Abstract
Background:
Nanoliposomal encapsulation of irinotecan (nal-IRI) with 5-fluorouracil and leucovorin (5-FU/LV) has shown a survival benefit for gemcitabine-pretreated patients with metastatic pancreatic adenocarcinoma (mPAC). The aim of this study was to evaluate the effectiveness and safety of nal-IRI with 5-FU/LV for use beyond second-line treatment after standard frontline therapy for mPAC.
Method:
This multicenter, retrospective, non-comparative observational study included mPAC patients who received nal-IRI plus 5-FU/LV as third- or later-line therapy after disease progression on first-line FOLFIRINOX (FFX) or gemcitabine plus nab-paclitaxel.
Results:
In all, 128 patients who received nal-IRI plus 5-FU/LV beyond second-line treatment between October 2017 and July 2021 were analyzed. Most patients (82%) received nal-IRI plus 5-FU/LV as a third-line treatment. The median overall survival (OS) was 4.9 months and the median progression-free survival (PFS) was 2.4 months. Patients with better Eastern Cooperative Oncology Group (ECOG) performance status experienced significantly longer OS (ECOG 0, 8.7 months; ECOG 1, 4.8 months; ECOG 2, 2.9 months; p < 0.001) and PFS (3.9 months; 2.1 months; 1.5 months; p = 0.019). Patients who had not been previously treated with FFX or had a time to progression of 7 months or more on FFX experienced longer OS and PFS than those who did not (6.1 months and 5.6 versus 4.1 months, p = 0.053; 3.6 months and 2.4 versus 2.1 months, p = 0.002). The most common adverse events were neutropenia (56%) and anemia (51%).
Conclusion:
Our real-world data indicated that nal-IRI plus 5-FU/LV can be effective not only as second-line therapy, but also as third-line or later-line treatment in selected patients. Nal-IRI plus 5-FU/LV may be particularly beneficial for the survival of patients that maintain good general condition or those with favorable prior experience to irinotecan.
Keywords: antineoplastic agents, carcinoma, irinotecan, liposomes, pancreatic ductal, survival
Introduction
Pancreatic cancer is the seventh leading cause of cancer deaths worldwide. It is predicted to be the second leading cause of cancer deaths in the United States by 2030. 1 Patients with pancreatic adenocarcinoma (PAC), the most common histological type of pancreatic cancer, have a poor prognosis with a 5-year survival rate of 9%. 2 Only less than 20% of patients have resectable PAC at initial diagnosis, and the majority of patients present with unresectable advanced stage pancreatic cancer. Systemic chemotherapy is the mainstay treatment strategy for unresectable PAC. However, options of chemotherapeutic regimens were limited until the emergence of FOLFIRINOX [FFX; combination of 5-fluorouracil (5-FU), leucovorin (LV), irinotecan, and oxaliplatin] and the gemcitabine plus nab-paclitaxel (GnP). Both regimens significantly improved response rates and survival benefits compared to sole gemcitabine treatment in their pivotal phase III studies, and have currently been considered as standard first-line treatments for patients with unresectable PAC.3–6
As approximately half of patients receive second-line treatment, PAC therapy should now be considered as a continuum care for patients who are fit, with second-line and even third-line treatments. 7 Patients who have undergone gemcitabine-based therapy may consider receiving fluoropyrimidine-based combination therapies as a subsequent therapy, and vice versa. 6 A combination of 5-FU, LV, and oxaliplatin (FOLFOX) or 5-FU, LV, and irinotecan (FOLFIRI) was used for rescue after gemcitabine-based therapy in the past decade. 8 Subsequent FFX or GnP can be considered as a second-line therapy for selected PAC patients in good general condition, resulting in favorable survival outcomes. 9 However, the number of high-quality data to establish a standard therapeutic regimen in the second-line setting is limited.
Recently, nanoliposomal encapsulation of irinotecan (nal-IRI) plus 5-FU/LV has shown an improvement of survival outcomes in the intent-to-treat population over 5-FU/LV in a global NAPOLI-1 phase III trial of patients with metastatic PAC following treatment with gemcitabine. The trial showed a medial overall survival (OS) of 6.1 months and progression-free survival (PFS) of 3.1 months in the nal-IRI plus 5-FU/LV group compared to 4.2 months and 1.5 months in the 5-FU/LV group. 10
In third-line setting, the only randomized evidence is identified in the NAPOLI-1 trial. Approximately 30% of the patients had received at least two prior lines of chemotherapy in the study. Although some retrospective studies reported real-world data of nal-IRI plus 5-FU/LV as second-line therapy in gemcitabine-refractory advanced PAC, data reporting efficacy beyond second-line therapy are scarce.11–13 To the best our knowledge, there is no real-world data solely comprised of patients with third- or later-line nal-IRI plus 5-FU/LV treatment. Therefore, the present multicenter, retrospective study is aimed to evaluate the effectiveness and safety of nal-IRI plus 5-FU/LV for use beyond second-line treatment after standard frontline therapy in patients with metastatic PAC (mPAC).
Methods
Patients
This multicenter, retrospective, non-comparative observational study included patients that received nal-IRI plus 5-FU/LV as third- or later-line therapy after disease progression on first-line FFX or GnP for unresectable PAC at two tertiary referral hospitals in Korea, between October 2017 and July 2021. In cases with tumor recurrence after previous pancreatectomy, the treatment line was counted after the start of palliative chemotherapy.
Demographic and clinicopathological data including performance status [Eastern Cooperative Oncology Group (ECOG)], tumor location, tumor size, site of metastatic lesions, date of diagnosis, date of disease progression, baseline laboratory findings, and detailed information of treatment were retrospectively collected and analyzed from the electronic medical records system. The cutoff date for data analysis was 11 November 2021. Survival data were obtained from the Ministry of the Interior and Safety of Korea. The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the institutional review boards of participating institutions (National Cancer Center, approval number NCC2021-0324; Seoul National University Hospital, approval number H-2110-173-1266).
Drugs were administered as described in the NAPOLI-1 trial (nal-IRI 70 mg/m2 as the irinotecan-free base, followed by LV 400 mg/m2, and 5-FU 2400 mg/m2 over 46 h, every 2 weeks). 10 Dose modification and schedule adjustment were determined according to the general condition of the individual patient and toxicities at the discretion of the treating physician. Tumor assessment was conducted prior to treatment and every 6–8 weeks by contrast enhanced computed tomography or magnetic resonance imaging. Tumor response was assessed by the clinician according to the Response Evaluation Criteria in Solid Tumors version 1.1. Adverse events were evaluated and graded in accordance with the Common Terminology Criteria for Adverse Events, version 4.0.
Objectives and statistical analysis
OS was defined as the period between the initiation of nal-IRI plus 5-FU/LV and death or last follow-up. PFS defined as the time from the date of initiating nal-IRI plus 5-FU/LV treatment to the date of disease progression, death of any cause, or loss to follow-up. Survival outcomes from the initiation of first-line therapy (the starting date of palliative FFX or GnP treatment) were also evaluated. The objective response rate (ORR) was defined as the percentage of patients with complete response or partial response. The disease control rate (DCR) was defined as ORR plus the rate of stable disease.
Descriptive statistics were expressed as median (range), number (percentage, %) as appropriate. Survival analysis was performed using the Kaplan–Meier method and compared by log-rank test. We performed subgroup analyses according to performance status, irinotecan exposure and time to progression (TTP), line of therapy, presence of dose reduction, first-line chemotherapy regimen, and sequential chemotherapy regimens. Patients previously treated with FFX were divided by TTP of 7 months, and this cutoff level was determined based on our previously reported median PFS of patients who were treated with FFX as first-line therapy. 9 Univariable analyses for survival outcomes were performed by the Cox proportional hazard regression model test. We fitted the multivariable model using pre-selected variables with univariable p value < 0.2. The final model was determined by performing backward selection with an elimination criterion of p value > 0.1. A p value < 0.05 was considered to be statistically significant. All statistical analyses were performed using SAS systems version 9.2 (SAS Institute, Inc, Cary, NC, USA) and SPSS 24.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Overall characteristics of patients treated with nal-IRI plus 5-FU/LV
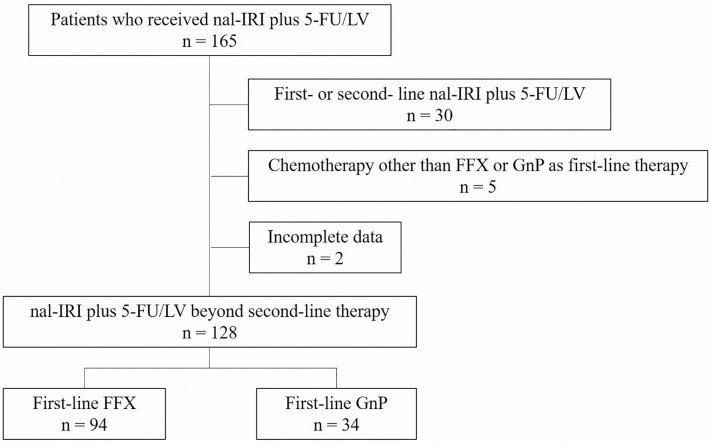
Among a total of 165 patients who received nal-IRI plus 5-FU/LV, 128 patients who received nal-IRI plus 5-FU/LV beyond second-line treatment were analyzed (Figure 1). Baseline demographics and clinicopathological characteristics are summarized in Table 1. Median age was 64 years (range, 37–79) and 81 patients (63%) were male. The majority of patients had ECOG performance status of 0 (23%) or 1 (71%). Liver was the most common metastatic site (68%) followed by peritoneum (31%). Curative-intent surgical resection and radiotherapy were previously performed in 51 (40%) and 37 (29%) patients, respectively.
Figure 1.
Flow chart of patients.
Table 1.
Baseline characteristics of patients.
| N = 128 (%) | |
|---|---|
| Age | 64 (37–79) |
| <65 | 68 (53.1%) |
| ⩾65 | 60 (46.9%) |
| Gender | |
| Male | 81 (63.3%) |
| Female | 47 (36.7%) |
| ECOG performance status | |
| 0 | 29 (22.7%) |
| 1 | 91 (71.1%) |
| 2 | 8 (6.3%) |
| Primary tumor location | |
| Head | 34 (26.6%) |
| Body | 43 (33.6%) |
| Tail | 51 (39.8%) |
| Site of metastatic lesions | |
| Liver | 87 (68.0%) |
| Lung | 29 (22.7%) |
| Peritoneum | 39 (30.5%) |
| Lymph node | 29 (22.7%) |
| Other | 8 (6.3%) |
| Median CA 19-9 (IU/mL) | 939 (2–13,160) |
| Previous surgery | 51 (39.8%) |
| Previous radiotherapy | 37 (28.9%) |
| Prior lines of palliative chemotherapy, median (range) | 2 (2–4) |
| 2 | 105 (82.0%) |
| 3 | 19 (14.8%) |
| 4 | 4 (3.1%) |
| Prior first-line palliative chemotherapy regimen | |
| FOLFIRINOX | 94 (73.4%) |
| Gemcitabine plus nab-paclitaxel | 34 (26.6%) |
| Prior exposure to irinotecan (FOLFIRINOX) | 99 (77.3%) |
| Controlled disease on FOLFIRINOX | 68 (53.1%) |
| TTP on FOLFIRINOX ⩾7 months | 47 (36.7%) |
ECOG, Eastern Cooperative Oncology Group; TTP, time to progression
Most patients (82%) received nal-IRI plus 5-FU/LV as third-line treatment. FFX (73%) was most commonly given as the first-line therapy prior to nal-IRI plus 5-FU/LV treatment. Prior administration of irinotecan was observed in 99 patients (77%), all of whom had been treated with FFX as first- or second-line therapy. Among patients with previous FFX treatments, 81 patients experienced controlled disease (partial response, 29; stable disease, 52 patients) and 18 patients had progressive disease on FFX treatment.
Treatment overview of nal-IRI plus 5-FU/LV
The dose delivery of nal-IRI plus 5-FU/LV beyond second-line therapy is presented in Table 2. The median number of cycles of chemotherapy was three (range, 1–26). In all, 36 patients (28%) received only one or two cycles of nal-IRI plus 5-FU/LV due to rapid disease progression, deterioration of general condition, or loss to follow-up. In total, 56 patients (44%) received a reduced dose of nal-IRI at the beginning or during treatment: the most common reduced dose amount was 56 mg/m2. One patient started nal-IRI plus 5-FU/LV treatment with 56 mg/m2 of nal-IRI and received a subsequent dose reduction to 47 mg/m2 due to adverse events.
Table 2.
Treatment overview.
| N = 128 (%) | |
|---|---|
| Number of cycles, median | 3 (1–26) |
| <3 | 36 (28.1%) |
| ⩾3 | 92 (71.9%) |
| Starting dose of nal-IRI | |
| 70 mg/m2 | 83 (64.8%) |
| 63 mg/m2 | 1 (0.8%) |
| 56 mg/m2 | 33 (25.8%) |
| 50 mg/m2 | 11 (8.6%) |
| Dose reduction during treatment | 12 (9.4%) |
| 56 mg/m2 | 4 (3.1%) |
| 50 mg/m2 | 7 (5.5%) |
| 47 mg/m2 | 1 (0.8%) |
| Subsequent chemotherapy after nal-IRI | |
| No | 75 (58.6%) |
| Yes | 53 (41.4%) |
naI-IRI, nanoliposomal encapsulation of irinotecan.
The effectiveness of treatment with nal-IRI plus 5-FU/LV is summarized in Table 3. Only five patients (4%) had a partial response and 56 patients (44%) a stable disease at the time of analysis, indicating a DCR of 48%. In all, 53 patients (41%) received subsequent chemotherapy after disease progression on nal-IRI plus 5-FU/LV treatment.
Table 3.
Effectiveness of treatment with nal-IRI plus 5-FU/LV.
| N = 128 (%) | |
|---|---|
| Best response | |
| Partial response | 5 (3.9%) |
| Stable disease | 56 (43.8%) |
| Progressive disease | 56 (43.8%) |
| Not evaluable | 11 (8.6%) |
| Response rate | |
| Objective response rate | 5 (3.9%) |
| DCR | 61 (47.7%) |
| 6-month survival rate, % (95% CI) | |
| OS rate | 41 (33–49) |
| PFS rate | 20 (12–28) |
| Median survival, months (95% CI) | |
| OS | 4.9 (4.2–5.6) |
| PFS | 2.4 (1.9–2.9) |
| Median survival from first-line therapy, months (95% CI) | |
| OS | 21.0 (18.4–23.6) |
| PFS | 18.3 (15.6–21.0) |
CI, confidence interval; DCR, disease control rate; 5-FU/LV, 5-fluorouracil and leucovorin; naI-IRI, nanoliposomal encapsulation of irinotecan; OS, overall survival; PFS, progression-free survival.
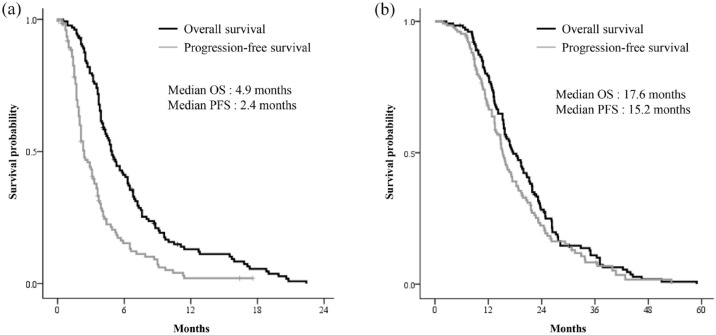
The survival analysis was performed based on 121 (95%) deaths and 108 (84%) disease progressions (Figure 2). The median OS was 4.9 months [95% confidence interval (CI): 4.2–5.6 months], and median PFS was 2.4 months (95% CI: 1.9–2.9 months). The 6-month OS and PFS rate was 41% (95% CI: 33–49%) and 20% (95% CI: 12–28%), respectively. The median OS and PFS from the start of the first-line therapy were 17.6 months (95% CI: 14.9–20.3 months) and 15.2 months (95% CI: 13.9–16.5 months).
Figure 2.
Kaplan–Meier survival analysis, patients who were treated with nal-IRI plus 5-FU/LV beyond second-line therapy: (a) from start of nal-IRI plus 5-FU/LV and (b) from start of first-line therapy.
5-FU/LV, 5-fluorouracil and leucovorin; naI-IRI, nanoliposomal encapsulation of irinotecan.
Survival outcomes in subgroups
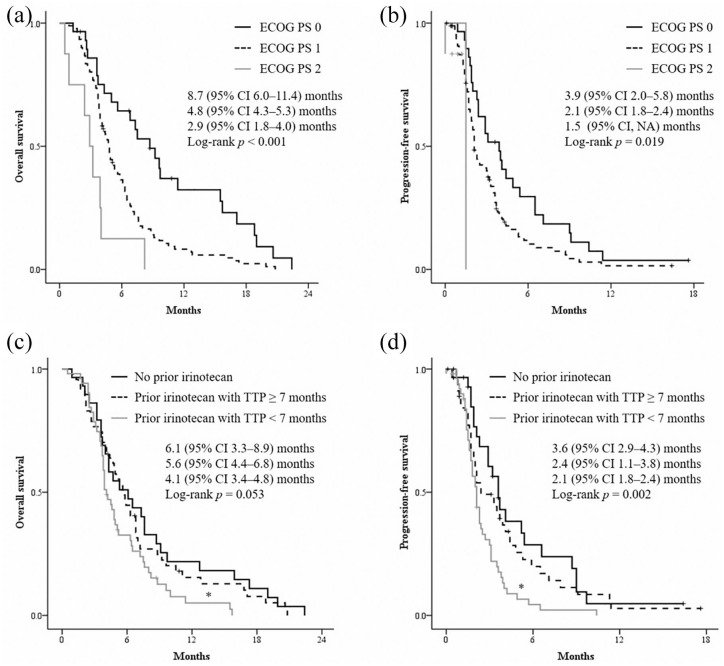
Patients with better ECOG performance status experienced significantly longer OS and PFS (log-rank p < 0.001 and log-rank p = 0.019, respectively) (Figure 3(a) and (b)). Patients who maintained a very good general condition (ECOG 0) had an excellent median OS of 8.7 months compared to 4.6 months in patients with ECOG 1–2 (p < 0.001). The median PFS was 3.9 months and 2.1 months, respectively (p = 0.018). Patients with ECOG 1 had longer OS of 4.8 months and PFS of 2.1 months than those with ECOG 2, OS 2.9 months (p = 0.019) and 1.5 months (p = 0.190).
Figure 3.
Kaplan–Meier survival analysis according to performance status: ECOG 0–2 (a, b), prior irinotecan exposure and response (c, d).
ECOG, Eastern Cooperative Oncology Group.
Prior irinotecan exposure and durability of chemotherapy were associated with survival outcomes. Patients with TTP less than 7 months on FFX treatment had worse OS and PFS compared to patients without prior irinotecan or with TTP greater than or equal to 7 months (p = 0.053 and p = 0.002, respectively) (Figure 3(c) and (d)). There were no significant differences in OS (p = 0.544) and PFS (p = 0.360) between patients without irinotecan exposure and those with a TTP of 7 months or more. Patients who experienced partial response on previous FFX treatment compared with patients with stable disease or disease progression had a median PFS of 3.5 versus 2.1 months (p = 0.021). The median OS was 5.0 versus 4.8 months (p = 0.417).
Prior lines of chemotherapy (third-line versus later-line therapy), chemotherapy dose reduction, and first-line palliative chemotherapy regimen (FFX versus GnP) were not associated with survival outcomes (Supplemental Figure 1A–F and Supplemental Figure 2A). According to the treatment sequence, the subsequent regimen of chemotherapy was not associated with survival outcomes (Supplemental Figure 1G–H). Such trends were maintained in analyses from the beginning of first-line therapy (Supplemental Figure 2B).
Prognostic factors for survival outcomes
The Cox proportional hazards regression analysis was performed to identify the factors affecting survival outcomes (Table 4). Absence of liver metastases and previous radiotherapy were significantly associated with better OS and PFS. In patients with prior irinotecan exposure, a trend of better prognosis as the irinotecan-free interval increased was observed in OS (HR: 0.97, 95% CI: 0.93–1.00; p = 0.071) and PFS (HR: 0.97, 95% CI: 0.93–1.00; p = 0.051). However, patients without irinotecan exposure were treated as missing because the irinotecan-free interval does not exist. Therefore, we analyzed the ‘prior exposure to irinotecan’ variable according to three categories. Patients with a TTP less than 7 months showed poor survival outcomes in the uni- and multivariable analyses. However, there were no statistical differences of OS and PFS between patients who were not previously exposed to irinotecan and those with a TTP greater than or equal to 7 months (HR: 1.16, 95% CI: 0.72–1.87; p = 0.547 and HR: 1.27, 95% CI: 0.75–2.15; p = 0.360, respectively).
Table 4.
Univariable and multivariable analyses of survival outcomes.
| OS | PFS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Sex (female) | 1.03 | 0.71–1.5 | 0.887 | 1.28 | 0.86–1.89 | 0.226 | ||||||
| Age ⩾65 | 1.09 | 0.76–1.56 | 0.656 | 1.06 | 0.73–1.55 | 0.769 | ||||||
| ECOG | ||||||||||||
| 0 | Ref | (<0.001) | Ref | (<0.001) | Ref | (0.024) | Ref | (0.009) | ||||
| 1 | 2.13 | 1.34–3.39 | 0.001 | 2.29 | 1.43–3.66 | 0.001 | 1.67 | 1.07–2.60 | 0.024 | 1.94 | 1.22–3.09 | 0.005 |
| 2 | 5.03 | 2.21–11.5 | <0.001 | 4.85 | 2.12–11.1 | <0.001 | 4.63 | 1.02–20.9 | 0.047 | 4.62 | 1.01–21.2 | 0.049 |
| Liver metastases | 1.55 | 1.07–2.26 | 0.022 | 1.68 | 1.13–2.52 | 0.011 | 2.29 | 1.49–3.51 | <0.001 | 2.75 | 1.76–4.30 | <0.001 |
| Previous surgery | 0.70 | 0.49–1.02 | 0.064 | 0.72 | 0.49–1.06 | 0.091 | ||||||
| Previous radiotherapy | 0.64 | 0.43–0.96 | 0.031 | 0.55 | 0.36–0.84 | 0.005 | 0.602 | 0.38–0.95 | 0.028 | |||
| First-line regimen (GnP versus FFX) | 0.96 | 0.64–1.44 | 0.84 | 0.83 | 0.53–1.30 | 0.409 | ||||||
| Chemotherapy dose reduction | 1.05 | 0.73–1.51 | 0.803 | 1.19 | 0.81–1.74 | 0.381 | ||||||
| Irinotecan-free survival | 0.97 | 0.93–1.00 | 0.075 | 0.97 | 0.93–1.00 | 0.051 | ||||||
| Prior exposure to irinotecan | ||||||||||||
| No prior irinotecan | Ref | (0.059) | (0.084) | Ref | (0.003) | Ref | (0.001) | |||||
| Prior irinotecan with TTP ⩾7 months | 1.17 | 0.73–1.89 | 0.516 | 1.22 | 0.75–1.98 | 0.416 | 1.27 | 0.75–2.14 | 0.376 | 1.84 | 1.06–3.21 | 0.031 |
| Prior irinotecan with TTP <7 months | 1.73 | 1.06–2.82 | 0.029 | 1.71 | 1.04–2.81 | 0.033 | 2.24 | 1.33–3.77 | 0.002 | 2.81 | 1.63–4.86 | <0.001 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; FFX, FOLFIRINOX; GnP, gemcitabine plus nab-paclitaxel; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
Safety
Adverse events during the nal-IRI plus 5-FU/LV treatment are listed in Table 5. The majority of patients (n = 97, 76%) experienced any grade of treatment-related adverse events. The most common adverse events were neutropenia (56%) and anemia (51%). Grade 3–4 adverse events frequently occurred in hematologic events: neutropenia (25%) and anemia (13%).
Table 5.
Adverse events of patients for any grade.
| Any grade (%) | Grade ⩾3 (%) | |
|---|---|---|
| Anemia | 65 (50.7%) | 16 (12.7%) |
| Neutropenia | 71 (55.5%) | 32 (25.4%) |
| Fatigue | 31 (24.2%) | 6 (4.7%) |
| Nausea | 34 (26.6%) | 2 (1.6%) |
| Vomit | 22 (17.1%) | 3 (2.3%) |
| Diarrhea | 19 (14.8%) | 1 (0.8%) |
| Abdominal pain | 13 (10.2%) | – |
| Oral mucositis | 10 (7.8%) | – |
Discussion
In real-world experience, nal-IRI plus 5-FU/LV is administered beyond second-line treatment due to the predominant use of first-line FFX treatment followed by second-line GnP, or the inverse.9,14 Therefore, this multicenter retrospective study evaluated the efficacy of third-line or later-line nal-IRI plus 5-FU/LV treatment in patients that previously received two effective frontline chemotherapy regimens. The median OS was 4.9 months and the median OS from the beginning of first-line therapy was 17.6 months. Nal-IRI plus 5-FU/LV resulted in a median PFS of 2.4 months and DCR of 48%. Patients with good performance status showed longer survival outcomes. Although prior irinotecan exposure was associated with poor survival, patients who had experienced favorable disease control with FFX treatment showed comparable survival outcomes to those without prior irinotecan exposure.
Regarding the effectiveness of nal-IRI plus 5-FU/LV treatment, survival outcomes in our study tend to be slightly shorter compared to those in previously reported real-world analyses (median OS 4.9 versus 5.3–9.4 months, median PFS 2.4 versus 2.5–3.8 months).12,13,15,16 Such discrepancies may result from the different characteristics of the enrolled patients. Patients who received a third-line or later-line treatment have been reported to have a worse prognosis. 12 In other studies, less than half of patients (23–42%) received nal-IRI plus 5-FU/LV as third-line therapy, compared to the majority of patients (82%) in our study. However, beyond second-line treatment, no significant difference in OS and PFS was found between third-line nal-IRI plus 5-FU/LV and later lines in this study. This result is in accordance with Glassman et al. that found no significant difference in survival between patients with third-line treatment or beyond third-line therapy. 15 Furthermore, more than half of the patients in the current study did not receive subsequent chemotherapy following failure of nal-IRI plus 5-FU/LV. Patients who received subsequent chemotherapy after nal-IRI treatment failure had a median OS of 7.6 months, whereas those without further subsequent therapy had a median OS of 3.7 months (data not shown here).
We observed a significantly longer OS and PFS in patients with good performance status. This is consistent with the final analysis of the NAPOLI-1 study and also the real-world experience of an East Asian cohort which showed better performance status to be associated with long-term survival.13,17 Patients that maintain a good general condition despite previous chemotherapies might have a chance to receive effective chemotherapy for a longer period of time. Therefore, it seems preferable to consider nal-IRI plus 5-FU/LV as late-line treatment in patients with good performance status (ECOG 0–1) for optimal clinical benefits.
FFX and GnP were commonly used as second-line therapy after receiving their crossover regimen in our previous retrospective cohort study. 9 Concerning the optimal treatment sequence, the results of our study demonstrate that the first-line chemotherapy regimen, FFX or GnP, was not associated with survival outcomes; this is consistent with other real-world data in Korea. 16 And we discovered that the sequential regimen of chemotherapy including second-line therapy (FFX followed by GnP or other; or GnP followed by FFX or other) prior to nal-IRI plus 5-FU/LV was not associated with survival. Therefore, nal-IRI and 5-FU/LV can be effectively utilized not only in second-line therapy, but also in later-line treatment regardless of the frontline chemotherapy regimen.
In this study, patients with prior irinotecan exposure experienced worse PFS in comparison to patients without previous exposure. The negative effect of prior irinotecan administration on survival outcomes with nal-IRI plus 5-FU/LV has already been reported, and the development of resistance to irinotecan was suggested as the potential cause.15,16 However, the number of patients who had previously received conventional irinotecan was small. Conversely, in this study, the vast majority of patients were previously treated with irinotecan-containing chemotherapy (i.e. FFX). Furthermore, we divided and classified patients who had been treated with FFX according to TTP. Although the cutoff level of 7 months is an arbitrary value based on our previous study, other studies including PRODIGE 4 trial have also reported median PFS as 6–7 months in patients with first-line FFX.3,18–20 Survival outcomes in patients that experienced favorable disease control on FFX treatment were comparable to those with no prior irinotecan exposure. This suggests that the response to prior conventional irinotecan-containing treatment could be a predictor factor for nal-IRI-based treatment. The irinotecan-free interval is also available potential predictive factors.21,22 And a correlation between irinotecan-free interval and survival outcomes was observed in the current study. Further investigation such as prospective, comparative cohort study is needed to clarify these prognostic factors.
This study has several limitations. First, its retrospective nature may result in a potential selection bias and lack of medical records including adverse events. We aimed to alleviate potential biases by collecting a relatively large number of patients from multicenter tertiary hospitals. This study, to the best of our knowledge, included the largest number of patients who received nal-IRI plus 5-FU/LV beyond second-line treatment compared with previously reported real-world analyses. 23 Second, immortal time bias can also affect the survival outcomes. We calculated survival outcomes from the date of initiating nal-IRI plus 5-FU/LV treatment to avoid time bias.24,25 However, time-lead bias still exists in the analysis of total survival because patients who received chemotherapy beyond second-line therapy could not have died prior to third-line therapy. The effectiveness of nal-IRI + 5-FU/LV as a third- or later-line therapy can be further clarified through comparative study deigns that include patients receiving other chemotherapy regimens or active symptom control. FFX and GnP regimens were considered as frontline treatment for unresectable pancreatic cancer, and each regimen was also considered as a subsequent line treatment. Median OS did not differ in patients with or without prior irinotecan (included in FFX) exposure in the current study. Therefore, nal-IRI plus 5-FU/LV may be notable, beyond second-line treatment option, and the data in this study can be meaningful in demonstrating the effectiveness of such lines of treatment. Patients that maintain good general condition or those with a favorable experience to prior irinotecan may particularly be beneficial for survival.
In conclusion, the effectiveness of nal-IRI plus 5-FU/LV beyond second-line treatment in real-world clinical practice was demonstrated in this multicenter retrospective study. The results presented here suggest that nal-IRI plus 5-FU/LV can be effective not only as second-line therapy, but also as a third-line or later line therapy in selected patients. Further investigations are necessary to identify patients that can benefit more from nal-IRI plus 5-FU/LV in various clinical settings.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221119539 for A real-world analysis of nanoliposomal-irinotecan with 5-fluorouracil and folinic acid as third- or later-line therapy in patients with metastatic pancreatic adenocarcinoma by Jung Won Chun, Sang Myung Woo, Sang Hyub Lee, Jin Ho Choi, Namyoung Park, Joo Seong Kim, In Rae Cho, Woo Hyun Paik, Woo Jin Lee, Ji Kon Ryu and Yong-Tae Kim in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors express sincere gratitude to Dr Ki Ho Yang and essayist Myeong Hee Choi for their support in this study. We thank Hyun Ii Lee, Lee Mee Young, Aeran Seo, and Doyeon Kim for helping with the data acquisition. The authors are grateful to Eun Young Park who is in charge of Biostatistics Collaboration Team in National Cancer Center for advice on statistical analysis.
Footnotes
ORCID iDs: Jung Won Chun  https://orcid.org/0000-0003-1964-7501
https://orcid.org/0000-0003-1964-7501
Sang Hyub Lee  https://orcid.org/0000-0003-2174-9726
https://orcid.org/0000-0003-2174-9726
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jung Won Chun, Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Goyang-si, Korea.
Sang Myung Woo, Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Goyang-si, Korea.
Sang Hyub Lee, Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea.
Jin Ho Choi, Division of Gastroenterology, Department of Internal Medicine, Liver Research Institute, College of Medicine, Seoul National University Hospital, Seoul National University, Seoul, Korea.
Namyoung Park, Division of Gastroenterology, Department of Internal Medicine, Liver Research Institute, College of Medicine, Seoul National University Hospital, Seoul National University, Seoul, Korea; Department of Gastroenterology, Kyung Hee University Hospital at Gangdong, Seoul, Korea.
Joo Seong Kim, Division of Gastroenterology, Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang-si, Korea; Department of Gastroenterology, Kyung Hee University Hospital at Gangdong, Seoul, Korea.
In Rae Cho, Division of Gastroenterology, Department of Internal Medicine, Liver Research Institute, College of Medicine, Seoul National University Hospital, Seoul National University, Seoul, Korea.
Woo Hyun Paik, Division of Gastroenterology, Department of Internal Medicine, Liver Research Institute, College of Medicine, Seoul National University Hospital, Seoul National University, Seoul, Korea.
Woo Jin Lee, Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Goyang-si, Korea.
Ji Kon Ryu, Division of Gastroenterology, Department of Internal Medicine, Liver Research Institute, College of Medicine, Seoul National University Hospital, Seoul National University, Seoul, Korea.
Yong-Tae Kim, Division of Gastroenterology, Department of Internal Medicine, Liver Research Institute, College of Medicine, Seoul National University Hospital, Seoul National University, Seoul, Korea.
Declarations
Ethics approval and consent to participate: Formal consent was waived because of the retrospective nature or this study, and which was approved by the Institutional Review Board (IRB). IRBs at Seoul National University Hospital (H-2110-173-1266) and National Cancer Center (NCC 2021-0324) approved the study. All individual-level data were anonymized and only available to authorized researchers. IRB approved and monitored safety and adequacy of data management. This study was performed in accordance with the Declaration of Helsinki.
Consent for publication: All authors have read the manuscript and approve its submission to Therapeutic Advances in Medical Oncology.
Author contribution(s): Jung Won Chun: Data curation; Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
Sang Myung Woo: Data curation; Investigation; Writing – review & editing.
Sang Hyub Lee: Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing.
Jin Ho Choi: Data curation; Investigation; Writing – review & editing.
Namyoung Park: Investigation; Writing – review & editing.
Joo Seong Kim: Investigation; Writing – review & editing.
In Rae Cho: Investigation; Writing – review & editing.
Woo Hyun Paik: Investigation; Writing – review & editing.
Woo Jin Lee: Investigation; Methodology; Writing – review & editing.
Ji Kon Ryu: Investigation; Methodology; Writing – review & editing.
Yong-Tae Kim: Investigation; Methodology; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was supported by grants from the National Cancer Center, Korea (Grant numbers 2212470-1, 2152460-1).
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The data sets used and/or analyzed during this study are available from the corresponding author on reasonable request.
References
- 1. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 2. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019; 10: 10–27. 20190226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 4. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26: v56–v68. [DOI] [PubMed] [Google Scholar]
- 6. Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021; 19: 439–457. [DOI] [PubMed] [Google Scholar]
- 7. Martín AM, Hidalgo M, Alvarez R, et al. From first line to sequential treatment in the management of metastatic pancreatic cancer. J Cancer 2018; 9: 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahma OE, Duffy A, Liewehr DJ, et al. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol 2013; 24: 1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chun JW, Lee SH, Kim JS, et al. Comparison between FOLFIRINOX and gemcitabine plus nab-paclitaxel including sequential treatment for metastatic pancreatic cancer: a propensity score matching approach. BMC Cancer 2021; 21: 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 11. Pellino A, Manai C, Merz V, et al. Observational retrospective evaluation of treatment with liposomal irinotecan plus fluorouracil/leucovorin for metastatic pancreatic cancer patients: an Italian large real-world analysis. J Clin Oncol 2020; 38: 660–660.31895607 [Google Scholar]
- 12. Kieler M, Unseld M, Bianconi D, et al. A real-world analysis of second-line treatment options in pancreatic cancer: liposomal-irinotecan plus 5-fluorouracil and folinic acid. Ther Adv Med Oncol 2019; 11: 1758835919853196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su Y-Y, Chiang N-J, Tsai H-J, et al. The impact of liposomal irinotecan on the treatment of advanced pancreatic adenocarcinoma: real-world experience in a Taiwanese cohort. Sci Rep 2020; 10: 7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JC, Woo SM, Shin DW, et al. Comparison of FOLFIRINOX and gemcitabine plus nab-paclitaxel for treatment of metastatic pancreatic cancer: Using Korean pancreatic cancer (K-PaC) registry. Am J Clin Oncol 2020; 43: 654–659. [DOI] [PubMed] [Google Scholar]
- 15. Glassman DC, Palmaira RL, Covington CM, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer 2018; 18: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoo C, Im H-S, Kim K-P, et al. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/leucovorin in patients with metastatic pancreatic adenocarcinoma: a study by the Korean Cancer Study Group. Ther Adv Med Oncol 2019; 11: 1758835919871126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang-Gillam A, Hubner RA, Siveke JT, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: final overall survival analysis and characteristics of long-term survivors. Eur J Cancer 2019; 108: 78–87. [DOI] [PubMed] [Google Scholar]
- 18. Chllamma MK, Cook N, Dhani NC, et al. FOLFIRINOX for advanced pancreatic cancer: the Princess Margaret Cancer Centre experience. Br J Cancer 2016; 115: 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeda O, Yokoyama Y, Yamaguchi J, et al. Real-world experience with FOLFIRINOX and gemcitabine plus nab-paclitaxel in the treatment of pancreatic cancer in Japan. Ann Oncol 2017; 28: x69. [Google Scholar]
- 20. Papneja N, Zaidi A, Chalchal H, et al. Comparisons of outcomes of real-world patients with advanced pancreatic cancer treated with FOLFIRINOX versus gemcitabine and nab-paclitaxel: a population-based cohort study. Pancreas 2019; 48: 920–926. [DOI] [PubMed] [Google Scholar]
- 21. Hebbar M, Di Fioré F, Conroy T, et al. Assessment of baseline clinical predictive factors of response to cetuximab-irinotecan in patients with irinotecan-refractory metastatic colorectal cancer. Oncology 2007; 73: 185–191. [DOI] [PubMed] [Google Scholar]
- 22. Rossini D, Lonardi S, Antoniotti C, et al. Treatments after progression to first-line FOLFOXIRI and bevacizumab in metastatic colorectal cancer: a pooled analysis of TRIBE and TRIBE2 studies by GONO. British Journal of Cancer 2021; 124: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frampton JE. Liposomal irinotecan: a review in metastatic pancreatic adenocarcinoma. Drugs 2020; 80: 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dekkers OM, Groenwold RHH. When observational studies can give wrong answers: the potential of immortal time bias. Eur J Endocrinol 2021; 184: E1–E4. [DOI] [PubMed] [Google Scholar]
- 25. Niemelä J, SyrjäLä H, Ohtonen P, et al. Chemotherapy improves survival after percutaneous biliary drainage in patients with pancreatic or biliary tract cancer with biliary obstruction. Anticancer Res 2021; 41: 2979–2984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221119539 for A real-world analysis of nanoliposomal-irinotecan with 5-fluorouracil and folinic acid as third- or later-line therapy in patients with metastatic pancreatic adenocarcinoma by Jung Won Chun, Sang Myung Woo, Sang Hyub Lee, Jin Ho Choi, Namyoung Park, Joo Seong Kim, In Rae Cho, Woo Hyun Paik, Woo Jin Lee, Ji Kon Ryu and Yong-Tae Kim in Therapeutic Advances in Medical Oncology