Abstract
Background and Hypothesis
Youth at clinical high-risk (CHR) for psychosis present with neuropsychological impairments relative to healthy controls (HC), but whether these impairments are distinguishable from those seen among putatively lower risk peers with other psychopathology remains unknown. We hypothesized that any excess impairment among CHR cohorts beyond that seen in other clinical groups is minimal and accounted for by the proportion who transition to psychosis (CHR-T).
Study Design
We performed a systematic review and meta-analysis of studies comparing cognitive performance among CHR youth to clinical comparators (CC) who either sought mental health services but did not meet CHR criteria or presented with verified nonpsychotic psychopathology.
Study Results
Twenty-one studies were included representing nearly 4000 participants. Individuals at CHR showed substantial cognitive impairments relative to HC (eg, global cognition: g = −0.48 [−0.60, −0.34]), but minimal impairments relative to CC (eg, global cognition: g = −0.13 [−0.20, −0.06]). Any excess impairment among CHR was almost entirely attributable to CHR-T; impairment among youth at CHR without transition (CHR-NT) was typically indistinguishable from CC (eg, global cognition, CHR-T: g = −0.42 [−0.64, −0.19], CHR-NT: g = −0.09 [−0.18, 0.00]; processing speed, CHR-T: g = −0.59 [−0.82, −0.37], CHR-NT: g = −0.12 [−0.25, 0.07]; working memory, CHR-T: g = −0.42 [−0.62, −0.22], CHR-NT: g = −0.03 [−0.14, 0.08]).
Conclusions
Neurocognitive impairment in CHR cohorts should be interpreted cautiously when psychosis or even CHR status is the specific clinical syndrome of interest as these impairments most likely represent a transdiagnostic vs psychosis-specific vulnerability.
Keywords: schizophrenia, transdiagnostic, neurocognitive, developmental psychopathology, clinical staging
Introduction
The clinical high-risk (CHR) framework offers a valuable opportunity to understand the early stages of psychosis and to intervene before a psychotic syndrome has fully crystalized. The CHR designation carries a strong valence for later psychosis outcome among help-seeking adolescents and young adults,1 but is also associated with mood, anxiety, and other disorders at the point of identification and longitudinally.2,3 Given that approximately 75% of youth at CHR do not develop psychosis but do experience persistent or emergent nonpsychotic disorders, questions have been raised about the extent to which putatively core neurobehavioral abnormalities observed in this population – especially without prospective knowledge of transition status – represent vulnerability to a psychotic disorder per se or a broader, transdiagnostic liability.4,5 These are critical and timely questions given the characteristic heterogeneity in CHR samples,6 the importance of predicting trajectories among high-risk youth,7 and the rapidly growing consensus that many psychiatric disorders share common risk factors and biopsychosocial features.8,9
Neuropsychological impairment represents an important area of inquiry in this context. It is well established that individuals at CHR show broad impairments in neurocognition and that these impairments are greatest among those later transitioning to psychosis.10 Although these findings may suggest that neurocognitive performance could serve as a biomarker for psychosis risk and provide clues about the neurodevelopmental aspects of illness, the extent to which such impairments carry specificity to psychosis vulnerability remains an open question.11 Evidence from the mood, anxiety, and general psychiatric disorder literatures demonstrates that these syndromes are commonly associated with a range of neuropsychological abnormalities.12–15 Thus, to contextualize cognitive impairment in CHR within the broader field of youth psychopathology, comparative studies are needed that examine performance in this group relative to help-seeking peers with nonpsychotic disorders.
To date, several studies have addressed the question of differential cognitive impairment in CHR youth vs non-psychotic clinical comparators (CC), but no quantitative summary of these findings is available. The results of independent studies seem to suggest that distinguishing CHR from CC is more difficult than distinguishing CHR from healthy controls (HC), but that those who transition to psychosis appear substantially more cognitively impaired at baseline, even relative to clinically diverse, help-seeking peers.16,17 Notably, among these studies, the characteristics of CC vary in terms of ascertainment and clinical presentation.18,19 Therefore, in addition to determining whether neuropsychological performance differs between CHR and CC in general, a similarly important research direction is to characterize cognitive similarities and differences across CHR and more specific clinical populations. Findings from this work would likely inform transdiagnostic and clinical staging models of developmental psychopathology.6
This study aimed to determine whether youth at CHR demonstrate more severe cognitive difficulties than seen in CC and whether any differential impairment depends on the transition status of those at CHR. Accordingly, we present what is to our knowledge the first systematic review and meta-analysis of neuropsychological performance in youth at CHR vs CC. We hypothesized that the CHR population broadly includes two groups of people: those with marked baseline cognitive deficits indicative of neurodevelopmental compromise and a trajectory toward psychosis, and those with milder cognitive deficits indicative of transdiagnostic psychopathology. Thus, we expected that any excess neurocognitive impairment in CHR beyond that observed in CC would be largely accounted for by CHR youth who later transitioned to psychosis, whereas the remainder of the cognitive deficit would be indistinguishable from the degree of impairment seen in CC. Given the possibility that cognitive deficits track a continuum of psychosis risk across clinical groups, we also planned to explore differential impairment across CC subgroups.
Methods
This protocol adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Supplementary Table S1) and Meta-analyses of Observational Studies in Epidemiology (Supplementary Table S2) reporting guidelines. The protocol was registered on the international prospective register of systematic reviews (ID CRD42021267259).
Search Strategy
We searched PubMed, Web of Science, and PsycINFO databases from inception to July 15, 2021, using the following terms: (psychosis OR ultra high risk OR clinical high risk OR ultra high risk OR clinical high-risk OR attenuated psycho* OR prodrom* OR psychotic OR schizo*) AND (control OR help-seeking OR clinical control OR psychiatric control) AND (cognit* OR neurocognit* OR neuropsych*). After excluding duplicates and articles with clearly unrelated titles and abstracts, two independent reviewers (ZBM, CR) inspected full texts of assembled studies and coded their eligibility (agreement was 95.1%). We also manually searched bibliographies of previous meta-analyses of neurocognition in CHR; bibliographies of studies included in our final sample; and for eligible publications by groups whose studies were only excluded for lacking neurocognitive data (ie, groups whose CHR work was identified as including CC and may therefore have published results of cognitive performance). Any eligibility discrepancies were resolved by group consensus through discussion.
Study Selection
Article inclusion criteria were (1) published in a peer-reviewed journal; (2) written in English; (3) included participants meeting internationally established CHR criteria (ultra-high risk or basic symptom criteria as determined by a gold-standard interview such as the Structured Interview for Psychosis-risk Syndromes or the Schizophrenia Proneness Instrument, respectively; more detail regarding CHR syndromes and assessment measures is provided in Supplementary Methods S1); (4) included a psychiatric CC group, the participants of which were assessed with a validated structured interview and either (a) sought mental health services but failed to meet CHR criteria (ie, were referred for CHR assessment due to suspected risk, or sought care at another mental health clinic; Supplementary Methods S1) or (b) presented with nonpsychotic psychopathology; and (3) reported neuropsychological data separately for groups of interest using formal, validated tests. Neurocognitive data from healthy controls (HC) were desired but not necessary and were included to contextualize cognitive performance in the clinical groups. Exclusion criteria included (1) reviews, editorials, abstracts, and dissertations; (2) studies lacking a CC group as defined above; (3) studies sampling CHR and CC participants exclusively from nonhelp-seeking environments; and (4) studies with overlapping samples from the same task. When overlapping samples included the same test data, the study with the largest sample size was used.
Data Extraction and Test Classification
Two independent reviewers (ZBM, CR) extracted data from original articles into a database (Supplementary Methods S2). Entries were cross-checked and discrepancies were reviewed in detail. Given the relatively limited literature on the present topic, rather than focusing on test-level data we classified tests into domains based on expert assessment (JMG). These included (1) global cognition, (2) executive functions (EF), (3) processing speed (PS), (4) working memory (WM), (5) episodic memory (EM), (6) fluency, (7) vigilance, (8) perceptual organization, and (9) premorbid intelligence. Where possible, we examined domain components, including EM (ie, verbal and visual memory) and fluency (ie, semantic and phonemic fluency). When a study reported multiple scores per test, multiple tests within the same domain, or data on ≥3 domains but no measure of global cognition, we pooled the data following prior meta-analyses and methodological guidelines (Supplementary Methods S3-S5).10,20 Global cognition included measures of IQ, composite scores from test batteries, and composite scores we created. This approach allowed meaningful group comparisons of several relevant cognitive domains richly populated with test data. The specific tests contributing to each domain are presented in Supplementary Table S3, and more detailed information regarding the methods used for pooling data is presented in Supplementary Table S4. When necessary, we requested data from corresponding authors.
To characterize the studies, we extracted additional demographic (age, gender), and clinical information (eg, cooccurring mood/anxiety disorder, antipsychotic medication use). Based on our prior review of the broader CHR-CC literature,4 we expected that the CC groups would generally comprise individuals with affective disorders or those seeking care at general psychiatric clinics (referred to collectively as youth with affective disorders and general psychopathology; ADGP), and individuals referred for CHR assessment but not meeting the diagnostic criteria (referred to as youth who were psychosis-risk referred, testing negative; PRTN). These groups both represent stringent comparators for determining the specificity of neuropsychological impairment within the CHR population given that they are drawn from similar (or identical) populations as those at CHR and, like CHR, present with broad psychopathology, yet do not carry the same degree of risk for later psychosis outcome as individuals meeting CHR criteria (Supplementary Methods S1).21 Of the 14 authors who were contacted, 11 provided additional data or protocol information (Supplementary Methods S2).
Bias and Quality Assessment
An investigator (ZBM) evaluated the risk of bias and quality of included studies using the Newcastle-Ottowa Scale for case-control and cohort studies.22 The scale includes 8 items assessed across three areas: selection, comparability, and exposure/outcome. The maximum score is 9. Because the comparison of interest in our study is CC, items concerning comparisons and control groups were scored with reference to this group.
Statistical Analysis
The primary effect size was Hedge’s g calculated with random effects. This model is well-suited for meta-analyses with small sample sizes and nontrivial heterogeneity.20 We examined the influence of individual studies on summary effects and variances by inspecting forest plots, performing one-study-removed analyses, and calculating standardized residuals. Studies were considered outliers when standardized residuals were larger than 3 in absolute value and/or when pooled effect size estimates were clearly altered in one-study-removed analyses.23
Our primary meta-analyses consisted of three sets of comparisons. First, to establish the presence of neuropsychological impairment in the CHR and CC groups within our sample, we calculated Hedge’s g for these groups relative to HC where HC data were available. Second, we calculated Hedge’s g for differences in neurocognitive performance between the CHR and CC groups, without considering CHR transition. Third, we calculated Hedge’s g separately for CHR participants with (CHR-T) vs without (CHR-NT) transition to psychosis relative to CC. In supplementary analyses, we inspected patterns of cognitive performance (vs HC) across ADGP, PRTN, CHR-T, CHR-NT to evaluate in more detail the extent to which cognitive impairment in youth follows a gradient of putative psychosis risk (Supplementary Methods S6). We also examined the influence of antipsychotic medication use (given that these agents may impair cognitive performance) and publication year (given evidence of declining rates of transition to psychosis in CHR cohorts in more recent years) on CHR-CC differences in cognitive functioning by performing meta-regressions with random effects (Supplementary Methods S6). We calculated effect sizes and regression coefficients when there were ≥3 studies with data for that analysis.
In two cases,24,25 researchers reported baseline and follow-up data from the same protocol in separate publications with different sample sizes. In these instances, we matched baseline neurocognitive data for CHR-T and CHR-NT with baseline data for CCs from the larger sample from that protocol.
Publication bias was assessed by inspecting funnel plot symmetry and using Egger’s test. Where there was evidence of publication bias, we adjusted effect size estimates using Duval and Tweedie’s trim and fill method. Heterogeneity was assessed using the Q statistic and I2 index. The I2 index was developed in part to be sensitive to heterogeneity in meta-analyses including relatively small sample sizes, and is proposed to broadly reflect low, medium, and high heterogeneity at values of 25%, 50%, and 75%, respectively.26 Analyses were conducted using Comprehensive Meta-Analysis, Version 3.
Results
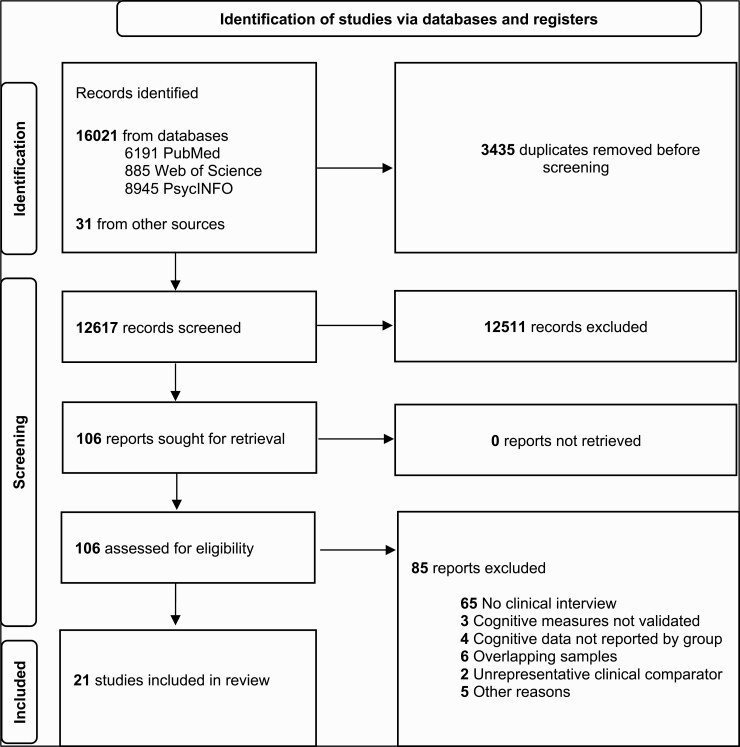
Of the 12 617 articles screened, 21 were included (figure 1), representing 1556 CHR, 1398 CC, and 973 HC. Seven studies reported cognitive data separately by transition status, representing 110 CHR-T and 553 CHR-NT. All studies enrolled individuals meeting ultra-high risk criteria and six also included participants meeting basic symptom criteria. The CC included PRTN (k = 8), individuals with affective syndromes (k = 5), and mixed clinical samples (k = 6). The average per-study age of participants was 20.4 for CHR, 20.9 for CC, and 22.2 for HC. Fifty six percent of the CHR groups, 54.3% of the CC groups, and 57.7% of the HC groups were female. These distributions are reflective of the general CHR literature and are similar to prior reviews of neuropsychological functioning in CHR.1, 10 Racial or ethnic data by group were available in only 5 studies. Using published data and additional data acquired from study authors, we were able to determine the proportion of patients with mood and/or anxiety disorders in 16 studies. Two of the remaining five were from the same protocol and another two reported outcome (ie, transition) data corresponding to studies for which these data were available. As shown in table 1, mood and anxiety disorder diagnoses were common and fairly evenly distributed across both groups. An average of 54.9% of CHR (median = 61.1%) and 51.5% of CC (median = 46.7%) presented with a cooccurring mood disorder, and an average of 39.6% (median = 37.1%) and 39.0% (median = 31.2%) respectively presented with a cooccurring anxiety disorder. Thus, CHR and CC samples were well matched on the presence of affective illness but differed with respect to their putative level of psychosis risk. Table 1, Supplementary Results S1, and Supplementary Table S5 further present study characteristics.
Fig. 1.
PRISMA flow diagram depicting study selection procedure.
Table 1.
Characteristics of the Included Studies
| Author & Year | CHR Description | CC Description | Sample Sizes | % Mood Disorders | % Anxiety Disorders | NOS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHR | CHR-T | CHR-NT | CC | HC | CHR | CC | CHR | CC | ||||
| Addington 201227 | UHR | PRTN | 172 | – | – | 100 | – | N/A | N/A | N/A | N/A | 7 |
| Carrión 201816 | UHR | PRTN | 205 | 12 | 163 | 89 | 60 | 50.7 | 34.8 | 42.9 | 25.8 | 7 |
| Grent-‘t-Jong, 202128 | UHR + BS | PRTN | 116 | 13 | 97 | 38 | 49 | 65 | 29 | 73 | 42 | 7 |
| Ilonen 201029 | UHR | General inpatient | 22 | – | – | 187 | – | 50 | 46.5 | 27.3 | 16 | 7 |
| Kindler 201830 | UHR + BS | PRTN | 29 | – | – | 18 | – | 24.1 | 44.4 | 31 | 16.7 | 7 |
| Koren 201931 | UHR | Mixed clinicalb | 22 | – | – | 42 | 34 | 13.6 | 31.0 | 13.6 | 31 | 7 |
| Koutsouleris 202117 | UHR + BS | Depression | 167 | 21 | 139 | 167 | 334 | 68.3 | 100 | 76.7 | 98.2 | 6 |
| Lincoln 201832 | UHR | Anxiety | 25 | – | – | 40 | 40 | 96 | 47.5 | 36 | 100 | 4 |
| Lindgren 201018 | UHR | Mixed clinicalb | 62 | – | – | 112 | 72 | 72.6 | 47.5 | 37.1 | 25 | 8 |
| a Lindgren 202124 | UHR | – | – | 9 | 38 | – | – | – | – | – | – | 8 |
| Liu 201519 | UHR | PRTN | 53 | 18 | 34 | 85 | 137 | 0 | 0 | 0 | 0 | 7 |
| Magaud 201033 | UHR | PRTN | 77 | – | – | 61 | – | N/A | N/A | N/A | N/A | 8 |
| Magaud 201434 | UHR | PRTN | 104 | – | – | 64 | – | N/A | N/A | N/A | N/A | 8 |
| Metzler 201435 | UHR + BS | Attenuated bipolar | 102 | – | – | 30 | 50 | 67.7 | 46.7 | 51 | 60 | 6 |
| a Metzler 201525 | UHR + BS | – | – | 12 | 60 | – | – | – | – | – | – | 6 |
| Millman 201736 | UHR | Mixed clinicalb | 14 | – | – | 34 | – | 57.1 | 54.3 | 57.8 | 31.4 | 7 |
| Romanowska 201837 | UHR | Mixed stage 1b (non-CHR)c | 80 | – | – | 23 | 71 | 11.9 | 30.4 | 29.8 | 47.8 | 7 |
| Salazar de Pablo 202038 | APS | General inpatient | 65 | – | – | 183 | – | 80 | 76 | 55.4 | 38.3 | 8 |
| Schulze 201239 | UHR | Depression | 47 | 25 | 22 | 34 | 76 | 48.9 | 100 | N/A | N/A | 8 |
| Simon 200740 | UHR + BS | PRTN | 69 | – | – | 49 | – | 41.9 | 44.9 | 14 | 6.2 | 7 |
| Szily 200941 | UHR | Depression | 26 | – | – | 42 | 50 | 100 | 100 | N/A | N/A | 7 |
Note: APS, attenuated psychosis syndrome; BS, basic symptom criteria; CC, clinical comparator; CHR, clinical high-risk for psychosis; CHR-NT, clinical high-risk for psychosis without later transition to psychosis; CHR-T, clinical high-risk for psychosis with later transition to psychosis; N/A, not available; NOS, Newcastle-Ottowa Scale; UHR, ultra-high risk criteria.
aFollow-up studies reporting transition data from same protocol represented in earlier study included here by the same first author. Here, cognitive data from CHR-T and CHR-NT were compared to CC data from the prior report to maximize power.
bMixed sample largely characterized by mood and anxiety disorders but also including trauma, attention deficit/hyperactivity, eating disorders, and other psychiatric concerns commonly seen in among help-seeking youth.
cStage 1b from the clinical staging model of psychopathology. Individuals with attenuated syndromes for severe nonpsychotic disorders (severe depression, bipolar disorder, borderline personality disorder).
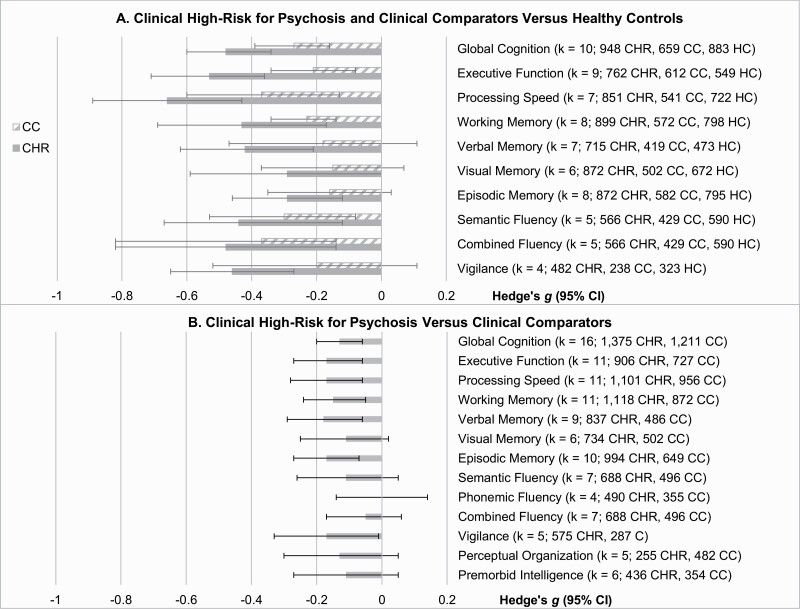
Neuropsychological Performance in CHR and CC Relative to HC
As expected, both CHR and CC showed substantial impairments in neuropsychological functioning relative to HC (figure 2a, Supplementary Table S6). Effect sizes ranged from −0.15 to −0.37 for CC and from −0.29 to −0.66 for CHR, suggesting small and small-to-medium sized impairments in CC and CHR, respectively. The index of global cognition, which likely represents the most reliable estimate of ability, was −0.27 for CC and −0.48 for CHR. These CHR estimates are similar to prior reviews,10 although estimates for CC appear somewhat smaller than has been reported in prior studies of other early-stage psychopathology, suggesting cognitive impairment among CC here could underestimate that seen in the broader youth psychiatric population.12,42–44
Fig. 2.
Panel A: Neurocognitive performance among individuals at CHR for psychosis and CCs, with HCs as the reference. Panel B: Neurocognitive performance among individuals at CHR for psychosis with CCs as the reference. Bars represent Hedge’s g effect size with 95% confidence intervals; more negative values indicate poorer performance relative to the reference group. CHR, clinical high-risk, CC, clinical comparator, HC, healthy control, CI, confidence interval.
Neuropsychological Performance in CHR Relative to CC
In contrast to the comparisons between CHR/CC and HC, differential impairment in CHR vs CC was minimal (figure 2b, Supplementary Table S7). The CHR group performed worse than the CC group on about half of the domains, but the magnitude of these effects was typically below −0.15 and never exceeded −0.18, consistently below the size conventionally indicating a small effect. The effect size estimate for global cognition was −0.13.
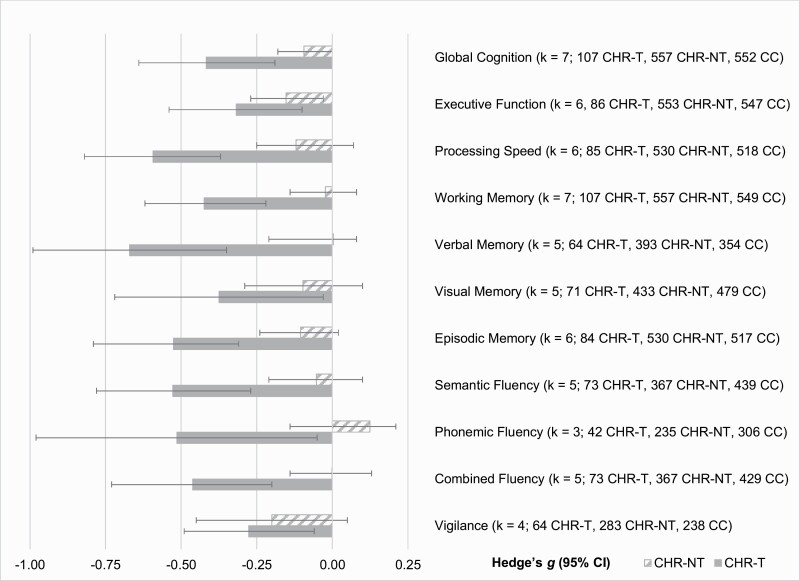
Neuropsychological Performance in CHR-T and CHR-NT Relative to CC
Importantly, we observed clear distinctions in effect sizes of neurocognitive functioning between CHR-T and CHR-NT when these groups were contrasted with CC. As shown in figure 3 and Supplementary Table S9, cognitive functioning in CHR-NT was typically comparable to CC whereas functioning in CHR-T was substantially more impaired: Effect sizes for CHR-T generally exceeded −0.40, whereas effect sizes for CHR-NT typically fell below −0.15. The general pattern and degree of impairment between CHR and CC was quite similar across domains, although WM, verbal memory, and fluency appeared to show the greatest CHR-T/CHR-NT divergence in terms of similarity to CC. Youth with CHR-NT showed somewhat greater EF impairment than CC, but the effect size estimate was only −0.15. The effect size representing global cognitive impairment relative to CC was −0.42 for CHR-T and −0.09 for CHR-NT.
Fig. 3.
Baseline neurocognitive functioning among individuals at clinical high-risk for psychosis plotted by whether they later developed a psychotic disorder, with baseline cognitive functioning of clinical comparators as the reference. Bars represent Hedge’s g effect size with 95% confidence intervals; more negative values indicate poorer performance. CHR-T, clinical high-risk with later transition to psychosis, CHR-NT, clinical high-risk without later transition to psychosis, CC, clinical comparator, CI, confidence interval.
Supplementary Analyses
Plotting effect sizes with HC as the reference group and ADGP, PRTN, CHR-T, and CHR-NT as the groups of interest suggested a severity gradient of performance such that HC > ADGP > PRTN > CHR-NT > CHR-T (Supplementary Results S3, Supplementary Figure S1, Supplementary Tables S10-S11). This was the case for global cognition, EF, PS, EM, and verbal memory. Too few studies were available to calculate effect sizes for visual memory, fluency or its subdomains, and vigilance. No effect of antipsychotic use or publication year on primary effect sizes was observed (Supplementary Results S4, Supplementary Tables S12-S14).
Study Quality, Heterogeneity, and Publication Bias
NOS scores ranged from 4–8 (median = 7; table 1). Heterogeneity was high when comparing CHR and CC participants to HC (Supplementary Table S6), but low or modest when comparing clinical groups to one another (Supplementary Results S4, Supplementary Table S7, Supplementary Table S9). There was no evidence of publication bias in comparisons of clinical groups with HC or in CHR-CC comparisons, although funnel plots and Egger’s test suggested an unexpected absence of studies reporting better performance in CHR-NT vs CC for verbal memory and phonemic fluency. Trim and fill adjustment suggested the true difference between these groups is likely even smaller than what was observed in published studies (Supplementary Table S9, Supplementary Figures S2-S36).
Discussion
This was to our knowledge the first systematic review and meta-analysis of neuropsychological performance among individuals with psychosis-risk syndromes vs putatively lower-risk peers with other psychopathology. There were two main findings: First, despite both groups showing neurocognitive deficits relative to healthy participants, differential impairment in CHR vs CC was minimal when the later transition status of CHR participants was not considered. Second, and related, any additional deficit seen in CHR was almost entirely attributable to the proportion of individuals who later developed psychosis. In this subgroup, impairment was markedly and consistently greater than all other clinical groups. These findings have conceptual implications for our understanding of neurocognitive performance in the CHR population as well as field-level implications for research design in early psychosis.
An important goal of high-risk psychiatric research is to improve our ability to identify young people with the greatest probability of experiencing adverse mental health outcomes later in life. Despite considerable advances, a major, current challenge is to characterize risk profiles that carry unique valence for a specific “exit syndrome” such as psychosis.7 Some evidence suggests that clinical trajectories of youth at CHR are characterized by greater rates of psychosis but similar or lower rates of nonpsychotic disorder when compared to CC.45,46 However, several independent studies have shown that individuals at CHR are more likely to experience nonpsychotic disorder over time than they are formal or attenuated psychosis, highlighting that psychosis-spectrum psychopathology represents only a fraction of the global psychiatric profile seen in CHR cohorts longitudinally.2,3,45,47 Importantly, in this study CHR and CC presented with very similar rates of mood (55% CHR, 52% CC) and anxiety diagnoses (39% CHR, 39% CC), meaning the nonpsychotic disorder likely contributing to the overall cognitive burden in CHR was well controlled relative to studies relying on HC alone.48 Our finding of highly comparable neurocognitive profiles among these groups therefore strongly suggests that neuropsychological impairment in CHR cohorts should not necessarily be assumed to reflect a psychosis-specific process – or even CHR status – particularly when future transition status is unknown. A more likely explanation is that these impairments reflect a transdiagnostic vulnerability to psychopathology that may or may not include a partially expressed liability for psychosis. If true, this represents a departure from conventional thinking about the meaning of neurocognitive deficits in the CHR population, which typically emphasizes their implications for psychosis, and suggests new ways of conceptualizing these early-stage phenomena are warranted.
The present findings have implications for our understanding of the neural substrates of cognitive deficits in emerging psychosis. Meta-analyses demonstrate that the neural networks underlying cognitive control are similarly affected across psychotic, affective, and other disorders.49,50 Notably, relative to those with nonpsychotic disorder, individuals with psychosis in these studies presented with greater but qualitatively similar impairment in the prefrontal cortex and anterior insula, structures frequently studied in the CHR population given their association with negative symptoms, cognition, and other important clinical phenomena.51–54 Considered alongside these findings, our observation of graded neuropsychological impairment across groups (including CC subgroups, with CHR-T always at the extreme end) potentially suggests that cognitive difficulties reflect neurodevelopmental risk for a range of disorders when moderate, but psychosis in particular when severe.15,55 Although the present study was not designed to examine brain structure or function directly, the findings add to a neurodevelopmental conceptualization of psychosis as a unique condition while situating CHR-associated cognitive impairments within the broader psychiatric literature.
Our findings also raise questions about environmental factors that may nonspecifically impact cognitive development in ways that increase risk for psychopathology. Recent work has highlighted that early deprivation of expected environmental input (eg, food security, academic stimulation) is associated with reduced EF capacity in youth,56 which may in turn increase risk for affective, psychotic, and developmental disorders.57 Attention to the role these risk factors play in cognitive development across high-risk groups may shed light on common environmental contributors to psychopathology, particularly among groups disproportionately exposed to such factors, including many racial-ethnic minority, lower income, and immigrant populations.58
A major methodological implication of this study is that identifying areas of neuropsychological overlap and divergence among the emerging psychopathologies requires a shift from reliance on HC as sole comparators in CHR research to protocols inclusive of both HC and CC. Clinical comparators offer a needed point of reference when seeking to understand processes that might be specifically associated with a diathesis for psychosis (or another syndrome) vs one that may represent a broader liability. Numerous studies have capitalized on this approach in adult psychopathology,59–61 but transdiagnostic work has yet to be widely adopted in the CHR field.62 Results generated from such research are more likely to clarify common and unique risk mechanisms and promote disseminable detection and treatment strategies than are those generated from studies relying on HC alone.4 More discussion is needed, however, regarding the optimal characteristics of CC across research contexts. In this study, PRTN appeared more cognitively alike CHR than did ADGP, suggesting PRTN may carry additional psychosis vulnerability that could inform our understanding of the disorder. This is consistent with epidemiological evidence suggesting that the presence of subtle psychotic experiences within nonpsychotic disorder represents a severity marker of multifactorial risk for psychopathology.63 Sample sizes for these analyses, however, were small, and the results should be interpreted cautiously. Nonetheless, our findings highlight that CC groups in CHR research support identification of both transdiagnostic and risk-specific aspects of psychopathology.
The distributions of age, gender, and cognitive functioning seen within the present CHR sample were comparable to prior reviews,10 suggesting our findings are representative of the broader CHR literature and are not merely a result of sampling bias. No effect of publication year or antipsychotic use was detected. Consistent with prior research,1 males tended to be overrepresented among CHR-T; although this could plausibly have influenced the results, available meta-analyses suggest no modifying effect of gender on relations between neuropsychological impairment and CHR status or transition.10 The demographic distributions were comparable among CC relative to the combined CHR group, but cognitive impairment within CC appeared somewhat smaller than is found in other reviews of adolescent and young adult psychopathology.42–44 Although there was little evidence of publication bias in the present meta-analysis, when bias was detected, it tended to suggest a lack of studies reporting greater cognitive impairment in CC vs CHR-NT. Together, these findings may suggest that the cognitive deficits seen among CC could underestimate that seen in the broader youth psychiatric literature, implying that neuropsychological differences between CHR and CC are even smaller than was observed here. This further underscores the transdiagnostic nature of cognitive difficulties and the need to consider the implications of this pattern for our understanding of impairment in CHR.
Limitations and Future Directions
Several study limitations warrant mention. First, although our review included 21 studies representing nearly 4000 participants, sample sizes varied across analyses, thus some domains (eg, perceptual organization) were insufficiently populated to be meta-analyzed across subgroups, and statistical power for moderator analyses in particular was low. Conclusions therefore should be limited to those domains for which adequate data were available, and the influence of possible modifiers of these effects (including those explored here as well as other relevant factors such as demographics and cooccurring disorders) deserves future attention. Second, we were unable to meta-analyze performance on specific cognitive tasks. Although our results are likely broadly applicable to cognitive functioning in the CHR population, it is plausible that some individual neuropsychological tests are capable of meaningfully differentiating CHR from CC. Third, although the main purpose of this study was to compare neuropsychological performance in CHR relative to a broad group of CC, sample size limitations temper firm conclusions about performance in CHR vs specific CC subgroups. Given that ADGP and PRTN (or other subgroups) may show meaningfully distinct clinical presentations and trajectories,21 further work in this area is needed.
The results of this study suggest a number of additional future directions. One important direction is to determine the pluripotentiality of neurocognition as it pertains to multiple diagnostic outcomes in CHR and other high-risk groups. Research has begun to show that risk factors for psychosis among those at CHR, notably maltreatment exposure, predict diagnostic changes beyond just psychotic disorder, including the onset of depressive and anxiety syndromes.64 The extent to which neuropsychological functioning follows a similar pattern has critical implications for neurodevelopmental models of psychosis as well as for early detection and prediction of clinical outcome. Given that some CC may be earlier in their course of illness than CHR and could later fulfill criteria for attenuated or threshold psychosis syndromes, a crucial component of this work will be tracking diagnostic changes in these groups longitudinally. Another important direction is to identify shared and distinct changes in cognitively relevant neural networks across CHR and other putatively high-risk populations.65 Additional future directions include research and discussion around the effects of ascertainment strategy (and associated risk of sampling bias) on transdiagnostic high-risk research,66 the experimental and analytic methods most appropriate for detecting shared and unique neurocognitive difficulties across groups (eg, tasks from experimental cognitive neuroscience,67 data-driven stratification approaches12), and how to interpret transdiagnostic patterns of results.68
Conclusions
Our basic conclusion from this study is that neuropsychological impairment in CHR cohorts should be interpreted cautiously when psychosis or even CHR status is the specific clinical syndrome of interest. Whereas marked baseline cognitive deficits in prospectively determined CHR-T may reflect a psychosis-specific vulnerability, impairments in CHR-NT – the majority of the CHR population – most likely reflect a transdiagnostic vulnerability, or more parsimoniously, one that is indistinguishable from that seen in other clinical groups. CHR research incorporating CC is essential for making this determination.
Funding
This work was funded by the National Institute of Mental Health (T32MH016259 to Z.B.M. and R01MH120090 to J.M.G.), the Andrew P. Merrill Memorial Research Fellowship (to Z.B.M.), and the Joseph and Susan Gatto Award for Excellence in Imaging, Drug Abuse, and Psychiatric Research (to Z.B.M.).
Supplementary Material
Acknowledgments
We thank the authors who produced the original research we review in this meta-analysis and the participants who made this research possible. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Zachary B Millman, Psychotic Disorders Division, McLean Hospital, Belmont, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
Caroline Roemer, Psychology Department, University of Maryland, Baltimore County, Baltimore, MD, USA.
Teresa Vargas, Department of Psychology, Northwestern University, Evanston, IL, USA.
Jason Schiffman, Department of Psychological Science, University of California, Irvine, CA, USA.
Vijay A Mittal, Department of Psychology, Northwestern University, Evanston, IL, USA.
James M Gold, Maryland Psychiatric Research Center, University of Maryland School of Medicine, Baltimore, MD, USA.
References
- 1. Salazar de Pablo G, Radua J, Pereira J, et al. Probability of transition to psychosis in individuals at clinical high risk: an updated meta-analysis. JAMA Psychiatry. 2021;78(9):970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry. 2015;172(3):249–258. [DOI] [PubMed] [Google Scholar]
- 3. Michel C, Ruhrmann S, Schimmelmann BG, Klosterkotter J, Schultze-Lutter F. Course of clinical high-risk states for psychosis beyond conversion. Eur Arch Psychiatry Clin Neurosci. 2018;268(1):39–48. [DOI] [PubMed] [Google Scholar]
- 4. Millman ZB, Gold JM, Mittal VA, Schiffman J. The critical need for help-seeking controls in clinical high-risk research. Clin Psychol Sci. 2019;7(6):1171–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Os J, Guloksuz SA. Critique of the “ultra-high risk” and “transition” paradigm. World Psychiatry. 2017;16(2):200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartmann JA, McGorry PD, Destree L, et al. Pluripotential risk and clinical staging: theoretical considerations and preliminary data from a transdiagnostic risk identification approach. Front Psychiatry. 2020;11:553578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGorry PD, Hartmann JA, Spooner R, Nelson B. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry. 2018;17(2):133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caspi A, Houts RM, Ambler A, et al. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin Birth Cohort Study. JAMA Netw Open. 2020;3(4):e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Catalan A, Salazar de Pablo G, Aymerich C, et al. Neurocognitive functioning in individuals at clinical high risk for psychosis: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(8):859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gold JM, Millman ZB, Dickinson D. Enhancing prediction of psychosis risk with cognitive measures: how do we get to there from here? JAMA Psychiatry. 2021;78(8):827–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crouse JJ, Chitty KM, Iorfino F, et al. Transdiagnostic neurocognitive subgroups and functional course in young people with emerging mental disorders: a cohort study. BJPsych Open. 2020;6(2):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139(1):81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moran TP. Anxiety and working memory capacity: a meta-analysis and narrative review. Psychol Bull. 2016;142(8):831–864. [DOI] [PubMed] [Google Scholar]
- 15. Abramovitch A, Short T, Schweiger A. The C factor: cognitive dysfunction as a transdiagnostic dimension in psychopathology. Clin Psychol Rev. 2021;86:102007. [DOI] [PubMed] [Google Scholar]
- 16. Carrion RE, Walder DJ, Auther AM, et al. From the psychosis prodrome to the first-episode of psychosis: no evidence of a cognitive decline. J Psychiatr Res. 2018;96:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindgren M, Manninen M, Laajasalo T, et al. The relationship between psychotic-like symptoms and neurocognitive performance in a general adolescent psychiatric sample. Schizophr Res. 2010;123(1):77–85. [DOI] [PubMed] [Google Scholar]
- 19. Liu CC, Hua MS, Hwang TJ, et al. Neurocognitive functioning of subjects with putative pre-psychotic states and early psychosis. Schizophr Res. 2015;164(1-3):40–46. [DOI] [PubMed] [Google Scholar]
- 20. Borenstein M, Hedges LV, Higgins JP, Rothstein HR.. Introduction to Meta-analysis. Chichester, West Sussex: John Wiley & Sons; 2021. [Google Scholar]
- 21. Lee TY, Lee J, Kim M, Choe E, Kwon JS. Can we predict psychosis outside the clinical high-risk state? A systematic review of non-psychotic risk syndromes for mental disorders. Schizophr Bull. 2018;44(2):276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii-x, 1–iii-x173. [DOI] [PubMed] [Google Scholar]
- 23. Hedges LV, Olkin I.. Statistical Methods for Meta-analysis. Orlando, Florida: Academic Press; 2014. [Google Scholar]
- 24. Lindgren M, Kuvaja H, Jokela M, Therman S. Predictive validity of psychosis risk models when applied to adolescent psychiatric patients. Psychol Med. 2021:1–12. [DOI] [PubMed] [Google Scholar]
- 25. Metzler S, Dvorsky D, Wyss C, et al. Changes in neurocognitive functioning during transition to manifest disease: comparison of individuals at risk for schizophrenic and bipolar affective psychoses. Psychol Med. 2015;45(10):2123–2134. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Addington J, Piskulic D, Perkins D, Woods SW, Liu L, Penn DL. Affect recognition in people at clinical high risk of psychosis. Schizophr Res. 2012;140(1-3):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grent-‘t-Jong T, Gajwani R, Gross J, et al. 40-Hz auditory steady-state responses characterize circuit dysfunctions and predict clinical outcomes in clinical high-risk for psychosis participants: a magnetoencephalography study. Biol Psychiatry. 2021;90(6):419–429. [DOI] [PubMed] [Google Scholar]
- 29. Ilonen T, Heinimaa M, Korkeila J, Svirskis T, Salokangas RK. Differentiating adolescents at clinical high risk for psychosis from psychotic and non-psychotic patients with the Rorschach. Psychiatry Res. 2010;179(2):151–156. [DOI] [PubMed] [Google Scholar]
- 30. Kindler J, Schultze-Lutter F, Hauf M, et al. Increased striatal and reduced prefrontal cerebral blood flow in clinical high risk for psychosis. Schizophr Bull. 2018;44(1):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koren D, Scheyer R, Stern Y, et al. Metacognition strengthens the association between neurocognition and attenuated psychosis syndrome: preliminary evidence from a pilot study among treatment-seeking versus healthy adolescents. Schizophr Res. 2019;210:207–214. [DOI] [PubMed] [Google Scholar]
- 32. Lincoln TM, Sundag J, Schlier B, Karow A. The relevance of emotion regulation in explaining why social exclusion triggers paranoia in individuals at clinical high risk of psychosis. Schizophr Bull. 2018;44(4):757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magaud E, Kebir O, Gut A, et al. Altered semantic but not phonological verbal fluency in young help-seeking individuals with ultra high risk of psychosis. Schizophr Res. 2010;123(1):53–58. [DOI] [PubMed] [Google Scholar]
- 34. Magaud E, Morvan Y, Rampazzo A, et al. Subjects at ultra high risk for psychosis have ‘heterogeneous’ intellectual functioning profile: a multiple-case study. Schizophr Res. 2014;152(2-3):415–420. [DOI] [PubMed] [Google Scholar]
- 35. Metzler S, Dvorsky D, Wyss C, et al. Neurocognitive profiles in help-seeking individuals: comparison of risk for psychosis and bipolar disorder criteria. Psychol Med. 2014;44(16):3543–3555. [DOI] [PubMed] [Google Scholar]
- 36. Millman ZB, Weintraub MJ, Bentley E, et al. Differential relations of locus of control to perceived social stress among help-seeking adolescents at low vs. high clinical risk of psychosis. Schizophr Res. 2017;184:39–44. [DOI] [PubMed] [Google Scholar]
- 37. Romanowska S, MacQueen G, Goldstein BI, et al. Neurocognitive deficits in a transdiagnostic clinical staging model. Psychiatry Res. 2018;270:1137–1142. [DOI] [PubMed] [Google Scholar]
- 38. Salazar de Pablo G, Guinart D, Cornblatt BA, et al. DSM-5 attenuated psychosis syndrome in adolescents hospitalized with non-psychotic psychiatric disorders. Front Psychiatry. 2020;11:568982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schulze C, Zimmermann R, Gschwandtner U, et al. Can cognitive deficits facilitate differential diagnosis between at-risk mental state for psychosis and depressive disorders? Early Interv Psychiatry. 2013;7(4):381–390. [DOI] [PubMed] [Google Scholar]
- 40. Simon AE, Cattapan-Ludewig K, Zmilacher S, et al. Cognitive functioning in the schizophrenia prodrome. Schizophr Bull. 2007;33(3):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szily E, Keri S. Anomalous subjective experience and psychosis risk in young depressed patients. Psychopathology. 2009;42(4):229–235. [DOI] [PubMed] [Google Scholar]
- 42. Goodall J, Fisher C, Hetrick S, Phillips L, Parrish EM, Allott K. Neurocognitive functioning in depressed young people: a systematic review and meta-analysis. Neuropsychol Rev. 2018;28(2):216–231. [DOI] [PubMed] [Google Scholar]
- 43. Bora E, Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophr Bull. 2015;41(5):1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allott K. Staging of Cognition in Psychiatric Illness. Cambridge: Clinical Staging in Psychiatry: Making Diagnosis Work for Research an d Treatment Cambridge University Press;2019:140–171. [Google Scholar]
- 45. Woods SW, PowersAR, 3rd, Taylor JH, et al. Lack of diagnostic pluripotentiality in patients at clinical high risk for psychosis: specificity of comorbidity persistence and search for pluripotential subgroups. Schizophr Bull. 2018;44(2):254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fusar-Poli P, Rutigliano G, Stahl D, et al. Long-term validity of the At Risk Mental State (ARMS) for predicting psychotic and non-psychotic mental disorders. Eur Psychiatry. 2017;42:49–54. [DOI] [PubMed] [Google Scholar]
- 47. Addington J, Piskulic D, Liu L, et al. Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophr Res. 2017;190:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hasmi L, Pries LK, Ten Have M, et al. What makes the psychosis “clinical high risk” state risky: psychosis itself or the co-presence of a non-psychotic disorder? Epidemiol Psychiatr Sci. 2021;30:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174(7):676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmidt A, Antoniades M, Allen P, et al. Longitudinal alterations in motivational salience processing in ultra-high-risk subjects for psychosis. Psychol Med. 2017;47(2):243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sharma A, Wolf DH, Ciric R, et al. Common dimensional reward deficits across mood and psychotic disorders: a connectome-wide association study. Am J Psychiatry. 2017;174(7):657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walton E, Hibar DP, van Erp TGM, et al. Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol Med. 2018;48(1):82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cannon TD, Chung Y, He G, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77(2):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hemager N, Plessen KJ, Thorup A, et al. Assessment of neurocognitive functions in 7-year-old children at familial high risk for schizophrenia or bipolar disorder: the Danish High Risk and Resilience Study VIA 7. JAMA Psychiatry. 2018;75(8):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johnson D, Policelli J, Li M, et al. Associations of early-life threat and deprivation with executive functioning in childhood and adolescence: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(11):e212511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. White LK, Moore TM, Calkins ME, et al. An evaluation of the specificity of executive function impairment in developmental psychopathology. J Am Acad Child Adolesc Psychiatry. 2017;56(11):975–982 e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anglin DM, Ereshefsky S, Klaunig MJ, et al. From womb to neighborhood: a racial analysis of social determinants of psychosis in the United States. Am J Psychiatry. 2021;178(7):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reininghaus U, Bohnke JR, Chavez-Baldini U, et al. Transdiagnostic dimensions of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). World Psychiatry. 2019;18(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smucny J, Barch DM, Gold JM, et al. Cross-diagnostic analysis of cognitive control in mental illness: insights from the CNTRACS consortium. Schizophr Res. 2019;208:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grisanzio KA, Goldstein-Piekarski AN, Wang MY, Rashed Ahmed AP, Samara Z, Williams LM. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry. 2018;75(2):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shah JL, Scott J, McGorry PD, et al. Transdiagnostic clinical staging in youth mental health: a first international consensus statement. World Psychiatry. 2020;19(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van Os J, Reininghaus U. Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry. 2016;15(2):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kraan TC, Velthorst E, Themmen M, et al. Child maltreatment and clinical outcome in individuals at ultra-high risk for psychosis in the EU-GEI High Risk Study. Schizophr Bull. 2018;44(3):584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McGorry P, Keshavan M, Goldstone S, et al. Biomarkers and clinical staging in psychiatry. World Psychiatry. 2014;13(3):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fusar-Poli P, Rutigliano G, Stahl D, et al. Deconstructing pretest risk enrichment to optimize prediction of psychosis in individuals at clinical high risk. JAMA Psychiatry. 2016;73(12):1260–1267. [DOI] [PubMed] [Google Scholar]
- 67. Gold JM, Corlett PR, Strauss GP, et al. Enhancing psychosis risk prediction through computational cognitive neuroscience. Schizophr Bull. 2020;46(6):1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Barch DM. What does it mean to be transdiagnostic and how would we know? Am J Psychiatry. 2020;177(5):370–372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.