Abstract
Patients with certain ABO classifications are at increased risk of certain types of malignancies. In the present study, a meta-analysis was performed to explore the association between the ABO blood group and the risk of lung cancer from an evidence-based medical perspective. The PubMed, Embase, Web of Science, Medline, China National Knowledge Infrastructure, Google Scholar, Science Direct and Wanfang databases were searched for relevant papers. Review Manger 5.4 was used to analyze the association between the ABO blood group and the risk of lung cancer. Trial Sequential Analysis (TSA) was used to determine whether the sample size of the meta-analysis was sufficient. A total of 29 studies were included in this paper. The results of the case-controlled studies showed that the proportion of patients with blood type A in patients with lung cancer was significantly higher than that in healthy individuals [odds ratio (OR), 1.10; 95% confidence interval (CI), 1.02-1.19]. Based on the subgroup analysis, type A blood showed heterogeneity in ethnicity and source of control (social or hospital). Additionally, type O blood was determined to be a protective factor for lung cancer in Caucasians (OR, 0.92; 95% CI, 0.85-0.99). TSA results suggested that there were sufficient participants in the case-controlled studies. Overall, the results of the cohort studies showed that the risk of lung cancer and blood type were weakly associated, and that the difference was not statistically significant. The case-controlled studies suggested that blood type A was associated with a higher risk of lung cancer. In addition, the analysis confirmed that Caucasians with type O blood had a lower risk of lung cancer. However, prospective cohort studies have not been able to draw this conclusion. Different experimental designs may have had a notable influence on the results obtained.
Keywords: lung cancer, ABO blood classification, meta-analysis
Introduction
Lung cancer is one of the leading causes of cancer morbidity and mortality in the world. According to GLOBOCAN estimates for 2020, lung cancer is the most common type of cancer in men and the third most common type of cancer in women (1). Furthermore, lung cancer has the highest cancer-associated death rate in men and the second highest cancer-associated death rate in women (1). The World Health Organization predicts that by 2025, the number of individuals with lung cancer in China will reach 1 million (2). Thus, lung cancer is a considerable public health concern.
The development of lung cancer is affected by several factors, such as environmental and genetic factors (3). Environmental factors include smoking, drinking, infection and exposure to ionizing radiation, amongst others (4). As environmental factors play such a strong role in lung cancer, less attention is paid to genetic factors. The ABO blood types are a very stable genetic trait. Reports have linked it to cancer risk (5–7); however, the molecular mechanisms involved are less clear. Blood group antigens may influence systemic inflammatory responses associated with malignancy (8–11). In addition, blood group antigens are expressed in several tissues, including certain malignant cells. However, there are some differences between ABO antigens expressed on the surface of malignant cells and those on normal tissues (12,13). This may influence the behaviors of the tumor cells, thereby promoting or inhibiting the proliferation of tumor cells (14).
The association between gastric cancer and blood type A was first noted by Aird et al (5) in 1953. Since this, a study by Hems (6) reported a correlation between breast cancer and type A blood, and a study by Vioque and Walker (7) also reported that type A blood was associated with an increased risk of pancreatic cancer in 1991. There have been several reports on the association between lung cancer and blood type. However, consistent conclusions have not been drawn. Urun et al (15) showed that non-O blood types were associated with an increased risk of lung cancer. However, Peng et al (16) reported that the occurrence of lung cancer was independent of blood type. Association studies with small sample sizes lack statistical power and may result in contradictory results. Based on the aforementioned points, a meta-analysis was conducted on the association between the ABO blood classification types and the occurrence of lung cancer.
Materials and methods
Search strategy
A comprehensive search of PubMed (pubmed.ncbi.nlm.nih.gov/), Embase (embase.com/landing?status=grey), Web of Science (webofscience.com), Medline (https://www.nlm.nih.gov/medline/index.html), China National Knowledge Infrastructure (CNKI, http://www.cnki.net/), Google Scholar (scholar.google.com), Science Direct (https://www.sciencedirect.com) and Wanfang databases (https://www. wanfangdata.com.cn/) was performed for studies published before February 1, 2022. The following English search strategy was used: (‘lung carcinoma’ OR ‘lung cancer’) AND ‘ABO’. A manual search was performed by reviewing a list of references in the retrieved studies. The studies were included if they were in English or Chinese only.
Eligibility criteria
The literature inclusion criteria were: i) Clear pathological diagnosis and ABO blood group typing; ii) case-controlled study or a cohort study; iii) the source and the raw data for the cases and controls were present; and iv) data on ethnicity, geographical distribution and publication year of the study were available.
The exclusion criteria were: i) Review articles and meta-analyses; ii) irrelevant or repetitive literature; iii) studies without a control group; and iv) studies with no useful data.
Data extraction
Information was extracted from all eligible studies by two reviewers independently. The information was then cross-checked to ensure no required data were missing. The following variables were extracted from each study: The year of publication, the name of the first author, the country of origin, the source of the control group (social means that the control group used routine patients attending health checkups or healthy blood donors from the area. Hospital means that the control group used non-cancer patients or patients attending health checkups from the same hospital as the experimental group), the study design, and the number of cases and controls with different ABO blood group types. If there was a disagreement in the extraction of information, it was discussed and reviewed with a third author. All the data presented in the study were agreed upon.
Study quality assessment
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included articles. Articles with a NOS score of ≥6 were considered high quality (17). The evaluation of case-controlled studies included selection (4 points), comparability (2 points) and exposure (3 points). The evaluation content of the cohort study included selection (4 points), comparability (2 points) and outcome (3 points).
Trial sequential analysis (TSA)
TSA was performed using TSA v0.9.5.10 Beta software developed by The Clinical Trial Center in Copenhagen, Denmark (18). In a case-controlled study, the OR was set to be reduced to 20% with a probability of type I error of A=0.05 and b=0.2 to estimate the required information size (RIS). If the cumulative Z value exceeded the RIS threshold, the result was considered statistically significant and the sample size was sufficient. If it did not exceed the RIS, the sample size was considered insufficient, suggesting that additional data were needed to draw the conclusion.
Statistical analysis
Case-controlled studies and cohort studies used odds ratios (ORs) and relative risks (RRs) with 95% confidence intervals (CIs) to assess the association between different blood types and lung cancer risk, respectively. Heterogeneity was assessed using I2 statistics and a χ2 test. I2>50% or P<0.10 was considered statistically significant heterogeneity. In cases where significant heterogeneity was detected, the random-effects model was used. Otherwise, the fixed-effect model was used. In this paper, funnel plots were used to identify publication bias. Each article was sequentially removed for sensitivity analysis to determine the impact and stability of merging OR or RR from individual studies. In addition, subgroup analysis was conducted for publication year, ethnicity, study type and source of control. P<0.05 was considered to indicate a statistically significant difference. All analyses were performed using Software Review Manager 5.4 (RevMan 5.4; Cochrane).
Results
Study selection and characteristics
According to the search strategy, 372 articles were identified from the PubMed, Embase, Web of Science, Medline, CNKI, Google Scholar, Science Direct and Wanfang databases. A total of 6 articles were identified through citation searching. After removal of duplications, the search returned 232 records. Finally, after further screening using the aforementioned inclusion and exclusion criteria, 29 studies (15,16,19–45) were eligible for evaluation of ABO blood types and lung cancer risk (Fig. 1). There were 26 case-controlled studies involving 12,598 patients with lung cancer and 3,299,927 healthy controls. The characteristics of the included studies are shown in Table I. Of these, 22 experiments were based on individuals of Chinese descent and 4 were based on individuals of Caucasian descent. In terms of selection of the control group, 20 studies were from the general populace and 6 studies were from healthy individuals in hospitals. Blood types were recorded for both the case and control groups in all studies. There were 3 cohort studies with 363,805 participants, and ultimately, 2,198 patients with lung cancer. The characteristics of the included studies are shown in Table II.
Figure 1.
Flowchart showing the search strategy for studies reporting on the ABO blood group and lung cancer susceptibility.
Table I.
Main characteristics of case-control studies included in the present meta-analysis.
| Lung cancer group, n | Control group, n | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author/s | Publication year | Area | Source of controla |
|
|
|||||||
| A | B | AB | O | A | B | AB | O | (Refs.) | ||||
| Xu et al | 2006 | China | Social | 10 | 14 | 3 | 17 | 1952 | 1211 | 434 | 1822 | (19) |
| Oguz et al | 2013 | Turkey | Social | 97 | 30 | 20 | 74 | 7756 | 2819 | 1316 | 5423 | (20) |
| Li et al | 2014 | China | Hospital | 357 | 279 | 83 | 373 | 648 | 492 | 168 | 670 | (21) |
| Sun and Zheng | 2001 | China | Social | 76 | 24 | 29 | 53 | 92 | 66 | 31 | 115 | (22) |
| Yang et al | 2000 | China | Social | 47 | 56 | 45 | 41 | 984 | 1060 | 344 | 909 | (23) |
| Li et al | 1995 | China | Social | 35 | 49 | 23 | 44 | 5979 | 7184 | 2189 | 5899 | (24) |
| Wang and Liang | 2000 | China | Social | 30 | 24 | 9 | 55 | 238 | 281 | 79 | 265 | (25) |
| Gao et al | 1998 | China | Social | 128 | 114 | 42 | 98 | 312 | 252 | 96 | 340 | (26) |
| Xiao et al | 2021 | China | Hospital | 297 | 276 | 74 | 256 | 342 | 259 | 81 | 379 | (27) |
| Feng and Ying | 2013 | China | Social | 164 | 122 | 37 | 140 | 9274 | 7986 | 2717 | 10542 | (29) |
| Chen et al | 2004 | China | Social | 230 | 270 | 50 | 346 | 11958 | 13979 | 2634 | 14848 | (30) |
| Tang et al | 2001 | China | Social | 29 | 58 | 11 | 45 | 23 | 36 | 13 | 49 | (32) |
| McConnell et al | 1954 | UK | Social | 312 | 55 | 31 | 379 | 406 | 81 | 32 | 481 | (33) |
| Peng et al | 2014 | China | Hospital | 306 | 265 | 69 | 367 | 4101 | 3308 | 975 | 4819 | (16) |
| Zhao et al | 1993 | China | Social | 45 | 51 | 11 | 69 | 1664 | 1712 | 406 | 2714 | (34) |
| Rennie and Haber | 1961 | Australia | Social | 90 | 18 | 3 | 107 | 11520 | 2910 | 900 | 14670 | (35) |
| Jiang and Wang | 1989 | China | Social | 92 | 62 | 22 | 112 | 6262 | 4672 | 1463 | 6781 | (36) |
| Pan et al | 2006 | China | Social | 382 | 268 | 93 | 399 | 771 | 727 | 251 | 714 | (37) |
| Liu et al | 2017 | China | Hospital | 41 | 30 | 15 | 29 | 24 | 33 | 7 | 34 | (39) |
| Zhang | 1990 | China | Social | 139 | 81 | 8 | 113 | 6382 | 4491 | 1581 | 7207 | (40) |
| Jin et al | 2000 | China | Hospital | 43 | 45 | 19 | 51 | 331 | 402 | 123 | 403 | (41) |
| Urun et al | 2013 | Turkey | Social | 896 | 354 | 167 | 627 | 1276032 | 493769 | 229554 | 1023528 | (15) |
| Liu et al | 2006 | China | Social | 97 | 46 | 9 | 67 | 3576 | 1870 | 824 | 3820 | (43) |
| Guo | 2001 | China | Social | 99 | 43 | 13 | 66 | 9270 | 6060 | 2463 | 10055 | (42) |
| Cai et al | 2006 | China | Hospital | 187 | 152 | 41 | 228 | 998 | 1087 | 297 | 1312 | (44) |
| Wang | 1993 | China | Social | 178 | 163 | 26 | 119 | 1484 | 1922 | 650 | 1597 | (45) |
Social means that the control group used routine patients attending health checkups or healthy blood donors from the area. Hospital means that the control group used non-cancer patients or patients attending health checkups from the same hospital as the experimental group.
Table II.
Main characteristics of cohort studies included in this meta-analysis.
Study quality
The quality of the included literature was evaluated according to the NOS. Finally, 29 high-quality studies were included. The 26 case-controlled studies included were of high quality (Table III). The 3 cohort studies were all of high quality as well (Table IV).
Table III.
Newcastle-Ottawa Scale scores for case-control studies.
| Selection | Exposure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| First author, year | Adequacy of case definition | Representativeness of the cases | Selection of controls | Definition of controls | Comparability cases/controls | Ascertainment of exposure | Same method of ascertainment | Non-response rate | Total scores | (Refs.) |
| Li et al, 2014 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 | (21) |
| Urun et al, 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (15) |
| Liu et al, 2017 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 | (39) |
| Oguz et al, 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (20) |
| Rennie and Haber, 1961 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (35) |
| McConnell et al, 1954 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (33) |
| Xiao et al, 2021 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 | (27) |
| Peng et al, 2014 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | (16) |
| Cai et al, 2006 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | (44) |
| Xu et al, 2006 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (19) |
| Feng and Ying, 2013 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (29) |
| Liu et al, 2006 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (43) |
| Pan et al, 2006 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (37) |
| Guo, 2001 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (42) |
| Tang et al, 2001 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (32) |
| Sun and Zheng, 2001 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (22) |
| Chen et al, 2004 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (30) |
| Gao et al, 1998 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (26) |
| Yang et al, 2000 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (23) |
| Jin et al, 2000 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 6 | (41) |
| Wang and Liang, 2000 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (25) |
| Jiang and Wang, 1989 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (36) |
| Zhang, 1990 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (40) |
| Zhao et al, 1993 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (34) |
| Wang, 1993 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (45) |
| Li et al, 1995 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (24) |
Table IV.
Newcastle-Ottawa Scale scores for cohort studies.
| Selection | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| First author, year | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposed | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | Total scores | (Refs.) |
| Huang et al, 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (28) |
| Hsiao et al, 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (31) |
| Sun et al, 2015 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (38) |
Meta-analyses of the case-controlled studies
Meta-analysis regarding blood type A
Based on the results of 26 case-controlled studies, the OR (CI; P-value) of type A blood and the risk of lung cancer was 1.10 (1.02-1.19; P=0.02). This showed that there was a difference in the distribution of type A blood between healthy individuals and patients with lung cancer (Fig. 2). The heterogeneity in the study was statistically significant (I2=67%; P<0.00001), and the random-effects model was used.
Figure 2.
Forest plot for meta-analysis of blood type A and lung cancer risk in the case-controlled studies. CI, confidence interval.
Meta-analyses regarding blood type B
Based on the results of 26 case-controlled studies, the OR of type B blood and the risk of lung cancer was 0.96 (0.89-1.04; P=0.30). This showed that there was no significant difference in the proportion of type B blood between healthy individuals and patients with lung cancer (Fig. 3). The heterogeneity in the study was statistically significant (I2=58%; P=0.0001), and the random-effects model was used.
Figure 3.
Forest plot for meta-analysis of blood type B and lung cancer risk in the case-controlled studies. CI, confidence interval.
Meta-analyses regarding blood type AB
Based on the results of 26 case-controlled studies, the OR of type AB blood and the risk of lung cancer was 0.96 (0.82-1.12; P=0.57). This showed that there was no significant difference in the proportion of type AB blood between healthy individuals and patients with lung cancer (Fig. 4). The heterogeneity in the study was statistically significant (I2=72%; P<0.00001), and the random-effects model was used.
Figure 4.
Forest plot for meta-analysis of blood type AB and lung cancer risk in the case-controlled studies. CI, confidence interval.
Meta-analyses regarding blood type O
Based on the results of 26 case-controlled studies, the OR of type O blood and the risk of lung cancer was 0.94 (0.86-1.02; P=0.14). This shows that there was no significant difference in the proportion of type AB blood between healthy individuals and patients with lung cancer (Fig. 5). The heterogeneity in the study was statistically significant (I2=72%; P<0.00001), and the random-effects model was used.
Figure 5.
Forest plot for meta-analysis of blood type O and lung cancer risk in the case-controlled studies. CI, confidence interval.
Sensitivity analyses
Sensitivity analysis was performed by removing each individual study in turn. The results showed that the combined results were not significantly affected by any specific individual, indicating that the combined results of the meta-analysis were reliable (Table V).
Table V.
Sensitivity analysis of the association between blood type A and lung cancer risk in the case-controlled studies.
| First author | Publication year | OR | (95%CI) | P-value | I2 % | (Refs.) |
|---|---|---|---|---|---|---|
| Cai et al | 2006 | 1.10 | 1.01-1.19 | 0.030 | 68 | (44) |
| Chen et al | 2004 | 1.11 | 1.03-1.21 | 0.008 | 66 | (30) |
| Feng and Ying | 2013 | 1.10 | 1.01-1.19 | 0.030 | 68 | (29) |
| Gao et al | 1998 | 1.10 | 1.02-1.20 | 0.020 | 69 | (26) |
| Guo | 2001 | 1.08 | 1.00-1.17 | 0.040 | 63 | (42) |
| Jiang and Wang | 1989 | 1.11 | 1.02-1.20 | 0.010 | 68 | (36) |
| Jin et al | 2000 | 1.10 | 1.02-1.20 | 0.020 | 69 | (41) |
| Li et al | 2014 | 1.11 | 1.02-1.20 | 0.020 | 68 | (21) |
| Li et al | 1995 | 1.11 | 1.03-1.21 | 0.008 | 67 | (24) |
| Liu et al | 2017 | 1.10 | 1.01-1.19 | 0.020 | 68 | (39) |
| Liu et al | 2006 | 1.09 | 1.01-1.18 | 0.030 | 67 | (43) |
| McConnell et al | 1954 | 1.11 | 1.02-1.20 | 0.010 | 68 | (33) |
| Oguz et al | 2013 | 1.11 | 1.02-1.20 | 0.010 | 68 | (20) |
| Pan et al | 2006 | 1.10 | 1.01-1.20 | 0.020 | 69 | (37) |
| Peng et al | 2014 | 1.11 | 1.02-1.21 | 0.010 | 67 | (16) |
| Rennie and Haber | 1961 | 1.10 | 1.01-1.20 | 0.020 | 69 | (35) |
| Sun and Zheng | 2001 | 1.09 | 1.01-1.18 | 0.030 | 67 | (22) |
| Tang et al | 2001 | 1.10 | 1.02-1.19 | 0.020 | 69 | (32) |
| Urun et al | 2013 | 1.10 | 1.01-1.21 | 0.030 | 69 | (15) |
| Wang | 1993 | 1.08 | 1.00-1.16 | 0.040 | 60 | (45) |
| Wang and Liang | 2000 | 1.11 | 1.02-1.20 | 0.010 | 68 | (25) |
| Xiao et al | 2021 | 1.11 | 1.02-1.20 | 0.020 | 69 | (27) |
| Xu et al | 2006 | 1.12 | 1.03-1.20 | 0.005 | 65 | (19) |
| Yang et al | 2000 | 1.11 | 1.03-1.21 | 0.007 | 67 | (23) |
| Zhang | 1990 | 1.09 | 1.01-1.18 | 0.030 | 66 | (40) |
| Zhao et al | 1993 | 1.11 | 1.02-1.20 | 0.020 | 69 | (34) |
Publication bias regarding blood type
Publication bias was assessed using funnel plots. The funnel diagram of the association between the ABO blood group and the risk of lung cancer is shown in Fig. 6. Funnel plots were mostly symmetric, and the corresponding points of the majority of data were within the 95% CI, indicating that publication bias had been adequately controlled.
Figure 6.
Funnel plot analysis of blood type. Funnel plot analysis of (A) blood type A, (B) blood type B, (C) blood type AB and (D) blood type O and lung cancer risk in the case-controlled studies. OR, odds ratio; SE, standard error.
Subgroup analysis
To assess the effect of each parameter on outcomes, subgroup analyses were performed based on ethnicity and the source of the control group (Table VI). In the subgroup analysis of ethnicity, blood type A was associated with the risk of lung cancer in patients from China (P=0.03), but was not associated with lung cancer risk in Caucasians (P=0.18). Blood type O was not associated with lung cancer risk in patients from China (P=0.14), but was associated with lung cancer risk in Caucasian patients (P=0.03). The other blood types did not show heterogeneity regarding ethnicity. In the subgroup analyses of the control source, type A blood was associated with the risk of lung cancer in the control groups that were from the general populace (P=0.04). In the control groups from healthy individuals in the hospital, there was no association with the risk of lung cancer (P=0.34). The other blood types did not show heterogeneity regarding the source of the control group.
Table VI.
Subgroup analysis of the association between ABO blood group and lung cancer risk in case-control studies.
| Test for heterogeneity | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Variable | n | Blood type | OR (95% CI) | P-value | I2, % | P-value | Analysis model |
| Ethnicity | |||||||
| Chinese | 22 | A | 1.12 (1.01-1.23) | 0.03 | 72 | <0.0001 | R |
| 22 | B | 0.96 (0.88-1.05) | 0.40 | 62 | <0.0001 | R | |
| 22 | AB | 0.93 (0.78-1.12) | 0.47 | 75 | <0.0001 | R | |
| 22 | O | 0.93 (0.83-1.03) | 0.14 | 75 | <0.0001 | R | |
| Caucasian | 4 | A | 1.05 (0.98-1.13) | 0.18 | 0 | 0.7300 | F |
| 4 | B | 1.02 (0.92-1.13) | 0.73 | 17 | 0.31 | F | |
| 4 | AB | 1.08 (0.94-1.25) | 0.27 | 0 | 0.42 | F | |
| 4 | O | 0.92 (0.85-0.99) | 0.03 | 41 | 0.17 | F | |
| Source of control | |||||||
| Social | 20 | A | 1.11 (1.00-1.23) | 0.04 | 72 | <0.0001 | R |
| 20 | B | 0.94 (0.86-1.03) | 0.21 | 52 | 0.0030 | R | |
| 20 | AB | 0.92 (0.75-1.13) | 0.45 | 78 | <0.0001 | R | |
| 20 | O | 0.94 (0.84-1.04) | 0.24 | 75 | <0.0001 | R | |
| Hospital | 6 | A | 1.04 (0.96-1.12) | 0.34 | 19 | 0.29 | F |
| 6 | B | 1.00 (0.84-1.18) | 0.97 | 71 | 0.004 | R | |
| 6 | AB | 0.96 (0.83-1.10) | 0.53 | 0 | 0.43 | F | |
| 6 | O | 0.94 (0.81-1.08) | 0.36 | 64 | 0.02 | R | |
F, fixed-effect model; R, random-effect model; OR, odds ratio; CI, confidence interval.
TSA
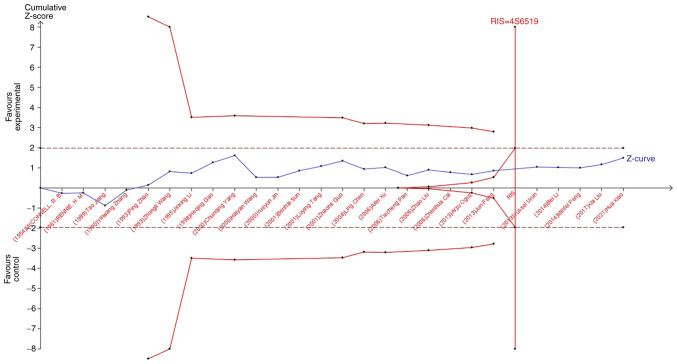
TSA was used to reduce the risk of type 1 error, and the RIS was evaluated by maintaining a 5% risk of type 1 error and a 20% relative risk reduction (80% power). As shown in Fig. 7, when studying the effects of blood type A on the occurrence of lung cancer, the sample size of study 21 (Jun Feng, 2013) crossed the TSA boundary and reached a positive conclusion in advance. This is consistent with previous meta-analysis results, suggesting that blood type A increases the risk of lung cancer. In the study of the influence of blood types B, O, and AB blood on the occurrence of lung cancer, the Z-curve did not cross the TSA boundary, but crossed the RIS line (Fig. 8, Fig. 9, Fig. 10). The results showed that blood types B, AB, and O had no effect on the occurrence of lung cancer. Moreover, the sample size was sufficient and no more case-controlled trials are required.
Figure 7.
Trial Sequential Analysis of the association between blood type A and the risk of lung cancer. The required information size was calculated based on a two-sided α=5% and β=15% (power 80%), and a relative risk reduction of 20%. RIS, required information size.
Figure 8.
Trial Sequential Analysis of the association between blood type B and the risk of lung cancer. The required information size was calculated based on a two-sided α=5% and β=15% (power 80%), and a relative risk reduction of 20%. RIS, required information size.
Figure 9.
Trial Sequential Analysis of the association between blood type AB and the risk of lung cancer. The required information size was calculated based on a two-sided α=5% and β=15% (power 80%), and a relative risk reduction of 20%. RIS, required information size.
Figure 10.
Trial Sequential Analysis of the association between blood type O and the risk of lung cancer. The required information size was calculated based on a two-sided α=5% and β=15% (power 80%), and a relative risk reduction of 20%. RIS, required information size.
Meta-analyses of cohort studies
Forest plot for meta-analysis
Based on the results of 3 cohort studies, the RR of blood type A and lung cancer was 1.05 (0.96-1.15; P=0.32), the RR of blood type B and lung cancer was 1.04 (0.94-1.14; P=0.47) the RR of blood type AB and lung cancer was 1.03 (0.88-1.20; P=0.71), and the RR of blood type O and lung cancer was 0.92 (0.85-1.01; P=0.08). This indicated that there was no statistically significant difference in blood type regarding the risk of lung cancer (Fig. 11). Heterogeneity was not statistically significant in the study, and a fixed-effect model was adopted.
Figure 11.
Forest plot for the meta-analysis of blood type and lung cancer risk. Forest plot for the meta-analysis of (A) blood type A, (B) blood type B, (C) blood type AB and (D) blood type O with lung cancer risk in the cohort study. CI, confidence interval.
Publication bias regarding the cohort studies
Due to the small number of included cohort studies, funnel plots were not used to assess publication bias.
Discussion
Lung cancer seriously affects the quality of life of patients. Thus, identifying similarities in the occurrence and development of lung cancer is key to identifying methods to reduce the incidence and mortality of affected patients. Since the discovery of the ABO blood group system by Landsteiner (46,47), >20 independent systems have been developed for human erythrocyte surface antigens. Due to its stable heritability, an increasing number of medical researchers are paying attention to its role in the occurrence and development of diseases (5–7). Multiple researchers have performed studies on the ABO blood group and the risk of lung cancer (15,16).
The present study comprehensively analyzed the influence of the ABO blood classification on the risk of lung cancer. By reviewing all eligible case-controlled studies, it was determined that blood type A was associated with the occurrence of lung cancer, and that this blood type may be a risk factor for lung cancer. The other blood types were not associated with the overall risk of lung cancer. In addition, to further explore the impact of ethnicity and source of control, subgroup analyses were performed. The results showed that type A blood was heterogeneous regarding ethnicity and source of control. These results were obtained when the study ethnicity was Chinese or the control group was from the social population. In addition, type O blood was determined to be a protective factor for lung cancer in Caucasian individuals. In Chinese individuals, type O blood had no effect on the prevalence of lung cancer. TSA results suggested that the sample size of the case-controlled study was sufficient; thus, additional case-controlled studies are not needed. Furthermore, the results from the cohort studies suggested that blood type was not associated with the risk of lung cancer.
The ABO blood group system consists of A and B antibodies and their corresponding antigens. The ABO blood type of can be determined by simply testing for the presence of antigens A or B in the blood. Individuals with type A blood have only A antigens on their red blood cells, and individuals with type B blood have only B antigens on their red blood cells. Individuals with type O blood have neither A nor B antigens in their red blood cells. Conversely, individuals with type AB have both A and B antigens. These antigens are present on the surface of red blood cells and also in several other tissues in the human body. The genes that determine ABO blood groups are located in the long arm of chromosome 9, region 3 and band 4 (9q34) (48). It was found that 9q34 contains the human DNA repair gene XPA, and proto-oncogene C-abl. If these genes are mutated or defective, they may cause tumor cell proliferation (49). Additionally, blood group antigen-associated glycosyltransferases encoded by the 9q34 gene can regulate intercellular adhesion and signal transduction (50). This may play an important role in immune monitoring of tumor cells and their sensitivity to apoptosis (51). On the other hand, the underlying mechanism associated with the ABO blood group and tumorigenesis also includes the inflammatory state of the body. Studies have identified an association between the ABO blood group and the circulating levels of TNF-α, soluble ICAM-1, e-selectin and p-selectin. The association was precisely found to be associated with the genotype of the A allele (8–10). This suggests that blood type A may influence inflammation throughout the body, leading to the development of cancer. Experimental study has also found that antigen A may improve immune escape capacity and prevent apoptosis (52). The aforementioned conclusions may underlie the increased incidence of patients with lung cancer with type A blood. The effect of ethnicity on the results may be due to the fact that lung cancer is caused by several factors. The incidence of lung cancer differs in different regions due to the different lifestyles of individuals. Furthermore, the ABO blood group affects several diseases. Therefore, the proportion of blood types in the control group from the hospital may differ from that of the total population, resulting in different results in the control groups from the different sources in this study.
The present study covered a wide range of subjects over a relatively large span of time. ABO blood group is a very stable genetic factor, which has not changed over decades. Therefore, the data from early studies are still valuable and can be included in this study. This meta-analysis provides a more accurate assessment of the association of the ABO blood type with lung cancer risk than previous studies. Additionally, the cohort study was added based on the inclusion of case-controlled studies. However, this analysis also has some limitations, as follows: i) Most of the studies included in the paper included patients of Chinese descent, thus there is a notable selection bias; ii) lung cancer has several different types of pathology, and different pathological types have different paths of pathogenesis (53); therefore, the study results may change when studying a specific pathological type of lung cancer; iii) case-controlled studies are observational studies that may have a selection bias due to incomplete randomization; iv) only a portion of the case-controlled studies retrieved in this paper corrected for traditional risk factors; therefore, the confounding effect of other risk factors cannot be completely controlled; and v) only the Chinese and English literature were included in this study, and the results may be affected by the inclusion of incomplete data.
In conclusion, the meta-analysis of the case-controlled studies analyzed in the present study suggest that patients with blood type A are at a higher risk of lung cancer. However, this result does not apply to Caucasians. In addition, this study also confirmed that Caucasians with type O blood have a lower risk of lung cancer. No association was found between other blood types and the prevalence of lung cancer. Differing study designs have a considerable impact on the research outcomes. The results of only three cohort studies showed that blood type was not associated with the risk of lung cancer. Larger and higher quality prospective studies recruiting patients from several international hospitals are required to better explore a more precise association between ABO blood group and the risk of lung cancer.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
PH, HY and ZT contributed to the conception and design of the study. HY, ZT, YZ and JS prepared the materials, collected the data and performed the analysis. HY drafted the manuscript. HY and ZT confirm the authenticity of all the raw data. All authors revised the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Zhao H, Suk R, Christiani DC. Genetic susceptibility to tobacco-related cancer. Oncogene. 2004;23:6500–6523. doi: 10.1038/sj.onc.1207811. [DOI] [PubMed] [Google Scholar]
- 3.Vachani A, Sequist LV, Spira A. AJRCCM: 100-Year anniversary. The shifting landscape for lung cancer: Past, present, and future. Am J Respir Crit Care Med. 2017;195:1150–1160. doi: 10.1164/rccm.201702-0433CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aird I, Bentall HH, Roberts JA. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1:799–801. doi: 10.1136/bmj.1.4814.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hems G. Epidemiological characteristics of breast cancer in middle and late age. Br J Cancer. 1970;24:226–234. doi: 10.1038/bjc.1970.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vioque J, Walker AM. Pancreatic cancer and ABO blood types: A study of cases and controls. Med Clin (Barc) 1991;96:761–764. (In Spanish) [PubMed] [Google Scholar]
- 8.Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, Rafferty I, Lauretani F, Murray A, Gibbs JR, Paolisso G, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs) PLoS Genet. 2008;4:e1000072. doi: 10.1371/journal.pgen.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, Nambi V, Bretler M, Smith NL, Peters A, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19:1863–1872. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson AD, Lopes-Virella MF, Waggott D, Boright AP, Hosseini SM, Carter RE, Shen E, Mirea L, Bharaj B, Sun L, et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29:1958–1967. doi: 10.1161/ATVBAHA.109.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi L, Cornelis MC, Kraft P, Jensen M, van Dam RM, Sun Q, Girman CJ, Laurie CC, Mirel DB, Hunter DJ, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet. 2010;19:1856–1862. doi: 10.1093/hmg/ddq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vowden P, Lowe AD, Lennox ES, Bleehen NM. The expression of ABH and Y blood group antigens in benign and malignant breast tissue: The preservation of the H and Y antigens in malignant epithelium. Br J Cancer. 1986;53:313–319. doi: 10.1038/bjc.1986.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauchen JA, Bergman SM, Hanson TA. Expression of A and B tissue isoantigens in benign and malignant lesions of the breast. Cancer. 1980;45:2149–2155. doi: 10.1002/1097-0142(19800415)45:8<2149::AID-CNCR2820450823>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clément M. ABH and Lewis histo-blood group antigens in cancer. APMIS. 2001;109:9–31. doi: 10.1111/j.1600-0463.2001.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 15.Urun Y, Utkan G, Cangir AK, Oksuzoglu OB, Ozdemir N, Oztuna DG, Kocaman G, Coşkun HŞ, Kaplan MA, Yuksel C, et al. Association of ABO blood group and risk of lung cancer in a multicenter study in Turkey. Asian Pac J Cancer Prev. 2013;14:2801–2803. doi: 10.7314/APJCP.2013.14.5.2801. [DOI] [PubMed] [Google Scholar]
- 16.Peng M, Yu S, Wang J, Wang D. Relationship between ABO blood group and risk of 8 kinds of malignant tumors. Chin J Health Inspection. 2014;24:811–813+823. (In Chinese) [Google Scholar]
- 17.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [ March 2; 2022 ];Appl Eng Agric. 2014 18:727–734. [Google Scholar]
- 18.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. 2nd edition. Copenhagen: Copenhagen Trial Unit; 2017. User Manual for Trial Sequential Analysis (TSA) pp. 1–119. [Google Scholar]
- 19.Xu A, He X, Wang W, Tang Y, Ping W. Relationship between ABO blood group and gastric cancer, liver cancer and lung cancer. J Clin Mil Med. 2006:722–723. (In Chinese) [Google Scholar]
- 20.Oguz A, Unal D, Tasdemir A, Karahan S, Aykas F, Mutlu H, Cihan YB, Kanbay M. Lack of any association between blood groups and lung cancer, independent of histology. Asian Pac J Cancer Prev. 2013;14:453–456. doi: 10.7314/APJCP.2013.14.1.453. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Tan B, Chen C, Zhao L, Qin L. Association between the ABO blood group and risk of common cancers. J Evid Based Med. 2014;7:79–83. doi: 10.1111/jebm.12098. [DOI] [PubMed] [Google Scholar]
- 22.Sun B, Zheng Y. ABO blood group typing in lung cancer patients. J Clin Pulmonology. 2001;2:30. (In Chinese) [Google Scholar]
- 23.Yang C, Zhang R, Zhou S, Wang Q. Discussion on the relationship between ABO blood group and lung cancer, liver cancer and gastric cancer. Clin Transfus Lab. 2000;1:11–12. (In Chinese) [Google Scholar]
- 24.Li J, Hu J, Wang M. The correlation between esophageal cancer, lung cancer and ABO blood group. Chin J Blood Transfus. 1995;1:45–46. (In Chinese) [Google Scholar]
- 25.Wang H, Liang X. Relationship between ABO blood group and 10 kinds of malignant tumors of Han nationality in Guangxi. Med Lit. 2000;5:643–644. (In Chinese) [Google Scholar]
- 26.Gao H, Zhao L, Wu S, Wang D, Li Y, Heyun S. Relationship between malignant tumor and ABO blood group. Chin J Prim Med. 1998;4:41–42. (In Chinese) [Google Scholar]
- 27.Xiao H, Xiang S, Shan Z, Bin X. Relationship between ABO blood group and different pathological types of lung cancer in southern Sichuan. Chin J Mod Med. 2021;31:98–102. (In Chinese) [Google Scholar]
- 28.Huang JY, Wang R, Gao YT, Yuan JM. ABO blood type and the risk of cancer-findings from the Shanghai cohort study. PLoS One. 2017;12:e0184295. doi: 10.1371/journal.pone.0184295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng J, Ying X. Correlation analysis between ABO blood group and lung cancer in Zhejiang Han people. Harbin Med. 2013;33:96–97. (In Chinese) [Google Scholar]
- 30.Chen L, Heng C, Shen W, Xiao X. Correlation analysis between ABO blood group and malignant tumor. Chin J Oncol. 2004;3:131–133. (In Chinese) [Google Scholar]
- 31.Hsiao LT, Liu NJ, You SL, Hwang LC. ABO blood group and the risk of cancer among middle-aged people in Taiwan. Asia Pac J Clin Oncol. 2015;11:e31–e36. doi: 10.1111/ajco.12253. [DOI] [PubMed] [Google Scholar]
- 32.Tang L, Ren Z, Zhou X, Su Z, Zhuang Z. ABO blood group and its interaction with smoking and lung cancer susceptibility. Chronic Dis Prev Control China. 2001;2:70–71. (In Chinese) [Google Scholar]
- 33.McConnell RB, Clarke CA, Downton F. Blood groups in carcinoma of the lung. Br Med J. 1954;2:323–325. doi: 10.1136/bmj.2.4883.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao P, Wu Q, Wang S. Lung cancer and ABO blood group relationship. Chin J Eugenics Genet. 1993:59–60. (In Chinese) [Google Scholar]
- 35.Rennie HM, Haber RW. Blood groups and carcinoma of the lung. Med J Aust. 1961;48:61–62. doi: 10.5694/j.1326-5377.1961.tb82571.x. [DOI] [PubMed] [Google Scholar]
- 36.Jiang T, Wang Z. ABO blood group distribution in 288 patients with lung cancer. J Chongqing Med Univ. 1989:220–221. (In Chinese) [Google Scholar]
- 37.Pan T, Zheng Z, Li J, Chen T, Wei X, Hu M, Liu L. Sixth National Thoracic and Cardiovascular Surgery Conference; Beijing, China: 2006. ABO blood group and lung cancer in Hubei province and literature review; p. 2. (In Chinese) [Google Scholar]
- 38.Sun W, Wen CP, Lin J, Wen C, Pu X, Huang M, Tsai MK, Tsao CK, Wu X, Chow WH. ABO blood types and cancer risk-a cohort study of 339,432 subjects in Taiwan. Cancer Epidemiol. 2015;39:150–156. doi: 10.1016/j.canep.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Chen X, Yang J, Guo R. Association of ABO blood groups with von Willebrand factor, factor VIII and ADAMTS-13 in patients with lung cancer. Oncol Lett. 2017;14:3787–3794. doi: 10.3892/ol.2017.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang YW. Preliminary analysis of ABO blood group distribution in cancer patients. J Hubei Med Coll. 1990:369–371. (In Chinese) [Google Scholar]
- 41.Jin YY, Wang ZY, Cheng CH. Relationship between ABO blood group and 3 common cancers. J Luoyang Med Coll. 2000;3:248. (In Chinese) [Google Scholar]
- 42.Guo ZH. Lung cancer and ABO blood group. Chin J Mod Med. 2001;1:61–63. (In Chinese) [Google Scholar]
- 43.Liu Z, Qiao Z, Huang P. Association between ABO blood group and lung cancer. Mod Med Health. 2006;14:2134–2135. (In Chinese) [Google Scholar]
- 44.Cai Z, Fang W, Qin J, Chen S, Lai K. Correlation between ABO blood group and malignant tumors in Guangzhou area. J Appl Med Technol. 2006;21:3727–3729. (In Chinese) [Google Scholar]
- 45.Wang Z. ABO blood group distribution analysis of lung cancer patients. Shaanxi Med Lab. 1993:242–243. (In Chinese) [Google Scholar]
- 46.Landsteiner K. ZTo know the antifermentative, lytic and agglutinative effects of blood serum and lymph. Centr Bakt Orig. 1900;27:357–362. (In German) [Google Scholar]
- 47.Landsteiner K. About agglutination symptoms of normal human blood. Wien Klin Wochschr. 1901;14:1132–1134. (In German) [Google Scholar]
- 48.Wagner FF, Flegel WA, Bittner R, Döscher A. Molecular typing for blood group antigens within 40 min by direct polymerase chain reaction from plasma or serum. Br J Haematol. 2017;176:814–821. doi: 10.1111/bjh.14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Z. Introduction to modern oncology. Med Res Lett. 2001:24. [Google Scholar]
- 50.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: Their changes associated with human cancer. Biochim Biophys Acta. 1999;1473:247–266. doi: 10.1016/S0304-4165(99)00183-X. [DOI] [PubMed] [Google Scholar]
- 51.Zhang S, Zhang HS, Cordon-Cardo C, Reuter VE, Singhal AK, Lloyd KO, Livingston PO. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997;73:50–56. doi: 10.1002/(SICI)1097-0215(19970926)73:1<42::AID-IJC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 52.Marionneau S, Le Moullac-Vaidye B, Le Pendu J. Expression of histo-blood group A antigen increases resistance to apoptosis and facilitates escape from immune control of rat colon carcinoma cells. Glycobiology. 2002;12:851–856. doi: 10.1093/glycob/cwf103. [DOI] [PubMed] [Google Scholar]
- 53.Sequist LV, Lynch TJ. EGFR tyrosine kinase inhibitors in lung cancer: An evolving story. Annu Rev Med. 2008;59:429–442. doi: 10.1146/annurev.med.59.090506.202405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.