This cross-sectional study investigates the prevalence of 24-hour activity pattern phenotypes in older adults and tests which phenotypes are associated with depression symptoms and cognitive performance.
Key Points
Question
What is the prevalence of 24-hour activity pattern phenotypes, those related to depression symptoms and cognitive performance, in older adults?
Findings
In this cross-sectional study of 1800 older adults, 4 subgroups were identified defined by earlier rising/robust patterns (37.6%), shorter activity duration/less modelable (32.6%), shorter active periods/very weak (9.8%), had later activity offset/very weak (20.0%). Compared with the early rising/robust group, both groups with weak rhythms had 2-fold odds of clinically significant depression symptoms and cognitive performance deficits.
Meaning
These data indicate that activity pattern disruption may be common in aging and may provide targets for tailored interventions.
Abstract
Importance
Evidence regarding the nature and prevalence of 24-hour activity pattern phenotypes in older adults, especially those related to depression symptoms and cognition, is needed to guide the development of targeted mechanism research and behavioral interventions.
Objectives
To identify subgroups of older adults with similar 24-hour activity rhythm characteristics and characterize associated depression symptoms and cognitive performance.
Design, Setting, and Participants
From January to March 2022, a cross-sectional analysis of the 2011-2014 National Health and Nutrition Examination and Survey (NHANES) accelerometer study was conducted. The NHANES used a multistage probability sample that was designed to be representative of noninstitutionalized adults in the US. The main analysis included participants 65 years or older who had accelerometer and depression measures weighted to represent approximately 32 million older adults.
Exposures
Latent profile analysis identified subgroups with similar 24-hour activity pattern characteristics as measured using extended-cosine and nonparametric methods.
Main Outcomes and Measures
Covariate-adjusted sample-weighted regressions assessed associations of subgroup membership with (1) depression symptoms defined as 9-Item Patient Health Questionnaire (PHQ-9) scores of 10 or greater (PHQ-9) and (2) having at least psychometric mild cognitive impairment (p-MCI) defined as scoring less than 1 SD below the mean on a composite cognitive performance score.
Results
The actual clustering sample size was 1800 (weighted: mean [SD] age, 72.9 [7.3] years; 57% female participants). Clustering identified 4 subgroups: (1) 677 earlier rising/robust (37.6%), (2) 587 shorter active period/less modelable (32.6%), (3) 177 shorter active period/very weak (9.8%), and (4) 359 later settling/very weak (20.0%). The prevalence of a PHQ-9 score of 10 or greater differed significantly across groups (cluster 1, 3.5%; cluster 2, 4.7%; cluster 3, 7.5%; cluster 4, 9.0%; χ2 P = .004). The prevalence of having at least p-MCI differed significantly across groups (cluster 1, 7.2%; cluster 2, 12.0%; cluster 3, 21.0%; cluster 4, 18.0%; χ2 P < .001). Five of 9 depression symptoms differed significantly across subgroups.
Conclusions and Relevance
In this cross-sectional study, findings indicate that approximately 1 in 5 older adults in the US may be classified in a subgroup with weak activity patterns and later settling, and approximately 1 in 10 may be classified in a subgroup with weak patterns and shorter active duration. Future research is needed to investigate the biologic processes related to these behavioral phenotypes, including why earlier and robust activity patterns appear protective, and whether modifying disrupted patterns improves outcomes.
Introduction
As a diurnal species, humans tend to be active during the solar day and rest at night. Disruption to this predictable 24-hour activity pattern does not appear to be an inevitable consequence of chronological aging and instead is linked with diseases such as depression and dementia in older adults (eg1,2). Loss of robust 24-hour activity patterns could theoretically be a contributor to, marker of, and/or consequence of underlying disease mechanisms. Research in this area has potential translational significance because activity patterns are exogenously modifiable and can have downstream effects including feedback on the biological clock.3,4 As such, experimental studies can target disease-linked activity patterns and evaluate whether doing so influences related pathological processes.
To target translational studies, evidence is needed regarding which 24-hour activity pattern phenotypes are common and related to major outcomes among older adults. We are aware of 2 large-scale studies that identified activity pattern characteristics as risk factors for future depression symptoms. These studies found that measures of activity pattern fragmentation5 (ie, higher intradaily variability indicating more “up and down” rather than maintaining consistent rest or activity) and robustness6 (eg, degree of conformity to a 24-hour cosine model) predicted future depression symptoms. Activity pattern characteristics have also been associated with future cognitive decline, including measures of low cross-daily stability,7 degraded temporal organization of activity,7,8,9,10 and poor fit to a 24-hour model.11,12 Further, activity pattern fragmentation correlates with dementia biomarkers13 including in preclinical samples.14,15 Recently, however, a prospective study found that activity pattern stability and fragmentation were not associated with future dementia risk16 and instead that a longer rest period characterized by earlier evening settling and fragmented sleep predicted dementia incidence.
Although past studies focused on measuring activity pattern disruption on continuous scales, it is not known how common disease-relevant 24-hour activity patterns are in the population. Typical 24-hour activity pattern disruption variables are correlated and conceptually tied, and it is highly plausible that one or more population subgroup/phenotype is enriched for activity pattern disruption as indicated by multiple 24-hour activity pattern variables. For instance, measures of intradaily variability and fractal dysregulation are conceptually tied and correlate strongly.17 Higher intraday variability also correlates moderately with lower cross-daily stability18 potentially owing to shared underpinnings (eg, weakened circadian signaling). Although 2 prior studies identified distinct 24-hour activity pattern phenotypes (subgroups that had similar activity pattern characteristics),19,20 these prior studies were limited in terms of sample generalizability and the breadth of measures examined.
Evidence from representative samples regarding the prevalence of activity pattern disruption would clarify the extent of activity pattern disruption as a public health problem in aging. Further, an understanding of which activity pattern phenotypes relate to depression symptoms and cognitive performance in older adults is needed to guide both mechanistic research (to subgroups in which these processes are overexpressed) and intervention development (regarding overall presentations to consider). We therefore aimed to (1) characterize 24-hour activity pattern phenotypes in a sample of older adults that was designed to be representative of the US population and (2) evaluate the health relevance of the behavioral subgroups identified by examining associations with depression symptoms and cognitive performance deficits. We used a data-driven clustering approach that parsed the sample into subgroups, or 24-hour activity pattern phenotypes, based on subgroups having similar values across a panel of activity pattern variables. Measures were derived from the 2 most common 24-hour approaches (nonparametric and extended-cosine based methods). Based on prior findings,19,20 we anticipated at least 3 subgroups with (1) earlier and robust patterns, (2) shorter active periods (longer resting duration), and (3) delayed timing and fragmented or unstable patterns.
Methods
Participants
This was a cross-sectional analysis, conducted from January to March 2022, of the 2011-2014 National Health and Nutrition Examination Survey (NHANES) sample. The NHANES sample was designed to represent the population of the US, excluding institutionalized civilians (eg, supervised care or custody) and active-duty military personnel or other citizens residing outside the 50 states and the District of Columbia. Analyses reported here are weighted following the NHANES Analytic Guidelines21 to represent the corresponding US civilian population age of 65 years and older (eAppendix, eFigure in the Supplement). All protocols were approved by the National Center for Health Statistics Ethics Review Board, and participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Measures
Accelerometer Data and Activity Patterns
Participants wore an accelerometer (GT3X+ [ActiGraph Corporation]) on their nondominant wrist continuously for 7 days. Data and metric extraction were previously described.22 For the cluster analysis, we included 6 measures as described further in the eAppendix of the Supplement: (1) activity onset time, (2) activity offset time, (3) the pseudo–F statistic (how well the model fits the observed data), (4) interdaily stability (cross-daily stability), (5) relative amplitude (rhythm strength), and (6) intradaily variability (rhythm fragmentation).
Depression Symptoms
Depression symptoms were measured with the 9-item Patient Health Questionnaire (PHQ-9). Analyses examined clinically significant depression symptoms defined using the standard cut point of PHQ-9 scores of 10 or greater (88% sensitivity and 88% specificity to a diagnosis of major depressive disorder23). Secondarily, we reported analyses using the individual PHQ-9 items as separate symptom-level outcomes.
Cognitive Tests
These NHANES waves included 3 tests assessing cognitive function. Full details are available online24 and in the eAppendix of the Supplement. To form a composite score reflecting overall cognitive performance, we summed then standardized normed individual test scores. We examined potential clinical relevance by defining the presence of at least psychometric mild cognitive impairment (p-MCI) as having total composite scores less than 1 SD below the mean.
Covariates
We selected covariates that could theoretically be related to both activity pattern subgroup and the outcomes without being on the causal pathway linking the 2 (ie, potential confounders). These were age (80 years or older recoded to 80 years per available NHANES data), sex, and race (Asian, Black, Hispanic, and White, other, and mixed race). In the NHANES, race is assessed using self-reported information. Race categories American Indian or Alaskan Native and Native Hawaiian or Pacific Islander are also included in NHANES data; however, owing to low numbers in these groups and the self-selected other group, they were combined in the White, other, and mixed category for this analysis. We additionally included the following covariates for models of cognitive performance variables: language of cognitive test administration and educational attainment (less than high school, high school, some college, college degree or more).
Statistical Analysis
We empirically derived subgroups that had similar activity pattern characteristics using a finite normal mixture modeling implemented with the R software package mclust (R Foundation).25 We entered the activity pattern measures specified previously and selected the best model fit based on the bayesian information criterion (BIC) considering models with different numbers of subgroups and covariance structures. While using BIC to select the optimal model, we also specified a priori that we would not select models that included small groups defined (considering the sample size) as less than 5% of the analytic sample. We reported the weighted prevalence of each subgroup in the optimal model, as well as their covariate and activity pattern characteristics. For descriptive purposes, activity pattern characteristics are expressed both on their original scales and after rescaling to a sample mean of 0 and SD of 1 to facilitate effect size comparisons.
We next used sample-weighted regression models to evaluate associations of subgroup membership with the following outcomes. Logistic regression was used for the outcomes of clinically significant depression symptoms and at least p-MCI (yes or no). Ordinal regression was used for the individual PHQ-9 depression symptom items (not at all, some days, more than half the days, almost every day). In these models of individual depression symptom items, we considered that there may potentially be false-positive results attributable to comparing multiple subgroups across 9 depression symptom items (eg, comparing 3 groups to a reference on 9 items would make 27 comparisons). Given the large sample size and potential for false positives, we only considered results to be statistically significant when the overall Wald χ2 was less than .05. All P values were 2-sided. Linear regression was used for the 3 continuous outcomes (performance on the cognitive tests). To facilitate effect size comparisons, these continuous outcomes were all standardized before the analysis.
Results
Activity Profiles Identifying With Clustering
The actual clustering sample size was 1800 (weighted: mean [SD] age, 72.9 [7.3] years; 57% female participants; 43% male participants). Participants from the following race and ethnicity categories were included (in weighted percentages): Asian (cluster 1, 4.1%; cluster 2, 2.1%; cluster 3, 3.4%; cluster 4, 3.4%), Black (cluster 1, 5.3%; cluster 2, 6.6%; cluster 3, 12.4%; cluster 4, 13.5%), Hispanic (cluster 1, 5.9%; cluster 2, 6.2%; cluster 3, 9.6%; cluster 4, 5.9%), and White, other, or mixed race and ethnicity (cluster 1, 84.8%; cluster 2, 85.1%; cluster 3, 74.6%; cluster 4, 77.3%). Participants wore an accelerometer for a mean (SD) recording length of 6.9 (0.2) days (range, 4-7 days). The BIC indicated that the optimal model had 4 subgroups and ellipsoidal covariance structures with equal shapes (eTables 1 and 2 in the Supplement). Activity patterns for participants illustrating each subgroup are shown in Figure 1. We labeled the largest subgroup (677 [37.6%]) cluster 1 early rising/robust because, on average, they had (1) an estimated activity onset before 7 am (approximately one-third of an SD earlier than average (Table), (2) a 15-hour active period, and (3) the most robust activity patterns as indicated on all measures.
Figure 1. Seven Days of Accelerometer Time Series Data From 4 Participants Illustrating the Subgroups Detected.
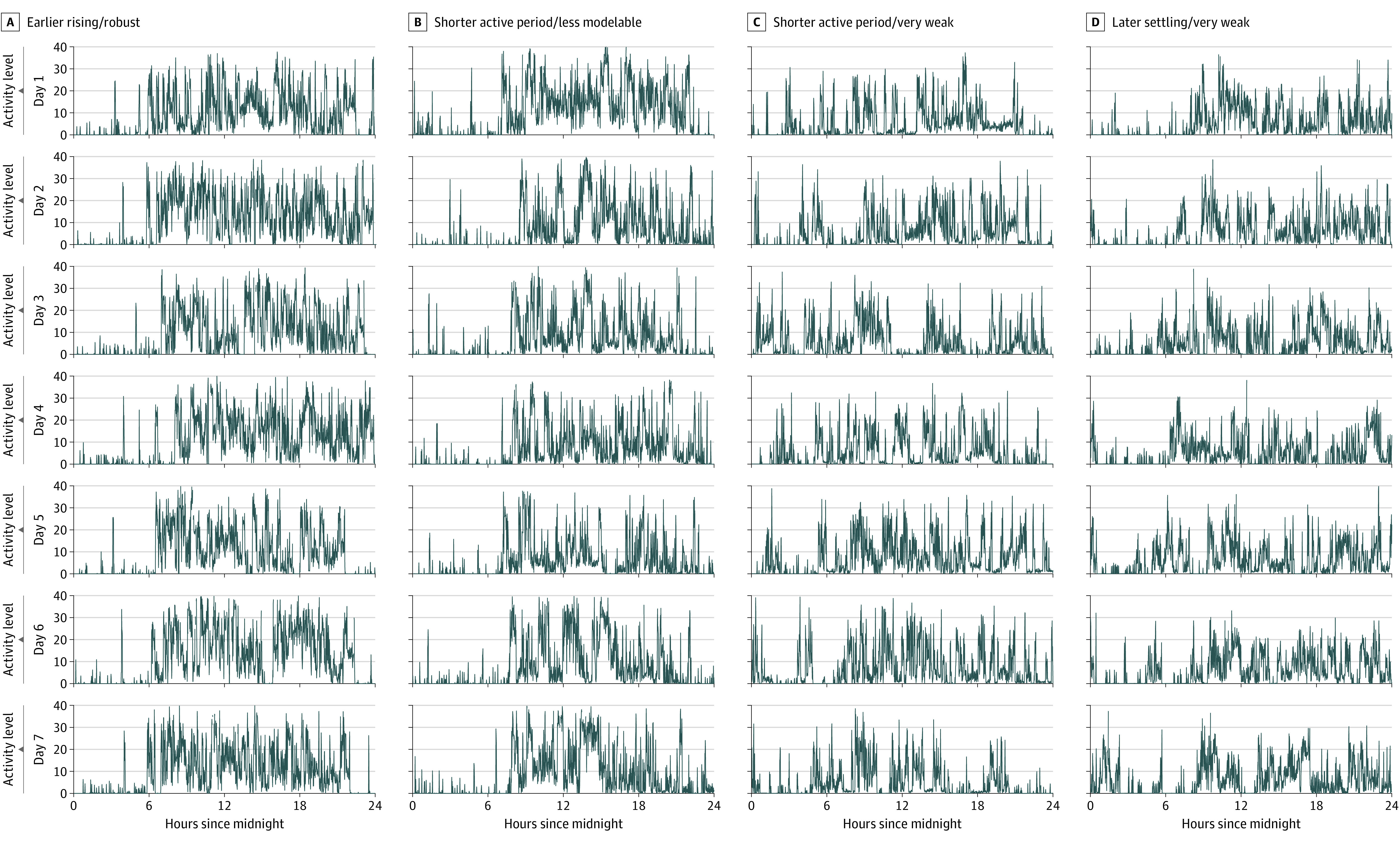
Each column includes data collected over 7 days from a single participant and includes the following periods: earlier rising/robust (A), shorter active period/less modelable (B), shorter active period/very weak (C), and later settling/very weak (D).
Table. Rest-Activity Rhythm Characteristics by Cluster (N = 1800).
| Characteristic | Cluster 1: earlier rising/robust | Cluster 2: shorter active period/less modelable | Cluster 3: shorter active period/very weak | Cluster 4: later settling/very weak |
|---|---|---|---|---|
| No. in cluster | 677 | 587 | 177 | 359 |
| Cluster prevalence, % | 37.6 | 32.63 | 9.83 | 19.96 |
| Sociodemographic factors | ||||
| Age, mean (SD), y | 72.3 (5.3) | 72.8 (5.3) | 74.0 (5.1) | 73.5 (5.6) |
| Sex, weighted % | ||||
| Female | 63.2 | 55.0 | 48.8 | 50.6 |
| Male | 36.8 | 45.0 | 51.2 | 49.4 |
| Race and ethnicity, weighted % | ||||
| Asian | 4.1 | 2.1 | 3.4 | 3.4 |
| Black | 5.3 | 6.6 | 12.4 | 13.5 |
| Hispanic | 5.9 | 6.2 | 9.6 | 5.9 |
| White/other/mixed | 84.8 | 85.1 | 74.6 | 77.3 |
| Education, weighted %a | ||||
| < High school | 15.4 | 20.4 | 29.2 | 20.2 |
| High school | 21.5 | 24.2 | 19.0 | 22.6 |
| Some college | 27.0 | 33.1 | 33.8 | 30.7 |
| College degree or more | 36.1 | 22.2 | 18.0 | 26.5 |
| Activity pattern factors, mean (SD), original units | ||||
| Activity onset timeb | 6.8 (0.8) | 7.6 (1.2) | 7.9 (1.9) | 7.1 (1.7) |
| Activity offset time | 22.0 (1.2) | 21.0 (1.6) | 19.8 (3.3) | 22.3 (1.8) |
| Active period duration, mean (SD), hc | 15.2 (1.1) | 13.4 (1.5) | 11.8 (3.2) | 15.2 (1.4) |
| Pseudo–F statistic | 5.8 (0.5) | 4.7 (0.7) | 3.9 (1.3) | 5.6 (0.6) |
| Amplitudec | 2.5 (0.3) | 2.4 (0.3) | 2.2 (0.5) | 2.2 (0.4) |
| Interdaily stability | 0.4 (0.1) | 0.4 (0.1) | 0.3 (0.1) | 0.3 (0.1) |
| Relative amplitude | 0.9 (0) | 0.9 (0) | 0.8 (0.1) | 0.8 (0.1) |
| Intradaily variability | 0.4 (0.1) | 0.4 (0.1) | 0.5 (0.1) | 0.5 (0.1) |
| Activity pattern factors, mean (SD), standardized units | ||||
| Activity onset time | −0.3 (0.6) | 0.3 (0.9) | 0.5 (1.4) | −0.1 (1.2) |
| Activity offset time | 0.2 (0.6) | −0.3 (0.8) | −0.9 (1.6) | 0.4 (0.9) |
| Active period duration, hc | 0.4 (0.5) | −0.4 (0.7) | −1.2 (1.5) | 0.4 (0.7) |
| Pseudo–F statistic | 0.6 (0.5) | −0.5 (0.7) | −1.3 (1.3) | 0.4 (0.6) |
| Amplitudec | 0.4 (0.7) | 0.2 (0.9) | −0.5 (1.4) | −0.4 (1.0) |
| Interdaily stability | 0.4 (0.8) | 0.1 (0.9) | −0.5 (1.2) | −0.4 (0.9) |
| Relative amplitude | 0.7 (0.3) | 0.4 (0.5) | −0.9 (1.4) | −0.7 (0.8) |
| Intradaily variability | −0.3 (0.7) | −0.2 (0.8) | 0.6 (1.4) | 0.4 (1.0) |
Self-reported other race and ethnicity indicates American Indian or Alaskan Native, Native Hawaiian or Pacific Islander.
Times are in 24-hour decimal time, eg, 6.5 = 06:30 am
Indicates that the variable was not included in the clustering, owing to high correlations and redundancy with others that were (shown for illustrative purposes).
The second largest subgroup (587 [32.6%]) was labeled cluster 2 shorter active period/less modelable because, on average, they were distinguished from the cluster 1 earlier rising/robust group by starting their daily activities later and settling down earlier (average active period duration = 13.4 hours), and they also had robustness measures higher than average except for the pseudo–F statistic (indicating that worse to a 24-hour extended cosine model).
The final 2 groups both had disrupted activity patterns as indicated by multiple measures (pseudo–F statistic, interdaily stability, intradaily variability, and relative amplitude). The smaller of these groups, which made up 9.8% (177 of 1800) of the sample, was labeled cluster 3 shorter active period/very weak as they had the shortest average active period length (11.8 hours).
The last group (359 [20.0%]) was labeled cluster 4 later settling/very weak based on them having the latest estimated activity offset times on average. Sample sociodemographic factors by subgroup are shown in the Table.
Depression Symptoms in the Activity Pattern Subgroups
The prevalence of a PHQ-9 score of 10 or greater differed across groups (cluster 1, 3.5%; cluster 2, 4.7%; cluster 3, 7.5%; cluster 4, 9.0%; χ2 P = .004) (Figure 2A). Overall, 5.4% of participants had PHQ-9 scores of 10 or greater. Age-, sex-, and race-adjusted odds of having a PHQ-9 score of 10 or more, compared with the cluster 1 earlier rising/robust group, were similar in the cluster 2 shorter active period/less modelable group (odds ratio [OR], 1.43; 95% CI, 0.76-2.67) and statistically higher in both the cluster 3 shorter active period/very weak group (OR, 2.34; 95% CI, 1.12-4.91) and the cluster 4 later settling/very weak group (OR, 2.91; 95% CI, 1.51-5.60).
Figure 2. Prevalence of Least Clinically Significant Depression and Psychometric Mild Cognitive Impairment (P-MCI) in the Activity Pattern Subgroups.
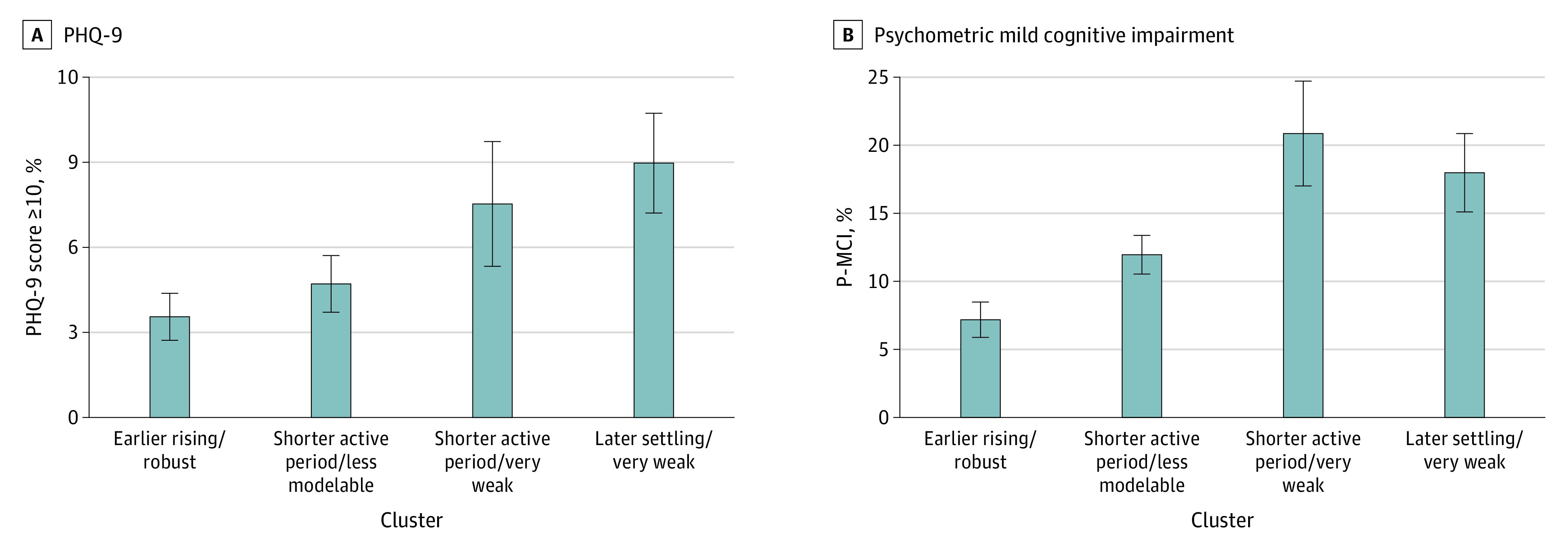
Prevalence data are from weighted estimates. SEs are shown, and P-MCI percentages include having at least psychometrically defined mild cognitive impairment. PHQ-9 indicates 9-item Patient Health Questionnaire.
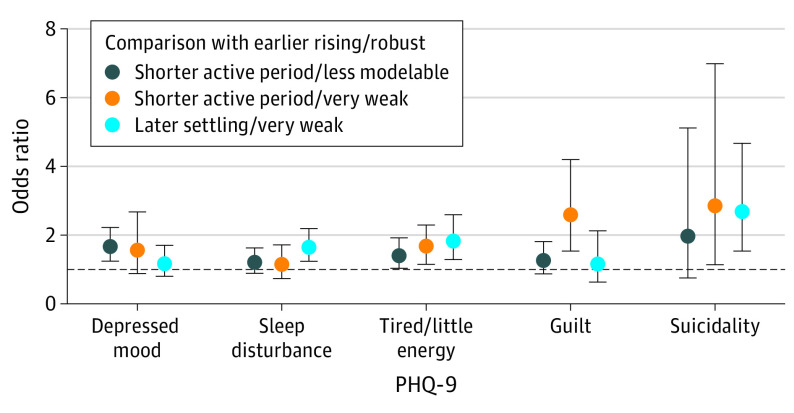
There were also statistically significant differences across groups in the levels of 5 of 9 individual depression symptoms (depressed mood, sleep disturbance, tired/little energy, guilt, suicidality). Compared with the cluster 1 earlier rising/robust group, ORs from ordinal regression and CIs (Figure 3) indicated that (1) both groups with shorter active periods reported greater depressed mood levels (although the 95% CI overlapped with 1 in the smallest of these groups), (2) the cluster 4 later settling/very weak group reported greater sleep disturbance levels, (3) all 3 groups reported greater tiredness levels, (4) only the cluster 3 shorter active period/very weak group reported greater guilt levels, and (5) both groups with weak activity patterns (clusters 3 and 4) reported greater suicidality levels.
Figure 3. Associations of Activity Pattern Subgroups With 5 of the 9 Individual Depression Symptoms Measured With the 9-Item Patient Health Questionnaire (PHQ-9).
Odds ratios and 95% CIs are shown from separate sample-weighted age-, sex-, and race and ethnicity–adjusted ordinal regression models. In order, the items shown are PHQ-9 items 2, 3, 4, 6, and 9.
Cognitive Test Performance in the Activity Pattern Subgroups
Note that there was a wide range of actual unweighted raw cognitive test scores indicating that the sample included participants across a range of cognitive statuses (eTable 3 in the Supplement). The prevalence of having at least p-MCI differed significantly across groups (cluster 1, 7.2%; cluster 2, 12.0%; cluster 3, 21.0%; cluster 4, 18.0%; χ2 P < .001) (Figure 2B). Compared with the cluster 1 earlier rising/robust group, the odds of having at least p-MCI (adjusted for age, sex, race, cognitive test administration language, and education) were higher in all 3 groups (cluster 2 shorter active period/less modelable group: OR, 1.84; 95% CI, 1.06-3.20; cluster 3 shorter active period/very weak group: OR, 3.36; 95% CI, 1.81-3.26; cluster 4 later settling/very weak group: OR, 2.76; 95% CI, 1.63-4.67).
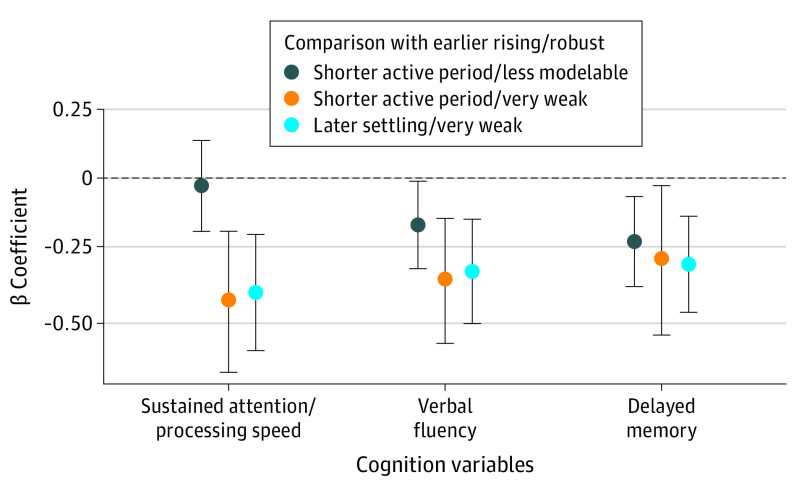
In terms of continuous cognitive performance outcomes, both groups with weak activity patterns also performed worse on all 3 tests (β coefficient > −0.25 and 95% CIs not overlapping with 0) (Figure 4). The cluster 2 shorter active period/less modelable group performed worse than the reference group on the delayed memory test only.
Figure 4. Associations Between the Activity Pattern Subgroups With the Normed Cognitive Performance Measures Expressed Continuously.
Established β coefficients and 95% CIs are shown from separate sample-weighted age-, sex-, race and ethnicity–, and education-adjusted linear regression models.
Comparison of Associations Effect Sizes for the Individual Activity Pattern Characteristics vs Subgroup Membership
Additional analyses confirmed that individual 24-hour activity pattern variables were associated with the outcomes independent of overall activity level (eTable 4 in the Supplement). Activity timing onset and level were independently associated with depression symptom severity, and several rhythm disruption indicators were independently associated with cognitive performance. Effect sizes were larger when comparing those of subgroup membership against any individual variable.
Discussion
This cross-sectional study identified 24-hour activity pattern phenotypes that are common among older adults in the US. Findings suggest that although activity pattern disruption was common (approximately 3 in 10 older adults), a substantial subgroup of older adults had earlier morning activity initiation and robust activity patterns. This earlier rising/robust subgroup had the lowest rates of depression and best cognitive performance. In contrast, activity pattern disruption was associated with more than twice the odds of clinically significant depression symptoms, including suicidal ideation, and p-MCI scores. These findings have potential translational implications, in part, because 24-hour activity patterns can be measured passively and objectively with accelerometers (including those in popular consumer wearables26,27). As such, scalable approaches could be developed to screen for those activity patterns associated with diseases. But moreover, activity patterns are exogenously modifiable and can have downstream effects including providing feedback on the biological clock.3,4 Therefore, the presentations of activity pattern disruption identified here can be targeted in future experimental trials to determine if modifying activity pattern disruption influences related mechanisms and disease outcomes.
These analyses of the NHANES study replicated and extended prior research in several ways. First, we replicated prior evidence that earlier rising times tended to co-occur with robust activity rhythms.20 Remarkably, both our prior study and present results indicated that 38% of older adults fit this profile of having early rising/robust activity patterns. We here confirm prior evidence that this subgroup had lower rates of clinically significant depression symptoms, and we extended existing knowledge by demonstrating this early rising/robust activity pattern subgroup also had the best average cognitive test performance. Second, our prior clustering results showed that approximately 40% of the sample had narrower active periods and intermediate values on activity pattern robustness measures. We here replicated and extended this finding by parsing 2 presentations with shortened active periods: 1 with narrower active periods but otherwise generally normative patterns (approximately 33% in NHANES) and another with narrower active periods and very weak patterns (approximately 10% in NHANES). Finally, and again remarkably like our prior data-driven clustering, we identified a subgroup of approximately 20% with very weak activity patterns and later activity offset timing.
Bidirectional relationships of disrupted activity patterns with both depression and neurocognitive disease mechanisms are plausible. Mood disturbances and cognitive deficits may involve reduced motivation, altered reward seeking, or impaired concentration/organization; theoretically, these factors could lead to the observed activity patterns. Activity patterns and depression or cognitive performance deficits may have common neurobiological underpinnings, eg, in dopaminergic dysfunction28 and/or white matter damage.29 It is also plausible that activity pattern disruption contributes to disease pathogenesis. Experimentally inducing circadian rhythm disruption affects brain structure in rodents,30 and experimentally inducing irregular circadian rhythms affects mood in humans.31 Glymphatic clearance of waste products from the brain follows a circadian rhythm.32 Because activity behaviors provide feedback to be integrated by the central clock,4 disrupted activity patterns theoretically would weaken or cause misaligned endogenous rhythms. In addition, disrupted activity patterns may reduce homeostatic drive, altogether altering sleep quality or architecture and related functions like emotional memory consolidation and glymphatic clearance of waste. In contrast, earlier and robust activity patterns could protect mood and brain health by facilitating or enhancing key functions like social and physical activity engagement and deep sleep.
Strengths and Limitations
This study had several strengths. Analyses used a large probability-based sample and sampling weights; therefore, subgroup prevalence rates can be considered fairly precise and generalizable to older adults in the US. The differences observed between the subgroups, both in activity pattern presentations and related symptoms or cognitive deficits, provided information which factors to target in future mechanistic and experimental studies. We observed that earlier timing and more robust patterns co-occurred. Compared with having activity pattern disruption, being in this earlier rising/robust subgroup was associated with lower rates of depression symptoms, tiredness, and better cognitive performance. In theory, regular and early rising times (and associated receipt of bright or sun light) can strengthen internal circadian timing signals and increase time being active and building homeostatic sleep drive. Experimental studies will be needed to determine if setting stable or early morning wake times strengthens weak activity rhythms and confers resilience to negative health outcomes like tiredness and depression. Given higher rates of cognitive deficits in both groups with weak activity patterns, many older adults may require support with planning and monitoring to achieve and maintain robust rhythms.
The observed differences between the 2 activity pattern subgroups with weak rhythms have implications for the development of future experimental studies. First, the later settling/weak group was the only group to report greater sleep disturbances. Activity persisting later into the night and sleep disturbances may be associated with elevated arousal that may be treatable; thus, this group may benefit from strategies (eg, stimulus control) that reduce activity or arousal around bedtime to improve sleep. Second, we noted that only the group with weak rhythms plus shorter active periods had higher levels of guilt. For patients with this profile, additional activating therapy may be required to increase the daily duration of activity engagement, and in particular, increase engagement with activities that promote positive self-evaluations.
This study also had some limitations. It was not designed to answer questions regarding which mechanisms link activity pattern disruption with mood or cognitive functioning or whether (or how) these processes are modifiable. Owing to potential residual (unmeasured) confounding, the associations detected here do not necessarily reflect causal relationships. Thus, our findings cannot attest to what caused the observed activity profiles, how stable they are over time, or what their consequences may be. The data reported here do not directly support inferences regarding the temporal relationships between biological mechanisms, activity profiles, or outcome. Future studies are needed to evaluate how 24-hour activity patterns relate to differences in sleep architecture, cognitive and social activity, and other putative mediators of healthy aging.
Conclusions
In this cross-sectional study, findings indicate that approximately 1 in 5 older adults in the US may be classified in a subgroup with weak activity patterns and later settling, and approximately 1 in 10 may be classified in a subgroup with weak patterns and shorter active duration. Future experimental studies are needed to determine if targeted treatments for activity rhythm disruption influence disease mechanisms and improve related outcomes.
eAppendix. Analytic Sample Size, Accelerometer Measures, and Cognition Measures
eFigure. Flowchart Illustrating How We Arrived at the Analytic Samples
eTable 1. Activity Pattern Subgroup Rates in Participants With and Without Cognitive Performance Test Data
eTable 2. Bayesian Information Criterion for Models of Different Variance Structures and Number of Groups
eTable 3. Sample Raw Unweighted Cognitive Test Performance Scores
eTable 4. Associations of Individual Activity Pattern Characteristics With the Main Outcomes Expressed Continuously
References
- 1.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer disease. Biol Psychiatry. 1990;27(6):563-572. doi: 10.1016/0006-3223(90)90523-5 [DOI] [PubMed] [Google Scholar]
- 2.Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Tiemeier H. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 2013;30(10):1223-1230. doi: 10.3109/07420528.2013.813528 [DOI] [PubMed] [Google Scholar]
- 3.Reebs SG, Mrosovsky N. Effects of induced wheel running on the circadian activity rhythms of Syrian hamsters: entrainment and phase response curve. J Biol Rhythms. 1989;4(1):39-48. doi: 10.1177/074873048900400103 [DOI] [PubMed] [Google Scholar]
- 4.Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21(6):482-493. doi: 10.1177/0748730406294627 [DOI] [PubMed] [Google Scholar]
- 5.de Feijter M, Kocevska D, Ikram MA, Luik AI. The bidirectional association of 24-h activity rhythms and sleep with depressive symptoms in middle-aged and elderly persons. Psychol Med. Published online August 11, 2021. doi: 10.1017/S003329172100297X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smagula SF, Ancoli-Israel S, Blackwell T, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group . Circadian rest-activity rhythms predict future increases in depressive symptoms among community-dwelling older men. Am J Geriatr Psychiatry. 2015;23(5):495-505. doi: 10.1016/j.jagp.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao Q, Sampson JN, LaCroix AZ, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group . Nonparametric parameters of 24-hour rest-activity rhythms and long-term cognitive decline and incident cognitive impairment in older men. J Gerontol A Biol Sci Med Sci. 2022;77(2):250-258. doi: 10.1093/gerona/glab275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu K, Riemersma-van der Lek RF, Patxot M, et al. Progression of dementia assessed by temporal correlations of physical activity: results from a 3.5-year, longitudinal randomized controlled trial. Sci Rep. 2016;6:27742. doi: 10.1038/srep27742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P, Yu L, Lim ASP, et al. Fractal regulation and incident Alzheimer disease in elderly individuals. Alzheimers Dement. 2018;14(9):1114-1125. doi: 10.1016/j.jalz.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Gao L, Gaba A, et al. Circadian disturbances in Alzheimer disease progression: a prospective observational cohort study of community-based older adults. Lancet Healthy Longev. 2020;1(3):e96-e105. doi: 10.1016/S2666-7568(20)30015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tranah GJ, Blackwell T, Stone KL, et al. ; SOF Research Group . Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722-732. doi: 10.1002/ana.22468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers-Soeder TS, Blackwell T, Yaffe K, et al. ; Osteoporotic Fractures in Men Study Research Group . Rest-activity rhythms and cognitive decline in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2018;66(11):2136-2143. doi: 10.1111/jgs.15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer R, Yu L, Schneider JA, Bennett DA, Buchman AS, Lim ASP. Disrupted rest-activity rhythms and cerebral small vessel disease pathology in older adults. Stroke. 2021;52(7):2427-2431. doi: 10.1161/STROKEAHA.120.030870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47(3):e148. doi: 10.1038/emm.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Someren EJW, Oosterman JM, Van Harten B, et al. Medial temporal lobe atrophy relates more strongly to sleep-wake rhythm fragmentation than to age or any other known risk. Neurobiol Learn Mem. 2019;160:132-138. doi: 10.1016/j.nlm.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 16.Lysen TS, Luik AI, Ikram MK, Tiemeier H, Ikram MA. Actigraphy-estimated sleep and 24-hour activity rhythms and the risk of dementia. Alzheimers Dement. 2020;16(9):1259-1267. doi: 10.1002/alz.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suibkitwanchai K, Sykulski AM, Perez Algorta G, Waller D, Walshe C. Nonparametric time series summary statistics for high-frequency accelerometry data from individuals with advanced dementia. PLoS One. 2020;15(9):e0239368-e0239368. doi: 10.1371/journal.pone.0239368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luik AI, Zuurbier LA, Direk N, Hofman A, Van Someren EJ, Tiemeier H. 24-hour activity rhythm and sleep disturbances in depression and anxiety: a population-based study of middle-aged and older persons. Depress Anxiety. 2015;32(9):684-692. doi: 10.1002/da.22355 [DOI] [PubMed] [Google Scholar]
- 19.Smagula SF, Boudreau RM, Stone K, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group . Latent activity rhythm disturbance subgroups and longitudinal change in depression symptoms among older men. Chronobiol Int. 2015;32(10):1427-1437. doi: 10.3109/07420528.2015.1102925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smagula SF, Krafty RT, Thayer JF, Buysse DJ, Hall MH. Rest-activity rhythm profiles associated with manic-hypomanic and depressive symptoms. J Psychiatr Res. 2018;102:238-244. doi: 10.1016/j.jpsychires.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen TC, Parker JD, Clark J, Shin HC, Rammon JR, Burt VL. National Health and Nutrition Examination Survey: estimation procedures, 2011-2014. Accessed January 15, 2022. https://www.cdc.gov/nchs/data/series/sr_02/sr02_177.pdf [PubMed]
- 22.Li J, Somers VK, Lopez-Jimenez F, Di J, Covassin N. Demographic characteristics associated with circadian rest-activity rhythm patterns: a cross-sectional study. Int J Behav Nutr Phys Act. 2021;18(1):107-107. doi: 10.1186/s12966-021-01174-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey: 2013-2014 data documentation, codebook, and frequencies. Accessed July 8, 2022. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CFQ_H.htm
- 25.Scrucca L, Fop M, Murphy TB, Raftery AE. mclust 5: Clustering, classification and density estimation using gaussian finite mixture models. R J. 2016;8(1):289-317. doi: 10.32614/RJ-2016-021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HA, Lee HJ, Moon JH, et al. Comparison of wearable activity tracker with actigraphy for sleep evaluation and circadian rest-activity rhythm measurement in healthy young adults. Psychiatry Investig. 2017;14(2):179-185. doi: 10.4306/pi.2017.14.2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smagula SF, Stahl ST, Krafty RT, Buysse DJ. Initial proof of concept that a consumer wearable can be used for real-time rest-activity rhythm monitoring. Sleep. 2022;45(3):zsab288. doi: 10.1093/sleep/zsab288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor WD, Zald DH, Felger JC, et al. Influences of dopaminergic system dysfunction on late-life depression. Mol Psychiatry. 2022;27(1):180-191. doi: 10.1038/s41380-021-01265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleh Y, Le Heron C, Petitet P, et al. Apathy in small vessel cerebrovascular disease is associated with deficits in effort-based decision-making. Brain. 2021;144(4):1247-1262. doi: 10.1093/brain/awab013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108(4):1657-1662. doi: 10.1073/pnas.1018375108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chellappa SL, Morris CJ, Scheer FAJL. Circadian misalignment increases mood vulnerability in simulated shift work. Sci Rep. 2020;10(1):18614. doi: 10.1038/s41598-020-75245-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hablitz LM, Plá V, Giannetto M, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11(1):4411. doi: 10.1038/s41467-020-18115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Analytic Sample Size, Accelerometer Measures, and Cognition Measures
eFigure. Flowchart Illustrating How We Arrived at the Analytic Samples
eTable 1. Activity Pattern Subgroup Rates in Participants With and Without Cognitive Performance Test Data
eTable 2. Bayesian Information Criterion for Models of Different Variance Structures and Number of Groups
eTable 3. Sample Raw Unweighted Cognitive Test Performance Scores
eTable 4. Associations of Individual Activity Pattern Characteristics With the Main Outcomes Expressed Continuously