Abstract
Many people with eating disorders (EDs) report symptoms of insomnia (i.e., frequent difficulty falling asleep, staying asleep, and/or early morning wakening) and sleep problems have been linked to alterations in eating behaviors; however, mechanisms of these bidirectional associations remain poorly understood and under researched. This is a problem because higher insomnia symptom severity is a risk factor for the onset and perpetuation of anxiety, mood, trauma, substance use disorders and, potentially, ED symptoms. Furthermore, insomnia symptoms may hinder recovery and increase relapse rates following successful psychotherapy. In this paper, we describe potential mechanisms underlying bidirectional associations between insomnia and eating psychopathology that may contribute to the etiology and maintenance of both disorders. We suggest novel directions for future research to characterize the association between dysregulated sleep and ED symptoms and to evaluate impacts of insomnia symptoms on relapse and recovery for people with co-occurring pathology. Finally, we discuss options for testing the incorporation of existing evidence-based treatments for insomnia disorder (e.g., Cognitive-Behavioral Therapy for Insomnia) with ED care. Overall, insomnia symptoms present a promising intervention point for ED treatment that has not been systematically tested, yet would be highly feasible to address in routine clinical care.
Keywords: Eating disorders, insomnia disorder, sleep
Emerging research suggests that insomnia symptoms are common in people with eating disorders (EDs) (e.g., Allison et al., 2016; Goel et al., 2020; KyungRan et al., 2010; Lombardo et al., 2014; Padez-Vieira & Afonso, 2016). To date, the majority of research on insomnia symptoms and EDs has focused on the prevalence and nature of sleep disturbance (e.g., delayed sleep onset, alterations in sleep architecture), with comparatively less focus on mechanisms (see reviews in Allison et al., 2016; Cooper et al., 2020; Lauer & Krieg, 2004). Although mechanisms linking sleep and eating behaviors have been studied in non-clinical and animal samples, the literature in people with EDs has been limited by smaller sample sizes and there is great opportunity for growth in this field of study. Thus, this review proposes a translational approach and offers applications for how it may apply to ED presentations, highlighting areas where more information is needed. To do so, we briefly explain the neurobiology of insomnia, highlight potential mechanisms linking insomnia and ED symptoms, and describe hypothesized pathways for insomnia to influence treatment outcomes. Finally, we describe several directions to guide future research on insomnia and eating pathology.
Understanding Mechanisms of Sleep in Insomnia
The psychobiologic processes controlling good sleep include two mechanisms: circadian processes (Process C) and homeostatic processes (Process S) (Perlis et al., 2011). In normal sleep, circadian processes contribute to varying levels of wakefulness and sleepiness throughout the day. Specifically, most individuals are alert in the morning after awakening until the “post-lunch dip” in the afternoon, after which alertness increases until it peaks around 7–9 PM in the evening. Following this point, alertness decreases and individuals become primed for sleep in the late evening. In terms of Process S, the homeostatic process (“sleep drive”) is directly related to time awake in a linear fashion, such that the longer an individual is awake, the stronger the sleep drive (i.e., the individual becomes more sleepy). When these processes are functioning in conjunction, they facilitate appropriate sleep onset and maintenance.
For individuals with insomnia disorder, these processes become interrupted, leading to difficulties with sleep onset and maintenance that occur three or more days per week for at least three months (American Psychiatric Association, 2013). Of note, although insomnia symptoms are among the diagnostic criteria for other psychological disorders, insomnia disorder is a distinct diagnosis (Harvey, 2001). People with insomnia disorder often engage in coping behaviors that dysregulate the circadian and homeostatic processes and maintain dysregulated sleep (Perlis et al., 2011). For example, they may “sleep in” to make up for poor sleep, which has the paradoxical effect of subsequently causing increased difficulty falling or staying asleep due to inability to build sleep drive, and disrupts circadian rhythms. Other common maladaptive behaviors include going to bed before one is actually sleepy to ensure “enough time” to fall asleep and napping to compensate for a night of poor sleep.
For those who do not meet full criteria for insomnia disorder, insomnia symptoms can often still cause clinically significant distress and impairment, including poorer quality of life and increased mental health symptoms (LeBlanc et al., 2007), suggesting the importance of examining the range of possible insomnia symptoms. Even one night of poor sleep (Short, Allan, et al., 2017) and/or acute insomnia can cause difficulties with cognitive-affective functioning, impact physiology, and cause significant distress and problems for daytime functioning (Ellis et al., 2012). However, chronic insomnia appears to be even more risky for the development of longer-term problems, such as suicide risk and mental health symptoms (Nadorff et al., 2013; Ohayon & Roth, 2003), potentially including EDs. This is likely because the impact of insomnia is cumulative over time, as maladaptive sleep-related behaviors become more ingrained and the impact of poor sleep on one’s physiology becomes chronic (e.g., et al., 2013).
It is likely that there is a bidirectional association between sleep and eating processes, such that eating pathology disrupts sleep and dysregulation in sleep influences eating behaviors (Linnaranta et al., 2020) and that this association is particularly problematic for people with EDs who may experience exacerbation and maintenance of ED pathology via dysregulated sleep. In the following paragraphs, we will discuss promising preliminary mechanisms underlying this bidirectional association (Figure 1). We propose a model in which acute symptoms of insomnia exacerbate problematic eating behaviors and ED behaviors cause alterations in sleep. Over time, this positive feedback loop results in cognitive, physiological, and behavioral changes that may further entrench individuals in EDs and result in insomnia disorder. This review should be taken as a starting point for inquiry and is not intended to encompass all theories of how insomnia and EDs may be related.
Figure 1: Model of Mechanisms Underlying Bidirectional Association between Insomnia and Eating Disorder Symptoms.
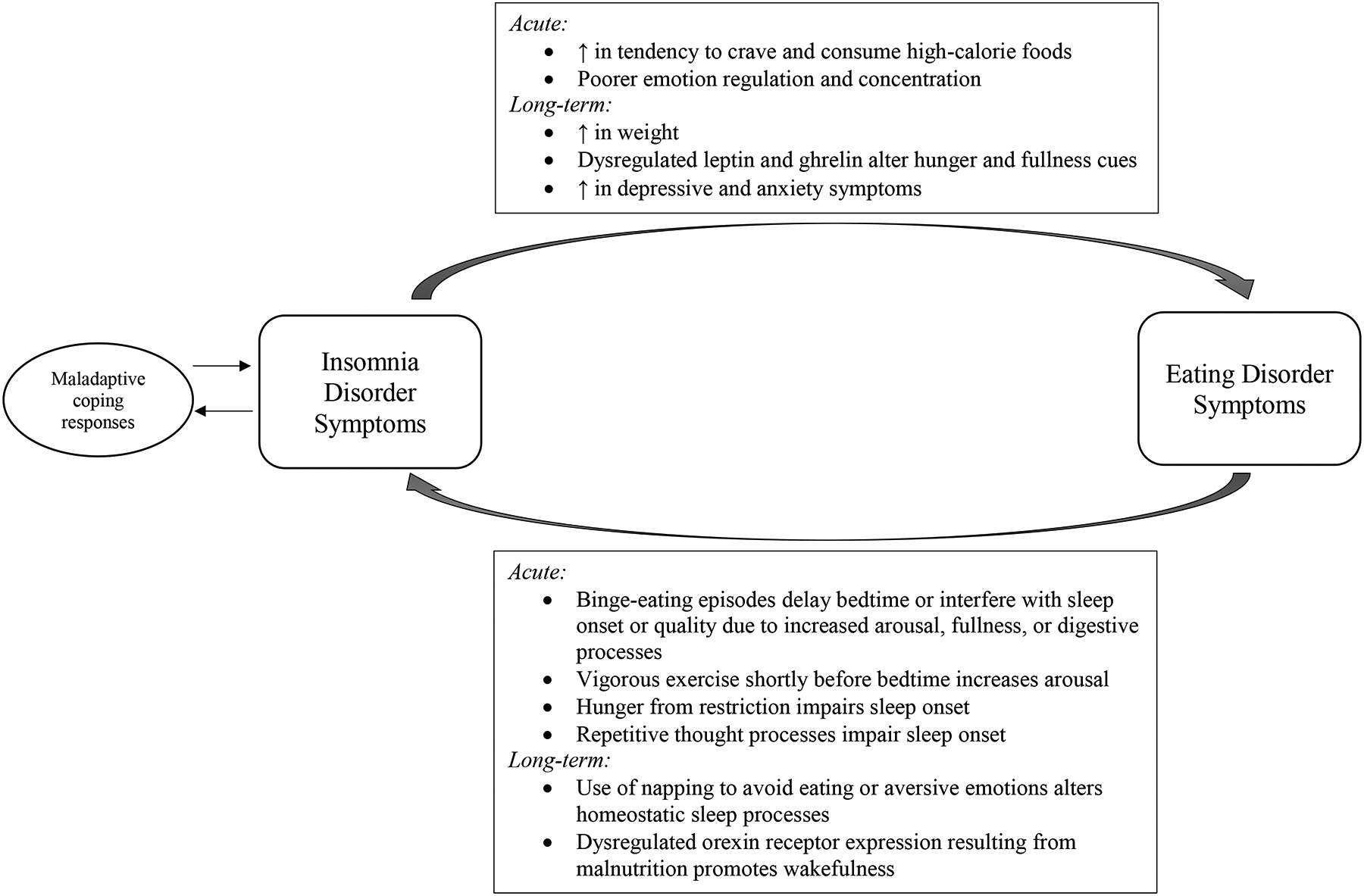
Acute symptoms of insomnia may exacerbate problematic eating behaviors and eating disorder behaviors may cause alterations in day-to-day sleep patterns. Over time, this positive feedback loop results in cognitive, physiological and behavioral changes that may further entrench individuals in eating disorders and result in insomnia disorder.
Potential Mechanisms Underlying Bidirectional Associations between ED Pathology and Insomnia Symptoms
ED Behaviors and Cognitions Disrupt Sleep Processes
Common ED behaviors may interfere with sleep processes; however, more research is needed to establish these claims as the literature has primarily examined eating behaviors in people without EDs, rather than clinical samples, and acute effects of poor sleep on behavior. For example, one study in a non-clinical sample found that vigorous exercise shortly before bedtime increases sleep-onset latency and decreases sleep efficiency (Stutz et al., 2019), which may be of particular relevance to individuals who exercise due to shape or weight concerns. Sleep may also be a means to avoid eating, aversive emotions, or distressing situations. For instance, people may nap or sleep in to avoid needing to eat meals or confronting stressors. This is problematic, because daytime napping decreases sleep load and results in less drive to fall asleep at appropriate bedtimes. Finally, binge-eating episodes, which are common in evenings (Schreiber-Gregory et al., 2013), may disrupt sleep cycles by delaying bedtime or interfering with sleep onset or quality due to increased arousal, fullness, or digestive processes. Similarly, restricting one’s eating may lead to difficulties with sleep onset due to hunger, or may facilitate sleep onset (including at unwanted times during the day) due to fatigue and malnutrition.
People with night-eating syndrome (NES), an ED characterized by recurrent eating after waking in the night or excessive eating after the nighttime meal (American Psychiatric Association, 2013), are at a higher risk for insomnia disorder (Vander Wal, 2012). Although not adapted as diagnostic criteria for DSM 5, insomnia occurring four days per week was proposed as a component of the diagnosis (Allison et al., 2010). NES has been proposed to function as a peripheral oscillator, causing delayed sleep phase (Kandeger et al., 2018). It is possible that over time, NES could entrench sleep habits that maintain and perpetuate insomnia, even in the absence of NES symptoms.
In terms of cognitive features of EDs, Harvey’s cognitive model of insomnia (Harvey, 2002) proposes that daytime negative affect is a core maintenance factor for insomnia. Specifically, individuals with insomnia have excessive negative affect and negatively toned cognitions during the day that contribute to increased arousal and trouble sleeping at night. Such cognitive and emotional patterns may be particularly problematic during the pre-sleep period, when individuals with insomnia often engage in negative repetitive thought. Indeed, those with insomnia often attribute their sleep difficulties with the inability to suppress pre-sleep cognitive activity (Harvey, 2003). Individuals with EDs frequently report high levels of worry and rumination (Prefit et al., 2019; Startup et al., 2013) and elevated negative affect is a risk factor for EDs (Stice et al., 2017). Thus, it is possible that global and/or ED-specific repetitive negative thought in the pre-sleep period contributes to the development and maintenance of insomnia in those with EDs. It is also possible that affective disorders commonly comorbid with EDs such as depression and anxiety could mediate associations between ED symptoms and insomnia. Indeed, cross-sectional studies have found that anxiety and depression symptoms mediated the association between insomnia and binge eating (Kenny et al., 2018) and between insomnia and ED psychopathology (Goel et al., 2020).
Finally, prolonged starvation likely impacts sleep processes through the dysregulation of orexin receptors. Orexins are neuropeptides that are hypothesized to increase during starvation to promote wakefulness and food-searching behavior (e.g., Willie et al., 2001). Indeed, increased orexin-A has been linked to poorer sleep in people with and without anorexia nervosa (Sauchelli et al., 2016). One explanation is that in individuals without malnutrition, glucose and leptin appropriately suppress orexins; however, in the case of chronic starvation, when glucose and leptin levels are altered, orexin neuronal firing becomes dysregulated, resulting in increased wakefulness.
Dysregulated Sleep Processes Contribute to ED Behaviors
Sleep dysregulation can also alter eating behaviors, thereby exacerbating ED symptoms. For instance, acute sleep deprivation is associated with an increased tendency to crave (Greer et al., 2013) and consume (De Leon & Hanlon, 2020) high-calorie foods in non-clinical samples. Furthermore, chronic insomnia has been associated with dysregulated levels of leptin and ghrelin (e.g., Motivala et al., 2009; Spiegel et al., 2004; Taheri et al., 2004), which are neuropeptides that regulate hunger and satiety and influence food consumption. Shorter sleep duration has been associated with increased weight gain in non-clinical samples (Lyytikäinen et al., 2011; Patel & Hu, 2008), although this finding has yet to be replicated with a sample with EDs. However, it is possible that people who experience such weight gain will then attempt to use unhealthy weight control behaviors, entering into a cycle of ED psychopathology. Further, sleep-related maladaptive behaviors could also contribute to ED symptoms. For example, individuals with insomnia may be awake when most others would be asleep, and get out of bed to eat as a way to cope with their insomnia. Overall, mechanisms linking insomnia to specific ED behaviors remain primarily hypothetical and understudied at this time and more research in this area is needed. Effects of Insomnia on ED Treatment Response and Recovery
Treatment Response
Understanding insomnia symptoms in EDs is important because insomnia-related behaviors may influence treatment outcomes. First, treatments such as Enhanced Cognitive Behavior Therapy (CBT-E) require clients to maintain a schedule of regular eating (Fairburn, 2008); however, disruptions in sleep may hamper the ability to follow this schedule. For example, sleeping in or daytime napping may cause clients to miss necessary feeding times. Furthermore, insomnia is associated with both objective and subjective impairments in cognitive performance, particularly related to negative emotional tasks (Goldstein & Walker, 2014; Wardle-Pinkston et al., 2019). Thus, clients with insomnia symptoms may experience impairment that interferes with their ability to engage with treatment, particularly tasks that are more effortful or distressing. In the case of ED treatment, this could include exposures, regular eating, and ED behaviors abstention. Indeed, individuals with insomnia have poorer distress tolerance and increased negative affect during frustrating or distressing tasks (Short et al., 2016). Finally, insomnia symptoms are risk factors for common co-occurring diagnoses with EDs (Hertenstein et al., 2019), such as mood, anxiety, and substance-use disorders, which could complicate treatment outcomes. Research on the notion that insomnia may be a risk factor for EDs is sparse. However, one study found that disturbed sleep predicted higher ED severity among ED inpatients after six months of treatment. This effect was mediated through an indirect effect of increased depressive symptoms (Lombardo et al., 2015).
Relapse and Recovery
Insomnia symptoms may increase relapse rates for psychological disorders after successful treatment (e.g., Babson et al., 2013; Chen et al., 2017; Manber et al., 2008; Ohayon & Roth, 2003; Short, Mathes, et al., 2017). As of yet, it is unknown if residual insomnia symptoms pose the same risks to sustained ED recovery following treatment, which is a critical question given the elevated rates of relapse in EDs. As insomnia is a separate disorder with its own maintenance factors, although treating comorbid disorders may reduce insomnia symptoms, residual insomnia is the norm after treatment for depression, anxiety, and posttraumatic stress (Belleville et al., 2010). This is likely because although CBT may reduce cognitive processes associated with poorer sleep (e.g., rumination, worry, increased negative affect), it does not typically address maintaining behaviors (e.g., extended time in bed, poor sleep hygiene). This is problematic because research in anxiety and mood disorders has found that insomnia symptoms are a risk factor for relapse after treatment of these conditions (e.g., Chen et al., 2017; Manber et al., 2008; Ohayon & Roth, 2003). Furthermore, insomnia symptoms are associated with a return to maladaptive behaviors, such as cannabis use (Babson et al., 2013) and tobacco use (Short, Mathes, et al., 2017) following quit attempts. Consequently, it is important to establish if insomnia symptoms similarly effect abstinence from ED behaviors following treatment. Innovative Directions for Research
An initial question for ED and sleep research is to characterize how sleep and ED behaviors interact with each other. This could be accomplished by combining wearable devices that track sleep/wake and physical activity with ecological momentary assessment of ED behaviors and subjective perceptions of sleep. Ideally, this would include mechanistic assessments of variables such as cognitive functioning, affect, and emotion dysregulation. This method could test questions such as if poor sleep is a risk factor for ED behaviors the next day or if binge eating at night results in dysregulated sleep. Furthermore, it could be used to evaluate if facets of sleep disturbance (e.g., insomnia, circadian dysfunction, inadequate sleep) are associated with specific ED symptoms. Several recent studies highlight such approaches to evaluating these questions. For example, one study utilized ecological momentary assessment along with actigraphy among youth with overweight/obesity, and found that longer sleep duration one night is associated with reduced consumption of solid fats, alcohol, and added sugar (Goldschmidt et al., 2020). Another study found that good sleep quality attenuated the impact of stress on unhealthy food consumption among Chinese workers (Liu et al., 2017), suggesting a more complex model of the impact of sleep on eating behaviors. Results from these types of analyses would allow researchers to identify potential points of intervention and could suggest timing of treatment interventions for sleep and eating behaviors.
At present, research examining actigraphy and sleep in EDs has primarily focused on characterizing the nature of sleep-wake cycles in small samples of people with BED (e.g., Linnaranta et al., 2020; Roveda et al., 2018; Tzischinsky et al., 2000; Tzischinsky & Latzer, 2006), with additional studies in AN (Latzer et al., 2001) and BN (Latzer et al., 1999). Although these methods have allowed for increased understanding of sleep-wake patterns, there is an urgent need to incorporate an assessment of ED behaviors to evaluate mechanisms of the association between sleep and eating behavior in EDs.
Experimental manipulations of sleep or ED behaviors within laboratory settings would also be valuable. For example, sleep deprivation, restriction, or extension paradigms could be used to examine whether sleep impacts engagement in ED behaviors, and whether this effect may be mediated by dysregulation of hormones related to appetite or satiety or other mechanisms (Irwin et al., 2016). Studies using partial sleep deprivation paradigms have found that partial sleep deprivation may decrease the consumption of nutritious foods (e.g., containing fiber) and increase daily snacks among young adults with and without BED symptoms (Cerolini et al., 2018). Furthermore, partial sleep deprivation increased food intake among healthy men (Hogenkamp et al., 2013) and good sleepers (Lombardo et al., 2020) highlighting the importance of assessing potential moderators of the relationship between sleep and eating behaviors. Another promising direction would be to examine the impact of experimental sleep deprivation on neural circuits related to ED behaviors. For example, Greer and colleagues (2013) found that sleep deprivation is associated with decreased activity in higher-order cortical brain regions and excess subcortical limbic reactivity, potentially resulting in the selection of foods high in weight gain potential. Alternatively, manipulations of ED behaviors (e.g., test meals) could be conducted prior to lab-based sleep studies to determine how such behaviors impact sleep and its architecture.
It is unknown how sleep problems influence treatment trajectories. First, it would be useful to establish if insomnia symptoms are associated with poorer treatment response (e.g., Lombardo et al., 2015). Second, there is limited knowledge about how ED treatments affect sleep behaviors. Although CBT tends to reduce insomnia severity over the course of treatment, a significant proportion of people do not experience full recovery from insomnia symptoms (e.g., Cousineau et al., 2016; Mason & Harvey, 2014). At present, no evidence-based treatment for EDs incorporates core principles of behavioral interventions for insomnia, such as stimulus control and sleep restriction and titration. Evaluating the impact of existing ED treatments on insomnia will determine the necessity of incorporating insomnia-specific interventions into clinical practice. Finally, it will be important to establish if residual insomnia symptoms are risk factors for relapse for people treated for EDs. To answer these questions, researchers could longitudinally track insomnia symptoms during and following treatment discharge using measures such as the Insomnia Severity Index (Morin et al., 2011), which is a brief seven-item measure with an empirically established cutoff for probable insomnia disorder.
If insomnia symptoms are a risk factor for poorer treatment response or increased relapse rates, then research into the treatment of insomnia in people with EDs holds great promise. Treatment staging research could evaluate whether it is beneficial to treat insomnia symptoms prior to, concurrently with, or following ED treatment. Fortunately, there are evidence-based treatments for insomnia that are well validated, relatively brief, and easy to administer even by non-professionals. For example, CBT for Insomnia (CBT-I) demonstrates strong evidence for reducing insomnia symptoms and maintaining treatment gains (van der Zweerde et al., 2019; van Straten et al., 2018). Additionally, it is a highly flexible treatment option and can be delivered using individual, guided self-help, or group formats; through telehealth, online, or in-person; and in periods as short as four weeks. Consequently, it is a highly promising treatment for integration with existing ED care. Although there have yet to be any published trials of evidence-based treatment of insomnia disorder in people with eating pathology, a recently published protocol using a single-case experimental design to treat residual insomnia symptoms following treatment for an ED is a promising first step towards addressing questions about the efficacy of treating insomnia in this population (Christensen et al., n.d.). Finally, more research on the associations between insomnia and EDs could reveal novel treatment targets to improve treatment outcomes for both disorders.
Conclusion
Overall, although researchers have evaluated insomnia as a transdiagnostic risk and maintenance factor for psychopathology, it remains understudied in eating pathology. Innovative research is necessary to characterize the bidirectional association between sleep and eating behaviors and to identify the risks posed by dysregulated sleep on ED treatment outcomes and relapse rates. With this knowledge, ED treatment developers can learn how to best incorporate evidence-based interventions for insomnia and improve recovery from EDs.
Acknowledgements:
KAC is supported by a TL1 postdoctoral fellowship awarded by Frontiers: University of Kansas Clinical and Translational Science Institute (# TL1TR002368) through a CTSA grant from NCATS. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the University of Kansas, NIH, or NCATS.
Footnotes
Conflict of Interest statement: The authors report no conflicts of interest.
Data Availability Statement:
No data were collected for this manuscript.
References
- Allison KC, Lundgren JD, O’Reardon JP, Geliebter A, Gluck ME, Vinai P, Mitchell JE, Schenck CH, Howell MJ, Crow SJ, Engel S, Latzer Y, Tzischinsky O, Mahowald MW, & Stunkard AJ (2010). Proposed diagnostic criteria for night eating syndrome. The International Journal of Eating Disorders, 43(3), 241–247. 10.1002/eat.20693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison KC, Spaeth A, & Hopkins CM (2016). Sleep and Eating Disorders. Current Psychiatry Reports, 18(10), 92. 10.1007/s11920-016-0728-8 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing. [Google Scholar]
- Babson KA, Boden MT, Harris AH, Stickle TR, & Bonn-Miller MO (2013). Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. Journal of Substance Abuse Treatment, 44(4), 438–443. [DOI] [PubMed] [Google Scholar]
- Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme M-E, & Marchand A (2010). The impact of cognitive-behavior therapy for anxiety disorders on concomitant sleep disturbances: A meta-analysis. In Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. Centre for Reviews and Dissemination; (UK: ). [DOI] [PubMed] [Google Scholar]
- Cerolini S, Rodgers RF, & Lombardo C (2018). Partial sleep deprivation and food intake in participants reporting binge eating symptoms and emotional eating: Preliminary results of a quasi-experimental study. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 23(5), 561–570. [DOI] [PubMed] [Google Scholar]
- Chen P-J, Huang CL-C, Weng S-F, Wu M-P, Ho C-H, Wang J-J, Tsai W-C, & Hsu Y-W (2017). Relapse insomnia increases greater risk of anxiety and depression: Evidence from a population-based 4-year cohort study. Sleep Medicine, 38, 122–129. 10.1016/j.sleep.2017.07.016 [DOI] [PubMed] [Google Scholar]
- Christensen KA, Forbush KT, Elliott BT, & Jarmolowicz DP (n.d.). A single-case multiple baseline design for treating insomnia in eating disorders: The TIRED study. International Journal of Eating Disorders, n/a(n/a). 10.1002/eat.23450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AR, Loeb KL, & McGlinchey EL (2020). Sleep and eating disorders: Current research and future directions. Current Opinion in Psychology, 34, 89–94. 10.1016/j.copsyc.2019.11.005 [DOI] [PubMed] [Google Scholar]
- Cousineau H, Marchand A, Bouchard S, Bélanger C, Gosselin P, Langlois F, Labrecque J, Dugas MJ, & Belleville G (2016). Insomnia Symptoms Following Treatment for Comorbid Panic Disorder With Agoraphobia and Generalized Anxiety Disorder. The Journal of Nervous and Mental Disease, 204(4), 267–273. 10.1097/NMD.0000000000000466 [DOI] [PubMed] [Google Scholar]
- De Leon AA, & Hanlon EC (2020). Chapter 23—Impact of Sleep Restriction on Food Intake and Food Choice. In Watson RR & Preedy VR (Eds.), Neurological Modulation of Sleep (pp. 217–228). Academic Press. 10.1016/B978-0-12-816658-1.00023-5 [DOI] [Google Scholar]
- Ellis JG, Perlis ML, Neale LF, Espie CA, & Bastien CH (2012). The natural history of insomnia: Focus on prevalence and incidence of acute insomnia. Journal of Psychiatric Research, 46(10), 1278–1285. [DOI] [PubMed] [Google Scholar]
- Fairburn CG (2008). Cognitive Behavior Therapy and Eating Disorders. Guilford Press. [Google Scholar]
- Goel NJ, Sadeh-Sharvit S, Trockel M, Flatt RE, Fitzsimmons-Craft EE, Balantekin KN, Monterubio GE, Firebaugh M-L, Wilfley DE, & Taylor CB (2020). Depression and anxiety mediate the relationship between insomnia and eating disorders in college women. Journal of American College Health, 0(0), 1–6. 10.1080/07448481.2019.1710152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Evans EW, Saletin JM, O’Sullivan K, Koren D, Engel SG, & Haedt-Matt A (2020). Naturalistic, multimethod exploratory study of sleep duration and quality as predictors of dysregulated eating in youth with overweight and obesity. Appetite, 146, 104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AN, & Walker MP (2014). The role of sleep in emotional brain function. Annual Review of Clinical Psychology, 10, 679–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer SM, Goldstein AN, & Walker MP (2013). The impact of sleep deprivation on food desire in the human brain. Nature Communications, 4(1), 2259. 10.1038/ncomms3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG (2001). Insomnia: Symptom or diagnosis? Clinical Psychology Review, 21(7), 1037–1059. [DOI] [PubMed] [Google Scholar]
- Harvey AG (2002). A cognitive model of insomnia. Behaviour Research and Therapy, 40(8), 869–893. 10.1016/S0005-7967(01)00061-4 [DOI] [PubMed] [Google Scholar]
- Harvey AG (2003). The Attempted Suppression of Presleep Cognitive Activity in Insomnia. Cognitive Therapy and Research, 27(6), 593–602. 10.1023/A:1026322310019 [DOI] [Google Scholar]
- Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, Jansson-Fröjmark M, Palagini L, Rücker G, Riemann D, & Baglioni C (2019). Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Medicine Reviews, 43, 96–105. 10.1016/j.smrv.2018.10.006 [DOI] [PubMed] [Google Scholar]
- Hogenkamp PS, Nilsson E, Nilsson VC, Chapman CD, Vogel H, Lundberg LS, Zarei S, Cedernaes J, Rångtell FH, Broman J-E, Dickson SL, Brunstrom JM, Benedict C, & Schiöth HB (2013). Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology, 38(9), 1668–1674. 10.1016/j.psyneuen.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, & Carroll JE (2016). Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological Psychiatry, 80(1), 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeger A, Egilmez U, Sayin AA, & Selvi Y (2018). The relationship between night eating symptoms and disordered eating attitudes via insomnia and chronotype differences. Psychiatry Research, 268, 354–357. 10.1016/j.psychres.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Kenny TE, Wijk MV, Singleton C, & Carter JC (2018). An examination of the relationship between binge eating disorder and insomnia symptoms. European Eating Disorders Review, 26(3), 186–196. 10.1002/erv.2587 [DOI] [PubMed] [Google Scholar]
- KyungRan K, YoungChul J, MiYeon S, NamKoong K, JoonKi K, & JungHyun L (2010). Sleep disturbance in women with eating disorder: Prevalence and clinical characteristics. Psychiatry Research, 176(1), 88–90. [DOI] [PubMed] [Google Scholar]
- Latzer Y, Tzischinsky O, & Epstein R (2001). Sleep-Wake Monitoring in Women Suffering From Anorexia Nervosa. Eating Disorders, 9(2), 159–166. 10.1080/10640260127713 [DOI] [PubMed] [Google Scholar]
- Latzer Y, Tzischinsky O, Epstein R, Klein E, & Peretz L (1999). Naturalistic sleep monitoring in women suffering from bulimia nervosa. International Journal of Eating Disorders, 26(3), 315–321. [DOI] [PubMed] [Google Scholar]
- Lauer CJ, & Krieg J-C (2004). Sleep in eating disorders. Sleep Medicine Reviews, 8(2), 109–118. 10.1016/S1087-0792(02)00122-3 [DOI] [PubMed] [Google Scholar]
- LeBlanc M, Beaulieu-Bonneau S, Mérette C, Savard J, Ivers H, & Morin CM (2007). Psychological and health-related quality of life factors associated with insomnia in a population-based sample. Journal of Psychosomatic Research, 63(2), 157–166. [DOI] [PubMed] [Google Scholar]
- Linnaranta O, Bourguignon C, Crescenzi O, Sibthorpe D, Buyukkurt A, Steiger H, & Storch K-F (2020). Late and Instable Sleep Phasing is Associated With Irregular Eating Patterns in Eating Disorders. Annals of Behavioral Medicine. 10.1093/abm/kaaa012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Song Y, Koopmann J, Wang M, Chang C-HD, & Shi J (2017). Eating your feelings? Testing a model of employees’ work-related stressors, sleep quality, and unhealthy eating. Journal of Applied Psychology, 102(8), 1237. [DOI] [PubMed] [Google Scholar]
- Lombardo C, Ballesio A, Gasparrini G, & Cerolini S (2020). Effects of acute and chronic sleep deprivation on eating behaviour. Clinical Psychologist, 24(1), 64–72. [Google Scholar]
- Lombardo C, Battagliese G, Baglioni C, David M, Violani C, & Riemann D (2014). Severity of insomnia, disordered eating symptoms, and depression in female university students. Clinical Psychologist, 18(3), 108–115. 10.1111/cp.12023 [DOI] [Google Scholar]
- Lombardo C, Battagliese G, Venezia C, & Salvemini V (2015). Persistence of poor sleep predicts the severity of the clinical condition after 6months of standard treatment in patients with eating disorders. Eating Behaviors, 18, 16–19. 10.1016/j.eatbeh.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Lyytikäinen P, Lallukka T, Lahelma E, & Rahkonen O (2011). Sleep problems and major weight gain: A follow-up study. International Journal of Obesity, 35(1), 109–114. 10.1038/ijo.2010.113 [DOI] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, Pedro-Salcedo MGS, Kuo TF, & Kalista T (2008). Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep, 31(4), 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason EC, & Harvey AG (2014). Insomnia before and after treatment for anxiety and depression. Journal of Affective Disorders, 168, 415–421. 10.1016/j.jad.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, & Ivers H (2011). The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep, 34(5), 601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ, Tomiyama AJ, Ziegler M, Khandrika S, & Irwin MR (2009). Nocturnal levels of ghrelin and leptin and sleep in chronic insomnia. Psychoneuroendocrinology, 34(4), 540–545. 10.1016/j.psyneuen.2008.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadorff M, Nazem S, & Fiske A (2013). Insomnia symptoms, nightmares, and suicide risk: Duration of sleep disturbance matters. Suicide and Life-Threatening Behavior, 43(2), 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, & Roth T (2003). Place of chronic insomnia in the course of depressive and anxiety disorders. Journal of Psychiatric Research, 37(1), 9–15. 10.1016/S0022-3956(02)00052-3 [DOI] [PubMed] [Google Scholar]
- Padez-Vieira F, & Afonso P (2016). Sleep disturbances in anorexia nervosa. Advances in Eating Disorders, 4(2), 176–188. 10.1080/21662630.2016.1175958 [DOI] [Google Scholar]
- Patel SR, & Hu FB (2008). Short Sleep Duration and Weight Gain: A Systematic Review. Obesity, 16(3), 643–653. 10.1038/oby.2007.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis M, Shaw PJ, Cano G, & Espie CA (2011). Models of insomnia. Principles and Practice of Sleep Medicine, 5, 850–850. [Google Scholar]
- Prefit A-B, Cândea DM, & Szentagotai-Tătar A (2019). Emotion regulation across eating pathology: A meta-analysis. Appetite, 143, 104438. 10.1016/j.appet.2019.104438 [DOI] [PubMed] [Google Scholar]
- Roveda E, Montaruli A, Galasso L, Pesenti C, Bruno E, Pasanisi P, Cortellini M, Rampichini S, Erzegovesi S, Caumo A, & Esposito F (2018). Rest-activity circadian rhythm and sleep quality in patients with binge eating disorder. Chronobiology International, 35(2), 198–207. 10.1080/07420528.2017.1392549 [DOI] [PubMed] [Google Scholar]
- Sauchelli S, Jiménez-Murcia S, Sánchez I, Riesco N, Custal N, Fernández-García JC, Garrido-Sánchez L, Tinahones FJ, Steiger H, Israel M, Baños RM, Botella C, de la Torre R, Fernández-Real JM, Ortega FJ, Frühbeck G, Granero R, Tárrega S, Crujeiras AB, … Fernández-Aranda F (2016). Orexin and sleep quality in anorexia nervosa: Clinical relevance and influence on treatment outcome. Psychoneuroendocrinology, 65, 102–108. 10.1016/j.psyneuen.2015.12.014 [DOI] [PubMed] [Google Scholar]
- Schreiber-Gregory DN, Lavender JM, Engel SG, Wonderlich SA, Crosby RD, Peterson CB, Simonich H, Crow S, Durkin N, & Mitchell JE (2013). Examining duration of binge eating episodes in binge eating disorder. International Journal of Eating Disorders, 46(8), 810–814. 10.1002/eat.22164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short NA, Allan NP, & Schmidt NB (2017). Sleep disturbance as a predictor of affective functioning and symptom severity among individuals with PTSD: An ecological momentary assessment study. Behaviour Research and Therapy, 97, 146–153. [DOI] [PubMed] [Google Scholar]
- Short NA, Babson KA, Schmidt NB, Knight CB, Johnson J, & Bonn-Miller MO (2016). Sleep and affective functioning: Examining the association between sleep quality and distress tolerance among veterans. Personality and Individual Differences, 90, 247–253. [Google Scholar]
- Short NA, Mathes BM, Gibby B, Oglesby ME, Zvolensky MJ, & Schmidt NB (2017). Insomnia symptoms as a risk factor for cessation failure following smoking cessation treatment. Addiction Research & Theory, 25(1), 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, & Cauter EV (2004). Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Annals of Internal Medicine, 141(11), 846–850. 10.7326/0003-4819-141-11-200412070-00008 [DOI] [PubMed] [Google Scholar]
- Startup H, Lavender A, Oldershaw A, Stott R, Tchanturia K, Treasure J, & Schmidt U (2013). Worry and rumination in anorexia nervosa. Behavioural and Cognitive Psychotherapy, 41(3), 301. [DOI] [PubMed] [Google Scholar]
- Stice E, Gau JM, Rohde P, & Shaw H (2017). Risk factors that predict future onset of each DSM–5 eating disorder: Predictive specificity in high-risk adolescent females. Journal of Abnormal Psychology, 126(1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz J, Eiholzer R, & Spengler CM (2019). Effects of Evening Exercise on Sleep in Healthy Participants: A Systematic Review and Meta-Analysis. Sports Medicine, 49(2), 269–287. 10.1007/s40279-018-1015-0 [DOI] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, & Mignot E (2004). Short Sleep Duration Is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLOS Medicine, 1(3), e62. 10.1371/journal.pmed.0010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzischinsky O, & Latzer Y (2006). Sleep–wake cycles in obese children with and without binge-eating episodes. Journal of Paediatrics and Child Health, 42(11), 688–693. 10.1111/j.1440-1754.2006.00952.x [DOI] [PubMed] [Google Scholar]
- Tzischinsky O, Latzer Y, Epstein R, & Tov N (2000). Sleep-wake cycles in women with binge eating disorder. International Journal of Eating Disorders, 27(1), 43–48. [DOI] [PubMed] [Google Scholar]
- van der Zweerde T, Bisdounis L, Kyle SD, Lancee J, & van Straten A (2019). Cognitive behavioral therapy for insomnia: A meta-analysis of long-term effects in controlled studies. Sleep Medicine Reviews, 48, 101208. 10.1016/j.smrv.2019.08.002 [DOI] [PubMed] [Google Scholar]
- van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, & Lancee J (2018). Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Medicine Reviews, 38, 3–16. 10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Vander Wal JS (2012). Night eating syndrome: A critical review of the literature. Clinical Psychology Review, 32(1), 49–59. 10.1016/j.cpr.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Wardle-Pinkston S, Slavish DC, & Taylor DJ (2019). Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Medicine Reviews, 48, 101205. 10.1016/j.smrv.2019.07.008 [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, & Yanagisawa M (2001). To Eat or to Sleep? Orexin in the Regulation of Feeding and Wakefulness. Annual Review of Neuroscience, 24(1), 429–458. 10.1146/annurev.neuro.24.1.429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were collected for this manuscript.


