Abstract
Cardiac xenotransplantation has been proposed to bridge the gap between supply and demand for patients with end-stage heart failure requiring transplantation. However, differences in pig anatomy compared with human anatomy require modification of the surgical approach. In addition, careful consideration should be given to size matching before transplantation. (Level of Difficulty: Advanced.)
Key Words: cardiac transplantation, chronic heart failure, genetic engineering
Abbreviations and Acronyms: CT, computed tomography; GHRKO, growth hormone receptor knockout; IVC, inferior vena cava; PA, pulmonary artery; RA, right atrium; SVC, superior vena cava
Central Illustration
The first genetically modified pig-to-human cardiac xenotransplantation was performed on January 7, 2022, from a 14-month-old 110-kg genetically altered source animal.1 To reduce the potential of a possible disparate size growth after transplantation, growth hormone receptor was knocked out (GHRKO).2 These source animals have been well characterized at 6 months of age. GHRKO source animals are healthy, and their hearts are physiologically and metabolically normal. However, experience with ages and weights beyond this age is lacking.3
Learning Objectives
-
•
To understand the differences in pig and human heart anatomy as it related to the surgical technique for cardiac xenotransplantation.
Anatomical Differences Between the Pig and Human Hearts
Although pig hearts are markedly similar to human hearts, there are subtle differences in the anatomy. We have found the diameter of the great vessels to be proportionally smaller in pigs than in humans, particularly for the ascending aorta and main pulmonary artery (PA). In addition, the length of the great vessels proximal to major branches is proportionally shorter in pigs than in humans. For the superior vena cava (SVC), this places the takeoff of the azygous vein closer to the superior cavoatrial junction. For the aorta, there are generally only 2 cerebral branches: the right and the left innominate arteries.
By contrast, the suprahepatic inferior vena cava (IVC) is longer in pigs than in humans. As opposed to humans, where the SVC and IVC enter the right atrium (RA) 180° relative to one another (in a straight line), in pigs the SVC and IVC enter at nearly 90° relative to one another.4 Pigs have a prominent hemiazygous vein that enters directly into the coronary sinus anterior to the left pulmonary veins. Pigs also have smaller atria relative to cardiac mass.
The right and left coronary ostia in pigs arise from the aortic root at 90° relative to one another, as opposed to humans, where it is typically 120 to 140°. In addition, the left anterior descending artery is rightward of the left ventricular apex in pigs instead of overlying it, as in humans. Differences in coronary anatomy become important for cardiac catheterization after cardiac xenotransplantation.
Approach to Procurement
Our team’s approach to cardiac procurement from pigs is similar to standard procurement in humans.5 A midline sternotomy is directed toward the sternocostal joints superiorly on the right. This is done to avoid the keel of the pig sternum superiorly, which courses ventrally at 90° and is thicker than a standard blade for our sternal saw.
Several anatomical differences need to be considered when procuring a pig heart. The length of the ascending aorta is particularly short in pigs; therefore, the cardioplegia catheter is placed just proximal to the origin of the right innominate artery. Once the cardioplegia catheter has been inserted, blood is drawn to prepare the 30 cc/kg, 1:4 blood:XVIVO heart solution (XVIVO Perfusion) for induction of arrest.
The entrance of the azygous vein into the SVC is often closer to the superior cavoatrial junction than in humans and is important to identify before encircling the SVC. Ties placed inferior to the azygous vein are avoided because of the proximity to the superior cavoatrial junction and the sinoatrial node. The aortic cross-clamp is placed across the aortic arch to accommodate a short ascending aorta. Dissection of the arch between the right and left innominate arteries is performed, and an umbilical tape is placed around the ascending aorta to aid in cross-clamping.
The suprahepatic cava is clamped external to the pericardium for right-sided decompression. The IVC is only partially transected to prevent outflow obstruction from the atrium because the IVC can retract posterior to the heart, given the angle of entrance to the RA. The left side is decompressed by transection of the right superior pulmonary vein. During procurement, careful attention to the hemizygous vein coming from the coronary sinus near the left pulmonary veins should be noted.
We use the XVIVO system for nonischemic perfusion of the donor heart. A dual-lumen cannula is placed into the transected aorta at the level of the left innominate artery. The mitral valve is stented open with a silicon semilunar tube fastened to the left atrial cuff to prevent left ventricular distention. The heart is maintained at 8°C, 20 mm Hg at a physiologic pH, and oxygenated until implantation with a blood mix of XVIVO perfusate.
Approach to Implantation
A biatrial implantation allowed accommodation for significant atrial size discrepancies resulting from this patient’s long-standing dilated cardiomyopathy and mitral regurgitation. It reduced the risk of caval kinking caused by malalignment from anatomical variations. Before implantation, final backbench preparation for biatrial implantation includes oversewing the entry site of the azygous vein, the SVC, and hemiazygous vein from the coronary sinus. The RA is opened from the posterior IVC superior toward the right atrial appendage.
In this recipient, the left atrium was nearly 3 times larger than in the donor heart. To accommodate for this mismatch, a wedge of recipient left atrial tissue was excised between the right and left superior pulmonary veins. The rest of the size mismatch was made up for by asymmetric suturing traveling less distance on the donor tissue than on the recipient. To aid in proper left atrial alignment on implantation, the previous suture line from the closure of the hemiazygous vein on the donor heart should align with the confluence of the left superior and inferior pulmonary veins in the recipient.
The recipient RA was similarly large in comparison with the donor RA. To accommodate for this mismatch, the donor right atriotomy was extended to the superior aspect of the right atrial appendage, and an asymmetric suturing technique was used. In addition to the circumferential mismatch, once the left atrium was anastomosed, the midpoints of the donor and recipient right atria were not aligned with significant right lateral position of the native atrium. To accommodate this, the recipient lateral wall and cavae were freed to reach the more medially fixed donor RA.
The PA mismatch was accommodated for with asymmetric suturing as well. Another option would be to use the donor PA beyond the bifurcation to have a larger rim of donor tissue to anastomose to the recipient main PA instead of sewing the donor main PA to the recipient main PA. The short PA in the pig would allow for this without a significant concern of having a long main PA after implantation in the recipient that could be prone to kinking.
The aortic mismatch was also significant. The opening in the aortic arch through the origin of the left innominate artery extended into the origin of the right innominate artery. This allowed for a larger circumference of donor aortic tissue to anastomose to the larger recipient aorta.
Observations Between GHRKO Source Animal Heart and Wild-Type Pigs
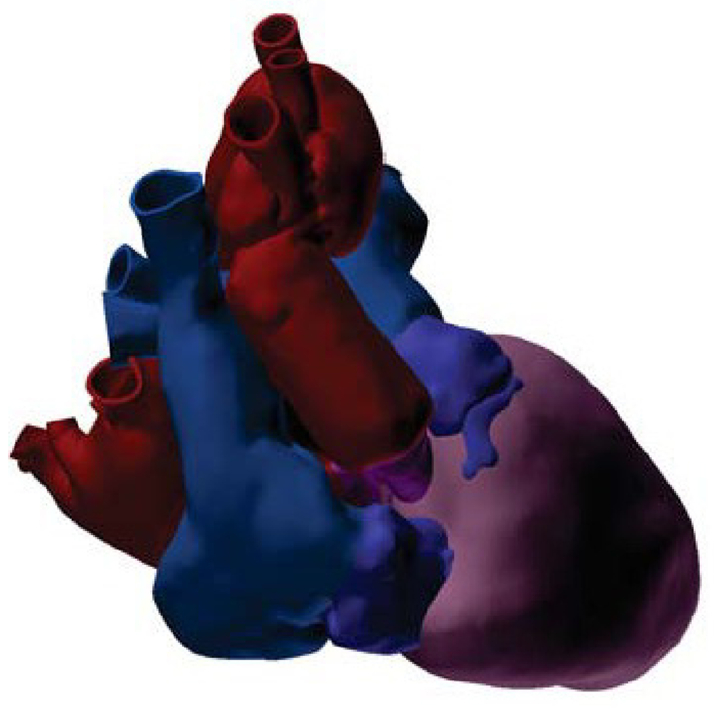
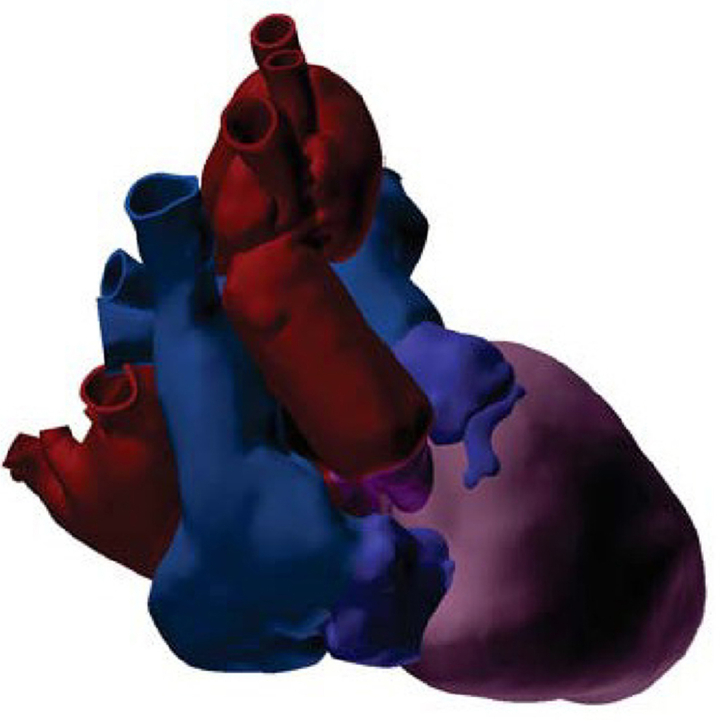
The GHRKO cardiac xenograft in the 90-kg recipient (Video 1) is demonstrated by a CT 3-dimensional reconstruction imaging 7 days after xenotransplantation (Figure 1). Despite the notable size mismatch at all 4 anastomotic sites, no significant gradients were observed on postoperative echocardiography.
Figure 1.
3-Dimensional Reconstruction
The cardiac xenograft is shown in the 90-kg recipient from CT 7 days after xenotransplantation. Ventricles shown in pink. Donor aorta and left atrium shown in purple. Recipient aorta and left atrium shown in red. Donor right atrium and pulmonary artery shown in dark blue. Recipient right atrium and pulmonary artery shown in light blue.
The cardiac xenograft for human transplantation weighed 328 g at procurement from a 110-kg 14-month adult GRKO pig. Xenograft measurements were obtained from a postimplantation CT scan because the graft could not be manipulated or measured between procurement and implantation. By our estimates, total ventricular mass was 232 gm, ascending aortic diameter was 16.6 mm, and pulmonary artery diameter was 19.3 mm.
By comparison, our evaluation of 7 adult wild-type Landrace pigs, averaging 100 kg, had a heart mass of 454 ± 32 g. Ventricular mass by weight was 321 ± 28 g. Ascending aortic diameter was 23 ± 1.5 mm, and pulmonary artery diameter was 24 ± 0.84 mm. Metrics are summarized between wild type, the GHRKO xenograft, and predicted recipient values in Table 1.
Table 1.
Heart Weight and Vessel Sizes
| WT Pigs | GHRKO Pig | Recipient | |
|---|---|---|---|
| Body weight (kg) | 100 | 110 | 85 |
| Heart weight (g) | 454 (397-492) | 328 | - |
| HW:BW ratio (g/kg) | 4.6 (4.0-4.9) | 2.98 | - |
| Total ventricular mass (g) | 321 (280-349) | 232a | 196.81b |
| VM:BW ratio (g/kg) | 3.2 (2.8-3.5) | 2.1 | 2.3 |
| LV mass (g) | 244c | 177c/163d | 171.84b |
| RV mass (g) | 76c | 55c | 24.97b |
| Ao diameter (mm) | 23 (20-24) | 16.6 | 38.2 |
| PA diameter (mm) | 24 (23-25) | 19.3 | 34.4 |
| Ao:VM ratio (mm/g) | 0.072 | 0.072 | - |
| PA:VM ratio (mm/g) | 0.075 | 0.083 | - |
Ao = ascending aorta; BW = body weight; GHRKO = growth hormone receptor knockout; HW = heart weight; LV = left ventricle; PA = pulmonary artery; RV = right ventricle; VM = ventricular mass; WT = wild-type.
Calculated on the basis of WT pig heart ratios.
Predicted on the basis of Reed et al.12
Predicted on the basis of Booth et al.11
Estimated on the basis of 3-dimensional volumetric reconstruction on CT.
Discussion
Anatomical differences between pig and human hearts necessitate modifications of the surgical approach for successful procurement and implantation of a pig heart xenograft into a human. One of the bigger challenges is appropriately sizing the donor pig heart to the recipient.6 Inasmuch as the ventricular mass data for the xenograft were not obtainable before transplantation, and that this was the first GHRKO pig xenograft with recognized limitations of use in this setting, we performed transesophageal echocardiography on the pig to assess the stroke volume and cardiac output.
Another consideration is the size of the great vessels relative to the size of the heart. From autopsy studies on healthy humans without pathological abnormalities, the average heart weight for men was found to be 331 ± 56.7 g (range, 188 to 575 g) and for women was 245 ± 51.8 g (range, 156 to 422 g).7,8 From CT studies of >4,000 adult humans, the average ascending aortic diameter is 33.2 ± 4.1 mm, with even the smallest subgroups (women <45 years of age with a body surface area <1.70 m2) averaging 28.4 ± 2.7 mm (range, 22.6 to 39.8 mm).9 Despite having an ascending aortic diameter of 16.5 mm, the implanted heart had no significant gradients across any of the valves or anastomoses in our recipient, and it generated adequate cardiac output.
Evidence on pig heart size in adult GHRKO pigs beyond 6 months of age is scarce. In published studies, their average weight at 6 months is 33.0 ± 1.5 kg. Their phenotype is noted to have increased adiposity, with markedly increased body fat (21.5% ± 0.7%, compared with age-matched wild-type pigs, which have 11.4% ± 0.5%).10 However, these data suggest that the body weight of GHRKO pigs may overestimate the heart size of the source animal for matching potential recipients for transplantation.
As these animals grow, transthoracic echocardiography becomes increasingly difficult because of the anatomy of the thoracic cavity, lungs overlying midline, thick sternum, and increasing adiposity of the GHRKO phenotype. It may be necessary to use screening transesophageal echocardiography for size matching before transplantation to overcome this limitation.
Conclusions
Successful cardiac xenotransplantation from porcine source animals is possible with careful consideration of the anatomical differences between porcine and human hearts, a modified approach to procurement and implantation, and careful consideration of the relative overestimation of ventricular mass when source animals are weight matched to recipients.
Funding Support and Author Disclosures
The genetically modified source animal was provided in-kind by Revivicor, Inc., Blacksburg, Virginia, USA. Funding for this study was provided by the University of Maryland Medical Center and School of Medicine, Baltimore, Maryland, USA. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Implanted Cardiac Xenograft, The cardiac xenograft is shown beating in situ in the recipient after implantation.
References
- 1.Griffith B.P., Goerlich C.E., Singh A.K., et al. Genetically modified porcine-to-human cardiac xenotransplantation. N Engl J Med. 2022;387:35–44. doi: 10.1056/NEJMoa2201422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goerlich CE, Griffith B, Hanna P, et al. The growth of xenotransplanted hearts can be reduced with growth hormone receptor knockout pig donors. J Thorac Cardiovasc Surg. Published online Sep 4, 2021. https://doi.org/10.1016/j.jtcvs.2021.07.051 [DOI] [PMC free article] [PubMed]
- 3.Hinrichs A., Riedel E.O., Klymiuk N., et al. Growth hormone receptor knockout to reduce the size of donor pigs for preclinical xenotransplantation studies. Xenotransplantation. 2021;28 doi: 10.1111/xen.12664. [DOI] [PubMed] [Google Scholar]
- 4.Crick S.J., Sheppard M.N., Ho S.Y., Gebstein L., Anderson R.H. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. 1998;193(Pt 1):105–119. doi: 10.1046/j.1469-7580.1998.19310105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goerlich CE, Griffith BP, Shah A, et al. A standardized approach to orthotopic (life-supporting) porcine cardiac xenotransplantation in a non-human primate model. Research Square. Published online December 15, 2021. https://doi.org/10.21203/rs.3.rs-1138842/v1
- 6.Yang T.-S. Size concerns regarding pig hearts for xenotransplantation. Xenotransplantation. 2006;13:12–13. doi: 10.1111/j.1399-3089.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 7.Molina D.K., DiMaio V.J.M. Normal organ weights in men: part I—the heart. Am J Forensic Med Pathol. 2012;33:362–367. doi: 10.1097/PAF.0b013e31823d298b. [DOI] [PubMed] [Google Scholar]
- 8.Molina D.K., DiMaio V.J.M. Normal organ weights in women: part I—the heart. Am J Forensic Med Pathol. 2015;36:176–181. doi: 10.1097/PAF.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 9.Wolak A., Gransar H., Thomson L.E.J., et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. J Am Coll Cardiol Img. 2008;1:200–209. doi: 10.1016/j.jcmg.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Hinrichs A., Kessler B., Kurome M., et al. Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver. Mol Metab. 2018;11:113–128. doi: 10.1016/j.molmet.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth N.H., Maaske C.A., Nielsen T.W. Ventricular weight ratios of normal swine and from swine with pulmonic stenosis. J Appl Physiol. 1966;21:1256–1260. doi: 10.1152/jappl.1966.21.4.1256. [DOI] [PubMed] [Google Scholar]
- 12.Reed R.M., Netzer G., Hunsicker L., et al. Cardiac size and sex-matching in heart transplantation: size matters in matters of sex and the heart. J Am Coll Cardiol HF. 2014;2:73–83. doi: 10.1016/j.jchf.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Implanted Cardiac Xenograft, The cardiac xenograft is shown beating in situ in the recipient after implantation.