Abstract
The unusual genetic properties of the non-chromosomal genetic elements, [URE3] and [PSI+] led to their being identified as prions (infectious proteins) of Ure2p and Sup35p, respectively. Ure2p and Sup35p, and now several other proteins, can form amyloid, a linear ordered polymer of protein monomers, with a part of each molecule, the prion domain, forming the core of this beta-sheet structure. Amyloid filaments passed to a new cell seed the conversion of the normal form of the protein to the same amyloid form. The cell’s phenotype is affected, usually from the deficiency of the normal form of the protein. Solid-state NMR studies indicate that the yeast prion amyloids are in-register parallel β-sheet structures, in which each residue (e.g. asparagine 35) forms a row along the filament long axis. The favorable interactions possible for aligned identical hydrophilic and hydrophobic residues are believed to be the mechanism for propagation of amyloid conformation. Thus, just as DNA mediates inheritance by templating its own sequence, these proteins act as genes by templating their conformation. Distinct isolates of a given prion have different biological properties, presumably determined by differences between the amyloid structures. Many lines of evidence indicate that the S. cerevisiae prions are pathological, disease agents, although the example of the [Het-s] prion of Podospora anserina shows that a prion can have beneficial aspects.
Keywords: prion, amyloid, Ure2p, Sup35p, [PSI+], [URE3], [PIN+], solid-state NMR, in-register parallel beta sheet
Summary:
>Certain yeast proteins can form self-propagating amyloid (a fibrous beta-sheet rich aggregate) that produces cellular defects, largely by inactivating the amyloid-forming protein.
>The self-propagation of the amyloids makes these infectious and heritable, so these proteins act as genes by templating their conformation, just as DNA templates its sequence.
>Chaperones play an important part in prion generation and propagation, and aggregate-collecting mechanisms also impinge on prion processes.
Introduction
Infectious elements in yeast generally appear as non-chromosomal genetic elements. Most differences between mated strains segregates 2+: 2- in meiosis, the pattern of a difference in a single chromosomal gene. However, if one parent in a genetic cross carries one of the yeast viruses and the other does not, all of the meiotic progeny will have the virus, a pattern called 4+ : 0 segregation. Yeast viruses (and prions) do not exit one cell into the environment and then enter another as is the case for human viruses or prions. [URE3] [1] and [PSI+] [2] are two long - known yeast non-chromosomal genetic elements whose basis was unknown. The similarity of the phenotype of [URE3] to that of mutants in the ure2 chromosomal gene, and the fact that URE2 is necessary for the propagation of [URE3] [3], first led us to suspect that [URE3] was actually a prion form of the Ure2 protein, an altered form of the Ure2p with the ability to catalyze the conversion of the normal form into the same altered (inactive) form [4]. Ure2p is a regulator of nitrogen catabolism, turning off the genes encoding enzymes and transporters needed for using poor nitrogen sources if a good nitrogen source was present in the medium [5, 6]. If [URE3] were indeed a prion, we reasoned that overproduction of Ure2p should increase the frequency with which the [URE3] non-chromosomal genetic element would arise, and this too proved to be true [4]. Various treatments can induce very frequent mutation of the mitochondrial DNA or curing of the yeast viruses: growth in the presence of guanidine [7] or ethidium bromide [8], for example, induces mutation in mitochondrial DNA and its loss. But while these mitochondrial DNA mutations or loss are irreversible, curing a prion should be reversible, since the prion-forming protein is still being made in the cell and should be able (rarely) to again convert to the prion form. Thus, reversible curability (not curability itself) is expected to be a trait of a prion [4]. Indeed, [URE3] can be cured by growth in the presence of low concentrations of guanidine, but, unlike guanidine induced mitochondrial DNA curing, that of [URE3] is reversible (at low frequency), again indicating it is a prion [4].
Interestingly, these three properties had already been shown for [PSI+] and the SUP35 gene [9–11], and we concluded that [PSI+] was likewise a prion of Sup35p [4]. Sup35p is a subunit of the translation termination factor, a protein whose activity is, unlike that of Ure2p, essential for growth of S. cerevisiae [12, 13].
The prion concept first arose as a leading hypothesis in studies of the uniformly lethal mammalian transmissible spongiform encephalopathies (TSEs)[14–16], but proving it was difficult because of the long incubation times for even infected rhodents. While the yeast prion systems can be non-lethal (but see below), show phenotypes unrelated to the TSE diseases and involve proteins with no sequence relation to the mammalian prion protein PrP, the yeast systems provided an important model in which it was possible to actually prove that proteins could be infectious elements, and quickly explore the mechanisms involved.
Most yeast prions are amyloid forms of a normally soluble protein.
Amyloid is a filamentous polymer of identical protein monomers that is characterized by a cross-beta structure (see next section). Amyloids play a prominent role in human degenerative diseases such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis. These neurodegenerative diseases, each quite common, are caused by deposits of amyloid, each of a specific protein or peptide, that cause damage to the brain. Type 2 (late onset) diabetes mellitus is associated with (though probably not caused by) deposits of amylin, a peptide that, like insulin, is made in the Islets of the pancreas. Senile amyloidosis is a very common disorder of the elderly due to deposition of amyloid filaments of transthyretin, a protein normally found in serum.
Perhaps with the many amyloidoses of humans it is not surprising that yeast has several amyloid diseases. A series of studies showed that Sup35p is aggregated in cells carrying the [PSI+] prion, that Ure2p is aggregated in [URE3] cells, and similarly for the other identified yeast prions (see Table 1) [17–19]. Recombinant Sup35p could form amyloid in vitro [20, 21] and introduction of this amyloid into yeast cells makes them prion positive [22, 23]. Similar results were later obtained for Ure2p and [URE3] [24, 25]. Thus these amyloid filaments are infectious.
Table 1.
Prions of S. cerevisiae and Podospora anserina.
| Prion | Protein | Normal Function |
Prion Phenotype |
Ref. |
|---|---|---|---|---|
| [URE3] | Ure2p | Nitrogen regulation | Inappropriate derepression of nitrogen catabolism genes; slow growth | [4] |
| [PSI+] | Sup35p | Translation termination | Readthrough of translation stop codons | [4] |
| [PIN+] | Rnq1p | Unknown | Rare cross – seeding of [PSI+] or other prions | [79] |
| [SWI+] | Swi1p | Chromatin remodeling | Inability to utilize non-fermentable carbon source and slow growth | [80] |
| [OCT+] | Cyc8p | Transcription repression | Derepressed transcription; flocculence | [81] |
| [MOT+] | Mot3p | Repressor of genes for anaerobic growth | Inappropriate derepression of anaerobic growth genes | [82] |
| [ISP+] | Sfp1p | Transcription factor | Decreased translation read through | [83] |
| [MOD+] | Mod5p | tRNA isopentenyl - transferase | Slow growth; resistance to azole anti-fungal drugs | [84] |
| [Het-s] | HET-s | Heterokaryon incompatibility | Heterokaryon incompatibility in Podospora anserina (a functional prion) | [63] |
| [BETA] | Prb1p | Vacuolar protease B | normal sporulation, survival in stationary phase (a functional prion) | [85] |
Prion variants.
A single prion protein sequence can form amyloids of different detailed structure resulting in different biological properties. These are called ‘prion variants’. Since prions are proteins acting as genes, prion variants can be thought of as different alleles of the protein gene. Each prion variant is (relatively) stable and propagates as cells divide. Prion variants were long known in mammalian prions, and were found in yeast prions soon after their discovery [25–27]. In yeast, variants are commonly distinguished based on whether their phenotype is ‘strong’ or ‘weak’, whether the variant is stably propagated or readily lost, response to excess or deficiency of certain chaperones, and transmission efficiency across interspecies or intraspecies barriers. The existence of variants is critical in understanding many aspects of prions, including structural studies, interactions of prions with other cellular components and the biological role of prions for yeast.
Prion domains, their structure and biological implications.
Only a part of each prion protein actually forms the amyloid structure, and this part is sufficient to transmit the prion trait [20, 24, 28, 29]. These ‘prion domains’ have been the focus of structural studies. The prion domains of Ure2p and Sup35p are the N-terminal ~70 residues and ~124 residues, respectively, although the size of the prion domain varies somewhat with prion variant (see below for discussion of prion variants). One particularly clear demonstration of the role of the prion domain is in Ure2p, whose C-terminal part is essential for its role in regulating nitrogen assimilation, and which has a glutathione peroxidase activity [30]. Remarkably, this activity is unaffected by amyloid formation, showing that the C-terminal domain is unchanged by amyloid formation, including its homodimer status [30]. The definition of amyloid includes the cross-beta structure, meaning that the filaments are rich in beta sheet, and that the beta strands run perpendicular to the long axis of the filament. Within this definition are several possible architectures, depending on the relation of the beta strands to eachother:
1) Antiparallel beta sheets have adjacent beta strands running in opposite directions. This is the most common type of beta sheet in monomeric proteins.
2) In parallel beta sheets, the peptide chains are oriented in the same direction. If a parallel beta sheet is in-register, each residue is aligned with the same residue in the molecule before and after it in the filament (see Fig. 1). Each molecule occupies a single 4.7 angstrom layer along the long axis of the filament. In-register parallel beta sheets are the most common architecture of pathologic amyloids (reviewed by [31]), and current evidence supports this form for the infectious amyloids of the prion domains of Sup35p, Ure2p and Rnq1p [32–34].
Figure 1.
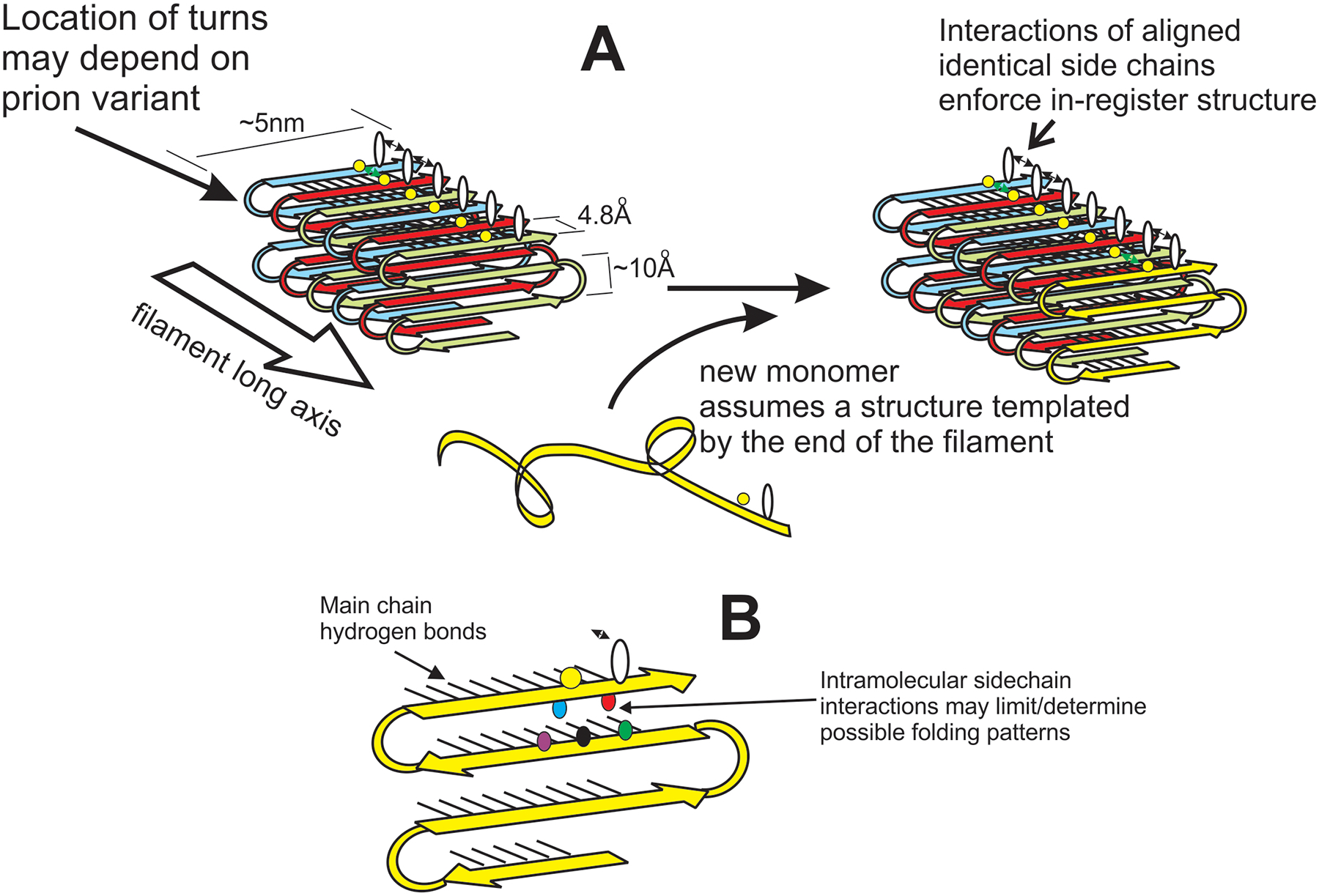
The in-register parallel architecture of yeast prion amyloid can explain templating of conformation. A. The unstructured prion domain of molecules newly joining the end of the amyloid filament are directed to assume the same conformation as molecules already in the filament by the favorable interactions between identical side chains that can only occur if the molecules are in-register [77]. B. Three interactions of an amino acid residue are shown. The black lines show the main chain hydrogen bonds of the β-sheet. The black double-headed arrow indicates the intermolecular side-chain interactions between identical residues (emphasized in A). Also shown are the interactions between non-identical side chains in the plane perpendicular to the long axis of the filaments. These interactions have been studied by X-ray crystallography of short peptides [78].
3) In a beta helix, each molecule occupies more than one layer along the long axis of the filament. Each molecule forms a helix of two or more turns. This architecture has been realized in the [Het-s] prion of Podospora anserina [35, 36].
The parallel in-register structure is maintained because of favorable interactions between identical amino acid side chains. Hydrophilic side chains can form a line of hydrogen bonds along the filament long axis and hydrophobic residues can favorably interact, but only if they are aligned. Aligned charged residues would repel eachother, but there are very few charged residues in these prion domains. These favorable interactions stabilize the in-register parallel structure, and direct a new monomer joining the ends of the filaments to assume the same conformation as that of the other monomers in the filament. Like the hydrogen bonding of complementary bases in DNA allows templating of sequence, these interactions of identical side chains allow templating of conformation.
Biology of yeast prions.
Prions arise stochastically, and not in response to specific environmental cues (an apparent exception will be discussed below). Thus, it would seem counterproductive to inactivate Ure2p at a random time, even though its normal function is to become inactive when good nitrogen sources are lacking in the medium, the appropriate time for this to happen. Similarly, allowing translational readthrough of all mRNAs by inativation of Sup35p does not seem to be a useful way to regulate gene expression. Nonetheless, there have been suggestions that the [PSI+] prion, and even [URE3], are beneficial to yeast. A report that [PSI+] made cells resistant to heat or high ethanol concentrations [37] was not reproduced in a subsequent study [38], and the advantages of [PSI+] reported in that study were not reproduced (using the same strains) in a further study [39]. A report of specific induction of [PSI+] generation by certain unfavorable conditions [40] was, again, not reproducible [41], but even in the original report, [PSI+] was usually unfavorable for growth under the conditions reported to induce its appearance [40], suggesting that this is not an adaptive response.
Even deadly viruses are found in natural populations because infectious agents can spread and outstrip the damage they do to their hosts. An advantageous infectious agent would surely be nearly universal in its distribution because infectivity and benefit to the host would be working together, instead of opposition. Thus, an infectious agent that is rare in the wild must be detrimental to its host. To examine the issue of whether yeast prions are adaptive or detrimental, we surveyed 70 wild yeast strains, and found that none carried either [URE3] or [PSI+], although each of the known selfish DNA/RNA plasmids and viruses of yeast were found in varying proportions of the wild isolates [42]. This implied that these prions, even their mildest variants, must be detrimental to their hosts. Another study confirmed the rarity of [PSI+] in the wild, but did not examine [URE3] [43]. This study did report frequent phenotypes of wild strains that were affected by growth on guanidine, and inferred that these phenotypes were due to unknown prions [43]. However, this report did not examine whether the phenotype was a result of guanidine’s known mutagenic effect on the mitochondrial DNA [7]. We were able to quantitate the detriment of acquiring a prion by comparing the frequency of the prions to that of 2 micron DNA, a selfish plasmid known to slow cell growth 1–3% [44–46], but nevertheless found in over half of wild isolates [42]. We find that the mildest forms of the [PSI+], [URE3] and [PIN+] prions must have a >1% detrimental effect on growth and/or survival to account for their limited occurence in the wild [41].
In trying to understand the overall impact of prion formation, one must consider the entire range of variants that can be formed. This range is as yet only beginning to be explored. In isolation of any prions, it is axiomatic that a lethal prion would not be recovered unless some special measures were taken to detect it. Sup35p is essential for growth, but the N-terminal prion domain is not essential. Low level expression of the essential C-terminal part of Sup35 lacking the prion domain allowed us to detect the formation of lethal [PSI+] prions [47]. Indeed, lethal and near - lethal variants of [PSI+] comprised more than half of total isolates, showing that [PSI+] generation is not generally a favorable event.
The method by which we isolated lethal [PSI+] variants implied that they were lethal because nearly all of the Sup35p joined the amyloid, leaving an insufficient amount for translation termination. This is not the mechanism of lethality of the TSE diseases, since the PrP protein is completely non-essential. Rather, the prion form of PrP has some toxic effect. Ure2p is likewise non-essential and, depending on the strain background, deletion of URE2 may not even slow cell growth. Using such a strain, we found that many [URE3] isolates were extremely toxic, slowing cell growth dramatically [47]. While the mechanism of [URE3] toxicity remains unclear, these results again show that acquisition of a yeast prion, like mutation of a chromosomal gene, is not generally a beneficial event.
The prion domains of Ure2p and Sup35p are perhaps misnamed as they have normal functions. The Sup35p prion domain is involved in the mRNA turnover process, interacting with polyA binding protein and the polyA degrading enzymes. In the absence of the Sup35p ‘prion domain’, turnover of all mRNAs is much slower [48]. The prion domain of Ure2p is necessary for the stability of Ure2p in vivo, and so plays an important role in the molecule [49]. Thus, these domains are not conserved for the purpose of prion formation. In fact, the Ure2p’s of Kluyveromyces lactis or Candida glabrata that have an N-terminal “prion domain”-like region cannot, in fact, form prions, even in their native hosts [50, 51]. Prion-forming ability appears to be sporadically arising, rather than conserved.
Sequence comparisons of Ure2p and Sup35p show that the prion domains change much more rapidly than does the remainder of either molecule [52–56]. These accumulated changes result in barriers to transmission of the [URE3] and [PSI+] prion between species [52, 55]. Even within the genus Saccharomyces there are barriers to transmission of [URE3] [57] and [PSI+] [58] based on sequence differences in the prion domains. There are even polymorphs in the Sup35p prion domain of wild S. cerevisiae isolates [54, 59, 60] that result in intra-species barriers to transmission of [PSI+] [60]. Indeed, the rare wild isolates of [PSI+] are sensitive to these barriers [60]. Just as in humans, where polymorphism at residue 129 of PrP results in a barrier to prion transmission [61], it is likely that these Sup35p polymorphisms were selected because of the transmission barrier they provide [60]. This suggests that prion acquisition is unfavorable, not advantageous.
The above is not to contend that no prions can be beneficial, nor that no yeast prions can be beneficial. We were the first to hail [62] a beneficial prion, the [Het-s] prion of the filamentous fungus Podospora anserina, which has a role in heterokaryon incompatibility, a normal function of filamentous fungi [63]. [Het-s] is striking in that it is present in 80% of wild strains of the appropriate chromosomal genotype, and forms only a single prion variant, as expected for a functional prion. This contrasts with the properties of those yeast prions investigated to date.
Prion Clouds.
Prions, like viruses and plasmids, are expected to be able to segregate during growth if they are mixed to begin with, and to mutate. Work on mammalian prions has suggested that prions exist as a ‘cloud’, a mixture of variants in one animal or cell [64, 65], and classic experiments with mouse scrapie showed that prions can mutate (without change in the protein sequence) when subjected to selective pressure [66].
We found that [PSI+] transmission to different polymorphs of Sup35p depends strongly on the prion variant examined [60]. A given [PSI+] clone can be a mixture of variants with different patterns of transmission, and simple mitotic growth results in segregation of clones with different transmission patterns [67]. However, extensive growth of a clone with one pattern results in production of clones, some of which have different patterns, indicating that there is variant mutation occurring as well [67]. The details of the experimental procedure were such that the changes were occurring without selection or interference with amyloid propagation. Interestingly, either ‘strong’ [PSI+] or ‘weak’ [PSI+] can have any of the four transmission phenotypes. Thus, yeast prions can exist as a cloud, a mixture of variants each of which is relatively stable, but that can interconvert over time under non-selective conditions. Further definition of the possible prion variant phenotypes and the mechanisms by which these phenotypes are produced will be an important area of future work.
Chaperones and Prions.
The involvement of chaperones, proteins that aid in the correct folding of other proteins, in prion phenomena began with Chernoff’s identification of Hsp104 as a gene whose overproduction could cure [PSI+], although [PSI+] was not then known to be a prion [68]. Either overproduction or deficiency of Hsp104 cures [PSI+] [69]. Hsp104 working together with Hsp70s and Hsp40s (chaperone families) break amyloid filaments producing new seeds, an essential step in prion propagation (reviewed by [70]). Other chaperones, co-chaperones and nucleotide exchange factors have also been found to play key roles in prion propagation (reviewed by [71])..
Btn2p and Cur1p and Prion aggregate collection.
A screen for proteins whose overproduction could cure the [URE3] prion produced two somewhat homologous proteins, Btn2p and Cur1p [72]. Deletion of both genes produced substantial effects on prion generation and increased prion stability, indicating that the normal levels of these proteins also affect prions [72]. In cells in the process of being cured of [URE3] by overproduced Btn2p, Ure2p and Btn2p were often co-localized in a single site, suggesting that sequestration of amyloid filaments might prevent one of the progeny cells from getting any seeds [72]. This was not observed in the case of curing of [URE3] by Cur1p. The involvement of the chaperone Hsp42 in aggregated protein sequestration was documented by Bukau’s group [73], and the interaction of Hsp42 with Btn2p (but not Cur1p) was shown by Malinovska et al. [74]. Several differences between Btn2p action and Cur1p action have been documented in spite of their sequence similarity. Mammalian cells have a centrosome-proximal structure at which aggregated proteins are collected [75]. In studying huntingin aggregation in yeast, an aggresome-like structure was also identified [76]. Further work will be needed to completely define the actions of these systems, and their relation to each other.
Perspectives.
In just under 20 years, the yeast prion field has reached a point where a great deal of information has accumulated and some of the central messages can be understood. However, there remain many very important areas that are largely unclear or controversial. The detailed structure of yeast prion variants has so far resisted all attempts at solution. What is the scope of prion variants, and how do they produce their many effects? The dramatic difference between a mild [PSI+] or [URE3] and a lethal form of the same prion suggests that there may be parallels in mammalian amyloidoses. While Alzheimer’s disease may be fatal, patients deceased from another cause are often found with extensive amyloidosis, but no brain damage. This is often taken as evidence that amyloid is not the toxic species, but it could well be that such patients have a mild amyloid variant, analogous to mild [PSI+] or mild [URE3] and a range of Abeta amyloid structures have been defined [31].
The functions of Btn2p and Cur1p and yeast aggresomes in dealing with aggregated proteins of various sorts remains to be elucidated. The many effects of elevated and depressed chaperones are far from understood. Yeast prions are largely pathogenic, but it remains possible that some functional yeast prions, like [Het-s] of Podospora anserina, will be found. Certainly the non-amyloid prion [BETA], the active form of vacuolar protease B, is beneficial to the cell. With an increasing number of prions identified in S. cerevisae, it seems likely that many prions that are not simply homologs of those found in this yeast will be found in other organisms if appropriate searches are made. It is perhaps most striking that proteins can act as genes - not often - but enough to make it interesting.
Acknowledgement:
This work was supported by the Intramural Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
References:
- 1.Lacroute F (1971) Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. J. Bacteriol. 106, 519–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox BS (1965) PSI, a cytoplasmic suppressor of super-suppressor in yeast. Heredity. 20, 505–521 [Google Scholar]
- 3.Aigle M and Lacroute F (1975) Genetical aspects of [URE3], a non-Mendelian, cytoplasmically inherited mutation in yeast. Molec. Gen. Genet. 136, 327–335 [DOI] [PubMed] [Google Scholar]
- 4.Wickner RB (1994) [URE3] as an altered URE2 protein: evidence for a prion analog in S. cerevisiae. Science. 264, 566–569 [DOI] [PubMed] [Google Scholar]
- 5.Cooper TG (2002) Transmitting the signal of excess nitrogen in Saccharomyces cerevisiae from the Tor proteins to th GATA factors: connecting the dots. FEMS Microbiol. Revs. 26, 223–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magasanik B and Kaiser CA (2002) Nitrogen regulation in Saccharomyces cerevisiae. Gene. 290, 1–18 [DOI] [PubMed] [Google Scholar]
- 7.Villa LL and Juliani MH (1980) Mechanism of rho- induction in Saccharomyces cerevisiae by guanidine hydrochloride. Mutat. Res. 7, 147–153 [DOI] [PubMed] [Google Scholar]
- 8.Goldring ES, Grossman LI, Krupnick D, Cryer DR and Marmur J (1970) The petite mutation in yeast: loss of mitochondrial DNA during induction of petites with ethidium bromide. J. Mol. Biol. 52, 323–335 [DOI] [PubMed] [Google Scholar]
- 9.Lund PM and Cox BS (1981) Reversion analysis of [psi-] mutations in Saccharomyces cerevisiae. Genet. Res. 37, 173–182 [DOI] [PubMed] [Google Scholar]
- 10.Chernoff YO, Derkach IL and Inge-Vechtomov SG (1993) Multicopy SUP35 gene induces de-novo appearance of psi-like factors in the yeast Saccharomyces cerevisiae. Curr. Genet. 24, 268–270 [DOI] [PubMed] [Google Scholar]
- 11.Doel SM, McCready SJ, Nierras CR and Cox BS (1994) The dominant PNM2− mutation which eliminates the [PSI] factor of Saccharomyces cerevisiae is the result of a missense mutation in the SUP35 gene. Genetics. 137, 659–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stansfield I and Tuite MF (1994) Polypeptide chain termination in Saccharomyces cerevisiae. Curr. Genet. 25, 385–395 [DOI] [PubMed] [Google Scholar]
- 13.Frolova L, LeGoff X, Rasmussen HH, Cheperegin S, Drugeon G, Kress M, Arman I, Haenni A-L, Celis JE, Philippe M, Justesen J and Kisselev L (1994) A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature. 372, 701–703 [DOI] [PubMed] [Google Scholar]
- 14.Alper T, Cramp WA, Haig DA and Clarke MC (1967) Does the agent of scrapie replicate without nucleic acid? Nature. 214, 764–766 [DOI] [PubMed] [Google Scholar]
- 15.Griffith JS (1967) Self-replication and scrapie. Nature. 215, 1043–1044 [DOI] [PubMed] [Google Scholar]
- 16.Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science. 216, 136–144 [DOI] [PubMed] [Google Scholar]
- 17.Paushkin SV, Kushnirov VV, Smirnov VN and Ter-Avanesyan MD (1996) Propagation of the yeast prion-like [psi+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 15, 3127–3134 [PMC free article] [PubMed] [Google Scholar]
- 18.Patino MM, Liu J-J, Glover JR and Lindquist S (1996) Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science. 273, 622–626 [DOI] [PubMed] [Google Scholar]
- 19.Edskes HK, Gray VT and Wickner RB (1999) The [URE3] prion is an aggregated form of Ure2p that can be cured by overexpression of Ure2p fragments. Proc. Natl. Acad. Sci. USA. 96, 1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King C-Y, Tittmann P, Gross H, Gebert R, Aebi M and Wuthrich K (1997) Prion-inducing domain 2–114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc. Natl. Acad. Sci. USA. 94, 6618–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glover JR, Kowal AS, Shirmer EC, Patino MM, Liu J-J and Lindquist S (1997) Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell. 89, 811–819 [DOI] [PubMed] [Google Scholar]
- 22.King CY and Diaz-Avalos R (2004) Protein-only transmission of three yeast prion strains. Nature. 428, 319–323 [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, Chien P, Naber N, Cooke R and Weissman JS (2004) Conformational variations in an infectious protein determine prion strain differences. Nature. 428, 323–328 [DOI] [PubMed] [Google Scholar]
- 24.Taylor KL, Cheng N, Williams RW, Steven AC and Wickner RB (1999) Prion domain initiation of amyloid formation in vitro from native Ure2p. Science. 283, 1339–1343 [DOI] [PubMed] [Google Scholar]
- 25.Brachmann A, Baxa U and Wickner RB (2005) Prion generation in vitro: amyloid of Ure2p is infectious. Embo J. 24, 3082–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG and Liebman SW (1996) Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics. 144, 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlumpberger M, Prusiner SB and Herskowitz I (2001) Induction of distinct [URE3] yeast prion strains. Mol Cell Biol. 21, 7035–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.TerAvanesyan A, Dagkesamanskaya AR, Kushnirov VV and Smirnov VN (1994) The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi+] in the yeast Saccharomyces cerevisiae. Genetics. 137, 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masison DC and Wickner RB (1995) Prion-inducing domain of yeast Ure2p and protease resistance of Ure2p in prion-containing cells. Science. 270, 93–95 [DOI] [PubMed] [Google Scholar]
- 30.Bai M, Zhou JM and Perrett S (2004) The yeast prion protein Ure2 shows glutathione peroxidase activity in both native and fibrillar forms. J Biol Chem. 279, 50025–50030 [DOI] [PubMed] [Google Scholar]
- 31.Tycko R (2011) Solid-state NMR studies of amyloid fibril structure. Annu. Rev. Phys. Chem. 62, 279–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shewmaker F, Wickner RB and Tycko R (2006) Amyloid of the prion domain of Sup35p has an in-register parallel β-sheet structure. Proc. Natl. Acad. Sci. USA. 103, 19754–19759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baxa U, Wickner RB, Steven AC, Anderson D, Marekov L, Yau W-M and Tycko R (2007) Characterization of β-sheet structure in Ure2p1–89 yeast prion fibrils by solid state nuclear magnetic resonance. Biochemistry. 46, 13149–13162 [DOI] [PubMed] [Google Scholar]
- 34.Wickner RB, Dyda F and Tycko R (2008) Amyloid of Rnq1p, the basis of the [PIN+] prion, has a parallel in-register β-sheet structure. Proc Natl Acad Sci U S A. 105, 2403–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritter C, Maddelein ML, Siemer AB, Luhrs T, Ernst M, Meier BH, Saupe SJ and Riek R (2005) Correlation of structural elements and infectivity of the HET-s prion. Nature. 435, 844–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siemer AB, Arnold AA, Ritter C, Westfeld T, Ernst M, Riek R and Meier BH (2006) Observation of highly flexible residues in amyloid fibrils of the HET-s prion. J. Am. Chem. Soc. 128, 13224–13228 [DOI] [PubMed] [Google Scholar]
- 37.Eaglestone SS, Cox BS and Tuite MF (1999) Translation termination efficiency can be regulated in Saccharomyces cerevisiae by environmental stress through a prion-mediated mechanism. EMBO J. 18, 1974–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.True HL and Lindquist SL (2000) A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature. 407, 477–483 [DOI] [PubMed] [Google Scholar]
- 39.Namy O, Galopier A, Martini C, Matsufuji S, Fabret C and Rousset C (2008) Epigenetic control of polyamines by the prion [PSI+]. Nat. Cell. Biol. 10, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 40.Tyedmers J, Madariaga ML and Lindquist S (2008) Prion switching in response to environmental stress. PLoS Biol. 6, e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly AC, Shewmaker FP, Kryndushkin D and Wickner RB (2012) Sex, prions and plasmids in yeast. Proc. Natl. Acad. Sci. USA. 109, E2683–E2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakayashiki T, Kurtzman CP, Edskes HK and Wickner RB (2005) Yeast prions [URE3] and [PSI+] are diseases. Proc Natl Acad Sci U S A. 102, 10575–10580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halfmann R, Jarosz DF, Jones SK, Chang A, Lancster AK and Lindquist S (2012) Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature. 482, 363–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Futcher AB and Cox BS (1983) Maintenance of the 2 μm circle plasmid in populations of Saccharomyces cerevisiae. J Bacteriol. 154, 612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mead DJ, Gardner DCJ and Oliver SG (1986) The yeast 2 μ plasmid: strategies for the survival of a selfish DNA. Mol. Gen. Genet. 205, 417–421 [DOI] [PubMed] [Google Scholar]
- 46.Futcher B, Reid E and Hickey DA (1988) Maintenance of the 2 micron circle plasmid of Saccharomyces cerevisiae by sexual transmission: an example of selfish DNA. Genetics. 118, 411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGlinchey R, Kryndushkin D and Wickner RB (2011) Suicidal [PSI+] is a lethal yeast prion. Proc. Natl. Acad. Sci. USA. 108, 5337–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoshino S, Imai M, Kobayashi T, Uchida N and Katada T (1999) The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3’-poly(A) tail of mRNA. J. Biol. Chem. 274, 16677–16680 [DOI] [PubMed] [Google Scholar]
- 49.Shewmaker F, Mull L, Nakayashiki T, Masison DC and Wickner RB (2007) Ure2p function is enhanced by its prion domain in Saccharomyces cerevisiae. Genetics. 176, 1557–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Safadi RA, Talarek N, Jacques N and Aigle M (2011) Yeast prions: could they be exaptations? The URE2/[URE3] system in Kluyveromyces lactis. FEMS Yeast Res. 11, 151–153 [DOI] [PubMed] [Google Scholar]
- 51.Edskes HK, Engel A, McCann LM, Brachmann A, Tsai H-F and Wickner RB (2011) Prion-forming ability of Ure2 of yeasts is not evolutionarily conserved. Genetics. 188, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santoso A, Chien P, Osherovich LZ and Weissman JS (2000) Molecular basis of a yeast prion species barrier. Cell. 100, 277–288 [DOI] [PubMed] [Google Scholar]
- 53.Chernoff YO, Galkin AP, Lewitin E, Chernova TA, Newnam GP and Belenkiy SM (2000) Evolutionary conservation of prion-forming abilities of the yeast Sup35 protein. Molec. Microbiol. 35, 865–876 [DOI] [PubMed] [Google Scholar]
- 54.Resende CG, Outeiro TF, Sands L, Lindquist S and Tuite MF (2003) Prion protein gene polymorphisms in Saccharomyces cerevisiae. Mol. Microbiol. 49, 1005–1017 [DOI] [PubMed] [Google Scholar]
- 55.Edskes HK and Wickner RB (2002) Conservation of a portion of the S. cerevisiae Ure2p prion domain that interacts with the full - length protein. Proc. Natl. Acad. Sci. USA. 99 (Suppl. 4), 16384–16391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baudin-Baillieu A, Fernandez-Bellot E, Reine F, Coissac E and Cullin C (2003) Conservation of the prion properties of Ure2p through evolution. Mol. Biol. Cell. 14, 3449–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edskes HK, McCann LM, Hebert AM and Wickner RB (2009) Prion variants and species barriers among Saccharomyces Ure2 proteins. Genetics. 181, 1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen B, Newnam GP and Chernoff YO (2007) Prion species barrier between the closely related yeast proteins is detected despite coaggregation. Proc Natl Acad Sci U S A. 104, 2791–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensen MA, True HL, Chernoff YO and Lindquist S (2001) Molecular population genetics and evolution of a prion-like protein in Saccharomyces cerevisiae. Genetics. 159, 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bateman DA and Wickner RB (2012) [PSI+] prion transmission barriers protect Saccharomyces cerevisiae from infection: intraspecies ‘species barriers’. Genetics. 190, 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mead S, Stumpf MP, Whitfield J, Beck JA, Poulter M, Campbell T, Uphill JB, Goldstein D, Alpers M, Fisher EM and Collinge J (2003) Balancing selection at the prion protein gene consistent with prehistoric kurulike epidemics. Science. 300, 640–643 [DOI] [PubMed] [Google Scholar]
- 62.Wickner RB (1997) A new prion controls fungal cell fusion incompatibility. Proc. Natl. Acad. Sci. USA. 94, 10012–10014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coustou V, Deleu C, Saupe S and Begueret J (1997) The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc. Natl. Acad. Sci. USA. 94, 9773–9778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collinge J and Clarke AR (2007) A general model of prion strains and their pathogenicity. Science. 318, 930–936 [DOI] [PubMed] [Google Scholar]
- 65.Li J, Mahal SP, Demczyk CA and Weissmann C (2011) Mutability of prions. EMBO Rep. 12, 1243–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimberlin RH, Cole S and Walker CA (1987) Temporary and permanent modifications to a single strain of mouse scrapie on transmission to rats and hamsters. J. Gen. Virol. 68, 1875–1881 [DOI] [PubMed] [Google Scholar]
- 67.Bateman D and Wickner RB (2013) The [PSI+] prion exists as a dynamic cloud of variants. Plos Genet. 9, e1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chernoff YO, Inge-Vechtomov SG, Derkach IL, Ptyushkina MV, Tarunina OV, Dagkesamanskaya AR and Ter-Avanesyan MD (1992) Dosage-dependent translational suppression in yeast Saccharomyces cerevisiae. Yeast. 8, 489–499 [DOI] [PubMed] [Google Scholar]
- 69.Chernoff YO, Lindquist SL, Ono B-I, Inge-Vechtomov SG and Liebman SW (1995) Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science. 268, 880–884 [DOI] [PubMed] [Google Scholar]
- 70.Reidy M and Masison DC (2011) Modulation and elimination of yeast prions by protein chaperones and co-chaperones. Prion. 5, 245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liebman SW and Chernoff YO (2012) Prions in yeast. Genetics. 191, 1041–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kryndushkin D, Shewmaker F and Wickner RB (2008) Curing of the [URE3] prion by Btn2p, a Batten disease-related protein. EMBO J. 27, 2725–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Specht S, Miller SBM, Mogk A and Bukau B (2011) Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J. Cell. Biol. 195, 617–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malinovska L, Kroschwald S, Munder MC, Richter D and Alberti S (2012) Molecular chaperones and stress-inducible protein-sorting factors coordinate the spaciotemporal distribution of protein aggregates. Mol. Biol. Cell. 23, 3041–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kopito R (2000) Aggresomes, inclusion bodies and protein aggregation. Trends in Cell Biol. 10, 524–530 [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Meriin AB, Zaarur N, Romanova NV, Chernoff YO, Costello CE and Sherman MY (2009) Abnormal proteins can form aggresome in yeast: aggresome-targeting signals and components of the machinery. FASEB J. 23, 451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wickner RB, Edskes HK, Bateman DA, Kelly AC, Gorkovskiy A, Dayani Y and Zhou A (2013) Amyloids and yeast prion biology. Biochemistry. 52, 1514–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R and Eisenberg D (2005) Structure of the cross-β spine of amyloid-like fibrils. Nature. 435, 773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Derkatch IL, Bradley ME, Hong JY and Liebman SW (2001) Prions affect the appearance of other prions: the story of [PIN]. Cell. 106, 171–182 [DOI] [PubMed] [Google Scholar]
- 80.Du Z, Park K-W, Yu H, Fan Q and Li L (2008) Newly identified prion linked to the chromatin-remodeling factor Swi1 in Saccharomyces cerevisiae. Nat. Genet. 40, 460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel BK, Gavin-Smyth J and Liebman SW (2009) The yeast global transcriptional co-repressor protein Cyc8 can propagate as a prion. Nat. Cell Biol. 11, 344–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alberti S, Halfmann R, King O, Kapila A and Lindquist S (2009) A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 137, 146–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rogoza T, Goginashvili A, Rodionova S, Ivanov M, Viktorovskaya O, Rubel A, Volkov K and Mironova L (2010) Non-mendelian determinant [ISP+] in yeast is a nuclear-residing prion form of the global transcriptional regulator Sfp1. Proc. Natl. Acad. Sci. USA. 107, 10573–10577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki G, Shimazu N and Tanaka M (2012) A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science. 336, 355–359 [DOI] [PubMed] [Google Scholar]
- 85.Roberts BT and Wickner RB (2003) A class of prions that propagate via covalent auto-activation. Genes Dev. 17, 2083–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]


