Abstract
Aims/Introduction
This study determined the prevalence and risk factors for diabetic peripheral neuropathy (DPN), painful DPN and diabetic foot ulceration (DFU) in patients with type 2 diabetes in secondary healthcare in Qatar, Kuwait and the Kingdom of Saudi Arabia.
Materials and Methods
Adults aged 18–85 years with type 2 diabetes were randomly enrolled from secondary healthcare, and underwent clinical and metabolic assessment. DPN was evaluated using vibration perception threshold and neuropathic symptoms and painful Diabetic Peripheral Neuropathy was evaluated using the Douleur Neuropathique 4 questionnaire.
Results
A total of 3,021 individuals were recruited between June 2017 and May 2019. The prevalence of DPN was 33.3%, of whom 52.2% were at risk of DFU and 53.6% were undiagnosed. The prevalence of painful DPN was 43.3%, of whom 54.3% were undiagnosed. DFU was present in 2.9%. The adjusted odds ratios for DPN and painful DPN were higher with increasing diabetes duration, obesity, poor glycemic control and hyperlipidemia, and lower with greater physical activity. The adjusted odds ratio for DFU was higher with the presence of DPN, severe loss of vibration perception, hypertension and vitamin D deficiency.
Conclusions
This is the largest study to date from the Middle East showing a high prevalence of undiagnosed DPN, painful DPN and those at risk of DFU in patients with type 2 diabetes, and identifies their respective risk factors.
Keywords: Diabetic foot ulceration, Diabetic peripheral neuropathy, Painful diabetic peripheral neuropathy
This is the largest prevalence study to date which has deployed standardized methodology in the Middle East to show a high prevalence of undiagnosed diabetic peripheral neuropathy (DPN), painful DPN and those at risk of diabetic foot ulceration (DFU). We have also identified obesity, poor glycemic control, hyperlipidemia, smoking cigarettes, and reduced physical activity as modifiable risk factors for DPN and severe loss of vibration perception, DPN, hypertension, and vitamin D deficiency as risk factors for DFU. These findings argue for a systematic approach to the diagnosis and management of risk factors for DPN.
INTRODUCTION
Diabetic peripheral neuropathy (DPN) can lead to significant morbidity, including painful DPN and diabetic foot ulceration (DFU) 1 . One in four patients with DFU are at risk of amputation 2 , and their risk of death is increased 2.5‐fold 3 . There is high variability in the prevalence of DPN depending on the population and diagnostic criteria of DPN of the study. The prevalence of DPN has been reported to be 17–53% in the Middle East and North Africa 4 , 5 , 27–32% in Europe 6 , 7 , 21–45% in the USA 8 , and 17–62% in China 9 . There is no approved treatment for DPN. 10 Identifying and managing the risk factors for DPN are key to delay or prevent the development of DPN 1 . DPN in type 2 diabetes is associated with age, duration of diabetes 11 , hyperglycemia 12 , obesity and hyperlipidemia 13 , 14 .
The American Diabetes Association advocates screening for DPN at diagnosis of type 2 diabetes and thereafter annually 1 . According to the 2013 IDF Diabetes Atlas, Qatar, Kuwait and the Kingdom of Saudi Arabia (KSA) were in the world's top 10 countries for the prevalence of diabetes (22.9–23.9%). However, these Gulf Arab states have undergone rapid healthcare transformation with the implementation of disease prevention strategies and provision of universal health coverage, and according to the 2019 IDF Diabetes Atlas, the prevalence of diabetes has been reduced by 7–10%. There is a need for a multicenter study to determine the prevalence and risk factors for DPN, painful DPN and DFU using a standardized methodology in the Middle East.
Using the same methods and diagnostic criteria, the present study determined the overall and individual prevalence and associated risk factors for DPN, painful DPN and DFU in patients with type 2 diabetes attending four major secondary healthcare (SHC) diabetes centers in Qatar, Kuwait and KSA.
MATERIALS AND METHODS
This was a cross‐sectional multicenter study. Individuals with type 2 diabetes aged between 18 and 85 years were randomly enrolled from the Diabetes Clinic in Hamad General Hospital and Al‐Wakra Hospital in Qatar, Dasman Diabetes Institute in Kuwait, and Obesity Endocrine and Metabolism Center at King Fahad Medical City in KSA. Exclusion criteria included type 1 diabetes, chemotherapy, severe vitamin B12 deficiency and hypothyroidism. Participants were enrolled on the day they attended the clinic for their diabetes review between June 2017 and May 2019. The present study received ethical approval from the Weill Cornell Medicine‐Qatar (WCM‐Q) IRB (IRB# 15–00078) and Hamad Medical Corporation (HMC) IRB (IRB# 16324/16) in Qatar, Dasman Center for Research and Treatment of Diabetes in Kuwait IRB (IRB# IRBH1 IORG0005964), and King Fahad Medical City in KSA IRB (IRB# IRB00010471). All participants consented to take part in the study. The study acted in accord to the tenets of the Declaration of Helsinki.
Demographic and clinical characteristics
Age, sex, diabetes duration, blood pressure, body weight, body mass index, glycated hemoglobin (HbA1c), lipid profile, vitamin B12 and D, thyroid‐stimulating hormone, free thyroxine, and medications were recorded.
Poor glycemic control was defined by a HbA1c ≥7% 15 . Hypertension was defined by a mean systolic blood pressure ≥140 mmHg or the use of blood pressure drugs 16 . Hyperlipidemia was defined by a total cholesterol ≥6.2 mmol/L, triglyceride ≥2.3 mmol/L or the use of statin, as described in the Mayo Clinic Guidelines. Obesity was classified as a body mass index ≥30 kg/m2 17 . Smoking cigarettes was defined by one or more cigarette/day over the past year. Physical activity was self‐reported and defined as ≥30 min walking per day, three or more times in a week over the past year. Vitamin D was defined as normal (≥30 ng/mL), mild deficiency (20–29 ng/mL), moderate deficiency (10–19 ng/mL) and severe deficiency (<10 ng/mL) 18 .
DPN, painful DPN and DFU assessments
Vibration perception threshold (VPT) ranging from 0 to 50 V was assessed using a neurothesiometer (Horwell; Scientific Laboratory Supplies, Wilford, UK) on the pulp of the large toe of both feet. The assessment was repeated three times and the mean value was recorded. Instead of using a 128‐Hz tuning fork, the cut‐off of ≥15 V was used for detecting impaired VPT 19 .
The diagnosis of DPN is principally a clinical one and in line with the American Diabetes Association recommendation 1 . DPN was defined based on one or more neuropathic symptom/s (numbness, burning pain, electric shocks, tingling, pins and needles and painful cold) and impaired VPT in the feet. Previously diagnosed DPN was self‐reported. High risk of DFU was defined as a VPT ≥25 V 19 .
Painful DPN was defined by the presence of neuropathic pain using the Douleur Neuropathique 4 (DN4) questionnaire based on ≥4/10 score 20 or the use of drugs for painful DPN. It has been validated for painful DPN (sensitivity 80%, specificity 92%) 20 and in the Arabic version 21 . It has three questions about pain description (burning, painful cold, electric shocks), four questions about abnormal sensations (tingling, pins and needles, numbness, itching) and three questions about neurological signs in the painful area (hypoesthesia to touch and pin prick, and allodynia to brushing). The questions are equally weighted, and 1 point is added for each positive answer. The DN4 was administered to the participant in English or Arabic. Previously diagnosed painful DPN was self‐reported.
DFU was defined based on a break in the epidermis or dermis of the foot as per the International Working Group on the Diabetic Foot guidelines. 22
Training on the use and interpretation of the neurothesiometer and DN4 questionnaire was given to all investigators.
Statistical analysis
The prevalence of DPN, undiagnosed DPN, high risk of DFU, DFU, painful DPN, undiagnosed painful DPN, and demographic and clinical characteristics were summarized as categorical variables using frequency distributions, and compared between Qatar, Kuwait and KSA using the χ2‐test.
In the binary logistic regression analysis, the independent variables were sex, age, diabetes duration, poor glycemic control, hyperlipidemia, hypertension, obesity, physical activity, smoking cigarettes and vitamin D deficiency, and the dependent variables were DPN, painful DPN and DFU. The variables with P ≤ 0.05 at the bivariate level were included in the multiple logistic regression. Adjusted odds ratios (AOR), 95% confidence intervals (CI) and P‐values are presented.
IBM SPSS (version 27; SPSS Inc, Armonk, NY, USA) was used for analyses. A two‐tailed P ≤ 0.05 was considered significant.
RESULTS
A total of 3,021 individuals with type 2 diabetes were recruited from four SHC centers in Qatar (n = 1,093), Kuwait (n = 1,168), and KSA (n = 760). Table 1 shows a comparison of demographic and clinical characteristics in patients with type 2 diabetes in SHC between Qatar, Kuwait and KSA.
Table 1.
Comparison of demographic and clinical characteristics in patients with type 2 diabetes in secondary healthcare between Qatar, Kuwait and the Kingdom of Saudi Arabia
| Gulf Arab states | Qatar (n = 1,093) | Kuwait (n = 1,168) | KSA (n = 760) | P‐value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 57.9 ± 11.7 | 52.4 ± 11.3† | 63.3 ± 9.9‡ | 57.1 ± 11.2§ | ≤0.0001 | ||||
| Duration of diabetes (years) | 14.4 ± 9.2 | 10.0 ± 7.7† | 18.0 ± 9.2‡ | 15.2 ± 8.3§ | ≤0.0001 | ||||
| Female, % (n) | 46.8 | 1,408/,3011 | 39.4† | 428/1,086 | 47.3 b | 552/1,167 | 56.5§ | 428/758 | ≤0.0001 |
| Poor glycemic control, % (n) | 72.9 | 2,025/2,776 | 67.2† | 666/991 | 71.0† | 746/1,051 | 83.5‡ | 613/734 | ≤0.0001 |
| Hyperlipidemia, % (n) | 75.3 | 2,135/2,835 | 73.2† | 738/,1008 | 69.3† | 751/1,083 | 86.8‡ | 646/744 | ≤0.0001 |
| Hypertension, % (n) | 65.4 | 1,926/2,945 | 64.3† | 669/1,040 | 60.0† | 691/1,151 | 75.1‡ | 566/754 | ≤0.0001 |
| Obesity, % (n) | 56.9 | 1,584/2,785 | 53.3† | 510/957 | 56.6†,‡ | 624/1,102 | 62.0‡ | 450/726 | ≤0.01 |
| Physical activity, % (n) | 33.7 | 932/2,763 | 38.2† | 326/854 | 28.5‡ | 332/1,163 | 36.7† | 274/746 | ≤0.0001 |
| Smoking, % (n) | 22.5 | 635/2,821 | 17.3† | 157/909 | 27.4‡ | 319/1,163 | 21.2† | 159/749 | ≤0.0001 |
| Vitamin D | |||||||||
| ≥30 ng/mL | 33.6 | 755/2,248 | 21.1† | 142/674 | 44.8‡ | 383/854 | 31.9§ | 230/720 | ≤0.0001 |
| 20–29 ng/mL | 33.2 | 747/2,248 | 37.5† | 253/674 | 32.3†,‡ | 276/854 | 30.3‡ | 218/720 | |
| 10–19 ng/mL | 29.2 | 656/2,248 | 37.1† | 250/674 | 19.3‡ | 165/854 | 33.5† | 241/720 | |
| <10 ng/mL | 4.0 | 90/2,248 | 4.3† | 29/674 | 3.5† | 30/854 | 4.3† | 31/720 | |
| Systolic blood pressure (mmHg) | 131.6 ± 25.2 | 132.5 ± 18.0† | 129.9 ± 33.4†,‡ | 132.7 ± 18.2† | <0.05 | ||||
| Diastolic blood pressure (mmHg) | 73.1 ± 11.8 | 78.2 ± 10.2† | 71.4 ± 11.1‡ | 69.0 ± 12.6§ | ≤0.0001 | ||||
| BMI (kg/m2) | 32.0 ± 7.3 | 31.5 ± 7.4† | 32.4 ± 7.5‡ | 32.3 ± 6.8†,‡ | ≤0.01 | ||||
| HbA1c (%) | 8.2 ± 1.9 | 8.1 ± 2.0† | 7.9 ± 1.5‡ | 8.9 ± 2.0§ | ≤0.0001 | ||||
| HbA1c (mmol/mol) | 66.5 ± 20.7 | 65.5 ± 21.9† | 62.4 ± 16.7‡ | 73.8 ± 22.1§ | ≤0.0001 | ||||
| Cholesterol (mmol/L) | 4.2 ± 1.3 | 4.4 ± 1.2† | 3.9 ± 1.4‡ | 4.1 ± 1.1§ | ≤0.0001 | ||||
| Triglyceride (mmol/L) | 1.7 ± 1.2 | 1.8 ± 1.2† | 1.5 ± 1.2‡ | 1.8 ± 1.3† | ≤0.0001 | ||||
| HDL (mmol/L) | 1.12 ± 0.41 | 1.05 ± 0.31† | 1.21 ± 0.48‡ | 1.05 ± 0.37† | ≤0.0001 | ||||
| LDL (mmol/L) | 2.36 ± 2.32 | 2.56 ± 0.94† | 2.15 ± 3.51‡ | 2.40 ± 0.92†,‡ | ≤0.0001 | ||||
| Vitamin D (ng/mL) | 26.4 ± 12.3 | 23.3 ± 11.2† | 29.5 ± 12.8‡ | 25.6 ± 12.0§ | ≤0.0001 | ||||
Variables are summarized using means and standard deviations for numeric variables and frequency distribution for categorical variables. Continuous and categorical variables were compared using anova and χ2, respectively. Symbols (†, ‡, §): rows with similar symbols are not statistically significant and different symbols are significantly different.
BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein
Prevalence of DPN, painful DPN and foot ulceration
The overall prevalence of DPN was 33.3%, of whom 53.6% were undiagnosed (Figure 1; Table S1). The lowest prevalence of DPN was in Qatar compared with KSA and Kuwait (23.9 vs 35.9 vs 40.3%, P ≤ 0.0001). Kuwait had the lowest percentage of undiagnosed patients with DPN compared with KSA and Qatar (35.5 vs 57.5 vs 82.3%, P ≤ 0.0001). A total of 52.2% of those with DPN were at high risk of DFU, with the lowest prevalence being in Qatar compared with Kuwait and KSA (40.0 vs 54.9% and 59.3%, P ≤ 0.0001).
Figure 1.
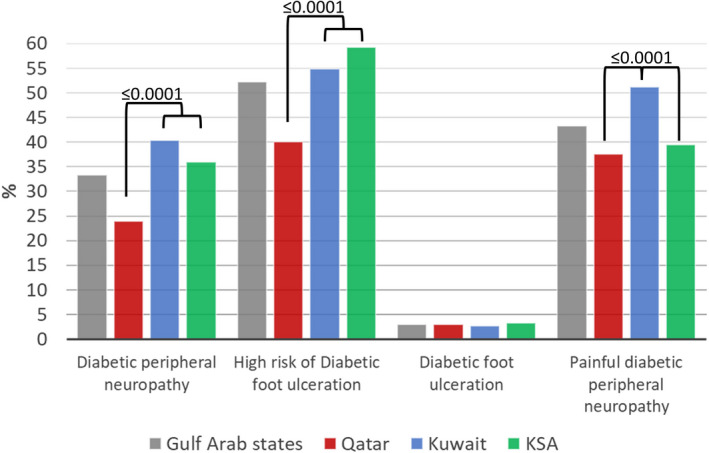
Prevalence of diabetic peripheral neuropathy, those at risk of foot ulceration, diabetic foot ulceration and painful diabetic peripheral neuropathy in patients with type 2 diabetes in secondary healthcare in Qatar, Kuwait and the Kingdom of Saudi Arabia (KSA). Variables were compared between countries using the χ2‐test. [Colour figure can be viewed at wileyonlinelibrary.com]
The highest percentage of patients with undiagnosed DPN was among those aged 20–50 years compared with those aged 51–60 years and >60 years (71.6 vs 56.8 and 48.2%, P ≤ 0.0001), and with type 2 diabetes for ≤10 years compared with those with 11–20 years and >20 years (70.4 vs 55.6 and 39.8%, P ≤ 0.0001).
The overall prevalence of painful DPN was 43.3%, of whom 54.3% were undiagnosed. Kuwait had the highest prevalence of painful DPN compared with Qatar and KSA (51.2 vs 37.5 and 39.5%, P ≤ 0.0001), but it also had the lowest percentage of patients not previously diagnosed with painful DPN (31.8 vs 71.5 and 75.7%, P ≤ 0.0001).
DFU was present in 2.9% of participants, and was comparable between countries (2.9 vs 2.6 vs 3.3%, P = 0.65).
Risk factors for DPN
The AOR for DPN increased 2.0–3.1‐fold with age (P ≤ 0.0001), 2.2–5.0‐fold with diabetes duration (P ≤ 0.0001), 2.0‐fold with obesity (P ≤ 0.0001), 1.6‐fold with poor glycemic control (HbA1c >7%; P ≤ 0.0001), 1.5‐fold with smoking cigarettes (P < 0.01) and 1.4‐fold with hyperlipidemia (P = 0.01), and decreased 1.7‐fold with physical activity (P ≤ 0.0001) and 1.4‐fold with being female (P ≤ 0.0001; Figure 2; Table 2). The prevalence of DPN differed significantly between countries, but after adjusting for confounders, this difference was lost (P = 0.27–0.79). Hypertension also lost its association with DPN after adjusting for confounders (P = 0.12).
Figure 2.
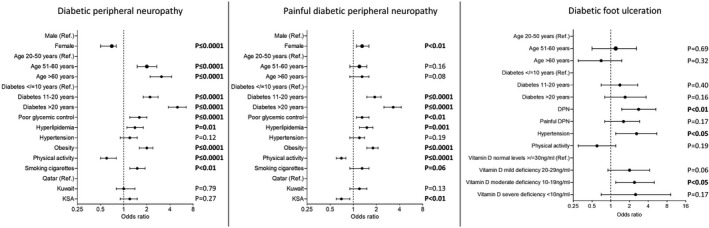
Predictors for diabetic peripheral neuropathy, painful diabetic peripheral neuropathy and diabetic foot ulceration in patients with type 2 diabetes in secondary healthcare in Qatar, Kuwait and Kingdom of Saudi Arabia (KSA). The variables with P ≤ 0.05 at the bivariate level were included in the multiple logistic regression. Adjusted odds ratios, 95% confidence intervals are presented.
Table 2.
Predictors for diabetic peripheral neuropathy in type 2 diabetes in secondary healthcare in Qatar, Kuwait and the Kingdom of Saudi Arabia
| Diabetic peripheral neuropathy | Gulf Arab states | Qatar | Kuwait | KSA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AOR | 95% CI | P‐value | AOR | 95% CI | P‐value | AOR | 95% CI | P‐value | AOR | 95% CI | P‐value | |
| Sex | ||||||||||||
| Male | 1 | 1 | 1 | 1 | ||||||||
| Female | 0.7 | 0.5–0.8 | ≤0.0001 | 0.6 | 0.4–0.9 | <0.05 | 0.6 | 0.4–0.8 | <0.01 | 0.9 | 0.6–1.4 | 0.69 |
| Age | ||||||||||||
| 20–50 years | 1 | 1 | 1 | 1 | ||||||||
| 51–60 years | 2.0 | 1.5–2.7 | ≤0.0001 | 2.1 | 1.2–3.5 | <0.01 | 1.6 | 0.9–3.0 | 0.13 | 1.9 | 1.2–3.2 | 0.01 |
| >60 years | 3.1 | 2.2–4.2 | ≤0.0001 | 2.7 | 1.5–4.8 | 0.001 | 3.1 | 1.7–5.6 | ≤0.0001 | 2.6 | 1.6–4.4 | ≤0.0001 |
| Diabetes duration | ||||||||||||
| ≤10 years | 1 | 1 | 1 | 1 | ||||||||
| 11–20 years | 2.2 | 1.8–2.8 | ≤0.0001 | 2.0 | 1.3–3.1 | <0.01 | 2.7 | 1.9–3.9 | ≤0.0001 | 1.8 | 1.2–2.8 | <0.01 |
| >20 years | 5.0 | 3.8–6.5 | ≤0.0001 | 7.1 | 3.6–13.7 | ≤0.0001 | 5.1 | 3.4–7.6 | ≤0.0001 | 4.8 | 2.9–8.1 | ≤0.0001 |
| Poor glycemic control | 1.6 | 1.2–2.0 | ≤0.0001 | 1.8 | 1.1–2.8 | <0.05 | 1.6 | 1.2–2.2 | <0.01 | 1.3 | 0.8–2.1 | 0.39 |
| Hyperlipidemia | 1.4 | 1.1–1.8 | 0.01 | 2.4 | 1.3–4.4 | <0.01 | 1.0 | 0.7–1.4 | 0.98 | 2.2 | 1.2–4.2 | 0.01 |
| Hypertension | 1.2 | 0.9–1.5 | 0.12 | 1.5 | 0.9–2.4 | 0.15 | 1.3 | 0.9–1.8 | 0.16 | 1.0 | 0.6–1.6 | 0.98 |
| Obesity | 2.0 | 1.6–2.4 | ≤0.0001 | 1.7 | 1.1–2.6 | <0.05 | 2.7 | 2.0–3.7 | <0.0001 | 1.4 | 0.9–2.0 | 0.12 |
| Physical activity | 0.6 | 0.5–0.8 | ≤0.0001 | 0.9 | 0.6–1.4 | 0.71 | 0.7 | 0.5–0.9 | <0.05 | 0.3 | 0.2–0.5 | <0.0001 |
| Smoking cigarettes | 1.5 | 1.2–1.9 | <0.01 | 0.9 | 0.5–1.6 | 0.64 | 2.0 | 1.4–2.9 | <0.0001 | 1.1 | 0.7–1.8 | 0.68 |
| Countries | ||||||||||||
| Qatar | 1 | |||||||||||
| Kuwait | 1.0 | 0.8–1.4 | 0.79 | |||||||||
| KSA | 1.2 | 0.9–1.5 | 0.27 | |||||||||
The multiple logistic regression model included all variables with P‐value of ≤0.05 at the bivariate level. Adjusted odds ratios (AOR), their corresponding 95% confidence intervals (CI) and P‐value are presented. KSA, Kingdom of Saudi Arabia.
Apart from age and diabetes duration, risk factors for DPN differed between countries. The risk factors with the highest odds ratio for DPN were diabetes duration >20 years (AOR 2.7, P = 0.001) and hyperlipidemia (AOR 2.4, P < 0.01) in Qatar, diabetes duration >20 years (AOR 5.1, P ≤ 0.0001) and obesity (AOR 2.7, P < 0.0001) in Kuwait, and >20 years of diabetes (AOR 4.8, P ≤ 0.0001) and physical activity (AOR 0.3, P < 0.0001) in KSA.
Risk factors for painful DPN
The AOR for painful DPN increased 1.9–3.3‐fold with diabetes duration (P ≤ 0.0001), 1.8‐fold with obesity (P ≤ 0.0001), 1.5‐fold with hyperlipidemia (P = 0.001), 1.3‐fold with poor glycemic control (P < 0.01) and 1.3‐fold with being female (P < 0.05), and decreased 1.4‐fold with physical activity (P ≤ 0.0001; Figure 2; Table 3). After adjusting for confounders, age (P = 0.08–0.16) and hypertension (P = 0.14) lost their association with painful DPN, but the association between countries and painful DPN remained significant (P < 0.01).
Table 3.
Predictors for painful diabetic peripheral neuropathy in type 2 diabetes in secondary healthcare in Qatar, Kuwait and the Kingdom of Saudi Arabia
| Painful diabetic peripheral neuropathy | Gulf Arab states | Qatar | Kuwait | KSA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AOR | 95% CI | P‐value | AOR | 95% CI | P‐value | AOR | 95% CI | P‐value | AOR | 95% CI | P‐value | |
| Sex | ||||||||||||
| Male | 1 | 1 | 1 | 1 | ||||||||
| Female | 1.3 | 1.1–1.6 | <0.01 | 1.5 | 1.0–2.3 | <0.05 | 1.2 | 0.9–1.5 | 0.35 | 1.4 | 0.9–2.2 | 0.08 |
| Age | ||||||||||||
| 20–50 years | 1 | 1 | 1 | 1 | ||||||||
| 51–60 years | 1.2 | 0.9–1.5 | 0.16 | 1.5 | 0.9–2.3 | 0.07 | 0.8 | 0.5–1.4 | 0.56 | 1.2 | 0.8–1.8 | 0.44 |
| >60 years | 1.3 | 0.9–1.6 | 0.08 | 2.1 | 1.3–3.3 | <0.01 | 1.0 | 0.6–1.6 | 0.94 | 0.9 | 0.5–1.4 | 0.54 |
| Diabetes duration | ||||||||||||
| ≤10 years | 1 | 1 | 1 | 1 | ||||||||
| 11–20 years | 1.9 | 1.5–2.3 | ≤0.0001 | 2.1 | 1.4–3.0 | <0.0001 | 1.9 | 1.4–2.6 | <0.0001 | 1.6 | 1.1–2.9 | <0.05 |
| >20 years | 3.3 | 2.5–4.2 | ≤0.0001 | 5.9 | 3.0–11.5 | <0.0001 | 3.0 | 2.1–4.3 | <0.0001 | 3.2 | 2.0–5.2 | <0.0001 |
| Poor glycemic control | 1.3 | 1.1–1.6 | <0.01 | 1.7 | 1.1–2.4 | <0.01 | 1.3 | 0.9–1.8 | 0.07 | 1.0 | 0.6–1.6 | 0.93 |
| Hyperlipidemia | 1.5 | 1.2–1.8 | 0.001 | 1.2 | 0.8–1.9 | 0.37 | 1.5 | 1.1–2.1 | <0.01 | 1.4 | 0.8–2.4 | 0.21 |
| Hypertension | 1.2 | 0.9–1.4 | 0.19 | 1.4 | 0.9–2.1 | 0.10 | 1.1 | 0.8–1.5 | 0.52 | 0.9 | 0.6–1.4 | 0.72 |
| Obesity | 1.8 | 1.5–2.1 | ≤0.0001 | 2.0 | 1.4–2.9 | <0.0001 | 2.0 | 1.6–2.7 | <0.0001 | 1.4 | 0.9–1.9 | 0.10 |
| Physical activity | 0.7 | 0.6–0.8 | ≤0.0001 | 0.8 | 0.6–1.2 | 0.36 | 0.7 | 0.5–0.9 | 0.01 | 0.5 | 0.4–0.8 | 0.001 |
| Smoking cigarettes | 1.3 | 0.9–1.6 | 0.06 | 1.8 | 1.1–3.0 | 0.01 | 1.2 | 0.9–1.6 | 0.33 | 1.1 | 0.7–1.8 | 0.68 |
| Countries | ||||||||||||
| Qatar | 1 | |||||||||||
| Kuwait | 1.2 | 0.9–1.5 | 0.13 | |||||||||
| KSA | 0.7 | 0.6–0.9 | <0.01 | |||||||||
The multiple logistic regression model included all variables with P‐value of ≤0.05 at the bivariate level. Adjusted odds ratios (AOR), their corresponding 95% confidence intervals (CI) and P‐value are presented. KSA, Kingdom of Saudi Arabia.
Risk factors for diabetic foot ulceration
The AOR for DFU increased 2.8‐fold with DPN (P < 0.01), 2.6‐fold with hypertension (P < 0.05) and 2.4‐fold with moderate vitamin D deficiency (P < 0.05; model 1), or 2.6‐fold with high risk of DFU (P = 0.01; model 2; Figure 2; Table S2). After adjusting for confounders, age (P = 0.32–0.69), diabetes duration (P = 0.16–0.40), painful DPN (P = 0.60) and physical activity (P = 0.19) lost their association with DFU.
Risk factors
The Kuwait cohort had the highest prevalence of those aged >60 years (62.2 vs 23.3% and 37.1%, P ≤ 0.0001), with type 2 diabetes duration >20 years (32.0 vs 8.1 and 20.9%, P ≤ 0.0001) and cigarette smokers (27.4 vs 17.3 and 21.2%, P ≤ 0.0001), and the lowest prevalence of those undertaking physical activity (28.5 vs 38.2 and 36.7%, P ≤ 0.0001; Table 1). The KSA cohort had the highest prevalence of those with poor glycemic control (83.5 vs 67.2 and 71.0%, P ≤ 0.0001), obesity (62.0 vs 53.3 and 56.6%, P ≤ 0.01) and hyperlipidemia (86.8 vs 73.2 and 69.9%, P ≤ 0.0001).
DISCUSSION
In the present cross‐sectional study of 3,021 patients with type 2 diabetes from SHC in Qatar, Kuwait and KSA, one in three patients had DPN, of whom one in two were at high risk of DFU, and yet over half of these patients had not been previously diagnosed. Similarly, painful DPN was present in one in three patients, and had not been previously diagnosed in half of them. Both DPN and painful DPN were associated with greater diabetes duration, obesity, poor glycemic control, hyperlipidemia and lower physical activity. DPN was associated with increasing age and smoking cigarettes, and although women had a lower risk of DPN, their risk of painful DPN was higher. The risk of DFU was higher in those with DPN, as was more severe neuropathy as evidenced by a higher VPT as well as hypertension and vitamin D deficiency.
We recently showed a higher‐than‐expected prevalence of DPN and painful DPN in Qatar 23 , 24 . We now extend these findings to show that the prevalence of DPN, painful DPN and risk for DFU differed significantly in SHC in Qatar, Kuwait and KSA using the same diagnostic methods, which might be attributed to differing management of risk factors. The overall prevalence of DPN was comparable to the UK (32%) 6 and Italy (31%) 7 , but relatively lower compared with Turkey (60%) 25 , Iran (49%) 26 , USA (45%) 8 and China (62%) 9 . This variability in the prevalence of DPN might be attributed to different study populations and diagnostic criteria of DPN used. These encouraging results might also reflect an increase in the number of healthcare centers providing universal health coverage and population‐based measures to reduce the prevalence of diabetes from 22.9% to 15.6% in Qatar, 23.1% to 12.2% in Kuwait and 23.9% to 15.8% in KSA between 2013 and 2019 according to the IDF Diabetes Atlas. These improvements might have impacted on the prevalence of painful DPN, as the current study shows a lower prevalence in KSA 4 and Kuwait 5 (39.5 vs 65.3 and 51.3%). The prevalence of DPN in the current study was higher than a previous study from KSA (35.9 vs 19.9%) 27 ; however, they defined DPN based on a higher vibration perception of ≥25 V compared with ≥15 V in the current study. The higher prevalence of painful DPN compared with DPN in the present study might be attributed to the criteria used to define these conditions. Painful DPN was defined according to DN4, whereas DPN was based on symptoms from DN4 and an elevated VPT (>15 V).
Our current study identified modifiable risk factors for DPN and painful DPN consistent with our previous findings. It identified that DPN was associated with physical activity, obesity and smoking cigarettes, painful DPN with physical activity, poor glycemic control and hyperlipidemia, and DFU with DPN, hypertension and moderate vitamin D deficiency. Indeed, poor glycemic control is associated with nerve damage 12 , and improving HbA1c has been associated with nerve repair in patients with DPN 28 . Obesity and hyperlipidemia are independently associated with DPN in type 2 diabetes patients 13 , 14 , and longitudinal studies have shown that weight, body mass index, high‐density lipoprotein and low‐density lipoprotein levels are associated with incident DPN 14 . In a cohort of obese patients with type 2 diabetes undergoing bariatric surgery, weight loss and an improvement in triglycerides was associated with an improvement in neuropathic symptoms and corneal nerve fiber regeneration 29 . Al Rashah et al. 30 also showed that the corneal nerve migration rate correlates with physical activity, and we have shown that the presence of insulin resistance limits nerve fiber regeneration after intensive glycemic control 31 . Balducci et al. 32 reported that aerobic exercise training was associated with a reduced incidence of impaired vibration perception and abnormal NCS. In this study, after adjusting for the modifiable risk factors, the prevalence of DPN did not differ between countries, suggesting that these factors are associated with DPN. Although active management of risk factors is advocated for all patients with type 2 diabetes, the present study shows a high heterogeneity in the management of risk factors between three Persian Gulf countries with mature health systems. Hence, more attention is required to implement DPN screening and better management of risk factors, particularly to encourage physical activity and reduce obesity.
Screening annually for DPN starting at diagnosis of type 2 diabetes is advocated by the American Diabetes Association 1 . The present study shows an alarmingly high prevalence of undiagnosed DPN (57.5–82.3%) and painful DPN (71.5–75.7%) in Qatar and KSA, and highlights the need for annual national screening for DPN 33 . Readily available and sensitive tests for diagnosing early DPN include VPT testing using a neurothesiometer, NerveCheck, 34 or sudomotor testing using Sudoscan or Neuropad 35 , and for painful DPN, questionnaires, such as the DN4 20 , Leeds Assessment of Neuropathic Symptoms and Signs pain scale, and Neuropathic Pain Symptom Inventory.
The worldwide prevalence of DFU is 6.3%, and varies from 1.5 to 16.6% 36 . The low prevalence of DFU in Qatar, Kuwait and KSA ranging from 2.6 to 3.3% is consistent with previous studies from the Middle East and North Africa region, showing a prevalence of 2.3% in KSA, 5.9% in Bahrain, 6.2% in Egypt and 4.2% in Jordan, compared to the USA (13.3%), Canada (14.8%), India (11.6%) and Belgium (16.6%). We confirm that the presence of DPN and loss of vibration perception are risk factors for DFU, in addition to prior history of DFU, foot deformities and peripheral vascular disease 37 , 38 .
The present study also shows that the odds ratio for DFU was 2.6‐fold higher in patients with hypertension, and 2.4‐fold higher in those with moderate vitamin D deficiency. A meta‐analysis of 801,985 patients with diabetes from 33 countries showed that the prevalence of hypertension was higher in patients with DFU compared to those without (63.4% vs 53.1%) 36 . Zubair et al. 39 reported that patients with DFU had significantly lower vitamin D levels compared to those without DFU. Vitamin D deficiency is associated with an increased susceptibility to wound infection 40 . Although the present study found no association between vitamin D deficiency and DPN, a meta‐analysis of 1,484 patients with type 2 diabetes showed that the odds of DPN was 2.7‐fold higher in those with vitamin D deficiency. 41 Our previous study 18 also showed that the odds ratio for painful DPN was 4.4‐fold higher with vitamin D insufficiency (<30 ng/mL), and 9.8‐fold higher with vitamin D deficiency (<20 ng/mL).
We acknowledge the outcomes of the present study are from patients attending SHC, which limits the generalizability of the results to all people with type 2 diabetes. We also acknowledge that VPT is a subjective psychophysical test, dependent on patient motivation, alertness and concentration 10 , and neurological evaluation of ankle reflexes might have improved the diagnosis of DPN. The cross‐sectional design of this study limits causal association between risk factors and DPN. However, this is the largest prevalence study to date that has deployed standardized methodology in the Gulf region to show that DPN, painful DPN and DFU occurs in 33.3, 43.3 and 2.9% of patients with type 2 diabetes attending SHC. Alarmingly, >50% of patients had not previously been diagnosed with DPN. We also identified obesity, poor glycemic control, hyperlipidemia, smoking cigarettes and reduced physical activity as modifiable risk factors for DPN and loss of vibration perception, and DPN, hypertension and vitamin D deficiency as risk factors for DFU. These findings argue for a systematic approach to the diagnosis and management of risk factors for DPN.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The research protocol was approved by Weill Cornell Medicine‐Qatar (WCM‐Q) IRB (IRB# 15–00078) and Hamad Medical Corporation (HMC) IRB (IRB# 16324/16) in Qatar, Dasman Center for Research and Treatment of Diabetes in Kuwait IRB (IRB# IRBH1 IORG0005964), and King Fahad Medical City in KSA IRB (IRB# IRB00010471).
Informed consent: All participants consented to take part in the study. The study acted in accordance with the tenets of the declaration of Helsinki.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Table S1 | Prevalence of diabetic peripheral neuropathy, painful diabetic peripheral neuropathy, undiagnosed diabetic peripheral neuropathy and painful Diabetic Peripheral Neuropathy foot ulceration, and those at risk of foot ulceration with type 2 diabetes in secondary healthcare in Qatar, Kuwait and the Kingdom of Saudi Arabia.
Table S2 | Predictors for diabetic foot ulceration in type 2 diabetes patients in secondary healthcare in Gulf Arab states.
ACKNOWLEDGMENTS
We thank the nurses and the physicians in the National Diabetes Centers, podiatry clinic and clinical laboratory at Dasman Diabetes Institute for their support, and all the participants for their efforts and commitment to be involved in the study.
Qatar National Research Fund, Grant BMRP‐5726113101. King Fahad Medical City, 17–057. This study received funding from Pfizer Gulf FZ LLC (W1230787) to purchase the neurothesiometers. The sponsor was not involved in any of the study activities.
[Correction added on May 17, 2022, after first online publication: Author name has been corrected as Imad Brema.]
REFERENCES
- 1. Pop‐Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the american diabetes association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apelqvist J, Agardh CD. The association between clinical risk factors and outcome of diabetic foot ulcers. Diabetes Res Clin Pract 1992; 18: 43–53. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017; 376: 2367–2375. [DOI] [PubMed] [Google Scholar]
- 4. Halawa MR, Karawagh A, Zeidan A, et al. Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr Med Res Opin 2010; 26: 337–343. [DOI] [PubMed] [Google Scholar]
- 5. Shehab D, Al‐Jarallah K, Abdella N, et al. Prospective evaluation of the effect of short‐term oral vitamin d supplementation on peripheral neuropathy in type 2 diabetes mellitus. Med Princ Pract 2015; 24: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Young MJ, Boulton AJ, MacLeod AF, et al. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993; 36: 150–154. [DOI] [PubMed] [Google Scholar]
- 7. Salvotelli L, Stoico V, Perrone F, et al. Prevalence of neuropathy in type 2 diabetic patients and its association with other diabetes complications: The Verona Diabetic Foot Screening Program. J Diabetes Complications 2015; 29: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 8. Mold JW, Vesely SK, Keyl BA, et al. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract 2004; 17: 309–318. [DOI] [PubMed] [Google Scholar]
- 9. Lu B, Yang Z, Wang M, et al. High prevalence of diabetic neuropathy in population‐based patients diagnosed with type 2 diabetes in the Shanghai downtown. Diabetes Res Clin Pract 2010; 88: 289–294. [DOI] [PubMed] [Google Scholar]
- 10. Malik RA. Wherefore art thou, o treatment for diabetic neuropathy? Int Rev Neurobiol 2016; 127: 287–317. [DOI] [PubMed] [Google Scholar]
- 11. Al‐Kaabi JM, Al‐Maskari F, Zoubeid T, et al. Prevalence and determinants of peripheral neuropathy in patients with type 2 diabetes attending a tertiary care center in the United Arab Emirates. J Diabetes Metab 2014; 5: 1–7. [Google Scholar]
- 12. Ferdousi M, Kalteniece A, Azmi S, et al. Diagnosis of neuropathy and risk factors for corneal nerve loss in type 1 and type 2 diabetes: a corneal confocal microscopy study. Diabetes Care 2021; 44: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and risk factors for diabetic neuropathy and painful diabetic neuropathy in primary and secondary healthcare in Qatar. J Diabetes Investig. 2021; 12: 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlesinger S, Herder C, Kannenberg JM, et al. General and abdominal obesity and incident distal sensorimotor polyneuropathy: insights into inflammatory biomarkers as potential mediators in the KORA F4/FF4 cohort. Diabetes Care 2019; 42: 240–247. [DOI] [PubMed] [Google Scholar]
- 15. American DA. 6. glycemic targets: standards of medical care in diabetes‐2019. Diabetes Care 2019; 42(Suppl 1): S61–S70. [DOI] [PubMed] [Google Scholar]
- 16. Moser M. World health organization‐international society of hypertension guidelines for the management of hypertension‐do these differ from the U.S. recommendations? Which guidelines should the practicing physician follow? J Clin Hypertens (Greenwich) 1999; 1: 48–54. [PubMed] [Google Scholar]
- 17. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: i‐xii, 1‐253. [PubMed] [Google Scholar]
- 18. Alam U, Petropoulos IN, Ponirakis G, et al. Vitamin D deficiency is associated with painful diabetic neuropathy. Diabetes Metab Res Rev 2021; 37: e3361. [DOI] [PubMed] [Google Scholar]
- 19. Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care 1994; 17: 557–560. [DOI] [PubMed] [Google Scholar]
- 20. Spallone V, Morganti R, D'Amato C, et al. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med 2012; 29: 578–585. [DOI] [PubMed] [Google Scholar]
- 21. Terkawi AS, Abolkhair A, Didier B, et al. Development and validation of Arabic version of the douleur neuropathique 4 questionnaire. Saudi J Anaesth 2017; 11(Suppl 1): S31–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Netten JJ, Bus SA, Apelqvist J, et al. Definitions and criteria for diabetic foot disease. Diabetes Metab Res Rev 2020; 36(Suppl 1): e3268. [DOI] [PubMed] [Google Scholar]
- 23. Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and management of diabetic neuropathy in secondary care in Qatar. Diabetes Metab Res Rev 2020; 36: e3286. [DOI] [PubMed] [Google Scholar]
- 24. Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and risk factors for painful diabetic neuropathy in secondary healthcare in Qatar. J Diabetes Investig. 2019; 10: 1558–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boru UT, Alp R, Sargin H, et al. Prevalence of peripheral neuropathy in type 2 diabetic patients attending a diabetes center in Turkey. Endocr J 2004; 51: 563–567. [DOI] [PubMed] [Google Scholar]
- 26. Kiani J, Moghimbeigi A, Azizkhani H, et al. The prevalence and associated risk factors of peripheral diabetic neuropathy in Hamedan, Iran. Arch Iran Med 2013; 16: 17–19. [PubMed] [Google Scholar]
- 27. Wang DD, Bakhotmah BA, Hu FB, et al. Prevalence and correlates of diabetic peripheral neuropathy in a Saudi Arabic population: a cross‐sectional study. PLoS One 2014; 9: e106935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ponirakis G, Abdul‐Ghani MA, Jayyousi A, et al. Effect of treatment with exenatide and pioglitazone or basal‐bolus insulin on diabetic neuropathy: a substudy of the Qatar Study. BMJ Open Diabetes Res Care 2020; 8: e001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adam S, Azmi S, Ho JH, et al. Improvements in diabetic neuropathy and nephropathy after bariatric surgery: a prospective cohort study. Obes Surg 2021; 31: 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al Rashah K, Pritchard N, Dehghani C, et al. Corneal nerve migration rate in a healthy control population. Optom Vis Sci 2018; 95: 672–677. [DOI] [PubMed] [Google Scholar]
- 31. Ponirakis G, Abdul‐Ghani MA, Jayyousi A, et al. Insulin resistance limits corneal nerve regeneration in patients with Type 2 diabetes undergoing intensive glycemic control. J Diabetes Investig. 2021; 12: 2002–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications 2006; 20: 216–223. [DOI] [PubMed] [Google Scholar]
- 33. Malik RA, Andag‐Silva A, Dejthevaporn C, et al. Diagnosing peripheral neuropathy in south East Asia: a focus on diabetic neuropathy. J Diabetes Investig 2020; 11: 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ponirakis G, Odriozola MN, Odriozola S, et al. NerveCheck: an inexpensive quantitative sensory testing device for patients with diabetic neuropathy. Diabetes Res Clin Pract 2016; 113: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ponirakis G, Petropoulos IN, Fadavi H, et al. The diagnostic accuracy of Neuropad for assessing large and small fibre diabetic neuropathy. Diabet Med 2014; 31: 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang P, Lu J, Jing Y, et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta‐analysis (dagger). Ann Med 2017; 49: 106–116. [DOI] [PubMed] [Google Scholar]
- 37. Sinwar PD. The diabetic foot management ‐ recent advance. Int J Surg 2015; 15: 27–30. [DOI] [PubMed] [Google Scholar]
- 38. Mariam TG, Alemayehu A, Tesfaye E, et al. Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients who attend the diabetic follow‐up clinic at the university of gondar referral hospital, North West Ethiopia, 2016: institutional‐based cross‐sectional study. J Diabetes Res 2017; 2017: 2879249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zubair M, Malik A, Meerza D, et al. 25‐Hydroxyvitamin D [25(OH)D] levels and diabetic foot ulcer: is there any relationship? Diabetes Metab Syndr 2013; 7: 148–153. [DOI] [PubMed] [Google Scholar]
- 40. van Etten E, Decallonne B, Bouillon R, et al. NOD bone marrow‐derived dendritic cells are modulated by analogs of 1,25‐dihydroxyvitamin D3. J Steroid Biochem Mol Biol 2004; 89‐90: 457–459. [DOI] [PubMed] [Google Scholar]
- 41. Lv WS, Zhao WJ, Gong SL, et al. Serum 25‐hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta‐analysis. J Endocrinol Invest 2015; 38: 513–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Prevalence of diabetic peripheral neuropathy, painful diabetic peripheral neuropathy, undiagnosed diabetic peripheral neuropathy and painful Diabetic Peripheral Neuropathy foot ulceration, and those at risk of foot ulceration with type 2 diabetes in secondary healthcare in Qatar, Kuwait and the Kingdom of Saudi Arabia.
Table S2 | Predictors for diabetic foot ulceration in type 2 diabetes patients in secondary healthcare in Gulf Arab states.


