Abstract
Background:
The NIH-funded Trial to Assess Chelation Therapy (TACT) randomized 1708 stable patients age ≥50 who were ≥6 months post myocardial infarction to 40 infusions of an edetate disodium-based regimen or placebo. In 633 patients with diabetes, edetate disodium significantly reduced the primary composite endpoint of mortality, recurrent myocardial infarction, stroke, coronary revascularization, or hospitalization for angina (hazard ratio [HR] 0.59, 95% confidence interval [CI] 0.440.79, p<0.001). The principal secondary endpoint of a composite of cardiovascular death, myocardial infarction, or stroke was also reduced (HR 0.60, 95% CI 0.39–0.91, p=0.017). It is unknown if the treatment effect differs by diabetes therapy.
Methods:
We grouped the subset of 633 patients with diabetes according to glucose-lowering therapy at time of randomization. The log-rank test was used to compare active therapy versus placebo. All treatment comparisons were performed using 2-sided significance tests at the significance level of 0.05 and were as randomized. Relative risks were expressed as HR with associated 95% CI, calculated using the Cox proportional hazards model.
Results:
There were 162 (25.7%) patients treated with insulin; 301 (47.5%) with oral hypoglycemics only; and 170 (26.8%) receiving no pharmacologic treatment for diabetes. Patients on insulin reached the primary endpoint more frequently than patients on no pharmacologic treatment [61 (38%) vs 49 (29%) (HR 1.56, 95% CI 1.07–2.27, p=0.022)] or oral hypoglycemics [61 (38%) vs 87 (29%) (HR 1.46, 1.05–2.03, p=0.024)]. The primary endpoint occurred less frequently with edetate disodium based therapy versus placebo in patients on insulin [19 (26%) vs 42 (48%) (HR 0.42, 95% CI 0.25–0.74, log-rank p=0.002)], marginally in patients on oral hypoglycemics [38 (25%) vs 49 (34%) (HR 0.66, 95% CI 0.43–1.01, log-rank p=0.041)], and no significant difference in patients not treated with a pharmacologic therapy [23 (25%) vs 26 (34%) (HR 0.69, 95% CI 0.39–1.20, log-rank p=0.225)]. The interaction between randomized intravenous treatment and type of diabetes therapy was not statistically significant (p=0.203).
Conclusions:
Edetate disodium treatment in stable, post-myocardial infarction patients with diabetes suggests that patients on insulin therapy at baseline may accrue the greatest benefit.
Keywords: diabetes, insulin, chelation, edetate disodium, myocardial infarction
INTRODUCTION
The global prevalence of diabetes in 2019 was estimated to be 463 million patients, 35.4 million in the United States1. It is expected that by 2040 there will be 72.1 million in the United States and 642 million worldwide. This accounts to 1 in 11 adults worldwide having diabetes and 1 in 8 in the United States. In the US more than 219,400 deaths were attributed to diabetes, almost two third of those deaths were in patients older than 60 years. Despite the advances in medical research that have identified effective medical therapy to reduce coronary events in diabetes, almost 60% of the patients with diabetes who died were attributed to coronary artery disease as a principal or contributing factor. Thus, there is a large residual risk of coronary disease in patients with diabetes. We previously reported important benefit for stable post-MI patients with diabetes treated with disodium ethylene diamine tetraacetic acid (edetate disodium, EDTA), in the National Institutes of Health (NIH)-funded Trial to Assess Chelation Therapy (TACT)2.
TACT enrolled 1708 post myocardial infarction patients and tested edetate disodium-based chelation versus placebo. Chelation, compared with placebo, resulted in a significant reduction in the primary composite endpoint of mortality, recurrent myocardial infarction, stroke, coronary revascularization, or hospitalization for angina. In the prespecified subgroup with diabetes (n=633), the chelation-based strategy reduced the primary endpoint by 41% (p=0.0002, 5-year NNT=6.5) and reduced total mortality by 43% (p=0.011, 5-year NNT=12)3. As a result of these findings, and because of the public health impacts of cardiovascular disease and diabetes, a more thorough understanding of the population of patients with diabetes that accrued the greatest benefit from chelation therapy would be important. For example, patients with diabetes who are at greatest risk would be patients treated with intensive insulin therapy4. Thus, we performed these post-hoc analyses to identify the relationship between clinically guided treatment for diabetes and the benefit of edetate disodium-based treatment.
METHODS
Trial Design
The design of TACT has been previously reported5. TACT was a randomized, double-blind, placebo-controlled, 2 X 2 factorial trial in which stable, post-myocardial infarction patients were randomly assigned to receive either 40 infusions of edetate disodium-based solution or intravenous placebo, and additionally an oral high-dose vitamin and mineral regimen or oral placebo (NCT00044213). Secure Web-based permuted block randomization was stratified by the clinical site (diabetes mellitus was not a stratification factor). The institutional review board at each clinical site approved the study, and patients provided written informed consent. An independent Data and Safety Monitoring Board reporting to the National Heart, Lung, and Blood Institute (NHLBI) and National Center for Complementary and Alternative Medicine (NCCAM) monitored the study.
Study Population
Patients were enrolled at 134 clinical sites in the United States and Canada between September 10, 2003 and October 4, 2010. TACT patients were 50 years or older with a history of myocardial infarction ≥6 weeks before enrollment. Major exclusion criteria were women of childbearing potential, a creatinine level >2.0 mg/dL, platelet count <100,000 per μL, abnormal liver function studies, blood pressure >160/100 mmHg, past intolerance to the infusion or vitamin components, prior edetate disodium treatment within 5 years, or revascularization within 6 months.
Patients with diabetes were a prespecified subgroup in the overall TACT trial2. Diabetes was defined as 1] self-reported diabetes, or 2] taking oral or insulin treatment for diabetes, or 3] having fasting blood glucose of ≥126 mg/dL at the time of enrollment. Of the 1708 TACT patients, 633 (37%) had diabetes at enrollment. Clinical practice calibrates the intensity of glucose lowering therapy based on the difficulty of attaining glycemic control. We therefore divided the 633 patients with diabetes at baseline according to their respective glucose-lowering therapy as: 1) insulin therapy, 2) oral hypoglycemic therapy only, or 3) no glucose-lowering therapy. Finally, in order to confirm that the concept of clinical calibration of diabetes treatment was accurate, and that patients treated with insulin demonstrated more advanced diabetes, we assessed fasting blood glucose, dipstick proteinuria, and estimated glomerular filtration (eGFR) rate across groups defined by diabetes therapy.
Treatment
The TACT active infusion consisted of 10 components: 3 g of disodium edetate disodium, adjusted downward based on eGFR; 7 g of ascorbic acid; 2 g of magnesium chloride; 100 mg of procaine hydrochloride; 2500 U of unfractionated heparin; 2 mEq of potassium chloride; 840 mg of sodium bicarbonate; 250 mg of pantothenic acid; 100 mg of thiamine; 100 mg of pyridoxine; and sterile water to make up 500 mL of solution.
The placebo solution consisted of 500 mL of normal saline and 1.2% dextrose (6 g total). Infusions were administered for ≥ 3 hours through a peripheral intravenous line weekly for 30 weeks, and then biweekly to bimonthly to complete 40 total infusions5.
All study patients received a daily low-dose vitamin regimen consisting of vitamin B6 25 mg, zinc 25 mg, copper 2 mg, manganese 15 mg and chromium 50 mcg, to prevent depletion of essential vitamins and minerals by the chelation regimen. The use of evidence-based medications for secondary prevention of coronary artery disease did not differ between the edetate disodium and placebo groups.
Follow-up
Median follow-up was 55 months. Patients were seen at baseline and at each infusion visit. Once patients completed the infusion phase, they were followed via quarterly telephone calls, annual clinic visits, and a final visit at the 5-year follow-up or at the end of the study, whichever occurred first. Laboratory evaluations included fasting blood glucose levels at baseline and throughout the infusion phase of the trial. Presence of proteinuria and eGFR at baseline were used for this analysis and compared in each of the diabetes treatment groups.
Endpoints
The primary endpoint was a composite of death from any cause, myocardial reinfarction, stroke, coronary revascularization, or hospitalization for angina. All endpoint events were reviewed and adjudicated by a clinical events committee blinded to the randomized treatment assignment.
Statistical Analysis
The present analyses compare edetate disodium-based chelation to placebo infusions and do not encompass the oral vitamins versus oral placebo comparisons. Subgroups based on diabetes treatment regimen were not prespecified and are considered exploratory and hypothesis-generating.
Baseline characteristics of patients were descriptively summarized using the median and interquartile range for continuous variables and frequencies and percentages for categorical variables. The comparison across groups defined by diabetes treatment at baseline were done using Kruskal-Wallis non-parametric analysis of variance for continuous variables and the conventional Chi-square test for categorical variables.
The log-rank test was used for comparing chelation versus placebo treatment arms with respect to the first occurrence of the primary and secondary clinical outcomes. All treatment comparisons were performed using 2-sided significance tests at the significance level of 0.05, including all patients assigned to their randomized treatment group (intention to treat). Cumulative event rates were calculated using the Kaplan–Meier method6. Relative risks were expressed as hazard ratio with associated 95% confidence intervals from a Cox proportional hazards model adjusted for oral vitamin treatment group7. A Cox model with an interaction between infusion therapy and the type of diabetes treatment was used to assess the effect of chelation therapy compared to placebo among groups by diabetes treatment. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
There were 633/1708 (37%) patients enrolled who met the criteria for diabetes and included in this analysis. Of these, 162 (25.7%) were on insulin treatment, 301 (47.5%) on oral hypoglycemics, and 170 (26.8%) on non-pharmacologic treatment at baseline for diabetes.
Baseline Characteristics
Patients on insulin were more obese, had a higher prevalence of congestive heart failure, lower HDL cholesterol levels, and higher triglyceride levels compared with patients on oral hypoglycemics or non-pharmacologic treatment (Table 1). Fasting blood glucose levels were also higher in patients treated with insulin than in those treated with oral hypoglycemics or non-pharmacologically. An eGFR less than 60 cc/min/1.73/m2 was more frequent in insulin-treated patients than in those not treated with insulin (43.2% vs 28.6% for oral hypoglycemic and 25.9% for those treated non-pharmacologically; p=0.001).
Table 1.
Baseline characteristics of patients according to diabetes therapy
| Insulin (n=162) | Oral therapy (N=301) | No diabetes therapy (N=170) | P–value | |
|---|---|---|---|---|
| Age (years) | 64.1 (58.6, 70.5) | 66.9 (61.2, 71.8) | 64.9 (58.5, 70.3) | 0.007 |
| Female | 43 (27%) | 49 (16%) | 27 (16%) | 0.014 |
| BMI | 33.5 (29.8,37.3) | 31.6 (28.0, 36.0) | 29.9 (26.8,34.2) | <0.001 |
| CHF | 58 (36%) | 61 (20%) | 26 (15%) | < 0.001 |
| Stroke | 17 (10%) | 23 (8%) | 11 (6%) | 0.378 |
| PAD | 43 (27%) | 65 (22%) | 28 (17%) | 0.099 |
| Hypertension | 131 (81%) | 238 (79%) | 125 (74%) | 0.228 |
| Hypercholesterolemia | 139 (86%) | 256 (86%) | 133 (81%) | 0.315 |
| Either CABG or PCI | 137 (85%) | 239 (79%) | 139 (82%) | 0.391 |
| Medications | ||||
| Aspirin | 141 (87%) | 255 (85%) | 135 (79%) | 0.145 |
| Beta-blocker | 127 (78%) | 219 (73%) | 131 (77%) | 0.339 |
| Statin | 127 (78%) | 231 (77%) | 121 (71%) | 0.258 |
| ACE or ARB | 128 (79%) | 225 (75%) | 107 (63%) | 0.002 |
| Laboratory Examinations | ||||
| Glucose (mg/dL) | 142 (105, 199) | 130 (107.0, 163.0) | 129 (112, 144) | 0.064 |
| Creatinine (mg/dL) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.3) | 0.013 |
| eGFR (mean± (std) (quartiles 75,25) | 64.5±18 (78, 49) | 70.8±18 (85, 58) | 70.6 ±17 (83, 59) | 0.0010 |
| eGFR < 60 (%) | 43.2 | 28.6 | 25.9 | 0.010 |
| Proteinuria (%)¶ | 20.8 | 7.1 | 10.2 | <0.0001 |
| Total cholesterol (mg/dL) | 158 (136, 188) | 159 (137, 193) | 167 (142, 198) | 0.131 |
| HDL (mg/dL) | 38 (33, 46.5) | 41 (36, 48.5) | 42.5 (36, 48.5) | 0.004 |
| LDL (mg/dL) | 77 (60, 103) | 80 (62, 111) | 90 (73, 114) | 0.004 |
| Triglycerides (mg/dL) | 176 (113, 262) | 157 (110, 221) | 142 (99, 196) | 0.035 |
CHF =congestive heart failure, PAD = peripheral artery disease, CABG =coronary artery bypass surgery, PCI = percutaneous coronary intervention. Descriptive statistics included percentages for discrete variables, and medians with 25th to 75th percentiles plus means with standard deviations for selected continuous variables. The p-values are based on a statistical comparison of the three groups of patients with diabetes in respect to each characteristic. Proteinuria was not available in 2.1% (13 patients) patients
Dipstick proteinuria was positive (1+ or higher) in 20.8% of the insulin-treated patients, compared with 7.1% of patients taking oral hypoglycemic and 10.2% of patients treated non-pharmacologically (p<0.0001).
Concomitant evidence-based medications
Evidence based cardiovascular medications for secondary prevention revealed patients on insulin were treated more frequently with angiotensin converting enzyme inhibitors and aldosterone receptor blockers, while no difference was observed in the rates of statin, aspirin, and beta-blocker use among diabetes-treatment groups (Table 1). This study was done before the availability of sodium glucose cotransporter 2 (SGLT-2) inhibitors.
Comparability across diabetes-treatment groups
More insulin-treated patients assigned to edetate disodium compared to placebo had a prior revascularization reported. In patients treated with oral hypoglycemics there was greater aspirin use in the edetate disodium chelation group compared to those in the placebo group. Finally, in patients with diabetes not treated pharmacologically, there was a higher prevalence of prior anterior myocardial infarction (Table 2).
Table 2.
Baseline characteristics of patients with diabetes randomized to edetate disodium chelation vs. placebo within each of the three diabetes treatment categories.
| Insulin | Oral hypoglycemic | No hypoglycemic therapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Edetate disodium (N=74) | Placebo (N=88) | P–value | Edetate disodium (N=155) | Placebo (N=146) | P–value | Edetate disodium (N=93) | Placebo (N=77) | P–value | |
| Age (years) | 64.6 (59.5, 71.4) | 63.7 (58, 70) | 0.23 | 66.8 (62, 72) | 67.1 (60.5, 71.5) | 0.52 | 63.1 (58.0, 68.2) | 67.4 (59.7, 72.2) | 0.019 |
| Female | 21 (28%) | 22 (25%) | 0.63 | 21 (14%) | 28 (19%) | 0.19 | 13 (14%) | 14 (18%) | 0.46 |
| BMI | 33 (29.4, 36.4) | 34.6 (31.3, 37.4) | 0.22 | 30.6 (27.7, 35.7) | 32.5 (28.9, 36.8) | 0.05 | 30.5 (27.6, 35.5) | 29.6 (26.1, 32.3) | 0.08 |
| CHF | 30 (41%) | 28 (32%) | 0.25 | 32 (21%) | 29 (20%) | 0.87 | 14 (15%) | 12 (16%) | 0.92 |
| Stroke | 11 (15%) | 6 (7%) | 0.09 | 12 (8%) | 11 (8%) | 0.95 | 3 (3%) | 8 (10%) | 0.068 |
| PAD | 19 (26%) | 24 (27%) | 0.82 | 32 (21%) | 33 (23%) | 0.63 | 18 (20%) | 10 (13%) | 0.25 |
| Hypertension | 56 (76%) | 75 (85%) | 0.12 | 126 (81%) | 112 (77%) | 0.33 | 69 (74%) | 56 (73%) | 0.83 |
| Either CABG or PCI | 69 (93%) | 68 (77%) | 0.005 | 126 (81%) | 113 (77%) | 0.40 | 76 (82%) | 63 (82%) | 0.98 |
| Anterior MI | 29 (39%) | 299 (33%) | 0.41 | 55 (35%) | 58 (40%) | 0.45 | 44 (47%) | 24 (31%) | 0.03 |
| Medications | |||||||||
| Aspirin | 65 (88%) | 76 (86%) | 0.78 | 138 (89%) | 117 (80%) | 0.032 | 75 (81%) | 60 (78%) | 0.66 |
| Beta-blocker | 61 (82%) | 66 (75%) | 0.25 | 114 (74%) | 105 (72%) | 0.75 | 73 (78%) | 58 (75%) | 0.63 |
| Statin | 59 (80%) | 68 (77%) | 0.71 | 121 (78%) | 110 (75%) | 0.58 | 67 (72%) | 54 (70%) | 0.78 |
| ACEi or ARB | 58 (78%) | 70 (80%) | 0.86 | 117 (75%) | 108 (74%) | 0.76 | 59 (63%) | 48 (62%) | 0.88 |
| Laboratory Examinations | |||||||||
| Glucose (mg/dL) | 133.5 (98, 192) | 149.5 (108, 202) | 0.25 | 129 (104, 163) | 130.5 (109, 165) | 0.60 | 129 (113, 143) | 129 (112, 149) | 0.80 |
| Creatinine (mg/dL) | 1.1 (0.9, 1.4) | 1.2 (1.0, 1.4) | 0.16 | 1.0 (0.9, 1.3) | 1.1 (0.9, 1.2) | 0.70 | 1.0 (0.9, 1.2) | 1.2 (0.9, 1.3) | 0.03 |
| Total cholesterol (mg/dL) | 149 (135, 181) | 161 (136, 194) | 0.20 | 156 (135, 191) | 163 (140, 200) | 0.11 | 165 (139, 194) | 172 (144, 206) | 0.34 |
| HDL (mg/dL) | 38 (34, 46) | 40 (32, 48) | 0.97 | 40 (34, 48) | 42.5 (36, 49) | 0.24 | 43 (36, 49) | 42 (37, 48) | 0.95 |
| LDL (mg/dL) | 75 (59, 100) | 82.5 (61, 115) | 0.22 | 76 (61, 99) | 83 (63, 115) | 0.13 | 89 (75, 113) | 93 (72, 115) | 0.80 |
| Triglycerides (mg/dL) | 193 (112, 251) | 174 (114, 271) | 0.77 | 158 (116, 220) | 157 (109, 221) | 0.86 | 135 (97, 179) | 161 (101, 216) | 0.24 |
CHF = congestive heart failure, PAD = peripheral arterial disease, CABG = coronary artery bypass surgery, PCI = percutaneous coronary intervention. Descriptive statistics included percentages for discrete variables, and medians with 25th to 75th percentiles plus means with standard deviations for continuous variables.
Primary endpoint by treatment of diabetes
Patients treated with insulin reached the primary endpoint more frequently than patients on oral hypoglycemic medications or non-pharmacologic therapy for diabetes 61 (38%), 87 (29%) and 49 (29%) respectively. The Kaplan-Meier estimates for the primary endpoint for patients on insulin therapy compared to patients with diabetes on non-pharmacologic therapy demonstrated a HR 1.56 (95% CI 1.07–2.27; p=0.022). Patients on oral hypoglycemic therapy were not significantly different with regards to reaching the primary endpoint when directly compared with those on non-pharmacotherapy for diabetes (HR 1.07; 95% CI 0.75–1.51; p=0.721). The Kaplan-Meier estimate for the primary endpoint for patients on insulin therapy compared with oral hypoglycemic therapy was significant (HR 1.46; 95% CI 1.05–2.03; p=0.024).
Primary clinical endpoints by infusion arm according to diabetes therapy
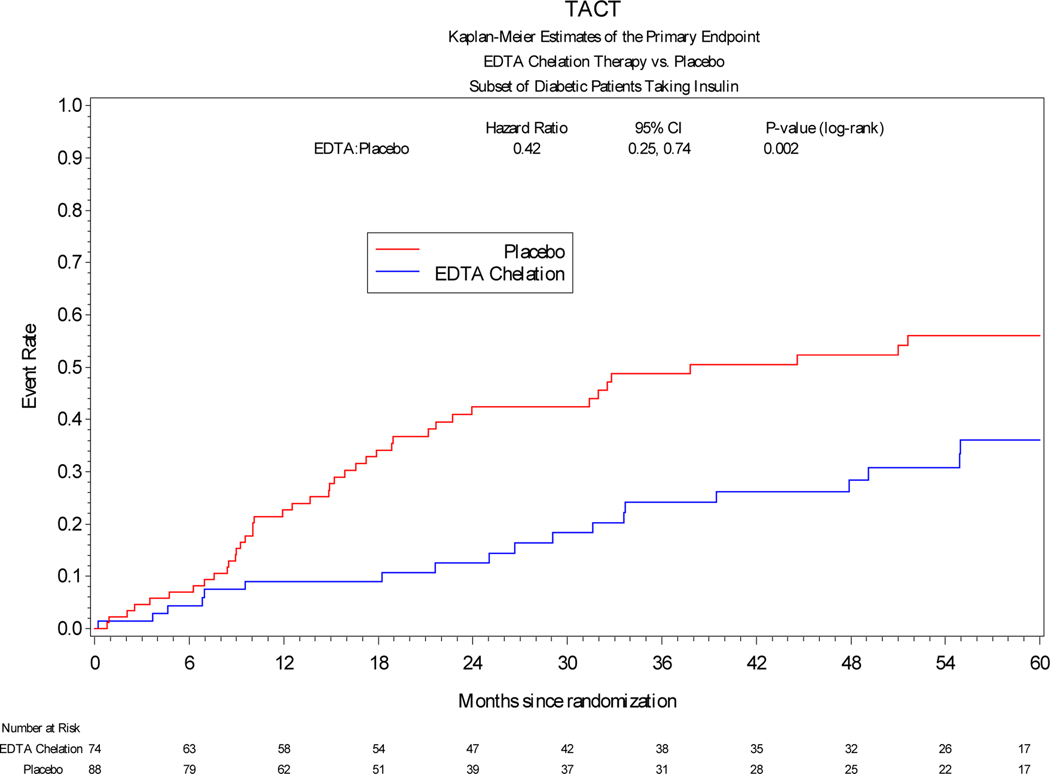
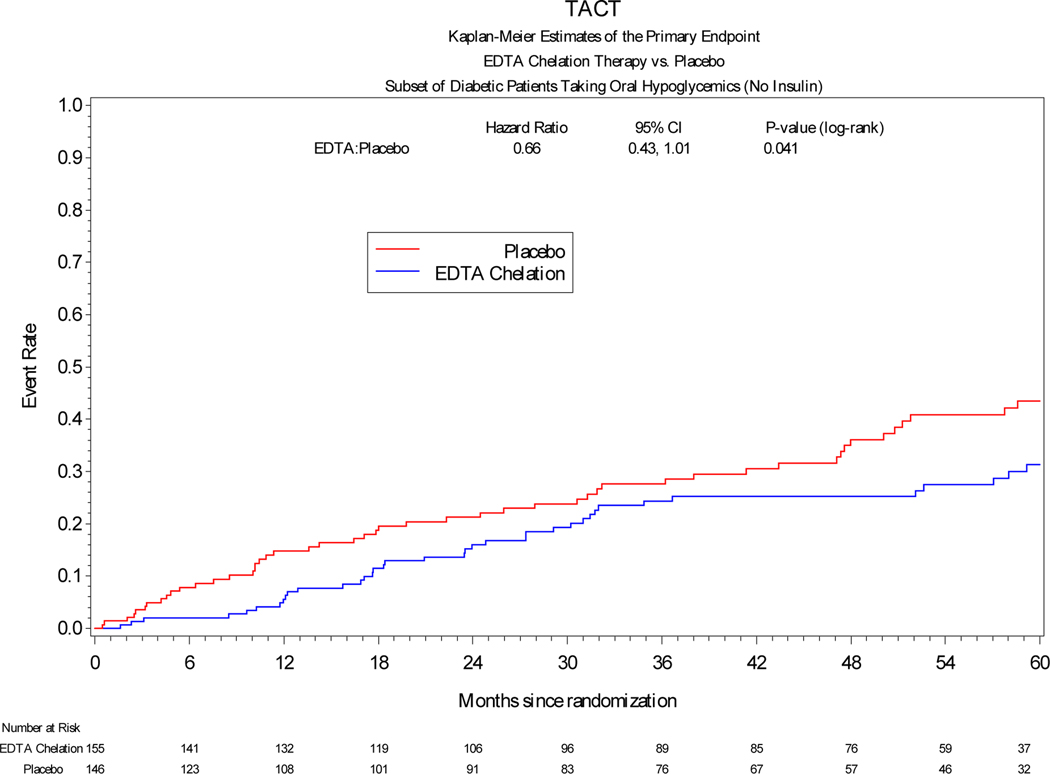
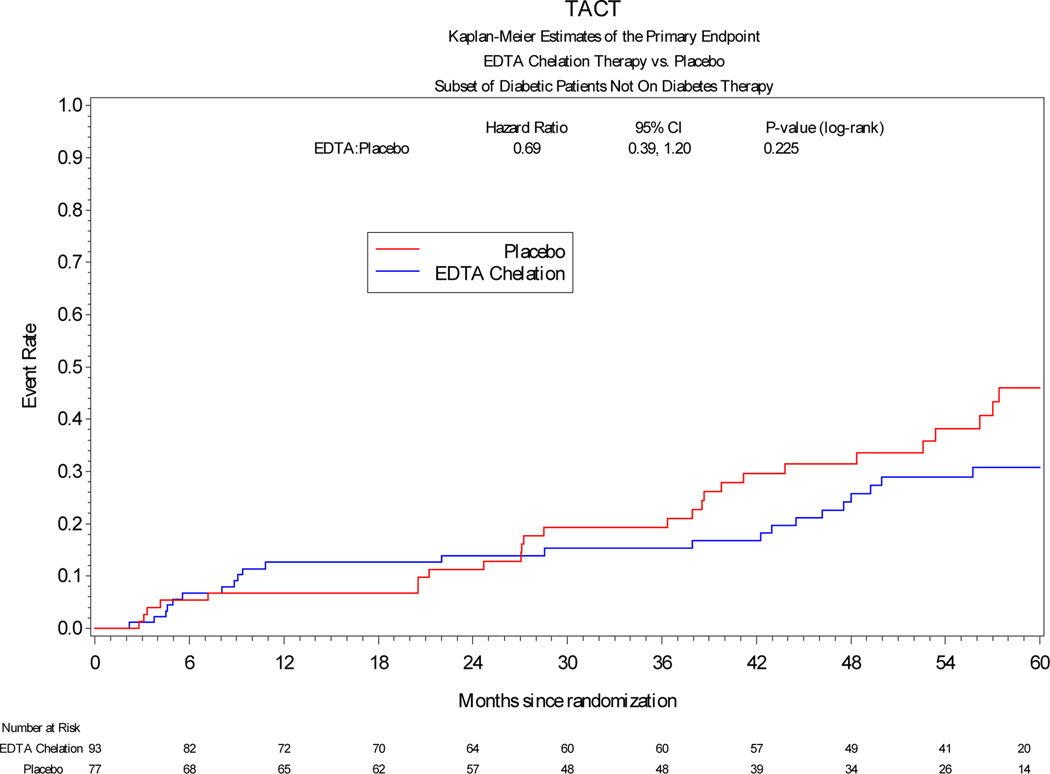
Among patients treated with insulin, the primary endpoint occurred significantly less frequently in patients receiving edetate disodium infusions vs placebo [19 (26%) vs 42 (48%), HR 0.42, 95% CI 0.25 to 0.74, log-rank p=0.002] (Table 3). The hazard ratio of the effect of active treatment on each of the components of the combined primary endpoint was directionally and quantitatively similar to the overall effect. Among patients treated with oral hypoglycemics, edetate disodium demonstrated a possible benefit compared with placebo [38 (25%) vs 49 (34%), HR 0.66, 95% CI 0.43–1.01, log-rank p=0.041]. Finally, among patients treated non-pharmacologically there was a directional effect favoring edetate disodium over placebo, but it was not statistically significant (HR 0.69, 95% CI 0.39–1.20, log-rank p=0.225). (Figure 1)
Table 3.
Primary endpoint and its individual components by infusion arm for patients with diabetes in the three groups according to their diabetes treatment.
| Insulin (N=162) | Oral hypoglycemic | No hypoglycemic therapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Edetate disodium (N=74) | Placebo (N=88) | Hazard Ratio (95% CI) | P–value | Edetate disodium (N=155) | Placebo (N=146) | Hazard Ratio (95% CI) | P–value | Edetate disodium (N=93) | Placebo (N=77) | Hazard Ratio (95% CI) | P–value | |
| Primary endpoint | 19 (26%) | 42 (48%) | 0.42 (0.25–0.74) | 0.002 | 38 (25%) | 49 (34%) | 0.66 (0.43–1.01) | 0.041 | 23 (25%) | 26 (34%) | 0.69 (0.39–1.20) | 0.225 |
| Death | 11 (15%) | 16 (18%) | 0.69 (0.32–1.50) | 0.359 | 12 (8%) | 22 (15%) | 0.46 (0.23–0.93) | 0.024 | 9 (10%) | 12 (16%) | 0.61 (0.26–1.44) | 0.265 |
| Myocardial Infarction | 4 (5%) | 12 (14%) | 0.37 (0.12–1.14) | 0.064 | 8 (5%) | 15 (10%) | 0.46 (0.19–1.08) | 0.071 | 4 (4%) | 3 (4%) | 1.12 (0.25–5.02) | 0.839 |
| Stroke | 0 | 1 (1%) | - | 0.289 | 3 (2%) | 1 (1%) | 2.50 (0.26–23.69) | 0.439 | 1 (1%) | 1 (1%) | 0.71 (0.04-11.31) | 0.806 |
| Coronary Revascularization | 10 (14%) | 21 (24%) | 0.49 (0.23–1.06) | 0.065 | 25 (16%) | 24 (16%) | 0.92 (0.53–1.61) | 0.732 | 13 (14%) | 17 (22%) | 0.57 (0.28–1.18) | 0.147 |
Figure 1:
Kaplan-Meier curves comparing edetate disodium chelation infusion groups versus placebo with respect to the primary endpoint in patients with diabetes in the three different subgroups according to their diabetes treatment.
Individual components of the primary endpoint
The comparison of active therapy with placebo across the 3 diabetes treatment groups is presented (Table 3). In brief, the sample size for all comparisons is small, and of course there are multiple comparisons presented. Nonetheless, the point estimate favors (HR <1) the edetate disodium group almost uniformly.
Interaction of diabetes treatment with chelation
The test for interaction to assess whether the effect of intravenous edetate disodium treatment was significantly different among the groups treated with insulin, hypoglycemic or non-pharmacologic therapy was not statistically significant (p=0.203).
DISCUSSION
We previously reported results from the diabetes subgroup of TACT, which demonstrated a marked, 41% (p=0.0002) relative risk reduction in combined cardiovascular outcomes, and a 43% reduction in all-cause death (p=0.011) with edetate disodium based infusions, a non-hypoglycemic treatment3. The present post-hoc analyses suggest, but do not confirm, a paradoxical gradient of benefit with edetate disodium-based therapy: those patients with the most severe diabetes, as defined by the requirement for insulin therapy at baseline, benefited the most (HR 0.42). The effect size of edetate disodium on patients with less severe diabetes, defined as not treated with insulin, was less marked. Patients treated with insulin indeed were more obese, had a higher prevalence of congestive heart failure, lower HDL cholesterol levels, and higher triglyceride levels compared with patients on oral hypoglycemics or non-pharmacologic treatment. Moreover, there are some suggestions that insulin-treated patients were more impacted by their diabetes, with higher glycemia, higher proteinuria, and a lower eGFR. Thus, we label the benefit paradoxical, since the patients with most severe disease demonstrated the greatest benefit from edetate disodium treatment. If future studies support these findings, the public health implications would be significant.
The mechanism by which edetate disodium chelation benefits patients with diabetes, particularly patients treated with insulin is unknown3. Edetate disodium does not appear to enhance control of glycemia. Although the overall TACT study did not collect glycated hemoglobin, there was absolutely no difference in fasting glucose during infusions between treatment groups in the overall diabetes study. Indeed, the likelihood that glycemic control would account for the marked reduction in events in insulin-treated patients seems implausible, since most of the large randomized clinical trials of glucose-lowering therapies in patients with type 2 diabetes and a history of cardiovascular disease have shown minimal to no effect on macrovascular endpoints, and in some instances, adverse outcomes8.
Edetate disodium is an artificial amino acid with a high affinity for divalent cations such as calcium, lead and cadmium. Epidemiologic data linking cadmium and lead exposure to cardiovascular disease are robust9. Lanphear et al in 2018 reported that low-level lead is associated with an increased risk of cardiovascular mortality10. Similarly, an increase in urinary cadmium levels has been associated with worsening vascular disease11–14. The adverse cardiovascular effects of cadmium may be enhanced by the presence of diabetes11–14. Hypotheses focus on oxidative stress and on the vulnerability of patients with diabetes to oxidative stress from toxic environmental pollutants, such as lead and cadmium. There is some evidence that toxic metals are atherogenic. Mechanisms may involve endothelial dysfunction by an increase in inflammation and reactive oxygen species11,16.
We have previously reviewed biological pathways of toxic metal-induced vascular disease17. Toxic metals increase oxidative stress by shared mechanisms, but each also poisons cellular metabolism through additional idiosyncratic processes. Lead exposure has been associated with an increased prevalence of chronic kidney disease18. Thus, metal chelation with a regimen of edetate disodium may reduce direct end-organ toxicity and decrease metal-catalyzed tissue oxidation by decreasing total body metal burden. These mechanisms may be particularly beneficial in patients with diabetes, who live with enhanced oxidative stress19. The diabetes literature suggests that complications of diabetes are partially mediated through the accumulation of advanced glycation end-products that upregulate the inflammatory cascade in target organs, promoting enhanced oxidative stress and accelerated atherosclerosis20. Insulin infusion in cultured renal cells increased SGLT-2 activity incorporating glucose into the cells via oxidative stress generation and potentiating apoptotic sensitivity of cells to advanced glycation end products21. Toxic metals may increase the formation of reactive oxygen species in a non-enzymatic reaction, enhancing the formation of advanced glycation end-products19. Moreover, cadmium and lead have been associated with epigenetic changes22,23. Thus, it is a plausible hypothesis that environmentally acquired toxic metals are vasculotoxic and patients with diabetes are a particularly vulnerable population.
The strong signal of benefit appears to focus the beneficial effect of edetate disodium chelation on patients with diabetes treated with insulin therapy, suggesting that the antioxidant effect of toxic metal chelation may represent an important emerging strategy. Based in this hypothesis a new confirmatory randomized clinical trial of edetate disodium chelation therapy in patients with diabetes and prior myocardial infarction (TACT 2) is ongoing with long-term follow-up, mechanistic components, and a biorepository.
This post-hoc data-driven analysis has many limitations. First, although the diabetes group in TACT was pre-specified, subgroups based on hypoglycemic strategies were not. TACT did not collect data on glycated hemoglobin, and a possible mild effect on glucose control cannot be excluded. The formal test of interaction of edetate disodium with hypoglycemic strategy was not significant, but the sample sizes were small. TACT was conducted before the availability of SGLT-2 inhibitors, so the interaction with these important agents could not be tested. Other limitations have been addressed in the main publication of TACT and in the pre-specified analyses of the overall diabetic TACT population2–3. The analyses presented here are interesting, but solely hypothesis-generating until reproduced.
Conclusion
These analyses suggest that edetate disodium-based infusions may have a more pronounced beneficial effect on insulin-treated post-myocardial infarction patients with more severe diabetic disease as defined by clinical treatment strategy. Although the implications are interesting, it is important to note that these subgroup analyses were not pre-specified. Thus, these results must be considered hypothesis generating, and requiring corroboration in future studies, such as the ongoing TACT2.
Highlights.
Patients with diabetes treated with insulin showed a marked benefit with edetate disodium treatment, less marked benefit in the less diseased patient with oral hypoglycemic therapy and similarly without pharmacotherapy for diabetes.
We published in 2014 that post-MI patients with diabetes demonstrated a large risk reduction in combined coronary events over 5 years when treated with edetate disodium-based chelation infusions. In this paper, we identify the relationship between clinically guided treatment for diabetes and the benefit of edetate disodium-based treatment.
In spite of greater atherosclerotic burden, post-MI patients with diabetes treated with insulin randomized to edetate disodium-based therapy demonstrated a significant reduction in combined cardiovascular events over 5 years.
Edetate disodium therapy should be considered for post-MI patients with insulin.
ACKNOWLEDGEMENTS
We are particularly indebted to the coordinators at the TACT sites that collected data and to the patients who agreed to participate in TACT.
FUNDING SOURCES
Supported by grants (U01 H092607) from the National Heart, Lung, and Blood Institute and (U01 AT001156) from the National Center for Complementary and Alternative Medicine, National Institutes of Health, Bethesda, MD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Center for Complementary and Alternative Medicine, or the National Institutes of Health.
Footnotes
Clinical Trial Registration: clinicaltrials.gov identifier:
http://clinicaltrials.gov/ct2/show/NCT00044213?term=TACT&rank=7 identifier Trial to Assess Chelation Therapy (TACT), NCT00044213
DISCLOSURES
There are no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157: DOI: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J, Lee KL; Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013; 309:1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escolar E, Lamas GA, Mark DB, Boineau R, Goertz C, Rosenberg Y, Nahin RL, Ouyang P, Rozema T, Magaziner A, Nahas R, Lewis EF, Lindblad L, Lee KL. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014. Jan;7(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddle MC, Ambrosius WT, Brillon DJ, et al. , and the Action to Control Cardiovascular Risk in Diabetes Investigators. Epidemiologic relationships between A1C and all-cause mortality during a median 3·4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010; 33: 983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Drisko JA, Lee KL. Design of the Trial to Access Chelation Therapy. Am Heart J. 2012;163(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 7.Cox DR. Regression models and life-tables (with discussion). J Royal Statist Soc B 1972;34:187–220. [Google Scholar]
- 8.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aneni EC, Escolar E, Lamas GA. Chronic Toxic Metal Exposure and Cardiovascular Disease: Mechanisms of Risk and Emerging Role of Chelation Therapy. Curr Atheroscler Rep. 2016. Dec;18(12):81. [DOI] [PubMed] [Google Scholar]
- 10.Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health. 2018;3:e177–84. [DOI] [PubMed] [Google Scholar]
- 11.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, et al. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep. 2013;15:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menke A, Muntner P, Silbergeld EK, et al. Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect. 2009;117:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking and increased risk of peripheral arterial disease. Circulation. 2004;109(25):3196–201. [DOI] [PubMed] [Google Scholar]
- 14.Ujueta F, Arenas IA, Diaz D, Yates T, Beasley R, Navas-Acien A, Lamas GA. Cadmium level and severity of peripheral artery disease in patients with coronary artery disease. Eur J Prev Cardi.2018. 10.1177/2047487318796585 [DOI] [PubMed] [Google Scholar]
- 15.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, et al. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep. 2013;15:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, Shen YH, Zeller I, Willeit J, Laufer G, Wick G, Kiechl S, Bernhard D. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol. 2009;29:1392–1398. [DOI] [PubMed] [Google Scholar]
- 17.Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J. 2014. Dec;168(6):812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Li L, Lee JP. Environmental-wide association study on CKD in the National Health and Nutrition Examination Study (1999–2016) [Meeting Abstract]. Kidney Week.2019.https://www.asnonline.org/education/kidneyweek/2019/programabstract.aspx?controlId=3232210. Accessed 3/31/20.
- 19.Frizzell N, Baynes JW. Chelation therapy: overlooked in the treatment and prevention of diabetes complications? Future Med Chem. 2013;5:1075–1078. [DOI] [PubMed] [Google Scholar]
- 20.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura N, Matsui T, Ishibashi Y, Yamagishi S. Insulin stimulates SGLT2-mediated tubular glucose absorption via oxidative stress generation. Diabetol Metab Syndr. 2015. May 24;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grau-Perez M, Pichler G, Galan-Chilet I, Briongos-Figuero LS, Rentero-Garrido P, Lopez-Izquierdo R, Navas-Acien A, Weaver V, García-Barrera T, Gomez-Ariza JL, Martín-Escudero JC, Chaves FJ, Redon J, Tellez-Plaza M. Urine cadmium levels and albuminuria in a general population from Spain: A gene-environment interaction analysis. Environ Int. 2017. May 27;106:27–36. [DOI] [PubMed] [Google Scholar]
- 23.Fragou D, Fragou A, Kouidou S, Njau S, Kovatsi L. Epigenetic mechanisms in metal toxicity. Toxicology Mechanisms and Methods. 2011;21(4):343–352. [DOI] [PubMed] [Google Scholar]