Abstract
Background and Hypothesis
People with severe mental illness (SMI) may experience excess mortality and inequitable treatment following acute coronary syndrome (ACS). However, cardioprotective pharmacotherapy and SMI diagnoses other than schizophrenia are rarely examined in previous reviews. We hypothesized that SMI including bipolar disorder (BD) is associated with increased post-ACS mortality, decreased revascularization, and cardioprotective medication receipt relative to those without SMI.
Study Design
We performed a meta-analysis to quantitatively synthesize estimates of post-ACS mortality, major adverse cardiac events (MACEs), and receipt of invasive coronary procedures and cardioprotective medications in patients with SMI, comprising schizophrenia, BD, and other nonaffective psychoses, relative to non-SMI counterparts. Subgroup analyses stratified by SMI subtypes (schizophrenia, BD), incident ACS status, and post-ACS time frame for outcome evaluation were conducted.
Study Results
Twenty-two studies were included (n = 12 235 501, including 503 686 SMI patients). SMI was associated with increased overall (relative risk [RR] = 1.40 [95% confidence interval = 1.21–1.62]), 1-year (1.68 [1.42–1.98]), and 30-day (1.26 [1.05–1.51]) post-ACS mortality, lower receipt of revascularization (odds ratio = 0.57 [0.49–0.67]), and cardioprotective medications (RR = 0.89 [0.85–0.94]), but comparable rates of any/specific MACEs relative to non-SMI patients. Incident ACS status conferred further increase in post-ACS mortality. Schizophrenia was associated with heightened mortality irrespective of incident ACS status, while BD was linked to significantly elevated mortality only in incident ACS cohort. Both schizophrenia and BD patients had lower revascularization rates. Post-ACS mortality risk remained significantly increased with mild attenuation after adjusting for revascularization.
Conclusions
SMI is associated with increased post-ACS mortality and undertreatment. Effective multipronged interventions are urgently needed to reduce these physical health disparities.
Keywords: schizophrenia, bipolar disorder/cardiovascular disease, myocardial infarction, major adverse cardiac events, coronary procedures
Introduction
Severe mental illness (SMI) including schizophrenia, bipolar disorder (BD), and other nonaffective psychoses (ONAP) affects approximately 2%–3% of the population1–3 and constitutes one of the major causes of disability worldwide.4 People with SMI have markedly elevated risk of premature death, with 2.5- to 3-fold increased rate of excess mortality5–7 and 11–18 years shorter lifespan relative to the general population.8,9 Literature has consistently shown that excess mortality associated with SMI is mainly attributable to natural causes, with cardiovascular disease (CVD) being one of the leading causes of death.10–13 Substantial evidence also indicated an increased risk of CVD, including coronary heart disease (CHD),14 among people with SMI compared to the general population.15
There has been a growing concern regarding physical health disparities experienced by SMI patients following acute coronary syndrome (ACS), a severe and potentially life-threatening CHD subtype16 composed of acute myocardial infarction and unstable angina which is associated with substantially elevated mortality risk relative to stable CHD and overall CVD. It is well-established that ACS is amenable to timely, evidence-based intervention of coronary revascularization including percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG).17,18 A large body of research has also revealed that prescription of cardioprotective medications after ACS constitutes an effective secondary prevention of ACS recurrence and other adverse CVD-related outcomes.17 Nonetheless, contrary to a significant decline in CHD-related mortality in developed countries during the past decades,19,20 such mortality rate among SMI patients was found to remain stably high or decrease to a much lesser degree than that of the general population,15,20 indicating that SMI patients have not benefited from the improved treatment. Previous meta-analyses demonstrated an increased risk of post-ACS mortality in patients with schizophrenia21 and with any mental disorders.22 Recent data have further revealed that SMI was associated with a higher rate of major adverse cardiac events (MACEs) other than mortality such as bleeding, stroke, and reinfarction.23–25 Critically, there is evidence suggesting that patients with CVD (including ACS) and coexisting schizophrenia are less likely to undergo cardiac procedures such as catheterization and coronary revascularization than those CVD patients without SMI.22,26,27 Moreover, schizophrenia patients exhibited lower prescription rate of cardioprotective medication treatment for CVD than those without SMI.27,28
Of note, although a number of meta-analyses have recently been conducted to assess post-ACS mortality and suboptimal cardiac care in SMI patients, several important methodological constraints merit attention. First, prior reviews focused either on SMI as a single category or schizophrenia only without taking into consideration other types of SMI, in particular BD.21,22,26,27 Second, thus far, there has been no synthesis of evidence on the risk of MACEs other than mortality following ACS among SMI patients. Third, the most recent meta-analytic review evaluated receipt of cardiac procedures and cardioprotective medications in schizophrenia patients with a more broadly defined CHD and CVD, respectively, rather than specifically targeting at the occurrence of ACS.27 To our knowledge, there has been no meta-analysis investigating treatment inequalities on cardioprotective medications after ACS in SMI patients. Fourth, until now, no pooled analyses have been conducted to delineate the effect of incident ACS status (ie, first-recorded ACS) on post-ACS mortality and likelihood of receiving cardiac care for SMI patients.21,22,26,27 This approach can avoid the confounding effect of past history of ACS on outcome evaluation. Furthermore, past meta-analyses did not clarify potential differential rates of cardiac treatment receipt within various time frames (eg, 30-day or 1-year) following ACS.22,26,27 Incorporation of specified post-ACS time frames into analysis, however, would enable a more refined estimation of patterns of undertreatment experienced by SMI patients, especially the receipt of coronary revascularization shortly after admission for ACS.
To this end, we conducted a systematic review and meta-analysis with an aim to provide updated summary estimates for the risk of mortality and other MACEs, as well as the quality of cardiac care after ACS in patients with SMI (comprising schizophrenia, BD, and ONAP) relative to the counterparts without SMI. Post-ACS receipt of both cardiac procedures (cardiac catheterization and coronary revascularization) and cardioprotective medications were examined. In addition, subgroup analyses stratified by specific cardiac procedures and cardioprotective drug classes, types of SMI (schizophrenia, BD), incident ACS status, and different post-ACS time frames for outcome evaluation were performed. The potential confounding effect of revascularization receipt on post-ACS mortality was adjusted to allow a more accurate estimation of mortality risk among SMI patients following ACS.
Methods
This study was conducted in accordance with guidelines of Meta-analysis of Observational Studies in Epidemiology29 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA; supplementary table S1).30 The study protocol was registered with PROSPERO (CRD42021270212). Since this meta-analysis was based on published data, the requirement for ethical approval was waived.
Search Strategy
We systematically searched 4 electronic databases (EMBASE, MEDLINE, PsycINFO, and Web of Science) for articles published from inception to July 31, 2021. Relevant articles were searched using the following keywords: (severe mental illness OR schizophrenia OR bipolar disorder) AND (acute coronary syndrome OR myocardial infarction) AND (mortality OR major adverse cardiac outcomes OR cardiac procedures OR revascularization OR cardiovascular pharmacotherapy). The full search strategy for each database is available in supplementary table S2. We also checked the references of all eligible articles and relevant review articles to identify additional studies for inclusion into the meta-analysis. Two reviewers (J.K.N.C. and R.S.T.C.) performed the searches independently and then compared the results.
Inclusion Criteria and Study Selection
Studies were included if they were English articles fulfilling the following criteria: (1) included patients with hospital admission for ACS comprising acute myocardial infarction and unstable angina; (2) patients aged 15 years or above with SMI (ie, schizophrenia, BD and ONAP) diagnosed according to ICD or DSM classification (any versions) or by validated diagnostic instruments that could be mapped to ICD/DSM criteria; and (3) reported risk ratios for mortality, any other MACEs, receipt of cardiac procedures, or prescription of cardioprotective medications in ACS patients with vs without SMI. We excluded conference abstracts, review studies, or articles reporting duplicate data.
Following the removal of duplicates, titles and abstracts of the identified articles were screened independently by 3 reviewers (R.S.T.C., J.W.Y.L., and C.H.) for potentially relevant studies. The full text of the publications identified at the screening stage was then evaluated independently by 2 reviewers (J.W.Y.L. and C.H.) using our selection criteria to determine the eligibility for inclusion in data synthesis, with disagreements being resolved by discussion with other members (W.C.C. and C.S.M.W.) of the research team.
Data Extraction and Risk of Bias Assessment
Data were extracted independently by 2 reviewers (J.K.N.C. and R.S.T.C.) from the included studies, and disagreements were resolved by consensus. Information extracted included: First author’s name, year of publication, study country and region, study design, time frame for outcome evaluation, sample size of the ACS cohort, age and sex of ACS patients, sample size of SMI patients, SMI subtypes, incident ACS status, and risk ratios (including odds ratios [ORs], hazard ratios [HRs], relative risk) with 95% confidence intervals (CIs) for mortality, other MACEs, cardiac procedures, or prescription of cardioprotective medications. Since the current study focused on schizophrenia, BD, and ONAP, studies including data of patients with other psychiatric diagnoses such as depression in the study sample of SMI were excluded. If studies reported outcome measures at multiple time points (ie, specified time frames), the results on mortality and MACEs at the longest time-point, and the results on receipt of cardiac procedure and cardioprotective medications at the shortest time-point would be selected for analysis of an overall estimate of that particular outcomes. Risk ratios of multivariable regression took precedence over those of univariate regression. If several adjusted risk ratios were reported for the same outcome, the one controlling for the most comprehensive set of covariates was chosen. We contacted authors to request risk ratios if only cumulative incidence of outcomes in SMI patients and comparison group were reported in the publication. Risk of bias was assessed independently by 2 reviewers (J.K.N.C. and R.S.T.C.) using Newcastle-Ottawa Scale, which addresses the following 3 domains: (1) selection (representativeness, selection of nonexposed cohort, ascertainment of exposure, outcome of interest was not present at baseline), (2) comparability (study controlled for covariates), and (3) outcome (assessment of outcomes, follow-up duration ≥3 years for mortality and other MACEs unless otherwise specified). Disagreements were resolved through consultation with other members (W.C.C. and C.S.M.W.) of the research team.
Statistical Analysis
The OR and HR would approximate RR when cumulative incidence of an outcome in the study population is <10%.31,32 Since cumulative incidence of mortality and other MACEs is rare in the study population of included studies, OR and HR were directly converted to RR for these outcomes as in a previous meta-analysis evaluating mortality risk in ACS patients with schizophrenia.21 Conversion between RR and OR in outcomes regarding receipt of cardiac procedures and prescriptions of cardioprotective medications were performed using the reported control event rate.31 Standard errors of RR and OR were estimated from their 95% CIs. RR and OR were then log-transformed into a normalized distribution. Random-effects meta-analytic models were conducted to generate pooled estimates of transformed RR and OR from included studies for each outcome (ie, mortality, other MACEs, receipt of cardiac procedures, and prescription of cardioprotective medications). Heterogeneity was assessed using the chi-square Cochran’s Q-test and I2 statistic (significant heterogeneity indicated by P < .05 and/or an I2 > 50%). Publication bias was examined using the funnel plot and Egger’s regression asymmetry test. Synthesized estimates subject to publication bias were further corrected for using Duval and Tweedie’s trim-and-fill procedure. We also performed 2 sets of sensitivity analyses to evaluate the stability of results on pooled estimates of RR and OR by (1) restricting the analyses to studies classified as fair or good quality in risk-of-bias assessment; and (2) sequentially omitting a study at a time (ie, leave-one-out analysis). Subgroup analyses were performed, stratified by (1) SMI subtypes (ie, schizophrenia and BD), and (2) specific types of other MACEs (bleeding, reinfarction, stroke), cardiac procedures (catheterization, PCI, CABG, and any revascularization [ie, PCI/CABG]), and cardioprotective medications (angiotensin-converting enzyme inhibitors [ACEI]/angiotensin receptor blockers [ARB], beta-blockers, statins, antiplatelet agents of aspirin, and clopidogrel). Supplementary analyses were carried out to evaluate influences of predefined study characteristics on risk of mortality and quality of medical care, including (1) specified time frame for outcome evaluation (30-day and 1-year mortality; receipt of cardiac procedures during index admission for ACS, ≤30 days post-ACS, or ≤30 days post-discharge), and (2) the incident ACS status (ie, first-diagnosed ACS). Regarding mortality, evidence adjusting for revascularization was additionally pooled based on the premise that inferior cardiac treatment may substantially increase post-ACS mortality risk in SMI patients. A minimum of 3 independent studies was required to justify pooling of estimates in each subgroup and supplementary analysis. Meta-analysis models were performed in R (version 4.0.2) with the metafor package. P < .05 was considered statistically significant.
Results
Study Selection
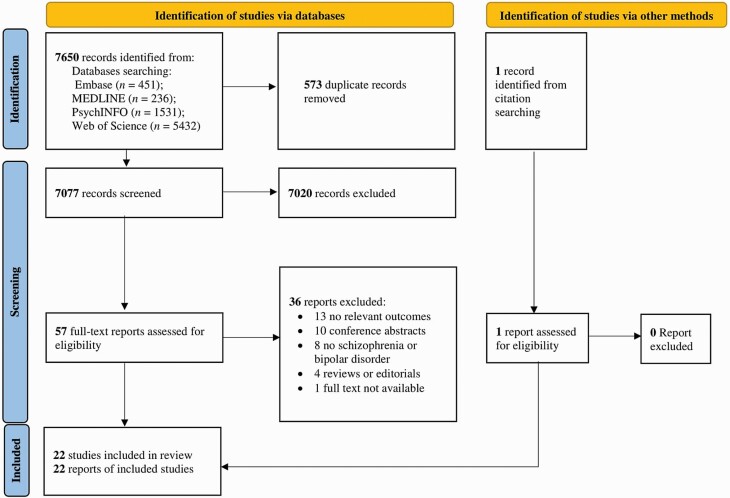
The PRISMA flow diagram describing the process of study identification and selection is shown in figure 1. Literature search identified 7650 articles, from which 7077 remained after removal of duplicates. Upon exclusion of irrelevant studies, we retrieved 57 full-text articles to be assessed for eligibility. One additional study was identified from citation searching. Of these, 36 were excluded mainly due to the following reasons: 13 did not report relevant outcomes; 10 were conference abstracts; and 8 included subjects without SMI or included mental disorders other than schizophrenia, BD, or ONAP in the definition of SMI. A total of 22 publications met inclusion criteria and were included in the current meta-analysis.
Fig. 1.
PRISMA flow chart for study selection.
Study Characteristics
Overall, the current meta-analysis included 12 235 501 patients with ACS (among which 503 686 had SMI diagnoses) from Australia,33 Canada,34–36 Denmark,24,37–39 Hong Kong,40 Sweden,41,42 Taiwan,43 United Kingdom,23,44 and the United States.25,45–51 One study reported OR of schizophrenia patients treated and untreated with cardioprotective medications relative to those without schizophrenia.39 We selected the estimate of schizophrenia patient subgroup treated with cardioprotective medications because a focus of our study was to evaluate the association of SMI with increased mortality in ACS patients adjusting for treatment-related characteristics including cardiac procedures and cardioprotective medications. All included studies were conducted in cohort design, except Wu et al.43 and Attar et al.24 which adopted nested case-control design. Diagnoses of schizophrenia, BD, and ONAP were all based on ICD criteria. Of the 22 included publications, 18, 9, and 3 studies reported post-ACS outcomes in patients with schizophrenia, BD, and ONAP, respectively. A total of 11 were conducted on patients with incident ACS. Characteristics of included studies are presented in table 1.
Table 1.
Characteristics of Included Studies
| Author, Year | Location | Design | Sample Size of ACS Patients | Sample Size of SMI Patients | Age (years) | Female Sex (%) | SMI Subtypes |
Diagnosis of SMI | Incident ACS | Mortality and Other MACEsa | Cardiac Proceduresa | Cardioprotective Medicationsa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Druss et al., 2000 | United States | Cohort | 113 653 | 188 | 75.5 | 53.4 | SCZ | ICD | No | 30-day mortality | Catheterization, PCI, CABG (inpatient) | NA |
| Young and Foster, 2000 | United States | Cohort | 354 195 | 1115 | NR | NR | SCZ | ICD | No | NA | Catheterization, PCI, CABG (inpatient) | NA |
| Druss et al., 2001 | United States | Cohort | 88 241 | 161 | 76.1 | 52.7 | SCZ | ICD | No | 1-year mortality | NA | ACEIs, beta-blockers (at discharge) |
| Lawrence et al., 2003 | Australia | Cohort | NR | NR | NR | NR | SCZ, BD, ONAPc | ICD | No | NA | Revascularization (time frame: NR) | NA |
| Jones and Carney, 2005 | United States | Cohort | 3368 | 1342 (NAPD)b | 54.2 | 23.8 | SCZ, ONAP | ICD | No | NA | PCI, CABG (inpatient or ≤30 days post-discharge) | NA |
| Kisely et al., 2009 | Canada | Cohort | 49 248 | 1285 (NAPD) | 65.4 | 42.0 | SCZ, ONAP | ICD | Yes | 28-day mortality, 1-year mortality | Catheterization, PCI, CABG (1-year post-ACS) | ACEIs, ARBs, beta-blockers, statins, clopidogrel (≤90 days post-discharge) |
| Laursen et al., 2009 | Denmark | Cohort | 605 649 | NR | NR | NR | SCZ, BD | ICD | No | NA | Revascularization (5-year post-ACS) | NA |
| Abrams et al., 2009 | United States | Cohort | 21 745 | 5225 | 68.5 | 2.0 | SCZ, BD | ICD | No | 30-day mortality | NA | NA |
| Kurdyak et al., 2012 | Canada | Cohort | 71 668 | 842 | 67.7 | 37.0 | SCZ | ICD | Yes | 30-day mortality | Revascularization (≤30 days post-ACS) | NA |
| Wu et al., 2013 | Taiwan | Nested case-control | 3361 | 591 (SCZ) 243 (BD) |
64.9 | 36.4 | SCZ, BD | ICD | Yes | 30-day mortality | Catheterization, revascularization (inpatient) | NA |
| Boden et al., 2015 | Sweden | Cohort | 209 592 | 541 (NAPD) 442 (BD) |
71.0 | 36.6 | SCZ, ONAP, BD | ICD | Yes | 30-day mortality, 1-year mortality | NA | NA |
| Sohn et al., 2015 | United States | Cohort | 42 416 | 16 140 | 65.9 | 39.3 | SCZ | ICD | No | Inpatient mortality | NA | NA |
| Schulman-Marcus et al., 2016 | United States | Cohort | 3 058 697 | 29 503 (Total) 12 590 (SCZ) 15 679 (BD) |
66.4 | 38.1 | SCZ, BD | ICD | Yes | 30-day mortality | PCI, CABG, revascularization (inpatient) | NA |
| Protty et al., 2017 | United Kingdom | Cohort | 57 668 | 236 (NAPD) | 70.3 | 41.7 | SCZ, ONAP | ICD | Yes | Mortality (5 years), MACEsd (5 years), stroke (5 years), reinfarction (5 years), bleeding (5 years) | NA | NA |
| Jakobsen et al., 2017 | Denmark | Cohort | 12 102 | 457 (Total) 64 (SCZ) 242 (BD) |
64.0 | 26.0 | SCZ, BD | ICD | No | 30-day mortality, mortality (11 years), MACEs (11 years), reinfarction (11 years) | Revascularization (≤30 days post-ACS) | ACEI, aspirin, beta-blockers, statin, clopidogrel, ticagrelor (1-year post-ACS) |
| Kugathasan et al., 2018 | Denmark | Cohort | 105 018 | 684 | 61.0 | 29.5 | SCZ | ICD | Yes | Mortality (21 years) | NA | NA |
| Attar et al., 2019 | Denmark | Nested case-control | 2178 | 726 | 61.2 | 38.0 | SCZ | ICD | Yes | Mortality (19 years), MACEs (19 years), reinfarction (19 years), stroke (19 years) | NA | NA |
| Mohamed et al., 2019 | United States | Cohort | 6 738 757 | 23 582 (SCZ), 41 362 (BD), 23 582 (ONAP) | 67.8 | 40.0 | SCZ, BD, ONAP | ICD | No | Mortality (inpatient), MACEs (inpatient), stroke (inpatient), bleeding (inpatient) | PCI,CABG (inpatient) | |
| Chang et al., 2020 | Hong Kong | Cohort | 67 692 | 703 (NAPD), 509 (SCZ), 194 (ONAP) | 71.2 | 37.9 | SCZ, ONAP | ICD | Yes | 30-day mortality, 1-year mortality | Catheterization, PCI, CABG, revascularization (≤30 days post-ACS) | ACEIs/ARBs, aspirin, beta-blockers, statins, clopidogrel (≤90 days post-discharge) |
| Attar et al., 2020 | Sweden | Cohort | 286 333 | 1008 | 71.3 | 36.0 | SCZ | ICD | No | Mortality (5 years), MACEs (5 years), reinfarction (5 years), stroke (5 years), heart failure (5 years), bleeding (5 years) | NA | ACEI/ARBs, aspirin, beta-blockers, statins, P2Y12 inhibitors (at discharge) |
| Hauck et al., 2020 | Canada | Cohort | 108 610 | 1145 | 68.0 | 36.4 | SCZ | ICD | Yes | 1-year mortality | NA | NA |
| Fleetwood et al., 2021 | United Kingdom | Cohort | 235 310 | 923 (SCZ) 642 (BD) |
69.1 | 41.7 | SCZ, BD | ICD | Yes | 30-day mortality, 1-year mortality, 5-year mortality, mortality (34 years) | Revascularization (≤30 days post-ACS) | NA |
Note: ACEI, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; ARB, angiotensin receptor blockers; BD, bipolar disorder; CABG, coronary artery bypass graft; MACE, major adverse cardiac events; NAPD, nonaffective psychotic disorders; NR, not reported; ONAP, other nonaffective psychoses; PCI, percutaneous coronary intervention; SCZ, schizophrenia; SMI, severe mental illness.
aOnly those reported outcome estimates (from individual studies) that were selected for the current meta-analyses are presented.
bNAPD comprised SCZ and ONAP.
cONAP included acute and transient psychotic disorders, delusional disorder, and other/unspecified nonorganic psychosis.
dMACEs included mortality and other MACEs.
Meta-analysis and Subgroup Analyses of Mortality and Other MACEs
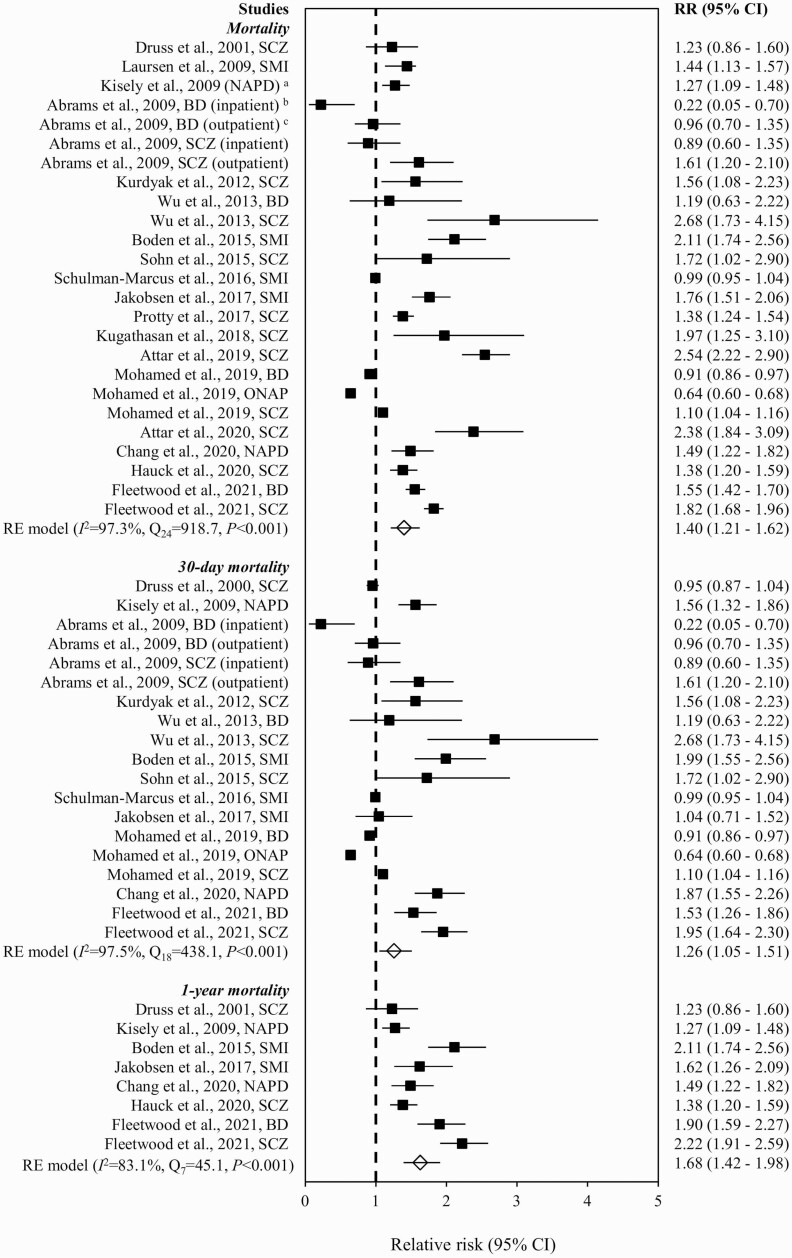
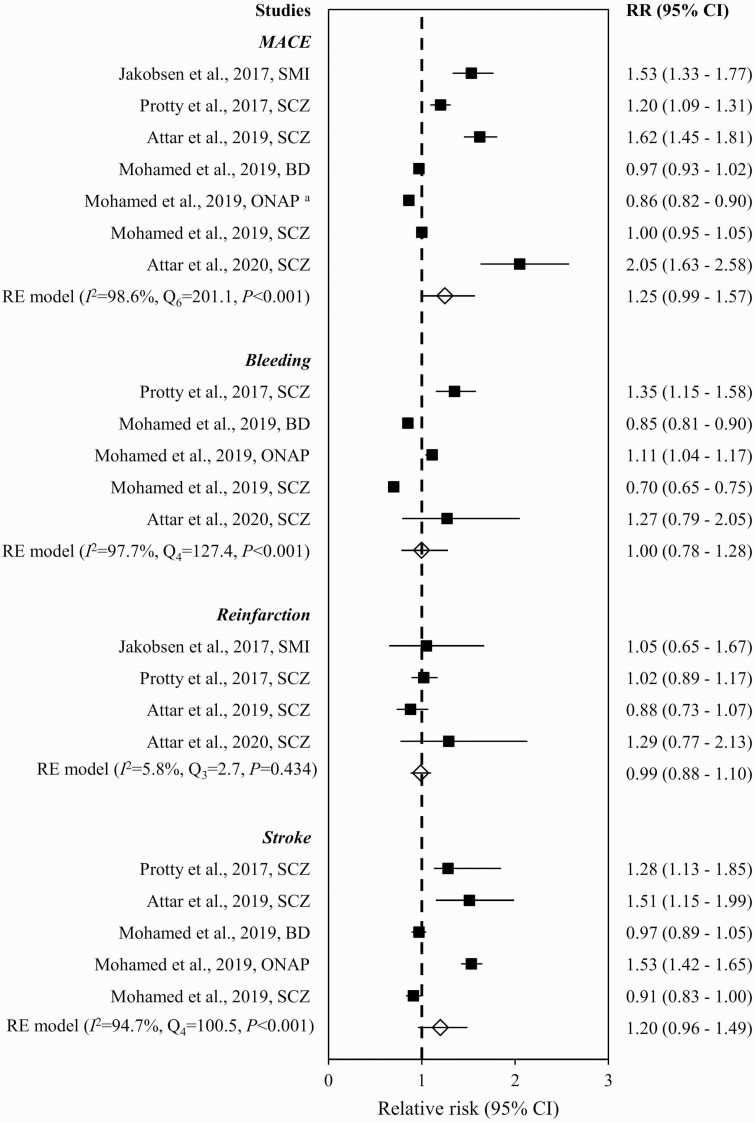
Figure 2 summarizes RR of mortality in ACS patients with vs without SMI in individual studies. The pooled RR showed that SMI was associated with increased risk of mortality (1.40 [95% CI = 1.21–1.62]), with significant heterogeneity (Q24 = 918.7, P < .001, I = 97.3%, k = 18). No publication bias was indicated by visual inspection of the funnel plot (ie, lack of asymmetry; supplementary figure S1) and nonsignificant result of Egger’s test (z = −0.7, P = .539). The synthesized estimate remained largely consistent with that in the original analysis when studies identified as of poor quality were excluded (1.36 [95% CI = 1.17–1.58]) and in the leave-one-out analysis (1.36–1.45). The elevated risk of mortality remained significant after controlling for receipt of revascularization (1.36 [1.06–1.74]; Q11 = 377.9, P < .001, I2 = 98.6%, k = 9; supplementary figure S2). Subgroup analyses stratified by specified time frame for mortality outcome evaluation demonstrated that risk of 30-day (RR = 1.26 [95% CI = 1.05–1.51]; Q18 = 438.1, P < .001, I2 = 97.5%, k = 12) and 1-year mortality (1.68 [1.42–1.98]; Q7 = 45.1, P < .001, I2 = 83.1%, k = 7) were significantly higher in SMI patients relative to those without SMI (figure 2). In stratified analyses by SMI subtypes, increased RR of mortality was observed in schizophrenia (1.60 [1.31–1.96]; Q13 = 376.6, P < .001, I2 = 97.1%, k = 13) but not BD (1.09 [0.84–1.40]; Q6 = 123.0, P < .001, I2 = 95.6%, k = 6) (supplementary figure S3). The pooled RR for mortality in SMI patients with incident ACS (1.74 [1.50–2.02]; Q12 = 105.9, P < .001, I2 = 90.6%, k = 10) was higher than that of the original analysis (supplementary figure S4). When both SMI subtypes and incident ACS status were considered, an elevated risk of mortality was observed in both schizophrenia (2.00 [1.63–2.45]; Q6 = 50.7, P < .001, I2 = 88.5%, k = 7) and BD (1.55 [1.42–1.69]; Q2 = 0.8, P = .685, I2 = 0.0%, k = 3) (supplementary figure S5). Subgroup analyses stratified by SMI subtypes and specified time frame for outcome evaluation found that schizophrenia (1.47 [1.15–1.88]; Q9 = 140.3, P < .001, I2 = 97.1%, k = 9), but not BD (1.05 [0.84–1.30]; Q6 = 34.9, P < .001, I2 = 90.9%, k = 6), patients showed higher risk of 30-day mortality relative to those without SMI (supplementary figure S6). Concerning other MACEs, the summary estimates of RR for any MACEs (1.25 [0.99–1.57]; Q6 = 201.1, P < .001, I2 = 98.6%, k = 5), bleeding (1.00 [0.78–1.28]; Q4 = 127.4, P < .001, I2 = 97.7%, k = 3), reinfarction (0.99 [0.88–1.10]; Q3 = 2.7, P = .434, I2 = 5.8%, k = 3), and stroke (1.20 [0.96–1.49]; Q4 = 100.5, P < .001, I2 = 94.7%, k = 3) were not significantly increased in SMI patients, relative to those without SMI (figure 3).
Fig. 2.
Risk of mortality in ACS patients with vs without severe mental illness. Note: ACS, acute coronary syndrome; BD, bipolar disorder; CI, confidence interval; NAPD, nonaffective psychotic disorders; ONAP, other nonaffective psychoses; RE, random effect; RR, relative risk; SCZ, schizophrenia; SMI, severe mental illness. aNAPD comprised schizophrenia (SCZ) and ONAP, whereas ONAP included acute and transient psychotic disorders, delusional disorder, and other/unspecified nonorganic psychosis. bPsychiatric diagnoses (BD, SCZ) were ascertained during index admission for ACS. cPsychiatric diagnoses (BD, SCZ) were ascertained in outpatient settings within 12 months before index admission for ACS.
Fig. 3.
Risk of MACEs in ACS patients with vs without severe mental illness. Note: ACS, acute coronary syndrome; BD, bipolar disorder; CI, confidence interval; MACE, major adverse cardiac events; NAPD, nonaffective psychotic disorders; ONAP, other nonaffective psychoses; RE, random effect; RR, risk ratios; SCZ, schizophrenia; SMI, severe mental illness. aONAP included acute and transient psychotic disorders, delusional disorder, and other/unspecified nonorganic psychosis.
Meta-analysis and Subgroup Analyses of Cardiac Procedures
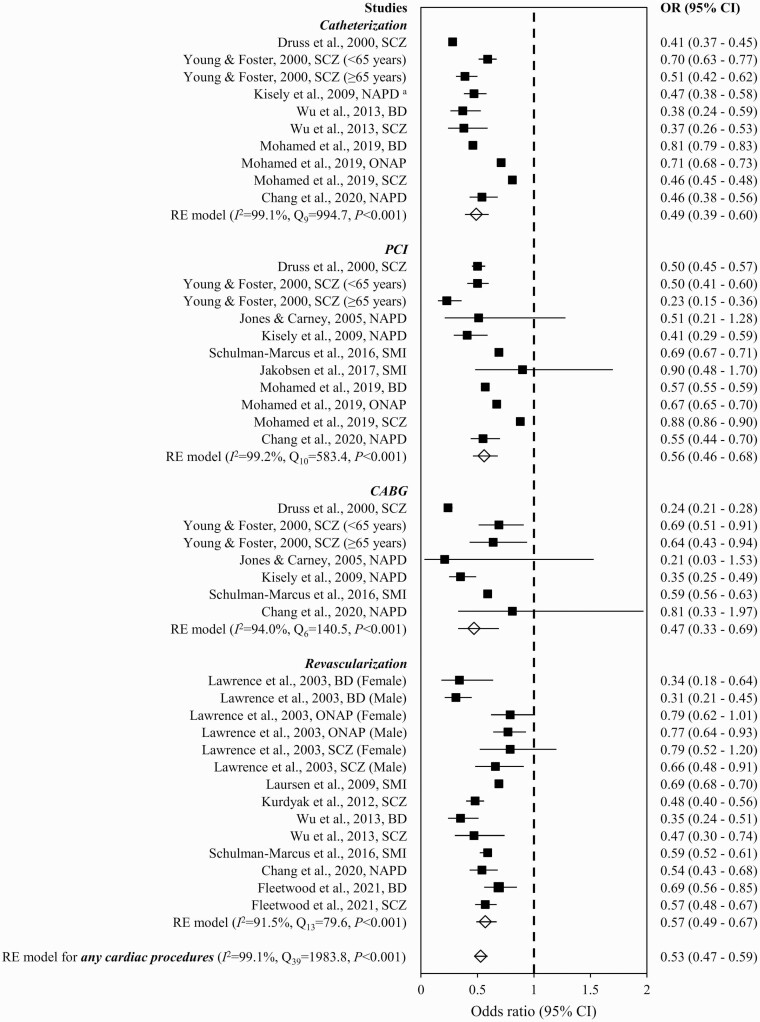
As shown in figure 4, ACS patients with SMI showed a significantly reduced estimate of overall OR for any cardiac procedures (0.53 [0.47–0.59]) compared to their counterparts without SMI. The OR among included studies were heterogeneous (Q39 = 1983.8, P < .001, I2 = 99.1%, k = 12). There is no evidence of publication bias as indicated by the symmetrical funnel plot inspected visually (supplementary figure S7) and nonsignificant result of Egger’s test (z = −1.6, P = .117). The estimate of overall OR was not significantly altered in sensitivity analyses, where studies regarded as poor quality in the risk-of-bias assessment were excluded (OR = 0.50 [95% CI = 0.45–0.57]) and in the leave-one-out analysis (OR = 0.51–0.54). Subgroup analyses stratified by types of cardiac procedures showed lower rates of catheterization (OR = 0.49 [0.39–0.60]; Q9 = 994.7, P < .001, I2 = 99.1%, k = 6), PCI (0.56 [0.46–0.68]; Q10 = 583.4, P < .001, I2 = 99.2%, k = 8), CABG (0.47 [0.33–0.69]; Q6 = 140.5, P < .001, I2 = 94.0%, k = 6), and any revascularization (0.57 [0.49–0.67]; Q13 = 79.6, P < .001, I2 = 91.5%, k = 7) in SMI patients than those without SMI (figure 4). In stratified analyses by SMI subtypes, the summary estimates of OR showed that both schizophrenia (0.43 [0.37–0.49]; Q19 = 349.4, P < .001, I2 = 96.8%, k = 9) and BD (0.74 [0.66–0.84]; Q7 = 57.2, P < .001, I2 = 96.4%, k = 5) patients received lower rate of any cardiac procedures than non-SMI counterparts (supplementary figure S8). Specified time frame of during index admission, ≤30 days post-ACS, or ≤30 days post-discharge (0.51 [0.45–0.58]; Q27 = 1912.8, P < .001, I2 = 99.0%, k = 10) and incident ACS status (0.47 [0.42–0.53]; Q11 = 25.6, P = .007, I2 = 60.2%, k = 5) exerted small effect on the synthesized estimate of OR for receipt of any cardiac procedures (supplementary figures S9 and S10). Concerning receipt of any revascularization, the pooled estimates of OR were consistent with that of the original analysis when specified time frame restricting to inpatient, ≤30 days post-ACS, or ≤30 days post-discharge and incident ACS status were considered (supplementary figures S11 and S12). Subgroup analyses stratified by SMI subtypes and restricting to specified time frame of inpatient, ≤30 days post-ACS, or ≤30 days post-discharge revealed lower likelihood of receiving revascularization after ACS in schizophrenia (0.46 [0.38–0.55]; Q11 = 241.4, P < .001, I2 = 96.9%, k = 8) and BD patients (0.75 [0.63–0.89]; Q3 = 32.8, P < .001, I2 = 96.3%, k = 4) than those without SMI (supplementary figure S13). Proportions of ACS patients with and without SMI who had received cardiac procedures in individual studies included in the meta-analysis are shown in supplementary table S4.
Fig. 4.
Receipt of cardiac procedures in ACS patients with vs without severe mental illness. Note: ACS, acute coronary syndrome; BD, bipolar disorder; CABG, coronary artery bypass grafting; CI, confidence interval; NAPD, nonaffective psychotic disorders; ONAP, other nonaffective psychoses; OR, odds ratio; PCI, percutaneous coronary intervention; RE, random effect; SCZ, schizophrenia; SMI, severe mental illness. aNAPD comprised schizophrenia and ONAP, whereas ONAP included acute and transient psychotic disorder, delusional disorder, and other/unspecified nonorganic psychosis.
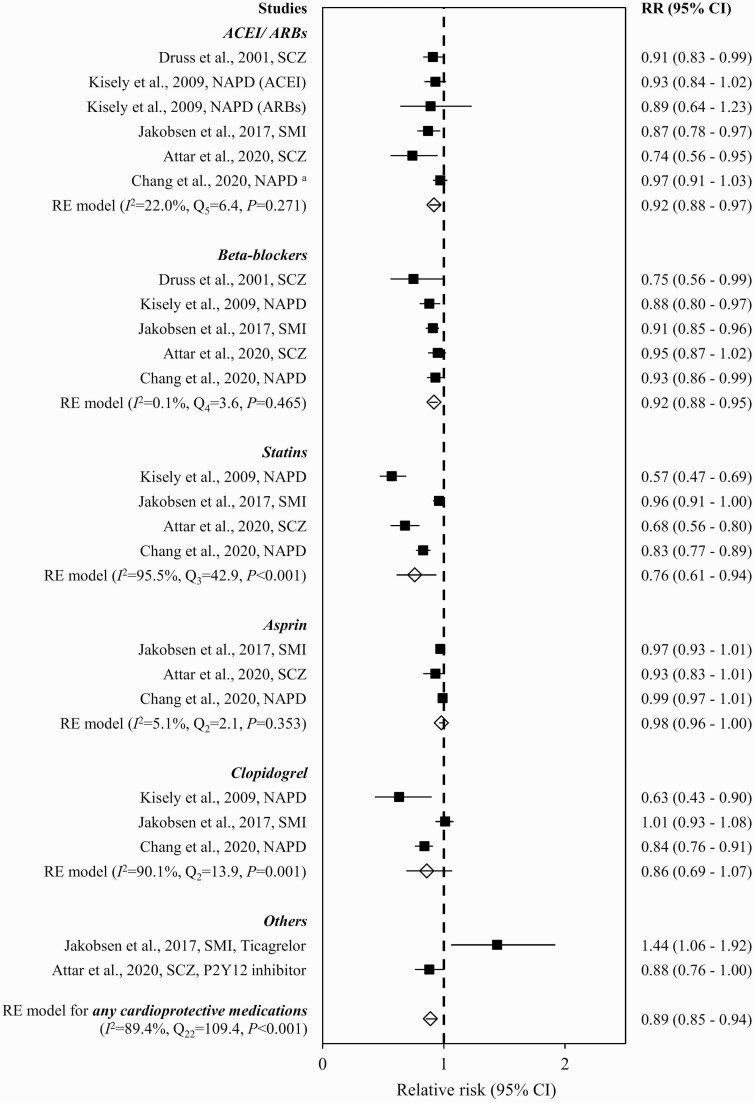
Meta-analysis and Subgroup Analyses of Cardioprotective Medications
The pooled estimate of RR demonstrated a lower prescription rate of cardioprotective medications within 1 year after ACS in SMI patients relative to non-SMI counterparts (0.89 [0.85–0.94]), with significant heterogeneity (Q22 = 109.4, P < .001, I2 = 89.4%, k = 5) (figure 5). Asymmetrical funnel plot based on visual inspection (supplementary figure S14) and significant result of Egger’s test (z = -3.5, P < .001) indicated the presence of a publication bias, which remained significant after correction with the trim-and-fill procedure (z = −3.5, P < .001). The leave-one-out analysis showed that the pooled RR was not influenced by a single trial (0.89–0.92). Subgroup analyses stratified by cardioprotective drug classes showed that SMI was associated with lower prescription rates of ACEI/ARBs (0.92 [95% CI = 0.88–0.97]; Q5 = 6.4, P = .271, I2 = 22.0%, k = 5), beta-blockers (0.92 [0.88–0.95]; Q4 = 3.6, P = .465, I2 = 0.1%, k = 5), and statins (0.76 [0.61–0.94]; Q3 = 42.9, P < .001, I2 = 95.5%, k = 4), but not aspirin (0.98 [0.96–1.00]; Q2 = 2.1, P = .353, I2 = 5.1%, k = 3) or clopidogrel (0.86 [0.69–1.07]; Q2 = 13.9, P = .001, I2 = 90.1%, k = 3) (figure 5). Incident ACS status exerted a small effect on the summary estimate of RR for the prescription rate of cardioprotective medications associated with SMI (0.86 [0.78–0.95]; Q9 = 70.8, P < .001, I2 = 93.1%, k = 2; supplementary figure S15). Proportions of ACS patients with and without SMI who had received cardioprotective medications in individual studies included in the meta-analysis are shown in supplementary table S5.
Fig. 5.
Prescription of cardioprotective medications in ACS patients with vs without severe mental illness. Note: ACEI, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; ARBs, angiotensin receptor blockers; CI, confidence interval; NAPD, nonaffective psychotic disorders; RE, random effect; RR, relative risk; SCZ, schizophrenia; SMI, severe mental illness. aNAPD comprised schizophrenia and ONAP, whereas ONAP included acute and transient psychotic disorders, delusional disorder, and other/unspecified nonorganic psychosis.
Risk of Bias Assessment
Of the 22 publications included in the current meta-analysis, 15, 4, and 3 studies were rated as of good, fair, and poor quality in the risk-of-bias assessment, respectively. Failure to adjust for covariates including demographic characteristics, baseline physical comorbidity, receipt of revascularization treatment, and prescription of cardioprotective medications was the most common methodological omission in analyses for mortality and other MACEs. Details of the quality assessment for individual studies are presented in supplementary table S3.
Discussion
To our knowledge, this is the first meta-analysis to comprehensively summarize data on the risk of mortality and other MACEs, as well as the likelihood of receiving invasive cardiac procedures and cardioprotective medication prescription following ACS in patients with coexisting SMI encompassing schizophrenia, BD, and ONAP relative to non-SMI counterparts. Our results indicated that SMI patients exhibited an increased risk of post-ACS mortality, with significantly elevated overall, 30-day, and 1-year death rates (ie, 40%, 26%, and 68% higher rates, respectively) relative to those without SMI. Subgroup analyses stratified by SMI subtypes showed that schizophrenia patients had 60% higher overall post-ACS mortality rate (and 53% increased 30-day mortality rate) than those without the disorder. This is consistent with a prior meta-analysis demonstrating that schizophrenia was associated with 66% raised post-ACS mortality rate,21 although several studies included in this pooled analysis incorporated ONAP in their diagnostic definition for schizophrenia23,41,52 and were therefore excluded from our analysis. Alternatively, the current study is the first to produce summary estimates on post-ACS mortality rate for patients with BD. We found lack of significant increase in overall and 30-day post-ACS mortality rates in BD patients relative to non-BD patients. Notably, when our analyses were restricted to studies examining incident ACS cohorts, we observed further increase in overall post-ACS mortality rate in SMI (RR: 1.74 vs 1.40) and schizophrenia (RR: 2.00 vs 1.60). Importantly, our findings revealed significantly increased overall mortality rate in BD patients presenting with incident ACS (RR: 1.55). This thus indicates differential impact of presence vs absence of past ACS episodes in estimating post-ACS mortality risk among SMI patients, and suggests that future investigation should take into account incident ACS status in post-ACS outcome analysis. Comparatively, BD was associated with lower magnitude of post-ACS mortality risk than schizophrenia. Such findings, however, should be treated with caution due to paucity of existing data on BD. In fact, previous reports assessing post-ACS mortality (and cardiac treatment) in SMI patients primarily focused on schizophrenia, and BD was understudied in this respect.53 More research evaluating mortality risk following ACS in BD patients should be conducted to verify our results. Conversely, SMI patients did not differ from non-SMI patients in the likelihood of experiencing any or specific MACEs (other than mortality) after ACS. Nonetheless, a small number of studies available for our analysis might preclude us from detecting subtle yet potentially significant between-group differences.
Our findings that SMI patients were significantly less likely to receive invasive cardiac procedures (47% lower receipt), including cardiac catheterization and coronary revascularization, than those without SMI following ACS are in keeping with previous meta-analyses showing lower revascularization rate in schizophrenia patients.22,26,27 Similar degrees of treatment inequalities were observed in our subgroup analyses stratified by modalities of cardiac procedures encompassing catheterization, revascularization, PCI, and CABG (ranged 43%–53% lower receipt), specified time frame of during index admission for ACS, 30 days post-ACS, or 30 days post-discharge (49% lower), and incident ACS status (53% lower). Both schizophrenia and BD were associated with lower likelihood of receiving any cardiac procedures (SCZ: 57% and BD: 26% lower receipt) and revascularization (SCZ: 54% and BD: 25% lower receipt) following ACS than those without the disorders, with such differential treatment gap being more pronounced in schizophrenia. Critically, our study extends previous findings of meta-analyses on suboptimal post-ACS cardiac care for SMI patients in terms of reduced receipt of revascularization by providing quantitative estimates indicating decreased prescription rates of cardioprotective medications following ACS relative to those without SMI. Specifically, our results revealed significantly lower prescription rates for any cardioprotective medications (11% lower receipt) as well as ACEIs/ARBs, beta-blockers, and statins (ranged 8%–24% lower) shortly after ACS among SMI patients relative to non-SMI patients. Analysis restricting to incident ACS cohort yielded similar estimate on reduced likelihood of any cardioprotective medication prescription. However, SMI patients did not differ from non-SMI counterparts in prescription rate for aspirin or clopidogrel. This null finding might partly be explained by a hierarchy within treatment, with antiplatelet agent being regarded as an essential cardioprotective medication (relative to other cardioprotective drug classes) for secondary prevention of recurrence of ACS and other cardiac events.54,55 In fact, earlier meta-analyses have also demonstrated inequitable cardioprotective medication treatment in SMI or schizophrenia patients, albeit in the presence of any coexisting physical diseases28 or broadly defined CVD.27 It is suggested that the association between SMI and inferior medical care could be explained by an array of patient, physician, and system factors.56 For instance, symptoms of SMI such as psychotic symptoms, diminished motivation, and cognitive dysfunction may impair patients’ ability of proper self-management for physical diseases including CVD, resulting in poorer treatment outcome.53 Stigmatizing attitudes commonly harbored by healthcare professionals in primary and specialist medical care settings towards people with SMI,57,58 their heightened tendency of misattributing physical symptoms to the manifestations of psychiatric disorders (ie, diagnostic overshadowing),59 and lack of service coordination between medical and psychiatric care56 are also regarded as important barriers to early recognition, timely and optimal management of physical diseases in SMI patients.
It is posited that suboptimal cardiac care may explain a substantial portion of excess mortality in SMI patients following ACS.60 A recent meta-analytic review found that the relationship between schizophrenia and raised post-ACS mortality became nonsignificant when 4 studies controlling for revascularization were pooled for analysis.21 On the contrary, our results, which were based on 9 studies with 12 SMI cohorts comprising also BD and ONAP patients, showed that the association between SMI and elevated post-ACS mortality adjusting for revascularization remained robust with mild attenuation (reduced adjusted OR: 1.40–1.36), suggesting that lower receipt of coronary interventional procedures is unlikely to substantially account for increased mortality associated with SMI. On the other hand, a small number of published studies on post-ACS prescription of cardioprotective medications in SMI patients precluded us from further adjusting pharmacotherapy in our meta-analytic models for a more refined estimation of post-ACS mortality risk. That said, a recent study revealed decreased post-ACS mortality risk, to a small degree, among patients with nonaffective psychotic disorders when cardioprotective medication prescription was adjusted in multivariate regression analysis.40 A Danish nationwide register-based study demonstrated that schizophrenia patients exposed to higher treatment intensity of cardioprotective medications displayed greater reduction in mortality risk following myocardial infarction.39 In particular, patients with schizophrenia who were treated with any combination of triple therapy had mortality rate similar to those observed in the general population (ie, patients on triple therapy after myocardial infarction but without preexisting schizophrenia).39 Further investigation is warranted to clarify differential and combined effects of inequitable revascularization and cardioprotective medication treatment on post-ACS mortality in SMI patients. Alternatively, it is suggested that excess mortality after cardiac events in SMI patients is likely multifactorial. Socioeconomic disadvantage and unhealthy lifestyle including physical inactivity, poor diet, smoking, and alcohol drinking, which are frequently associated with SMI, may contribute to elevated mortality risk.53 Use of antipsychotics, the mainstay treatment for SMI, is a risk factor of QTc interval prolongation61,62 which may induce cardiac arrhythmia (esp. torsade de pointes) and increase mortality risk following ACS. Literature also showed that patients with psychotic disorders had lower physical healthcare utilization,63 including cardiologist services,35 and were less likely to receive guideline-recommended screening and monitoring of CVD risk factors and metabolic parameters than the general population.27,64 This in turn may result in delayed initiation of treatment, more advanced ACS presentation with greater severity, and consequent heightened mortality rate for SMI patients. In line with these findings, a recent study even noted underdiagnosis of CVD prior to CVD-related death among schizophrenia patients.65
Given that factors contributing to excess post-ACS mortality and inferior cardiac care among SMI patients are multiple and intertwined, adoption of multilevel intervention framework66 comprising preventative measures for CVD development is thus recommended for outcome improvement. Individual-level strategies include promotion of patients’ physical health literacy and lifestyle interventions addressing modifiable cardiometabolic risk factors such as regular exercise, smoking cessation, and weight reduction. Symptom control for SMI should be optimized to enhance patients’ self-management ability for physical conditions, with judicious use of antipsychotics (ie, avoid polypharmacy and high-dose treatment unless clinically indicated) to minimize risk of metabolic syndrome and cardiotoxicity. System- and physician-focused interventions consisting of strategies to reduce stigma towards SMI patients with decreased diagnostic overshadowing, implementation of regular guideline-concordant monitoring of cardiometabolic parameters, and facilitation of coordinated psychiatric and physical healthcare delivery.53 These measures would lower the likelihood of underdiagnosis and undertreatment of CVD in SMI patients. Additionally, as evidence indicates that cardiometabolic abnormalities have already emerged in the initial stage of SMI,67–69 early intervention targeting at CVD risk factors in this critical illness period70 could lower the risk for subsequent poor cardiometabolic trajectories and excess CVD-related mortality. Development of cardiometabolic risk prediction algorithms that are specific to SMI populations would further facilitate identification of patients at high risk for adverse CVD outcomes and delivery of individualized interventions to prevent avoidable morbidity and mortality.71,72
Several limitations should be noted in interpreting the study result. First, most of our pooled analyses showed high heterogeneity, as is often the case in meta-analyses of large-scale observational studies.73 We selected estimates derived from the fully adjusted models of included individual studies for our meta-analyses to minimize the effect of potential confounders on the associations between SMI and various post-ACS outcomes. We also performed subgroup analyses stratified by SMI subtypes, incident ACS status, and specified time frame for outcome evaluation to identify potential sources of heterogeneity. Nonetheless, there is a risk of utilizing data from adjusted models which may be overadjusted, resulting in lowering the likelihood to detect true effect differences between SMI and non-SMI patients with ACS. In addition, other potential contributors to the heterogeneity such as social deprivation, psychotropic medication treatment, and lifestyle behaviors could not be examined in the current meta-analysis as these variables were rarely reported in previous studies. Second, pooled analyses for BD, specific categories of MACEs, and individual cardioprotective drug classes were conducted on a limited number of studies. These findings should be comprehensively reevaluated when more studies have been published in this respect. Third, as the vast majority of the included studies were conducted in Western countries (with only 2 of the 22 included studies being conducted in Asia), our reported summary estimates may not be generalizable to other geographical regions due to significant cross-regional variation in sociocultural context, healthcare systems, and population health indices.74,75 Fourth, we were unable to conduct additional analyses to further clarify the association between SMI and post-ACS mortality by controlling for differential mortality risk conferred by SMI per se (ie, independent of ACS, as SMI is associated with excess mortality) due to paucity of existing data in this regard. Further research comparing mortality rates of SMI patients experiencing ACS with controls from the general population experiencing ACS, as well as SMI patients and controls without ACS would help delineate such confounding effect.20 Fifth, as we only included articles published in English, our meta-analysis may therefore miss those studies which were potentially eligible but published in other languages. Lastly, the current study aimed to quantify mortality risk and quality of cardiac care following ACS in SMI patients relative to non-SMI counterparts. We were not able to address possible mechanisms underlying elevated post-ACS mortality rates (though revascularization receipt was also adjusted) and inferior treatment for SMI. Future investigation is therefore needed to identify these mechanisms and to clarify their differential contributions to adverse post-ACS outcomes.
In conclusion, this meta-analysis indicates that SMI patients experienced increased mortality rate, reduced receipt of any cardiac procedures and revascularization, as well as lower prescription rate of cardioprotective medications following ACS relative to those without SMI. The relationship between SMI and elevated post-ACS mortality remained significant, albeit mildly attenuated, after adjustment for revascularization. SMI patients presenting with incident ACS demonstrated further increase in differential mortality rate. Schizophrenia and BD were associated with raised post-ACS mortality rate and lower likelihood of receiving cardiac procedures and revascularization, with the magnitude of excess mortality and inequitable treatment receipt being more pronounced in the former than the latter. Our findings thus affirm poorer post-ACS outcomes and inferior cardiac treatment for SMI patients, and underscore an urgent need to adequately address physical health disparities experienced by this vulnerable population. Future research should also systematically evaluate relevant moderators and mediators for heightened CVD-related mortality among SMI patients so as to optimize treatment strategies with consequent outcome improvement.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Joe Kwun Nam Chan, Department of Psychiatry, LKS Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong.
Ryan Sai Ting Chu, Department of Psychiatry, LKS Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong.
Chun Hung, Department of Psychiatry, LKS Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong.
Jenny Wai Yiu Law, Department of Psychiatry, LKS Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong.
Corine Sau Man Wong, School of Public Health, LKS Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong.
Wing Chung Chang, Department of Psychiatry, LKS Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong; State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Pokfulam, Hong Kong.
Funding
The study was supported by the Hong Kong Research Grants Council (grant number: 17127417). The funders had no role in study design, data collection, data analysis, interpretation of the data, manuscript preparation, or journal submission.
Conflict of Interest
The authors declared no conflicts of interest in relation to the subject of this study.
References
- 1. Chang WC, Wong CSM, Chen EYH, et al. Lifetime prevalence and correlates of schizophrenia-spectrum, affective, and other non-affective psychotic disorders in the Chinese adult population. Schizophr Bull. 2017;43:1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McIntyre RS, Berk M, Brietzke E, et al. Bipolar disorders. Lancet. 2020;396:1841–1856. [DOI] [PubMed] [Google Scholar]
- 3. Jauchar S, Johnstone M, McKenna P. Schizophrenia. Lancet. 2022;399:473–486. [DOI] [PubMed] [Google Scholar]
- 4. GBD. 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oakley P, Kisely S, Baxter A, et al. Increased mortality among people with schizophrenia and other non-affective psychotic disorders in the community: a systematic review and meta-analysis. J Psychiatr Res. 2018;102:245–253. [DOI] [PubMed] [Google Scholar]
- 6. Ali S, Santomauro D, Ferrari AJ, Charlson F. Excess mortality in severe mental disorders: a systematic review and meta-regression. J Psychiatr Res. 2022;149:97–105. [DOI] [PubMed] [Google Scholar]
- 7. Correll CU, Solmi M, Croatto G, et al. Mortality in people with schizophrenia: a systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry. 2022;21:248–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hjorthoj C, Sturup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4:295–301. [DOI] [PubMed] [Google Scholar]
- 9. Chan JKN, Tong CHY, Wong CSM, Chen EYH, Chang WC. Life expectancy and years of potential life lost in bipolar disorder: systematic review and meta-analysis. Br J Psychiatry. 2022; doi: 10.1192/bjp.2022.19. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170:324–333. [DOI] [PubMed] [Google Scholar]
- 11. Jayatilleke N, Hayes RD, Dutta R, et al. Contributions of specific causes of death to lost life expectancy in severe mental illness. Eur Psychiatry. 2017;43:109–115. [DOI] [PubMed] [Google Scholar]
- 12. Chan JKN, Wong CSM, Yung NCL, Chen EYH, Chang WC. Excess mortality and life-years lost in people with bipolar disorder: an 11-year population-based cohort study. Epidemiol Psychiatr Sci. 2021;30:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yung NCL, Wong CSM, Chan JKN, Chen EYH, Chang WC. Excess mortality and life-years lost in people with schizophrenia and other non-affective psychoses: an 11-year population-based cohort study. Schizophr Bull. 2021;47:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. GBD. 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–e155. [DOI] [PubMed] [Google Scholar]
- 18. Lüscher TF. Myocardial revascularization: guideline-based therapy today. Eur Heart J. 2019;40:75–78. [DOI] [PubMed] [Google Scholar]
- 19. Moran AE, Forouzanfar MH, Roth GA, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kugathasan P, Laursen TM, Grøntved S, Jensen SE, Aagaard J, Nielsen RE. Increased long-term mortality after myocardial infarction in patients with schizophrenia. Schizophr Res. 2018;199:103–108. [DOI] [PubMed] [Google Scholar]
- 21. Shao M, Tian H, Wang L, Jiang D, Ji F, Zhuo C. Mortality risk following acute coronary syndrome among patients with schizophrenia: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109737. [DOI] [PubMed] [Google Scholar]
- 22. Mitchell AJ, Lawrence D. Revascularization and mortality rates following acute coronary syndromes in people with severe mental illness: comparative meta-analysis. Br J Psychiatry. 2011;198:434–441. [DOI] [PubMed] [Google Scholar]
- 23. Protty MB, Lacey A, Smith D, Hannoodee S, Freeman P. Increased morbidity, mortality and length of in-hospital stay for patients with acute coronary syndrome with pre-morbid psychiatric diagnoses. Int J Cardiol. 2017;236:5–8. [DOI] [PubMed] [Google Scholar]
- 24. Attar R, Valentin JB, Freeman P, Andell P, Aagaard J, Jensen SE. The effect of schizophrenia on major adverse cardiac events, length of hospital stay, and prevalence of somatic comorbidities following acute coronary syndrome. Eur Heart J Qual Care Clin Outcomes. 2019;5:121–126. [DOI] [PubMed] [Google Scholar]
- 25. Mohamed MO, Rashid M, Farooq S, et al. Acute myocardial infarction in severe mental illness: prevalence, clinical outcomes, and process of care in U.S. hospitalizations. Can J Cardiol. 2019;35:821–830. [DOI] [PubMed] [Google Scholar]
- 26. Shao M, Zhuo C, Cao X, et al. Reduced rate of revascularization in schizophrenic patients with acute myocardial infarction: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109870. [DOI] [PubMed] [Google Scholar]
- 27. Solmi M, Fiedorowicz J, Poddighe L, et al. Disparities in screening and treatment of cardiovascular diseases in patients with mental disorders across the world: systematic review and meta-analysis of 47 observational studies. Am J Psychiatry. 2021;178:793–803. [DOI] [PubMed] [Google Scholar]
- 28. Mitchell AJ, Lord O, Malone D. Differences in the prescribing of medication for physical disorders in individuals with v without mental illness: meta-analysis. Br J Psychiatry. 2012;201:435–443. [DOI] [PubMed] [Google Scholar]
- 29. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 30. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:19. [DOI] [PubMed] [Google Scholar]
- 32. George A, Stead TS, Ganti L. What’s the risk: differentiating risk ratios, odds ratios, and hazard ratios? Cureus. 2020;12:e10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lawrence DM, Holman CDJ, Jablensky AV, Hobbs MST. Death rate from ischaemic heart disease in western Australian psychiatric patients 1980–1998. Br J Psychiatry. 2003;182:31–36. [DOI] [PubMed] [Google Scholar]
- 34. Kisely S, Campbell LA, Wang Y. Treatment of ischaemic heart disease and stroke in individuals with psychosis under universal healthcare. Br J Psychiatry. 2009;195:545–550. [DOI] [PubMed] [Google Scholar]
- 35. Kurdyak P, Vigod S, Calzavara A, Wodchis WP. High mortality and low access to care following incident acute myocardial infarction in individuals with schizophrenia. Schizophr Res. 2012;142:52–57. [DOI] [PubMed] [Google Scholar]
- 36. Hauck TS, Liu N, Wijeysundera HC, Kurdyak P. Mortality and revascularization among myocardial infarction patients with schizophrenia: a population-based cohort study. Can J Psychiatry. 2020;65:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laursen TM, Munk-Olsen T, Agerbo E, Gasse C, Mortensen PB. Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry. 2009;66:713–720. [DOI] [PubMed] [Google Scholar]
- 38. Jakobsen L, Terkelsen CJ, Christiansen EH, et al. Severe mental illness and clinical outcome after primary percutaneous coronary intervention. Am J Cardiol. 2017;120:550–555. [DOI] [PubMed] [Google Scholar]
- 39. Kugathasan P, Horsdal HT, Aagaard J, Jensen SE, Laursen TM, Nielsen RE. Assocation of secondary preventive cardiovascular treatment after myocardial infarction with mortality among patients with schizophrenia. JAMA Psychiatry. 2018;75:1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang WC, Chan JKN, Wong CSM, Hai JSH, Or PCF, Chen EYH. Mortality, revascularization, and cardioprotective pharmacotherapy after acute coronary syndrome in patients with psychotic disorders: a population-based cohort study. Schizophr Bull. 2020;46:774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boden R, Molin E, Jernberg T, Kieler H, Lindahl B, Sundstrom J. Higher mortality after myocardial infarction in patients with severe mental illness: a nationwide cohort study. J Intern Med. 2015;277:727–736. [DOI] [PubMed] [Google Scholar]
- 42. Attar R, Wester A, Koul S, et al. Higher risk of major adverse cardiac events after acute myocardial infarction in patients with schizophrenia. Open Heart. 2020;7:e001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu SI, Chen SC, Juang JJM, et al. Diagnostic procedures, revascularization, and inpatient mortality after acute myocardial infarction in patients with schizophrenia and bipolar disorder. Psychosom Med. 2013;75:52–59. [DOI] [PubMed] [Google Scholar]
- 44. Fleetwood K, Wild SH, Smith DJ, et al. Severe mental illness and mortality and coronary revascularization following a myocardial infarction: a retrospective cohort study. BMC Med. 2021;19:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Druss BG, Bradford DW, Rosenheck RA, Radford MJ, Krumholz HM. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506–511. [DOI] [PubMed] [Google Scholar]
- 46. Young JK, Foster DA. Cardiovascular procedures in patients with mental disorders. JAMA. 2000;283:3198–3199. [PubMed] [Google Scholar]
- 47. Druss BG, Bradford D, Rosenheck RA, Radford MJ, Krumholz HM. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58:565–572. [DOI] [PubMed] [Google Scholar]
- 48. Jones LE, Carney CP. Mental disorders and revascularization procedures in a commercially insured sample. Psychosom Med. 2005;67:568–576. [DOI] [PubMed] [Google Scholar]
- 49. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Psychiatric comorbidity and mortality after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:213–220. [DOI] [PubMed] [Google Scholar]
- 50. Sohn M, Moga DC, Talbert J. Mental disorder comorbidity and in-hospital mortality among patients with acute myocardial infarction. Geriatr Ment Health Care. 2015;3:7–11. [Google Scholar]
- 51. Schulman-Marcus J, Goyal P, Swaminathan RV, et al. Comparison of trends in incidence, revascularization, and in-hospital mortality in ST-elevation myocardial infarction in patients with versus without severe mental illness. Am J Cardiol. 2016;117:1405–1410. [DOI] [PubMed] [Google Scholar]
- 52. Plomondon ME, Ho PM, Wang L, et al. Severe mental illness and mortality of hospitalized ACS patients in the VHA. BMC Health Serv Res. 2007;7:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nielsen RE, Banner J, Jensen SE. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol. 2021;18:136–145. [DOI] [PubMed] [Google Scholar]
- 54. Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 56. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10:138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vistorte AOR, Ribeiro WS, Jaen D, Jorge MR, Evans-Lacko S, Mari JJ. Stigmatizing attitudes of primary care professionals towards people with mental disorders: a systematic review. Int J Psychiatry Med. 2018;53:317–338. [DOI] [PubMed] [Google Scholar]
- 58. Giandinoto JA, Stephenson J, Edward KL. General hospital health professionals’ attitudes and perceived dangerous towards patients with comorbid mental and physical health conditions: systematic review and meta-analysis. Int J Ment Health Nurs. 2018;27:942–955. [DOI] [PubMed] [Google Scholar]
- 59. Shefer G, Henderson C, Howard LM, Murray J, Thornicroft G. Diagnostic overshadowing and other challenges involved in the diagnostic process of patients with mental illness who present in emergency departments with physical symptoms—a qualitative study. PLoS One. 2014;9:e111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhai DS, Lang Y, Dong GP, et al. QTc interval lengthening in first-episode schizophrenia (FES) patients in the earliest stages of antipsychotic treatment. Schizophr Res. 2017;179:70–74. [DOI] [PubMed] [Google Scholar]
- 62. Elliott A, Mork TJ, Hojlund M, et al. QTc interval in patients with schizophrenia receiving antipsychotic treatment as monotherapy or polypharmacy. CNS Spectr. 2018;23:278–283. [DOI] [PubMed] [Google Scholar]
- 63. Swildens W, Termorshuizen F, de Ridder A, Smeets H, Engelhard IM. Somatic care with a psychotic disorder: lower somatic health care utilization of patients with a psychotic disorder compared to other patient groups and to controls without a psychiatric diagnosis. Adm Policy Ment Health. 2016;43:650–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42:125–147. [DOI] [PubMed] [Google Scholar]
- 65. Heiberg IH, Jacobsen BK, Balteskard L, et al. Undiagnosed cardiovascular disease prior to cardiovascular death in individuals with severe mental illness. Acta Psychiatr Scand. 2019;139:558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71:1350–1363. [DOI] [PubMed] [Google Scholar]
- 68. Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith J, Griffiths LA, Band M, Horne D. Cardiometabolic risk in first episode psychosis patients. Front Endocrinol. 2020;11:564240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gates J, Killackey E, Phillips L, Álvarez-Jiménez M. Mental health starts with physical health: current status and future directions of non-pharmacological interventions to improve physical health in first-episode psychosis. Lancet Psychiatry. 2015;2:726–742. [DOI] [PubMed] [Google Scholar]
- 71. Osborn DPJ, Hardoon S, Omar RZ, et al. Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry. 2015;72:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Perry BI, Osimo EF, Upthegrove R, et al. Development and external validation of the psychosis metabolic risk calculator (PsyMetRiC): a cardiometabolic risk prediction algorithm for young people with psychosis. Lancet Psychiatry. 2021;8:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Metelli S, Chaimani A. Challenges in meta-analyses with observational studies. Evid Based Ment Health. 2020;23:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kandula N, Ahmed M, Dodani S, et al. Cardiovascular disease & cancer risk among South Asians: impact of sociocultural influences on lifestyle and behavior. J Immigr Minor Health. 2019;21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jilani MH, Javed Z, Yahya T, et al. Social determinants of health and cardiovascular disease: current state and future directions towards healthcare equity. Curr Atheroscler Rep. 2021;23:55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.