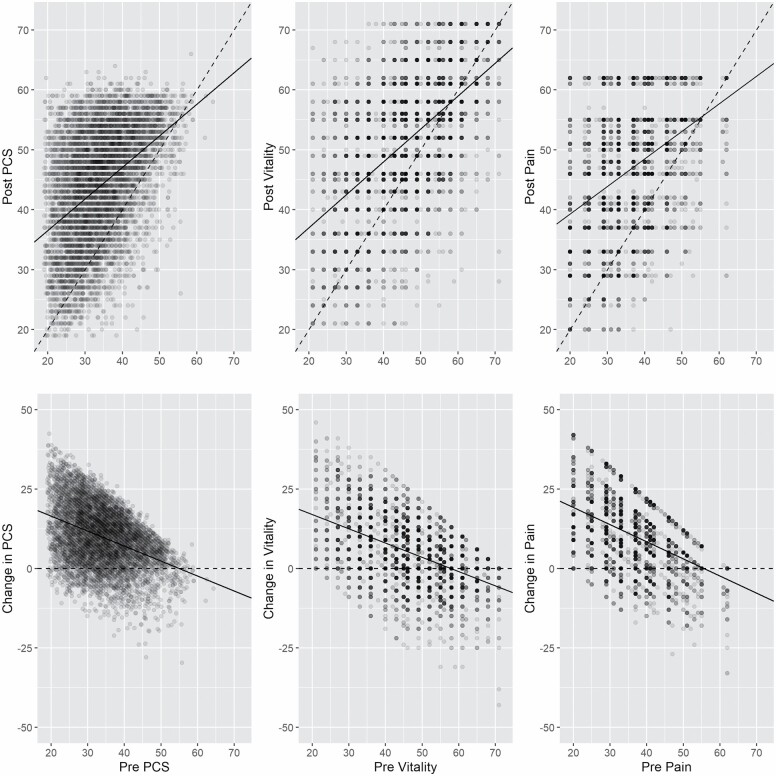
The results for change from preoperative to postoperative levels in PCS, BP, and VT are represented in Table 3. Larger, positive regression coefficients indicate better recovery, negative values, worse. Figure 2 shows the relationship between post-TKR function and pre-TKR function, as well as the relationship between the change (post-TKR minus pre-TKR) in function and pre-TKR function.
Table 3.
Results on the Change From Preoperative to Postoperative Levels in PCS, Bodily Pain, and Vitality
| PCS | Bodily Pain | Vitality | ||
|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | ||
| Intercept | 13.22*** (12.46 to 13.99) | 11.42*** (10.61 to 12.23) | 6.44*** (6.75 to 7.14) | |
| Baseline value | −0.52*** (−0.55 to −0.50) | −0.58*** (−0.61 to −0.55) | −0.46*** (−0.48 to −0.44) | |
| Age | Per 5 years | −0.99*** (−1.16 to −0.82) | −0.30*** (−0.48 to −0.12) | −0.58*** (−0.73 to −0.42) |
| Gender (ref = Female) | Male | 0.47* (0.01 to 0.93) | 0.96*** (0.47 to 1.44) | −0.11 (−0.54 to 0.31) |
| Race (ref = White) | Black | −1.76*** (−2.72 to −0.80) | −1.63** (−2.63 to −0.63) | −0.61 (−1.47 to 0.25) |
| Other | −0.044 (−1.56 to 1.47) | −0.77 (−2.36 to 0.82) | −0.34 (−1.70 to 1.02) | |
| Education (ref = High school or less) | Post-high school or more | 0.41 (−0.07 to 0.88) | 0.21 (−0.29 to 0.72) | 0.46* (0.02 to 0.89) |
| Other | 0.32 (−1.16 to 1.79) | 0.53 (−1.03 to 2.10) | 0.31 (−1.03 to 1.65) | |
| Income (ref = ≤$45 000) | >$45 000 | 0.97*** (0.45 to 1.49) | 1.25*** (0.69 to 1.80) | 0.77** (0.29 to 1.25) |
| Not reported | 0.63 (−0.03 to 1.29) | 0.79* (0.10 to 1.48) | 0.62* (0.02 to 1.22) | |
| Marital status (ref = Married or as married) | Widowed, separated or divorced | 0.068 (−0.47 to 0.60) | 0.29 (−0.27 to 0.86) | −0.12 (−0.61 to 0.36) |
| Never married | −1.31* (−2.41 to −0.21) | −0.07 (−1.24 to 1.10) | −1.45** (−2.46 to −0.45) | |
| BMI | Per 5 unit | −0.70*** (−0.90 to −0.51) | −0.21* (−0.41 to 0.00) | −0.43*** (−0.61 to −0.26) |
| Comorbidity (ref = 0) | 1 | −1.57*** (−2.11 to −1.04) | −1.14*** (−1.70 to −0.58) | −0.73** (−1.22 to −0.24) |
| 2–5 | −3.19*** (−3.86 to −2.52) | −2.79*** (−3.49 to −2.08) | −1.74*** (−2.35 to −1.13) | |
| ≥6 | −2.22*** (−2.88 to −1.56) | −1.78*** (−2.48 to −1.08) | −1.25*** (−1.86 to −0.65) | |
| Smoking history (ref = Never smoked) | Other | −0.28 (−0.70 to 0.14) | −0.28 (−0.73 to 0.16) | −0.075 (−0.46 to 0.31) |
Notes: BMI = body mass index; PCS = physical component summary. (a) Age = (age-60)/5, BMI = (bmi-25)/5. (b) Significance codes: ***p < .001, **p < .01, *p < .05.
Figure 2.
Association between pre-TKR and post-TKR scores and between pre-TKR score and post-TKR change for PCS, BP, and VT. The top 3 panels show the association between pre-TKR and post-TKR scores of PCS, BP, and VT. The bottom 3 panels show the association between the change (post-TKR minus pre-TKR) and the pre-TKR scores. Pre = preoperative; Post = postoperative; TKR = total knee replacement; PCS = physical component summary; BP = bodily pain; VT = vitality.
Higher preoperative baseline value was associated with a lower mean change in function in PCS of −0.52 (−0.55 to −0.50, p < .001), BP of −0.58 (−0.61 to −0.55, p < .001), and VT of −0.46 (−0.48 to −0.44, p < .001). The negative associations between the change in function and preoperative function are also evident from the bottom 3 panels of Figure 2.
Higher age was associated with a lower mean change in function in PCS of −0.99 per 5 years (−1.16 to −0.82, p < .001), BP of −0.30 (−0.48 to −0.12, p < .001), and VT of −0.58 (−0.73 to −0.42, p < .001). Male gender was associated with a higher mean change in function in PCS of 0.47 (0.01 to 0.93, p = .05) and in BP of 0.96 (0.47 to 1.44, p < .001), but no association was evident with VT. Race identified as Black was associated with a lower mean change in function in PCS of −1.76 (−2.72 to −0.80, p < .001) and in BP of −1.63 (−2.63 to −0.63, p = .001), while the composite category of other race was not associated with a significant mean change. Race was not significantly associated with the mean change in function in VT score. Education post-high school or more was also associated with a higher mean change in function in VT compared to education reported as high school or less, 0.46 (0.02 to 0.89, p = .041), but no association was evident with PCS or BP. Household income reported as more than $45 000 was associated with a higher mean change in function postoperatively in PCS 0.97 (0.45 to 1.49, p < .001), BP 1.25 (0.69 to 1.80, p < .001), and VT 0.77 (0.29 to 1.25, p = .002). Household income not reported was also associated with a higher mean change in function in BP and VT, though no association was evident with PCS. Marital status of never married was associated with a significantly lower mean change in function in PCS of −1.31 (−2.41 to −0.21, p = .020) and in VT of −1.45 (−2.46 to −0.45, p = .005). Marital status was not associated with a significant mean change in function for BP.
Higher BMI was associated with a lower mean change in function for PCS of −0.70 per 5 kg/m2 (−0.90 to −0.51, p < .001), BP of −0.21 (−0.41 to 0.00, p = .048), and VT of −0.43 (−0.61 to −0.26, p ≤ .001). Using mean change in function between preoperative and postoperative PCS, BP, or VT score as a measure of resilience, CCI was associated with less resilient outcomes for all 3 measures. As given in Table 3, those with comorbidities had decreased recovery of PCS compared to those with no comorbidity. History of smoking was not significantly associated with a mean change in function in outcome for PCS, BP, or VT.
Discussion
We built this construct of resilience to provide a simple, practical measure that can translate resilience from the theory of complex systems to the clinical and epidemiological setting. Our resilience construct has several uses. Clinically, we can help surgical teams to more accurately counsel patients and direct interventions to those at risk for poor outcomes. Translationally, we can define a resilience phenotype that serves as a starting point for research into the causes of resilience (along the spectrum from sociology to physiology).
A major strength of the current study is that we were able to incorporate an individual’s baseline function measured prior to their surgical stressor. This is not the case in some other studies of physical resilience, in part due to the unplanned nature of the stressor/intervention under investigation (eg, hip fracture, acute respiratory infections) (12). The availability of presurgical function allowed us to construct a novel phenotype for physical resilience, while also demanding distinct interpretive considerations.
As expected, age, BMI, increased comorbidity, and income are likely important in influencing resilience. These factors were consistently associated with resilience in the aggregated PCS as well as its subcomponents BP and VT. Our findings are consistent with prior research and support the clinical plausibility of our resilience construct (6,30–32). One important clinical correlate is that this may highlight the need to more carefully monitor or provide different rehabilitation and “prehabilitation” strategies to subgroups with higher risk. Higher baseline scores on PCS, BP, and VT were also associated with slightly increased risk for nonresilience outcomes—a finding discussed at greater length below.
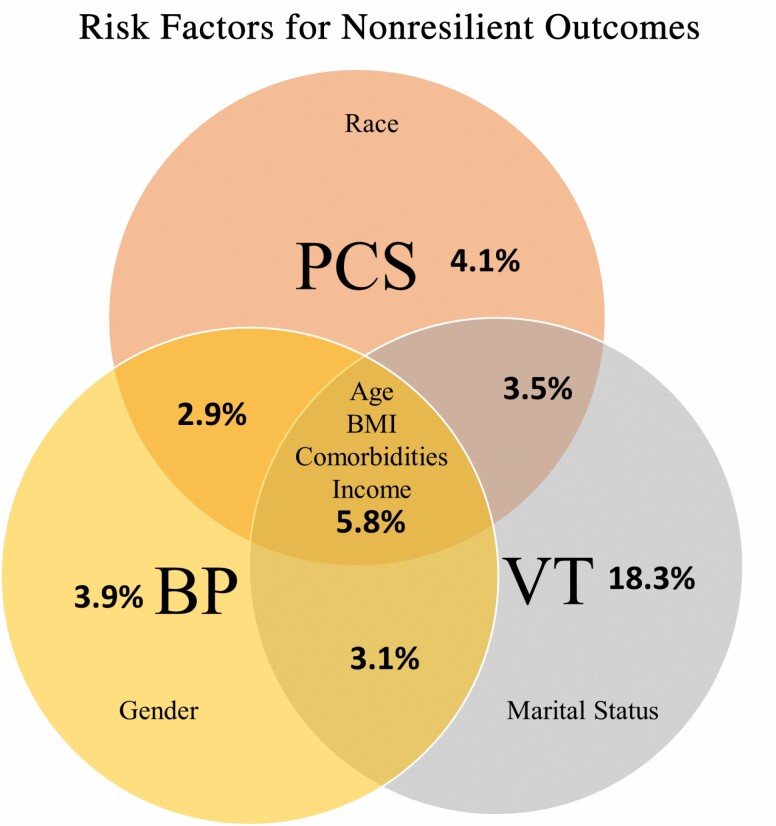
Several sociodemographic factors had inconsistent associations with resilience across PCS and the 2 subscales including self-identified race, gender, and marital status. These sociodemographic factors possibly relate to quality of life and its subdomains in a complex manner—capturing the interacting effects of financial status, health literacy, environmental/occupational exposures, social support, and health care access. Interpretation here must also be qualified by the small number of people in these categories, such as those who identified as never married or with a race other than Black or White. The complete set of protective and risk factors and the respective domains in which they were significant are represented in Figure 3.
Figure 3.
Venn diagram representing factors predicting nonresilience outcomes and percentage of participants affected for each SF-36 measure. Percentages of patients categorized as nonresilient across outcomes investigated. PCS = physical component summary; BP = bodily pain; VT = vitality; BMI = body mass index.
This study yielded some subtle or unexpected points that suggest potentially important issues to be considered in the study and clinical translation of physical resilience. We noted double the probability of physically nonresilient response in the subdomain of VT compared to overall PCS and BP domains. The implications of this are unclear but could relate to longer-term outcomes and survival. Indeed, prior work has demonstrated that lower VT scores are associated with increased risk for adverse outcomes such as job loss, hospitalization, and mortality (33). Also, gender differences were noted in BP. This could be attributable to differences in pain perception between older men and women. Gender differences in reported pain and function have been previously reported in other studies and need to be further explored with attention both to biological and sociocultural contexts (34).
As noted above, higher baseline functional status was associated with a lower recovery—an interesting finding consistent with prior studies of risk factors for poor improvement in quality of life metrics in the setting of cardiac surgery (35,36). However, this is likely a spurious finding which should be interpreted cautiously. In our analyses, we adjusted for presurgical function for 2 reasons: baseline adjustment is a common practice in longitudinal studies, and the model with baseline function had a substantially larger coefficient of determination (R2) compared to a model without it. Prestressor function or baseline function is a special type of variable in the analysis of resilience. It plays a dual role, both as an independent variable (as a determinant of response to the stressor) and as part of the definition of the dependent variable—because resilience is defined as a contrast between the pre- and poststressor levels of function. Therefore, the negative association of prestressor function with the degree of recovery arises at least in part from the mathematical nature of the regression model which constrains the coefficient of prestressor function to be negative—the negative slope of the best-fit lines in the bottom 3 panels of Figure 2 illustrates this. This phenomenon is aptly termed mathematical coupling and has been discussed in prior literature (37). Another, related, challenge is that of regression to the mean. People with high (low) pre-TKR values are more likely to regress toward their lower (higher) true value at a later time, even in the absence of any impact due to TKR. This would induce a bias in the relationship between the true change score and the baseline value (38). Thus, it is challenging to extract valid clinical or physiological interpretations when both mathematical coupling and regression to the mean are present. Having a control group and collecting multiple measurements pre- and postsurgery can help overcome these 2 challenges and allow us to draw proper inference regarding the impact of baseline function on postsurgical gains (37,38).
A limitation to the current study is the collection of follow-up data at a single time point at 1-year after surgery. More frequently sampled information on the recovery of function after TKR would be preferred, but was not pragmatically feasible for a large registry such as the FORCE-TJR in which the primary outcome at 12 months was selected to align with CMS quality endpoints and well as an expected course of clinical recovery. Therefore, any postprocedural perturbation in the outcome variable during the weeks and months immediately after surgery was not captured. In relation to the wider literature on physical resilience, this means that we are only able to characterize participant resilience in terms of their recovery “plateau” (maximal functional recovery poststressor) and not their recovery “slope” (the rate at which maximal functional recovery is attained poststressor). The relatively high frequency of good outcomes in TKR also represents a limitation, as the stress may not be sufficient to test the physiological capacities for recovery in the majority of patients (in contrast to more severe stressors such as bone marrow transplantation or hip fracture). Another limitation is that we did not investigate the interrelationship between PCS and its subdomains, BP and VT. This is an important topic for future investigation.
In summary, we have presented a novel measure of resilience—particularly well-suited to the epidemiological setting. We identified risk factors for nonresilient outcomes that, while not surprising, support the clinical plausibility of our model. Benefits to our specific construct of resilience include its simplicity, its flexible application to various outcomes of interest, and its tolerance for the relatively coarse timescale of measurement that are often available in epidemiologic or large cohort studies. Future work should address those features that restrict its applicability (eg, accounting for mathematical coupling and regression to mean, identification of proxy variables for baseline function, adaptations to allow application to studies with more time points), and further validation is needed for comparability of preserved high function and improved function within a single “resilient” category. Practical resilience measures can help standardize the study of resilience as a clinical phenomenon with the ultimate aim of identifying interventions to improve outcomes for older adults faced with physiological stressors.
Acknowledgments
We thank the FORCE-TJR Cohort study team and AHRQ P50HS018910. We would also like to acknowledge Dr. Hua Zheng, Assistant Professor from University of Massachusetts, for her assistance with data preparation.
Contributor Information
Thomas Laskow, Division of Geriatric Medicine and Gerontology, Johns Hopkins University, Baltimore, Maryland, USA.
Jiafeng Zhu, Division of Geriatric Medicine and Gerontology, Johns Hopkins University, Baltimore, Maryland, USA.
Brian Buta, Division of Geriatric Medicine and Gerontology, Johns Hopkins University, Baltimore, Maryland, USA.
Julius Oni, Department of Orthopaedic Surgery, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Frederick Sieber, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Karen Bandeen-Roche, Division of Geriatric Medicine and Gerontology, Johns Hopkins University, Baltimore, Maryland, USA; Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Jeremy Walston, Division of Geriatric Medicine and Gerontology, Johns Hopkins University, Baltimore, Maryland, USA.
Patricia D Franklin, Institute for Public Health and Medicine at Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
Ravi Varadhan, Division of Biostatistics and Bioinformatics, Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland, USA.
Funding
This study was supported by the National Institute on Aging (grants UH2AG056933 and T32AG058527 [to T.L.]).
Conflict of Interest
None declared.
References
- 1. Kane RL, Saleh KJ, Wilt TJ, Bershadsky B. The functional outcomes of total knee arthroplasty. J Bone Joint Surg Am. 2005;87(8):1719–1724. doi: 10.2106/JBJS.D.02714 [DOI] [PubMed] [Google Scholar]
- 2. Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373(17):1597–1606. doi: 10.1056/NEJMoa1505467 [DOI] [PubMed] [Google Scholar]
- 3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222 [DOI] [PubMed] [Google Scholar]
- 4. Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100(17):1455–1460. doi: 10.2106/JBJS.17.01617 [DOI] [PubMed] [Google Scholar]
- 5. Wylde V, Blom AW, Whitehouse SL, Taylor AH, Pattison GT, Bannister GC. Patient-reported outcomes after total hip and knee arthroplasty: comparison of midterm results. J Arthroplasty. 2009;24(2):210–216. doi: 10.1016/j.arth.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 6. Kuperman EF, Schweizer M, Joy P, Gu X, Fang MM. The effects of advanced age on primary total knee arthroplasty: a meta-analysis and systematic review. BMC Geriatr. 2016;16:41. doi: 10.1186/s12877-016-0215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirkland JL, Stout MB, Sierra F. Resilience in aging mice. J Gerontol A Biol Sci Med Sci. 2016;71(11):1407–1414. doi: 10.1093/gerona/glw086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hadley EC, Kuchel GA, Newman AB; Workshop Speakers and Participants . Report: NIA Workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci. 2017;72(7):980–990. doi: 10.1093/gerona/glx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varadhan R, Walston JD, Bandeen-Roche K. Can a link be found between physical resilience and frailty in older adults by studying dynamical systems? J Am Geriatr Soc. 2018;66(8):1455–1458. doi: 10.1111/jgs.15409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colón-Emeric C, Pieper CF, Schmader KE, et al. Two approaches to classifying and quantifying physical resilience in longitudinal data. J Gerontol A Biol Sci Med Sci. 2020;75(4):731–738. doi: 10.1093/gerona/glz097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71(4):489–495. doi: 10.1093/gerona/glv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colón-Emeric C, Whitson HE, Pieper CF, et al. Resiliency groups following hip fracture in older adults. J Am Geriatr Soc. 2019;67(12):2519–2527. doi: 10.1111/jgs.16152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill A-M, Ross-Adjie G, McPhail SM, et al. Incidence, risk factors and the healthcare cost of falls postdischarge after elective total hip and total knee replacement surgery: protocol for a prospective observational cohort study. BMJ Open. 2016;6:11139. doi: 10.1136/bmjopen-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao M, Wang F, Shi L, Xue Q. Interleukin-6 serum concentration in the elderly undergoing total knee arthroplasty: a potential predictor of the early postoperative knee joint range of motion. J Back Musculoskelet Rehabil. 2020;33(1):35–40. doi: 10.3233/BMR-181460 [DOI] [PubMed] [Google Scholar]
- 15. Chan HY, Sultana R, Yeo SJ, Chia SL, Pang HN, Lo NN. Comparison of outcome measures from different pathways following total knee arthroplasty. Singapore Med J. 2018;59(9):476–486. doi: 10.11622/smedj.2018011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bade MJ, Wolfe P, Zeni JA, Stevens-Lapsley JE, Snyder-Mackler L. Predicting poor physical performance after total knee arthroplasty. J Orthop Res. 2012;30(11):1805–1810. doi: 10.1002/jor.22140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fang M, Noiseux N, Linson E, Cram P. The effect of advancing age on total joint replacement outcomes. Geriatr Orthop Surg Rehabil. 2015;6(3):173–179. doi: 10.1177/2151458515583515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nilsdotter AK, Toksvig-Larsen S, Roos EM. Knee arthroplasty: are patients’ expectations fulfilled? A prospective study of pain and function in 102 patients with 5-year follow-up. Acta Orthop. 2009;80(1):55–61. doi: 10.1080/17453670902805007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elmallah RD, Jauregui JJ, Cherian JJ, Pierce TP, Harwin SF, Mont MA. Effect of age on postoperative outcomes following total knee arthroplasty. J Knee Surg. 2016;29(8):673–678. doi: 10.1055/s-0036-1571428 [DOI] [PubMed] [Google Scholar]
- 20. Liebs TR, Herzberg W, Roth-Kroeger AM, Rüther W, Hassenpflug J. Women recover faster than men after standard knee arthroplasty. Clin Orthop Relat Res. 2011;469(10):2855–2865. doi: 10.1007/s11999-011-1921-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsonga T, Michalopoulou M, Kapetanakis S, et al. Risk factors for fear of falling in elderly patients with severe knee osteoarthritis before and one year after total knee arthroplasty. J Orthop Surg (Hong Kong). 2016;24(3):302–306. doi: 10.1177/1602400306 [DOI] [PubMed] [Google Scholar]
- 22. Laucis NC, Hays RD, Bhattacharyya T. Scoring the SF-36 in orthopaedics: a brief guide. J Bone Joint Surg Am. 2015;97(19):1628–1634. doi: 10.2106/JBJS.O.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Busija L, Osborne RH, Nilsdotter A, Buchbinder R, Roos EM. Magnitude and meaningfulness of change in SF-36 scores in four types of orthopedic surgery. Health Qual Life Outcomes. 2008;6:55. doi: 10.1186/1477-7525-6-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ward MM, Guthrie LC, Alba MI. Clinically important changes in short form 36 health survey scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res (Hoboken). 2014;66(12):1783–1789. doi: 10.1002/acr.22392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Franklin PD, Allison JJ, Ayers DC. Beyond joint implant registries: a patient-centered research consortium for comparative effectiveness in total joint replacement. JAMA. 2012;308(12):1217–1218. doi: 10.1001/jama.2012.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pivec R, Issa K, Given K, et al. A prospective, longitudinal study of patient satisfaction following total knee arthroplasty using the Short-Form 36 (SF-36) survey stratified by various demographic and comorbid factors. J Arthroplasty. 2015;30(3):374–378. doi: 10.1016/j.arth.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 28. Zeileis A. Object-Oriented Computation of Sandwich Estimators. Vol 16. Foundation for open access statistics;2006. http://www.jstatsoft.org/ [Google Scholar]
- 29. R Core Team. R: A Language and Environment for Statistical Computing. R foundation for statistical computing;2020. [Google Scholar]
- 30. Ultee KHJ, Tjeertes EKM, Bastos Gonçalves F, et al. The relation between household income and surgical outcome in the Dutch setting of equal access to and provision of healthcare. PLoS One. 2018;13(1):e0191464. doi: 10.1371/journal.pone.0191464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pozzobon D, Ferreira PH, Blyth FM, Machado GC, Ferreira ML. Can obesity and physical activity predict outcomes of elective knee or hip surgery due to osteoarthritis? A meta-analysis of cohort studies. BMJ Open. 2018;8(2):e017689. doi: 10.1136/bmjopen-2017-017689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Podmore B, Hutchings A, van der Meulen J, Aggarwal A, Konan S. Impact of comorbid conditions on outcomes of hip and knee replacement surgery: a systematic review and meta-analysis. BMJ Open. 2018;8(7):e021784. doi: 10.1136/bmjopen-2018-021784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bjorner JB, Wallenstein GV, Martin MC, et al. Interpreting score differences in the SF-36 Vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23(4):731–739. doi: 10.1185/030079907x178757 [DOI] [PubMed] [Google Scholar]
- 34. Elboim-Gabyzon M, Rozen N, Laufer Y. Gender differences in pain perception and functional ability in subjects with knee osteoarthritis. ISRN Orthop. 2012;2012:413105. doi: 10.5402/2012/413105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bishawi M, Hattler B, Almassi GH, et al. ; VA #517 Randomized On/Off Bypass (ROOBY) Study Group . Preoperative factors associated with worsening in health-related quality of life following coronary artery bypass grafting in the Randomized On/Off Bypass (ROOBY) trial. Am Heart J. 2018;198:33–38. doi: 10.1016/j.ahj.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 36. Rijnhart-De Jong H, Haenen J, Bol Raap G, et al. Determinants of non-recovery in physical health-related quality of life one year after cardiac surgery: a prospective single centre observational study. J Cardiothorac Surg. 2020;15:234. doi: 10.1186/s13019-020-01273-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tu YK, Maddick IH, Griffiths GS, Gilthorpe MS. Mathematical coupling can undermine the statistical assessment of clinical research: illustration from the treatment of guided tissue regeneration. J Dent. 2004;32(2):133–142. doi: 10.1016/j.jdent.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 38. Hayes RJ. Methods for assessing whether change depends on initial value. Stat Med. 1988;7(9):915–927. doi: 10.1002/sim.4780070903 [DOI] [PubMed] [Google Scholar]