Abstract
Endoplasmic reticulum (ER)-associated degradation (ERAD) is a mechanism against ER stress, wherein unfolded/misfolded proteins accumulated in the ER are transported to the cytosol for degradation by the ubiquitin-proteasome system. The ER resident E3 ubiquitin ligase HRD1 has been identified as a key ERAD factor that directly catalyzes ubiquitin conjugation onto the unfolded or misfolded proteins for proteasomal degradation. The abnormally increased HRD1 expression was discovered in rheumatoid synovial cells, providing the first evidence for HRD1 dysregulation involved in human inflammatory pathogenesis. Further studies shown that inflammatory cytokines involved in rheumatoid pathogenesis including IL-1β TNF-α, IL-17 and IL-26 induce HRD1 expression. Recent studies using mice with tissue-specific targeted deletion of HRD1 gene have revealed important functions of HRD1 in immune regulation and inflammatory diseases. HRD1 has been shown critical for dendritic cell expression of antigens to both CD4 and CD8 T cells. Both TCR and costimulatory receptor CD28 signaling induces HRD1 expression, which promotes T cell clonal expansion and IL-2 production. Together with the fact that HRD1 is required for maintaining the stability of regulatory T cell (Treg) stability, HRD1 appears to fine tone T cell immunity. In addition, HRD1 is involved in humoral immune response by regulating early B cell development and maintaining B cell survival upon recognition of specific antigen. HRD1 appears to target its substrates for ubiquitination through, either ERAD-dependent or -independent, at least two distinct molecular mechanisms in a cell or tissue specific manner to achieve its physiological functions. Dysregulation of HRD1 expression and/or it functions are involved in autoimmune inflammatory diseases in particular rheumatoid arthritis and lupus. Here, we review current findings on the mechanism of HRD1 protein in immune regulation and the involvement of HRD1 in the pathogenesis of autoimmune inflammatory diseases.
Keywords: HRD1, ubiquitination, ER stress, signaling transduction, autoimmunity
Folding and maturation of proteins in eukaryotic cells occur in the endoplasmic reticulum (ER). Proteins that fail to acquire their proper structure are retained within the ER and eventually degraded to prevent toxicity by the accumulation of misfolded proteins in the secretory pathway. This turnover is mediated by a mechanism known as ER-associated degradation (ERAD). ERAD is one of the quality control systems in the cell to maintain ER homeostasis and adjust ER capacity in response to environmental cues (1). ERAD requires three steps, substrate transportation from the ER to the cytoplasm (dislocation), ubiquitination by specific ubiquitin ligases and proteolysis by proteasome in cytoplasm (2). The ER chaperone, BiP, plays a critical role to recognize the misfolded proteins (3). The ER-resident nucleotide exchange factor Grp170 triggers nucleotide exchange of BiP (binding immunoglobulin protein) to generate ATP-BiP. ATP-BiP disengages from the misfolded substrate, enabling it to retrotranslocate to the cytosol for their degradation through ubiquitin-dependent pathways (3–5). Impaired ERAD functions lead to accumulation of aberrant proteins within the ER and triggers the unfolded protein response (UPR) or ER stress. The UPR pathways are mediated by three major ER transmembrane protei factors, including protein kinase R-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme-1α (IRE1α), and activating transcription factor 6 (ATF6) (6–8). IRE1α functions as a protein kinase and endoribonuclease. IRE1α executes an unconventional splicing of the mRNA encoding XBP-1 by removing a 26-base intron (9, 10). Spliced XBP-1 mRNA encodes a potent transcription factor X–box protein 1 (Xbp-1s) that activates the expression of several genes involved in protein folding, secretion and degradation(11).
HRD1 is an ERAD ubiquitin ligase.
Several ER-resident E3 ubiquitin ligases have been identified so far (2), with hydroxymethylglutaryl reductase degradation protein 1 (HRD1) as the best characterized in regulating ERAD. HRD1 has a six-transmembrane domain and a RING-H2 motif (Fig. 1), it forms a complex with an ER-resident single-transmembrane protein Hrd3 in yeast or a suppressor/enhancer of Lin-12-like (SEL1L) in mammals, for the degradation of a subset of misfolded proteins in the ER (12, 13) (Fig. 1B). In yeast, Hrd3 is stable protein required for the E3 activity of HRD1 (14). Unlike Hrd3, human SEL1L is an unstable protein, which is restored by the association with HRD1 to trigger its E3 ligase activity (15). In addition, HRD1 interacts with Derlin family proteins to form an ERAD complex with SEL1L (16). The scaffold protein Herp is required for forming an active retrotranslocation complex containing HRD1, SEL1L, and Derlin 2 for ERAD (17). Similar to most of the E3 ubiquitin ligases, HRD1 can catalyze autoubiquitination, and this autoubiquitination of HRD1 plays an essential role in retrotranslocating the misfolded protein from ER lumen to cytoplasm (18, 19).
Figure 1. HRD1 structure and its substrates.
HRD1 protein has six N-terminal transmembrane domains (TMDs), a RING finger domain, and C-terminal proline-rich (PR) cytoplasmic tail. The HRD1 binding proteins, functional consequences of their interactions and possible disease associations are highlighted.
HRD1 C-terminus is involved in recruitment of signaling molecules.
Accumulated evidences have discovered that the C-terminus cytoplasmic tail of HRD1 is critical for its physiological functions by recruiting a variety of signaling molecules or transcription factors for ubiquitination-mediated degradation. HRD1 has been shown to interact with the tumor suppressor p53 through its linker region between the sixth transmembrane domain and RING finger, and consequently catalyzes p53 ubiquitination (20). Recent studies have demonstrated that HRD1 is a critical regulator in metabolic regulation and energy expenditure. HRD1 destructs the thermogenic coactivator peroxisome proliferator-activated receptor coactivator (PGC)-1β through ubiquitination-mediated degradation. As a consequence, HRD1 deletion resulted in upregulation of PGC-1β target genes and increased mitochondrion number, respiration, and basal energy expenditure in adipose tissue (21). Our laboratory has revealed that mice with HRD1 deletion specifically in the liver display increased energy expenditure and are resistant to high fat diet (HFD)-induced obesity, liver steatosis and insulin resistance due to the elevated activation of pathways for glycolysis and fatty acid oxidation (22). Unexpectedly, liver specific HRD1 gene ablation results in the fragmentation of biological circadian rhythm, which is controlled by hippocampus, indicating that hepatic HRD1 controls the biological clock through a circulating factor. Indeed, HRD1 directly targets CREBH for ubiquitination-mediated degradation to regulate the hepatokine FGF21 production to maintain the circadian rhythm (23). Through analysis of the pro-opiomelanocortin (POMC) neuron-specific SEL1 deficient mice, Kim et al discovered that the SEL1/HRD1 complex is required for POMC maturation in preventing mice from age-associated obesity (24). In addition, HRD1 ERAD is required for vasopressin prohormone processing and systemic water homeostasis (25, 26), these studies reveal critical pathophysiological HRD1 functions in tissue and organ-specific manners (Fig. 1). More recently, gene ontology analyses identified a variety of potential HRD1 substrates, many of which function in pathways involved in stress adaptation or immune surveillance (27, 28).
Regulation of HRD1 expression.
As a critical E3 ubiquitin ligase involved in endoplasmic reticulum-associated degradation, it is not surprising that HRD1 is induced by ER stress response. The ER stress downstream transcription factor Xbp-1 spliced form can directly bind to the promoter region of HRD1 and promotes HRD1 mRNA transcription (29, 30). Interestingly, we have discovered that HRD1 controls the protein expression of IRE1α through ubiquitination-mediated degradation to suppress synovial cell apoptosis during rheumatoid arthritis pathogenesis, as well as to maintain intestinal homeostasis (31, 32). Since IRE1α is essential for Xbp-1 mRNA splicing to activate Xbp-1 transcriptional activation, these studies identify a feedback loop in regulating HRD1 expression: on one hand, IRE1α activation induces Xbp-1 mRNA splicing to activate its transcriptional function for the expression of ER stress responsive genes including HRD1; on the other hand, HRD1 destructs IRE1α to suppress Xbp-1 transcriptional activity. In addition to Xbp-1, the MAPK kinase ERK1/2 pathway can be activated upon inflammatory cytokine stimulation, which induces the activation of ETS transcription factor for HRD1 gene transcription (33). At the post-transcription level, it has been shown that the targeted deletion of the adaptor protein SEL1 in the ERAD complex largely diminishes HRD1 expression possibly due to auto-ubiquitination-mediated degradation (32, 34). In addition, the ER resident ubiquitin specific peptidase USP19 has been identified as a HRD1 deubiquitinase that stabolizes HRD1 protein (35). Therefore, HRD1 expression is tightly controlled at both transcriptional and post-translational levels at different pathophysiological settings.
HRD1 IS A CRITICAL MULTITASKING IMMUNE REGULATOR.
A variety of studies have shown important roles of ER stress response in regulating both innate and adaptive immune response at both physiological and pathological settings (5, 36, 37). The involvement of the altered ERAD protein quality control and/or unfolded protein response contribute to autoimmunity has been elegantly reviewed by Barrera et al (38). This review focuses on one of the ERAD ubiquitin ligases HRD1 in immune regulation and autoimmunity.
HRD1 in antigen presentation.
Early evidence suggests that the ubiquitin pathway is involved in the presentation of viral antigens to MHC class I, because facilitation of viral protein proteolysis by fusion with ubiquitin results in the presentation of viral antigen to CD8 T cells (39). Further studies using cells with a temperature-sensitive defect in ubiquitin conjugation demonstrated that ubiquitin-mediated protein degradation is required for presentation of antigen to MHC I restricted CD8 T cells (40). Meanwhile, type I interferon induces a subclass of proteasomes that contain two MHC-encoded subunits, LMP2 and LMP7 to promote peptide antigen presentation by MHC I (41, 42). The first evidence to indicate that ERAD is involved in regulating the antigen presentation by MHC I is the observation of MHC I protein co-localization with artificial antigen in the ER (43). Huang et al showed for the first time that the ER-resident E3 ubiquitin ligase HRD1 functions as a positive regulator for the peptide antigen presentation by MHC I molecules (44). MHC class I heavy chains that fail to achieve their native conformation in complex with both β2-microglobulin and peptide are targeted for ER-associated degradation. In addition, HRD1 and its E2 ubiquitin conjugation enzyme UBE2J1 regulate the homeostatic regulation of MHC class I assembly and expression by directly promoting ubiquitin-conjugation onto the misfolded MHC I molecules (45, 46). Therefore, ERAD-mediated MHC I degradation plays an important role in the homeostatic regulation of MHC class I assembly and expression by dendritic cells (Fig. 2). In addition to HRD1, the E3 ubiquitin ligase TMEM129 has recently joined in the action of ERAD-mediated regulation MHC I expression through its recruitment onto ER by Derlin 1 (47).
Figure 2. Illustration of HRD1 functions in DCs and T cells.
HRD1 targets MHC class I proteins for degradation as a quality control mechanism for MHC class I cell surface expression. Meanwhile, dendritic HRD1 C-terminus interacts with the transcription suppresser Blimp1 for ubiquitination-mediated degradation, which consequently activates CIITA for MHC class II transcription. HRD1 also functions as an E3 ubiquitin ligase to promote p27kip1 destruction for T cell clonal expansion upon TCR and CD28 stimulation.
Surprisingly, analysis of the phenotypes of CD11c-restriced HRD1 deficient mice, Yang et al observed a selective reduction of MHC II expression by CD11c+ dendritic cells (DCs) (36). Interestingly, HRD1 directly recognizes Blimp1, a transcription co-suppresser that inhibits the MHC II transcription activator (CIITA) transcriptional activity (48, 49), as a substrate for ubiquitination and degradation (36). As a consequence, loss of HRD1 functions leads to the elevated Blimp1 expression to suppress MHC-II transcription and MHC-II mediated antigen presentation to CD4 T cells (36) (Fig. 2). Therefore, HRD1 appears to regulate antigen presentation through two distinct molecular mechanisms: First to regulate MHC-I assembly in the ER by destructing the misfolded β2-microglobulin through ERAD, and second to control MHC II gene transcription through catalyzing Blimp1 ubiquitination and degradation in an ERAD-independent manner (Fig. 2).
HRD1 is an E3 ligase that positively regulates T cell activation.
It has been reported that Xbp-1, a transcription factor of HRD1(30), is induced and activated by TCR and IL-2 stimulation in CD8 T cells. Inactivation of Xbp-1 resulted in impaired CD8 T cell immune response to viral infection (50). More recently, Kemp et al has observed that targeted deletion of IRE1α, an ER stress sensor that activates Xbp-1, impairs CD4 T cell differentiation toward Th2 (51). As Xbp-1 is a transcription factor in promoting HRD1 gene expression, these studies imply a possibility that HRD1 functions as a possible positive regulator of T cell immunity. Indeed, Xu et al recently discovered that genetic suppression of HRD1 impairs T cell activation and differentiation. HRD1 appears to positively regulate T cell clonal expansion by targeting p27Kip1, a cyclin-dependent kinase inhibitor for ubiquitination and degradation. Loss of HRD1 expression results in accumulated p27kip1 protein expression and G1/0 cell cycle arrest upon TCR/CD28 ligation (37). Therefore, HRD1 is a TCR/CD28-dependent E3 ubiquitin ligase of p27Kip1 in promoting the clonal expansion during early T cell activation (Fig. 2).
In addition to promoting T cell growth, HRD1 functions are essential for IL-2 production and CD4 T cell differentiation toward both Th1 and Th17 (37). However, HRD1 appears to regulate cytokine production by CD4 T cells independent of its activity in catalyzing p27Kip1 ubiquitination and degradation, because further deletion of p27Kip1 in HRD1-null T cells, while rescued T cell proliferation, failed to restore IL-2, IFN-γ and IL-17 production (37). It has also been shown that the transcriptional co-suppresser Blimp1 inhibits T cell production of cytokines including IL-2, IFN-γ and IL-17(52, 53). Given the fact that HRD1 targets Blimp1 for ubiquitination and degradation in DCs (36), it is speculated that HRD1 may regulate T cell cytokine production through Blimp1 ubiquitination and degradation.
HRD1 fine-tunes T cell immunity through maintaining Treg stability.
In the inflammatory environment, the suppressive regulatory T cells (Tregs) are often unstable and fail to maintain their suppressive functions (54). Since inflammatory cytokines induce the ER stress responses, in particular the activation of IRE1α pathway (55), we reasoned whether the ER stress response is involved in Treg instability. Indeed, treatment with a pharmacological ER stress inducer dramatically destabilized Treg cells while an ER stress inhibitor largely blocked inflammatory cytokine-induced Treg instability. The HRD1 ERAD pathway plays a critical role in FoxP3 expression, stability, and immune suppressive functions. Conditional genetic deletion of HRD1 specifically in Tregs results in a significant reduction in FoxP3 expression during polarization in HRD1fl/fl-FoxP3cre Treg with an increase in IRE1α, but not in PERK or ATF6, activity. More importantly, treatment with either the ER stress inhibitor or the IRE1α inhibitor could rescue HRD1-null Tregs from inflammatory cytokine-induced Treg destabilization. Therefore, HRD1 fine-tunes T cell immune homeostasis through promoting T cell activation and Treg stabilization (56).
HRD1 plays critical role at two distinct stages in B cell immunity.
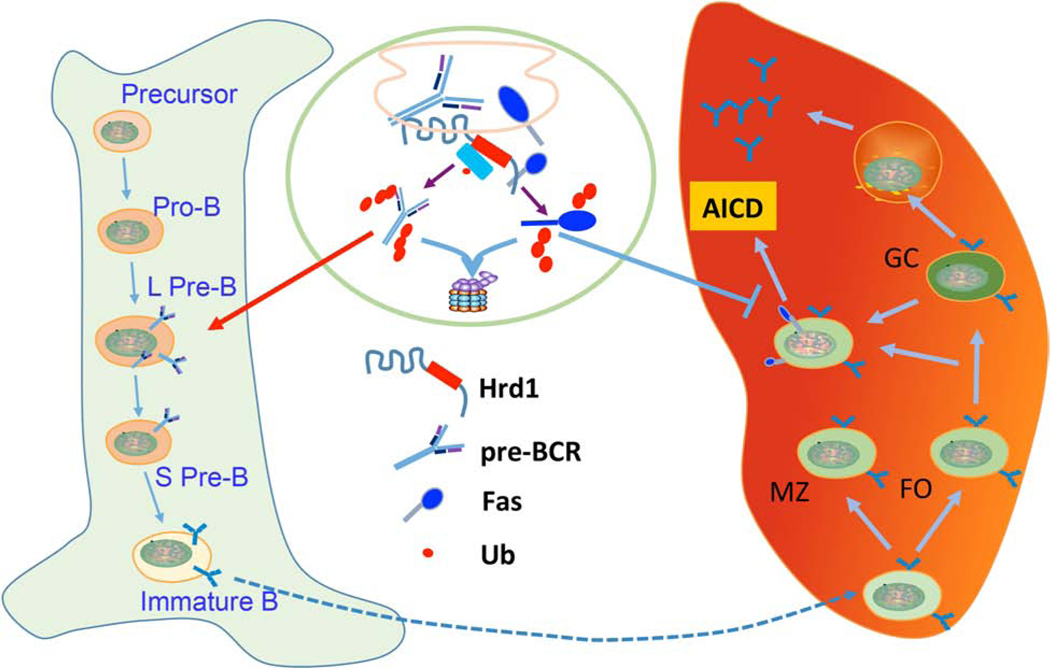
Activation of IRE1α-Xbp-1 pathway has been detected during B cell development and activation stages (57). Recent studies by Ji et al report that B cell-specific loss of SEL1L, an ER adaptor that forms a complex with HRD1 for ERAD (58), leads to a severe developmental block during transition from large to small pre-B cells. Mechanistically, SEL1L selectively recognizes and targets the pre-B cell receptor (pre-BCR) for proteasomal degradation. The pre-BCR complex accumulates both intracellularly and at the cell surface in SEL1L-deficient pre-B cells, leading to persistent pre-BCR signaling and pre-B cell proliferation. Our laboratory has observed a similar B cell developmental defect in B cell-specific HRD1 knockout mice (33). Therefore, the SEL1L-HRD1 complex recognizes pre-BCR complex as an ERAD substrate in regulation B cell development (Fig. 3).
Figure 3. HRD1 regulates B cell immunity at two distinct stages.
HRD1 ERAD plays a critical role in quality control of pre-BCR processing, specifically during the transition from large (L-) to small pre-B (S PreB) cell stage during B cell development in bone marrow. The immature B cells then home to the spleen and HRD1 protects B cells from the activation-induced apoptosis through targeting the death receptor Fas for ubiquitination and proteasomal degradation.
During the activation phase, HRD1 plays an important role in protecting B cells from activation-induced apoptosis because loss of HRD1 functions results in the increased B cell death upon either BCR or LPS stimulation (57). While HRD1 has been defined as an anti-apoptotic factor to suppress ER stress-induced cell death, HRD1 appears to inhibit activation induced B cell death through an ER stress-independent mechanism. HRD1 deletion does not cause increased ER stress response and further deletion of CHOP (59, 60), a downstream transcription factor in regulating ER stress-induced cell death, could not rescue HRD1-null B cells from the activation-induced apoptosis (57). Also, further IRE1α deletion could not rescue HRD1-null B cells from activation-induced B cell apoptosis (57, 61). Importantly, the expression of Fas, the death receptor that is required for the activation-induced B cell death, is upregulated at the protein but not mRNA levels in HRD1-null B cells, implying that HRD1 targets the death receptor Fas for degradation to protect B cells from activation induced B cell apoptosis. In fact, HRD1 interacts with Fas and catalyzes Fas ubiquitination and degradation. HRD1 recognizes pre-BCR complex and Fas through two distinct regions. Interaction with pre-BCR complex is mediated by the N-terminal region of HRD1, which binds to the misfolded proteins for ubiquitination and degradation. In contrast, the C-terminal proline-rich region of HRD1 is required for its interaction with Fas (57) (Fig. 1). It is likely that HRD1 regulates B cell development and activation through two distinct molecular mechanisms (Fig. 3).
HRD1 in regulating the innate immune response:
In addition to its important roles in antigen presentation, HRD1 was identified to function as a critical positive regulator that enhances TLR-induced inflammatory cytokine production in macrophages during bacterial infection. At the molecular level, HRD1 specifically targets the deubiquitinase USP15 to attenuate USP15-mediated iKBα stabilization and consequently leads to NF-kB activation, linking, for the first time, ERAD functions to microbial infection (62). In addition, HRD1 has been shown as an ubiquitin ligase for degradation of CD155, the ligand of CD226 in tumor microenvironment in hepatic cell carcinoma. Since, CD226 is a critical receptor for NK cell functions, HRD1-mediated suppression of CD155 expression impairs the NK cell immunity to liver cancer (63).
HRD1 IN AUTOIMMUNE INFLAMMATORY DISEASES.
Rheumatoid Arthritis:
Through an immunoscreening using antirheumatoid synovial cell antibody, Amano et al observed that HRD1 expression is significantly uprogelated in synovial fibroblasts from patients with rheumatoid arthritis (RA) and renamed HRD1 to Synoviolin. They further showed that mice overexpressing this HRD1 developed spontaneous arthropathy. Conversely, reduced expression of HRD1 protected mice from collagen-induced arthritis (64). Further studies by our laboratory demonstrated that the proinflammatory cytokines IL-1β and TNF-α are responsible for inducing HRD1 expression in mouse synovial fibroblasts (65, 66). In addition, both IL-17 and IL-26, two pathogenic cytokines in RA disease development and progression were found to induce HRD1 expression during RA development (65, 67). Therefore, cytokine-induced HRD1 expression is a critical pathogenic factor in RA through the anti-apoptotic functions of HRD1.
While the underlying molecular mechanisms remain largely unknown, it has been speculated that elevated HRD1 expression promotes RA development and progression through its anti-apoptotic activity of suppressing the death of synovial fibroblasts during synovitis (64). HRD1 achieves its anti-apoptotic functions through at least three distinct molecular mechanisms: First, our laboratory has discovered that HRD1 protects ER stress induced cell death through targeting the ER stress sensor IRE1α for ubiquitination and degradation (31, 61). Since IRE1α leads to caspase-12 cleavage and triggers cell apoptosis (68), HRD1 suppresses synovial cell apoptosis through targeting IRE1α for degradation. Second, HRD1 promotes cell cycle progression and survival by targeting tumor suppressor gene p53 for ubiquitination (20). Recently, our laboratory has shown that HRD1 protects the activation-induced B cell apoptosis through its E3 ligase activity in marking the death receptor Fas for ubiquitination-mediated degradation. Given that both HRD1 and Fas are ubiquitously expressed and involved in regulating the death of a variety types of cells, HRD1-mediated Fas degradation could be a common molecular mechanism in the death pathway unrestricted to B cells (57). In addition, HLA-B27 misfolded heavy chain dimers is involved inflammatory arthritis by inducing the unfolded protein response. Interestingly, HRD1 ERAD can recognize the misfolded HLA-B27 for degradation and consequently suppresses HLA-B27 induced inflammation (69, 70).
A partial suppression of HRD1 protects mice from collagen-induced arthritis, implying that HRD1 is a potential therapeutic target for RA. Interestingly, a HRD1 specific inhibitor, LS-102, which inhibits the E3 ligase catalytic activities of HRD1 has been identified by a high throughput screening (71). Of note, LS-102 suppresses the proliferation of rheumatoid synovial cells, and significantly reduces the severity of disease in a mouse model of RA (71). Therefore, even though further investigations are needed, it is an optimistic expectation for the use of HRD1 inhibitors to treat human RA as well as other types of autoimmune inflammatory diseases.
Similar to HRD1, the E3 ubiquitin ligase Ro52/Trim21 is targeted as an autoantigen in systemic lupus erythematosus, rheumatoid arthritis and Sjögren’s syndrome. Polymorphisms in the Ro52 gene have been linked to these autoimmune conditions (72–76). Of note, TRIM21 has been indented as binding partner of Fc fragment and it functionally interacts with IgG1 and regulates its quality control via the ERAD system (77–79). It will be interesting to further elucidate the crosstalk between TRIM21 and HRD1 in autoimmune pathogenesis.
Other types of autoimmune inflammatory diseases:
A possible link between HRD1 and human multiple sclerosis is seen in the increase of HRD1 expression in T cells from patients with multiple sclerosis (MS) (37). This elevated HRD1 expression plays critical roles in human T cell activation and is possibly involved in immune tolerance. In mice, targeted deletion of HRD1 specifically in T cells largely protects mice from myelin-oligodendrocyte-glycoprotein-induced experimental autoimmune encephalomyelitis, a mouse autoimmune central nervous system disease that resembles human MS. In addition, the reduced expression or loss-of-function mutations in the Fas protein or its ligand lead to lupus-like systemic autoimmune diseases in mice and humans (80–83). Fas-mediated cell death has been known as a critical mechanism to eliminate unwanted autoreactive B cells. The fact that HRD1 targets Fas to protect B cells from activation induced apoptosis, provides a possible link between HRD1 and lupus pathogenesis. Important work is waiting to be done in elucidating HRD1-mediated Fas ubiquitination and degradation in lupus and other B cell-mediated autoimmune diseases.
PERSPECTIVES AND FUTURE DIRECTIONS.
How isHRD1-mediated ubiquitination regulated?
Much progress has been made during the last few decades to understand how HRD1-mediated ubiquitination and degradation of misfolded proteins after translocation from ER lumen to cytoplasmic compartment during ERAD occurs. Interestingly, several recent studies have revealed that HRD1-mediated ubiquitination of certain proteins, including p53 (20), Nrf2 (84), Blimp1 (36), p27Kip1(37) and Fas (57), uncouples with extensive UPR response, implying an ERAD-independent molecular mechanism in protein degradation. The N-terminal ER lumen domain is often required for the recognition of the misfolded protein substrates during ERAD. In contrast, HRD1 recognizes p53, Nrf2, Blimp1, p27Kip1 and Fas through its C-terminal cytoplasmic region (Fig. 1A). In this case, the specific extracellular stimuli rather than pharmacological ER stress inducers such as tunicamycin can induce HRD1 interaction with its targeting proteins. For example, TCR and CD28 stimulation enhances HRD1 interaction with p27Kip1 in T cells, and LPS stimulation triggers Blimp1 interaction with HRD1 in DCs in regulating MHC-II transcription (36, 37). It remains a molecular puzzle underlying how extracellular signaling regulates HRD1 interaction with its non-ERAD substrates to achieve its physiological functions.
How does HRD1 target nuclear transcription factors for ubiquitination?
One of the interesting recent discoveries is that the ER spanning E3 ubiquitin ligase targets several transcription factors for ubiquitination-mediated degradation. p53 was the first identified transcription factor substrate of HRD1, which predominantly co-localizes with HRD1 in the perinuclear regions of cells, but not in their nuclei, suggesting a cytoplasmic recognition of p53 by HRD1. The subcellular interactions of HRD1 with other nuclear factors including Nrf1, Nrf2, Blimp1 and PGC-1β has not been studied further. Considering the fact that HRD1 is an ER transmembrane protein (Fig. 1), it is unlikely that HRD1 interacts with these transcription factors in the nucleus. However, bioinformatics analysis of the HRD1 amino acid sequence reveals two putative cleavage sites for both Site-1 protease (S1P) and Site-2 protease (S2P) (Fig. 1), two ER resident proteases that cleave ATF6 in regulating ER stress response. Importantly, both sites are located in the region between the RING finger and the 6th transmembrane domain of HRD1, where cleavage results in the production of a protein with an E3 ligase catalytic RING finger and a long C-terminal proline-rich cytoplasmic tail. Therefore, it is possible that this cleaved HRD1 may function as a nuclear E3 ligase for degradation of its nuclear substrates. More recently, ERAD machinery has been shown to control inner nuclear membrane protein quality, suggesting a possibility that HRD1 targets nuclear proteins at the inner nuclear membrane (85).
Highlights.
HRD1 is an ER-resident ubiquitin ligase known to regulate ERAD.
HRD1 regulates both T cell and B cell immunity
HRD1 is involved in antigen presentation process
The altered HRD1 expression is associated with autoimmune inflammatory diseases
ACKNOWLEDGEMENTS
We thank Fang lab members for critical reading of the manuscript and constructive suggestions during our research. This work was supported by National Institutes of Health (NIH) R01 grants (AI079056, AI108634, AR006634 and CA232347) to D.F.
Abbreviations:
- HRD1
Hydroxymethylglutaryl reductase degradation protein 1
- ER
Endoplasmic reticulum
- ERAD
ER-associated degradation
- IL-1β
Interleukin 1 beta
- TNF-α
Tumor necrosis factor alpha
- BiP
Binding immunoglobulin protein
- XBP-1
X-box protein 1
- RING
Really interesting new gene
- SEL1
Suppressor/enhancer of Lin-1-like
- HFD
High fat diet
- POMC
pro-opiomelanocortin
- IRE1α
Inositol-requiring enzyme 1 alpha
- PERK
Protein kinase R-like endoplasmic reticulum kinase
- ATF6
Activating transcription factor 6
- CREBH
cAMP-responsive element-binding protein H
- FGF21
Fibroblast growth factor 21
- RA
Rheumatoid arthritis
- LMP2
Latent membrane protein 2
- MHC
Major histocompatibility complex
- CIITA
Class II transcription activator
- TCR
T cell receptor
- BCR
B cell receptor
- Skp2
S-phase kinase associated protein 2
- CD28
Cluster of differentiation 28
- DC
Dendritic cells
- Blimp1
Beta-interferon gene positive regulatory domain I-binding factor
- Tfh
T follicular help cells
- Treg
T regulatory cells
- FoxP3
folk head box protein 3
- TRAF2
(tumor necrosis factor receptor (TNFR)-associated factor-2)
- JNK
c-Jun n-terminal kinases
- MS
Multiple sclerosis
- IFN-γ
Interferon gamma
- Nrf2
Nuclear factor erythroid 2–related factor 2
- T2D
type 2 diabetes
Footnotes
Disclosure of Conflicts of Interest: The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feldman M, and van der Goot FG 2009. Novel ubiquitin-dependent quality control in the endoplasmic reticulum. Trends Cell Biol 19:357–363. [DOI] [PubMed] [Google Scholar]
- 2.Mehnert M, Sommer T, and Jarosch E. 2010. ERAD ubiquitin ligases: multifunctional tools for protein quality control and waste disposal in the endoplasmic reticulum. Bioessays 32:905–913. [DOI] [PubMed] [Google Scholar]
- 3.Kozutsumi Y, Segal M, Normington K, Gething MJ, and Sambrook J. 1988. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332:462–464. [DOI] [PubMed] [Google Scholar]
- 4.Behnke J, Mann MJ, Scruggs FL, Feige MJ, and Hendershot LM 2016. Members of the Hsp70 Family Recognize Distinct Types of Sequences to Execute ER Quality Control. Molecular cell 63:739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue T, and Tsai B. 2016. The Grp170 nucleotide exchange factor executes a key role during ERAD of cellular misfolded clients. Molecular biology of the cell 27:1650–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang K, and Kaufman RJ 2006. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66:S102–109. [DOI] [PubMed] [Google Scholar]
- 7.Lee AH, and Glimcher LH 2009. Intersection of the unfolded protein response and hepatic lipid metabolism. Cell Mol Life Sci 66:2835–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotamisligil GS 2010. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140:900–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, and Ron D. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96. [DOI] [PubMed] [Google Scholar]
- 10.Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, and Kaufman RJ 2001. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107:893–903. [DOI] [PubMed] [Google Scholar]
- 11.Sha H, He Y, Yang L, and Qi L. 2011. Stressed out about obesity: IRE1alpha-XBP1 in metabolic disorders. Trends Endocrinol Metab 22:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampton RY, Gardner RG, and Rine J. 1996. Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Molecular biology of the cell 7:2029–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordallo J, Plemper RK, Finger A, and Wolf DH 1998. Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Molecular biology of the cell 9:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vashistha N, Neal SE, Singh A, Carroll SM, and Hampton RY 2016. Direct and essential function for Hrd3 in ER-associated degradation. Proceedings of the National Academy of Sciences of the United States of America 113:5934–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Hoewyk D. 2016. Defects in endoplasmic reticulum-associated degradation (ERAD) increase selenate sensitivity in Arabidopsis. Plant Signal Behav:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuberth C, and Buchberger A. 2005. Membrane-bound Ubx2 recruits Cdc48 to ubiquitin ligases and their substrates to ensure efficient ER-associated protein degradation. Nat Cell Biol 7:999–1006. [DOI] [PubMed] [Google Scholar]
- 17.Huang CH, Chu YR, Ye Y, and Chen X. 2014. Role of HERP and a HERP-related protein in HRD1-dependent protein degradation at the endoplasmic reticulum. J Biol Chem 289:4444–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldridge RD, and Rapoport TA 2016. Autoubiquitination of the Hrd1 Ligase Triggers Protein Retrotranslocation in ERAD. Cell 166:394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson BG, Glaser ML, Rapoport TA, and Baldridge RD 2019. Cycles of autoubiquitination and deubiquitination regulate the ERAD ubiquitin ligase Hrd1. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamasaki S, Yagishita N, Sasaki T, Nakazawa M, Kato Y, Yamadera T, Bae E, Toriyama S, Ikeda R, Zhang L, Fujitani K, Yoo E, Tsuchimochi K, Ohta T, Araya N, Fujita H, Aratani S, Eguchi K, Komiya S, Maruyama I, Higashi N, Sato M, Senoo H, Ochi T, Yokoyama S, Amano T, Kim J, Gay S, Fukamizu A, Nishioka K, Tanaka K, and Nakajima T. 2007. Cytoplasmic destruction of p53 by the endoplasmic reticulum-resident ubiquitin ligase ‘Synoviolin’. EMBO J 26:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita H, Yagishita N, Aratani S, Saito-Fujita T, Morota S, Yamano Y, Hansson MJ, Inazu M, Kokuba H, Sudo K, Sato E, Kawahara K, Nakajima F, Hasegawa D, Higuchi I, Sato T, Araya N, Usui C, Nishioka K, Nakatani Y, Maruyama I, Usui M, Hara N, Uchino H, Elmer E, Nishioka K, and Nakajima T. 2015. The E3 ligase synoviolin controls body weight and mitochondrial biogenesis through negative regulation of PGC-1beta. EMBO J 34:1042–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J, Yuan Y, Chen L, Xu Y, Zhang Y, Wang Y, Yang Y, Peek CB, Diebold L, Yang Y, Gao B, Jin C, Melo-Cardenas J, Chandel NS, Zhang DD, Pan H, Zhang K, Wang J, He F, and Fang D. 2018. ER-associated ubiquitin ligase HRD1 programs liver metabolism by targeting multiple metabolic enzymes. Nat Commun 9:3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei J, Chen L, Li F, Yuan Y, Wang Y, Xia W, Zhang Y, Xu Y, Yang Z, Gao B, Jin C, Melo-Cardenas J, Green RM, Pan H, Wang J, He F, Zhang K, and Fang D. 2018. HRD1-ERAD controls production of the hepatokine FGF21 through CREBH polyubiquitination. EMBO J 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim GH, Shi G, Somlo DR, Haataja L, Song S, Long Q, Nillni EA, Low MJ, Arvan P, Myers MG Jr., and Qi L. 2018. Hypothalamic ER-associated degradation regulates POMC maturation, feeding, and age-associated obesity. J Clin Invest 128:1125–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bichet DG, and Lussier Y. 2017. Mice deficient for ERAD machinery component Sel1L develop central diabetes insipidus. J Clin Invest 127:3591–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi G, Somlo DRM, Kim GH, Prescianotto-Baschong C, Sun S, Beuret N, Long Q, Rutishauser J, Arvan P, Spiess M, and Qi L. 2017. ER-associated degradation is required for vasopressin prohormone processing and systemic water homeostasis. J Clin Invest 127:3897–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KA, Hammerle LP, Andrews PS, Stokes MP, Mustelin T, Silva JC, Black RA, and Doedens JR 2011. Ubiquitin ligase substrate identification through quantitative proteomics at both the protein and peptide levels. J Biol Chem 286:41530–41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye Y, Baek SH, Ye Y, and Zhang T. 2018. Proteomic characterization of endogenous substrates of mammalian ubiquitin ligase Hrd1. Cell Biosci 8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto K, Suzuki N, Wada T, Okada T, Yoshida H, Kaufman RJ, and Mori K. 2008. Human HRD1 promoter carries a functional unfolded protein response element to which XBP1 but not ATF6 directly binds. Journal of biochemistry 144:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang K, Wang S, Malhotra J, Hassler JR, Back SH, Wang G, Chang L, Xu W, Miao H, Leonardi R, Chen YE, Jackowski S, and Kaufman RJ 2011. The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J 30:1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao B, Lee SM, Chen A, Zhang J, Zhang DD, Kannan K, Ortmann RA, and Fang D. 2008. Synoviolin promotes IRE1 ubiquitination and degradation in synovial fibroblasts from mice with collagen-induced arthritis. EMBO Rep 9:480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun S, Shi G, Sha H, Ji Y, Han X, Shu X, Ma H, Inoue T, Gao B, Kim H, Bu P, Guber RD, Shen X, Lee AH, Iwawaki T, Paton AW, Paton JC, Fang D, Tsai B, Yates JR 3rd, Wu H, Kersten S, Long Q, Duhamel GE, Simpson KW, and Qi L. 2015. IRE1alpha is an endogenous substrate of endoplasmic-reticulum-associated degradation. Nat Cell Biol 17:1546–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Kong S, Zhang Y, Melo-Cardenas J, Gao B, Zhang Y, Zhang DD, Zhang B, Song J, Thorp E, Zhang K, Zhang J, and Fang D. 2018. The endoplasmic reticulum-resident E3 ubiquitin ligase Hrd1 controls a critical checkpoint in B cell development in mice. J Biol Chem 293:12934–12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun S, Shi G, Han X, Francisco AB, Ji Y, Mendonca N, Liu X, Locasale JW, Simpson KW, Duhamel GE, Kersten S, Yates JR 3rd, Long Q, and Qi L. 2014. Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival. Proc Natl Acad Sci U S A 111:E582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada K, Kato M, and Nakamura N. 2016. USP19-Mediated Deubiquitination Facilitates the Stabilization of HRD1 Ubiquitin Ligase. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H, Qiu Q, Gao B, Kong S, Lin Z, and Fang D. 2014. Hrd1-mediated BLIMP-1 ubiquitination promotes dendritic cell MHCII expression for CD4 T cell priming during inflammation. J Exp Med 211:2467–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Zhao F, Qiu Q, Chen K, Wei J, Kong Q, Gao B, Melo-Cardenas J, Zhang B, Zhang J, Song J, Zhang DD, Zhang J, Fan Y, Li H, and Fang D. 2016. The ER membrane-anchored ubiquitin ligase Hrd1 is a positive regulator of T-cell immunity. Nat Commun 7:12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrera MJ, Aguilera S, Castro I, Gonzalez S, Carvajal P, Molina C, Hermoso MA, and Gonzalez MJ 2018. Endoplasmic reticulum stress in autoimmune diseases: Can altered protein quality control and/or unfolded protein response contribute to autoimmunity? A critical review on Sjogren’s syndrome. Autoimmun Rev 17:796–808. [DOI] [PubMed] [Google Scholar]
- 39.Townsend A, Bastin J, Gould K, Brownlee G, Andrew M, Coupar B, Boyle D, Chan S, and Smith G. 1988. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. The Journal of experimental medicine 168:1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalek MT, Grant EP, Gramm C, Goldberg AL, and Rock KL 1993. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature 363:552–554. [DOI] [PubMed] [Google Scholar]
- 41.Gaczynska M, Rock KL, and Goldberg AL 1993. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature 365:264–267. [DOI] [PubMed] [Google Scholar]
- 42.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, and Goldberg AL 1994. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761–771. [DOI] [PubMed] [Google Scholar]
- 43.Raposo G, van Santen HM, Leijendekker R, Geuze HJ, and Ploegh HL 1995. Misfolded major histocompatibility complex class I molecules accumulate in an expanded ER-Golgi intermediate compartment. The Journal of cell biology 131:1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang L, Marvin JM, Tatsis N, and Eisenlohr LC 2011. Cutting Edge: Selective role of ubiquitin in MHC class I antigen presentation. Journal of immunology 186:1904–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burr ML, Cano F, Svobodova S, Boyle LH, Boname JM, and Lehner PJ 2011. HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation. Proc Natl Acad Sci U S A 108:2034–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burr ML, van den Boomen DJ, Bye H, Antrobus R, Wiertz EJ, and Lehner PJ 2013. MHC class I molecules are preferentially ubiquitinated on endoplasmic reticulum luminal residues during HRD1 ubiquitin E3 ligase-mediated dislocation. Proc Natl Acad Sci U S A 110:14290–14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Boomen DJ, Timms RT, Grice GL, Stagg HR, Skodt K, Dougan G, Nathan JA, and Lehner PJ 2014. TMEM129 is a Derlin-1 associated ERAD E3 ligase essential for virus-induced degradation of MHC-I. Proceedings of the National Academy of Sciences of the United States of America 111:11425–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piskurich JF, Lin KI, Lin Y, Wang Y, Ting JP, and Calame K. 2000. BLIMP-I mediates extinction of major histocompatibility class II transactivator expression in plasma cells. Nature immunology 1:526–532. [DOI] [PubMed] [Google Scholar]
- 49.Steimle V, Otten LA, Zufferey M, and Mach B. 1993. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell 75:135–146. [PubMed] [Google Scholar]
- 50.Kamimura D, and Bevan MJ 2008. Endoplasmic reticulum stress regulator XBP-1 contributes to effector CD8+ T cell differentiation during acute infection. Journal of immunology 181:5433–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kemp KL, Lin Z, Zhao F, Gao B, Song J, Zhang K, and Fang D. 2013. The serine-threonine kinase inositol-requiring enzyme 1alpha (IRE1alpha) promotes IL-4 production in T helper cells. J Biol Chem 288:33272–33282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, and Crotty S. 2011. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34:932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta S, Anthony A, and Pernis AB 2001. Stage-specific modulation of IFN-regulatory factor 4 function by Kruppel-type zinc finger proteins. J Immunol 166:6104–6111. [DOI] [PubMed] [Google Scholar]
- 54.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, and Rudensky AY 2010. Stability of the regulatory T cell lineage in vivo. Science 329:1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiu Q, Zheng Z, Chang L, Zhao YS, Tan C, Dandekar A, Zhang Z, Lin Z, Gui M, Li X, Zhang T, Kong Q, Li H, Chen S, Chen A, Kaufman RJ, Yang WL, Lin HK, Zhang D, Perlman H, Thorp E, Zhang K, and Fang D. 2013. Toll-like receptor-mediated IRE1alpha activation as a therapeutic target for inflammatory arthritis. EMBO J 32:2477–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Y, Melo-Cardenas J, Zhang Y, Gau I, Wei J, Montauti E, Zhang Y, Gao B, Jin H, Sun Z, Lee SM, and Fang D. 2019. The E3 ligase Hrd1 stabilizes Tregs by antagonizing inflammatory cytokine-induced ER stress response. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong S, Yang Y, Xu Y, Wang Y, Zhang Y, Melo-Cardenas J, Xu X, Gao B, Thorp EB, Zhang DD, Zhang B, Song J, Zhang K, Zhang J, Zhang J, Li H, and Fang D. 2016. Endoplasmic reticulum-resident E3 ubiquitin ligase Hrd1 controls B-cell immunity through degradation of the death receptor CD95/Fas. Proc Natl Acad Sci U S A 113:10394–10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji Y, Kim H, Yang L, Sha H, Roman CA, Long Q, and Qi L. 2016. The Sel1L-Hrd1 Endoplasmic Reticulum-Associated Degradation Complex Manages a Key Checkpoint in B Cell Development. Cell reports 16:2630–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang XZ, Lawson B, Brewer JW, Zinszner H, Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM, and Ron D. 1996. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Molecular and cellular biology 16:4273–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang XZ, and Ron D. 1996. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science 272:1347–1349. [DOI] [PubMed] [Google Scholar]
- 61.Sun S, Shi G, Sha H, Ji Y, Han X, Shu X, Ma H, Inoue T, Gao B, Kim H, Bu P, Guber RD, Shen X, Lee AH, Iwawaki T, Paton AW, Paton JC, Fang D, Tsai B, Yates Iii JR, Wu H, Kersten S, Long Q, Duhamel GE, Simpson KW, and Qi L. 2015. IRE1alpha is an endogenous substrate of endoplasmic-reticulum-associated degradation. Nature cell biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y, Qiu Y, Chen P, Chang H, Guo L, Zhang F, Ma L, Zhang C, Zheng X, Xiao J, Zhong R, Han L, Xu X, Zhang Y, Li D, Zhong G, Boyton R, Huang Y, He Y, Hu R, Wei B, and Wang H. 2019. ER-localized Hrd1 ubiquitinates and inactivates Usp15 to promote TLR4-induced inflammation during bacterial infection. Nat Microbiol. [DOI] [PubMed] [Google Scholar]
- 63.Gong J, Fang L, Liu R, Wang Y, Xing J, Chen Y, Zhuang R, Zhang Y, Zhang C, Yang A, Zhang X, Jin B, and Chen L. 2014. UPR decreases CD226 ligand CD155 expression and sensitivity to NK cell-mediated cytotoxicity in hepatoma cells. Eur J Immunol 44:3758–3767. [DOI] [PubMed] [Google Scholar]
- 64.Amano T, Yamasaki S, Yagishita N, Tsuchimochi K, Shin H, Kawahara K, Aratani S, Fujita H, Zhang L, Ikeda R, Fujii R, Miura N, Komiya S, Nishioka K, Maruyama I, Fukamizu A, and Nakajima T. 2003. Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes & development 17:2436–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corvaisier M, Delneste Y, Jeanvoine H, Preisser L, Blanchard S, Garo E, Hoppe E, Barre B, Audran M, Bouvard B, Saint-Andre JP, and Jeannin P. 2012. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol 10:e1001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao B, Calhoun K, and Fang D. 2006. The proinflammatory cytokines IL-1beta and TNF-alpha induce the expression of Synoviolin, an E3 ubiquitin ligase, in mouse synovial fibroblasts via the Erk1/2-ETS1 pathway. Arthritis Res Ther 8:R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toh ML, Gonzales G, Koenders MI, Tournadre A, Boyle D, Lubberts E, Zhou Y, Firestein GS, van den Berg WB, and Miossec P. 2010. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLoS One 5:e13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, and Ron D. 2000. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666. [DOI] [PubMed] [Google Scholar]
- 69.Colbert RA, Tran TM, and Layh-Schmitt G. 2014. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol 57:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guiliano DB, Fussell H, Lenart I, Tsao E, Nesbeth D, Fletcher AJ, Campbell EC, Yousaf N, Williams S, Santos S, Cameron A, Towers GJ, Kellam P, Hebert DN, Gould KG, Powis SJ, and Antoniou AN 2014. Endoplasmic reticulum degradation-enhancing alpha-mannosidase-like protein 1 targets misfolded HLA-B27 dimers for endoplasmic reticulum-associated degradation. Arthritis Rheumatol 66:2976–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yagishita N, Aratani S, Leach C, Amano T, Yamano Y, Nakatani K, Nishioka K, and Nakajima T. 2012. RING-finger type E3 ubiquitin ligase inhibitors as novel candidates for the treatment of rheumatoid arthritis. International journal of molecular medicine 30:1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hennig J, Bresell A, Sandberg M, Hennig KD, Wahren-Herlenius M, Persson B, and Sunnerhagen M. 2008. The fellowship of the RING: the RING-B-box linker region interacts with the RING in TRIM21/Ro52, contains a native autoantigenic epitope in Sjogren syndrome, and is an integral and conserved region in TRIM proteins. Journal of molecular biology 377:431–449. [DOI] [PubMed] [Google Scholar]
- 73.Espinosa A, Dardalhon V, Brauner S, Ambrosi A, Higgs R, Quintana FJ, Sjostrand M, Eloranta ML, Ni Gabhann J, Winqvist O, Sundelin B, Jefferies CA, Rozell B, Kuchroo VK, and Wahren-Herlenius M. 2009. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med 206:1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oke V, and Wahren-Herlenius M. 2012. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmun 39:77–82. [DOI] [PubMed] [Google Scholar]
- 75.Racanelli V, Prete M, Musaraj G, Dammacco F, and Perosa F. 2011. Autoantibodies to intracellular antigens: generation and pathogenetic role. Autoimmun Rev 10:503–508. [DOI] [PubMed] [Google Scholar]
- 76.Rhodes DA, and Isenberg DA 2017. TRIM21 and the Function of Antibodies inside Cells. Trends Immunol 38:916–926. [DOI] [PubMed] [Google Scholar]
- 77.James LC, Keeble AH, Khan Z, Rhodes DA, and Trowsdale J. 2007. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc Natl Acad Sci U S A 104:6200–6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keeble AH, Khan Z, Forster A, and James LC 2008. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci U S A 105:6045–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahata M, Bohgaki M, Tsukiyama T, Kondo T, Asaka M, and Hatakeyama S. 2008. Ro52 functionally interacts with IgG1 and regulates its quality control via the ERAD system. Mol Immunol 45:2045–2054. [DOI] [PubMed] [Google Scholar]
- 80.Wu J, Wilson J, He J, Xiang L, Schur PH, and Mountz JD 1996. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. The Journal of clinical investigation 98:1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, and Nagata S. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314–317. [DOI] [PubMed] [Google Scholar]
- 82.Vaishnaw AK, Toubi E, Ohsako S, Drappa J, Buys S, Estrada J, Sitarz A, Zemel L, Chu JL, and Elkon KB 1999. The spectrum of apoptotic defects and clinical manifestations, including systemic lupus erythematosus, in humans with CD95 (Fas/APO-1) mutations. Arthritis and rheumatism 42:1833–1842. [DOI] [PubMed] [Google Scholar]
- 83.Horiuchi T, Nishizaka H, Yasunaga S, Higuchi M, Tsukamoto H, Hayashi K, and Nagasawa K. 1999. Association of Fas/APO-1 gene polymorphism with systemic lupus erythematosus in Japanese. Rheumatology 38:516–520. [DOI] [PubMed] [Google Scholar]
- 84.Wu T, Zhao F, Gao B, Tan C, Yagishita N, Nakajima T, Wong PK, Chapman E, Fang D, and Zhang DD 2014. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev 28:708–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buchanan BW, Mehrtash AB, Broshar CL, Runnebohm AM, Snow BJ, Scanameo LN, Hochstrasser M, and Rubenstein EM 2019. Endoplasmic reticulum stress differentially inhibits endoplasmic reticulum and inner nuclear membrane protein quality control degradation pathways. J Biol Chem 294:19814–19830. [DOI] [PMC free article] [PubMed] [Google Scholar]