Abstract
Treatment-pattern data suggest that some patients with multiple sclerosis (MS) in the Kingdom of Saudi Arabia (KSA) may not be receiving optimal treatment. A virtual meeting of ten expert Saudi neurologists, held on October 23, 2020, discussed unmet needs in relapsing–remitting MS (RRMS), and the role of ofatumumab as a suitable treatment in the KSA. Multiple unmet needs were identified: poor quality of life, with high rates of depression and anxiety; a negative impact of MS on work ability; treatment choices that may compromise efficacy for safety or vice versa; inconvenient or complex dosage regimens; and limited access to patient education and support. Early use of highly effective disease-modifying treatments (DMTs) results in better patient outcomes than starting with less effective treatments and downstream escalation, but this strategy may be underutilized in the KSA. B cells are important in MS pathogenesis, and treatments targeting these may improve clinical outcomes. Ofatumumab differs from other B cell–depleting therapies, being a fully human monoclonal antibody that binds to CD20 at a completely separate site from the epitope bound by ocrelizumab, and being administered by subcutaneous injection. When compared with teriflunomide in two randomized, phase 3 clinical trials in patients with RRMS, ofatumumab was associated with significant reductions in annualized relapse rates, rates of confirmed disability worsening, and active lesions on magnetic resonance imaging. The incidence of adverse events, including serious infections, was similar with the two treatments. Ofatumumab is a valuable first- or second-line treatment option for RRMS in the KSA, particularly for patients who would benefit from highly effective DMTs early in the disease course, and for those who prefer the convenience of self-injection. Future research will clarify the position of ofatumumab in RRMS treatment, and comparative cost data may support the broad inclusion of ofatumumab in formularies across the KSA.
Keywords: B cells, Multiple sclerosis, Ofatumumab, Relapsing–remitting multiple sclerosis
Key Summary Points
| Data from the Kingdom of Saudi Arabia (KSA) suggest that some patients with relapsing-remitting multiple sclerosis (RRMS) may not be optimally treated because some physicians delay the use of highly effective disease-modifying treatments. |
| A virtual meeting of expert Saudi neurologists identified a number of unmet needs for patients with RRMS in the KSA, including treatment choices that must compromise efficacy for safety or vice versa and inconvenient or complex dosage regimens. |
| B cells play an important role in MS pathogenesis, and treatments that target B cells, including ofatumumab, improve clinical outcomes in RRMS patients. |
| In clinical trials, ofatumumab significantly reduced relapse rates, confirmed disability worsening, and active lesions on magnetic resonance imaging compared with teriflunomide, with no increase in the incidence of adverse events, including serious infections. |
Introduction
The prevalence of multiple sclerosis (MS) in the Kingdom of Saudi Arabia (KSA) is approximately 40.4 persons per 100,000 in the general population and 61.95 per 100,000 Saudi nationals [1]. Moreover, the prevalence of MS in the Arabian Gulf has been increasing at a rate of 2.3% per year since 1986 [2]. In fact, the prevalence of MS, and its growth, in the Middle East are comparable with epidemiological patterns in Western countries, like the United States of America (USA) and Canada [2]. These patterns reflect changes in genetic susceptibility and environmental triggers over time, but nevertheless, there are some potential risk factors that may be more prevalent in the Middle Eastern region than in the West, such as parental consanguinity and regional armed conflicts [2, 3].
MS is characterized by a highly variable and mostly unpredictable onset and natural history [4, 5]. Of the three main phenotypes of MS, by far the most common is the relapsing–remitting subtype (RRMS), in which patients experience acute relapses interspersed with periods of full or partial recovery or stable clinical status [6]. Estimates indicate that approximately 74% of MS patients in the Middle East and 65–75% of MS patients in the KSA have RRMS [7–9]. In 2017–2018, the estimated annualized relapse rate (ARR) in the KSA was 0.70 [10].
Monoclonal antibody (mAb) therapy options for RRMS include treatments that target the trafficking of leukocytes into the central nervous system (CNS), namely natalizumab, CD52 receptors on T-cells (alemtuzumab), and the CD20 receptor protein on B cells (ocrelizumab, ofatumumab, and rituximab1) [11, 12]. The most recently approved agent is ofatumumab (Kesimpta®; Novartis), which was approved for the treatment of RRMS in the USA in August 2020, in Europe in March 2021 and in the KSA in September 2021. As the number of biologic treatment options expands, so does the complexity of treatment decision-making in MS.
Compared with starting MS treatment with less effective therapy and then escalating therapy as the disease worsens, the early use of highly effective disease-modifying treatments (DMTs) has been shown to be associated with better patient outcomes [13, 14]. However, this strategy may be underutilized in the KSA.
Therefore, to support physicians managing patients with MS, particularly those in the KSA, a virtual meeting of ten experts in the management of MS from across the KSA was held on October 23, 2020; this advisory board meeting was sponsored by Novartis Pharmaceuticals Corporation. The aim was to investigate the unmet needs in patients with RRMS, with a focus on the KSA, and review the role of ofatumumab in the treatment paradigm. This article describes the discussions and outcomes of that meeting. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Attendee Survey
Prior to the meeting, the ten experts completed a questionnaire about the unmet needs in patients with RRMS and the role of B cell-directed therapy in their management in the KSA. The survey consisted of 12 statements and participants were asked to rate their agreement with each statement on a five-point Likert scale (i.e., strongly disagree, disagree, neutral, agree, or strongly agree). It should be noted that the survey included a small number of participants (i.e., the ten experts), and its results represent only the opinions of these participants and are not intended to replace scientific evidence.
The participants’ responses are shown in Table 1. There was general agreement (60%) that there are still unmet needs in the treatment of RRMS, and 60% of respondents agreed that the complexity and side effects of existing treatments may contribute to poor adherence. None of the respondents strongly disagreed with these statements. Similarly, 70% of respondents agreed that early treatment of RRMS is important to improve long-term disease outcomes. The majority of respondents agreed that selective targeting of B cells is a long-term treatment strategy for MS based on the fact that B cells are significant drivers of the pathogenesis of MS. Overall, 80% of respondents agreed with the hypothesis that the low rate of infections seen during B cell therapy indicates that immunosurveillance is preserved during treatment. All respondents agreed or strongly agreed that they consider patient preferences when making treatment decisions in RRMS.
Table 1.
Responses to the pre-meeting survey
| Responses (%) | |||||
|---|---|---|---|---|---|
| Strongly agree | Agree | Neutral | Disagree | Strongly disagree | |
| Unmet needs in RRMS | 23 | 63 | 10 | 3 | 0 |
| There is still an unmet medical need in the treatment of RRMS | 40 | 60 | 0 | 0 | 0 |
| Some of the most effective medications that suppress disease activity also have severe safety and tolerability issues | 10 | 90 | 0 | 0 | 0 |
| Complexity and side effect profile of current treatment options result in poor treatment adherence | 20 | 40 | 30 | 10 | 0 |
| Early treatment of RRMS | 65 | 27.5 | 7.5 | 0 | 0 |
| The regenerative potential of the brain is limited and becomes less effective with age | 50 | 30 | 20 | 0 | 0 |
| Early treatment in RRMS is important for better long-term disease outcomes | 80 | 20 | 0 | 0 | 0 |
| Earlier intervention with highly effective DMTs results in better long-term disease control | 60 | 40 | 0 | 0 | 0 |
| “Time is Brain”: MS damages the whole brain, and damage begins from the start of the disease | 70 | 20 | 10 | 0 | 0 |
| Selective B cell targeting | 25 | 65 | 10 | 0 | 0 |
| B cells are an appropriate target for a long-term treatment strategy in patients with RRMS | 30 | 60 | 10 | 0 | 0 |
| Selective targeting of B cells is a long-term treatment strategy | 30 | 60 | 10 | 0 | 0 |
| With B cell therapy, low rates of infection in clinical trials support the hypothesis of preserved immune surveillance | 10 | 80 | 10 | 0 | 0 |
| B cells are drivers of MS pathogenesis | 30 | 60 | 10 | 0 | 0 |
| I involve my patients’ preference when it comes to making treatment decisions | 60 | 40 | 0 | 0 | 0 |
Text in bold indicates main survey topics and the mean overall levels of agreement for each topic
DMT disease-modifying treatment, MS multiple sclerosis, RRMS relapsing–remitting MS
Unmet Needs in RRMS
Poor Quality of Life
Research shows that patients with MS have impaired quality of life (QoL), including those in the KSA [15–17], and worsening disability is associated with deterioration of QoL [18]. RRMS has a negative impact on patients’ QoL by interfering with their ability to work, undertake leisure activities, and participate fully in the usual life roles [19–21]. A high proportion of patients with MS have depression and/or anxiety [22], and depression is associated with poor QoL in MS [17, 23]. Epidemiological studies from Saudi Arabia demonstrated that the prevalence of depression in patients with MS, including milder symptoms, ranged between 64 and 90% [24, 25]. In addition, 51% of MS patients in a study conducted in KSA had moderate-to-severe anxiety [24]. The prevalence of both anxiety and depression are higher in patients with poorer health status [24]. In addition to depression, fatigue, disability, and cognitive function impairment also have a major impact on QoL in patients with MS [17, 23, 26, 27].
DMT Efficacy
Due to perceived safety concerns with highly efficacious treatments, many RRMS patients are started on a low-efficacy treatment [28]. The expert panel discussed how low-efficacy treatments may result in more relapses, faster disease worsening and disability progression, as previously reported [28–30]. Therefore, there is a choice of treatment strategy between early intensive treatment with potentially more serious adverse events but a better disease course, or reserving the high-efficacy treatments for later in the disease course, reducing potential toxicity but risking more rapid disease progression. Faced with this choice, most patients with MS are willing to accept the risk of adverse events in exchange for slower disease progression and preserving functional status [31]. However, in the KSA, those patients with mild-to-moderate disease activity (either radiologically or by relapse activity) start with low-efficacy treatment and then escalate to high-efficacy DMTs. This is particularly the case outside of MS centers, where MS patients mostly see general neurologists. There is also considerable variability with regard to access to medications in the KSA. Most of the high-efficacy DMTs are only offered at government hospitals, and these hospitals may limit the number of patients eligible to receive them. Each hospital determines its own formulary, and national guidelines on which treatments to use at which stage of the disease are currently pending publication.
Inconvenient Dosage Regimens and Administration
Treatment schedules for DMTs can be complicated, and many require parenteral administration by intravenous (IV) infusion, subcutaneous (SC), or intramuscular (IM) injection (Table 2) [32–45]. Most IV infusions are administered less often than SC or IM injections during maintenance therapy, which can be convenient for patients and physicians, but IV infusions are often time-consuming to administer, and in some cases require premedication and/or a post-infusion observation period. Because of the risk of infusion-related reactions, the IV regimens must be administered at an infusion center that is capable of patient monitoring. Patients receiving alemtuzumab or ocrelizumab require premedication with steroids and/or antihistamines, and possibly antipyretics. The requirement to visit a hospital for treatment is a particular concern during the current COVID-19 pandemic.
Table 2.
Administration/dosage regimens for approved RRMS treatments
| Disease-modifying treatment | Route of administration | Adult dose |
|---|---|---|
| Alemtuzumab [42] | IV infusion | 12 mg/day for 5 consecutive days, then 12 mg/day for 3 consecutive days every 12 months for a total of up to four courses |
| Cladribine [41] | PO | 10–20 mg/day for 4–5 days in week 1 and 2 of months 1 and 2 in years 1 and 2 |
| Dimethyl fumarate [40] | PO | 120–240 mg twice daily |
| Fingolimod [43] | PO | 0.5 mg once daily |
| Glatiramer acetate [45] | SC | 20 mg once daily or 40 mg three times weekly |
| Interferon β-1a [32] | IM | 30 μg once a week |
| Interferon β-1a [38] | SC | 22–44 μg three times weekly |
| Interferon β-1b [33, 34] | SC | 62.5–250 μg every other day |
| Natalizumab [35] | IV infusion | 300 mg every 4 weeks |
| Ocrelizumab [37] | IV infusion | 300 mg then 300 mg 2 weeks later, then 600 mg every 6 months |
| Ofatumumab [36] | SC | 20 mg per week for first three doses and then 20 mg monthly |
| Pegylated interferon β- 1a [39] | SC or IM | 63 mg on day 1, 94 mg on day 14, and then 125 mg on day 28 and every 2 weeks thereafter |
| Teriflunomide [44] | PO | 14 mg once daily |
IM intramuscular, IV intravenous, PO oral, SC subcutaneous
Agents administered by SC or IM injection may be administered at home by the patient, a caregiver, or a home-care nurse. Oral agents offer some convenience to patients since no injections or infusions are required, but only teriflunomide, fingolimod, and siponimod have a simple once-daily dosing regimen. Regimen complexity and the route of administration may be important factors in patient adherence to treatment, especially when long-term treatment is required, and the patient’s cognition and/or dexterity are impaired [46].
Access to Patient Education and Support
The expert panel noted that there are limited social platforms for MS patients in Arabic, and that a national patient-centered advocacy or support organization could be a useful resource for MS patients in the KSA. Such an organization would help to communicate updated disease information to the MS community and the wider general public, enhancing the knowledge base of patients with MS about treatment options, their pros and cons, and the importance of early diagnosis and treatment.
Treatment Considerations
Early Treatment
During the early course of MS, peripheral immune activation predominates, and the influx of immune cells (e.g., B cells and cytotoxic CD8 T cells) drives the pathogenic changes in the CNS [47]. However, later in the disease course, the principal source of the immunologic response is the resident CNS cells, such as astrocytes and microglia [47]. During the early phase of MS, compensatory mechanisms counteract some of the peripheral immune cell activation, accounting for the relapsing/remitting nature of MS symptoms. Although neurodegeneration continues to occur, the signs may be subtle or masked by these compensatory repair mechanisms. However, as compensatory mechanisms and functional reserves exhaust over time, the impact of neurodegeneration becomes increasingly apparent as disease activity becomes consistently progressive [4, 48]. The expert panel agreed that targeting early inflammation provides an opportunity to improve treatment outcomes by preventing damage to the CNS, but this requires physicians to be willing and able to implement high-efficacy therapies early in the disease course.
In some cases, it may be challenging to confirm the diagnosis of MS. A delayed diagnosis can impact the initiation of treatment, highlighting the importance of educating primary care physicians about the need to promptly refer patients to a neurologist if they have signs and symptoms suggestive of MS [49]. The anecdotal experience of the expert panel indicates that diagnostic delays are indeed a common issue in the KSA, where there is a lack of awareness of MS signs and symptoms among patients and physicians.
Even after the MS diagnosis, patients may not start treatment immediately. According to data from the USA, only 35% of newly diagnosed MS patients start DMTs within the first few years (median 2.4 years) of the diagnosis, and those who do start DMT begin about 6 months after MS is diagnosed [50]. Yet, the evidence suggests that earlier initiation of DMTs is associated with a slower rate of disability progression [51]. Delaying DMT increases the risk of progression to significant disability (Expanded Disability Status Scale [EDSS] of 4) by 7.4% per year [51]. In addition, brain atrophy, and subsequently loss of function, may begin early and continue as the disease progresses if untreated. The deterioration of cognitive function, increasing disability, and worsening fatigue negatively impact QoL in MS patients [49]. Therefore, treating inflammation in early MS with effective DMTs could preserve brain function and QoL for patients, as well as delay conversion to a progressive disease course [28, 49].
Historically, the ‘start low and go slow’ escalation approach was a common treatment strategy for MS, in which patients began treatment with low-efficacy and low-risk treatments and switched to high-efficacy agents when relapses occurred or signs of disease activity developed [52]. However, there is growing interest in developing strategies that initiate highly effective treatments earlier [52], based on evidence that early and aggressive treatment reduces the risk of relapses [28]. Real-world clinical data have shown that initial use of highly effective DMTs (i.e., fingolimod, alemtuzumab, or natalizumab) significantly delayed the progression to secondary progressive MS compared with less effective agents (i.e., glatiramer acetate or interferon-β) [30]. The high use of these less effective DMTs in the KSA [10] suggests that the optimal treatment strategy is underutilized.
The Role of B Cells in RRMS
B lymphocytes are an integral part of the adaptive immune system, protecting the body from pathogens by recognizing antigens and producing plasma cells that make antibodies. There is growing evidence for the role of B cells in the pathogenesis of MS.
MS is characterized by autoantibody production. Oligoclonal bands of immunoglobulin G (IgG) are present in the cerebrospinal fluid (CSF) of more than 95% of patients with MS, and B cells in the CSF have been shown to be the source of oligoclonal IgG [53]. In addition, IgG antibodies directed against the myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein are frequently present in the blood and CSF of patients with MS [54]. Plasma cells are present in high numbers in chronic or subacute MS plaques, mostly in the perivascular spaces but also in the parenchyma, suggesting that autoantibody processing occurs there [55, 56]. In addition, some patients with advanced, secondary progressive MS (SPMS) develop ectopic follicles in their meninges that are comprised of B cells and plasma cells [53]. SPMS patients with these meningeal B cell follicle-like structures show greater inflammation, more grey matter pathology, and worse outcomes compared with SPMS patients without these follicle-like structures [57].
In addition to B cells being involved in adaptive immunity, they have also been shown to act as antigen-presenting cells to promote autoimmune T-cell responses, independently of MOG antibody production, in experimental MS models [58, 59]. T cells and macrophages are activated by antigen presentation and inflammatory cytokine (e.g., IL-6, IL-12) secretion by B cells [56].
Further evidence of the role of B cells in the pathogenesis of MS is provided by clinical studies with B cell-depleting therapies (i.e., rituximab, ocrelizumab, and ofatumumab), which have shown clinical benefits, including reductions in brain inflammation and relapse rates in patients with MS [60–64].
The Role of Ofatumumab in the Treatment of RRMS
Ofatumumab is a fully human IgG1κ mAb directed against the CD20 transmembrane phosphoprotein expressed on B cells. CD20 is highly expressed in B cells in a number of conditions, including B cell hematologic malignancies [65, 66], and has a role in their differentiation into plasma cells [67].
CD20 has a number of attributes that make it an attractive treatment target in MS. First, CD20 has a propensity to remain on the cell surface without internalization after interaction with an mAb [68]. The interaction of CD20 and mAb leads to B cell lysis through antibody-dependent cell-mediated and complement-dependent cytotoxicities [68]. CD20 is exclusively a cell-surface protein that does not circulate freely in serum, meaning that mAbs can target CD20-expressing cells exclusively without competition from circulating antigens [68].
B cell-depleting therapies used in the treatment of MS differ in molecular structure, sequence composition, binding site, and mechanism of B cell depletion. Rituximab is a chimeric mAb that is approved for use in B cell hematologic malignancies, but not for MS, although it has been studied in MS patients [61, 69, 70]. Ocrelizumab is an anti-CD20 mAb that is derived from mice but humanized, and is approved for use in MS [37]. Ocrelizumab and rituximab bind to an overlapping but similar epitope on CD20, and are administered by IV infusion at doses of 600 or 1000 mg, respectively, every 6 months [67].
Ofatumumab differs from these agents in several ways. Ofatumumab is a fully human mAb that binds to the small and large extracellular loops on CD20 [36, 67], which is a completely different epitope from the binding site of rituximab and ocrelizumab (Fig. 1) [71, 72]. These separate binding sites likely explain some of the differential pharmacologic properties of these agents [73]. Ofatumumab is administered as a SC injection of 20 mg, with weekly administration for the first 3 weeks and monthly administration thereafter [36].
Fig. 1.
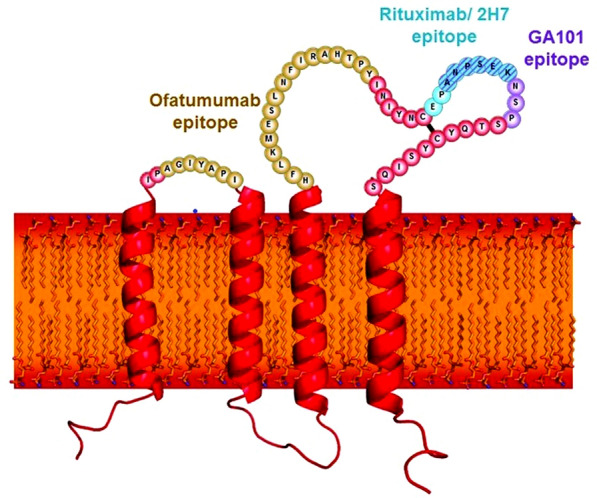
Epitopes on the CD20 transmembrane receptor and the binding sites of ocrelizumab (OCR), rituximab (RTX), and ofatumumab (OMB) [71].
Reproduced from Klein et al. [71], with permission from Taylor & Francis Ltd. www.tandfonline.com
Animal studies have reported transient peripheral B cell depletion and lymphocytopenia in neonates exposed to anti-CD20 B cell depleting antibodies in utero [36]. While there are limited data on the developmental risks associated with ofatumumab use in pregnant women, females of reproductive potential have been advised to use effective contraception while receiving ofatumumab and for at least 6 months after the last dose, unless the potential benefit to the mother outweighs the potential risk to the fetus. For neonates exposed to ofatumumab in utero, avoidance of live or live-attenuated vaccine administration is recommended until recovery of B cell counts [36].
Ofatumumab Phase 3 Clinical Data
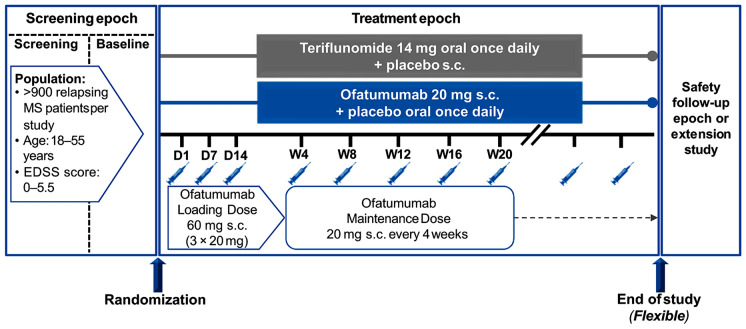
Based on phase 2 data, two phase 3 studies (ASCLEPIOS I and II) were conducted in patients aged 18–55 years with active RRMS and a baseline EDSS ranging between 0 and 5.5 [64]. These two studies were identical in design: both were multicenter, double-blind, double-dummy studies in which patients were randomized to treatment with oral teriflunomide or SC ofatumumab for up to 30 months (Fig. 2). Teriflunomide was chosen as the comparator to limit the injection burden for patients during double-blind assessment, since having interferon-β as a comparator would require frequent injections in both groups. The primary endpoint was ARR in each study, and secondary endpoints included the pooled rate of disability worsening, confirmed at 3 and 6 months, and disability improvement confirmed at 6 months [64]. Serum neurofilament light chain levels and MRI endpoints, including the number of Gd + T1-weighted lesions, new or enlarging T2-weighted lesions, and brain volume, were also evaluated in each study [64].
Fig. 2.
Design of the identical ASCLEPIOS I and II studies [64]
Overall, 927 patients were enrolled in ASCLEPIOS I and 955 were included in ASCLEPIOS II [64]. The patients’ demographic and clinical characteristics were similar in the two studies. Mean patients’ age was 38 and 39 years in both studies, and females comprised 68% of patients in ASCLEPIOS I and 66% in ASCLEPIOS II. Patients had been diagnosed with MS for a mean of 8 years, and 60–62% had previously received DMTs, most commonly interferons and glatiramer acetate. The mean number of relapses in the past 12 months was 1.2 and 1.3, consistent with the requirement for patients to have active RRMS at enrolment, and the mean EDSS score was 2.9 and 3.0 [64].
ASCLEPIOS Findings
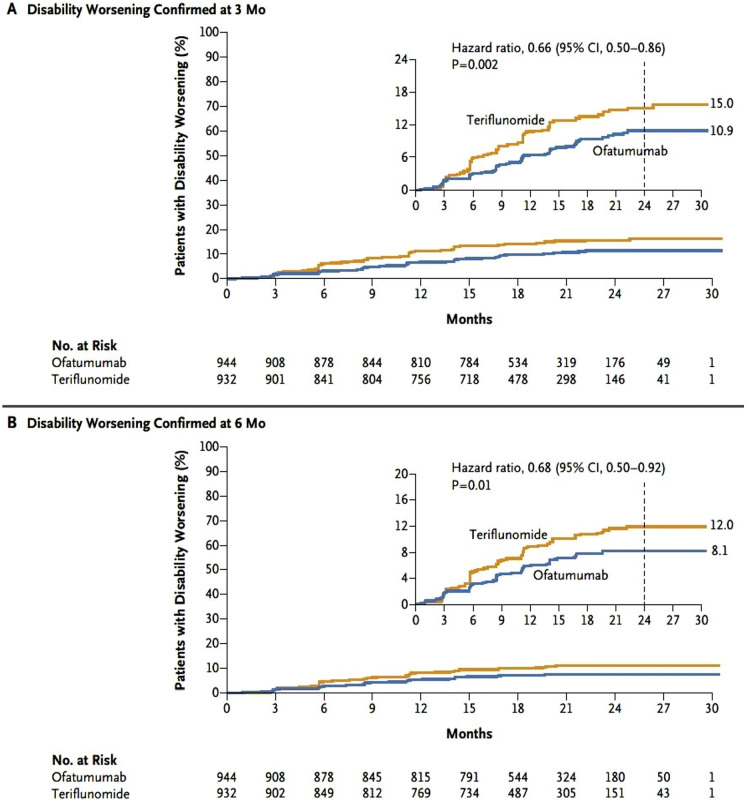
Over 30 months of treatment, the ARR was 0.11 with ofatumumab and 0.22 with teriflunomide in ASCLEPIOS I (rate ratio 0.49 [95% confidence interval (CI) 0.37–0.65]; p < 0.001), and 0.10 with ofatumumab and 0.25 with teriflunomide in ASCLEPIOS II (rate ratio 0.42 [95% CI 0.31–0.56]; p < 0.001), representing a relative reduction in ARR with ofatumumab versus teriflunomide of 51% in ASCLEPIOS I and 58% in ASCLEPIOS II [64]. In the pooled analysis of data from both studies, ofatumumab reduced the risk of confirmed disability worsening compared with teriflunomide by 34% at 3 months (p = 0.002) and by 32% at 6 months (p = 0.01; Fig. 3). Overall, 11% of patients receiving ofatumumab and 8.1% receiving teriflunomide showed a reduction in disability (improvement) confirmed at 6 months, but the between-group difference was not statistically significant (p = 0.09) [64].
Fig. 3.
Kaplan–Meier estimate of disability worsening confirmed at A 3 months or B 6 months during treatment with ofatumumab or teriflunomide [64]. CI confidence interval.
From Hauser et al. [64]. Copyright© 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society
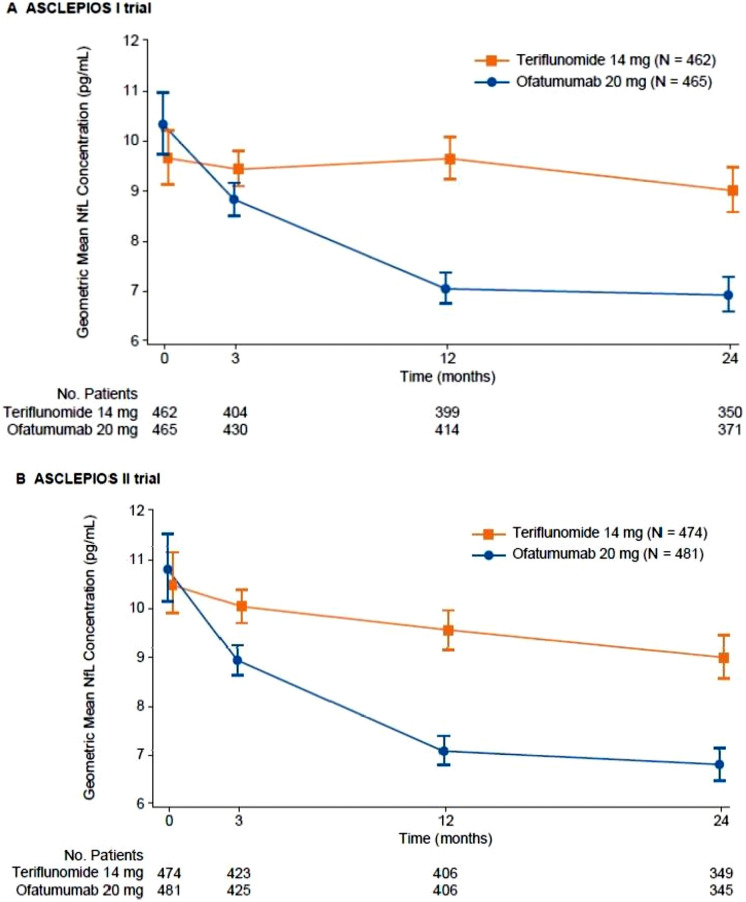
In each study, there was a significantly lower number of Gd + T1-weighted lesions per scan in the ofatumumab group than in the teriflunomide group, with a mean of 0.01 (95% CI 0.01–0.02) versus 0.45 (95% CI 0.36–0.58) Gd + T1-weighted lesions per scan in ASCLEPIOS I, respectively, and 0.03 (95% CI 0.02–0.05) versus 0.51 (95% CI 0.40–0.66) lesions per scan, in ASCLEPIOS II, respectively (p < 0.001 for both comparisons) [64]. The number of new or enlarging T2-weighted lesions per year was also significantly lower with ofatumumab versus teriflunomide in ASCLEPIOS I (mean 0.72 [95% CI 0.61–0.85] vs. 4.00 [95% CI 3.47–4.61] lesions per year; p < 0.001) and ASCLEPIOS II (mean 0.64 [95% CI 0.55–0.75] vs. 4.15 [95% CI 3.64–4.74] lesions per year; p < 0.001). The rate of brain volume loss tended to be lower in the ofatumumab group (− 0.28% or − 0.29% loss per year) than in the teriflunomide group (− 0.35% loss per year in both studies), but the between-group difference was not statistically significant in either study. Serum neurofilament light chain levels decreased with ofatumumab relative to teriflunomide, with significant differences apparent from the first measurement at month 3 (Fig. 4) [64]. Post hoc analyses of pooled ASCLEPIOS I and II data demonstrated consistent treatment benefits on clinical (ARR) and disability (3- or 6-month confirmed disability worsening) outcomes across subgroups defined by baseline characteristics, including age, gender, bodyweight, EDSS, the number of relapses prior to the study, the number of Gd + T1-weighted lesions, and previous treatment with DMTs [74].
Fig. 4.
Changes in serum neurofilament light chain levels during the ASCLEPIOS I and II studies [64]. NfL neurofilament light chain.
From Hauser et al. [64]. Copyright© 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society
The effect of ofatumumab versus teriflunomide on achieving no evidence of disease activity (NEDA-3) was investigated using pooled ASCLEPIOS I and II study data, including ARR, disability worsening, and Gd + T1-weighted lesions [75]. The odds of achieving NEDA-3 were significantly higher with ofatumumab than teriflunomide in both the first (months 0–12) and second (months 12–24) year of study treatment. In ofatumumab-treated patients, the odds of achieving NEDA-3 were threefold higher from months 0–12 (odds ratio [OR] 3.36 [95% CI 2.67–4.21]; p < 0.001) and eightfold higher from months 12–24 (OR 8.09 [95% CI 6.26–10.45]; p < 0.001) than in teriflunomide-treated patients in the same time periods [75].
Ofatumumab dosed at 20 mg led to rapid B cell depletion in the ASCLEPIOS I and II studies, with B cell counts of less than 10 cells/μl by week 2 and 0 cells/μl sustained between week 4 and week 96 in the ofatumumab group in the pooled population, whereas in the teriflunomide groups, B cell counts ranged between 150 and 220 cells/μl throughout the observation period [74]. However, this did not appear to affect the risk of infection, since the rate of any infections or serious infections were similar in both treatment groups [64]. A reduction from baseline in IgG levels was observed until week 36, followed by a recovery in IgG levels, and a reduction in IgM levels from baseline until week 120 was seen in both treatment groups. The proportion of patients who experienced infections was higher (45.5 vs. 36.4%) in the ofatumumab versus teriflunomide group after the first drop of IgG levels [76]. Among patients who showed a drop in IgM/IgG levels below the lower limit of normal (LLN), the rate of grade 3 or serious infections remained low in both treatment groups in the ASCLEPIOS trials. Nasopharyngitis and urinary tract infection were the most common infections after the first drop in IgM/IgG levels to below the LLN. Most of the infections reported were non-serious in nature, and were mild-to-moderate in severity. Overall, there was no apparent association between decreased Ig levels and increased rate of serious/non-serious infections in RRMS patients treated with ofatumumab, and no fatal, life-threatening or opportunistic infections were observed [64, 76]. The rate of malignancy was low (0.2–0.6%) and similar in ofatumumab and teriflunomide recipients in both studies [64]. Systemic injection-related reactions (e.g., headache, flushing) were reported in 20.2% of patients in the ofatumumab group and 15.0% in the teriflunomide group [64]. The only marked difference between the treatment groups was observed after the first injection, when systemic reactions occurred in 14.4% of patients receiving ofatumumab and 7.5% receiving teriflunomide. With subsequent injections, the incidence of systemic reactions was low and similar in both groups, and almost all of these systemic injection-related events (99.7%) were mild-to-moderate in severity [64].
ALITHIOS was a phase 3b open-label long-term safety study that enrolled 1969 patients who had completed ASCLEPIOS I/II, APLIOS or APOLITOS and who continued to receive ofatumumab (n = 1292) or switched from teriflunomide to ofatumumab (n = 677) [77]. Ofatumumab exposure, expressed as median (range) time at risk, was 35.6 (0–59.7) months in the continuous ofatumumab group and 24.2 (1.2–30.6) months in the switch group. The risk of serious infections or malignancies did not increase with long-term ofatumumab exposure of up to 4 years (5197.9 patient-years), and no new safety signals were detected [78].
Future Studies
Further research on ofatumumab is ongoing, including open-label extensions studies, phase 3b and 4 studies, studies in pediatric patients, studies assessing QoL and patient satisfaction, and evaluations of switching from other DMTs to ofatumumab. Data from these studies will help to determine the position of ofatumumab in MS treatment strategies, while future real-world studies could demonstrate the effects and patterns of use of ofatumumab during the routine care of patients with RRMS.
The Future of Ofatumumab in Clinical Practice in the KSA
Ofatumumab has several features which may make it an attractive treatment option for patients with RRMS, including being the only B cell-depleting therapy that patients can self-administer at home with a low dosing regimen (0.4 ml). Thus far, clinical trials and real-world safety data are promising, and suggest that the fully human mAb structure may limit the immunogenicity of ofatumumab compared with a chimeric structure.
The expert panel considered that ofatumumab offers a valuable new treatment option for first- or second-line treatment of RRMS. Some patients do not need an aggressive treatment strategy, but may still benefit from a high-efficacy treatment, such as that provided by ofatumumab. Similarly, the safety profile makes it an appealing option for many patients.
The expert panel believed that some patients are comfortable with self-injection at home, and would appreciate that the monthly schedule of ofatumumab involves fewer injections than interferon-β treatment and does not require hospital visits. Others, however, may prefer in-hospital IV infusions since ocrelizumab, for instance, is only administered every 6 months. The availability of both treatments gives patients more options. However, ofatumumab may not be widely available in the KSA because of the variability between formularies across the country. Many formularies in the KSA favor inclusion of ocrelizumab because it can be used for both RRMS and primary progressive MS. Similarly, some patients may not have access because of eligibility restrictions or reimbursement constraints. It was also noted by the panel that a study comparing the costs of ocrelizumab and ofatumumab could be pivotal in determining access to ofatumumab for Saudi patients.
Conclusions
There are many unmet needs in the treatment of RRMS in the KSA, most of which are common to other nations around the world. While the early use of high-efficacy treatment is now becoming the standard of care to delay disability progression, this strategy may be underutilized in the KSA. Ofatumumab is the first fully human B cell-depleting treatment for RRMS that can be administered by SC injection. Data available to date show high efficacy relative to teriflunomide and no increased risk of adverse events or infections. As a result, Saudi neurologists may consider ofatumumab as a first- or second-line treatment for patients with RRMS who would benefit from a high-efficacy treatment. Data from ongoing research will further clarify the role of ofatumumab in the treatment of RRMS in the KSA and around the world.
Acknowledgements
Funding
Sponsorship for the advisory board meeting, medical writing assistance, and the journal’s Rapid Service Fee were provided by Novartis Pharmaceuticals Corporation. The authors received no honoraria related to the development of this publication.
Medical Writing Assistance
Medical writing support, including assisting authors with the development of the manuscript drafts and incorporation of comments, was provided by Catherine Rees, of Springer Healthcare Communications, and was supported by Novartis Pharmaceuticals Corporation, according to Good Publication Practice guidelines (https://www.ismpp.org/gpp3).
Author Contributions
Crafting this manuscript from the report on the content of the advisory board was an original idea from Foziah Alshamrani. Hazem Wahba developed the concept for the article, organized the advisory board, developed the pre-meeting survey, and was a co-moderator on the advisory board. Yaser Al Malik was the lead moderator of the advisory board, and Matthew Craner was a presenter at the meeting. The rest of authors are ordered alphabetically as they contributed equally. Fawzi A. Babtain, Foziah Alshamrani, Mona M. Alkhawajah, Nora Alfugham, Rumaiza H. Al-Yafeai, Salman Aljarallah, Seraj Makkawi, and Shireen Qureshi had similar participatory roles in the advisory board meeting and contributed their insights, based on their local clinical practice and experience, to the discussions that formed the basis of the article. Marina Ziehn also participated in the advisory board and assisted, reviewed, and was involved in finalizing the manuscript.
Disclosures
Matthew Craner, Nora Alfugham, Salman Aljarallah, Mona M. Alkhawajah, Yaser Al Malik, Foziah Alshamrani, Rumaiza H. Al-Yafeai, Fawzi A. Babtain, Seraj Makkawi, and Shireen Qureshi received an honorarium for attending the advisory board meeting on which this article is based, but have no other conflicts to declare. Marina Ziehn and Hazem Wahba are employees of Novartis Pharma AG.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article.
Footnotes
Rituximab is not currently approved as a treatment for MS but is commonly used off-label for reducing relapse risk. Any mention of rituximab for the treatment of RRMS in this manuscript refers to the personal opinions of the experts/advisors and its off-label use in their clinical practice.
References
- 1.AlJumah M, Bunyan R, Al Otaibi H, et al. Rising prevalence of multiple sclerosis in Saudi Arabia, a descriptive study. BMC Neurol. 2020;20(1):49. doi: 10.1186/s12883-020-1629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etemadifar M, Nikanpour Y, Neshatfar A, Mansourian M, Fitzgerald S. Incidence and prevalence of multiple sclerosis in Persian Gulf area: a systematic review and meta-analysis. Mult Scler Relat Disord. 2020;40:101959. doi: 10.1016/j.msard.2020.101959. [DOI] [PubMed] [Google Scholar]
- 3.AlJumah M, Otaibi HA, Al Towaijri G, et al. Familial aggregation of multiple sclerosis: results from the national registry of the disease in Saudi Arabia. Mult Scler J Exp Transl Clin. 2020;6(4):2055217320960499. doi: 10.1177/2055217320960499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krieger SC, Sumowski J. New insights into multiple sclerosis clinical course from the topographical model and functional reserve. Neurol Clin. 2018;36(1):13–25. doi: 10.1016/j.ncl.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935–943. doi: 10.1016/j.apmr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Antel J, Antel S, Caramanos Z, Arnold DL, Kuhlmann T. Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol. 2012;123(5):627–638. doi: 10.1007/s00401-012-0953-0. [DOI] [PubMed] [Google Scholar]
- 7.Heydarpour P, Khoshkish S, Abtahi S, Moradi-Lakeh M, Sahraian MA. Multiple sclerosis epidemiology in Middle East and North Africa: a systematic review and meta-analysis. Neuroepidemiology. 2015;44(4):232–244. doi: 10.1159/000431042. [DOI] [PubMed] [Google Scholar]
- 8.Nazish S, Shahid R, Zafar A, et al. Clinical presentations and phenotypic spectrum of multiple sclerosis at a university hospital in Saudi Arabia. J Clin Neurol. 2018;14(3):359–365. doi: 10.3988/jcn.2018.14.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Abdullah MS, Siddiqui AF. Demographic and disease characteristics of multiple sclerosis in the Southwest Region of Saudi Arabia. Neurosciences (Riyadh) 2018;23(4):320–325. doi: 10.17712/nsj.2018.4.20180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moradi N, Sharmin S, Malpas C, et al. Utilization of multiple sclerosis therapies in the Middle East over a decade: 2009–2018. CNS Drugs. 2021;35:1097–1106. doi: 10.1007/s40263-021-00833-w. [DOI] [PubMed] [Google Scholar]
- 11.Buc M. New biological agents in the treatment of multiple sclerosis. Bratisl Lek Listy. 2018;119(4):191–197. doi: 10.4149/BLL_2018_035. [DOI] [PubMed] [Google Scholar]
- 12.Gensicke H, Leppert D, Yaldizli O, et al. Monoclonal antibodies and recombinant immunoglobulins for the treatment of multiple sclerosis. CNS Drugs. 2012;26(1):11–37. doi: 10.2165/11596920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307–316. doi: 10.1016/S1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen CS, Flemmen H, Broch L, et al. Early high efficacy treatment in multiple sclerosis is the best predictor of future disease activity over 1 and 2 years in a Norwegian population-based registry. Front Neurol. 2021;12:693017. doi: 10.3389/fneur.2021.693017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algahtani HA, Shirah BH, Alzahrani FA, Abobaker HA, Alghanaim NA, Manlangit JS., Jr Quality of life among multiple sclerosis patients in Saudi Arabia. Neurosciences (Riyadh) 2017;22(4):261–266. doi: 10.17712/nsj.2017.4.20170273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alhazzani AA, Alqahtani MS, Alahmari MS, et al. Quality of life assessment among multiple sclerosis patients in Saudi Arabia. Neurosciences (Riyadh) 2018;23(2):140–147. doi: 10.17712/nsj.2018.2.20170335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goksel Karatepe A, Kaya T, Gunaydn R, Demirhan A, Ce P, Gedizlioglu M. Quality of life in patients with multiple sclerosis: the impact of depression, fatigue, and disability. Int J Rehabil Res. 2011;34(4):290–298. doi: 10.1097/MRR.0b013e32834ad479. [DOI] [PubMed] [Google Scholar]
- 18.Miller A, Dishon S. Health-related quality of life in multiple sclerosis: the impact of disability, gender and employment status. Qual Life Res. 2006;15(2):259–271. doi: 10.1007/s11136-005-0891-6. [DOI] [PubMed] [Google Scholar]
- 19.Zwibel HL, Smrtka J. Improving quality of life in multiple sclerosis: an unmet need. Am J Manag Care. 2011;17(Suppl 5):S139–S145. [PubMed] [Google Scholar]
- 20.Hakim EA, Bakheit AM, Bryant TN, et al. The social impact of multiple sclerosis–a study of 305 patients and their relatives. Disabil Rehabil. 2000;22(6):288–293. doi: 10.1080/096382800296755. [DOI] [PubMed] [Google Scholar]
- 21.Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4(3):189–201. doi: 10.2165/11591150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331–341. doi: 10.1016/j.jns.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt S, Jostingmeyer P. Depression, fatigue and disability are independently associated with quality of life in patients with multiple sclerosis: results of a cross-sectional study. Mult Scler Relat Disord. 2019;35:262–269. doi: 10.1016/j.msard.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Bahathig A, Alblowi MA, Alhilali AA, et al. The prevalence and association of depression and anxiety with multiple sclerosis in Riyadh, Saudi Arabia: a cross-sectional study. Cureus. 2020;12(12):e12389. doi: 10.7759/cureus.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alhussain H, Aldayel AA, Alenazi A, Alowain F. Multiple sclerosis patients in Saudi Arabia: prevalence of depression and its extent of severity. Cureus. 2020;12(2):e7005. doi: 10.7759/cureus.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdulla FA, Albagmi FM, Al-Khamis FA. Factors that influence quality of life in patients with multiple sclerosis in Saudi Arabia. Disabil Rehabil. 2022;44(17):4775–4783. doi: 10.1080/09638288.2021.1919929. [DOI] [PubMed] [Google Scholar]
- 27.Gil-Gonzalez I, Martin-Rodriguez A, Conrad R, Perez-San-Gregorio MA. Quality of life in adults with multiple sclerosis: a systematic review. BMJ Open. 2020;10(11):e041249. doi: 10.1136/bmjopen-2020-041249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merkel B, Butzkueven H, Traboulsee AL, Havrdova E, Kalincik T. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Rev. 2017;16(6):658–665. doi: 10.1016/j.autrev.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Binquet C, Quantin C, Le Teuff G, Pagliano JF, Abrahamowicz M, Moreau T. The prognostic value of initial relapses on the evolution of disability in patients with relapsing-remitting multiple sclerosis. Neuroepidemiology. 2006;27(1):45–54. doi: 10.1159/000094380. [DOI] [PubMed] [Google Scholar]
- 30.Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175–187. doi: 10.1001/jama.2018.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson FR, Van Houtven G, Ozdemir S, et al. Multiple sclerosis patients' benefit-risk preferences: serious adverse event risks versus treatment efficacy. J Neurol. 2009;256(4):554–562. doi: 10.1007/s00415-009-0084-2. [DOI] [PubMed] [Google Scholar]
- 32.European Medicines Agency. Avonex (interferon beta-1a) summary of product characteristics. 2019. https://www.ema.europa.eu/en/documents/overview/avonex-epar-medicine-overview_en.pdf. Accessed 28 Aug 2021.
- 33.European Medicines Agency. Betaferon (interferon beta-1b) summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/betaferon-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 34.European Medicines Agency. Extavia (interferon beta-1b) summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/extavia-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 35.European Medicines Agency. Tysabri (natalizumab) summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/tysabri-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 36.European Medicines Agency. Kesimpta (ofatumumab) summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 37.European Medicines Agency. Ocrevus (ocrelizumab) summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 38.European Medicines Agency. Rebif (interferon beta-1a) summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/rebif-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 39.European Medicines Agency. Plegridy (pegylated interferon beta-1a) summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/plegridy-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 40.European Medicines Agency. Tecfidera (dimethyl fumarate) summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/tecfidera-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 41.European Medicines Agency. Mavenclad (cladribine) summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 42.European Medicines Agency. Lemtrada (alemtuzumab) summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 43.European Medicines Agency. Gilenya (fingolimod) summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/gilenya-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 44.European Medicines Agency. Aubagio (teriflunomide) summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/aubagio-epar-product-information_en.pdf. Accessed 28 Aug 2021.
- 45.US Food and Drug Administration. Copaxone (glatiramer acetate injection), for subcutaneous use. Prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020622s110lbl.pdf. Accessed 28 Aug 2021.
- 46.Remington G, Rodriguez Y, Logan D, Williamson C, Treadaway K. Facilitating medication adherence in patients with multiple sclerosis. Int J MS Care. 2013;15(1):36–45. doi: 10.7224/1537-2073.2011-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Correale J, Marrodan M, Ysrraelit MC. Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines. 2019;7(1):14. doi: 10.3390/biomedicines7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoonheim MM, Geurts JJ, Barkhof F. The limits of functional reorganization in multiple sclerosis. Neurology. 2010;74(16):1246–1247. doi: 10.1212/WNL.0b013e3181db9957. [DOI] [PubMed] [Google Scholar]
- 49.Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9(Suppl 1):S5–S48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Kern DM, Cepeda MS. Treatment patterns and comorbid burden of patients newly diagnosed with multiple sclerosis in the United States. BMC Neurol. 2020;20(1):296. doi: 10.1186/s12883-020-01882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kavaliunas A, Manouchehrinia A, Stawiarz L, et al. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult Scler. 2017;23(9):1233–1240. doi: 10.1177/1352458516675039. [DOI] [PubMed] [Google Scholar]
- 52.Stankiewicz JM, Weiner HL. An argument for broad use of high efficacy treatments in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2020;7(1):e636. doi: 10.1212/NXI.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008;7(9):852–858. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- 54.Reindl M, Linington C, Brehm U, et al. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain. 1999;122(Pt 11):2047–2056. doi: 10.1093/brain/122.11.2047. [DOI] [PubMed] [Google Scholar]
- 55.Disanto G, Morahan JM, Barnett MH, Giovannoni G, Ramagopalan SV. The evidence for a role of B cells in multiple sclerosis. Neurology. 2012;78(11):823–832. doi: 10.1212/WNL.0b013e318249f6f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol. 2012;8(11):613–623. doi: 10.1038/nrneurol.2012.203. [DOI] [PubMed] [Google Scholar]
- 57.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(Pt 9):2755–2771. doi: 10.1093/brain/awr182. [DOI] [PubMed] [Google Scholar]
- 58.Molnarfi N, Schulze-Topphoff U, Weber MS, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210(13):2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol. 2003;171(1):462–468. doi: 10.4049/jimmunol.171.1.462. [DOI] [PubMed] [Google Scholar]
- 60.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 61.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 62.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 63.Sorensen PS, Lisby S, Grove R, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology. 2014;82(7):573–581. doi: 10.1212/WNL.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 64.Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 65.Ginaldi L, De Martinis M, D'Ostilio A, Marini L, Quaglino D. Changes in antigen expression on B lymphocytes during HIV infection. Pathobiology. 1998;66(1):17–23. doi: 10.1159/000027990. [DOI] [PubMed] [Google Scholar]
- 66.Ginaldi L, De Martinis M, Matutes E, Farahat N, Morilla R, Catovsky D. Levels of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin Pathol. 1998;51(5):364–369. doi: 10.1136/jcp.51.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Florou D, Katsara M, Feehan J, Dardiotis E, Apostolopoulos V. Anti-CD20 agents for multiple sclerosis: spotlight on ocrelizumab and ofatumumab. Brain Sci. 2020;10(10):758. doi: 10.3390/brainsci10100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 69.Hawker K, O'Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 70.Naegelin Y, Naegelin P, von Felten S, et al. Association of rituximab treatment with disability progression among patients with secondary progressive multiple sclerosis. JAMA Neurol. 2019;76(3):274–281. doi: 10.1001/jamaneurol.2018.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klein C, Lammens A, Schafer W, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2013;5(1):22–33. doi: 10.4161/mabs.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Touil I, Perrot C, Elain G, Weckbecker G. Ofatumumab and ocrelizumab differentially induced human primary B-cell lysis by complement-dependent cytotoxicity [abstract LB325] Mult Scler J. 2019;25(Suppl 1):162–163. [Google Scholar]
- 73.Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177(1):362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 74.Wiendl H, Hauser SL, Bar-Or A et al., editors. Effect of ofatumumab on B-cell depletion and efficacy outcomes: subgroup analysis from the pooled phase 3 ASCLEPIOS I and II trials [abstract EPR3101 plus poster]. 6th Congress of the European Academy of Neurology; 2020 May 23–26; Virtual meeting.
- 75.Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab vs teriflunomide in relapsing multiple sclerosis: analysis of No Evidence of Disease Activity (NEDA-3) from ASCLEPIOS I and II trials [abstract LB62] Eur J Neurol. 2020;27(Suppl 1):1289–1290. [Google Scholar]
- 76.de Seze J, Bar-Or A, Correale J, et al. Effect of ofatumumab on serum immunoglobulin levels and infection risk in relapsing multiple sclerosis patients from the phase 3 ASCLEPIOS I and II trials [abstract LB82] Eur J Neurol. 2020;27(Suppl 1):1295–1296. [Google Scholar]
- 77.Hauser SL, Cross AH, Winthrop K, et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler. 2022;28(10):1576–1590. doi: 10.1177/13524585221079731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hauser SL, Cross AH, Winthrop K, et al. Long-term safety of ofatumumab in patients with relapsing multiple sclerosis (S14.004) Neurology. 2022;98(18 Supplement):2481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.