Abstract
We corrected the previously published sequence for the regB gene, which encodes a histidine sensor kinase in Rhodobacter capsulatus. The deduced RegB amino acid sequence has an additional putative transmembrane domain at the N terminus. Analysis of RegB-PhoA and RegB-LacZ fusion proteins supports a topology model for RegB with six membrane-spanning domains.
Facultatively phototrophic bacteria, such as Rhodobacter capsulatus, perform aerobic respiration as long as oxygen is available. A decrease in oxygen tension below a threshold value results in the formation of a photosynthetic apparatus which is used to generate energy through anoxygenic photosynthesis (6). This demands the coordinated expression of many genes encoding pigment-binding proteins, enzymes for pigment synthesis, and regulatory proteins. A two-component system comprising the sensor kinase RegB and the response regulator RegA is involved in oxygen-dependent regulation of photosynthesis genes in R. capsulatus (reviewed in references 2 and 3). Dependent on the external oxygen signal, RegB undergoes autophosphorylation at a histidine residue and in turn phosphorylates an aspartate residue of RegA (13, 22). RegA binds to DNA sequences upstream of the puf and puc promoters and activates transcription (7, 11, 16). The puf and puc operons encode pigment-binding proteins of the reaction center and of the two light-harvesting complexes and are part of a cluster of photosynthesis genes (20). It is not known which signal (molecular oxygen or the redox state of other cellular components) is sensed by RegB and what the mechanism of sensing is. In order to learn more about the mechanism of sensing it is important to determine the membrane topology of RegB. Mosley et al. (22) suggested a model with five membrane-spanning regions for this sensor kinase based on the DNA sequence they published. In this model the N terminus of RegB is placed in the periplasm, which is an uncommon topology among bacterial sensor kinases.
Correction of the sequence of the regB gene.
In an attempt to test the model of Mosley et al. (22), we constructed a number of regB-lacZ and regB-phoA fusions. During sequencing of these constructs we noticed an additional C in the 5′ region of regB, which was missed in the sequence published previously. If translation of the regB gene started at the ATG proposed by Mosley et al. (22), the addition of the C would result in a reading frame shift and the deduced amino acid sequence would no longer be similar to bacterial sensor kinases. Indeed, the paper of Mosley et al. (22) did not present any experimental evidence for the translational start of RegB, and the ATG selected by these authors is not preceded by a strong ribosome binding site. In the corrected RegB sequence, four ATG codons could function as translational start site, all of them upstream of the ATG proposed by Mosley et al. In order to determine the start site of RegB translation, we fused DNA sequences which extended to different positions of the putative RegB-coding sequence to the lacZ gene and quantified the β-galactosidase activity of the fusion proteins in R. capsulatus. The positions of the fusions and the β-galactosidase activities are shown in Fig. 1. Our data strongly suggest the start of translation of RegB to be at the two adjacent ATGs (Fig. 1). The putative ribosome binding site for the two ATGs was compared to the ribosome binding sequence which was deduced from the R. capsulatus 16S rRNA sequence. When we compared the deduced amino acid sequence of the corrected regB gene to that of the R. sphaeroides homologue prrB (9), it became obvious that the correction extends the similarity between the two proteins to the N terminus (Fig. 1).
FIG. 1.
Alignment of the amino acid sequences of the RegB protein from R. capsulatus as previously published (22), of the RegB protein as deduced from our corrected DNA sequence, and RegB homologue PrrB (9) of R. sphaeroides. The positions of fusion to the lacZ gene and the β-galactosidase activities of the fusion proteins (where synthesized) in R. capsulatus are indicated. The strategy for construction of the gene fusions is identical to the strategy used for the other regB-lacZ fusions as described in the text and shown in Fig. 2. Vertical lines identify identical amino acids, and asterisks indicate similar amino acids. The putative ribosome binding site is underlined.
Furthermore, when we cloned a DNA fragment harboring the regB sequence with the additional C into the KpnI-Ecl136 sites of plasmid pRK415 (15) and transferred the resulting construct, pRK4RegB1, by conjugation (17) into the regB mutant strain CSM01 (22), spectral analysis of extracts from equal amounts of cells revealed that plasmid pRK4RegB1 was able to restore wild-type levels of photosynthetic complexes the strain (data not shown). This demonstrates that the additional C nucleotide was not an artifact of PCR amplification.
Implication of the corrected RegB sequence for its orientation in the membrane.
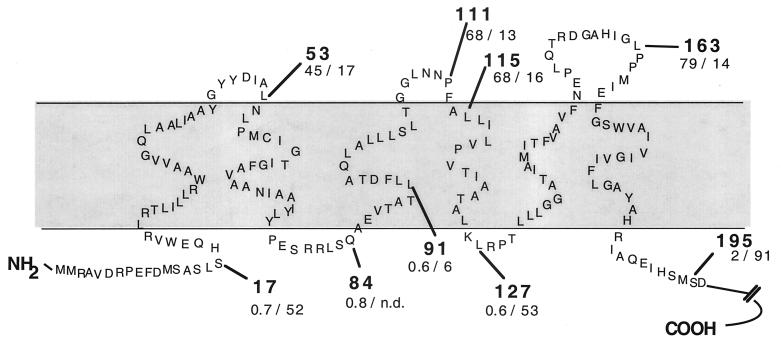
By the addition of the C nucleotide the predicted sequence of the RegB protein was extended by 19 amino acids (Fig. 1). When the complete corrected RegB sequence was analyzed, some computer programs (HMMTOP [26], TopPred 2 [27], ProtScale [18], and Split [14]) predicted six membrane-spanning segments with the N terminus in the cytoplasm (Fig. 2), although some others (PHDhtm [10] and Sosui [25]) predicted five membrane-spanning segments with the N terminus in the periplasmic space. All computer programs give similar results for the prediction of four transmembrane domains extending from positions 28 ± 2 to 47 ± 2, 56 ± 2 to 75 ± 1, 131 ± 1 to 150 ± 3, and 163 ± 2 to 186 ± 1 (positions refer to amino acid residues of the corrected RegB sequence as shown in Fig. 2).
FIG. 2.
Schematic representation of the membrane topology of the sensor kinase RegB. The bold numbers give the position of the last amino acid of RegB, which was fused to LacZ (first amino acid Ala) or to PhoA (first amino acid Ser). Two additional amino acids have been introduced by the cloning procedure. The units of activity for β-galactosidase and alkaline phosphatase in E. coli are given below the number for the fusion position. The activities measured in parental strains were subtracted, and average values from at least three measurements were calculated. E. coli cultures were grown semiaerobically in Luria-Bertani medium to late exponential phase. n.d., not determined.
Analysis of the membrane topology of RegB.
In order to test this model, we used the approach of studying RegB-PhoA and RegB-LacZ fusion proteins (8, 19). For creation of the RegB-PhoA fusions we cloned the 1.4-kb BamHI fragment from plasmid pSWFII (8) comprising the phoA gene into the BamHI site of plasmid pRK415 (15). We amplified DNA fragments comprising 36 nucleotides upstream of the new regB start codon and sequences of different lengths from the 5′ part of regB by PCR using plasmid pRK4RegB1 as a template. The PCR primers introduced new restriction sites which were used to insert the regB fragments into the HindIII and XbaI sites of the pRK415 derivative containing the phoA gene. Our cloning strategy allowed the in-frame fusion of regB to phoA and transcription by the lac promoter of plasmid pRK415. The positions of the fusions between RegB and PhoA are indicated in Fig. 2.
For the construction of the RegB-LacZ fusions we amplified DNA fragments spanning the same sequences as used for the PhoA fusions. By using different primers for the PCR, we created different restriction sites which allowed cloning of the PCR products into the KpnI and HindIII sites of plasmid pPHU236 (12). This cloning strategy created in-frame fusions to the lacZ gene. These constructs gave no β-galactosidase activity in Escherichia coli or R. capsulatus, indicating that no promoter for the regB gene is present on the amplified DNA fragment. In order to allow analysis of our fusion constructs as well in R. capsulatus as in E. coli, we cloned a PCR product containing the aph promoter (amplified from plasmid pUC4-KIXX [1] with primers 5′GAAAGCAGGTACCTTGCA and 5′CAGATCTGGTACCCCTGC) into the KpnI sites of the pPHU236 derivatives harboring the regB-lacZ fusion genes.
We analyzed all regB-phoA fusions in E. coli strain CC118 (19), which does not harbor an endogenous alkaline phosphatase. RegB-PhoA fusion plasmids were also transferred into R. capsulatus strain 37b4, but only very low activities of alkaline phosphatase were detected (data not shown). This is surprising, since prrB-phoA fusions yielded even higher levels of alkaline phosphatase in R. sphaeroides than in E. coli and the lac promoter is known to be expressed in R. capsulatus (24) (expression of RegB from plasmid pRK4RegB1 is described in this paper). However, it was previously shown that the alkaline phosphatase activities in E. coli reflect the values obtained in Rhodobacter and that proteins fold in a very similar manner in both bacterial species (23). The regB-lacZ fusions were expressed in E. coli strain MC1061 [5] as well as in R. capsulatus. The activities determined for the different fusion proteins (4, 21) expressed in E. coli and R. capsulatus are given in Fig. 2. For fusion proteins with the β-galactosidase extending into the periplasm, the activities determined for R. capsulatus were in the same range as determined for E. coli. Fusion proteins with the β-galactosidase extending into the cytoplasm gave three- to ninefold-higher activity in R. capsulatus than in E. coli. Our results clearly support the model, which is in agreement with the topology analysis performed for the R. sphaeroides PrrB protein (23).
Our results are not in accordance with any alternative model, including the five-transmembrane-helix model proposed earlier (22), and strongly suggest that the membrane topologies of the R. capsulatus RegB protein and the R. sphaeroides PrrB protein show high similarity. These models now provide a basis for future experiments designed to determine the RegB domains involved in sensing of an oxygen-dependent signal.
Nucleotide sequence accession number.
The corrected regB sequence has been assigned GenBank accession number AF189160.
Acknowledgments
We thank Carl Bauer for providing strain CSM01.
This work was supported by the Deutsche Forschungsgemeinschaft (K1 563/5-3) and by Fonds der Chemischen Industrie.
REFERENCES
- 1.Barany F. Two-codon insertion mutagenesis of plasmid genes by using single-stranded hexameric oligonucleotides. Proc Natl Acad Sci USA. 1985;82:4202–4206. doi: 10.1073/pnas.82.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer C E. Regulation of photosynthesis gene expression. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1221–1234. [Google Scholar]
- 3.Bauer C E, Bird T H. Regulatory circuits controlling photosynthesis gene expression. Cell. 1996;85:5–8. doi: 10.1016/s0092-8674(00)81074-0. [DOI] [PubMed] [Google Scholar]
- 4.Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975;96:307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 5.Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 6.Drews G, Imhoff J F. Phototrophic purple bacteria. In: Shively J M, Barton L L, editors. Variations in autotrophic life. New York, N.Y: Academic Press Ltd.; 1991. pp. 51–97. [Google Scholar]
- 7.Du S, Bird T H, Bauer C E. DNA binding characteristics of RegA. A constitutively active anaerobic activator of photosynthesis gene expression in Rhodobacter capsulatus. J Biol Chem. 1998;273:18509–18513. doi: 10.1074/jbc.273.29.18509. [DOI] [PubMed] [Google Scholar]
- 8.Ehrmann M, Boyd D, Beckwith J. Genetic analysis of membrane protein topology by a sandwich gene fusion approach. Proc Natl Acad Sci USA. 1990;87:7574–7578. doi: 10.1073/pnas.87.19.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eraso J M, Kaplan S. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J Bacteriol. 1995;177:2695–2706. doi: 10.1128/jb.177.10.2695-2706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fariselli P, Casadio R. Transmembrane helices predicted at 95% accuracy. Protein Sci. 1995;4:521–533. doi: 10.1002/pro.5560040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemschemeier S K, Kirndörfer M, Ebel U, Klug G. Transcriptional regulation of puf and puc expression in Rhodobacter capsulatus by the DNA binding protein RegA. In: Peschek G A, Löffelhardt W, Schmetterer G, editors. The phototrophic procaryotes. New York, N.Y: Plenum Press; 1999. pp. 127–130. [Google Scholar]
- 12.Hübner P, Willison J C, Vignais P M, Bickle T A. Expression of regulatory nif genes in Rhodobacter capsulatus. J Bacteriol. 1991;173:2993–2999. doi: 10.1128/jb.173.9.2993-2999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue K, Kouadio J K, Mosley C S, Bauer C E. Isolation and in vitro phosphorylation of sensory transduction components controlling anaerobic induction of light harvesting and reaction center gene expression in Rhodobacter capsulatus. Biochemistry. 1995;34:391–396. doi: 10.1021/bi00002a002. [DOI] [PubMed] [Google Scholar]
- 14.Juretic D, Zucic D, Lucic B, Trinajstic N. Preference functions for prediction of membrane-buried helices in integral membrane proteins. Comput Chem. 1998;4:279–294. doi: 10.1016/s0097-8485(97)00070-3. [DOI] [PubMed] [Google Scholar]
- 15.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 16.Kirndörfer M, Jäger A, Klug G. Integration host factor affects the oxygen-regulated expression of photosynthesis genes in Rhodobacter capsulatus. Mol Gen Genet. 1998;258:297–305. doi: 10.1007/s004380050734. [DOI] [PubMed] [Google Scholar]
- 17.Klug G, Drews G. Construction of a gene bank of Rhodopseudomonas capsulata using a broad host range cloning system. Arch Microbiol. 1984;139:319–325. doi: 10.1007/BF00408373. [DOI] [PubMed] [Google Scholar]
- 18.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 19.Manoil C. Analysis of protein localization by use of gene fusions with complementary properties. J Bacteriol. 1990;172:1035–1042. doi: 10.1128/jb.172.2.1035-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981;146:1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 22.Mosley C S, Suzuki J Y, Bauer C E. Identification and molecular genetic characterization of a sensor kinase responsible for coordinately regulating light harvesting and reaction center gene expression in response to anaerobiosis. J Bacteriol. 1994;176:7566–7573. doi: 10.1128/jb.176.24.7566-7573.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouchane S, Kaplan S. Topology analysis of the membrane-localized redox-responsive sensor kinase PrrB from Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1999;274:17290–17296. doi: 10.1074/jbc.274.24.17290. [DOI] [PubMed] [Google Scholar]
- 24.Pollich M, Klug G. Identification and sequence analysis of genes involved in late steps of cobalamin (vitamin B12) synthesis in Rhodobacter capsulatus. J Bacteriol. 1995;177:4481–4487. doi: 10.1128/jb.177.15.4481-4487.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takatsugu H, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 26.Tusnády G E, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol. 1998;283:489–506. doi: 10.1006/jmbi.1998.2107. [DOI] [PubMed] [Google Scholar]
- 27.von Heijne G. Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992;255:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]