This cohort study investigates the association between risk factors for atrial fibrillation and patient sex.
Key Points
Question
Are women at lower risk for atrial fibrillation (AF) after accounting for other AF risk factors and do risk factors for AF differ for men vs women?
Findings
In this cohort study of 25 119 participants within the Vitamin D and Omega-3 Trial (VITAL) Rhythm Study, women were at greater risk for developing AF than men when height and weight, rather than body mass index, were controlled for in multivariable models. AF risk factors were similar for women vs men.
Meaning
Results suggest that for a given height and weight, women are at higher risk for incident AF than men, and primary prevention with risk factor modification should be equally effective; these findings emphasize the need for AF prevention in women.
Abstract
Importance
Women have a lower incidence of atrial fibrillation (AF) compared with men in several studies, but it is unclear whether this sex difference is independent of sex differences in prevalent cardiovascular disease (CVD), body size, and other risk factors.
Objective
To examine sex differences in AF incidence and whether AF risk factors differ by sex in a contemporary cohort of men and women without prevalent CVD.
Design, Setting, and Participants
This was a prospective cohort analysis within the Vitamin D and Omega-3 Trial (VITAL) Rhythm Study, a randomized trial that examined the effect of vitamin D and ω-3 fatty acid supplementation on incident AF among men 50 years or older and women 55 years or older without a prior history of prevalent AF, CVD, or cancer at baseline. Data were analyzed from September 29, 2020, to June 29, 2021.
Exposures
Sex, height, weight, body mass index (BMI), body surface area (BSA), and other AF risk factors at study enrollment.
Main Outcomes and Measures
Incident AF confirmed by medical record review.
Results
A total of 25 119 individuals (mean [SD] age, 67.0 [7.1] years; 12 757 women [51%]) were included in this study. Over a median (IQR) follow-up of 5.3 (5.1-5.7) years, 900 confirmed incident AF events occurred among 12 362 men (495 events, 4.0%) and 12 757 women (405 events, 3.2%). After adjustment for age and treatment assignment, women were at lower risk for incident AF than men (hazard ratio [HR], 0.68; 95% CI, 0.59-0.77; P < .001). The inverse association between female sex and AF persisted after adjustment for race and ethnicity, smoking, alcohol intake, hypertension, diabetes (type 1, type 2, gestational), thyroid disease, exercise, and BMI (HR, 0.73; 95% CI, 0.63-0.85; P <.001). However, female sex was positively associated with AF when height (HR, 1.39; 95% CI, 1.14-1.72; P = .001), height and weight (HR 1.49, 95% CI, 1.21-1.82; P <.001), or BSA (HR, 1.25; 95% CI, 1.06-1.49; P = .009) were substituted for BMI in the multivariate model. In stratified models, risk factor associations with incident AF were similar for women and men.
Conclusions and Relevance
In this cohort study, findings suggest that after controlling for height and/or body size, women without CVD at baseline were at higher risk for AF than men, suggesting that sex differences in body size account for much of the protective association between female sex and AF. These data underscore the importance of AF prevention in women.
Introduction
Atrial fibrillation (AF) is the most prevalent arrhythmia in the world and is associated with an increased risk of stroke, heart failure, and overall mortality.1 As the population ages, the number of patients with AF is expected to burgeon. Similar to other cardiovascular disorders, there are important sex-specific differences in the epidemiology and outcomes of AF.1,2,3,4,5 Women have lower rates of AF than men but are more likely to suffer adverse sequalae of the disease, including stroke, heart failure, and death.4,6,7,8 Therefore, understanding risk factors that may affect the burden of AF differentially between women and men is of considerable importance.
Body size,9,10,11,12 height,13 and adiposity,14,15,16,17,18 which significantly vary by sex, are important contributors to AF risk11; thus, they may underlie, in part, the observed sex differences in AF risk (eFigure in the Supplement). Data from observational studies that enrolled populations in the 1970s to 1980s suggest that body mass index (BMI) may have a stronger relation to AF in men,5,12 whereas height may have a stronger association with AF in women.18 A single prospective study suggested that this strong association between height and AF may mediate the sex differences in AF risk in an older adult population.11 However, data on sex differences in AF risk in contemporary populations are lacking.
In the present study, we examined sex differences in incident AF risk in a contemporary cohort of men and women without prior cardiovascular disease (CVD) enrolled in the Vitamin D and Omega-3 Trial (VITAL) Rhythm study, a substudy of the randomized VITAL trial.19,20,21 We then evaluated the contribution of anthropometric measures and other AF risk factors to sex differences in incident AF risk and examined whether AF risk factors differ for women vs men.
Methods
Study Design and Participants
VITAL is a large US-based, randomized, primary prevention, placebo-controlled trial that used a 2 × 2 design to assess the effect of vitamin D3 (2000 IU per day) and marine ω-3 fatty acids (840 mg of ω-3 fatty acids, including eicosapentaenoic acid, 460 mg, and docosahexaenoic acid, 460 mg) on the primary prevention of CVD and cancer in 25 871 participants enrolled between November 11, 2011, and March 27, 2014, in the US.19,20 The VITAL Rhythm study (a substudy of VITAL) tested the association of long-term administration of marine ω-3 fatty acids and vitamin D with incident AF.21 Men 50 years or older and women 55 years or older without a prior history of CVD or cancer were eligible for the study. Patients who reported a prior diagnosis of AF at baseline were excluded from VITAL Rhythm and the present analysis. To comply with National Institutes of Health reporting requirements, information on self-reported race and ethnicity was collected on the screening questionnaire, which included fixed categories of race (American Indian or Alaskan Native, Asian or Pacific Islander, Black or African American, White, and other or unknown) and ethnicity (Hispanic or Latino, not Hispanic or Latino, and other or unknown). Baseline questionnaires collected data on clinical and lifestyle risk factors including anthropometric measures of height and weight. In a subcohort of VITAL participants, in-clinic assessments of height and weight were performed at the Clinical and Translational Science Center (CTSC). All participants were recruited and enrolled directly by mail and provided written informed consent. The trial was approved by the institutional review board of Partners HealthCare–Brigham and Women’s Hospital and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Incident AF Ascertainment and Confirmation
Incident AF was identified through 2 methods in the VITAL Rhythm study.21 First, participants self-reported new AF diagnoses on annual follow-up questionnaires. Second, the VITAL population was linked to claims data from the Centers for Medicare and Medicaid Services, and International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 427.31 and Tenth Revision (ICD-10) diagnosis code I48.91 for AF and ICD-9 diagnosis code 427.32 and ICD-10 diagnosis code I48.92 for atrial flutter were ascertained. For all participants with an incident AF diagnosis, either by self-report or Centers for Medicare and Medicaid Services linkage, we requested permission to obtain medical records pertaining to the AF diagnosis. An end point committee consisting of cardiologists confirmed AF events according to predefined criteria.21,22 Electrocardiogram (ECG) evidence of AF or a physician’s report outlining a diagnosis of AF were required for confirmation. Only AF events confirmed by medical record review are included in the analyses. Presentation characteristics at time of AF diagnosis were abstracted from the medical record where available. Pattern of AF at the time of diagnosis was classified in accordance with the latest American College of Cardiology/American Heart Association/Heart Rhythm Society and European Society of Cardiology guidelines.23,24
Statistical Analysis
For the entire cohort, baseline characteristics are presented as mean (SD) or median (IQR) for continuous variables and percentages for categorical variables, stratified by sex, and compared using analysis of variance and χ2 tests as appropriate. In the CTSC subcohort, Spearman correlations were estimated for self-reported and measured height and weight to provide estimates for validity of the self-reported measures. In the entire cohort, multivariable Cox proportional hazards models were used to determine the association between sex and AF after adjustment for various risk factors. The proportionality assumption was tested for the full Cox models using interaction terms with log time, and no violation was found. To assess the contributions of AF risk factors and body size measures to the observed association between sex and AF, sequential multivariable models were constructed. The first controlled for age, sex, and trial treatment assignment. The second (base model) additionally controlled for common AF risk factors including diabetes, thyroid disease, hypertension, average alcohol intake (categorized by frequency on a weekly or daily level), smoking status (as never smoker, former smoker, or current smoker), and weekly leisure-time physical activity (categorized as tertiles of total weekly metabolic equivalent task [MET] hours). Third, each anthropometric measure (BMI, calculated as weight in kilograms divided by height in meters squared; height in inches; weight in pounds; and body surface area [BSA] in meters squared) calculated as [(weight in kilograms × height in centimeters) / 3600]½ was separately added to this base model. An additional model including both height and weight was also constructed. To determine whether AF risk factor associations differed between men and women, sex-stratified models were performed, and sex-interaction terms were included in the full multivariable model.
Several secondary sensitivity analyses were conducted to determine result reproducibility of the multivariable models examining the association between sex and AF. First, because the age enrollment criteria in VITAL differed for men (age ≥50 years) and women (age ≥55 years), we conducted a sensitivity analysis restricting the population to those 55 years or older. Second, given the major sex differences in height distribution, sensitivity analysis limited to a height range that included a sizable representation of both sexes (64-68 in; to convert height to centimeters, multiply by 2.54) was performed. Third, to determine the sensitivity of the result to extreme values in height, overall population-derived quintiles were substituted for continuous height in the multivariable models. Fourth, sex-specific spline modeling was used to compare the multivariable hazard of incident AF in women compared with men across ranges of BMI and height. Finally, in order to adjust for height within each sex separately, sex-specific z scores (standard scores) for height were calculated for each participant as the number of SDs from the sex-specific mean height, and these z scores were substituted for crude height in the multivariable models.
All analyses were performed using SAS, version 9.4 for Windows (SAS Institute). A 2-sided P < .05 was used to define statistical significance. Data were analyzed from September 29, 2020, to June 29, 2021.
Results
Baseline Population
A total of 25 119 individuals (mean [SD] age, 67.0 [7.1] years; 12 757 women [51%]; 12 362 men [49%]) were included in this study. Participants identified with the following race and ethnicity categories: 215 American Indian or Alaskan Native (0.9%), 382 Asian or Pacific Islander (2%), 5052 Black (20%), 999 Hispanic (4%), 17 425 White (69%), and 506 other or unknown (2%) race and ethnicity (Table 1). On average, compared with men, women were older (per study enrollment protocol; mean [SD], 68.1 [6.8] years vs 66.0 [7.1] years), more often of Black race and ethnicity (3125 [25%] vs 1927 [16%]), and had a higher prevalence of hypertension (6845 [54%] vs 6066 [49%]) and thyroid disorders (2321 [19%] vs 602 [5%]). Conversely, compared with men, women were less likely to have ever smoked cigarettes (never smoked, 6865 [55%] vs 5970 [49%]), drank less alcohol (never or <1 drink, 5988 [48%] vs 3670 [30%]), exercised less (lowest MET tertile, 4714 [37.3%] vs 3581 [29.3%]), and reported a lower income (<$50 000 per year, 5177 [46%] vs 3120 [28%]). All body size parameters differed significantly between men and women. Compared with men, women had a higher mean BMI (mean [SD], 28.4 [6.6] vs 27.8 [4.7]), lower mean height (mean [SD], 64.4 [2.8] in vs 70.2 [2.8] in), lower mean weight (mean [SD], 167.5 [40.0] lb vs 194.8 [36.7] lb; to convert weight to kilograms, multiply by 0.45), and lower mean BSA (mean [SD], 1.85 [0.2] m2 vs 2.08 [0.2] m2). Self-reported height and weight were both highly correlated with measured values in the CTSC cohort (height, ρ = 0.96; weight, ρ = 0.97). Accuracy was slightly greater among women (height, ρ = 0.95; weight, ρ = 0.97) compared with men (height, ρ = 0.91; weight, ρ = 0.94), with both sexes overestimating height and underestimating weight to a small degree.
Table 1. Baseline Characteristics for the Complete Vitamin D and Omega-3 Trial Study Populationa.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All participants | Men | Women | |
| No. (%) | 25 119 | 12 362 (49) | 12 757 (51) |
| Age, mean (SD), y | 67.0 (7.1) | 66.0 (7.1) | 68.1 (6.8) |
| Age categories, y | |||
| <65 | 9696 (39) | 5732 (46) | 3964 (31) |
| 65-<75 | 12 267 (49) | 5321 (43) | 6946 (54) |
| 75+ | 3156 (13) | 1309 (11) | 1847 (15) |
| Race and ethnicity | |||
| Black | 5052 (20) | 1927 (16) | 3125 (25) |
| White | 17 425 (69) | 8995 (73) | 8430 (66) |
| Otherb | 2642 (11) | 1440 (12) | 1202 (9) |
| Body mass index, mean (SD)c | 28.1 (5.7) | 27.8 (4.7) | 28.4 (6.6) |
| Height, mean (SD), in | 67.2 (4.0) | 70.2 (2.8) | 64.4 (2.8) |
| Weight, mean (SD), lb | 180.9 (40.8) | 194.8 (36.7) | 167.5 (40.0) |
| Body surface area, mean (SD), m2 | 1.96 (0.3) | 2.08 (0.2) | 1.85 (0.2) |
| Average alcohol use, drink/week | |||
| Never or <1 | 9658 (39) | 3670 (30) | 5988 (48) |
| 1-6 | 8638 (35) | 4223 (35) | 4415 (35) |
| 1 | 2892 (12) | 1723 (14) | 1169 (9) |
| 2+ | 3509 (14) | 2529 (21) | 980 (8) |
| Annual income | |||
| <$50 000 | 8297 (37) | 3120 (28) | 5177 (46) |
| $50 000-$120 000 | 10 224 (45) | 5378 (48) | 4846 (43) |
| >$120 000 | 4079 (18) | 2803 (25) | 1276 (11) |
| Smoker | |||
| Never | 12 835 (52) | 5970 (49) | 6865 (55) |
| Former | 10 111 (41) | 5291 (44) | 4820 (38) |
| Current | 1798 (7) | 913 (8) | 885 (7) |
| Diabetes | 3442 (14) | 1666 (14) | 1776 (14) |
| Hypertension | 12911 (52) | 6066 (49) | 6845 (54) |
| Thyroid conditions | 2923 (12) | 602 (5) | 2321 (19) |
| Weekly total MET, hd | |||
| Lowest tertile | 8295 (33.3) | 3581 (29.3) | 4714 (37.3) |
| Middle tertile | 8298 (33.4) | 4019 (32.8) | 4279 (33.9) |
| Highest tertile | 8282 (33.3) | 4637 (37.9) | 3645 (28.8) |
| Randomized to vitamin D | 12 553 (50.0) | 6169 (49.9) | 6384 (50.0) |
| Randomized to ω-3 | 12 542 (49.9) | 6174 (49.9) | 6368 (49.9) |
Abbreviation: MET, metabolic equivalent task.
SI conversion factor: To convert height to centimeters, multiply by 2.54; to convert weight to kilograms, multiply by 0.45.
Categorical variables are listed as absolute No. (%) of population.
Other race and ethnicity includes 215 American Indian or Alaskan Native (0.9%), 382 Asian or Pacific Islander (2%), 999 Hispanic (4%), 506 other or unknown (2%), and 540 missing (2%).
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Weekly MET hours were calculated from participant responses to survey questions about their weekly leisure time physical activity.
Incident AF Population
In total, 900 participants in VITAL Rhythm had confirmed incident AF over a median (IQR) of 5.3 (5.1-5.7) years of follow-up (eTable 1 in the Supplement). Among 12 362 men, 495 AF events (4.0%) occurred; among 12 757 women, 405 AF events (3.2%) occurred. At 5 years, the corresponding age-adjusted cumulative incidence of AF was 5.7 events per 1000 person-years for women compared with 8.0 events per 1000 person-years for men (absolute rate difference, 2.3; 95% CI, 1.4-3.3 events per 1000 person-years). The mean (SD) age of participants who developed AF was 74.4 (7.2) years, and 405 (45%) were women. Women with incident AF differed in their initial presentation. Among participants with information on symptoms and/or AF pattern at the time of diagnosis, women were more likely to have symptoms at time of AF diagnosis (77% in women [284 of 369] vs 63% in men [273 of 435]) and, compared with men, tended to present with paroxysmal (65% [259 of 396] vs 56% [267 of 476]) rather than persistent (28% [109 of 396] vs 32% [153 of 476]) or permanent (7% [28 of 396] vs 12% [56 of 476] P for sex interaction = .008) forms of AF.
Sex and Incident AF Risk
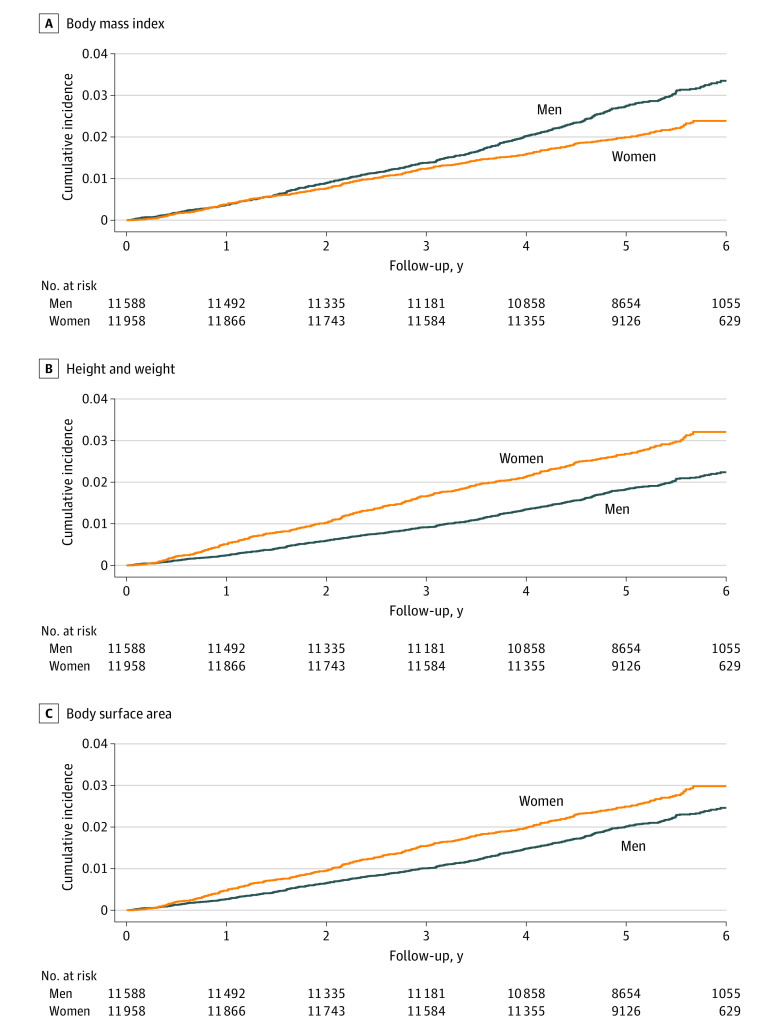
Women had a 32% lower risk of incident AF than men (hazard ratio [HR], 0.68; 95% CI, 0.59-0.77; P < .001) (Table 2) after adjusting for age and treatment assignment. This sex difference was attenuated but remained significant in the multivariable model that included nonanthropometric AF risk factors (HR, 0.75; 95% CI, 0.65-0.86; P < .001). Although BMI was associated with AF, further adjustment for BMI on top of these risk factors did not materially alter the association between female sex and AF (HR, 0.73; 95% CI, 0.63-0.85; P < .001). When height and weight were substituted for BMI in the multivariable model, female sex became positively associated with AF (HR, 1.49; 95% CI, 1.21-1.82; P < .001). This result was primarily attributable to adjustment for height (HR, 1.39; 95% CI, 1.14-1.70; P = .001) compared with weight (HR, 1.05; 95% CI, 0.90-1.23; P = .56). After adjustment for BSA, women continued to have a greater risk of incident AF compared with men (HR, 1.25; 95% CI, 1.06-1.49; P = .009). Sex-stratified cumulative incidence curves adjusting for BMI, height and weight, and BSA are displayed in Figure 1, respectively.
Table 2. Sex-Specific Risk for Incident Atrial Fibrillation for Women Compared With Men.
| Cox proportional hazards model | Hazard ratio (95% CI) | P value |
|---|---|---|
| Age, trial treatment group | 0.68 (0.59-0.77) | <.001 |
| Base modela | 0.75 (0.65-0.86) | <.001 |
| Base model,a body mass index | 0.73 (0.63-0.85) | <.001 |
| Base model,a weight | 1.05 (0.90-1.23) | .56 |
| Base model,a height | 1.39 (1.14-1.70) | .001 |
| Base model,a height, weight | 1.49 (1.21-1.82) | <.001 |
| Base model,a body surface area | 1.25 (1.06-1.49) | .009 |
Base Cox proportional model adjusted for trial treatment group, age at randomization, race, average alcohol use per day, history of smoking, thyroid disease, diabetes, hypertension, and amount of weekly leisure time physical activity.
Figure 1. Cumulative Incidence of Incident Atrial Fibrillation During the Vitamin D and Omega-3 Trial, Stratified by Sex, Adjusting for Different Body Size Surrogates.
Sex-stratified cumulative incidence of atrial fibrillation, adjusted for body mass index (A), height and weight (B), and body surface area (C), as well as age, trial treatment assignment, race and ethnicity, thyroid disease, hypertension, diabetes, physical activity, alcohol intake, and smoking.
Sensitivity Analyses
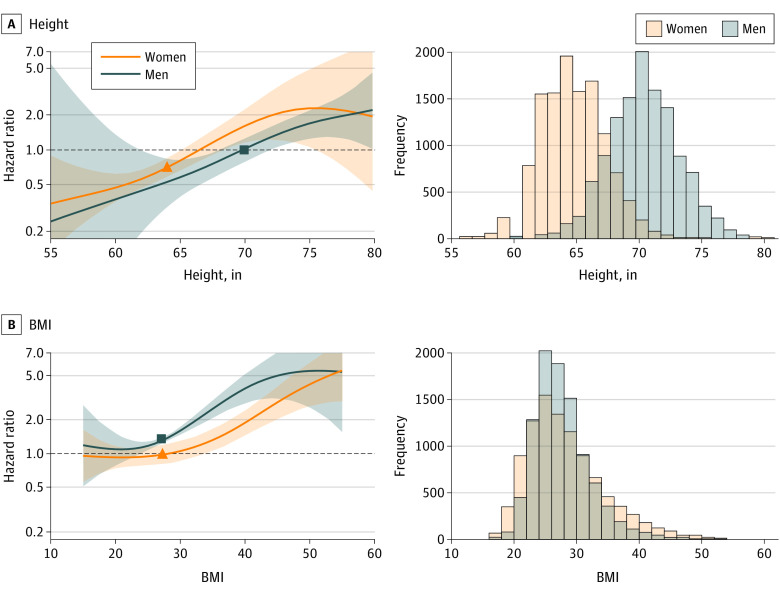
Several sensitivity analyses were performed to explore the association between female sex and AF in height-adjusted models. First, results were similar when limited to participants 55 years and older (eTable 2 in the Supplement), the age range at which both sexes were enrolled in the trial. Second, female sex remained positively associated with AF risk (HR, 1.57; 95% CI, 1.16-2.11; P = .003) when the population was restricted to a height range between 64 to 68 in, where there was significant representation by both sexes (3188 men, 7056 women; 41% of the total population). Results were also similar in models that controlled for height in quintiles. In sex-specific spline models (Figure 2A), the higher relative hazard of incident AF in women compared with men persisted over the full range of height, although CIs overlapped. This association was reversed when considering BMI rather than height (Figure 2B). When comparing the HR for incident AF at sex-specific median heights, the HR appeared lower in women than in men (Figure 2A). Similarly, when sex-specific height and weight z scores were used to adjust for height within each sex separately, the positive association between female sex and AF reverted back to the inverse association found in crude models that did not include height and weight (HR, 0.78; 95% CI 0.67-0.90; P = <.001).
Figure 2. Risk of Incident Atrial Fibrillation in the Study Population, Adjusting for Height and Body Mass Index (BMI).
Spline modeling of the hazard ratios for incident atrial fibrillation, stratified by sex, across the range of height (A) and BMI (B) in the study population. Histograms show the distribution of height and BMI in the study population. Sex-specific median values of height and BMI for women and men are represented by triangles and squares in each graphic, respectively. BMI is calculated as weight in kilograms divided by height in meters squared.
SI conversion factor: To convert height to centimeters, multiply by 2.54.
Risk Factors for Incident AF in Women vs Men
In sex-stratified Cox models, age, height, weight, BMI, and hypertension were all significantly associated with an increased risk of incident AF in women and men (Table 3). Black race was associated with lower AF incidence in both sexes (men: HR, 0.25; 95% CI, 0.15-0.41; women: HR, 0.29; 95% CI, 0.20-0.44; P for interaction = .14). The magnitude of the associations for BMI and alcohol intake and AF were greater in men compared with women, but the test for interaction was not significant. Other AF risk factor associations were also generally similar across men and women, except for diabetes, which was associated with a lower multivariable adjusted risk of incident AF only in women (HR, 0.65; 95% CI, 0.46-0.93; P for sex interaction = .03). When BMI was substituted for height and weight in the multivariable model, the interaction between sex and diabetes was no longer significant.
Table 3. Predictors of Incident Atrial Fibrillation Stratified by Sex in a Multivariate Model Accounting for Height and Weight or Body Mass Index.
| Characteristic | Men | Women | P value for sex interaction | ||
|---|---|---|---|---|---|
| No. (%) | HR (95% CI)a | No. (%) | HR (95% CI)a | ||
| Mean age at randomization, y | |||||
| <65 | 5396 (47) | 1 [Reference] | 3726 (31) | 1 [Reference] | .97 |
| 65-<75 | 5003 (43) | 2.41 (1.90-3.06) | 6542 (55) | 2.31 (1.63-3.28) | |
| 75+ | 1189 (10) | 5.90 (4.46-7.81) | 1690 (14) | 5.49 (3.76-8.03) | |
| Race and ethnicity | |||||
| Black | 1732 (15) | 0.25 (0.15-0.41) | 2844 (24) | 0.29 (0.20-0.44) | .14 |
| White | 8524 (74) | 1 [Reference] | 8015 (67) | 1 [Reference] | |
| Other | 1332 (11) | 0.76 (0.55-1.05) | 1099 (9) | 0.63 (0.42-0.95) | |
| BMI (per kg/m2 increase)b | NA | 1.07 (1.04-1.09) | NA | 1.04 (1.02-1.05) | .06 |
| Height (per inch increase) | 1.06 (1.02-1.10) | 1.08 (1.04-1.12) | .51 | ||
| Weight (per pound increase) | 1.01 (1.01-1.01) | 1.01 (1.00-1.01) | .69 | ||
| Average alcohol use, drink/wk | |||||
| Never or <1 | 3489 (30) | 1 [Reference] | 5647 (47) | 1 [Reference] | .58 |
| 1-6 | 4046 (35) | 1.19 (0.93-1.52) | 4229 (35) | 1.02 (0.81-1.30) | |
| 1 | 1635 (14) | 1.25 (0.92-1.68) | 1140 (10) | 1.18 (0.83-1.66) | |
| 2+ | 2418 (21) | 1.53 (1.17-1.99) | 942 (8) | 1.19 (0.82-1.72) | |
| Smoker | |||||
| Never | 5686 (49) | 1 [Reference] | 6521 (55) | 1 [Reference] | .29 |
| Former | 5046 (44) | 1.10 (0.91-1.33) | 4593 (38) | 1.28 (1.04-1.58) | |
| Current | 856 (7) | 0.83 (0.51-1.35) | 844 (7) | 1.04 (0.62-1.75) | |
| Diabetes | 1538 (7) | 1.13 (0.87-1.46) | 1611 (7) | 0.65 (0.46-0.93) | .03 |
| Hypertension | 5677 (49) | 1.33 (1.10-1.62) | 6398 (54) | 1.64 (1.31-2.05) | .33 |
| Leisure time physical activity (weekly total MET hours) | |||||
| Lowest tertile | 3360 | 1 [Reference] | 4403 | 1 [Reference] | .14 |
| Middle tertile | 3818 | 1.03 (0.81-1.31) | 4074 | 1.01 (0.79-1.28) | |
| Highest tertile | 4410 | 1.23 (0.97-1.55) | 3481 | 0.91 (0.69-1.19) | |
Abbreviations: BMI, body mass index; HR, hazard ratio; MET, metabolic equivalent task; NA, not applicable.
SI conversion factor: To convert height to centimeters, multiply by 2.54; to convert weight to kilograms, multiply by 0.45.
Multivariable models simultaneously adjusted for trial treatment group assignment, height, weight, and all listed variables except BMI.
BMI substituted for height and weight, all other variables the same and is calculated as weight in kilograms divided by height in meters squared.
Discussion
In this contemporary, prospective, observational cohort study of more than 25 000 individuals without prior CVD or AF with equal representation of women and men and overrepresentation of Black participants, findings suggest that women were at lower risk for the development of incident AF than men in age-adjusted and multivariate models including BMI, a measure of adiposity. When crude measures of height and weight or BSA were substituted for BMI, the association reversed direction, and female sex was associated with a higher risk for AF. In sex-stratified analyses, all anthropometric risk factors, including BMI, were significantly associated with incident AF in women and men. Other AF risk factors such as age, race and ethnicity, and hypertension were significantly associated with AF in both sexes, and definitive evidence for sex differences in risk factor associations with AF was not found.
Large epidemiologic studies have shown that women appear to have both a lower incidence and lifetime risk of AF compared with men.1 For example, in the Framingham Heart Study, the most recent assessments of age-adjusted incidence rates of AF from 1998 to 2007 were 8.6 events per 1000 person-years in women and 13.4 events per 1000 person-years in men.1 In the present study, which was performed in the subsequent decade (2011-2018) among individuals without CVD at baseline, women were again at lower age-adjusted cumulative incidence of AF (5.7 events per 1000 person-years for women and 8.0 events per 1000 person-years for men). The lower overall incidence of AF in our cohort compared with prior epidemiologic studies is likely attributable to the exclusion of patients with established CVD, which elevates risk of AF.25,26 The persistence of the sex difference in AF incidence in this population without CVD argues that sex differences in prevalent CVD do not entirely account for sex-differences in AF incidence observed in prior studies.
Although prior studies have demonstrated a higher incidence of AF in men compared with women,1,27,28 the Cohorts for Heart and Aging Research in Genomic Epidemiology for Atrial Fibrillation (CHARGE-AF) risk prediction model derived and replicated in various US and European cohorts did not find that sex improved AF risk prediction after accounting for other AF risk factors, including height.29 In the Cardiovascular Health Study, control for height attenuated the elevated risk of incident AF in men compared with women.30 In our contemporary cohort without CVD, adjustment for height not only attenuated—but reversed—the protective association between female sex and AF. When we accounted for the differences in height within each sex separately using sex-specific standardized z scores, the positive association between female sex and AF was no longer seen. These data, in combination with prior studies, suggest that sex differences in height account for much of the lower AF risk in women. Our results also raise the possibility that at a given height, women without CVD may have a higher risk of developing AF than men. However, it is important to recognize the marked sex difference in height distributions when interpreting this finding, such that a given height may be extreme in one sex but not in the other. There are also sex differences in the association of height with BMI, such that these measures are negatively correlated in women as opposed to positively correlated in men.31 The latter association might account for the greater magnitude of the BMI and AF association in men observed here and reported in the literature.5,12
There are potential biologic pathways through which women might have a higher risk for developing AF at a given body size compared with men. In 1 magnetic resonance imaging study involving 60 patients with AF, female sex was associated with higher levels of atrial fibrosis measured as a percentage of the left atrial wall compared with men.32 Because body size and height are correlated with left atrial size,33,34 these data raise the possibility that for a given height and similarly sized atrium, there may be a higher degree of atrial fibrosis in women compared with men. Compared with men, women may also be more likely to manifest left atrial dilation and/or dysfunction resulting in AF.35,36 In addition, because height is inversely associated with CVD,37 it is feasible that the elevation in AF risk in women observed after controlling for height may be specific to patient populations without CVD.
Although AF risk factors were generally similar between men and women in this study, the clinical presentation differed. Compared with men, women were more likely to be symptomatic and less likely to present with persistent forms of AF at the time of incident AF diagnosis. In the recently reported Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET), women were similarly more likely to be symptomatic at the time of initial presentation,38 and several studies have reported more symptoms and reduced quality of life in women with prevalent AF than in men.39,40,41 In the Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial, a large-scale randomized trial of AF ablation vs drug therapy, women were also more likely to be symptomatic and to have paroxysmal forms of AF at the time of enrollment.42 Paroxysmal forms of AF tend to be more symptomatic, and these sex differences in the ability to sustain AF could be attributable to differences in left atrial electrophysiology.43,44 However, there may be systematic biases that could result in differential detection of AF (symptomatic and/or asymptomatic) between women and men which could account for these findings.43,44 Large-scale population level screening studies will help answer these questions and allow for better strategies for AF detection for men and women.
Strengths and Limitations
Strengths of this study included the large, contemporary, racially diverse population of more than 25 000 participants comprising 50% women and 20% Black participants. AF ascertainment was performed using 2 complementary methods, and incident AF outcomes were rigorously adjudicated by medical record review. There were also important limitations in this study to consider. First, the data must be interpreted in the context of the study design as a secondary analysis of a randomized clinical trial. Second, the study population was older, with an average age of 67 years at randomization and 74 years at the time of incident AF diagnosis. Therefore, generalizability of these findings to younger individuals may be limited. Third, individuals with underlying CVD were excluded from the trial; thus, these findings may not be generalizable to patients with established CVD. Fourth, ascertainment of AF was based solely on clinical diagnoses, which would be expected to be dependent, at least to some degree, on health care utilization and/or likelihood of undergoing ECGs or rhythm monitoring during health care evaluations. Thus, the lack of protocolized AF screening in all study patients could manifest as a systematic bias if 1 sex was more likely to seek health care and/or receive an ECG and/or cardiac monitor, either owing to symptoms or for other reasons. Fifth, we used self-reported height and weight leading to some misclassification, which based on our validation data would be expected to minimal and unlikely to account for the findings observed.
Conclusions
In this large, contemporary, cohort study of women and men without CVD at baseline, findings suggest that women had a lower risk of developing incident AF; however, after controlling for height, female sex was associated with a higher risk for AF. These data suggest that sex differences in body size account for much of the previously reported protective association between female sex and AF and underscore the importance of AF prevention in women.
eFigure. Schematic Showing the Proposed Relationship Between Sex, Anthropometric Measures, and Atrial Fibrillation
eTable 1. Baseline and AF Characteristics for Individuals Who Developed Incident AF
eTable 2. Sensitivity Analyses for Adjusted Sex-Specific Risk of Incident AF for Females Compared With Males, Restricting to Key Subgroups
References
- 1.Schnabel RB, Yin X, Gona P, et al. Fifty-year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154-162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagres N, Nieuwlaat R, Vardas PE, et al. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on Atrial Fibrillation. J Am Coll Cardiol. 2007;49(5):572-577. doi: 10.1016/j.jacc.2006.10.047 [DOI] [PubMed] [Google Scholar]
- 3.Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol. 2016;13(6):321-332. doi: 10.1038/nrcardio.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madan N, Itchhaporia D, Albert CM, Aggarwal NT, Volgman AS. Atrial fibrillation and heart failure in women. Heart Fail Clin. 2019;15(1):55-64. doi: 10.1016/j.hfc.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Peters SAE, Woodward M. Established and novel risk factors for atrial fibrillation in women compared with men. Heart. 2019;105(3):226-234. doi: 10.1136/heartjnl-2018-313630 [DOI] [PubMed] [Google Scholar]
- 6.Ball J, Carrington MJ, Wood KA, Stewart S; SAFETY Investigators . Women vs men with chronic atrial fibrillation: insights from the Standard vs Atrial Fibrillation Specific Management Study (SAFETY). PLoS One. 2013;8(5):e65795. doi: 10.1371/journal.pone.0065795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946-952. doi: 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- 8.Barillas-Lara MI, Monahan K, Helm RH, et al. Sex-specific prevalence, incidence, and mortality associated with atrial fibrillation in heart failure. JACC Clin Electrophysiol. 2021;7(11):1366-1375. doi: 10.1016/j.jacep.2021.02.021 [DOI] [PubMed] [Google Scholar]
- 9.Andersen K, Rasmussen F, Neovius M, Tynelius P, Sundström J. Body size and risk of atrial fibrillation: a cohort study of 1.1 million young men. J Intern Med. 2018;283(4):346-355. doi: 10.1111/joim.12717 [DOI] [PubMed] [Google Scholar]
- 10.Feng T, Vegard M, Strand LB, et al. Weight and weight change and risk of atrial fibrillation: the HUNT study. Eur Heart J. 2019;40(34):2859-2866. doi: 10.1093/eurheartj/ehz390 [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg MA, Patton KK, Sotoodehnia N, et al. The impact of height on the risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;33(21):2709-2717. doi: 10.1093/eurheartj/ehs301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnussen C, Niiranen TJ, Ojeda FM, et al. ; BiomarCaRE Consortium . Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the Biomarcare Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation. 2017;136(17):1588-1597. doi: 10.1161/CIRCULATIONAHA.117.028981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marott JL, Skielboe AK, Dixen U, Friberg JB, Schnohr P, Jensen GB. Increasing population height and risk of incident atrial fibrillation: the Copenhagen City Heart Study. Eur Heart J. 2018;39(45):4012-4019. doi: 10.1093/eurheartj/ehy367 [DOI] [PubMed] [Google Scholar]
- 14.Aune D, Sen A, Schlesinger S, et al. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(3):181-192. doi: 10.1007/s10654-017-0232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball J, Løchen ML, Wilsgaard T, et al. Sex differences in the impact of body mass index on the risk of future atrial fibrillation: insights from the longitudinal population-based Tromsø Study. J Am Heart Assoc. 2018;7(9):e008414. doi: 10.1161/JAHA.117.008414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee NA, Giulianini F, Geelhoed B, et al. Genetic obesity and the risk of atrial fibrillation: causal estimates from mendelian randomization. Circulation. 2017;135(8):741-754. doi: 10.1161/CIRCULATIONAHA.116.024921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol. 2010;55(21):2319-2327. doi: 10.1016/j.jacc.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118(5):489-495. doi: 10.1016/j.amjmed.2005.01.031 [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert CM, Cook NR, Pester J, et al. Effect of marine omega-3 fatty acid and vitamin D supplementation on incident atrial fibrillation: a randomized clinical trial. JAMA. 2021;325(11):1061-1073. doi: 10.1001/jama.2021.1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;300(21):2489-2496. doi: 10.1001/jama.2008.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.January CT, Wann LS, Alpert JS, et al. ; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071-2104. doi: 10.1161/CIR.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 24.Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 25.Kwok MK, Schooling CM. Mendelian randomization study on atrial fibrillation and cardiovascular disease subtypes. Sci Rep. 2021;11(1):18682. doi: 10.1038/s41598-021-98058-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840-844. doi: 10.1001/jama.271.11.840 [DOI] [PubMed] [Google Scholar]
- 27.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85-93. doi: 10.1161/CIRCOUTCOMES.111.962688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123(14):1501-1508. doi: 10.1161/CIRCULATIONAHA.110.009035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2(2):e000102. doi: 10.1161/JAHA.112.000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karas MG, Yee LM, Biggs ML, et al. Measures of body size and composition and risk of incident atrial fibrillation in older people: the Cardiovascular Health Study. Am J Epidemiol. 2016;183(11):998-1007. doi: 10.1093/aje/kwv278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperrin M, Marshall AD, Higgins V, Renehan AG, Buchan IE. Body mass index relates weight to height differently in women and older adults: serial cross-sectional surveys in England (1992-2011). J Public Health (Oxf). 2016;38(3):607-613. doi: 10.1093/pubmed/fdv067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cochet H, Mouries A, Nivet H, et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol. 2015;26(5):484-492. doi: 10.1111/jce.12651 [DOI] [PubMed] [Google Scholar]
- 33.Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. Left atrial volume and geometry in healthy aging: the Cardiovascular Health Study. Circ Cardiovasc Imaging. 2009;2(4):282-289. doi: 10.1161/CIRCIMAGING.108.826602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41(6):1036-1043. doi: 10.1016/S0735-1097(02)02981-9 [DOI] [PubMed] [Google Scholar]
- 35.Proietti M, Raparelli V, Basili S, Olshansky B, Lip GY. Relation of female sex to left atrial diameter and cardiovascular death in atrial fibrillation: the AFFIRM trial. Int J Cardiol. 2016;207:258-263. doi: 10.1016/j.ijcard.2016.01.169 [DOI] [PubMed] [Google Scholar]
- 36.Yoshida K, Obokata M, Kurosawa K, Sorimachi H, Kurabayashi M, Negishi K. Effect of sex differences on the association between stroke risk and left atrial anatomy or mechanics in patients with atrial fibrillation. Circ Cardiovasc Imaging. 2016;9(10):e004999. doi: 10.1161/CIRCIMAGING.116.004999 [DOI] [PubMed] [Google Scholar]
- 37.Khetan AK, Leong DP, Gupta R, et al. Variations in the association of height with mortality, cardiovascular disease and cancer in low-, middle- and high-income countries. Int J Epidemiol. 2021;dyab268. doi: 10.1093/ije/dyab268 [DOI] [PubMed] [Google Scholar]
- 38.Willems S, Borof K, Brandes A, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J. 2022;43(12):1219-1230. doi: 10.1093/eurheartj/ehab593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccini JP, Simon DN, Steinberg BA, et al. ; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients . Differences in clinical and functional outcomes of atrial fibrillation in women and men: 2-year results from the ORBIT-AF Registry. JAMA Cardiol. 2016;1(3):282-291. doi: 10.1001/jamacardio.2016.0529 [DOI] [PubMed] [Google Scholar]
- 40.Blum S, Muff C, Aeschbacher S, et al. Prospective assessment of sex-related differences in symptom status and health perception among patients with atrial fibrillation. J Am Heart Assoc. 2017;6(7):e005401. doi: 10.1161/JAHA.116.005401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds MR, Lavelle T, Essebag V, Cohen DJ, Zimetbaum P. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152(6):1097-1103. doi: 10.1016/j.ahj.2006.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo AM, Zeitler EP, Giczewska A, et al. ; CABANA Investigators . Association between sex and treatment outcomes of atrial fibrillation ablation vs drug therapy: results from the CABANA trial. Circulation. 2021;143(7):661-672. doi: 10.1161/CIRCULATIONAHA.120.051558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linde C, Bongiorni MG, Birgersdotter-Green U, et al. ; ESC Scientific Document Group . Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. 2018;20(10):1565–, 1565ao.. doi: 10.1093/europace/euy067 [DOI] [PubMed] [Google Scholar]
- 44.Gillis AM. Atrial fibrillation and ventricular arrhythmias: sex differences in electrophysiology, epidemiology, clinical presentation, and clinical outcomes. Circulation. 2017;135(6):593-608. doi: 10.1161/CIRCULATIONAHA.116.025312 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Schematic Showing the Proposed Relationship Between Sex, Anthropometric Measures, and Atrial Fibrillation
eTable 1. Baseline and AF Characteristics for Individuals Who Developed Incident AF
eTable 2. Sensitivity Analyses for Adjusted Sex-Specific Risk of Incident AF for Females Compared With Males, Restricting to Key Subgroups