Background:
The ASPREE (ASPirin in Reducing Events in the Elderly) trial was a randomized, double-blind, placebo-controlled primary prevention trial of aspirin in 19 114 community-dwelling persons aged 70 years and older (≥65 years in U.S. racial minorities). The results of the trial demonstrated that aspirin had no benefit for disability-free survival, prevention of cardiovascular disease events, or prevention of incident cancer, and increased risk for major bleeding and all-cause mortality (1-3). These findings were interpreted by some as being relevant only to aspirin initiation and not aspirin discontinuation (4). The availability of evidence to inform the risks (for example, forgone cardiovascular protection) and benefits (for example, decreased risk for major hemorrhage) from aspirin cessation among older adults is timely, given updated guideline recommendations regarding aspirin use and clinical uncertainty (5).
Objective:
To investigate the effect of aspirin cessation versus continuation on disability-free survival and other clinical outcomes in a post hoc analysis of ASPREE participants who were regularly taking aspirin before trial enrollment.
Methods and Findings:
We included participants who reported taking aspirin 2 or more days per week at enrollment in the trial. We compared those randomly assigned to placebo (cessation) with those randomly assigned to aspirin (continuation). The primary outcome was a composite of all-cause mortality, incident dementia, or persistent physical disability (“disability-free survival” [1]). Secondary outcomes were all-cause mortality, major adverse cardiovascular events, any cardiovascular event, major hemorrhage, and incident cancer (1-3). All outcomes were adjudicated.
Cox proportional hazards regression was used to analyze outcomes. Subgroup analyses were conducted by age (<75 years and ≥75 years), race (White and non-White), and years of pretrial aspirin exposure (<5 years and ≥5 years).
The Table (page 762) shows baseline characteristics of the entire ASPREE population and the group for this analysis. Of 19 114 recruited participants, 2094 (11%) reported aspirin use before trial entry, of whom 1714 reported taking aspirin 2 or more days per week. Due to higher rates of pretrial aspirin use, U.S. participants were overrepresented in this analysis (40.6%) relative to the entire ASPREE study (12.6%).
Table.
Baseline Characteristics of All ASPREE Participants and Those Taking Regular Aspirin (2 or More Days Per Week) Before Enrollment, by Randomly Assigned Treatment
| Characteristic | All ASPREE Participants | Participants Included in This Study | ||
|---|---|---|---|---|
| Total | Placebo (Cessation) |
Aspirin (Continuation) | ||
| Participants, n | 19 114 | 1714 | 841 | 873 |
| Age | ||||
| Median (IQR), y | 74.0 (71.6–77.7) | 74.4 (71.8–78.4) | 74.5 (71.8–78.3) | 74.3 (71.6–78.5) |
| ≥75 y, n (%) | 7951 (41.6) | 777 (45.3) | 385 (45.8) | 392 (44.9) |
| Female, n (%) | 5373 (56.4) | 941 (54.9) | 447 (53.2) | 494 (56.6) |
| Country, n (%) | ||||
| Australia | 16 703 (87.4) | 1018 (59.4) | 505 (60.1) | 513 (58.8) |
| United States | 2411 (12.6) | 696 (40.6) | 336 (40.0) | 360 (41.2) |
| Ethnicity/race, n (%)* | ||||
| White | 17 450 (91.3) | 1335 (77.9) | 659 (78.4) | 676 (77.4) |
| Black | 901 (4.7) | 262 (15.3) | 131 (15.6) | 131 (15.0) |
| Hispanic/Latino | 488 (2.6) | 90 (5.3) | 40 (4.8) | 50 (5.7) |
| Other | 275 (1.4) | 27 (1.6) | 11 (1.3) | 16 (1.8) |
| Mean BMI (SD), kg/m2 | 28.1 (4.8) | 28.8 (5.0) | 28.6 (5.0) | 28.9 (5.0) |
| Smoking status, n (%) | ||||
| Never | 10 580 (55.4) | 892 (52.0) | 443 (52.7) | 449 (51.4) |
| Previous | 7799 (40.8) | 750 (43.8) | 356(42.3) | 394 (45.1) |
| Current | 735 (3.8) | 72 (4.2) | 42 (5.0) | 30 (3.4) |
| Diabetes mellitus, n (%)† | 2057 (10.8) | 325 (19.0) | 164 (19.5) | 161 (18.4) |
| Hypertension, n (%)‡ | 14 213 (74.4) | 1354 (79.0) | 660 (78.5) | 694 (79.5) |
| Dyslipidemia, n (%)§ | 12 467 (65.2) | 1096 (63.9) | 548 (65.2) | 548 (62.8) |
| Chronic kidney disease, n (%)∥ | 4920 (25.7) | 488 (28.5) | 254 (30.2) | 234 (26.8) |
| CVD risk factors, n (%)¶ | ||||
| 0–1 | 8678 (45.4) | 704 (41.1) | 339 (40.3) | 365 (41.8) |
| 2 | 8808 (46.1) | 750 (43.8) | 374 (44.5) | 376 (43.1) |
| 3–4 | 1628 (8.5) | 260 (15.2) | 128 (15.2) | 132 (15.1) |
| Personal history of cancer, n (%) | 1827 (19.2) | 347 (20.3) | 160 (19.0) | 187 (21.4) |
| Use of statins, n (%) | 5987 (31.3) | 719 (42.0) | 355 (42.2) | 364 (41.7) |
| Pretrial aspirin use, n (%)** | ||||
| Dose, mg | ||||
| ≤100 | – | 1519 (88.6) | 741 (88.1) | 778 (89.1) |
| 325 | – | 94 (5.5) | 45 (5.4) | 49 (5.6) |
| 500 | – | 17 (1.0) | 9 (1.1) | 8 (0.9) |
| Unknown | – | 84 (4.9) | 46 (5.5) | 38 (4.4) |
| Regimen, d/wk | ||||
| 2–5 | – | 292 (17.0) | 145 (17.2) | 147 (16.8) |
| ≥6 | – | 1422 (83.0) | 696 (82.8) | 726 (83.2) |
| Duration, y | ||||
| <1 | – | 345 (20.1) | 175 (20.8) | 170 (19.5) |
| 1–4 | – | 679 (39.6) | 337 (40.1) | 342 (39.2) |
| 5–9 | – | 370 (21.6) | 178 (21.2) | 192 (22.0) |
| ≥10 | – | 320 (18.7) | 151 (18.0) | 169 (19.4) |
ASPREE = ASPirin in Reducing Events in the Elderly; BMI = body mass index; CVD = cardiovascular disease; IQR = interquartile range.
Ethnicity/race “Other” includes Australian Aborigine/Torres Strait Islander, Native American, more than 1 race, Native Hawaiian/Pacific Islander, and those who were not Hispanic and who did not state their ethnicity/race.
Diabetes mellitus is defined from self-report or fasting blood glucose level ≥7 mmol/L (≥126 mg/dL) or on treatment of diabetes mellitus.
Hypertension is defined as “on treatment” for high blood pressure or blood pressure >140/90 mm Hg at study entry.
Dyslipidemia is defined as those taking cholesterol-lowering medications or serum cholesterol ≥5.5 mmol/L (≥212 mg/dL; Australia) and ≥6.2 mmol/L (≥240 mg/dL; United States) or low-density lipoprotein cholesterol >4.1 mmol/L (>160 mg/dL).
Chronic kidney disease is defined as having an estimated glomerular filtration rate <60 mL/min/1.73 m2 or urinary albumin-creatinine ratio ≥30 mg/g.
Cardiovascular risk factors include the following 4 conditions: hypertension, diabetes, dyslipidemia, and current smoking.
Data regarding prior aspirin use pretrial were collected by investigators from participants (before being enrolled) via a questionnaire with the following questions (response options in parentheses): 1) Immediately prior to your involvement in ASPREE, were you taking aspirin regularly? (yes; no); if yes, 2) What dosage? (≤100 mg, 325 mg, 500 mg, unknown); 3) How often? (≤1 once per week, 2–5 days per week, ≥6 days per week); and 4) For how long? (<1, 1–4, 5–9, 10+ year(s)).
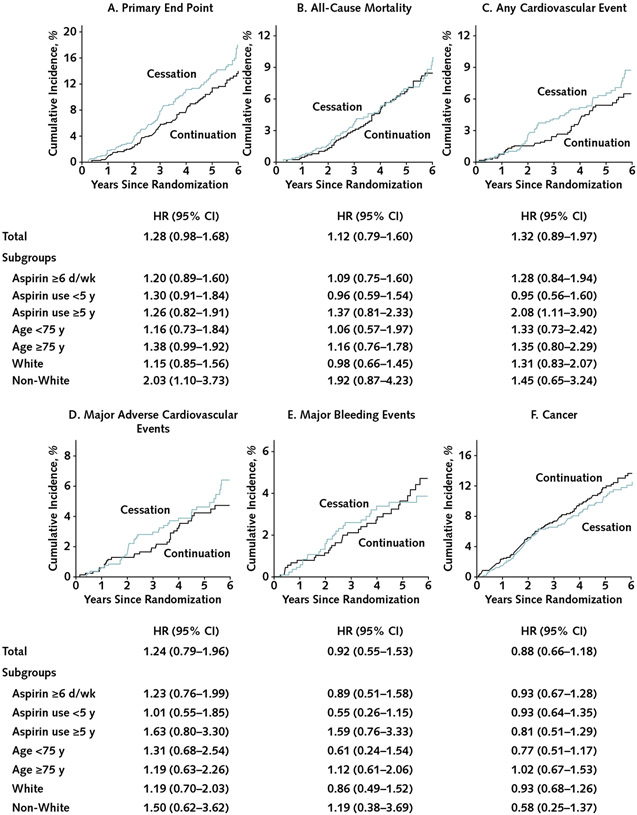
Over a median follow-up of 4.9 years, evidence of an increased risk for the primary outcome for aspirin cessation versus continuation was weak, and this appeared to be confined to non-White participants (Figure [page 763]). Overall, 116 of 841 participants (13.8%) in the cessation group and 97 of 873 (11.1%) in the continuation group experienced the primary end point (incidence rate, 29.8 vs. 23.4 per 1000 person-years); the hazard ratio for cessation versus continuation was 1.28 (95% CI, 0.98 to 1.68). When the analysis was restricted to those taking prior aspirin 6 or more days per week, the hazard ratio for cessation versus continuation was 1.20 (CI, 0.89 to 1.60). Although it was not associated with any secondary outcome, aspirin cessation appeared to increase cardiovascular disease events among those who reported taking aspirin for 5 years or longer. Age had no evident impact on the effect of aspirin cessation.
Figure. Cumulative incidence of outcomes compared between randomly assigned groups in all participants taking regular aspirin before trial entry and in subgroups by duration of use, race, and age at baseline.
HR= hazard ratio for cessation versus continuation. A–B. Kaplan–Meier failure function was applied to present cumulative incidence of the (A) primary end point and (B) all-cause mortality. C–F. The plots were constructed from the cumulative incidence predicted on the basis of separate models in the aspirin groups (cessation, n = 1841; continuation, n = 873), taking into account competing risk of non-end point death for: (C) any cardiovascular event (a composite of coronary heart disease death, nonfatal myocardial infarction, stroke, noncoronary cardiac or vascular death, or hospitalization for heart failure), (D) major adverse cardiovascular events (a composite of coronary heart disease death, nonfatal myocardial infarction, or ischemic stroke), (E) major bleeding events, and (F) incident cancer. The graphs were truncated at year 6 because only 2 participants reached year 7. All end points were analyzed as time to first event satisfying the end point definition. Overall, 116 participants (13.8%) in the cessation group and 97 (11.1%) in the continuation group experienced the primary end point (incidence rate, 29.8 vs. 23.4 per 1000 person-years); 64 versus 60 participants, respectively, experienced all-cause death (incidence rate, 7.6 vs. 6.9 per 1000 person-years); 55 versus 44 experienced a cardiovascular event (6.5 vs. 5.0 per 1000 person-years); 40 versus 34 experienced a major adverse cardiovascular event (4.8 vs. 3.9 per 1000 person-years); 28 versus 32 experienced major bleeding (3.3 vs. 3.7 per 1000 person-years); and 84 versus 100 experienced incident cancer (10.0 vs. 11.5 per 1000 person-years).
Discussion:
Aspirin cessation through random assignment to placebo among prior regular aspirin users gave rise to increased rates of the primary end point and cardiovascular disease events compared with continuation through random assignment to aspirin, but the CIs were wide and the findings were therefore inconclusive. No substantial increased risks for major hemorrhage and cancer were seen with continued aspirin use. Absence of an effect on hemorrhage may relate to self-selection for tolerance among aspirin users. Noting that the analyses are likely underpowered, our findings cannot conclusively demonstrate clear harm or benefit of either cessation or continuation of aspirin in older adults.
We previously performed subgroup analyses from the main trial by prior aspirin use; they can be found elsewhere (1-3). However, these analyses were based on any previous aspirin use. We restricted our analyses to regular aspirin users to address the question of the effects of aspirin cessation in said users. Our findings were consistent among those taking aspirin for at least 6 days per week.
Our study does have limitations. A 4-week placebo run-in phase for the trial occurred before randomization, which meant a gap in continuous exposure for those randomly assigned to aspirin. In addition, the post hoc study design within an ASPREE subgroup reduced statistical power for this exploratory analysis.
Considering our study limitations of power and likely self-selection for tolerance, we cannot definitively address the question of the safety of aspirin cessation in older populations without a clinical indication for its use. This would require a well-designed study with a large sample size. However, given the community sampling frame and the findings of the main study, at this time, a pragmatic recommendation may be to consider aspirin cessation in those with a large medication burden with due caution.
Acknowledgment:
The authors acknowledge the ASPREE Investigator Group (https://aspree.org) for their contributions to the original trial.
Grant Support:
The ASPREE (ASPirin in Reducing Events in the Elderly) trial was supported by grant U01AG029824 from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health, by grants 334047 and 1127060 from the National Health and Medical Research Council of Australia, and by Monash University and the Victorian Cancer Agency.
Footnotes
Disclosures: Disclosures can be viewed at http://www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M21-3823.
Contributor Information
Mark R. Nelson, Menzies Institute for Medical Research, University of Tasmania, Hobart, Tasmania, and School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Galina Polekhina, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Robyn L. Woods, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Christopher M. Reid, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, and School of Public Health, Curtin University, Perth, Western Australia, Australia.
Andrew M. Tonkin, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Rory Wolfe, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Anne M. Murray, Berman Center for Outcomes & Clinical Research, Minneapolis Medical Research Foundation, Hennepin County Medical Center, Minneapolis, and Division of Geriatrics, Department of Medicine, Hennepin County Medical Center and University of Minnesota, Minneapolis, Minnesota.
Brenda Kirpach, Berman Center for Outcomes & Clinical Research, Minneapolis Medical Research Foundation, Hennepin County Medical Center, Minneapolis, Minnesota.
Michael E. Ernst, Department of Pharmacy Practice and Science, College of Pharmacy and Department of Family Medicine, Carver College of Medicine, The University of Iowa, Iowa City, Iowa.
Jessica E. Lockery, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, and Translational Immunology and Nanotechnology Research Program, School of Health and Biomedical Sciences, RMIT University, Bundoora, Victoria, Australia.
Raj C. Shah, Department of Family Medicine and Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, Illinois.
Nigel Stocks, Discipline of General Practice, The University of Adelaide, Adelaide, South Australia, Australia.
Suzanne G. Orchard, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Zhen Zhou, Menzies Institute for Medical Research, University of Tasmania, Hobart, Tasmania, Australia.
Data Sharing Statement:
The data may be made available at the discretion of the principal investigators of the ASPREE trial (Australia: Prof. John McNeil, john.mcneil@monash.edu; United States: Prof. Anne Murray, amurray@bermancenter.org) on request.
References
- 1.McNeil JJ, Nelson MR, Woods RL, et al. ; ASPREE Investigator Group. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018; 379:1519–1528. doi: 10.1056/NEJMoa1803955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeil JJ, Wolfe R, Woods RL, et al. ; ASPREE Investigator Group. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNeil JJ, Woods RL, Nelson MR, et al. ; ASPREE Investigator Group. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379:1499–1508. doi: 10.1056/NEJMoa1800722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes A, McEvoy JW, Halvorsen S. “Doctor, should I keep taking an aspirin a day?”. N Engl J Med. 2019;380:1967–1970. doi: 10.1056/NEJMclde1903004 [DOI] [PubMed] [Google Scholar]
- 5.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data may be made available at the discretion of the principal investigators of the ASPREE trial (Australia: Prof. John McNeil, john.mcneil@monash.edu; United States: Prof. Anne Murray, amurray@bermancenter.org) on request.