ABSTRACT
Aims/Introduction
Gestational diabetes (GDM) is characterized by low‐grade systemic inflammation, which manifests as changes in the levels of cytokines in the blood. We aimed to investigate plasma immune mediators during gestational weeks 23–28 in 213 women at risk for GDM, and to find associations between GDM and its complications.
Materials and Methods
We quantified the levels of adipokines: adiponectin, leptin, plasminogen activator inhibitor‐1 and resistin; chemokines: C‐C motif chemokine ligand 2 (CCL2), CCL4, C‐X‐C motif chemokine ligand 8 (CXCL8) and CXCL10; and cytokines: granulocyte‐macrophage colony‐stimulating factor, interferon‐γ, interleukin (IL)‐1β, soluble (s)IL‐1RI, IL‐2, sIL‐2Ra, IL‐4, IL‐5, IL‐6, IL‐7, IL‐10, IL‐12(p70), IL‐13, IL‐15, IL‐17A, IL‐17F, IL‐21, IL‐22, IL‐23, IL‐27, transforming growth factor (TGF)‐β1, TGF‐β2, TGF‐β3, tumor necrosis factor‐α and soluble tumor necrosis factor receptor 2 using the Milliplex®MAP Magnetic Bead assay on Luminex®200™, and compared the results with clinical data from pregnancy and post‐partum follow up.
Results
Lower levels of adiponectin and higher levels of CCL2 (Wilcoxon test, P = 3.4E‐03 and P = 0.03, respectively) were found in women with GDM. IL‐27 levels were associated with lower odds of GDM (adjusted logistic regression 0.90, P = 2.4E‐03), and showed a risk association with glutamic acid decarboxylase autoantibody positivity (adjusted odds ratio 1.13, P = 2.8E‐03). Similarly, higher IL‐22 levels increased the odds of glutamic acid decarboxylase autoantibody positivity (adjusted odds ratio 4.23, P = 0.04). TGF‐β1 was associated with post‐partum fasting glucose levels, and CCL4 with post‐partum C‐peptide levels (linear regression, P = 0.04 and P = 0.01, respectively). Women who developed pregnancy complications had higher levels of CXCL10 and CCL4 (linear regression, P = 7.0E‐04 and P = 0.01, respectively).
Conclusions
Plasma adiponectin and CCL2 concentrations distinguish women with GDM. IL‐27 and IL‐22 levels might select women with an autoimmune reaction, whereas increased TGF‐β1 and CCL4 are associated with post‐partum glucose and insulin metabolism.
Keywords: Cytokines, Gestational diabetes, Plasma
33 plasma immune mediators during gestational weeks 23‐28 in women at risk for gestational diabetes (GDM) were measured and their levels were compared to find associations between GDM development and its complications during delivery and also during post‐partum follow‐up. We found that IL‐27 and IL‐22 levels may select women with an autoimmune reaction, while increased TGF‐β1 and CCL4 are associated with post‐partum glucose and insulin metabolism.

INTRODUCTION
Gestational diabetes (GDM) is currently the most common complication of pregnancy, affecting approximately 16.7% of total live births with an increasing trend 1 . It poses multiple threats to mother and child during pregnancy, and also impacts their life afterwards. GDM increases the risk for premature delivery, pre‐eclampsia and birth of a macrosomic child. Additionally, GDM poses an increased risk for developing diabetes later in life 2 .
Obesity, previous birth of a macrosomic child or previous GDM are the main risk factors for GDM development during ongoing pregnancy. GDM is diagnosed using a glucose tolerance test (GTT) during the second trimester of pregnancy. However, a major problem connected with GDM diagnosis is the lack of consensus for the diagnostic criteria for GDM or treatment regimens 3 . Diagnosing GDM according to the International Association of Diabetes and Pregnancy Study Groups criteria, combined with advice on diet and exercise, has led to overall reduction in the incidence of serious pregnancy complications due to hyperglycemia. However, this has increased the number of women who have to be under close surveillance, which represents a growing burden on the public healthcare systems 4 . Searching for GDM biomarkers and identification of risk factors for pregnancy complications or future diabetes development have been suggested as the highest priority research areas in this field 5 .
There is increasing evidence about immune system dysregulation in GDM characterized by chronic low‐grade systemic inflammation 6 , 7 , 8 . Elevated levels of cytokines during pregnancy can worsen glucose intolerance and increase the risk for adverse perinatal outcome 9 . Most studies in this field have focused mainly on comparing women with GDM with healthy pregnant women. However, increased pro‐inflammatory milieu, characteristic to GDM patients, might instead be associated with GDM risk factors, and thereby mask the true relationships between cytokines and GDM 10 , 11 . Therefore, the aim of the present study was to compare women with GDM risk factors and to assess differences in a wide panel of peripheral blood immune mediators in regard to GDM diagnosis, as well as associations between pregnancy and post‐partum complications.
MATERIALS AND METHODS
Study population
The study was approved by the Ethics Review Committee on Human Research of the University of Tartu (Estonia; 229/M‐16, 23.09.2013 and 254/M‐16, 21.12.2015) and complied with the documents of the Declaration of Helsinki. Written informed consent was obtained from all participants.
A cohort of 213 Caucasian women with singleton pregnancy from the Women's Clinic of Tartu University Hospital, Estonia, between November 2013 and March 2015 were consecutively recruited in the study. All participants belonged to the GDM risk group according to the Estonian Gynecologists' Society guidelines, with at least one of the following: pre‐pregnancy body mass index ≥30 kg/m2; history of GDM; history of impaired glucose tolerance; history of unexplained fetal death; first‐degree relative with diabetes; previous delivery of a newborn >4,500 g; and history of polycystic ovary syndrome 12 . The participants were referred to a 2‐h GTT test with 75 g glucose after overnight fast at gestational weeks 23–28. A total of 60 women were diagnosed with GDM based on the International Association of Diabetes and Pregnancy Study Groups Consensus Panel criteria 13 . The remaining 153 women formed the non‐GDM group who served as the reference group. Pre‐existing diabetes was excluded by detection of normal fasting serum glucose level (≤6.0 mmol/L) during the first trimester of pregnancy. Women who showed symptoms of an ongoing active infection or self‐reported a recent infection within the past month were excluded from the study. Gestational age was determined by ultrasound. At the time of recruitment, all women were asked to participate in the follow‐up study. Post‐partum samples were obtained from 202 (94.8%) participants, 49 with GDM, during a visit to the Women's Clinic at 6 weeks up to 1 year after delivery.
To minimize the circadian variation in immune mediator levels, all peripheral blood samples were collected from overnight fast blood in the morning of the same day of the GTT. The blood samples were processed within the subsequent 2 h and stored at −40°C for serum or −80°C for plasma until further analysis. The blood chemistry tests at both time points were measured using Cobas p 501 (Roche, Rotkreuz, Switzerland) for blood glucose levels, Cobas e 601 for C‐peptide levels and Cobas c 501 for other analytes at the United Laboratories of Tartu University Hospital. The other analytes were C‐reactive protein, cholesterol (including high‐density lipoprotein and low‐density lipoprotein) and triglycerides. Cholesterol and triglycerides were only measured from post‐partum samples.
Detection of autoantibodies
Diabetes‐related autoantibodies against glutamic acid decarboxylase 65 kDa (GADA, islet cell antigen 2 and zinc transporter 8 were detected in pregnancy serum samples using commercial enzyme‐linked immunosorbent assay kits (RSR Ltd., Cardiff, UK) according to the manufacturer's instructions. The cut‐off levels were 5 U/mL for glutamic acid decarboxylase autoantibodies, 7.5 U/mL for islet cell antigen 2 autoantibodies and 15 U/mL for zinc transporter 8. Our laboratory participated with the autoantibody tests used in the present study in the Islet Autoantibody Standardization Program (IASP).
Immune mediator measurements
Ethylenediaminetetraacetic acid‐treated blood plasma was used to measure the level of immune mediators in multiplex analysis, using the Milliplex®MAP Magnetic Bead assay according to the manufacturer's recommendation (Millipore, Billerica, MA, USA). Altogether, 33 analytes were measured by using the following seven assays: (i) Adipokine Magnetic Bead Panel 1 for detecting adiponectin, resistin and plasminogen activator inhibitor‐1 (PAI‐1); (ii) Adipokine Magnetic Bead Panel 2 for leptin; (iii) Cytokine/Chemokine Magnetic Bead Panel for C‐C motif chemokine ligand 2 (CCL2; monocyte chemoattractant protein‐1), CCL4 (macrophage inflammatory protein‐1β) and C‐X‐C motif chemokine ligand 10 (CXCL10; IP‐10); (iv) High Sensitivity T Cell Magnetic Bead Panel for granulocyte‐macrophage colony‐stimulating factor, interferon‐γ, interleukin (IL)‐1β, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, CXCL8 (IL‐8), IL‐10, IL‐12(p70), IL‐13, IL‐17A, IL‐21, IL‐23 and tumor necrosis factor (TNF)α; (v) Soluble Cytokine Receptor Magnetic Bead Panel for soluble (s)IL‐1RI, sIL‐2Rα and soluble TNFRII; (vi) Th17 Magnetic Bead Panel for IL‐15, IL‐17F, IL‐22 and IL‐27; and (vii) transforming growth factor (TGF)‐β 1,2,3 Magnetic Bead Kit for measuring the activated forms of TGF‐β1, TGF‐β2 and TGF‐β3. The kits used allowed to obtain the intra‐assay variation coefficients of <10% and inter‐assay variation coefficients of <20% for all assays. The xMAP Technology in Luminex 200™ (Luminex Corp., Austin, TX, USA) was used for detection in accordance with the manufacturer's protocols. The minimal detection limit varied for each cytokine and ranged from 0.2 to 60 pg/mL. A five‐point standard curve was generated on each plate.
Statistical analysis
Statistical analysis was carried out using R (version 1.2.1335; The R Foundation for Statistical Computing, Vienna, Austria). The χ2‐test and Wilcoxon rank sum test were used to determine differences between the study groups in the baseline characteristics for categorical and continuous variables, respectively. Spearman’s rank correlation was used to find correlations between the measured analytes. Immune mediator concentration differences between the study groups, as well as the associations between these mediators and different parameters, were analyzed with multivariate linear and logistic regression models adjusted for age, gestational week and the season when the blood sample was taken, if not stated otherwise. A P‐value of <0.05 was considered significant.
RESULTS
Patients' baseline characteristics
The characteristics of the GDM (n = 60) and non‐GDM (n = 153) groups are presented in Table 1 and Table 2. The study groups did not differ in age, gestational week at GTT, pre‐pregnancy body mass index or seasonal distribution of the blood draw. Compared with normoglycemic individuals, patients with GDM presented with higher glucose, C‐peptide and C‐reactive protein levels, and they had had more pregnancies (Table 1). Their glucose and C‐reactive protein levels were also higher during post‐partum follow up (Table 2). No differences were detected between the medians for the study groups in the other pregnancy‐ and delivery‐related characteristics or in the presence of autoantibodies. The 5% loss of study participants to follow up did not lead to significant bias.
Table 1.
Anthropometric and additional medical characteristics for the study groups
| Clinical parameters | GDM group (n = 60) | Non‐GDM group (n = 153) | P‐value |
|---|---|---|---|
| Median ± SD | Median ± SD | ||
| Age (years) | 32.00 ± 5.35 | 30.00 ± 5.25 | 0.10 |
| Gestational week | 26.50 ± 1.63 | 26.29 ± 1.61 | 0.08 |
| Pre‐pregnancy BMI (kg/m2) | |||
| Underweight (BMI <19), % (n/N) | 5% (3/60) | 5% (8/153) | 0.35 |
| Normal weight (BMI 19–24), % (n/N) | 35% (21/60) | 44% (68/153) | |
| Overweight (BMI 25–29), % (n/N) | 32% (19/60) | 33% (50 /153) | |
| Obesity (BMI >30), % (n/N) | 28% (17/60) | 18% (27/153) | |
| Season of blood draw | |||
| Spring, % (n/N) | 37% (22/60) | 30% (46/153) | 0.30 |
| Summer, % (n/N) | 22% (13/60) | 18% (28/153) | |
| Fall, % (n/N) | 23% (14/60) | 20% (31/153) | |
| Winter, % (n/N) | 18% (11/60) | 31% (48/153) | |
| Gravidity (including the index pregnancy) | 3 ± 1.78 | 2 ± 1.45 | 0.03 |
| Parity | 1 ± 1.14 | 1 ± 1.00 | 0.06 |
| Fasting glucose (mmol/L) | 5.1 ± 0.44 | 4.40 ± 0.28 | 2.2E‐16 |
| 1‐h glucose (mmol/L) | 9.70 ± 1.75 | 6.70 ± 1.46 | 2.6E‐16 |
| 2‐h glucose (mmol/L) | 7.25 ± 1.35 | 5.70 ± 1.78 | 7.5E‐14 |
| C‐peptide (nmolL) | 0.84 ± 0.30 | 0.65 ± 0.22† | 1.7E‐05 |
| C‐reactive protein (mg/L) | 4.0 ± 2.88 | 3.0 ± 2.96 | 0.02 |
| IA‐2 (>7.5 U/mL), % (n/N) | 0% (0/60) | 1% (1/153) | 0.53 |
| GADA (>5 U/mL), % (n/N) | 3% (2/60) | 5% (8/153) | 0.56 |
| ZnT8 (>15 U/mL), % (n/N) | 0% (0/60) | 3% (4/153) | 0.21 |
| Previous delivery <37 gestational weeks, % (n/N) | 7% (3/41) | 5% (5/100) | 0.47 |
| Previous delivery >42 gestational weeks, % (n/N) | 0% (0/41) | 3% (3/100) | 0.47 |
| Previous interfered delivery, % (n/N)‡ | 17% (7/41) | 20% (20/100)† | 0.67 |
| Previous macrosomia (>4,500 g), % (n/N) | 15% (6/41) | 14% (14/100) | 0.93 |
Continuous variables are analyzed with Welch’s two‐sample t‐test. Categorical variables are analyzed with the χ2‐test. †Data missing for one study participant. ‡Delivery by cesarean section or vacuum extraction. BMI, body mass index; GADA, 65 kDa glutamic acid decarboxylase autoantibodies; GDM, gestational diabetes; IA‐2, islet cell antigen 2 autoantibodies; SD, standard deviation; ZnT8, zinc transporter 8 autoantibodies.
Table 2.
Delivery and post‐partum characteristics for the study groups
| Delivery parameters† | GDM group (n = 60) | Non‐GDM group (n = 153) | P‐value |
|---|---|---|---|
| Median ± SD | Median ± SD | ||
| Apgar 1 score | 9 ± 1.17 | 9 ± 1.20 | 0.37 |
| Apgar 5 score | 9 ± 0.76 | 9 ± 1.30 | 0.11 |
| Premature birth (<37 weeks), % (n/N) | 8% (4/49) | 6% (9/153) | 0.57 |
| Macrosomia (>4,500 g,) % (n/N) | 2% (1/49) | 5% (8/153) | 0.34 |
| Sex of child (male), % (n/N) | 63% (31/49) | 57% (87/153) | 0.43 |
| Post‐partum parameters† | |||
| Fasting glucose (mmol/L) | 4.90 ± 0.49 | 4.70 ± 0.51‡ | 4.8E‐04 |
| C‐peptide (nmol/L) | 0.66 ± 0.25‡ | 0.59 ± 0.27 | 0.35 |
| C‐reactive protein (mg/L) | 2.0 ± 5.55 | 1.0 ± 2.96‡ | 0.02 |
| Cholesterol (mmol/L) | 5.2 ± 1.10 | 5.2 ± 0.88 | 0.46 |
| HDL cholesterol (mmol/L) | 1.73 ± 0.54 | 1.77 ± 0.46 | 0.93 |
| LDL cholesterol (mmol/L) | 3.36 ± 1.00 | 3.19 ± 0.83 | 0.53 |
| Triglycerides (mmol/L) | 0.86 ± 0.44 | 0.75 ± 0.43‡ | 0.94 |
Continuous variables are analyzed with Welch’s two‐sample t‐test. Categorical variables are analyzed with the χ2‐test. †The gestational diabetes (GDM) group data from this period is only available for 49 women due to loss of follow up. ‡Data missing for one study participant. HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SD, standard deviation.
Comparison of immune mediator concentrations between the study groups
Immune mediator levels (medians, 1st–3rd quantile) are shown in Table 3. As these levels were not normally distributed, the Wilcoxon rank sum test with continuity correction was used for comparisons. In the GDM group, the levels of adiponectin were lower (P = 3.4E‐03) and CCL2 levels higher (P = 0.03) compared with the non‐GDM group. The levels of sIL‐1RI, IL‐4, IL‐17F and TGF‐β3 were below the minimal detection limit, and were left out of further analysis.
Table 3.
Plasma immune mediator levels for the study groups
| Cytokines (pg/mL) | Concentrations in GDM (N = 60) | Concentrations in Non‐GDM (N = 153) | P‐value | ||
|---|---|---|---|---|---|
| Median | Interquartile range | Median | Interquartile range | ||
| Adipokines (ng/mL) | |||||
| Adiponectin | 14.44 | 10.71–23.54 | 21.43 | 14.43–30.51 | 3.4E‐03 |
| Leptin | 29.30 | 22.88–40.12 | 25.83 | 15.71–37.18 | 0.12 |
| PAI‐1 | 24.42 | 20.38–27.63 | 24.05 | 19.12–32.32 | 0.80 |
| Resistin | 30.89 | 25.47–34.74 | 29.26 | 22.79–40.28 | 0.56 |
| Chemokines (pg/mL) | |||||
| CCL2 | 174.22 | 139.21–220.99 | 157.16 | 121.22–197.08 | 0.03 |
| CCL4 | 15.76 | 9.49–27.07 | 15.51 | 9.18–23.15 | 0.30 |
| CXCL8 | 3.68 | 2.68–5.56 | 3.80 | 2.76–5.91 | 0.68 |
| CXCL10 | 341.0 | 252.60–463.80 | 317.30 | 250.18–405.45 | 0.27 |
| Cytokines (pg/mL) | |||||
| GM‐CSF | 46.49 | 21.59–88.65 | 48.62 | 27.00–75.71 | 0.74 |
| IL‐1β | 0.80 | 0.19–2.09 | 1.02 | 0.28–1.90 | 0.71 |
| sIL‐1RI | 0 | 0 | 0 | 0 | 0.64 |
| IL‐2 | 1.21 | 0–3.16 | 0.83 |
0–2.13 |
0.22 |
| IL‐2RA | 688.30 | 549.10–977.50 | 684.23 | 546.97–926.30 | 0.61 |
| IL‐4 | 0 | 0–0 | 0 | 0–0 | 0.71 |
| IL‐5 | 3.09 | 1.74–4.07 | 3.24 | 1.85–4.50 | 0.40 |
| IL‐6 | 1.63 | 0.82–2.41 | 1.60 | 1.0–2.27 | 0.80 |
| IL‐7 | 2.95 | 0.00–6.38 | 3.90 | 0–7.47 | 0.29 |
| IL‐10 | 6.13 | 2.83–12.32 | 6.69 | 1.34–11.30 | 0.17 |
| IL‐12(p70) | 4.63 | 2.35–6.80 | 4.86 | 2.62–7.07 | 0.61 |
| IL‐13 | 7.68 | 3.22–13.09 | 9.08 | 5.20–15.53 | 0.20 |
| IL‐15 | 38.18 | 18.00–65.71 | 36.11 | 17.43–61.67 | 0.78 |
| IL‐17A | 8.16 | 3.83–13.81 | 8.94 | 4.13–16.56 | 0.51 |
| IL‐17F | 0.02 | 0–0.05 | 0.02 | 0–0.05 | 0.65 |
| IL‐21 | 0.74 | 0–2.61 | 0.68 | 0–2.09 | 0.73 |
| IL‐22 | 0.88 | 0.47–1.14 | 0.80 | 0.47–1.11 | 0.51 |
| IL‐23 | 112.45 | 6.94–317.84 | 138.20 | 17.34–287.69 | 0.96 |
| IL‐27 | 7.19 | 6.22–9.77 | 7.91 | 6.48–11.90 | 0.13 |
| IFN‐γ | 15.53 | 9.30–21.95 | 16.11 | 11.34–24.22 | 0.17 |
| sTNFRII | 8.0 | 7.16–9.77 | 7.85 | 6.94–9.19 | 0.59 |
| TNFα | 3.26 | 2.25–6.03 | 4.15 | 2.57–6.53 | 0.39 |
| TGF‐β1 | 6.56 | 4.80–11.94 | 6.72 | 4.53–12.45 | 0.85 |
| TGF‐β2 | 560.0 | 402.50–818.90 | 496.1 | 402.50–802.30 | 0.95 |
| TGF‐β3 | 0 | 0–56.83 | 0 | 0–56.83 | 0.35 |
P‐value from Wilcoxon rank sum test with continuity correction. CCL, C‐C motif chemokine ligand; CXCL, C‐X‐C motif chemokine ligand; GDM, gestational diabetes; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IFN‐γ, interferon‐gamma; IL, interleukin; PAI‐1, plasminogen activator inhibitor one; sTNFR, soluble tumor necrosis factor receptor; TGF‐β, transforming growth factor beta; TNFα, tumor necrosis factor alpha.
Correlations between immune mediator concentrations
Using Spearman's rank correlation, we noted both positive and negative correlations between the measured immune mediators (Figure 1). The four cytokines showing the highest number of strong correlations (r > 0.5, P < 0.05) with the other immune mediators were IL‐1β, IL‐5, IL‐12(p70) and IL‐23 (Figure 2). Interestingly, strong negative correlations were only detected between TNFα, and IL‐15 and IL‐22. Of the adipokines, only PAI‐1 showed strong correlations with resistin and TGF‐β1; of the chemokines, only CXCL8 correlated strongly with IL‐1β, IL‐2, IL‐5 and IL‐12(p70).
Figure 1.
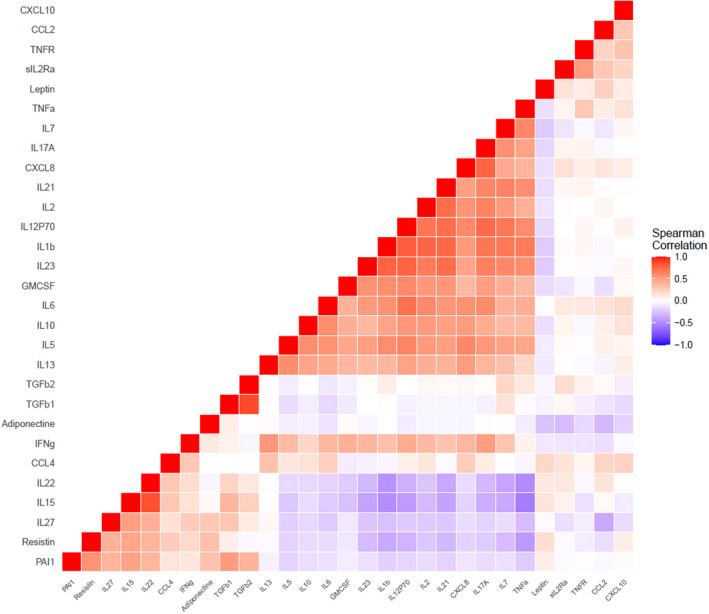
Spearman’s rank correlation matrix for measured immune mediators. CCL, C‐C motif chemokine ligand; CXCL, C‐X‐C motif chemokine ligand; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IFN‐γ, interferon‐gamma; IL, interleukin; PAI‐1, plasminogen activator inhibitor one; sTNFR, soluble tumor necrosis factor receptor; TGF‐β, transforming growth factor beta; TNFα, tumor necrosis factor alpha. Red color indicates positive correlations; blue color indicates negative correlations. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
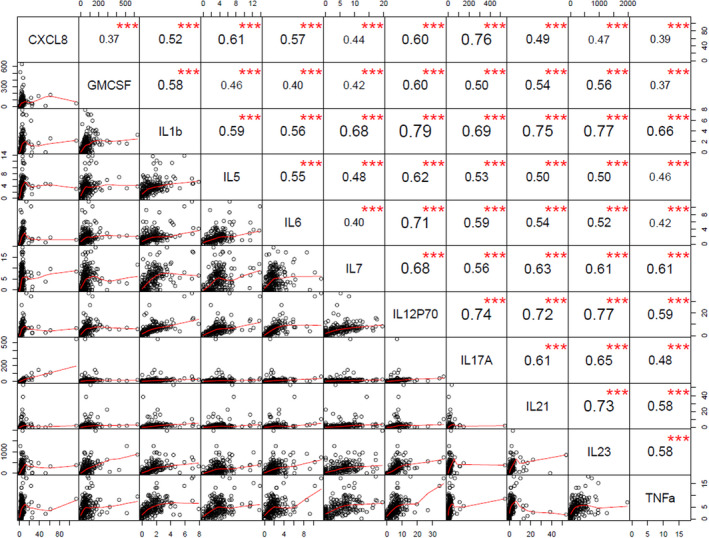
Spearman’s rank correlation matrix with correlation coefficient and statistical significance for the top four cytokines: interleukin (IL)‐1β, IL‐5, IL‐12(p70) and IL‐23, that showed the highest number of strong correlations (r > 0.5, P < 0.05) with the other immune mediators. CXCL, C‐X‐C motif chemokine ligand; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; TNFα, tumor necrosis factor alpha. ***P < 0.001. [Colour figure can be viewed at wileyonlinelibrary.com]
Associations between immune mediators and confounding variables
As another goal of the present study, we analyzed whether the measured immune mediators were associated with the clinical characteristics of GDM. For this, we applied linear regression models with stepwise regression. In Table 4, only the results from models with adjusted (a)R 2 > 15%, P < 0.01 are presented, along with information about model adjustments. With increasing age, the levels of IL‐22 increased (β = 0.02; P = 0.02). Only IL‐27 levels increased with gestational week (β = 0.82; P = 0.02). Overweight and obesity were associated with higher levels of CCL2 (overweight β = 58.4; P = 8.0E‐04; obesity β = 80.0; P = 6.9E‐04) and leptin (overweight β = 9.6; P = 4.4E‐05; obesity β = 18.6; P = 1.3E‐11). Some of the immune mediator levels also depended on the season of the blood draw. For example, compared with summer, IL‐22 levels were higher in spring (β = 0.3; P = 0.03), fall (β = 0.6; P = 9.3E‐05) and winter (β = 0.29; P = 4.6E‐02). IL‐27 levels were significantly higher in spring (β = 6.3; p = 5.2E‐06), whereas CCL2 levels were significantly higher in fall (β = 58.0; P = 8.2E‐05).
Table 4.
Associations between immune mediators and confounding variables
| Clinical parameters | Leptin | Resistin | CCL2 | CCL4 | CXCL10 | IL‐22 | IL‐27 | TGF‐β1 |
|---|---|---|---|---|---|---|---|---|
| Age (years) | * | |||||||
| Gestational week | ** | |||||||
| Pre‐pregnancy BMI (kg/m2) Normal weight (BMI 19–24) = ref | ||||||||
| Underweight (BMI <19) | * | |||||||
| Overweight (BMI 25–29) | *** | ** | ||||||
| Obesity (BMI >30) | *** | *** | ||||||
| GDM diagnosis | * | |||||||
| Presence of GADA (U/mL) | ** | *** | ||||||
| Parity | * | |||||||
| Previous delivery >42 gestational weeks | *** | ** | ** | |||||
| Previous interfered delivery† | ** | |||||||
| Delivery parameters | ||||||||
| Premature birth (<37 gestational weeks) | *** | ** | ||||||
| Apgar score at 1st minute | * | |||||||
| Apgar score at 5th minute | ** | |||||||
| Child diagnosed with hyperbilirubinemia or infection | * | |||||||
| Macrosomic child (>4,500 g) | *** | |||||||
| Sex of child (male) | * | |||||||
| Post‐partum parameters | ||||||||
| Fasting glucose (mmol/L) | * | |||||||
| C‐peptide (nmol/L) | ** | |||||||
| C‐reactive protein (mg/L) | *** | ** | ||||||
| Model adjusted R 2 | 22.4%‡ | 16.0%‡ | 18.6%§ | 29.5%§ | 16.0%§ | 15.3%§ | 19.6%§ | 15.3%§ |
| Model adjusted P‐value | 3.7E‐10 | 5.8E‐06 | 2.3E‐07 | 5.2E‐08 | 2.6E‐04 | 2.0E‐04 | 6.6E‐09 | 1.3E‐03 |
Light shaded boxes show increasing effect. Dark shaded boxes show decreasing effect. *P < 0. 5; **P < 0.01; ***P < 0.001. †Delivery by cesarean section or vacuum extraction. ‡Model adjusted for maternal age, gestational week, season of blood draw and pre‐pregnancy body mass index (BMI). §Model adjusted for maternal age, gestational week and season of blood draw. CCL, C‐C motif chemokine ligand; CXCL, C‐X‐C motif chemokine ligand; GADA, 65 kDa glutamic acid decarboxylase autoantibodies; GDM, gestational diabetes; IL, interleukin; TGF‐β, transforming growth factor beta.
The presence of a GDM diagnosis showed an inverse association with IL‐27 levels (β = −2.6; P = 0.02). According to unadjusted logistic regression, lower levels of IL‐27 reduced the odds of GDM, odds ratio (OR) 0.95 (2.75–97.5% confidence interval [CI] 0.90–0.99, P = 0.04). This association remained significant after adjusting for confounding factors, adjusted OR 0.93 (2.75–97.5% CI 0.88–0.98, P = 0.02, adjusted for maternal age, gestational week, season of the blood draw, presence of GADA). CCL2 and adiponectin did not influence the odds of GDM, OR 1.00 (2.75–97.5% CI 0.99–1.01, P = 0.09) and OR 0.98 (2.75–97.5% CI 0.96–1.00, P = 0.10), respectively, even after adjusting for confounding variables (data not shown).
None of the measured immune mediators was associated with GTT glucose levels during pregnancy. Higher TGFβ1 and CCL4 levels at the second trimester of pregnancy associated positively with post‐partum fasting glucose (β = 3.3; P = 0.04) and C‐peptide levels (β = 20.0; P = 0.01), respectively.
When the GADA test result was analyzed as a continuous variable, positive associations with IL‐27 (β = 0.3; P = 7.6E‐05) and IL‐22 (β = 0.02; P = 0.01) were found. According to the unadjusted logistic regression model, higher levels of IL‐27 increased the odds of GADA being positive, OR 1.10 (2.75–97.5% CI 1.03–1.17, P = 4.4E‐03), even after adjustment for confounding factors, adjusted OR 1.13 (2.75–97.5% CI 1.05–1.24, P = 2.8E‐03, adjusted for maternal age, gestational week, season of the blood draw and GDM diagnosis). Similarly, IL‐22 levels increased the odds of a positive GADA test result, OR 3.18 (2.75–97.5% CI 1.25–8.24, P = 0.01), which increased after adjustment for confounding factors, adjusted OR 4.23 (2.75–97.5% CI 1.13–18.16, P = 0.04, adjusted for maternal age, gestational week, timeliness of the previous delivery and season of the blood draw).
Of the pregnancy complications, premature birth was associated with higher levels of CCL4 (β = 20.3; P = 0.01) and resistin (β = 150.4; P = 9.4E‐06). Birth of a macrosomic child was associated with higher levels of CXCL10 (β = 291.6; P = 7.0E‐04). Women carrying male fetuses had lower levels of CXCL10 (β = −93.1; P = 0.02).
DISCUSSION
The present study aimed to compare changes in plasma immune mediator profiles for women of the GDM risk group who had developed GDM and those who had not. We also characterized associations between the immune mediators and covariates. In general, only adiponectin and CCL2 levels were different between the two study groups, and lower IL‐27 levels showed reduced odds of GDM. Another important finding was that higher levels of IL‐27 together with IL‐22 were associated with increased odds of GADA positivity. In addition, women with pregnancy complications had higher levels of CCL4, resistin and CXCL10.
The established higher levels of CCL2 in women with GDM, and the association between CCL2 and body mass index are consistent with previous findings. CCL2 has been associated with the development of type 2 diabetes and insulin resistance, as it plays a role in β‐cell failure 14 , 15 , 16 . CCL2 has also been associated with the birth of a small for gestational age baby 17 . However, we were not able to confirm this finding based on our cohort, which was biased toward GDM (associated with a macrosomic fetus). We detected lower levels of adiponectin in women with GDM, as reported in many other studies 18 , 19 , 20 . Adiponectin has been linked with insulin sensitization, and a decline in adiponectin levels might show adipose tissue dysfunction, which can precede GDM 20 . Maternal hypoadiponectinemia could also be an important risk factor for type 2 diabetes development later in life 21 . Although both CCL2 and adiponectin levels were different between the two study groups, they did not point to risk associations with GDM, showing that the detected difference might be not due to the diagnosis of GDM, but rather to the GDM‐associated risk factors.
To our knowledge, IL‐27 has not been previously studied in relation to GDM, but has been implied in the context of pathologies of autoimmune diseases 22 . Lower levels of IL‐27, which showed a reduced risk for GDM in the present study, have been found to exert a protective effect on pancreatic β‐cell survival, whereas higher levels have been linked with type 1 diabetes development 23 , 24 . It is therefore interesting to note that higher levels of IL‐27 in the present study were characteristic of women who were positive for GADA, irrespective of their GDM diagnosis, and might hint to its role in promoting β‐cell autoimmunity. The presence of GADA can increases the risk for type 1 diabetes development later in life 2 , 25 . Although GADA was the most common antibody in the present study, the number of participants who tested positive for GADA was too small (3% in GDM, 5% in non‐GDM) to be able to draw a general conclusion. Still, it can be hypothesized that there are women in the whole GDM risk group who do not develop classical GDM (type 2 diabetes‐similar GDM), but who nevertheless are at an increased risk of developing type 1 diabetes later in life, due to triggering factors during pregnancy 10 . These women require different treatment, and their post‐partum maternal prognosis is different from that expected in type 2 diabetes‐similar GDM 10 .
Another interesting finding was the association of higher IL‐22 levels with the presence of GADA. Similar to IL‐27, IL‐22 has not been previously associated with GDM, but has rather been studied in connection with type 1 diabetes and its complications. Honkanen et al. 26 reported increased levels of IL‐22 in type 1 diabetes patients. Knoop et al. 27 also detected higher production of IL‐22 in recent onset also patients. Elevated IL‐22 levels could be another marker for pancreatic self‐destruction in these women, who later develop type 1 diabetes. As the diagnosis of GDM through GTT mostly focuses on insulin‐resistant GDM, pregnant women with a propensity for autoimmune diabetes are often left out of close medical monitoring 10 . Therefore, further studies should evaluate the predictive role of IL‐27 and IL‐22 levels together with pancreatic islet‐specific autoantibody detection for type 1 diabetes development in women of the GDM risk group, to identify, without type 2 diabetes prejudice, women with a predisposition to autoimmune diabetes.
TGF‐β1 is a multifunctional cytokine, including an important regulator of the immune system with a mainly immunosuppressive effect 28 . Hyperglycemia is the key inducer of TGF‐β1 expression, owing to which elevated concentrations of TGF‐β1 have been measured in patients with type 2 diabetes and in women with previous GDM 28 , 29 . Contrary to expectations, the present study did not detect differences in TGF‐β1 levels between GDM and non‐GDM participants. This finding supports the study of Lygnos et al., 30 who suggested that pregnancy itself is the main reason for increased TGF‐β1 levels. Nevertheless, the TGF‐β1 pathway is one of the major pathways associated with the pathogenesis of GDM 31 , 32 . TGF‐β1 signaling regulates the gene expression pathways involved in β‐cell function 33 . Also, higher TGF‐β1 levels in patients with recent‐onset type 1 diabetes are associated with reduced β‐cell functionality 34 , suggesting that impairments in TGF‐β1 signaling might lead to diabetes development. Therefore, the present finding of the positive association between higher TGF‐β1 levels during pregnancy and higher post‐partum fasting blood glucose levels might be predictive of the beginning of β‐cell failure in these women. In addition, we were able to confirm the positive correlation of TGF‐β1 with PAI‐1. TGF‐β1 is an important stimulator of PAI‐1 production 29 . Both of them are associated with insulin resistance and are involved in renal malfunctions 35 , which further implies the hazard that higher TGF‐β1 levels during pregnancy could predict future diabetes‐related complications in these women. On the contrary, CCL4 has been shown to exert a protective role for pancreatic β‐cells, and thereby a preventive influence on diabetes progression 36 . The present result of a positive association between CCL4 and post‐partum C‐peptide levels is consistent with the suggestions that CCL4 helps to maintain β‐cell function 37 .
The present study provides an exciting opportunity to improve our knowledge of the role of CXCL10 in GDM, since as far as we know, only Wang et al. 38 have studied CXCL10 in GDM. Higher CXCL10 levels have previously been associated also with pre‐eclampsia 39 . We showed that higher levels of CXCL10 were associated with the birth of a macrosomic child. Together, these results suggest that CXCL10 could be used as a predictive marker for pregnancy complications in GDM. Interestingly, lower CXCL10 levels were characteristic of mothers carrying male fetuses. Cytokines and chemokines have been linked with fetal sex, although the exact mechanism of the effect on the fetus is not clear. One possible explanation could be that male fetuses have been described with a more pro‐inflammatory profile 40 , 41 .
There are still numerous conflicting data on the association between maternal cytokine levels and pregnancy complications. The present finding of a positive association between higher CCL4 levels and preterm delivery supports the results from a number of previous studies reviewed by Polettini et al. 42 We detected a positive association between resistin and preterm labor, which does not coincide with earlier findings and requires further validation 43 . This inconsistency might be due to differences in the study groups involved, as well as due to methodological discrepancies. Some reports are based on healthy pregnant women without diabetes, owing to which the role of these immune mediators can be different in the case of low‐grade inflammation characteristic of the women of the GDM risk group 6 .
The present study had several strengths. First, the inclusion of a wide range of immune mediators helped gain a better overview of the pro‐ and anti‐inflammatory branches of the immune system relevant to GDM. Many of the analytes had not been previously assessed in GDM. Therefore, the present study adds novel information on the complexity of GDM‐related immunological dysregulation. Second, the consistent and strict collection, and immediate careful processing and storage of the blood samples helped minimize the variability of immune mediator levels as a result of secondary influences. Third, we provided further proof that immune mediator levels can be dependent on individuals' age and gestational week, as well as to the season of the blood draw, and should therefore always be checked and adjusted for in statistical analysis. The generalizability of the present results, however, is subject to limitation. Because of practical constraints, the present study only included plasma samples from gestational weeks starting from the second trimester, when GDM usually manifests. A study design including pre‐pregnancy samples or samples from the first trimester would have allowed better monitoring of the immune mediator changes in patients who remained normoglycemic compared with women who developed GDM.
In conclusion, cytokines can give early hints about changes in the complex immune system's balance. As such, they could help discriminate women in the GDM risk group who are closer to diabetes development than others. We showed that lower adiponectin and higher CCL2 plasma concentrations were characteristic of women in the GDM risk group who had developed GDM. Changes in TGF‐β1 and CCL4 levels might predict the future risk for diabetes in these women. Higher IL‐27 and IL‐22 levels, together with positivity for GADA, can be useful markers to identify women with a higher risk for type 1 diabetes development. Additionally, CXCL10 and CCL4 could be markers for pregnancy complications in women in the GDM risk group.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The protocol for this research project has been approved by the Ethics Review Committee on Human Research of the University of Tartu (Estonia), Approval No. 229/M‐16, 23.09.2013 and 254/M‐16, 21.12.2015.
The study conforms to the provisions of the Declaration of Helsinki.
Informed consent: All informed consent was obtained from the participants.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
The authors are grateful to midwife Mrs Laura Lauren from the Women's Clinic, Tartu University Hospital, for interviewing the participants and for collecting the blood samples, to Ms Helis Janson, Ms Kirsti Alnek and Ms Hanna Sepp from the Department of Immunology, University of Tartu, for laboratory assistance in the handling of the biomaterial. This study was supported by the Estonian Research Council (institutional research funding IUT20‐43, PRG712) and the European Regional Development Fund.
Contributor Information
Aili Tagoma, Email: aili.tagoma@ut.ee.
Kadri Haller‐Kikkatalo, Email: kadrihk@gmail.com.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 2021. [Internet]. 10th ed. Available from: www.diabetesatlas.org Accessed December 20, 2021.
- 2. Auvinen A‐M, Luiro K, Jokelainen J, et al. Type 1 and type 2 diabetes after gestational diabetes: a 23 year cohort study. Diabetologia 2020; 63: 2123–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sandu C, Bica C, Salmen T, et al. Gestational diabetes ‐ modern management and therapeutic approach (review). Exp Ther Med 2021; 21: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 2018; 19: 3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gan WZ, Ramachandran V, Lim CSY, et al. Omics‐based biomarkers in the diagnosis of diabetes. J Basic Clin Physiol Pharmacol 2020; 31: 20190120. [DOI] [PubMed] [Google Scholar]
- 6. Lekva T, Norwitz ER, Aukrust P, et al. Impact of systemic inflammation on the progression of gestational diabetes mellitus. Curr Diab Rep 2016; 16: 26. [DOI] [PubMed] [Google Scholar]
- 7. Yang Y, Liu L, Liu B, et al. Functional defects of regulatory T cell through interleukin 10 mediated mechanism in the induction of gestational diabetes mellitus. DNA Cell Biol 2018; 37: 278–285. [DOI] [PubMed] [Google Scholar]
- 8. Sifnaios E, Mastorakos G, Psarra K, et al. Gestational diabetes and T‐cell (Th1/Th2/Th17/Treg) immune profile. In Vivo 2019; 33: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wedekind L, Belkacemi L. Altered cytokine network in gestational diabetes mellitus affects maternal insulin and placental–fetal development. J Diabetes Complications 2016; 30: 1393–1400. [DOI] [PubMed] [Google Scholar]
- 10. Haller‐Kikkatalo K, Uibo R. Clinical recommendations for the use of islet cell autoantibodies to distinguish autoimmune and non‐autoimmune gestational diabetes. Clin Rev Allergy Immunol 2016; 50: 23–33. [DOI] [PubMed] [Google Scholar]
- 11. Siddiqui S, Waghdhare S, Jha S, et al. Role of immunological markers in gestational diabetes mellitus‐a brief review. Diabetes Metab Syndr Clin Res Rev 2019; 13: 2983–2985. [DOI] [PubMed] [Google Scholar]
- 12. Kirss A, Lauren L, Rohejärv M, et al. Gestational diabetes: risk factors and prevalence at the Women's Clinic of Tartu University Hospital 2012–2013. (Eesti Arst) Estonian Med J 2015; 94: 75–82 (Estonain). [Google Scholar]
- 13. Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: Response to Weinert. Diabetes Care 2010; 33: e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Telejko B, Kuzmicki M, Zonenberg A, et al. Circulating monocyte chemoattractant protein‐1 in women with gestational diabetes. Folia Histochem Cytobiol 2007; 45(Suppl 1): S153–S156. [PubMed] [Google Scholar]
- 15. Klein K, Satler M, Elhenicky M, et al. Circulating levels of MCP‐1 are increased in women with gestational diabetes. Prenat Diagn 2008; 28: 845–851. [DOI] [PubMed] [Google Scholar]
- 16. Corrêa‐Silva S, Alencar AP, Moreli JB, et al. Hyperglycemia induces inflammatory mediators in the human chorionic villous. Cytokine 2018; 111: 41–48. [DOI] [PubMed] [Google Scholar]
- 17. Kumarathasan P, Williams G, Bielecki A, et al. Characterization of maternal plasma biomarkers associated with delivery of small and large for gestational age infants in the MIREC study cohort. PloS One 2018; 13: e0204863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsai P‐J, Yu C‐H, Hsu S‐P, et al. Maternal plasma adiponectin concentrations at 24 to 31 weeks of gestation: negative association with gestational diabetes mellitus. Nutrition 2005; 21: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 19. Ott R, Stupin JH, Melchior K, et al. Alterations of adiponectin gene expression and DNA methylation in adipose tissues and blood cells are associated with gestational diabetes and neonatal outcome. Clin Epigenetics 2018; 10: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dereke J, Nilsson C, Strevens H, et al. Pregnancy‐associated plasma protein‐A2 levels are increased in early‐pregnancy gestational diabetes: a novel biomarker for early risk estimation. Diabet Med 2020; 37: 131–137. [DOI] [PubMed] [Google Scholar]
- 21. Mehmood S, Ye C, Connelly PWH, et al. Rising plasminogen activator inhibitor‐1 and hypoadiponectinemia characterize the cardiometabolic biomarker profile of women with recent gestational diabetes. Cardiovasc Diabetol 2018; 17: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parackova Z, Vrabcova P, Zentsova I, et al. Enhanced STAT3 phosphorylation and PD‐L1 expression in myeloid dendritic cells indicate impaired IL‐27Ralpha signaling in type 1 diabetes. Sci Rep 2020; 10: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang R, Han G, Wang J, et al. The pathogenic role of interleukin‐27 in autoimmune diabetes. Cell Mol Life Sci 2008; 65: 3851–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alnek K, Kisand K, Heilman K, et al. Increased blood levels of growth factors, proinflammatory cytokines, and Th17 cytokines in patients with newly diagnosed type 1 diabetes. PloS One 2015; 10: e0142976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nilsson C, Ursing D, Törn C, et al. Presence of GAD antibodies during gestational diabetes mellitus predicts type 1 diabetes. Diabetes Care 2007; 30: 1968–1971. [DOI] [PubMed] [Google Scholar]
- 26. Honkanen J, Nieminen JK, Gao R, et al. IL‐17 immunity in human type 1 diabetes. J Immunol 2010; 185: 1959–1967. [DOI] [PubMed] [Google Scholar]
- 27. Knoop J, Gavrisan A, Kuehn D, et al. GM‐CSF producing autoreactive CD4+ T cells in type 1 diabetes. Clin Immunol 2018; 188: 23–30. [DOI] [PubMed] [Google Scholar]
- 28. Herder C, Zierer A, Koenig W, et al. Transforming growth factor‐beta1 and incident type 2 diabetes: results from the MONICA/KORA case‐cohort study, 1984‐2002. Diabetes Care 2009; 32: 1921–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yener S, Demir T, Akinci B, et al. Transforming growth factor‐beta 1 levels in women with prior history of gestational diabetes mellitus. Diabetes Res Clin Pract 2007; 76: 193–198. [DOI] [PubMed] [Google Scholar]
- 30. Lygnos MC, Pappa KI, Papadaki HA, et al. Changes in maternal plasma levels of VEGF, bFGF, TGF‐beta1, ET‐1 and sKL during uncomplicated pregnancy, hypertensive pregnancy and gestational diabetes. In Vivo 2006; 20: 157–163. [PubMed] [Google Scholar]
- 31. Qian Y, Sun H, Xiao H, et al. Microarray analysis of differentially expressed genes and their functions in omental visceral adipose tissues of pregnant women with vs. without gestational diabetes mellitus. Biomed Rep 2017; 6: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tagoma A, Alnek K, Kirss A, et al. MicroRNA profiling of second trimester maternal plasma shows upregulation of miR‐195‐5p in patients with gestational diabetes. Gene 2018; 672: 137–142. [DOI] [PubMed] [Google Scholar]
- 33. Lin H‐M, Lee J‐H, Yadav H, et al. Transforming growth factor‐beta/Smad3 signaling regulates insulin gene transcription and pancreatic islet beta‐cell function. J Biol Chem 2009; 284: 12246–12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pham MN, Kolb H, Battelino T, et al. Fasting and meal‐stimulated residual beta cell function is positively associated with serum concentrations of proinflammatory cytokines and negatively associated with anti‐inflammatory and regulatory cytokines in patients with longer term type 1 diabetes. Diabetologia 2013; 56: 1356–1363. [DOI] [PubMed] [Google Scholar]
- 35. Krag S, Nyengaard JR, Wogensen L. Combined effects of moderately elevated blood glucose and locally produced TGF‐beta1 on glomerular morphology and renal collagen production. Nephrol Dial Transplant 2007; 22: 2485–2496. [DOI] [PubMed] [Google Scholar]
- 36. Pfleger C, Kaas A, Hansen L, et al. Relation of circulating concentrations of chemokine receptor CCR5 ligands to C‐peptide, proinsulin and HbA1c and disease progression in type 1 diabetes. Clin Immunol 2008; 128: 57–65. [DOI] [PubMed] [Google Scholar]
- 37. Meagher C, Arreaza G, Peters A, et al. CCL4 protects from type 1 diabetes by altering islet β‐cell–targeted inflammatory responses. Diabetes 2007; 56: 809–817. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Yu H, Liu F, et al. Analysis of key genes and their functions in placental tissue of patients with gestational diabetes mellitus. Reprod Biol Endocrinol 2019; 17: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gotsch F, Romero R, Friel L, et al. CXCL10/IP‐10: a missing link between inflammation and anti‐angiogenesis in preeclampsia? J Matern Fetal Neonatal Med 2007; 20: 777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chow SSW, Craig ME, Jones CA, et al. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine 2008; 44: 78–84. [DOI] [PubMed] [Google Scholar]
- 41. Enninga EAL, Nevala WK, Creedon DJ, et al. Fetal sex‐based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol 2015; 73: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Polettini J, Cobo T, Kacerovsky M, et al. Biomarkers of spontaneous preterm birth: a systematic review of studies using multiplex analysis. J Perinat Med 2017; 45: 71–84. [DOI] [PubMed] [Google Scholar]
- 43. Kominiarek MA, Gambala CT, Sutherland M, et al. Adipokinins in pregnancies at risk of preterm delivery. Gynecol Endocrinol 2016; 32: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


