Abstract
Background:
Cardiac magnetic resonance (CMR) provides excellent temporal and spatial resolution, tissue characterization, and flow measurements. This enables major advantages when guiding cardiac invasive procedures compared with X-ray fluoroscopy or ultrasound guidance. However, clinical implementation is limited due to limited availability of technological advancements in magnetic resonance imaging (MRI) compatible equipment. A systematic review of the available literature on past and present applications of interventional MR and its technology readiness level (TRL) was performed, also suggesting future applications.
Methods:
A structured literature search was performed using PubMed. Search terms were focused on interventional CMR, cardiac catheterization, and other cardiac invasive procedures. All search results were screened for relevance by language, title, and abstract. TRL was adjusted for use in this article, level 1 being in a hypothetical stage and level 9 being widespread clinical translation. The papers were categorized by the type of procedure and the TRL was estimated.
Results:
Of 466 papers, 117 papers met the inclusion criteria. TRL was most frequently estimated at level 5 meaning only applicable to in vivo animal studies. Diagnostic right heart catheterization and cavotricuspid isthmus ablation had the highest TRL of 8, meaning proven feasibility and efficacy in a series of humans.
Conclusion:
This article shows that interventional CMR has a potential widespread application although clinical translation is at a modest level with TRL usually at 5. Future development should be directed toward availability of MR-compatible equipment and further improvement of the CMR techniques. This could lead to increased TRL of interventional CMR providing better treatment.
Keywords: cardiac catheterization, interventional cardiovascular magnetic resonance, technology readiness level
Introduction
Use of cardiac magnetic resonance (CMR) has increased dramatically due to its many advantages.1–7 CMR is the gold standard for quantifying heart chamber dimensions, volumes and ejection fraction due to its excellent temporal and spatial resolution. It provides soft tissue characterisation, perfusion imaging for detecting ischemia, and flow measurements for quantifying shunt volumes or valve regurgitation fractions. CMR can obtain images in any plane and does not use ionizing radiation.
Although X-ray fluoroscopy is the primary modality in cardiac interventions due to its widespread availability and good visualization of versatile catheters and interventional devices, which are commercially available, 8 use of CMR to guide procedures pre-, intra-, and postprocedurally is increasing because of its many advantages.1–7 However, clinical translation is hindered by the lack of technological advancements in MR compatible equipment.
The aim of this review is to investigate the clinical implementation of CMR in cardiac interventions by evaluating the technology readiness level (TRL). The TRL methodology was developed by National Aeronautics and Space Administration (NASA) to define several levels of ‘readiness’ for emerging technologies, and is still used by several organizations such as the US Department of Defense and the European Space Agency. In medical literature, it has been used for assessment of robotic gastro-intestinal endoscopy9,10 and of several devices designed to improve healthcare for elderly.11–13
Methods
An extensive literature search was performed using the PubMed database. The search included the following PubMed search and Mesh terms:
‘Magnetic Resonance Imaging, Interventional’[Mesh] OR ‘interventional magnetic resonance imaging’[tiab]
‘Cardiac Catheterization’[Mesh] OR ‘Cardiac Catheterization’[tiab] OR ‘Coronary Angiography’[Mesh] OR ‘Coronary Angiography’[tiab] OR ‘Percutaneous Coronary Intervention’[Mesh] OR ‘Percutaneous Coronary Intervention’[tiab]
‘Cardiac Catheterization’[Mesh] OR ‘Cardiac Catheterization’[tiab] AND ‘Biopsy’[Mesh] OR ‘Image-Guided Biopsy’[Mesh] OR ‘Myocardial biopsy’[tiab] OR ‘Endomyocardial biopsy’[tiab]
‘Cardiac Electrophysiology’[Mesh] OR ‘Cardiac Electrophysiology’[tiab] OR ‘Pulmonary Vein Isolation’[tiab] OR ‘Catheter Ablation’[tiab] OR ‘Catheter Ablation’[Mesh]
‘Atrial Fibrillation’[Mesh] OR ‘Atrial Fibrillation’[tiab] OR ‘Arrhythmias, Cardiac’[Mesh] OR ‘cardiac arrhythmias’[tiab]
Inclusion criteria for the selected papers were: the paper had to be peer-reviewed, written in English language, published before June 2021 and concern interventional CMR rather than diagnostic CMR. The results of the initial search were screened by title and abstract for eligibility. After screening, the full text was read for eligibility. Finally, references were crosschecked for additional papers.
The following subcategories were made: technical aspects, electrophysiology (EP) procedures, right-sided cardiac catheterization and congenital procedures, coronary procedures, valvular procedures, and other procedures. The advancement toward clinical translation was assessed by evaluating the TRL. For this review, the nine TRL levels used by the US Government Accountability Office (GAO) 14 were modified as shown in Table 1. The subcategory technical aspects comprise papers describing technical developments without clinical applications and were therefore not assigned a TRL.
Table 1.
TRL levels based on the US Government Accountability Office (GAO). 14
| TRL level based on US-GAO | Description based on US-GAO | Modified TRL level description as applied in this review |
|---|---|---|
| TRL 1 Basic principles observed and reported |
Published research identifying problem/possible technology | Proposed hypothesis of using interventional CMR |
| TRL 2 Technology concept and/or application formulated |
Papers of analytic studies. Supporting analyses providing
scientific information and data to develop research proposals |
Preprocedural CMR used to guide interventions |
| TRL 3 Analytical and experimental critical function and/or characteristic proof of concept |
Proof of concept in laboratory, publication of results | Ex vivo phantom studies |
| TRL 4 Component and/or breadboard validation in lab |
Proof of concept and safety demonstrated in ex vivo animal bowel | Ex vivo animal studies |
| TRL 5 Component and/or breadboard validation in relevant environment |
Evidence of device being equivalent to predicate device (FDA
510(k)) ready for clinical trials |
In vivo animal studies |
| TRL 6 (Sub)System model or prototype demonstration in relevant environment |
Clinical trials conducted in small number of humans | Proven feasibility in single human case |
| TRL 7 System prototype demonstration in operational environment |
Clinical safety and effectiveness trials in operational
environment. Determination of short-term adverse events and risks associated with the device. Final design validated |
Proven efficacy in single human case |
| TRL 8 System completed and qualified through test and demonstration |
FDA 510(k) or equivalent approved | Proven feasibility and efficacy in series of humans |
| TRL 9 System proven through successful mission operations |
The device is being marketed. Postmarketing studies | Widespread clinical translation |
CMR, cardiac magnetic resonance; GAO, Government Accountability Office; TRL, technology readiness level; FDA, Food and Drug Administration.
Results
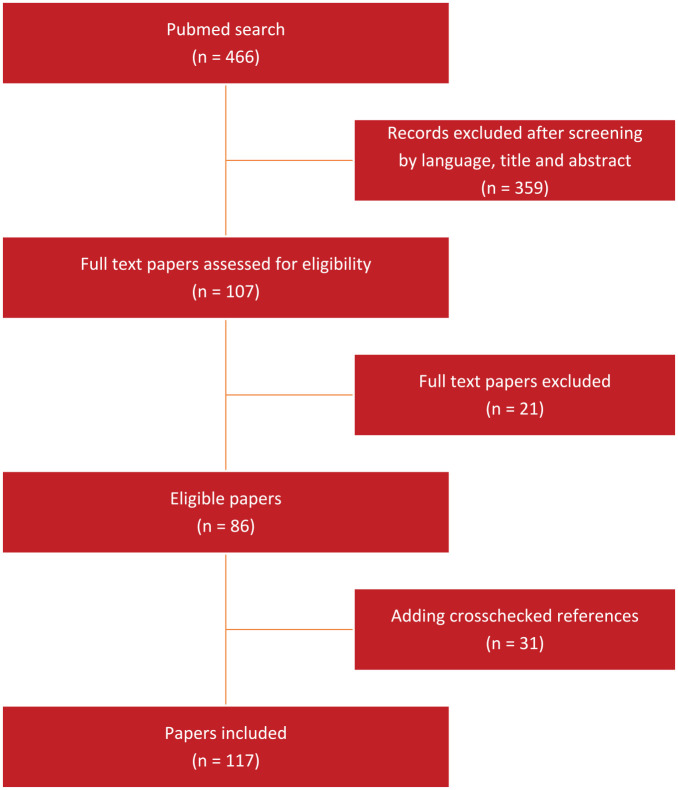
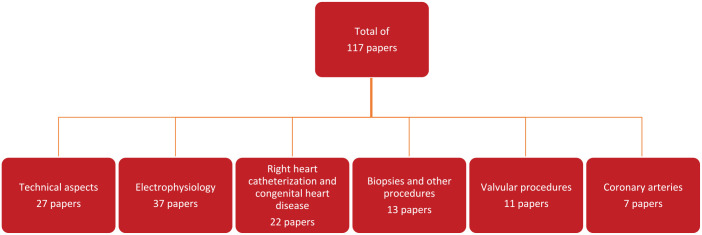
The literature search returned 466 papers. After screening by language, title, and abstract 359 papers were excluded. These were mostly papers regarding extra-cardiac procedures. After reading the full text of the remaining papers for eligibility, a further 21 papers were excluded, mostly because the topic was diagnostic CMR rather than interventional. After crosschecking the references of these papers, 31 subsequent papers were included leading to a total of 117 papers. Figure 1 shows the PRISMA figure of the literature search. Figure 2 shows the division in subcategories, which are described in the subsections below. Table 2 lists the TRL levels of the various subcategories.
Figure 1.
Flowchart depicting the selection of papers.
Figure 2.
Flowchart depicting the various subcategories.
Table 2.
Cardiac procedures divided into subcategories with their respective TRLs.
| Procedure | TRL level | References |
|---|---|---|
| Electrophysiology | ||
| Right atrial flutter ablation | TRL 8 | Grotthoff et al. 15 |
| Left atrium ablation | TRL 5 | Grotthoff et al., 16 Schmidt et al. 17 |
| Right ventricle ablation | TRL 5 | Dukkipati et al., 18 Lardo et al. 19 |
| Left ventricle ablation | TRL 5 | Toupin et al., 20 Mukherjee et al., 21 Krahn et al. 22 |
| AV-node ablation | TRL 5 | Schmidt et al. 17 |
| Congenital procedures | ||
| Diagnostic right and left heart catheterization | TRL 8 | Veeram Reddy et al., 23 Ratnayaka et al., 24 Pushparajah et al., 25 Knight et al., 26 Velasco Forte et al., 27 Meierhofer et al. 28 |
| Ventricular septal defect closure, treatment of pulmonary artery stenosis and aortic coarctation | TRL 6 | Grant et al. 4 |
| Atrial septal defect closure | TRL 5 | Ratnayaka et al., 29 Rickers et al. 30 |
| Cavopulmonary shunt | TRL 5 | Ratnayaka et al. 31 |
| Valvular procedures | ||
| Transcatheter aortic valve replacement | TRL 5 | Kuehne et al., 32 Horvath et al., 33 McVeigh et al., 34 Miller et al., 35 Miller et al., 36 Horvath et al., 37 Horvath et al. 38 |
| Pulmonary valve stenosis balloon dilatation | TRL 6 | Tzifa et al. 39 |
| Coronary procedures | ||
| Diagnostic catheterization | TRL 5 | Green et al., 40 Zhang et al., 41 Qiu et al., 42 Spuentrup et al., 43 Serfaty et al., 44 Heidt et al. 45 |
| Percutaneous coronary intervention | TRL 5 | Spuentrup et al. 43 |
| Other procedures | ||
| Endomyocardial biopsy | TRL 5 | Behm et al., 46 Rogers et al., 47 Unterberg-Buchwald et al. 48 |
| Intramyocardial injections | TRL 5 | Carlsson and Saeed, 49 Krombach et al., 50 Tomkowiak et al., 51 van Es et al. 52 |
| Pericardiocentesis | TRL 5 | Halabi et al. 53 |
TRL, technology readiness level.
Technical aspects
Most papers in this category focused on development of MR compatible equipment and disposables such as catheters, which is usually the limiting factor for procedures not achieving a higher TRL.
Catheters can be visualized in the MR image either by active or passive tracking. Some papers focused on passive tracking, a technique were instruments can be discerned due to their material characteristics. Most contain some form of para- or ferromagnetic materials. With optimized imaging protocols, these devices can be discerned from the surrounding anatomical structures.54–58 Other papers focus on balloon angiographic catheters that can enhance visualization by inflating them with carbon dioxide or a gadolinium contrast-doped solution.58–60
Passive tracking has some limitations. The imaging plane must be frequently manipulated to keep the device within the field of view and in plane. 59 Furthermore, the narrow imaging planes often allow only one device to be projected at a time, where many cardiac interventional procedures require more than one catheter or device at once.56,59,61
More recent papers report on active tracking, a technique which is based on coils or other detectors incorporated into the catheter. These are electronically connected to the scanner and by using standard MR read-out techniques, the exact three-dimensional position of the coils can be determined. The catheter position can then be overlaid on the MR images. This method provides better visualization and easier localization of the instruments compared with passive tracking, but requires the tracked device to be modified.16,58,62–65 In contrast to most passive tracking algorithms, in active tracking, the imaging plane can be automatically adjusted to the catheter. A major limitation is that the connection to external circuits via a long wire in a strong magnetic field makes it prone to induction of an electrical current or heating.56,57,61,66,67
Nazarian et al. 55 found that active tracking requires fewer imaging planes compared with passive tracking. Also, the robustness of active tracking of micro coils may be further enhanced by using dephasing magnetic field gradient pulses applied orthogonal to the frequency encoding gradient pulse used in conventional MR tracking. 62
Furthermore, several papers reported about the important safety issues involved with using instruments and devices under real-time MR guidance. One of the most important issues is heating of conductive objects.67,68 Inductive loops, formed by equipment leads, for example, can potentially cause unwanted tissue burns. Long structures such as guidewires or actively tracked catheters that use long conductive wires may also be inadvertently heated.66,69 Temperatures up to 74°C have been observed in nitinol guidewires. 59
However, several methods exist to minimize these issues, most importantly adjusting instrument design by decoupling possible resonant circuits, using alternative nonconductive components, for example, Kevlar or interrupting the metallic segments to reduce radiofrequency (RF)-induced heating.68,69 Because not all effects can be fully eliminated, the MR sequence can be optimized to cause less RF dissipation, for example, by using lower flip angles and more RF-efficient gradient echo spiral imaging.23,69–72
Electrophysiology
EP was one of the most advanced fields regarding implementation of interventional CMR, specifically the cavotricuspid isthmus ablation, which has a TRL of 8. Most other EP procedures have a TRL of 5. Of the 116 selected papers, 37 described studies on MR-guided catheter ablations, which is the most of all subcategories.15–22,55,64,73–99
Catheter ablation uses RF or cryothermal energy to disable the heart tissue, which is believed to cause arrhythmias. The disadvantage of current X-ray fluoroscopy-guided catheter ablation is the disappointing clinical success rate on the long-term, due to either technical failure (e.g. failure to form durable transmural lesions) or pathophysiological causes (e.g. incorrect selection of ablation targets). Especially information on pre-existing scar and peri-procedural lesion visualization are potential advantages of MR.
Real-time MR-guided catheter ablation in animals has been described as early as 2000. 19 Several groups describe MR-guided RF catheter ablation in the left ventricle,20–22 right ventricle,18,19 left atrium, 17 and right atrium. 81 Cryo-ablation was described as well.83,86 In addition, Grothoff et al. 16 described active catheter tracking and left atrial access via transseptal puncture under MR guidance, which Raval et al. 100 had also described in a non-EP procedure. Aside from RF ablation in the left atrium, pacing and activation mapping were also performed in the coronary sinus. These are all crucial elements for future real-time MR guidance of atrial fibrillation (AF) ablation, during which pacing and activation mapping are frequently used. Authors describe that preprocedural anatomic imaging might be needed in the case of active tracking for adequate visualization of surrounding structures. 16 Furthermore, left atrial electrograms did not allow recording of very low amplitude signals. These were filtered out by the signal filtering that was used to prevent artifacts from the surrounding environment. This was a limitation for His-electrograms, which must be able to detect very low amplitude signals and distinguish them from artifacts. 16 Nevertheless, the prospect of real-time MR-guided left atrial ablation is promising.
Several human clinical studies have been performed, showing the feasibility of MR-guided catheter ablations for strictly anatomically defined ablations. Due to the benefits of interventional CMR for tissue characterization and lesion visualization, a future benefit is foreseen for substrate-guided ablations. Nazarian et al., 55 in 2008, described the first MR-guided placement of catheters and recording of intracardiac electrograms in the right atrium, right ventricle, and bundle of His in humans (N = 2) using prototype passively tracked catheters. In 2013, Sommer et al. 99 described a series of five patients who underwent atrial flutter ablation, AV-nodal re-entry tachycardia ablation, or an electrophysiological study under X-ray fluoroscopy guidance. Afterwards, patients underwent a real-time MR-guided procedure where catheters were placed in the right atrium and the right ventricle using passive catheter tracking. Intracardiac electrograms were successfully recorded and simple programmed stimulation maneuvers were performed. In this case, commercially available MR-compatible catheters were used (Vision™, Imricor Medical Systems, Burnsville, MN, USA). Grothoff et al. 15 described the first real-time MR-guided cavotricuspid isthmus ablations in a series of patients (N = 10). Complete conduction block was achieved in one patient. This was partly due to the study protocol limiting the procedure time in the MR-room to 90 min. Nevertheless, in the other nine patients, only limited additional ablation pulses were required under X-ray fluoroscopy guidance to gain complete isthmus block. In 2016, the same group described the first cavotricuspid isthmus ablations in humans using active catheter tracking in combination with passive tracking, which enhanced catheter navigation. 64 Complete bidirectional block was achieved in 50% of cases (three out of six patients). In a follow-up study 94 of 30 patients, MR-guided cavotricuspid isthmus ablation was achieved in 93% compared with 100% in the control group. One recurrence of AF (3%) occurred in the control group. This validation proving feasibility, safety, and efficacy of cavotricuspid isthmus ablation in humans warrants a TRL of 8, which is among the highest score of all categories that are mentioned in this review.
Right heart catheterization and congenital cardiology
Along with cavotricuspid isthmus ablation, diagnostic right heart catheterization too is well advanced regarding implementation of interventional CMR, also achieving a TRL of 8. Most other congenital procedures had a TRL of 5.
In children with congenital heart disease, cardiac catheterization is often required for diagnosis, treatment, and follow-up. Therefore, MR-guided catheterization is beneficial.24,101–103 Its excellent resolution enables the sometimes complex congenital anatomy to be well visualized, while also enabling measurements of pulmonary artery pressure and left atrial or wedge pressure. MR phase-contrast enables calculation of the pulmonary vascular resistance, although arrhythmias and turbulent flow are major constraints.104,105
Several research groups assessed the feasibility of MR-guided right heart catheterization in phantoms, animals, and patients.23–26,60,102,104–108 In 2003, Razavi et al. 102 was among the first to describe diagnostic right heart catheterization under real-time MR guidance. Pulmonary artery pressure, wedge pressure, pulmonary artery and aortic or left atrial oxygen saturations were measured. Pulmonary vascular resistance was calculated using the Fick method and using MR flow measurements. Catheters were visualized by passive tracking and enhanced by attaching a CO2-filled balloon to the tip. In 2015, the group of Pushparajah et al. 25 described a cohort of 149 patients with congenital heart disease who underwent 167 catheterizations for pulmonary vascular resistance assessment (including the procedures from the 2003 publication) 102 using either only MR guidance or a combination of MR and X-ray fluoroscopy guidance, firmly establishing its feasibility. While this group used conventional guidewires under X-ray fluoroscopy when necessary, Veeram Reddy et al. 23 demonstrated the use of an MR-conditional guidewire for right and left heart catheterization successfully in 24 out of 25 cases. Meierhofer et al. 28 also describe successful use of MR-conditional guidewires for right and left heart catheterization and pressure measurement in great vessels in 23 out of 25 cases of patients with congenital heart disease.
Feasibility of MR-guided transcatheter closure of atrial and ventricular septal defects (ASD/VSD) was described in several animal studies, achieving a TRL of 5.29,30,104,107,109,110
Rickers et al. 30 showed the first feasibility of MR-guided closure of ASD in seven swines. The shunt was visualized using MR angiography after administration of gadolinium-gadopentetate dimeglumine using T1-weighted gradient echo sequences, and the size of the defect was measured using orthogonal short- and long-axis images. An Amplatzer Septal Occluder device to close the ASD was then attached to a custom-designed nitinol delivery cable and loaded into the delivery sheath to facilitate deployment. Postmortem examination showed good correlation of the ASD size when an antenna guide wire crossing the ASD had been used, allowing for a smaller field-of-view without aliasing artifacts. MR-guided VSD-closure was described in animals by Ratnayaka et al. 29 Furthermore, Raval et al. 100 described transseptal puncture under real-time MR guidance using a custom-made actively tracked needle. The feasibility of transseptal puncture can be useful in a wide variety of procedures.
Finally, Ratnayaka et al. 31 described one of the most complicated MR-guided interventions. In 15 swines, a transcatheter cavopulmonary anastomosis and a shunt were created using novel, purpose-built devices after commercially available endografts were tested but provided suboptimal distal anastomosis. While the advantages of performing this procedure via a transcatheter route are further enhanced when done under MR guidance, they have not yet translated to human studies or clinical practice (TRL 5).
Valvular procedures
Feasibility of MR-guided valvular procedures has been tested in animal studies mostly since 2004, 111 especially in the aortic valve position, and thereby achieving a TRL of 5.
Real-time MR guidance of transcatheter aortic valve implantation has several possible advantages over X-ray fluoroscopy guidance and angiography. It allows precise positioning, and immediate assessment of the function of the prosthesis using flow measurements.112,113 After the initial proof of concept in 2004, several other in vivo animal studies have been performed.32–38 It has been shown that the commercially available Medtronic CoreValve® prosthesis can be used under real-time MR guidance, with small modifications made to the delivery device, of which the metal braiding had to be removed due to severe susceptibility artifacts.35,112,113
However, clinical implementation has been limited by several aspects, being the costs and time required to implement an interventional CMR catheterization suite, and safety aspects such as MR-incompatible implants in patients. The only valvular procedures performed under real-time MR guidance in humans and thereby achieving a TRL of 6, were described by Tzifa et al. 39 After first performing 20 balloon dilatations in five swines using a nonmetallic guidewire and passive catheter tracking, balloon dilatation of pulmonary valve stenosis was performed in two humans. Although both procedures took approximately 3 h, both patients had significant reduction of the gradient over the pulmonary valve and no complications were reported.
Coronary artery catheterization and interventions
Coronary artery catheterization and intervention under real-time MR guidance has been performed in a limited number of animal studies,40–45 achieving a TRL of 5. Spuentrup et al. 43 described successful engagement of both coronary artery ostia in seven pigs using passive tracking. Stent placement was successful in 10 of 11 coronary arteries. Limitations of this study included the incorporation of nondiseased coronary arteries and, with a passive tracking approach, the guidewire tips could not be visualized in distal parts of coronary arteries.
A comparison between X-ray fluoroscopy and real-time MR guidance was made by Green et al. 40 A stenosis was surgically created in the proximal left circumflex coronary artery in nine swines. Conventional X-ray fluoroscopy-guided coronary catheterization was performed 3 weeks after surgery to objectify the stenosis. On the MR-scanner, the same femoral arterial sheath was used for left coronary artery catheterization, using an actively tracked guidewire and the same angiographic catheter, which led to successful intubation of the left coronary artery in eight out of nine cases. This showed an excellent correlation with an intra-class correlation coefficient of 0.955.
MR-guided coronary interventions have hardly made any progress since 2008 due to the small size of the coronary arteries and the lack of MR-compatible materials with sufficient stiffness, guiding stability, and MR visibility. In the most recent animal study performed by Heidt et al., 45 the authors still had to rely on custom-designed catheters.
Other procedures
Several other cardiac catheterization procedures requiring intracardiac or pericardial access were described, predominantly in animal studies and therefore achieving a TRL of 5.
Few groups46–48 investigated the use of MR guidance for endomyocardial biopsies, as the diagnostic yield of these biopsies is often dependent on the distribution of disease. The largest study investigating the use of real-time MR guidance for endomyocardial biopsies was performed by Rogers et al. 47 In five swines, late gadolinium enhancement MRI was performed 3 weeks after inducing myocardial infarction by obstructing an obtuse marginal branch. X-ray fluoroscopy and real-time MR-guided biopsies were taken, and the diagnostic yield of the MR-guided biopsies (63 of 77 biopsies, 82%) was significantly better than that of the X-ray fluoroscopy-guided biopsies (49 of 87 biopsies, 56%). This suggests MR guidance of endomyocardial biopsies can improve the diagnostic yield compared with X-ray fluoroscopy guidance.
In animal studies, intramuscular injection of bone marrow stem cells into myocardial infarct border zones has shown functional myocardial recovery, but convincing feasibility has not been shown in humans. Hatt et al. 114 suggested this might partly be due to the delivery method and target location. Intramyocardial injections have been performed successfully under real-time MR guidance in animals by several authors.50–52 However, the limited clinical application of stem cell therapy in ischemic heart disease limits the requirement of interventional CMR for this purpose.
Halabi et al. 53 described real-time MR-guided pericardiocentesis in 12 swines with either no effusion, small (50 ml), moderate (100 ml) or large effusion (150 ml) using commercially available passive access titanium needles (CMR Puncture Needle, 18G × 15 cm, Philips Invivo, Schwerin, Germany) via a subxiphoid access route. It was successful in all cases.
Feasibility of pericardiocentesis in an MR-environment is essential, since cardiac tamponade is a known complication of various cardiac procedures. Damaging delays may be prevented when pericardiocentesis can be performed in the same environment.
Discussion
A detailed systematic review of the available literature on interventional CMR for cardiac procedures was performed and TRL of these procedures was assessed. Although several limitations still need to be overcome to further advance and improve clinical translation, interventional cardiac MRI is a highly promising technique. Particularly in diagnostic right heart catheterization and cavotricuspid isthmus ablation, the technique has advanced toward clinical translation over the past 10 years, reaching a TRL of 8. Other procedures have mostly been done in animal studies, achieving a TRL of 5.
To date, technical aspects like catheter tracking and heating of instruments are still major limitations. Passive tracking often only shows part of the device, is time-consuming and is problematic when multiple devices need to be visualized at the same time. Active tracking provides more convenient visualization, but requires substantial device modification and is prone to heating. The lack of an MR-compatible defibrillator and the need to acquire new technical expertise are other factors preventing widespread clinical translation of MR-guided procedures. Nevertheless, the possibility of adding the strong diagnostic features of CMR (e.g. high spatial and temporal resolution, excellent anatomic visualization, tissue characterization, and flow measurements) to interventional procedures is very valuable, with the advantage of not exposing patient and staff to ionizing radiation. Furthermore, Campbell-Washburn et al. 115 have described how low-field-strength MRI (0.55 T) reduces heating, which allowed conventional metallic guidewires to be used in right heart catheterization in seven cases, while efficient spiral image acquisitions enabled good image quality. Diagnostic right heart catheterization is especially beneficial in children with congenital heart disease, who often have to undergo multiple assessments of pulmonary artery pressures and pulmonary vascular resistance and benefit most from avoiding ionizing radiation. This is a particular area that might benefit from low-field-strength interventional CMR.
In EP, clinical translation of interventional CMR has been achieved in right-sided procedures. Real-time MR-guided cavotricuspid isthmus ablation in atrial flutter has been described in a series of 30 patients 94 and was shown to be equally effective as X-ray fluoroscopy guidance. Left-sided ablation is still limited by the lack of an MR-compatible needle available for transseptal puncture. Lesion visualization in the left atrium is also impeded by the difficulty of imaging the thin atrial wall with the currently available resolution, although this also applies to the right atrium where substantial progress had been made. Nevertheless, this area would particularly benefit from tissue characterization, as it could give immediate feedback on the efficacy of the procedure because of the ability to ensure ablation lesions are interconnected and transmural, leading to a higher success rate of AF ablation and less re-do procedures. Conventional T1 and T2 mapping, especially when using contrast enhancement for T1-weighted imaging, are also useful to determine reversibility of RF-lesions. 22
Another area that could benefit from interventional CMR is endomyocardial biopsy, since diagnostic yield is affected by disease distribution. Small animal studies have already shown a superior diagnostic yield for MR-guided interventions compared with X-ray fluoroscopy-guided interventions.47,48 However, to date, MR-compatibility of bioptomes is a limiting factor.
Valvular and coronary procedures have mostly been performed in animal studies, and clinical translation in these areas appears not imminent for a longer period.
Conclusion
Our results show that diagnostic right heart catheterization and cavotricuspid isthmus ablation have the highest TRL in interventional CMR, achieving a TRL of 8. These procedures are clinically feasible and have several advantages over conventional X-ray fluoroscopy guidance. Other procedures, like left-sided RF-ablation and endomyocardial biopsy might benefit greatly from the features of interventional CMR (e.g. tissue characterization and lesion visualization), but have so far been performed almost exclusively in animal studies, therefore achieving a TRL of 5. Further clinical translation of such procedures is dependent on the development of MR-compatible instruments, but we believe that once this is achieved, interventional CMR will greatly contribute to improving success rates of left-sided ablation and diagnostic yield of endomyocardial biopsies.
Acknowledgments
The authors report no acknowledgements.
Footnotes
ORCID iD: Sophie C. Rier  https://orcid.org/0000-0001-5881-4937
https://orcid.org/0000-0001-5881-4937
Contributor Information
Sophie C. Rier, Cardiology Division, Department of Cardiology, Haga Teaching Hospital, Els Borst-Eilersplein 275, Postbus 40551, The Hague 2504 LN, The Netherlands.
Suzan Vreemann, Department of Cardiology, Haga Teaching Hospital, The Hague, The Netherlands Siemens Healthineers Nederland B.V., Den Haag, The Netherlands.
Wouter H. Nijhof, Siemens Healthineers Nederland B.V., Den Haag, The Netherlands
Vincent J.H.M. van Driel, Department of Cardiology, Haga Teaching Hospital, The Hague, The Netherlands
Ivo A.C. van der Bilt, Department of Cardiology, Haga Teaching Hospital, The Hague, The Netherlands
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Sophie C. Rier: Conceptualization; Investigation; Methodology; Project administration; Visualization; Writing – original draft; Writing – review & editing.
Suzan Vreemann: Conceptualization; Methodology; Writing – review & editing.
Wouter H. Nijhof: Conceptualization; Methodology; Writing – review & editing.
Vincent J.H.M. van Driel: Conceptualization; Methodology; Writing – review & editing.
Ivo A.C. van der Bilt: Conceptualization; Methodology; Project administration; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Behar JM, Sieniewicz B, Mountney P, et al. Image integration to guide wireless endocardial LV electrode Implantation for CRT. JACC Cardiovasc Imaging 2017; 10: 1526–1528. [DOI] [PubMed] [Google Scholar]
- 2. Fagan TE, Truong UT, Jone PN, et al. Multimodality 3-dimensional image integration for congenital cardiac catheterization. Methodist Debakey Cardiovasc J 2014; 10: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glöckler M, Halbfaβ J, Koch A, et al. Multimodality 3D-roadmap for cardiovascular interventions in congenital heart disease – a single-center, retrospective analysis of 78 cases. Catheter Cardio Inte 2013; 82: 436–442. [DOI] [PubMed] [Google Scholar]
- 4. Grant EK, Kanter JP, Olivieri LJ, et al. X-ray fused with MRI guidance of pre-selected transcatheter congenital heart disease interventions. Catheter Cardio Inte 2019; 94: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nageotte SJ, Lederman RJ, Ratnayaka K. MRI catheterization: ready for broad adoption. Pediatr Cardiol 2020; 41: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salden OAE, van den Broek HT, van Everdingen WM, et al. Multimodality imaging for real-time image-guided left ventricular lead placement during cardiac resynchronization therapy implantations. Int J Cardiovasc Imaging 2019; 35: 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suntharos P, Setser RM, Bradley-Skelton S, et al. Real-time three dimensional CT and MRI to guide interventions for congenital heart disease and acquired pulmonary vein stenosis. Int J Cardiovasc Imaging 2017; 33: 1619–1626. [DOI] [PubMed] [Google Scholar]
- 8. Ratnayaka K, Faranesh AZ, Guttman MA, et al. Interventional cardiovascular magnetic resonance: still tantalizing. J Cardiovasc Magn Res 2008; 10: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marlicz W, Ren X, Robertson A, et al. Frontiers of robotic gastroscopy: a comprehensive review of robotic gastroscopes and technologies. Cancers (Basel) 2020; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tapia-Siles SC, Coleman S, Cuschieri A. Current state of micro-robots/devices as substitutes for screening colonoscopy: assessment based on technology readiness levels. Surg Endosc 2016; 30: 404–413. [DOI] [PubMed] [Google Scholar]
- 11. Lapierre N, Neubauer N, Miguel-Cruz A, et al. The state of knowledge on technologies and their use for fall detection: a scoping review. Int J Med Inform 2018; 111: 58–71. [DOI] [PubMed] [Google Scholar]
- 12. Liu L, Stroulia E, Nikolaidis I, et al. Smart homes and home health monitoring technologies for older adults: a systematic review. Int J Med Inform 2016; 91: 44–59. [DOI] [PubMed] [Google Scholar]
- 13. Rossi S, Lisini Baldi T, Aggravi M, et al. Wearable haptic anklets for gait and freezing improvement in Parkinson’s disease: a proof-of-concept study. Neurol Sci 2020; 41: 3643–3651. [DOI] [PubMed] [Google Scholar]
- 14. U.S. Government Accountability Office. Technology readiness assessment guide, 2020, https://www.gao.gov/products/gao-20-48g
- 15. Grothoff M, Piorkowski C, Eitel C, et al. MR imaging-guided electrophysiological ablation studies in humans with passive catheter tracking: initial results. Radiology 2014; 271: 695–702. [DOI] [PubMed] [Google Scholar]
- 16. Grothoff M, Gutberlet M, Hindricks G, et al. Magnetic resonance imaging guided transatrial electrophysiological studies in swine using active catheter tracking – experience with 14 cases. Eur Radiol 2017; 27: 1954–1962. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt EJ, Mallozzi RP, Thiagalingam A, et al. Electroanatomic mapping and radiofrequency ablation of porcine left atria and atrioventricular nodes using magnetic resonance catheter tracking. Circ Arrhythm Electrophysiol 2009; 2: 695–704. [DOI] [PubMed] [Google Scholar]
- 18. Dukkipati SR, Mallozzi R, Schmidt EJ, et al. Electroanatomic mapping of the left ventricle in a porcine model of chronic myocardial infarction with magnetic resonance-based catheter tracking. Circulation 2008; 118: 853–862. [DOI] [PubMed] [Google Scholar]
- 19. Lardo AC, McVeigh ER, Jumrussirikul P, et al. Visualization and temporal/spatial characterization of cardiac radiofrequency ablation lesions using magnetic resonance imaging. Circulation 2000; 102: 698–705. [DOI] [PubMed] [Google Scholar]
- 20. Toupin S, Bour P, Lepetit-Coiffé M, et al. Feasibility of real-time MR thermal dose mapping for predicting radiofrequency ablation outcome in the myocardium in vivo. J Cardiovasc Magn Res 2017; 19: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mukherjee RK, Roujol S, Chubb H, et al. Epicardial electroanatomical mapping, radiofrequency ablation, and lesion imaging in the porcine left ventricle under real-time magnetic resonance imaging guidance-an in vivo feasibility study. Europace 2018; 20: f254–f262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krahn PRP, Singh SM, Ramanan V, et al. Cardiovascular magnetic resonance guided ablation and intra-procedural visualization of evolving radiofrequency lesions in the left ventricle. J Cardiovasc Magn Res 2018; 20: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veeram Reddy SR, Arar Y, Zahr RA, et al. Invasive cardiovascular magnetic resonance (iCMR) for diagnostic right and left heart catheterization using an MR-conditional guidewire and passive visualization in congenital heart disease. J Cardiovasc Magn Res 2020; 22: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ratnayaka K, Kanter JP, Faranesh AZ, et al. Radiation-free CMR diagnostic heart catheterization in children. J Cardiovasc Magn Res 2017; 19: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pushparajah K, Tzifa A, Bell A, et al. Cardiovascular magnetic resonance catheterization derived pulmonary vascular resistance and medium-term outcomes in congenital heart disease. J Cardiovasc Magn Res 2015; 17: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knight DS, Kotecha T, Martinez-Naharro A, et al. Cardiovascular magnetic resonance-guided right heart catheterization in a conventional CMR environment - predictors of procedure success and duration in pulmonary artery hypertension. J Cardiovasc Magn Res 2019; 21: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Velasco Forte MN, Roujol S, Ruijsink B, et al. MRI for Guided Right and Left Heart Cardiac Catheterization: A Prospective Study in Congenital Heart Disease. J Magn Reson Imaging 2021; 53: 1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meierhofer C, Belker K, Shehu N, et al. Real-time CMR guidance for intracardiac and great vessel pressure mapping in patients with congenital heart disease using an MR conditional guidewire – results of 25 patients. Cardiovasc Diagn Ther 2021; 11: 1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ratnayaka K, Saikus CE, Faranesh AZ, et al. Closed-chest transthoracic magnetic resonance imaging-guided ventricular septal defect closure in swine. JACC Cardiovasc Interv 2011; 4: 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rickers C, Jerosch-Herold M, Hu X, et al. Magnetic resonance image-guided transcatheter closure of atrial septal defects. Circulation 2003; 107: 132–138. [DOI] [PubMed] [Google Scholar]
- 31. Ratnayaka K, Rogers T, Schenke WH, et al. Magnetic resonance imaging-guided transcatheter cavopulmonary shunt. JACC Cardiovasc Interv 2016; 9: 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuehne T, Saeed M, Higgins CB, et al. Endovascular stents in pulmonary valve and artery in swine: feasibility study of MR imaging-guided deployment and postinterventional assessment. Radiology 2003; 226: 475–481. [DOI] [PubMed] [Google Scholar]
- 33. Horvath KA, Mazilu D, Guttman M, et al. Midterm results of transapical aortic valve replacement via real-time magnetic resonance imaging guidance. J Thorac Cardiovasc Surg 2010; 139: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McVeigh ER, Guttman MA, Lederman RJ, et al. Real-time interactive MRI-guided cardiac surgery: aortic valve replacement using a direct apical approach. Magn Reson Med 2006; 56: 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller JG, Li M, Mazilu D, et al. Real-time magnetic resonance imaging-guided transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2016; 151: 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller JG, Li M, Mazilu D, et al. Robot-assisted real-time magnetic resonance image-guided transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2016; 151: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Horvath KA, Mazilu D, Kocaturk O, et al. Transapical aortic valve replacement under real-time magnetic resonance imaging guidance: experimental results with balloon-expandable and self-expanding stents. Eur J Cardiothorac Surg 2011; 39: 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horvath KA, Mazilu D, Cai J, et al. Transapical sutureless aortic valve implantation under magnetic resonance imaging guidance: acute and short-term results. J Thorac Cardiovasc Surg 2015; 149: 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tzifa A, Krombach GA, Krämer N, et al. Magnetic resonance-guided cardiac interventions using magnetic resonance-compatible devices: a preclinical study and first-in-man congenital interventions. Circ Cardiovasc Interv 2010; 3: 585–592. [DOI] [PubMed] [Google Scholar]
- 40. Green JD, Omary RA, Schirf BE, et al. Comparison of X-ray fluoroscopy and interventional magnetic resonance imaging for the assessment of coronary artery stenoses in swine. Magn Reson Med 2005; 54: 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang S, Rafie S, Chen Y, et al. In vivo cardiovascular catheterization under real-time MRI guidance. J Magn Reson Imaging 2006; 24: 914–917. [DOI] [PubMed] [Google Scholar]
- 42. Qiu B, Gao F, Karmarkar P, et al. Intracoronary MR imaging using a 0.014-inch MR imaging-guidewire: toward MRI-guided coronary interventions. J Magn Reson Imaging 2008; 28: 515–518. [DOI] [PubMed] [Google Scholar]
- 43. Spuentrup E, Ruebben A, Schaeffter T, et al. Magnetic resonance – guided coronary artery stent placement in a swine model. Circulation 2002; 105: 874–879. [DOI] [PubMed] [Google Scholar]
- 44. Serfaty JM, Yang X, Foo TK, et al. MRI-guided coronary catheterization and PTCA: a feasibility study on a dog model. Magn Reson Med 2003; 49: 258–263. [DOI] [PubMed] [Google Scholar]
- 45. Heidt T, Reiss S, Krafft AJ, et al. Real-time magnetic resonance imaging – guided coronary intervention in a porcine model. Sci Rep 2019; 9: 8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Behm P, Gastl M, Jahn A, et al. CMR-guidance of passively tracked endomyocardial biopsy in an in vivo porcine model. Int J Cardiovasc Imaging 2018; 34: 1917–1926. [DOI] [PubMed] [Google Scholar]
- 47. Rogers T, Ratnayaka K, Karmarkar P, et al. Real-time magnetic resonance imaging guidance improves the diagnostic yield of endomyocardial biopsy. JACC Basic Transl Sci 2016; 1: 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Unterberg-Buchwald C, Ritter CO, Reupke V, et al. Targeted endomyocardial biopsy guided by real-time cardiovascular magnetic resonance. J Cardiovasc Magn Res 2017; 19: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carlsson M, Saeed M. Intracoronary injection of contrast media maps the territory of the coronary artery: an MRI technique for assessing the effects of locally delivered angiogenic therapies. Acad Radiol 2008; 15: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 50. Krombach GA, Pfeffer JG, Kinzel S, et al. MR-guided percutaneous intramyocardial injection with an MR-compatible catheter: feasibility and changes in T1 values after injection of extracellular contrast medium in pigs. Radiology 2005; 235: 487–494. [DOI] [PubMed] [Google Scholar]
- 51. Tomkowiak MT, Klein AJ, Vigen KK, et al. Targeted transendocardial therapeutic delivery guided by MRI-x-ray image fusion. Catheter Cardio Inte 2011; 78: 468–478. [DOI] [PubMed] [Google Scholar]
- 52. van Es R, van den Broek HT, van der Naald M, et al. Validation of a novel stand-alone software tool for image guided cardiac catheter therapy. Int J Cardiovasc Imaging 2019; 35: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Halabi M, Faranesh AZ, Schenke WH, et al. Real-time cardiovascular magnetic resonance subxiphoid pericardial access and pericardiocentesis using off-the-shelf devices in swine. J Cardiovasc Magn Res 2013; 15: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barkhausen J, Kahn T, Krombach GA, et al. White paper: interventional MRI: current status and potential for development considering economic perspectives, part 1: general application. Rofo 2017; 189: 611–623. [DOI] [PubMed] [Google Scholar]
- 55. Nazarian S, Kolandaivelu A, Zviman MM, et al. Feasibility of real-time magnetic resonance imaging for catheter guidance in electrophysiology studies. Circulation 2008; 118: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pushparajah K, Chubb H, Razavi R. MR-guided cardiac interventions. Top Magn Reson Imaging 2018; 27: 115–128. [DOI] [PubMed] [Google Scholar]
- 57. Susil RC, Yeung CJ, Halperin HR, et al. Multifunctional interventional devices for MRI: a combined electrophysiology/MRI catheter. Magn Reson Med 2002; 47: 594–600. [DOI] [PubMed] [Google Scholar]
- 58. Velasco Forte MN, Pushparajah K, Schaeffter T, et al. Improved passive catheter tracking with positive contrast for CMR-guided cardiac catheterization using partial saturation (pSAT). J Cardiovasc Magn Res 2017; 19: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miquel ME, Hegde S, Muthurangu V, et al. Visualization and tracking of an inflatable balloon catheter using SSFP in a flow phantom and in the heart and great vessels of patients. Magn Reson Med 2004; 51: 988–995. [DOI] [PubMed] [Google Scholar]
- 60. Ratnayaka K, Faranesh AZ, Hansen MS, et al. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J 2013; 34: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rogers T, Lederman RJ. Interventional CMR: clinical applications and future directions. Curr Cardiol Rep 2015; 17: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dumoulin CL, Mallozzi RP, Darrow RD, et al. Phase-field dithering for active catheter tracking. Magn Reson Med 2010; 63: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 63. Ertürk MA, Sathyanarayana Hegde S, Bottomley PA. Radiofrequency ablation, MR thermometry, and high-spatial-resolution MR parametric imaging with a single, minimally invasive device. Radiology 2016; 281: 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hilbert S, Sommer P, Gutberlet M, et al. Real-time magnetic resonance-guided ablation of typical right atrial flutter using a combination of active catheter tracking and passive catheter visualization in man: initial results from a consecutive patient series. Europace 2016; 18: 572–577. [DOI] [PubMed] [Google Scholar]
- 65. Wang P, Unal O. Motion-compensated real-time MR thermometry augmented by tracking coils. J Magn Reson Imaging 2015; 41:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jacquier A, Higgins CB, Saeed M. MR imaging in assessing cardiovascular interventions and myocardial injury. Contrast Media Mol Imaging 2007; 2:1–15. [DOI] [PubMed] [Google Scholar]
- 67. Sonmez M, Saikus CE, Bell JA, et al. MRI active guidewire with an embedded temperature probe and providing a distinct tip signal to enhance clinical safety. J Cardiovasc Magn Res 2012; 14: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Basar B, Rogers T, Ratnayaka K, et al. Segmented nitinol guidewires with stiffness-matched connectors for cardiovascular magnetic resonance catheterization: preserved mechanical performance and freedom from heating. J Cardiovasc Magn Res 2015; 17: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yildirim KD, Basar B, Campbell-Washburn AE, et al. A cardiovascular magnetic resonance (CMR) safe metal braided catheter design for interventional CMR at 1.5 T: freedom from radiofrequency induced heating and preserved mechanical performance. J Cardiovasc Magn Res 2019; 21: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bell JA, Saikus CE, Ratnayaka K, et al. A deflectable guiding catheter for real-time MRI-guided interventions. J Magn Reson Imaging 2012; 35: 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Campbell-Washburn AE, Rogers T, Basar B, et al. Positive contrast spiral imaging for visualization of commercial nitinol guidewires with reduced heating. J Cardiovasc Magn Res 2015; 17: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tse ZT, Dumoulin CL, Clifford GD, et al. A 1.5T MRI-conditional 12-lead electrocardiogram for MRI and intra-MR intervention. Magn Reson Med 2014; 71: 1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bhagirath P, van der Graaf M, Karim R, et al. Interventional cardiac magnetic resonance imaging in electrophysiology: advances toward clinical translation. Circ Arrhythm Electrophysiol 2015; 8: 203–211. [DOI] [PubMed] [Google Scholar]
- 74. Bisbal F, Fernández-Armenta J, Berruezo A, et al. Use of MRI to guide electrophysiology procedures. Heart 2014; 100: 1975–1984. [DOI] [PubMed] [Google Scholar]
- 75. Chubb H, Harrison JL, Weiss S, et al. Development, preclinical validation, and clinical translation of a cardiac magnetic resonance – electrophysiology system with active catheter tracking for ablation of cardiac arrhythmia. JACC Clin Electrophysiol 2017; 3: 89–103. [DOI] [PubMed] [Google Scholar]
- 76. Dickfeld T, Tian J, Ahmad G, et al. MRI-guided ventricular tachycardia ablation: integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol 2011; 4: 172–184. [DOI] [PubMed] [Google Scholar]
- 77. Eitel C, Hindricks G, Grothoff M, et al. Catheter ablation guided by real-time MRI. Curr Cardiol Rep 2014; 16: 511. [DOI] [PubMed] [Google Scholar]
- 78. Eitel C, Piorkowski C, Hindricks G, et al. Electrophysiology study guided by real-time magnetic resonance imaging. Eur Heart J 2012; 33: 1975. [DOI] [PubMed] [Google Scholar]
- 79. Godeschalk-Slagboom CJ, van der Geest RJ, Zeppenfeld K, et al. Cardiac MRI visualization for ventricular tachycardia ablation. Int J Comput Assist Radiol Surg 2012; 7: 753–767. [DOI] [PubMed] [Google Scholar]
- 80. Guttman MA, Tao S, Fink S, et al. Acute enhancement of necrotic radio-frequency ablation lesions in left atrium and pulmonary vein ostia in swine model with non-contrast-enhanced T(1) -weighted MRI. Magn Reson Med 2020; 83: 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hoffmann BA, Koops A, Rostock T, et al. Interactive real-time mapping and catheter ablation of the cavotricuspid isthmus guided by magnetic resonance imaging in a porcine model. Eur Heart J 2010; 31: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ipek EG, Nazarian S. Cardiac magnetic resonance for prediction of arrhythmogenic areas. Trends Cardiovasc Med 2015; 25: 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kholmovski EG, Coulombe N, Silvernagel J, et al. Real-time MRI-guided cardiac cryo-ablation: a feasibility study. J Cardiovasc Electrophysiol 2016; 27: 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kolandaivelu A, Lardo AC, Halperin HR. Cardiovascular magnetic resonance guided electrophysiology studies. J Cardiovasc Magn Res 2009; 11: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kolandaivelu A, Zviman MM, Castro V, et al. Noninvasive assessment of tissue heating during cardiac radiofrequency ablation using MRI thermography. Circ Arrhythm Electrophysiol 2010; 3: 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lichter J, Kholmovski EG, Coulombe N, et al. Real-time magnetic resonance imaging-guided cryoablation of the pulmonary veins with acute freeze-zone and chronic lesion assessment. Europace 2019; 21: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Malchano ZJ, Neuzil P, Cury RC, et al. Integration of cardiac CT/MR imaging with three-dimensional electroanatomical mapping to guide catheter manipulation in the left atrium: implications for catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2006; 17: 1221–1229. [DOI] [PubMed] [Google Scholar]
- 88. Nordbeck P, Beer M, Köstler H, et al. Cardiac catheter ablation under real-time magnetic resonance guidance. Eur Heart J 2012; 33: 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nordbeck P, Hiller KH, Fidler F, et al. Feasibility of contrast-enhanced and nonenhanced MRI for intraprocedural and postprocedural lesion visualization in interventional electrophysiology: animal studies and early delineation of isthmus ablation lesions in patients with typical atrial flutter. Circ Cardiovasc Imaging 2011; 4: 282–294. [DOI] [PubMed] [Google Scholar]
- 90. Nordbeck P, Quick HH, Ladd ME, et al. Real-time magnetic resonance guidance of interventional electrophysiology procedures with passive catheter visualization and tracking. Heart Rhythm 2013; 10: 938–939. [DOI] [PubMed] [Google Scholar]
- 91. Oduneye SO, Pop M, Shurrab M, et al. Distribution of abnormal potentials in chronic myocardial infarction using a real time magnetic resonance guided electrophysiology system. J Cardiovasc Magn Res 2015; 17: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Okada DR, Wu KC. Applications of cardiac MR imaging in electrophysiology: current status and future needs. Magn Reson Imaging Clin N Am 2019; 27: 465–473. [DOI] [PubMed] [Google Scholar]
- 93. Paetsch I, Jahnke C, Hilbert S, et al. Cardiovascular magnetic resonance-guided electrophysiological interventions: radiofrequency ablation of typical atrial flutter. Circ Cardiovasc Imaging 2017; 10: e005780. [DOI] [PubMed] [Google Scholar]
- 94. Paetsch I, Sommer P, Jahnke C, et al. Clinical workflow and applicability of electrophysiological cardiovascular magnetic resonance-guided radiofrequency ablation of isthmus-dependent atrial flutter. Euro Heart J Cardiovas Imag 2019; 20: 147–156. [DOI] [PubMed] [Google Scholar]
- 95. Piorkowski C, Grothoff M, Gaspar T, et al. Cavotricuspid isthmus ablation guided by real-time magnetic resonance imaging. Circ Arrhy Electrophy 2013; 6: e7–e10. [DOI] [PubMed] [Google Scholar]
- 96. Ranjan R, Kholmovski EG, Blauer J, et al. Identification and acute targeting of gaps in atrial ablation lesion sets using a real-time magnetic resonance imaging system. Circ Arrhythm Electrophysiol 2012; 5: 1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reiter T, Gensler D, Ritter O, et al. Direct cooling of the catheter tip increases safety for CMR-guided electrophysiological procedures. J Cardiovasc Magn Res 2012; 14: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saremi F, Tafti M. The role of computed tomography and magnetic resonance imaging in ablation procedures for treatment of atrial fibrillation. Semin Ultrasound CT MR 2009; 30: 125–156. [DOI] [PubMed] [Google Scholar]
- 99. Sommer P, Grothoff M, Eitel C, et al. Feasibility of real-time magnetic resonance imaging-guided electrophysiology studies in humans. Europace 2013; 15: 101–108. [DOI] [PubMed] [Google Scholar]
- 100. Raval AN, Karmarkar PV, Guttman MA, et al. Real-time MRI guided atrial septal puncture and balloon septostomy in swine. Catheter Cardio Inte 2006; 67: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Deutsch N, Swink J, Matisoff AJ, et al. Anesthetic considerations for magnetic resonance imaging-guided right-heart catheterization in pediatric patients: a single institution experience. Paediatr Anaesth 2019; 29: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Razavi R, Hill DL, Keevil SF, et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet (London, England) 2003; 362: 1877–1882. [DOI] [PubMed] [Google Scholar]
- 103. Saikus CE, Lederman RJ. Interventional cardiovascular magnetic resonance imaging: a new opportunity for image-guided interventions. JACC Cardiovasc Imaging 2009; 2: 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kuehne T, Yilmaz S, Schulze-Neick I, et al. Magnetic resonance imaging guided catheterisation for assessment of pulmonary vascular resistance: in vivo validation and clinical application in patients with pulmonary hypertension. Heart 2005; 91: 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Muthurangu V, Atkinson D, Sermesant M, et al. Measurement of total pulmonary arterial compliance using invasive pressure monitoring and MR flow quantification during MR-guided cardiac catheterization. Am J Physiol Heart Circ Physiol 2005; 289: H1301–H1306. [DOI] [PubMed] [Google Scholar]
- 106. Bietenbeck M, Florian A, Chatzantonis G, et al. Introduction of a CMR-conditional cardiac phantom simulating cardiac anatomy and function and enabling training of interventional CMR procedures. Sci Rep 2019; 9: 19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kuehne T, Yilmaz S, Steendijk P, et al. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: in vivo validation and clinical application in patients with pulmonary hypertension. Circulation 2004; 110: 2010–2016. [DOI] [PubMed] [Google Scholar]
- 108. Rogers T, Ratnayaka K, Khan JM, et al. CMR fluoroscopy right heart catheterization for cardiac output and pulmonary vascular resistance: results in 102 patients. J Cardiovasc Magn Res 2017; 19: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Barbash IM, Saikus CE, Faranesh AZ, et al. Direct percutaneous left ventricular access and port closure: pre-clinical feasibility. JACC Cardiovasc Interv 2011; 4: 1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Halabi M, Ratnayaka K, Faranesh AZ, et al. Transthoracic delivery of large devices into the left ventricle through the right ventricle and interventricular septum: preclinical feasibility. J Cardiovasc Magn Res 2013; 15: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kuehne T, Yilmaz S, Meinus C, et al. Magnetic resonance imaging-guided transcatheter implantation of a prosthetic valve in aortic valve position: feasibility study in swine. J Am Coll Cardiol 2004; 44: 2247–2249. [DOI] [PubMed] [Google Scholar]
- 112. Kahlert P, Eggebrecht H, Plicht B, et al. Towards real-time cardiovascular magnetic resonance-guided transarterial aortic valve implantation: in vitro evaluation and modification of existing devices. J Cardiovasc Magn Res 2010; 12: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kahlert P, Parohl N, Albert J, et al. Towards real-time cardiovascular magnetic resonance guided transarterial CoreValve implantation: in vivo evaluation in swine. J Cardiovasc Magn Res 2012; 14: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hatt CR, Jain AK, Parthasarathy V, et al. MRI-3D ultrasound-X-ray image fusion with electromagnetic tracking for transendocardial therapeutic injections: in-vitro validation and in-vivo feasibility. Comput Med Imaging Graph 2013; 37: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 2019; 293: 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]