ABSTRACT
Aims/Introduction
Some women develop type 1 diabetes during pregnancy or immediately after delivery. However, the underlying pathophysiology remains largely unknown, probably because of the lack of a suitable animal model. In this study, we administered pregnant NOD mice with an anti‐CD25 antibody to reduce regulatory T cells along with poly I:C and examined the onset of diabetes.
Materials and Methods
Anti‐CD25 antibody and poly I:C were intraperitoneally administered to mated female NOD mice. Mice delivered within 3 weeks after the treatment, and the onset of diabetes during pregnancy or within 6 weeks after delivery was examined. Some mice were killed 1 week after treatment, and their spleen and pancreas were excised to examine the expression levels of cytokines and for histological examination.
Results
Half of the mice treated with anti‐CD25 antibody plus poly I:C developed diabetes, as compared with none of the poly I:C‐injected mice (P < 0.05). The ratios of interleukin‐18/forkhead box P3 and granzyme B/forkhead box P3 were higher in the pancreas of anti‐CD25 antibody plus poly I:C‐treated mice than in the pancreas of control mice. The insulitis score decreased in the pancreas of anti‐CD25 antibody plus poly I:C‐injected pregnant NOD mice.
Conclusions
We describe the use of anti‐CD25 antibody to reduce regulatory T cells and poly I:C as a Toll‐like receptor 3 stimulator to mimic viral infection in a pregnant NOD mouse, which can be used as a model of ‘pregnancy‐related’ type 1 diabetes.
Keywords: NOD mouse, Pregnancy, Type 1 diabetes
A system of anti‐CD25 antibody, to reduce regulatory T cells, plus poly I:C, as a TLR3 stimulator to mimic viral infection, in pregnant NOD mice can be used as a model of pregnancy‐related type 1 diabetes.

INTRODUCTION
Type 1 diabetes is an autoimmune disease that affects the pancreatic β‐cells. It is well known that some women develop type 1 diabetes during pregnancy or immediately after delivery. Most cases of type 1 diabetes during pregnancy are the fulminant type rather than the acute‐onset type 1 . If fulminant type 1 diabetes occurs during pregnancy, nearly 70% of fetuses die 1 . However, the underlying pathophysiology remains largely unknown, probably because of the lack of a suitable animal model.
We have previously reported that the reduction in the regulatory T‐cell population and the activation of Toll‐like receptor 3 (TLR3) through poly I:C to mimic viral infection in an autoimmune diabetes model, but not in a pregnant state, resulted in a phenotype similar to that of fulminant type 1 diabetes in humans 2 . Therefore, in the present study, we administered pregnant NOD mice with an anti‐CD25 antibody to reduce regulatory T cells along with poly I:C and examined the onset of diabetes.
MATERIALS AND METHODS
Mice
In the present study, 8‐ to 16‐week‐old NOD female mice were crossed with male NOD mice. One week after mating, an anti‐CD25 antibody (0.5 mg/mouse, PC‐61.5.3; BioCell, Lebanon, NH, USA) and poly I:C (200 μg/mouse; Sigma‐Aldrich, Tokyo, Japan) were intraperitoneally administered to mated female mice. In a previous study, a single injection of the anti‐CD25 antibody PC‐61 was found to reduce the proportion of regulatory T cells in vivo 3 . We confirmed that the splenic expression level of forkhead box P3 (Foxp3) was lower after single treatment with anti‐CD25 antibody than after control treatment (1.71 vs 6.64). Mice delivered within 3 weeks after the treatment, and the onset of diabetes during pregnancy or within 6 weeks after delivery was examined. Pregnant mice treated with poly I:C, but without anti‐CD25 antibody, served as the control group, because it is known that prior infection occurs in 70% of fulminant type 1 diabetes cases during or immediately after pregnancy in humans 1 . Pregnant NOD mice without any treatment or on only anti‐CD25 antibody treatment were also used as controls. Some mice were killed 1 week after treatment, and their spleen and pancreas were excised to examine cytokine expression levels. A portion of the resected pancreas was used for histological examination. To evaluate the pregnancy‐specific immunological changes that contribute to the type 1 diabetes phenotype, non‐pregnant age‐matched female NOD mice were used as controls.
All animals were maintained under specific pathogen‐free conditions in the Laboratory Animal Section of Saitama Medical University according to the guidelines for animal welfare (approval No.2979). After two consecutive positive readings of glucosuria based on a urine strip, blood glucose measurements were carried out using a blood glucose meter (Terumo, Shinjuku, Japan). Mice with a blood glucose concentration ≥250 mg/dL were considered to have diabetes.
Cytokine expression
Total ribonucleic acid was extracted from the pancreas and spleen using an RNeasy Mini Kit (Qiagen, Hilden, Germany). DNase treatment was carried out according to the manufacturer's protocol, as previously described 2 . The extracted ribonucleic acid was reverse‐transcribed using the Not I‐d(T)18 primer and a first‐strand complementary deoxyribonucleic acid synthesis kit according to the manufacturer's instructions 2 . Semi‐quantitative reverse‐transcriptase polymerase chain reaction (RT–PCR) was carried out for interferon (IFN)‐γ, interleukin (IL)‐2, IL‐4, IL‐10, IL‐18, IL‐12p40, transforming growth factor (TGF)‐β, Foxp3, granzyme B and glyceraldehyde 3‐phosphate dehydrogenase (internal control) using an ABI Prism 7,700 sequence detector (Applied Biosystems, Tokyo, Japan). Primer and probe sequences were used as previously described 2 . All reactions were carried out using TaqMan Universal MasterMix (Applied Biosystems). Messenger ribonucleic acid expression levels were normalized to that of the glyceraldehyde 3‐phosphate dehydrogenase PCR product amplified from the same sample ([sample PCR product/glyceraldehyde 3‐phosphate dehydrogenase PCR product] × constant).
Pancreatic histology
The pancreas harvested from each mouse was fixed in 3.7% paraformaldehyde and embedded in paraffin. Thin sections, 100‐μm apart, were stained with hematoxylin–eosin to grade insulitis and mononuclear cell infiltration into the exocrine area. Insulitis was graded according to the following criteria: grade 0, no insulitis; grade 1, peri‐insulitis with mononuclear cell infiltration in <20% of the area of each islet; grade 2, moderate insulitis with mononuclear cell infiltration in 20–50% of the area of each islet; and grade 3, severe insulitis with mononuclear cell infiltration in >50% of the area of each islet (Figure S1).
Mononuclear cell infiltration into the exocrine area was graded as follows: grade 0, no infiltration of the periductal region; grade 1, infiltration of several mononuclear cells in the periductal region; grade 2, mononuclear cells clustering in the periductal region, but not in the limiting plate; grade 3, focal acinar infiltration; and grade 4, widespread periductal and acinar infiltration or regeneration of ducts, as previously described 2 (Figure S2). Thin sections were stained with the following monoclonal antibodies to identify infiltrating cell types in the pancreas: anti‐mouse CD4 (H129.19; BD Biosciences), anti‐mouse CD8a (53–6.7), anti‐mouse CD19 (ID3), anti‐mouse CD11b (M1/70), anti‐mouse CD11c and natural killer group 2D (NKG2D; 191,004; R&D Systems, Minneapolis, MN, USA). The stained tissues were examined using fluorescence microscopy.
Statistical analysis
All statistical analyses were carried out using the IBM SPSS statistical software version 23 (SPSS Inc., Chicago, IL, USA). Results are presented as the mean ± standard error of the mean. Fisher's exact test was used to compare the incidence of diabetes and histological grades. Other mean values among the groups were compared using the Mann–Whitney U‐test or, in some instances, analysis of variance.
RESULTS
The incidence of diabetes increased in pregnant NOD mice injected with anti‐CD25 antibody plus poly I:C
First, we examined the onset of diabetes during pregnancy or 6 weeks after delivery. As shown in Figure 1, 50% (7/14) of mice injected with anti‐CD25 antibody plus poly I:C developed diabetes as compared with none of the mice injected with poly I:C alone (0/9, P < 0.05). Only one mouse developed diabetes during pregnancy after anti‐CD25 antibody plus poly I:C treatment; most mice developed diabetes after delivery.
Figure 1.

Diabetes incidence in treated pregnant NOD mice. Solid line: anti‐CD25 antibody + poly I:C injection group (n = 14); dotted line: poly I:C‐only injection group (n = 9). *P < 0.05. [Colour figure can be viewed at wileyonlinelibrary.com]
The ratios of IL‐18/Foxp3 and granzyme B/Foxp3 were higher in the pancreas of pregnant NOD mice injected with anti‐CD25 antibody plus poly I:C
We then examined the cytokine expression levels in the pancreas of anti‐CD25 antibody plus poly I:C‐injected mice 1 week after treatment and compared them with those from poly I:C‐injected mice without anti‐CD25 antibody. As shown in Figure 2, the ratio of IL‐18/Foxp3 ratio was higher in the pancreas of anti‐CD25 antibody plus poly I:C‐treated mice than in the pancreas of poly I:C‐injected mice (1.22 ± 0.07 vs 0.98 ± 0.09, P = 0.06). Furthermore, a significantly higher granzyme B/Foxp3 ratio was observed in the pancreas of anti‐CD25 antibody plus poly I:C‐treated mice than in the pancreases of poly I:C‐injected mice (1.13 ± 0.14 vs 0.66 ± 0.09, P < 0.05). However, IL‐18, granzyme B and Foxp3 expression levels in the pancreas were not significantly different between the two groups. Considering the pancreatic expression of other cytokines, IL‐4 level in the anti‐CD25 antibody plus poly I:C treatment group was significantly lower than that in the poly I:C treatment group (0.99 ± 0.25 vs 1.53 ± 0.73, P < 0.05), whereas no significant difference was observed in the expression levels of other cytokines examined (Table 1).
Figure 2.
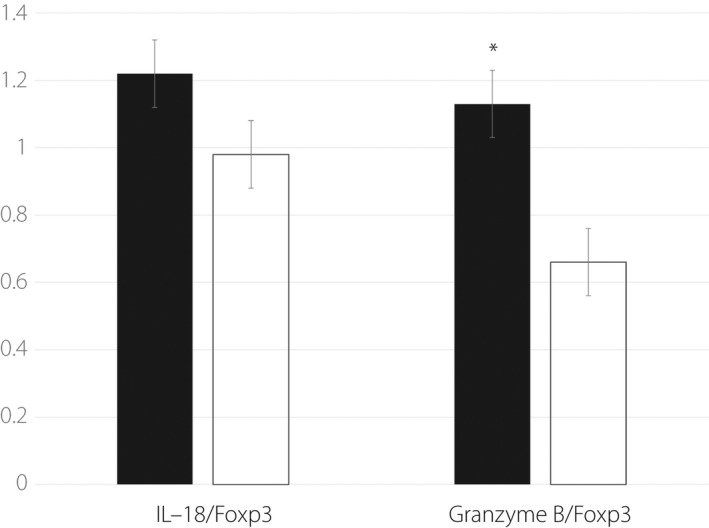
Interleukin (IL)‐18/forkhead box P3 (Foxp3) and granzyme B/Foxp3 ratios in the pancreas of treated pregnant NOD mice. Closed bar: anti‐CD25 antibody + poly I:C injection group (n = 5); open bar: poly I:C only injection group (n = 5). *P < 0.05.
Table 1.
Cytokine expression levels in treated pregnant NOD mice
| IFN‐gamma | IL‐2 | IL‐4 | IL‐10 | IL‐18 | IL‐12 p40 | TGF‐beta | Foxp3 | Granzyme B | |
|---|---|---|---|---|---|---|---|---|---|
| Pancreas | |||||||||
| Anti‐CD25 Ab + poly (I:C) | 4.45 ± 3.61 | 3.16 ± 1.91 | 0.99 ± 0.25* | 2.15 ± 0.82 | 2.10 ± 1.03 | 1.37 ± 1.06 | 1.60 ± 0.85 | 1.66 ± 0.82 | 2.24 ± 1.24 |
| Poly (I:C) only | 0.63 ± 0.47 | 1.20 ± 0.73 | 1.53 ± 0.73 | 0.36 ± 0.20 | 0.50 ± 0.29 | 0.57 ± 0.39 | 0.36 ± 0.22 | 0.53 ± 0.31 | 0.43 ± 0.28 |
| Spleen | |||||||||
| Anti‐CD25 Ab + poly (I:C) | 1.03 ± 0.70 | 2.42 ± 1.57 | 52.2 ± 32,5 | 0.48 ± 0.14 | 0.97 ± 0.19* | 19.0 ± 14.7 | 0.93 ± 0.08* | 2.65 ± 1.26 | 1.73 ± 0.44 |
| Poly (I:C) only | 0.21 ± 0.08 | 0.58 ± 0.22 | 24.5 ± 18.4 | 0.29 ± 0.06 | 0.41 ± 0.07 | 10.8 ± 9.15 | 0.45 ± 0.04 | 1.38 ± 0.62 | 0.87 ± 0.24 |
P < 0.05. Ab, antibodies; Foxp3, forkhead box 3; IFN, interferon; IL, interleukin; TGF, transforming growth factor.
In contrast, no significant difference was observed in the ratios of IL‐18/Foxp3 and granzyme B/Foxp3 in the spleen from anti‐CD25 antibody plus poly I:C‐treated mice and poly I:C‐treated mice (0.67 ± 0.23 vs 0.49 ± 0.13, 0.93 ± 0.20 vs 0.80 ± 0.15, respectively). The levels of IL‐18 and TGF‐β were significantly higher in anti‐CD25 antibody plus poly I:C‐injected mice than in poly I:C‐injected mice (0.97 ± 0.19 vs 0.41 ± 0.07, 0.93 ± 0.08 vs 0.45 ± 0.04, P < 0.05, respectively, Table 1).
As shown in Table 2, there were no significant differences in the expression levels of the splenic cytokines analyzed, except for IL‐18, between anti‐CD25 antibody plus poly I:C‐treated non‐pregnant NOD mice and poly I:C‐treated non‐pregnant NOD mice. Furthermore, no significant difference was observed in the ratios of IL‐18/Foxp3 and granzyme B/Foxp3 in the pancreas and spleen between these two groups (pancreas 0.73 ± 0.29 vs 0.51 ± 0.10 and 0.73 ± 0.17 vs 1.00 ± 0.14; spleen 1.11 ± 0.14 vs 1.41 ± 0.24 and 2.00 ± 0.50 vs 1.52 ± 0.36, respectively). These results suggest that the immunological changes observed in pregnant NOD mice are pregnancy specific.
Table 2.
Cytokine expression levels in treated non‐pregnant NOD mice
| IFN‐gamma | IL‐2 | IL‐4 | IL‐10 | IL‐18 | IL‐12 p40 | TGF‐beta | Foxp3 | Granzyme B | |
|---|---|---|---|---|---|---|---|---|---|
| Pancreas | |||||||||
| Anti‐CD25 Ab + poly (I:C) | 19.5 ± 8.42 | 3.44 ± 2.39 | 6.42 ± 4.02 | 5.02 ± 4.44 | 5.76 ± 5.00 | 8.36 ± 4.23 | 2.98 ± 0.85 | 4.50 ± 2.90 | 3.05 ± 1.91 |
| Poly (I:C) only | 14.0 ± 2.20 | 2.15 ± 0.49 | 3.00 ± 0.88 | 1.23 ± 0.16 | 1.03 ± 0.19 | 5.92 ± 0.68 | 1.38 ± 0.15 | 2.04 ± 0.29 | 2.11 ± 0.50 |
| Spleen | |||||||||
| Anti‐CD25 Ab + poly (I:C) | 0.76 ± 0.10 | 0.79 ± 0.26 | 25.2 ± 16.7 | 1.02 ± 0.24 | 0.77 ± 0.08* | 4.60 ± 2.70 | 0.71 ± 0.05 | 0.75 ± 0.14 | 1.32 ± 0.14 |
| Poly (I:C) only | 1.32 ± 0.20 | 0.76 ± 0.15 | 2.84 ± 0.63 | 1.31 ± 0.35 | 1.38 ± 0.11 | 1.66 ± 0.21 | 0.79 ± 0.07 | 1.04 ± 0.09 | 1.47 ± 0.19 |
P < 0.05. Ab, antibodies; Foxp3, forkhead box 3; IFN, interferon; IL, interleukin; TGF, transforming growth factor.
The insulitis score decreased in the pancreas of pregnant NOD mice injected with anti‐CD25 antibody plus poly I:C
We then examined the pancreatic histology of anti‐CD25 antibody plus poly I:C‐injected mice 1 week after treatment, and compared it with that of the poly I:C‐injected mice. The insulitis score was lower in the pancreas of mice from the anti‐CD25 antibody plus poly I:C treatment group than in the pancreas of mice injected with poly I:C only (1.11 ± 0.25 vs 1.84 ± 0.25, P < 0.05; Figure 3, Figure S3).
Figure 3.

Lower insulitis score observed in the pancreas of anti‐CD25 antibody plus poly I:C‐injected pregnant NOD mice. *P < 0.05.
In non‐pregnant NOD female mice, the insulitis score in the pancreas was lower for those injected with anti‐CD25 antibody plus poly I:C than for the mice injected with poly I:C only (1.06 ± 0.15 vs 1.68 ± 0.13, P < 0.05).
Considering the degree of mononuclear cell infiltration into the exocrine area, no significant difference was observed between the two groups (2.2 ± 0.4 vs 2.2 ± 0.7; Figure S4).
Among the non‐pregnant NOD female mice, the degree of mononuclear cell infiltration into the exocrine area of the pancreas was significantly lower in the anti‐CD25 antibody plus poly I:C injection group than in the poly I:C only injection group (0.50 ± 0.29 vs 2.20 ± 0.20, P < 0.05).
To quantify each cell type, we examined the proportion of different cell types that occupied >50% of the islets using immunohistochemistry. There was no significant difference between anti‐CD25 antibody + poly I:C‐treated pregnant NOD mice and poly I:C‐treated pregnant NOD mice in the population of different cell types (CD4 7.1 vs 30.0%; CD8 7.1 vs 0.0%; CD19 7.1 vs 30.0%; CD11b 0.0 vs 0.0%; CD11c 7.1 vs 30.0%; NK 0.0 vs 0.0%), although CD8 T cells were only observed in anti‐CD25 antibody + poly I:C‐treated pregnant NOD mice. In non‐pregnant NOD female mice also, no significant difference was observed in the levels of different cell types between the anti‐CD25 antibody + poly I:C and poly I:C treatment groups (CD4 9.1 vs 0.0%; CD8 18.2 vs 18.2%; CD19 18.2 vs 18.2%; CD11b 0.0 vs 0.0%; CD11c 18.2 and 18.2%; NK 0.0 vs 0.0%), although CD4 T cells were detected only in anti‐CD25 antibody + poly I:C‐treated non‐pregnant NOD mice.
Role of poly I:C in pregnancy‐related diabetes in NOD mice
To clarify the role of poly I:C in pregnancy‐related diabetes in NOD mice, we compared the incidence of diabetes between poly I:C‐treated pregnant NOD mice (dotted line in Figure S5) and pregnant NOD mice without treatment (solid line in Figure S5). As shown in Figure S5, the incidence of diabetes was higher in untreated pregnant NOD mice (37.5%; P = 0.057 by Fischer's exact test) at 6 weeks post‐delivery. The insulitis score was higher in poly I:C‐treated pregnant NOD mice than in untreated pregnant NOD mice (Figure S6; P = 0.07). We examined the proportion of each cell type that occupied >50% of the islets. CD4 and CD8 T cells were observed in 30.0 and 0.0% of the islets from the poly I:C‐treated pregnant NOD mice, and 45.5and 45.5% of the islets from the untreated pregnant NOD mice, respectively. No CD8 T cells were detected in the poly I:C‐treated pregnant NOD mice. Furthermore, the ratios of IL‐18/Foxp3 and granzyme B/Foxp3 in the pancreas were significantly higher in untreated pregnant NOD mice than in poly I:C‐treated pregnant NOD mice (Figure S7; P < 0.05 and P < 0.01, respectively), although the expression levels of IL‐18, granzyme B and Foxp3 in the pancreas were not significantly different between the groups (Table S1). These results suggest that poly I:C treatment in pregnant NOD mice induces a regulatory dominant response in the pancreas, resulting in the suppression of diabetes onset.
We then compared the incidence of diabetes between anti‐CD25 antibody plus poly I:C‐treated pregnant NOD mice and anti‐CD25 antibody‐treated pregnant NOD mice. As shown in Figure S8, although not significant, the incidence of diabetes was higher in the anti‐CD25 antibody plus poly I:C treatment group (50.0%) than in the anti‐CD25 antibody only treatment group (28.6%) at 6 weeks post‐delivery. There was no significant difference in the insulitis score between the two groups (Figure S9). We examined the proportion of each T‐cell type that occupied >50% of the islets. CD4 and CD8 T cells were observed in 7.1 and 7.1% of the islets from the anti‐CD25 antibody + poly I:C treatment group, and 46.7 and 46.7% of the islets from the anti‐CD25 antibody treatment group, respectively (P < 0.05). However, a significantly higher IFN‐γ/IL‐4 ratio in the pancreas was observed in the mice treated with anti‐CD25 antibody + poly I:C than in those treated with only anti‐CD25 antibody (Figure S10; P < 0.05). There was no significant difference in cytokine expression levels between the two groups (Table S2).
These results suggest that poly I:C treatment in anti‐CD25 antibody‐injected pregnant NOD mice seems to induce a higher Th1/Th2 dominant response in the pancreas, resulting in the acceleration of diabetes onset as compared to anti‐CD25 antibody injection.
DISCUSSION
In some cases, new‐onset type 1 diabetes is reported during pregnancy or after delivery; however, the etiology remains largely unknown, probably due to the lack of a suitable animal model. Here, we describe the use of an anti‐CD25 antibody to reduce the number of regulatory T cells and use of poly I:C as a TLR3 stimulator to mimic a viral infection in pregnant NOD mice.
In the present study, we injected an anti‐CD25 antibody into pregnant NOD mice only once; one may argue that the reduction in the regulatory T cell population might be transient. It has been reported that the reduction in the number of regulatory T cells by anti‐CD25 antibodies might last approximately 2–3 weeks 4 . Therefore, we believe that it might be sufficient to change the balance between effector T cells and regulatory T cells during pregnancy, because the pregnancy period in mice is <3 weeks.
In poly I:C‐only injected pregnant NOD mice without anti‐CD25 antibody, no onset of diabetes was observed. Poly I:C has been reported to induce both effector and regulatory T cells in NOD mice 3 . We assumed that TLR3 stimulation without reduction in the population of regulatory T cells can mediate a protective effect against diabetes onset; therefore, the effect of poly I:C injection and virus‐like stimulation in this model might have induced a dominant response in the regulatory T‐cell function. As described in the Results section, we compared the cytokine profiles in the pancreas between pregnant NOD mice treated with poly I:C and untreated pregnant NOD mice, and observed poly I:C treatment‐induced regulatory dominant response in the pancreas of pregnant NOD mice.
In human fulminant type 1 diabetes patients during pregnancy, 60–70% of patients experience a prior infection. Considering the data obtained herein, we believe that the reduction in regulatory T cells might be important for inducing diabetes onset after viral infection during pregnancy. We cannot conclude that an active infection state can induce a reduction in regulatory T‐cell population or that the primarily reduced regulatory T‐cell number and/or function might be important to cause diabetes onset in the pregnant state, which should be addressed in a future study.
In the present study, we observed higher IL‐18/Foxp3 and granzyme B/Foxp3 ratios in the pancreas of poly I:C‐injected pregnant NOD mice treated with anti‐CD25 antibody, but this was not the case in non‐pregnant NOD mice. This result suggests that our observation in this system is a pregnancy‐specific phenomenon. In our previous report 2 , higher IL‐18 and granzyme B levels were observed in the pancreas of a regulatory T cell‐deficient CD28 knockout NOD mouse, which represents the fulminant type 1 diabetes‐like phenotype in humans. Regarding IL‐18, we have previously reported that systemic induction of IL‐18 in NOD mice results in diabetes progression 5 . We believe that IL‐18, granzyme B and Foxp3 are the key factors in both pregnancy and non‐pregnancy states; however, the balance rather than the factors themselves seems to be important during pregnancy.
We observed higher expression of TGF‐β in the spleen, but not in the pancreas of poly I:C‐injected pregnant NOD mice with anti‐CD25 antibody. The immune regulation by TGF‐β is important in local lesions 6 , and the suppression of TGF‐β signaling results in β‐cell regeneration 7 . β‐Cell proliferation (or replication) is important during pregnancy 8 ; therefore, we assumed that TGF‐β might suppress β‐cell proliferation in this system and contribute to diabetes onset, although further investigation is warranted to confirm this hypothesis.
A lower insulitis score was observed in poly I:C‐injected pregnant NOD mice treated with anti‐CD25 antibody. This observation along with the higher IL‐18/Foxp3 and granzyme B/Foxp3 ratios, and lower IL‐4 expression in the pancreas of anti‐CD25 + poly I:C‐injected pregnant NOD mice than in poly I:C‐only injected pregnant NOD mice indicated that the regulatory (and/or suppressor) function seemed to be dominant in the pancreas of only poly I:C‐injected pregnant NOD mice.
To investigate the role of poly I:C in this model, we compared poly I:C‐treated pregnant NOD mice with untreated pregnant NOD mice, and found a higher incidence of diabetes in untreated pregnant NOD mice. Furthermore, the ratios of IL‐18/Foxp3 and granzyme B/Foxp3 in the pancreas were significantly higher for the untreated group than for the poly I:C‐treated group. Therefore, poly I:C treatment in pregnant NOD mice seems to induce a regulatory dominant response in the pancreas, resulting in the suppression of diabetes onset. In contrast, we compared the incidence of diabetes in pregnant NOD mice treated with either anti‐CD25 antibody plus poly I:C or anti‐CD25 antibody only. Although not significantly different, the incidence of diabetes was higher in the anti‐CD25 antibody plus poly I:C group than in the anti‐CD25 antibody group at 6 weeks after delivery. Furthermore, a significantly higher IFN‐γ/IL‐4 ratio was observed in the pancreas of the mice from the anti‐CD25 antibody plus poly I:C treatment group than in the pancreas of the anti‐CD25 antibody only treatment group. Thus, poly I:C treatment along with anti‐CD25 antibody injection in pregnant NOD mice seems to induce a higher Th1/Th2 dominant response in the pancreas, as compared with anti‐CD25 antibody‐only treatment. This phenomenon seems to accelerate the onset of diabetes.
As aforementioned, poly I:C treatment resulted in paradoxical outcomes with or without anti‐CD25 antibody treatment. It was previously suggested that a viral mimic (poly I:C treatment) could either suppress or promote the development of autoimmune diabetes, depending on the model system 9 . Therefore, we believe that both anti‐CD25 antibody and poly I:C treatment are necessary to accelerate autoimmunity during pregnancy.
In the current study, very few mice developed diabetes during pregnancy; in most mice, the onset of diabetes was observed after delivery. Therefore, the current system might serve as a model of ‘postpartum’ type 1 diabetes. We are now using CD28 knockout NOD mice that have very few regulatory T cells to establish a system specific for type 1 diabetes during pregnancy. Our current pilot data showed that the incidence of diabetes in poly I:C‐treated pregnant CD28 knockout NOD mice within 6 weeks post‐delivery (within 10 weeks post‐poly I:C treatment) was 85.7% (6/7), and five out of six (83.3%) mice developed diabetes during pregnancy (unpublished data). The combination of this NOD system and the CD28 knockout NOD system will provide clues regarding the pathophysiology of type 1 diabetes onset during pregnancy and after delivery.
DISCLOSURE
The authors declare no conflict of interest.
Approval of research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: animals were maintained under specific pathogen‐free conditions in the Laboratory Animal Section of Saitama Medical University, according to the guidelines for animal welfare (approval No.2979).
Supporting information
Figure S1 | Grading of insulitis.
Figure S2 | Grading of mononuclear cell infiltration into the exocrine area.
Figure S3 | Distribution of insulitis score.
Figure S4 | Distribution of mononuclear cell infiltration into the exocrine area.
Figure S5 | Incidence of diabetes in treated pregnant NOD mice. Solid line: untreated group (n = 16); dotted line: poly I:C‐only injected group (n = 9). *P = 0.057.
Figure S6 | A lower insulitis score was observed in the pancreas of pregnant NOD mice without treatment than in the pancreas of those treated with poly I:C only. *P = 0.07.
Figure S7 | Interleukin‐18/Foxp3 and granzyme B/Foxp3 ratios in the pancreas of pregnant NOD mice. Closed bar: untreated group (n = 5); open bar: poly I:C only injection group (n = 5). *P < 0.05, **P < 0.01.
Figure S8 | Incidence of diabetes in treated pregnant NOD mice. Solid line: anti‐CD25 antibody only injection group (n = 14); dotted line: anti‐CD25 antibody plus poly I:C injection group (n = 14).
Figure S9 | A similar insulitis score was observed in the pancreas from anti‐CD25 antibody plus poly I:C‐injected pregnant NOD mice and anti‐CD25 antibody‐injected pregnant NOD mice.
Figure S10 | Interferon‐γ/interleukin‐4 (IFNγ/IL‐4) ratio in the pancreas of pregnant NOD mice. Closed bar: anti‐CD25 antibody plus poly I:C injection group (n = 5); open bar: anti‐CD25 antibody injection group (n = 5). *P < 0.05.
Figure S11 | Representative figure of the immunohistochemistry.
Table S1 | Cytokine expression levels in pregnant NOD mice. *P < 0.05.
Table S2 | Cytokine expression levels in treated pregnant NOD mice.
ACKNOWLEDGMENTS
Part of this work was supported by Saitama Medical University. We thank Ms Tokuko Suzuki for her technical assistance.
REFERENCES
- 1. Shimizu I, Makino H, Osawa H, et al. Association of fulminant type 1 diabetes with pregnancy. Diabetes Res Clin Pract 2003; 62: 33–38. [DOI] [PubMed] [Google Scholar]
- 2. Tada A, Shimada A, Yamada T, et al. A mimic of viral double‐stranded RNA triggers fulminant type 1 diabetes‐like syndrome in regulatory T cell‐deficient autoimmune diabetic mouse. J Immunol 2011; 187: 4947–4953. [DOI] [PubMed] [Google Scholar]
- 3. Fukushima K, Abiru N, Nagayama Y, et al. Combined insulin B:9‐23 self‐peptide and polyinosinic‐polycytidylic acid accelerate insulitis but inhibit development of diabetes by increasing the proportion of CD4+Foxp3+ regulatory T cells in the islets in non‐obese diabetic mice. Biochem Biophys Res Commun 2008; 367: 719–724. [DOI] [PubMed] [Google Scholar]
- 4. Ellis JS, Wan X, Braley‐Mullen H. Transient depletion of CD4+ CD25+ regulatory T cells results in multiple autoimmune diseases in wild‐type and B‐cell‐deficient NOD mice. Immunology 2013; 139: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oikawa Y, Shimada A, Kasuga A, et al. Systemic administration of IL‐18 promotes diabetes development in young nonobese diabetic mice. J Immunol 2003; 171: 5865–5875. [DOI] [PubMed] [Google Scholar]
- 6. Grewal IS, Grewal KD, Wong FS, et al. Expression of transgene encoded TGF‐beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun 2002; 19: 9–22. [DOI] [PubMed] [Google Scholar]
- 7. Dhawan S, Dirice E, Kulkarni RN, et al. Inhibition of TGF‐β Signaling promotes human pancreatic β‐cell replication. Diabetes 2016; 65: 1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 2010; 16: 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong FS, Wen L. IFN‐alpha can both protect against and promote the development of type 1 diabetes. Ann N Y Acad Sci 2008; 1150: 187–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Grading of insulitis.
Figure S2 | Grading of mononuclear cell infiltration into the exocrine area.
Figure S3 | Distribution of insulitis score.
Figure S4 | Distribution of mononuclear cell infiltration into the exocrine area.
Figure S5 | Incidence of diabetes in treated pregnant NOD mice. Solid line: untreated group (n = 16); dotted line: poly I:C‐only injected group (n = 9). *P = 0.057.
Figure S6 | A lower insulitis score was observed in the pancreas of pregnant NOD mice without treatment than in the pancreas of those treated with poly I:C only. *P = 0.07.
Figure S7 | Interleukin‐18/Foxp3 and granzyme B/Foxp3 ratios in the pancreas of pregnant NOD mice. Closed bar: untreated group (n = 5); open bar: poly I:C only injection group (n = 5). *P < 0.05, **P < 0.01.
Figure S8 | Incidence of diabetes in treated pregnant NOD mice. Solid line: anti‐CD25 antibody only injection group (n = 14); dotted line: anti‐CD25 antibody plus poly I:C injection group (n = 14).
Figure S9 | A similar insulitis score was observed in the pancreas from anti‐CD25 antibody plus poly I:C‐injected pregnant NOD mice and anti‐CD25 antibody‐injected pregnant NOD mice.
Figure S10 | Interferon‐γ/interleukin‐4 (IFNγ/IL‐4) ratio in the pancreas of pregnant NOD mice. Closed bar: anti‐CD25 antibody plus poly I:C injection group (n = 5); open bar: anti‐CD25 antibody injection group (n = 5). *P < 0.05.
Figure S11 | Representative figure of the immunohistochemistry.
Table S1 | Cytokine expression levels in pregnant NOD mice. *P < 0.05.
Table S2 | Cytokine expression levels in treated pregnant NOD mice.


