Abstract
Purpose
Breast cancer (BC) patients who achieve a favorable residual cancer burden (RCB) after neoadjuvant chemotherapy (NACT) have an improved recurrence-free survival. Those who have an unfavorable RCB will have gone through months of ineffective chemotherapy. No ideal method exists to predict a favorable RCB early during NACT. Diffuse optical tomography (DOT) is a novel imaging modality that uses near-infrared light to assess hemoglobin concentrations within breast tumors. We hypothesized that the 2-week percent change in DOT-measured hemoglobin concentrations would associate with RCB.
Methods
We conducted an observational study of 40 women with stage II–IIIC BC who received standard NACT. DOT imaging was performed at baseline and 2 weeks after treatment initiation. We evaluated the associations between the RCB index (continuous measure), class (categorical 0, I, II, III), and response (RCB class 0/I = favorable, RCB class II/III = unfavorable) with changes in DOT-measured hemoglobin concentrations.
Results
The RCB index correlated significantly with the 2-week percent change in oxyhemoglobin [HbO2] (r = 0.5, p = 0.003), deoxyhemoglobin [Hb] (r = 0.37, p = 0.03), and total hemoglobin concentrations [HbT] (r = 0.5, p = 0.003). The RCB class and response significantly associated with the 2-week percent change in [HbO2] (p ≤ 0.01) and [HbT] (p ≤ 0.02). [HbT] 2-week percent change had sensitivity, specificity, positive, and negative predictive values for a favorable RCB response of 86.7, 68.4, 68.4, and 86.7%, respectively.
Conclusion
The 2-week percent change in DOT-measured hemoglobin concentrations was associated with the RCB index, class, and response. DOT may help guide NACT for women with BC.
Keywords: Breast cancer, Neoadjuvant chemotherapy, Diffuse optical tomography, Imaging
Introduction
Neoadjuvant chemotherapy (NACT) results in a similar survival as adjuvant therapy for women with operable breast cancer [1, 2]. However, it also allows for breast conserving surgery in patients who would have otherwise required a mastectomy [1, 3, 4], and provides an in vivo assessment of tumor responsiveness to treatment. Residual cancer burden (RCB) measures the response of breast tumors to NACT. The RCB index is a continuous measure derived from the primary tumor dimensions, cellularity of the tumor bed, and axillary nodal burden. In a multivariate model, Symmans et al. [5]. found that the RCB index was independently prognostic of disease-free survival, with a twofold increase in relapse risk for each unit increase. The RCB index can be subdivided into RCB class, a categorical measure where RCB-0 is defined as a pathologic complete response (pCR), RCB-I is defined as an RCB index greater than 0–1.36, RCB-II as an RCB index 1.37–3.28, and RCB-III as an RCB index greater than 3.28. The RCB class can be further grouped into favorable responders (RCB class 0/I), or unfavorable responders (RCB class II/III) based on distant relapse rates.
Patients who do not achieve a favorable RCB response are exposed to months of ineffective treatment and unnecessary side effects. A method to assess early tumor response could provide physicians the ability to make evidence-based changes in treatment. Unfortunately, there is no clear and cost-effective way to predict which patient will respond well to NACT. Physical exam, mammography, ultrasound (US), magnetic resonance imaging (MRI), and positron emission tomography with computed tomography (PET–CT) are either insensitive to early response to NACT, expensive to perform serially, require IV contrast, or expose patients to ionizing radiation and/or radioactive isotopes [6–10].
Diffuse optical tomography (DOT) is a non-invasive, functional, three-dimensional imaging modality that quantitatively measures near-infrared light absorption and scattering to determine the tissue concentration of oxyhemoglobin [HbO2], deoxyhemoglobin [Hb], total hemoglobin [HbT], and water fraction (WF). Vascular changes precede measurable structural changes in mouse tumor models [11], suggesting that DOT could characterize breast tumor response to NACT and may do so earlier than other modalities. A number of studies demonstrate that responders have statistically significant changes in DOT parameters after initiation of NACT compared to non-responders [12–14]. Several limitations of these prior optical imaging studies include the use of hand-held operator-dependent devices that decrease reproducibility and do not allow for a full three-dimensional tomographic assessment of the entire breast, as well as the use of non-uniform chemotherapy. We hypothesized that early changes in DOT parameters after 2 weeks of NACT would predict the 20-week response to NACT as measured by the RCB.
Methods
Study population
We conducted an observational study of 40 women. The main inclusion criteria were women over the age of 18 with clinical stage II to IIIC invasive breast cancer, scheduled to undergo NACT with 12 weeks of weekly taxane (paclitaxel or nab-paclitaxel) followed by four cycles of doxorubicin (A) and cyclophosphamide (C) given every 2 weeks with growth factor support. Additional biologic therapies, such as trastuzumab for patients with human epidermal growth factor 2 + (HER2+) disease, were allowed if clinically indicated. Hormone receptor (HR) and HER2 status were defined as per ASCO–CAP guidelines from biopsy specimens prior to treatment. The institutional review board at Columbia University Medical Center determined the DOT imager to be a low-risk device and approved the study protocol. Informed consent was obtained from all patients prior to their enrollment.
Study procedures
Upon enrollment, each participant had a baseline assessment including complete history, physical exam, breast tumor size (mm) measurement with calipers, breast MRI (with and without intravenous contrast unless contraindicated), and mammogram. Subjects were followed at 2-week intervals with clinic visits, physical exams, and tumor sizes measurements with physical exam. DOT evaluation for determination of [HbO2], [Hb], [HbT], and WF in the affected breast occurred before initiation of chemotherapy and after 2 weeks of taxane chemotherapy. Mammograms and MRI were performed at baseline and at completion of NACT before surgery.
The DOT imager used in this study has been previously described [15]. DOT imaging was performed by placing each subjects’ breasts into the DOT imager where light from four laser diodes (wavelengths: 765, 808, 827, and 905 nm) were sequentially coupled into fibers contacting both the affected and healthy breasts. Transmitted light intensities were collected by fibers coupled to individual silicon photodiodes. The measured data were then processed to produce three-dimensional optical maps, from which values for [HbO2], [Hb], [HbT], and WF were determined. The DOT images were examined by JEG without prior knowledge of tumor location and areas suspicious for tumor were identified. Using custom software in MATLAB (MathWorks, Natick, MA), the maximum value (M) of the chromophore concentration was identified within a 2 cm radius, and a standard deviation (SD) of the volume was calculated. The region of interest was defined as all the voxels with value Vi that satisfied the condition M−SD ≤ Vi ≤ M. The weighted average of the ROI was taken as the measured chromophore concentration.
Final pathology specimens (mastectomy or lumpectomy) were scored according to Symmans et al. [5]. (http://www3.mdanderson.org/app/medcalc/index.cfmpagename=jsconvert3) for the RCB index (continuous), RCB class (0, I, II, III), and a dichotomized RCB response (RCB class 0 or I: favorable response; RCB class II or III: unfavorable response).
Outcome measures
The primary outcome was the association between the 2-week percent change from baseline of DOT-derived parameters ([HbO2], [Hb], [HbT], and WF) and pathologic response as assessed by the RCB response. We also assessed complete response rates as seen on MRI and mammography, comparing those obtained at baseline and those obtained after NACT and prior to surgery. A complete response for either imaging modality was defined as the absence of breast masses or abnormal appearing lymph nodes seen on the pre-surgical evaluation.
Statistical analysis
Descriptive statistics were generated for all data collected. For continuous variables, means, medians, standard deviations, and interquartile ranges were computed. For categorical and discrete variables, frequency tables were made.
Correlation analysis, ANOVA testing, and Wilcoxon rank sum tests were used to evaluate the relationship between the 2-week changes in DOT-derived parameters and the RCB index, class, and response, respectively. Sensitivities, specificities, positive (PPV) and negative predictive values (NPV) were calculated, and receiver operator curves were generated for each DOT-derived parameter, mammogram, and MRI. Statistical analyses were performed using SAS 9.4 Software (SAS Institute Inc., Cary, NC). Receiver operator curves were generated and the areas under the curve (AUC) were calculated using MATLAB.
Results
We enrolled 40 subjects between June 2011 and September 2015. Of the 40 subjects, 6 were not evaluable for the primary outcome. One subject was lost to follow-up after her first dose of chemotherapy, 1 subject received concurrent chemotherapy with radiation prior to surgery, 2 subjects missed DOT imaging at the 2-week time point, and 2 subjects had DOT data that were not evaluable due to instrumentation failure during their imaging session.
Of the 34 evaluable patients (Table 1), the mean age was 49.9 years (standard deviation: SD 11.3) and mean BMI was 30 (SD 6.2). Fifteen subjects were pre-menopausal, while 19 were post-menopausal. The majority of patients had invasive ductal carcinoma (n = 30), and four had invasive lobular carcinoma. Twenty-three subjects had high-grade disease, 10 had intermediate-grade disease, and one had low-grade disease. Most patients had HR+/HER2− disease (n = 17), followed by HR+/HER2+ (n = 9), HR−/HER2−, (n = 5), and HR−/HER2+ breast cancer (n = 3).
Table 1.
Demographics and Results of RCB data for the 34 subjects enrolled on study, received NACT, and had data from their 2-week DOT evaluation
| Characteristic | |
|---|---|
| Age (mean ± SD) | 49.9 years ± 11.3 |
| BMI (mean ± SD) | 30 ± 6.2 |
| Tumor histologic subtype | |
| Invasive ductal carcinoma (n, %) | 30 (88%) |
| Invasive lobular carcinoma (n, %) | 4 (12%) |
| Hormone/HER2 status | |
| HR+/HER2− (n, %) | 17 (50%) |
| HR+/HER2+ (n, %) | 9 (26.5%) |
| HR−/HER2+ (n, %) | 3 (8.8%) |
| HR−/HER2− (n, %) | 5 (14.7%) |
| RCB class | |
| RCB 0 (n, %) | 13 (38.2%) |
| RCB 1 (n, %) | 2 (5.9%) |
| RCB 2 (n, %) | 13 (38.2%) |
| RCB 3 (n, %) | 6 (17.6%) |
BMI body mass index, HR hormone receptor; estrogen and progesterone receptor staining >1% of cells was classified as HR positive, HER2 human epidermal growth factor receptor 2, RCB residual cancer burden
Responses to NACT by RCB class included 13 subjects with RCB 0 (pCR), 2 subjects with RCB-I, 13 subjects with RCB-II, and 6 subjects with RCB-III (Table 1). Figure 1 shows representative DOT images for a subject who had a favorable RCB response and another who did not.
Fig. 1.
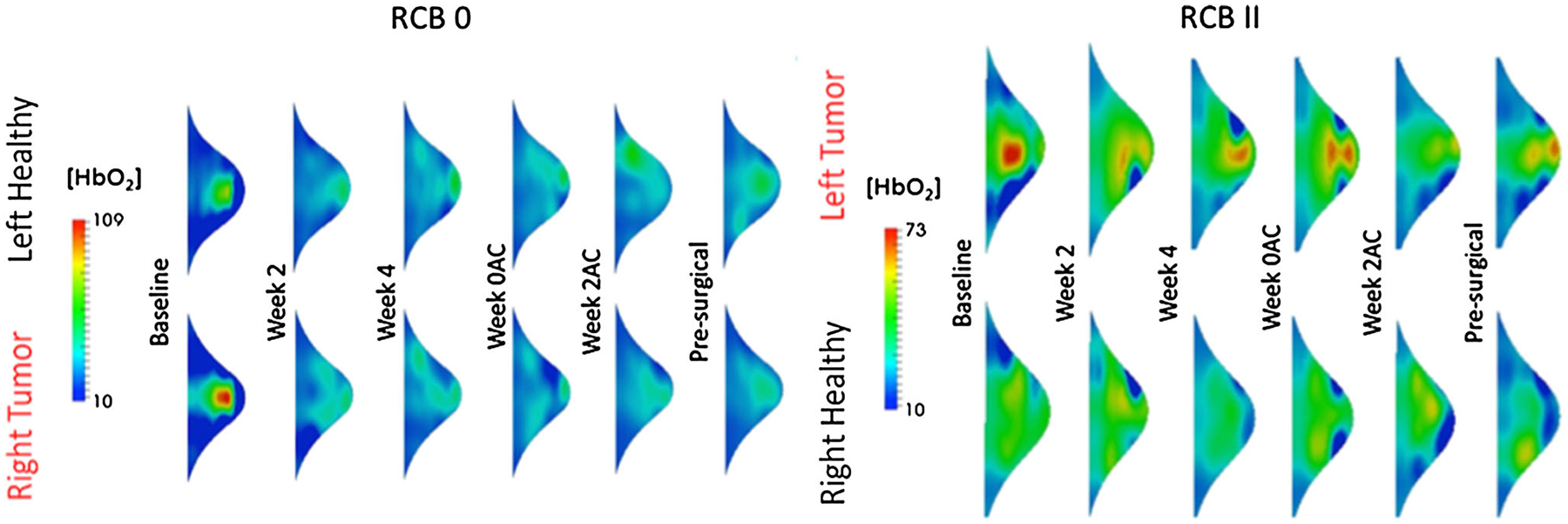
Representative data from two patients who received the same NACT, one had a pathologic complete response (RCB 0) and the other had an unfavorable response to NACT (RCB 2). At 2 weeks after the initiation of chemotherapy, we see a decline in [HbO2], while in the subject who did not have a good response, we see persistently high levels of this chromophores
The RCB index (continuous measure) was statistically significantly correlated with the 2-week percent change in [HbO2] (r = 0.5, p = 0.003), [Hb] (r = 0.37, p = 0.03), and [HbT] (r = 0.5, p = 0.003), but did not correlate significantly with the 2-week percent change in WF (r = 0.095, p = 0.59) (Fig. 2). The RCB index did not correlate with baseline DOT measurements (data not shown). The RCB class (categorical variable) was found to be statistically significantly associated with the 2-week percent change in [HbO2] (p = 0.01) and [HbT] (p = 0.02), but did not associate significantly with the 2-week percent change in [Hb] (p = 0.15) or WF (p = 0.75) (Fig. 3a; See Online Appendix for subject level data). Finally, the RCB response (dichotomized variable) was also significantly associated with the 2-week percent change in [HbO2] (p = 0.004), [Hb] (p = 0.05), and [HbT] (p = 0.003), but not with WF (p = 0.89) (Fig. 3b). Receiver operator curves (ROC) were generated from the data evaluating RCB response. The ROC for the 2-week percent change in [HbT] had the highest AUC at 0.807 (Online Appendix). Using the Youden index for the 2-week percent change for [HbT], the calculated sensitivity, specificity, positive, and negative predictive values were 86.7, 68.4, 68.4, and 86.7%, respectively.
Fig. 2.
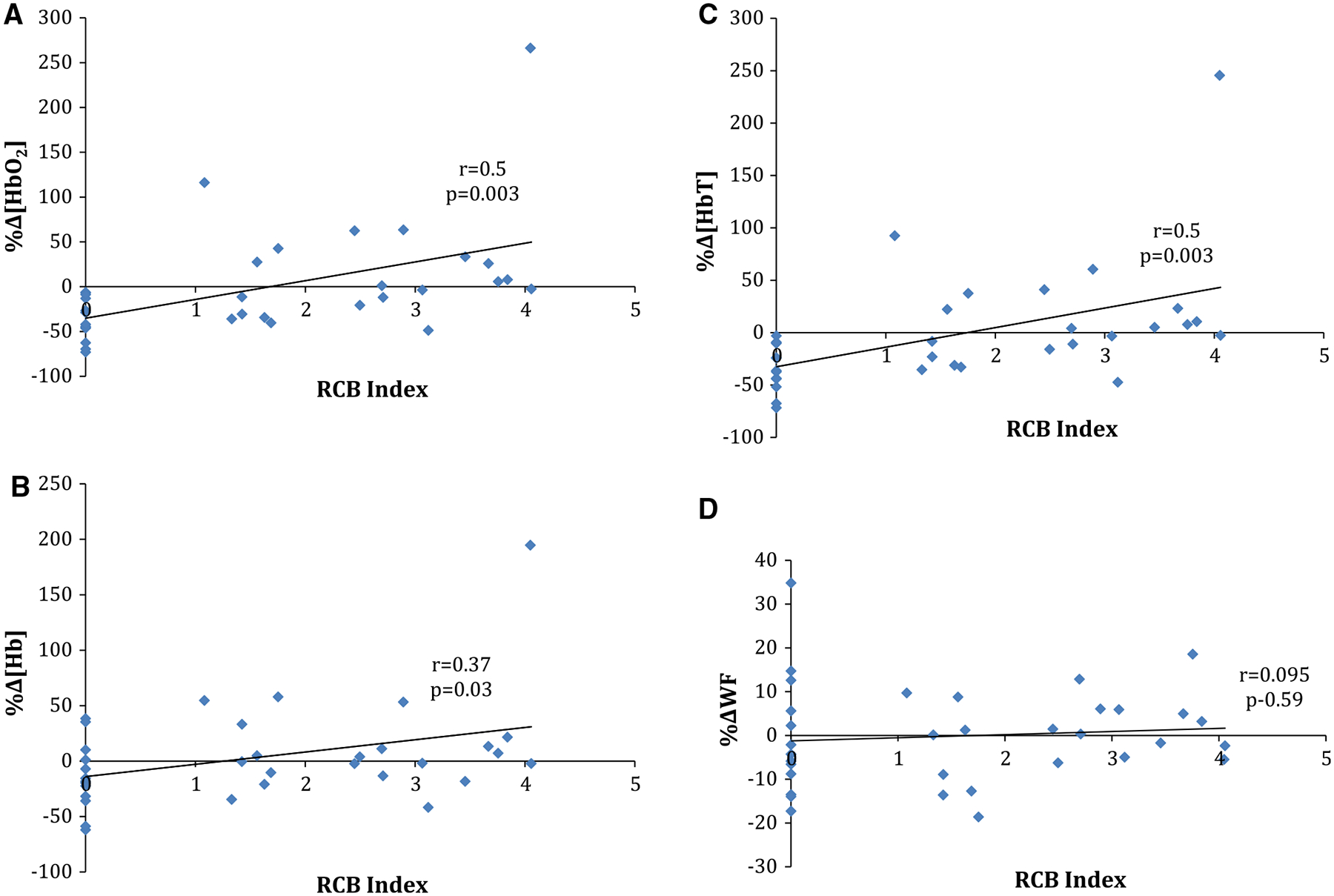
Correlation of the RCB index (continuous measure) with the 2-week percent change in a oxyhemoglobin concentration, b deoxyhemoglobin concentration, c total hemoglobin concentration, and d water fraction
Fig. 3.

a Two-week percent change in DOT parameter and RCB class showed a significant association between the 2-week percent change in [HbO2] (p = 0.01) and [HbT] (p = 0.02), but did not associate significantly with the 2-week percent change in [Hb] (p = 0.15) or WF (p = 0.75); b Two-week percent change in DOT parameter and response versus no response by dichotomized RCB score found an association with RCB score and the 2-week percent change in [HbO2] (p = 0.004) and [HbT] (p = 0.003), but not with [Hb] (p = 0.052) or WF (p = 0.89). Error bars represent standard deviations
There were 15 subjects who had repeat mammograms and 18 who had repeat MRI scans following NACT and prior to surgery. Repeat MRI and mammograms were done after completion of NACT rather than 2 weeks into treatment due to cost. Thirty-two subjects were followed with physical exam measurements of their breast tumors every 2 weeks. Subjects who were not imaged prior to surgery declined repeat mammography and/or MRI. Mammography had a sensitivity of 57%, specificity of 75%, PPV and NPV of 67% to predict a favorable RCB response (RCB 0/I), while the values for MRI were 62, 80, 71 and 72%, respectively. Physical exam sensitivity was 83%, however, specificity was only 41%, with a PPV of 50% and NPV of 78%. The AUC for MRI was 0.71, for mammogram was 0.66, and for physical exam was 0.66 (Online Appendix).
Discussion
We found that serial imaging of breast tumors in women receiving NACT with DOT was possible. We were able to recruit 40 subjects and successfully perform serial DOT assessments in 34 subjects receiving NACT. There were statistically significant declines in DOT-measured [HbO2], [Hb], and [HbT] 2 weeks after starting chemotherapy in subjects who had a favorable RCB response compared to those who did not. Sensitivity, specificity, PPV, and NPV for DOT in predicting a favorable RCB response were 86.7, 68.4, 68.4, and 86.7%, respectively. The ROC for the 2-week percent change in [HbT] to predict a favorable RCB had an AUC of 0.807.
A number of studies have evaluated the use of optical spectroscopy or optical tomography to assess breast cancers. Some have focused on the use of DOT for diagnostic purposes to improve the accuracy of breast cancer diagnoses in conjunction with other imaging modalities such as ultrasound or MRI [16, 17]. Other studies have evaluated the use of DOT as a way to evaluate the biologic activity of breast tumors. Ueda et al. noted that optical imaging was associated with more mitotically active tumors with higher FDG uptake on PET imaging [18]. Other evaluations of optical tomography have also noted significant associations with tissue vascularity, larger tumor size, and tissue-based biomarkers including HR and HER2 status [19–21].
The correlation between DOT and biologic markers of tumor aggressiveness led to studies looking at whether DOT can predict response to NACT. Many of these studies have evaluated the ability of DOT, or similar technology, to predict a pCR. The largest and most recent of these studies, by Ueda et al., recruited 100 subjects to receive NACT with 84 of them undergoing diffuse optical spectroscopic imaging. Similar to our study, they found a larger decline in tumor hemoglobin levels in subjects who had a complete response compared to those who did not. While the sensitivity of this approach for predicting a pCR was 81.2%, the specificity was only 47%, with a PPV of 26.5% and NPV of 91.4% [22]. Other earlier studies have similarly found statistically significant declines in hemoglobin levels in patients who went on to have a pCR compared to those who did not [12–14, 22–27].
Prior optical imaging studies such as that by Ueda et al., have used hand-held, operator-dependent devices to image the breast. This approach can decrease the reproducibility of the evaluations and may account for the issue of limited sensitivity and specificity. Hand-held devices also do not allow for a full three-dimensional tomographic assessment of the entire breast, as our machine is able to do. Prior studies also used a variety of chemotherapy regimens, while ours used a uniform standard regimen (12 weeks of taxane followed by 8 weeks of doxorubicin and cyclophosphamide) for all enrolled patients, allowing for a more standardized evaluation. Finally, almost all prior studies used pCR as the outcome. While pCR is a good predictor of survival, it does not allow for a more graded evaluation of response. Zhu et al. addressed this issue by assessing response with the Miller–Payne grading system, which allows for less than a pCR for breast tumors [17]. One problem with this system, however, is that it does not account for disease in the regional lymph nodes as does the RCB assessment [28]. Recently published guidelines recommend use of the RCB system to quantify residual disease in clinical trials [29].
Diffuse optical tomography measurements occurred at baseline and at 2 weeks after starting NACT, while MRI and mammographic assessments took place at baseline and prior to surgery. A direct comparison of sensitivity, specificity, PPV and NPV, and RCB outcomes can therefore not be made across the different imaging modalities. The comparison between DOT and MRI or mammography would have been possible if repeat MRI and mammographic imaging took place 2 weeks after starting chemotherapy, however, this was not feasible due to cost constraints. MRI has been evaluated to monitor breast cancers during NACT and was shown to be effective in monitoring HR−/HER2− or HER2-positive disease, but was found to be inaccurate for HR+/HER2− breast cancer [30]. Furthermore, MRI is expensive, requires the injection of intravenous contrast, is time consuming, and can be uncomfortable to those who experience claustrophobia, making it less appealing as an imaging modality for this purpose.
While our study complements the findings of prior studies, additional investigations to confirm our findings and further explore this technology are needed. Strategies to increase contrast within the breast, such as changing blood flow with simple maneuvers such as breath holds or using intravenous chromophores, may improve the ability of DOT to detect more subtle changes. Whether DOT has better predictability for different subtypes of breast tumors is also an area of interest. As DOT changes were detected as soon as 2 weeks following the initiation of NACT, it provides an early time point in treatment to allow for changes to a patient’s chemotherapy regimen. Whether intervening early in the course of NACT will result in improved pathologic response, disease-free and overall survival is still not clear. Prospective studies to use DOT response as a guide to change chemotherapy regimens for those who do not have a favorable response will eventually help to fully realize the potential of NACT and this emerging technology.
Conclusions
Using DOT imaging to monitor women receiving NACT for breast cancer, we were able to distinguish statistically significant declines in hemoglobin concentrations between favorable and unfavorable responders. At considerably lower cost compared to breast MRI or PET–CT, and with no ionizing radiation or intravenous contrast needed, DOT promises to be a reliable, easy to administer, low cost, comfortable, and safe early predictor of tumor response.
Supplementary Material
Funding
This study was funded by Grants from the Komen Foundation for the Cure Post Doctoral Fellowship Research Grant to EAL, National Science Foundation Integrative Graduate Education and Research Traineeship program on Optical Techniques for Actuation, Sensing, and Imaging of Biological Systems at Columbia University to JEG, and the Natural Sciences and Engineering Research Council of Canada (NSERC) to MF. This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant No. KL2 TR000081 to KK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported in part by the Witten Family Fund.
Abbreviations
- A
Doxorubicin
- BC
Breast cancer
- BMI
Body mass index
- C
Cyclophosphamide
- CT
Computerized tomography
- DOT
Diffuse optical tomography
- [Hb]
Deoxyhemoglobin concentration
- [HbO2]
Oxyhemoglobin concentration
- [HbT]
Total hemoglobin concentration
- HER2
Human epidermal growth factor receptor 2
- HR
Hormone receptor
- MRI
Magnetic resonance imaging
- NACT
Neoadjuvant chemotherapy
- nm
Nanometer
- NPV
Negative predictive value
- pCR
Pathologic complete response
- PET
Position emission tomography
- PPV
Positive predictive value
- RCB
Residual cancer burden
- ROI
Region of interest
- SD
Standard deviation
- T
Paclitaxel
- US
Ultrasound
- WF
Water fraction
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-017-4150-7) contains supplementary material, which is available to authorized users.
Conflict of interest All authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A et al. (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16(8):2672–2685 [DOI] [PubMed] [Google Scholar]
- 2.Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, Dorval T, Palangie T, Jouve M, Beuzeboc P et al. (1994) Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer 30A(5):645–652 [DOI] [PubMed] [Google Scholar]
- 3.Makris A, Powles TJ, Ashley SE, Chang J, Hickish T, Tidy VA, Nash AG, Ford HT (1998) A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol 9(11):1179–1184 [DOI] [PubMed] [Google Scholar]
- 4.Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, Dilhuydy JM, Bonichon F (1999) Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol 10(1):47–52 [DOI] [PubMed] [Google Scholar]
- 5.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M et al. (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422 [DOI] [PubMed] [Google Scholar]
- 6.Brix G, Lechel U, Glatting G, Ziegler SI, Munzing W, Muller SP, Beyer T (2005) Radiation exposure of patients undergoing wholebody dual-modality 18F-FDG PET/CT examinations. J Nucl Med 46(4):608–613 [PubMed] [Google Scholar]
- 7.Buck AK, Herrmann K, Stargardt T, Dechow T, Krause BJ, Schreyogg J (2010) Economic evaluation of PET and PET/CT in oncology: evidence and methodologic approaches. J Nucl Med Technol 38(1):6–17 [DOI] [PubMed] [Google Scholar]
- 8.Chagpar AB, Middleton LP, Sahin AA, Dempsey P, Buzdar AU, Mirza AN, Ames FC, Babiera GV, Feig BW, Hunt KK et al. (2006) Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg 243(2):257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schott AF, Roubidoux MA, Helvie MA, Hayes DF, Kleer CG, Newman LA, Pierce LJ, Griffith KA, Murray S, Hunt KA et al. (2005) Clinical and radiologic assessments to predict breast cancer pathologic complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat 92(3):231–238 [DOI] [PubMed] [Google Scholar]
- 10.Zhu Q, Tannenbaum S, Hegde P, Kane M, Xu C, Kurtzman SH (2008) Noninvasive monitoring of breast cancer during neoadjuvant chemotherapy using optical tomography with ultrasound localization. Neoplasia 10(10):1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flexman ML, Hernandez SL, Huang J, Johung TJ, Kim HK, Lee J, Vlachos F, Yamashiro DJ, Kandel J, Hielscher AH (2010) Early detection of tumor vascular response to anti-angiogenic drugs with optical tomography. Optical Society of America (OSA) topical meeting, Miami, Florida, 13 April 2010 [Google Scholar]
- 12.Cerussi A, Hsiang D, Shah N, Mehta R, Durkin A, Butler J, Tromberg BJ (2007) Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc Natl Acad Sci USA 104(10):4014–4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soliman H, Gunasekara A, Rycroft M, Zubovits J, Dent R, Spayne J, Yaffe MJ, Czarnota GJ (2010) Functional imaging using diffuse optical spectroscopy of neoadjuvant chemotherapy response in women with locally advanced breast cancer. Clin Cancer Res 16(9):2605–2614 [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, Cheng SQ, Ji HF, Wang JS, Xu HT, Zhang GQ, Pang D (2010) Evaluation of protein pigment epithelium-derived factor (PEDF) and microvessel density (MVD) as prognostic indicators in breast cancer. J Cancer Res Clin Oncol 136:1719–1727 [DOI] [PubMed] [Google Scholar]
- 15.Flexman ML, Khalil MA, Al Abdi R, Kim HK, Fong CJ, Desperito E, Hershman DL, Barbour RL, Hielscher AH (2011) Digital optical tomography system for dynamic breast imaging. J Biomed Opt 16(7):076014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Q, Huang M, Chen N, Zarfos K, Jagjivan B, Kane M, Hedge P, Kurtzman SH (2003) Ultrasound-guided optical tomographic imaging of malignant and benign breast lesions: initial clinical results of 19 cases. Neoplasia 5(5):379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Q, Ricci A Jr, Hegde P, Kane M, Cronin E, Merkulov A, Xu Y, Tavakoli B, Tannenbaum S (2016) Assessment of functional differences in malignant and benign breast lesions and improvement of diagnostic accuracy by using US-guided diffuse optical tomography in conjunction with conventional US. Radiology. doi: 10.1148/radiol.2016151097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueda S, Nakamiya N, Matsuura K, Shigekawa T, Sano H, Hirokawa E, Shimada H, Suzuki H, Oda M, Yamashita Y et al. (2013) Optical imaging of tumor vascularity associated with proliferation and glucose metabolism in early breast cancer: clinical application of total hemoglobin measurements in the breast. BMC Cancer 13:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung SH, Feldman MD, Martinez D, Kim H, Putt ME, Busch DR, Tchou J, Czerniecki BJ, Schnall MD, Rosen MA et al. (2015) Macroscopic optical physiological parameters correlate with microscopic proliferation and vessel area breast cancer signatures. Breast Cancer Res 17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MJ, Su MY, Yu HJ, Chen JH, Kim EK, Moon HJ, Choi JS (2016) US-localized diffuse optical tomography in breast cancer: comparison with pharmacokinetic parameters of DCE-MRI and with pathologic biomarkers. BMC Cancer 16(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao M, Jiang Y, Zhu Q, You S, Li J, Wang H, Lai X, Zhang J, Liu H, Zhang J (2015) Diffuse optical tomography of breast carcinoma: can tumor total hemoglobin concentration be considered as a new promising prognostic parameter of breast carcinoma? Acad Radiol 22(4):439–446 [DOI] [PubMed] [Google Scholar]
- 22.Ueda S, Yoshizawa N, Shigekawa T, Takeuchi H, Ogura H, Osaki A, Saeki T, Ueda Y, Yamane T, Kuji I et al. (2016) Near-infrared diffuse optical imaging for early prediction of breast cancer response to neoadjuvant chemotherapy: a comparative study using FDG-PET/CT. J Nucl Med 57(8):1189–1195 [DOI] [PubMed] [Google Scholar]
- 23.Jiang S, Pogue BW, Carpenter CM, Poplack SP, Wells WA, Kogel CA, Forero JA, Muffly LS, Schwartz GN, Paulsen KD et al. (2009) Evaluation of breast tumor response to neoadjuvant chemotherapy with tomographic diffuse optical spectroscopy: case studies of tumor region-of-interest changes. Radiology 252(2):551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang S, Pogue BW, Kaufman PA, Gui J, Jermyn M, Frazee TE, Poplack SP, DiFlorio-Alexander R, Wells WA, Paulsen KD (2014) Predicting breast tumor response to neoadjuvant chemotherapy with diffuse optical spectroscopic tomography prior to treatment. Clin Cancer Res 20(23):6006–6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueda S, Roblyer D, Cerussi A, Durkin A, Leproux A, Santoro Y, Xu S, O’Sullivan TD, Hsiang D, Mehta R et al. (2012) Baseline tumor oxygen saturation correlates with a pathologic complete response in breast cancer patients undergoing neoadjuvant chemotherapy. Cancer Res 72(17):4318–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu C, Vavadi H, Merkulov A, Li H, Erfanzadeh M, Mostafa A, Gong Y, Salehi H, Tannenbaum S, Zhu Q (2016) Ultrasound-guided diffuse optical tomography for predicting and monitoring neoadjuvant chemotherapy of breast cancers: recent progress. Ultrason Imaging 38(1):5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Q, DeFusco PA, Ricci A Jr, Cronin EB, Hegde PU, Kane M, Tavakoli B, Xu Y, Hart J, Tannenbaum SH (2013) Breast cancer: assessing response to neoadjuvant chemotherapy by using US-guided near-infrared tomography. Radiology 266(2):433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, Schofield A, Heys SD (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12(5):320–327 [DOI] [PubMed] [Google Scholar]
- 29.Bossuyt V, Provenzano E, Symmans WF, Boughey JC, Coles C, Curigliano G, Dixon JM, Esserman LJ, Fastner G, Kuehn T et al. (2015) Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol 26(7):1280–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loo CE, Straver ME, Rodenhuis S, Muller SH, Wesseling J, Vrancken Peeters MJ, Gilhuijs KG (2011) Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. J Clin Oncol 29(6):660–666 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


