Abstract
Background
To examine the association between regular use of proton pump inhibitors and the risk of incident dementia, including dementia subtypes, and whether the association differs between APOE genotypes.
Methods
Based on a prospective analysis of data from the UK Biobank, 501,002 individuals (female, 54.4%) aged between 40 and 70 years, who had no prevalent dementia at baseline, were enrolled between 2006 and 2010 and followed up to 2018. We compared all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) incidence rates between proton pump inhibitor users and non-users by the Cox proportional hazard model.
Results
During 4,438,839 person-years of follow-up (median length of follow-up, 9.0 years), there were 2505 incident cases of all-cause dementia, including 932 cases of AD and 524 cases of VaD. The incident rate of all-cause dementia among proton pump inhibitor users was 1.06 events per 1000 person-years, compared with 0.51 events per 1000 person-years among non-users. After adjustment for multiple confounders and indications, the hazard ratios (HRs) of the proton pump inhibitor users were 1.20 (95% CI, 1.07–1.35) for incident all-cause dementia, 1.23 (95% CI, 1.02–1.49) for incident AD, and 1.32 (95% CI, 1.05–1.67) for incident VaD. In addition, the association between proton pump inhibitor use and all-cause dementia differed by APOE genotype (P for interaction = 0.048). Among APOE ε4 heterozygotes, the fully adjusted HR of proton pump inhibitor use was 1.46 (95% CI, 1.22–1.75) and 1.68 (95% CI, 1.36–2.07), especially for individuals aged 65 years and older.
Conclusions
The finding of this large population-based cohort study indicates that the use of proton pump inhibitors is associated with an increased risk of incident dementia, particularly among APOE ε4 heterozygotes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02478-y.
Keywords: Proton pump inhibitors, Dementia, Alzheimer’s disease, APOE, Amyloid-β
Background
Proton pump inhibitors (PPIs) are widely used in the treatment of gastric acid-related disorders such as peptic ulcer disease, gastroesophageal reflux disease (GERD), non-steroidal anti-inflammatory drug (NSAID)-associated ulcers, and eradication of Helicobacter pylori [1]. In many countries, including the UK, some PPIs are available for over-the-counter purchase, which increases public accessibility. Furthermore, PPIs are often prescribed in and out of the hospital for incorrect indications or long-term use that does not meet guidelines [2, 3]. With the increasing use of PPIs [4], more attention has been paid to the research on its side effects [5]. A series of studies have reported associations between PPI use and cardiovascular disease [6, 7], fracture [8], kidney disease [9], infections [10], and diabetes [11], but the association with dementia is controversial [12].
Dementia is characterized by inexorably progressive impairment in cognitive and independent living functions. Alzheimer’s disease (AD), vascular dementia (VaD), Lewy body, and frontotemporal dementia are the most common pathologies. It is estimated that there were 35.6 million dementia patients worldwide in 2010, and the number may reach 65.7 million in 2030 [13]. Meanwhile, the worldwide costs of dementia were estimated at $818 billion in 2015 [14]. To prevent dementia, reducing risk factor exposure is vital in the circumstance of limited treatment. Several cohort studies reported the association between PPI use and all-cause dementia or AD among the elderly, and the hazard ratios (HRs) of PPI users were 1.38 to 1.44 [15, 16]. However, other studies showed conflicting conclusions, and most of these studies did not observe any associations [17–22]. Therefore, the association between PPI use and dementia is still uncertain.
PPIs are aimed to reduce the gastric acid secretion of the parietal cell by inhibiting (H(+), K(+))-ATPase [23]. Similar enzymes are also found in microglia lysosomes [24], and the lysosomal acidic environment is essential for amyloid-β (Aβ) clearance, the disorder of which may lead to neurodegeneration and dementia [25]. A study reveals that PPIs may increase Aβ deposition in the mouse brain by affecting the β- and γ-secretases [26]. However, precision measurement of Aβ metabolism in a large population would be difficult. A measurable proxy for Aβ is required to infer whether the PPIs promote dementia via affecting Aβ metabolism.
Apolipoprotein E (Apo-E) is a primary cholesterol carrier involved in lipid transport, and APOE ε4 alleles are the main genetic risk factors for AD and dementia due to their reduced capacity for Aβ transport [27]. APOE ε4 may also promote AD by reducing the ability of astrocytes to remove toxic fatty acids from the extracellular milieu [28]. Possible mechanisms for the potential association between PPIs and dementia and whether PPIs can interact with APOE require evidence from population-based studies.
To further explicitly whether regular PPI use is associated with incident all-cause dementia and pathological specific dementia (AD and VaD), we conducted a large prospective cohort study in the UK Biobank. Furthermore, we also tried to explore the differences in the associations among different APOE ε4 genotypes, a potential regulatory gene of Aβ metabolism, to suggest biological mechanisms.
Results
Participant characteristics
Table 1 presents the baseline characteristics of eventually included participants stratified by PPI users or non-users. Of the 501,002 individuals (mean [SD] age, 56.5 [8.1] years), 272,605 (54.4%) were female and 53,735 (10.7%) were regular PPI users (Fig. 1). The regular users were slightly older, had higher BMIs, more smoking exposure, less alcohol consumption, and more comorbidity and regular drug use.
Table 1.
Baseline characteristics
| Characteristics | No. (%)a | |
|---|---|---|
| PPI non-users | PPI users | |
| Total | 447,267 | 53,735 |
| Age (years), mean (SD) | 56.2 (8.1) | 59.4 (7.3) |
| Sex | ||
| Female | 243,253 (54.4) | 29,352 (54.6) |
| Male | 204,014 (45.6) | 24,383 (45.4) |
| Ethnicity | ||
| White | 422,581 (94.5) | 51,240 (95.4) |
| Others | 24,686 (5.5) | 2495 (4.6) |
| Education | ||
| Higher | 220,614 (49.3) | 20,767 (38.6) |
| Upper secondary | 39,176 (8.8) | 3660 (6.8) |
| Lower secondary | 92,519 (20.7) | 11,203 (20.8) |
| Vocational | 22,670 (5.1) | 3563 (6.6) |
| Others | 72,288 (16.2) | 14,542 (27.1) |
| Household income (£) | ||
| <18,000 | 99,929 (22.3) | 18,700 (34.8) |
| 18,000–30,999 | 114,099 (25.5) | 14,956 (27.8) |
| 31,000–51,999 | 117,645 (26.3) | 11,560 (21.5) |
| 52,000–100,000 | 91,382 (20.4) | 6918 (12.9) |
| >100,000 | 24,212 (5.4) | 1601 (3.0) |
| Townsend deprivation index, median [interquartile range] | −2.2 [−3.7, 0.5] | −1.8 [−3.5, 1.2] |
| Body mass index (kg/m2), mean (SD) | 27.2 (4.7) | 29.1 (5.1) |
| Regular physical activity | ||
| No | 185,029 (41.4) | 25,213 (46.9) |
| Yes | 262,238 (58.6) | 28,522 (53.1) |
| Smoking status | ||
| Never | 249,076 (55.7) | 25,226 (46.9) |
| Former | 150,929 (33.7) | 22,604 (42.1) |
| Current | 47,262 (10.6) | 5905 (11.0) |
| Alcohol consumption (g/day) | ||
| 0 | 107,342 (24.0) | 17,191 (32.0) |
| 0.01–13.99 | 167,958 (37.6) | 18,331 (34.1) |
| 14–27.99 | 96,157 (21.5) | 9685 (18.0) |
| ≥28 | 75,810 (16.9) | 8528 (15.9) |
| Occupational exposure | ||
| Rarely/never | 355,224 (79.4) | 43,967 (81.8) |
| Sometimes | 57,614 (12.9) | 5383 (10.0) |
| Often | 34,429 (7.7) | 4385 (8.2) |
| Health conditions | ||
| Hypertension | 110,673 (24.7) | 22,237 (41.4) |
| Coronary heart disease | 16,831 (3.8) | 7377 (13.7) |
| Diabetes | 20,061 (4.5) | 5362 (10.0) |
| High cholesterol | 49,626 (11.1) | 11,814 (22.0) |
| Stroke | 5395 (1.2) | 1802 (3.4) |
| Traumatic brain injury | 1393 (0.3) | 222 (0.4) |
| Depression | 22,812 (5.1) | 5294 (9.9) |
| Anxiety | 5539 (1.2) | 1160 (2.2) |
| Sleep apnea | 1240 (0.3) | 368 (0.7) |
| Cancer | 33,592 (7.5) | 5739 (10.7) |
| GERD | 5183 (1.2) | 15,758 (29.3) |
| Barrett’s esophagus | 237 (0.1) | 1231 (2.3) |
| Gastroduodenal ulcer | 2765 (0.6) | 2840 (5.3) |
| Regular use of supplement or drugs | ||
| Statin | 68,147 (15.2) | 18,474 (34.4) |
| Antihypertensive drugs | 83,931 (18.8) | 19,782 (36.8) |
| Anticholinergic drugs | 38,032 (8.5) | 11,747 (21.9) |
| Benzodiazepines | 2424 (0.5) | 1013 (1.9) |
| z-Hypnotics | 1458 (0.3) | 611 (1.1) |
| Aspirin | 56,812 (12.7) | 12,648 (23.5) |
| Non-aspirin NSAIDs | 128,374 (28.7) | 20,366 (37.9) |
| Multivitamin | 139,534 (31.2) | 17,859 (33.2) |
| H2RAs | 7533 (1.7) | 1921 (3.6) |
| APOE genotype | ||
| APOE ε4 −/− | 310,111 (71.5) | 37,676 (72.3) |
| APOE ε4 +/− | 113,253 (26.1) | 13,280 (25.5) |
| APOE ε4 +/+ | 10,367 (2.4) | 1169 (2.2) |
Abbreviations: PPI proton pump inhibitor, SD standard deviation, GERD gastroesophageal reflux disease, NSAIDs non-steroidal anti-inflammatory drugs, H2RAs H2 receptor antagonists, APOE apolipoprotein E
aAll variables globally significantly different between groups at P < 0.001, except for sex (P = 0.299)
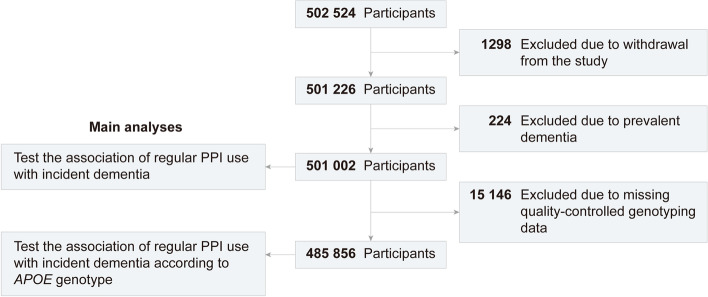
Fig. 1.
Flowchart of participant enrolment. Abbreviations: PPI, proton pump inhibitor; APOE, apolipoprotein E
Associations of PPI use with dementia outcomes
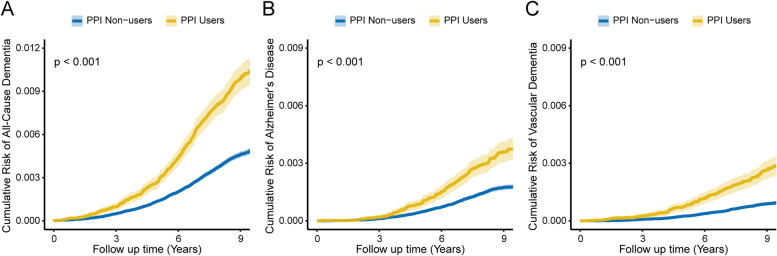
Over 4,438,839 person-years of follow-up (median [interquartile range] length of follow-up, 9.0 [8.3–9.5] years), there were 2505 incident cases of all-cause dementia, including 932 cases of AD and 524 cases of VaD. The incident rate of all-cause dementia among PPI users was 1.06 events per 1000 person-years, compared with 0.51 events per 1000 person-years among non-users. The basic multivariable models found significant associations between PPI use and increased all-cause and cause-specific dementia risks (Table 2). After additional adjustment for clinical indications, the HRs of the PPI users were 1.20 (95% confidence interval [CI], 1.07–1.35; P = 0.001) for incident all-cause dementia, 1.23 (95% CI, 1.02–1.49; P = 0.031) for incident AD, and 1.32 (95% CI, 1.05–1.67; P = 0.017) for incident VaD. Figure 2 shows the cumulative risk of incident all-cause and cause-specific dementia in each PPI use status during follow-up (all P < 0.001).
Table 2.
Associations of regular PPI use with incident dementia
| Outcomes | PPI non-users (n = 447,267) | PPI users (n = 53,735) | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|---|---|
| No. of events (%) | No. of events (%) | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| All-cause dementia | 2008 (0.45) | 497 (0.92) | 1.49 (1.35–1.65) | <0.001 | 1.17 (1.05–1.29) | 0.003 | 1.20 (1.07–1.35) | 0.001 |
| Alzheimer’s disease | 752 (0.17) | 180 (0.33) | 1.41 (1.20–1.66) | <0.001 | 1.19 (1.00–1.41) | 0.045 | 1.23 (1.02–1.49) | 0.031 |
| Vascular dementia | 392 (0.09) | 132 (0.25) | 1.99 (1.63–2.42) | <0.001 | 1.32 (1.07–1.62) | 0.009 | 1.32 (1.05–1.67) | 0.017 |
Abbreviations: PPI proton pump inhibitor, HR hazard ratio, CI confidence interval
aModel 1: Cox proportional hazards regression adjusted for age and sex
bModel 2: Cox proportional hazards regression adjusted for model 1 and ethnicity, education, household income, Townsend deprivation index, smoking status, alcohol consumption, physical activity, BMI, occupational exposure, hypertension, coronary heart disease, diabetes, high cholesterol, stroke, traumatic brain injury, depression, anxiety, sleep apnea, cancer, and regular use of medications (statin, antihypertensive drugs, anticholinergic drugs, benzodiazepines, z-hypnotics, aspirin, non-aspirin NSAIDs, and multivitamin)
cModel 3: Cox proportional hazards regression adjusted for model 2 and GERD, Barrett’s esophagus, gastroduodenal ulcer, and regular H2RAs use
Fig. 2.
The cumulative risk of incident all-cause dementia (A), Alzheimer’s disease (B), and vascular dementia (C) according to regular PPI use. Abbreviation: PPI, proton pump inhibitor
Subgroup analyses
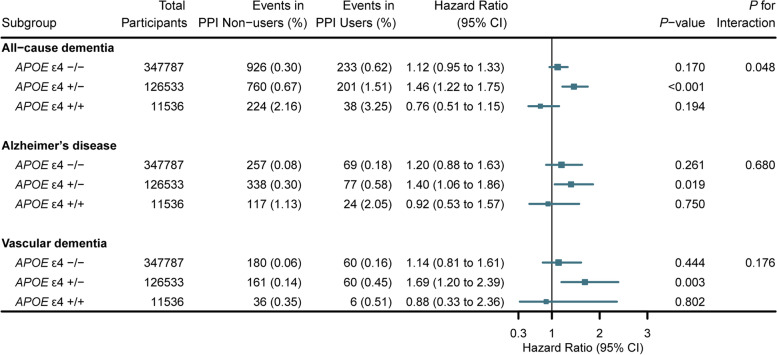
To verify whether the APOE gene played a role as an effect modifier in the PPI use and dementia associations, we conducted subgroup analysis in different APOE ε4 genotypes by the fully adjusted model and tested the interactions. The association between PPI use and incident all-cause dementia was observed particularly among the APOE ε4 heterozygous (+/−) population (HR, 1.46; 95% CI, 1.22–1.75; P < 0.001), and the interaction was statistically significant (P for interaction = 0.048; Fig. 3). Additional file 1: Fig. S1 shows the cumulative risk of incident dementia in each PPI use status among different APOE ε4 genotype groups, and Additional file 1: Fig. S2 shows the combined effects of PPI use and APOE ε4 on the risk of dementia.
Fig. 3.
Association of regular PPI use with incident dementia stratified by APOE genotype. The vertical line indicates the reference value of 1. Estimated effects were based on the fully adjusted model. Abbreviations: PPI, proton pump inhibitor; APOE, apolipoprotein E; HR, hazard ratio; CI, confidence interval
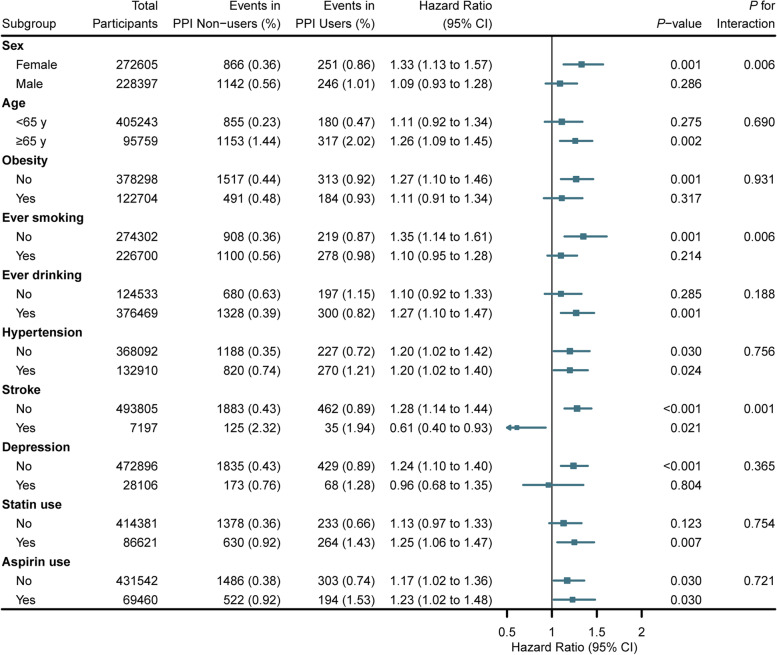
Furthermore, we conducted subgroup analyses according to other potential modifying factors. Regular PPI use and all-cause dementia associations were stronger among females, never smokers, and participants without stroke (all P for interaction < 0.05; Fig. 4). The combined effects of PPI use and significant modifying factors are shown in Additional file 1: Fig. S3. In addition, the associations of regular PPI use with cause-specific dementia were strong among females for AD and participants without stroke for VaD (all P for interaction < 0.05; Additional file 1: Fig. S4, S5). When each PPI was analyzed separately (Additional file 1: Table S3), the associations with all-cause dementia persisted in lansoprazole (HR, 1.26; 95% CI, 1.07–1.48; P = 0.007).
Fig. 4.
Association of regular PPI use with incident all-cause dementia stratified by potential risk factors. The vertical line indicates the reference value of 1. Estimated effects were based on the fully adjusted model. Abbreviations: PPI, proton pump inhibitor; HR, hazard ratio; CI, confidence interval
Sensitivity analyses
We excluded participants younger than 65 years at baseline for sensitivity analysis to verify the effect modifier role of APOE ε4. The results showed that the interaction between PPI use and APOE ε4 genotype was still present for all-cause dementia (P for interaction = 0.012; Additional file 1: Fig. S6), and the HR of PPI users among APOE ε4 heterozygote was 1.68 (95% CI, 1.36–2.07; P < 0.001). Also, in the APOE ε4 heterozygotes, the HRs of PPI users were 1.55 (95% CI, 1.12–2.15; P = 0.008) and 1.80 (95% CI, 1.21–2.68; P = 0.004) for incident AD and VaD, respectively. However, interaction tests did not reach the statistically significant level (P for interaction > 0.05).
Results showed no significant change in PPI use and incident dementia associations when we excluded participants who developed dementia outcomes within the first 2 years of follow-up (Additional file 1: Table S4), excluded participants with missing values for covariates (Additional file 1: Table S5), and excluded participants who developed outcomes that were recorded on the death register data only (Additional file 1: Table S6). We included all covariates and used logistic regression to construct propensity scores with a c-statistic of 0.815 (95% CI, 0.813–0.817). The propensity score matching analysis results were consistent with the main model (Table 3).
Table 3.
Associations of regular PPI use with incident dementia in propensity score matching model
| Outcomes | PPI non-users (n = 107,470) | PPI users (n = 53,735) | PSM model 3a | |
|---|---|---|---|---|
| No. of events (%) | No. of events (%) | HR (95% CI) | P-value | |
| All-cause dementia | 907 (0.84) | 497 (0.92) | 1.22 (1.08–1.37) | 0.001 |
| Alzheimer’s disease | 321 (0.30) | 180 (0.33) | 1.25 (1.02–1.52) | 0.028 |
| Vascular dementia | 223 (0.21) | 132 (0.25) | 1.30 (1.03–1.65) | 0.028 |
Abbreviations: PPI proton pump inhibitor, PSM propensity score matching, HR hazard ratio, CI confidence interval
aPropensity scores were derived from logistic regression, which included age, sex, ethnicity, education, household income, Townsend deprivation index, smoking status, alcohol consumption, physical activity, BMI, occupational exposure, hypertension, coronary heart disease, diabetes, high cholesterol, stroke, traumatic brain injury, depression, anxiety, sleep apnea, cancer, GERD, Barrett’s esophagus, gastroduodenal ulcer, and regular use of medications (statin, antihypertensive drugs, anticholinergic drugs, benzodiazepines, z-hypnotics, aspirin, non-aspirin NSAIDs, multivitamin, and H2RA use)
Discussion
In this population-based prospective cohort study of half a million participants, we found that regular PPI use was associated with an increased risk of incident all-cause dementia, AD, and VaD. Meanwhile, we found an interaction between PPI use and APOE ε4 genotype for all-cause dementia, and the association was more significant among APOE ε4 heterozygotes.
Our results were consistent with the previous studies that reported the association between PPI use and increased risk of dementia [15, 16, 29]. The study, which followed 70,000 participants over 75 years of age for 7 years, showed that PPI users had 1.44 times the risk of incident dementia as non-users [16]. Another study of more than 15,000 participants over 40 years of age without prevalent dementia followed for 8.44 years found that the adjusted HR for PPI users was 1.22 [29]. However, a separate series of observational studies reported that the associations were absent [19–22]. For example, a prospective study including over 70,000 participants showed that PPI use was not associated with dementia [21]. A systematic review and meta-analysis pooling 11 observational studies did not observe the association between short-term PPI use and dementia [30]. After that, the current study of more than 500,000 participants suggests that significant associations with incident dementia were still emerging with regular PPI use after adjusting for a wide range of lifestyle, comorbidity, and clinical indications. To our knowledge, this is the most extensive prospective study of PPI-dementia associations in the general population while providing some validation of the possible biological mechanisms of the association. Therefore, this study offers high-quality population-based evidence to assess the side effects accompanying regular PPI use.
Aβ aggregation to form plaques triggers neuronal dysfunction and death in the brain, which is the critical pathological feature of AD [31]. Studies of mouse models showed that PPIs might cross the blood-brain barrier [32, 33] and exacerbate Aβ production [26] to promote the development of dementia. Another mechanism was that the PPIs increase the accumulation of fibrillar Aβ by inhibiting the acidification of the degradation process in microglia [34, 35]. Aβ clearance from the brain requires the involvement of membrane cholesterol, and glial-derived APOE is a critical cholesterol transporter in the brain [31]. APOE ε4 is a determining risk factor of AD by promoting Aβ aggregation, associated with a 4-fold increased risk for a single allele [36]. We unprecedentedly reported the interaction between PPI use and APOE ε4 genotype in dementia risk. Compared to the APOE ε4 noncarrier, the risk of dementia among ε4 heterozygotes may be further amplified with regular PPI use. We speculated that PPIs might affect Aβ metabolism and synergize with the APOE ε4 to promote Aβ accumulation and increase dementia risk. PPIs may reduce lysosomal acidification by inhibiting V-ATPase activity, which is critical for Aβ clearance [25, 37].
Notably, the association between PPI use and dementia was not presented among APOE ε4 homozygotes. The APOE ε4 homozygote is a validated risk factor with significant effects, and its HRs of all-cause dementia and AD were 6.93 (95% CI, 6.05–7.92; P < 0.001) and 12.91 (95% CI, 10.59–15.75; P < 0.001) in this study. We hypothesized that the ε4 homozygotes are more likely with high loading of Aβ level, which may mask the relatively modest effects of PPI use by the mechanism like the epistasis effect [38]. When we investigated the combined effect of PPI use and APOE ε4, the results showed a significantly increased risk of dementia in ε4 homozygotes, regardless of whether they used PPI or not (Additional file 1: Fig. S2). In addition, PPIs may promote dementia by inducing vitamin B12 deficiency [39] or inhibiting choline acetyltransferase [40], but this has not been verified in this study.
Sex factors play an unavoidable role in the development of dementia. This study showed that the PPI-dementia association was more pronounced in females. Previous studies reported that females are more likely to develop dementia due to carrying APOE ε4 [41], which may be explained by the increased sensitivity of females to Aβ [42]. Thus, based on the hypothesis that PPIs promote dementia by increasing Aβ accumulation, we speculated that PPIs would synergize with the high Aβ sensitivity to increase the risk of dementia among females. In addition, the results of the subgroup analysis also suggested that the association between PPI use and dementia was more substantial in the non-smokers and participants without stroke. Smoking and stroke are often concomitant with cerebral oxidative stress and vascular inflammation, which are potential mechanisms for increased risk of AD [43]. Meanwhile, functional studies on primary human tissues and animal models showed PPIs had antioxidant and anti-inflammatory properties [44]. Therefore, we speculate that PPI use may neutralize the risk effect of smoking and stroke.
Our results showed that the association between different types of PPIs and dementia might differ, with lansoprazole being associated with dementia with greater strength than omeprazole at a relatively close statistical power. Consistent with earlier studies, results based on the AD cell model showed that the increase in Aβ levels after lansoprazole stimulation was more pronounced than omeprazole [29]. Lansoprazole also profoundly limits the retention of spatial information and the capacity to manipulate remembered memory to develop a strategy and execute a complex task [45]. In addition, there were more adverse effects of headaches after lansoprazole use [46]. Therefore, we believe that attention should be paid to the potential differences in PPIs in the nervous system.
Our study has several significant strengths, including the prospective population-based study design, the large sample size, and detailed information on related covariates, which provided adequate confounding adjustment and robust statistical power. In addition, individual genotype data set the stage for investigating drug-gene interactions. Thus, we demonstrated that PPI use and dementia associations might vary across APOE ε4 genotypes for the first time.
Some limitations should also be considered. First, PPI use was self-reported at baseline, and accurate dosage, duration, and validation by other sources were lacking. These may lead to recall bias and obscure within-group heterogeneity. This issue obstructed us from performing further analyses on these important factors. The primary exposure was based on data from a single baseline assessment only, and it cannot be excluded that a few participants only used the PPIs for a short period around the survey. Second, PPI use was not randomly assigned. Although we corrected for as many confounding factors and clinical indications as available, there may still be unmeasured confounding. Third, dementia consists of a complex set of symptomatic, and there may be diagnostic inaccuracies through ICD coding in electronic health records, while information on severity may be lost [47]. Due to the high under-diagnosis in the natural population, defining dementia based on hospital admissions and death registers may lead to missed diagnoses, and recorded dementia in these systems is often in an advanced stage. Besides, participants with comorbidities and prescription of PPI may have more contact with the health system and thus have a greater chance of being diagnosed with dementia. Fourth, considering the interpretability of the biological mechanisms, only one genetic risk factor, APOE ε4, was included in this study. In contrast, dementia and AD have complex genetic susceptibility factors, and the Aβ metabolism has complex regulatory mechanisms, and these may be the effect modifiers on the role of PPIs. Fifth, the UK Biobank study population may have intrinsic characteristics and limit the generalization of the results to other populations or nations.
Conclusions
In conclusion, this population-based cohort study showed that regular PPI use was associated with an increased risk of incident all-cause dementia, AD, and VaD. Moreover, there was a significant interaction between PPI use and APOE ε4 genotype for dementia, and the association was most prominent in APOE ε4 heterozygotes. This study reveals prospective evidence and a potential mechanism for an association between PPI use and dementia, which requires further controlled trials and experimental studies to verify the causal relationship.
Methods
Study design
The UK Biobank study recruited more than 500,000 participants aged 40 to 70 years from the general population throughout the UK between 2006 and 2010 [48]. Participants provided information on health-related aspects through extensive baseline questionnaires, verbal interviews, and physical measurements. Participants were excluded if they withdrew from the study (n = 1298) and had prevalent dementia (n = 224). Then, we excluded 15,146 participants due to missing quality-controlled genotype data for subsequent analysis (Fig. 1).
Ascertainment of exposure
The regular use of medications was collected through a verbal interview by a trained nurse at the baseline. “Regular” was defined as most days of the week for the past 4 weeks [49]. Data on short-term medication use, such as a 1-week course of antibiotics and medications they have recently stopped taking, were not recorded. Dosage and duration of medication use were not recorded in the UK Biobank. However, a repeat assessment conducted in 2012–2013 that included 20,346 participants showed 91.2% were consistent with their PPI use at baseline. PPIs mainly included omeprazole, esomeprazole, pantoprazole, lansoprazole, and rabeprazole. We combined the use of these drugs and defined regular PPI use as a dichotomous variable (yes or no).
APOE genotyping
UK Biobank participants were genotyped using two genotyping arrays: UK BiLEVE or UK Biobank Axiom arrays. Following single nucleotide polymorphism (SNP) and sample quality controls, directly genotyped data were then imputed centrally by the UK Biobank based on the 1000 Genomes Phase 3, UK 10K haplotype, and Haplotype Reference Consortium reference panels [50]. APOE genotype was defined by two SNPs, rs429358 and rs7412. As APOE ε4 is a recognized genetic risk factor for dementia and AD mainly by affecting Aβ metabolism [31], we divided the population into APOE ε4 noncarriers (−/−), heterozygotes (+/−), and homozygotes (+/+) [51].
Ascertainment of incident dementia
Data on defining dementia, including all-cause dementia, AD, and VaD, were obtained from the UK Biobank baseline assessment data, linked hospital admission data, and death register data. Diagnoses were recorded using the International Classification of Diseases (ICD) coding system (Additional file 1: Table S1) [52]. Participants with the incident disease were identified as having a primary and secondary diagnosis in hospital admission records or underlying and secondary causes of death from morbidity records post the date of baseline assessment. We calculated the follow-up time from the date of attendance until the date of first diagnosis, date of death, or February 25, 2018, for Wales and England, and February 28, 2017, for Scotland, whichever occurred first.
Covariates
To control for potential confounding factors, we included the following covariates: sociodemographic characteristics (age, sex, ethnicity, education, household income, and Townsend deprivation index), lifestyle habits (smoking status, alcohol consumption, physical activity, body mass index [BMI], and occupational exposure), comorbidities (hypertension, coronary heart disease, diabetes, high cholesterol, stroke, traumatic brain injury, depression, anxiety, sleep apnea, cancer, GERD, Barrett’s esophagus, and gastroduodenal ulcer), and regular use of drugs or supplements (statin, antihypertensive drugs, anticholinergic drugs, benzodiazepines, z-hypnotics, aspirin, non-aspirin non-steroidal anti-inflammatory drugs [NSAIDs], multivitamin, and H2 receptor antagonists [H2RAs]). The Townsend deprivation index was used as an indicator of socioeconomic status and is provided directly by the UK Biobank [53]. Alcohol consumption was calculated based on the US Dietary Guidelines for Americans 2015–2020 [54]. Regular physical activity was calculated based on the validated International Physical Activity Questionnaire and categorized into three groups: regular, some, or no regular physical activity [55]. Information on medical history and use of drugs was collected via verbal interview at baseline. Anticholinergic drugs were defined by the Anticholinergic Cognitive Burden (ACB) scale and previous reports [56].
Statistical analyses
Baseline characteristics of the participants were summarized across regular PPI users as numbers (percentage [%]) for categorical variables, mean (standard deviation [SD]) for normally distributed variables, or median (interquartile range) for skewed variables. The cumulative incident dementia outcomes were measured by the Kaplan-Meier method, and the differences between PPI users and PPI non-users were compared with the log-rank test. The analyses were conducted among the whole population and each APOE ε4 genotype group. To maximize the statistical power, we performed multiple imputations with chained equations (MICE) to assign missing covariate values. Detailed information on the number of missing covariates is shown in Additional file 1: Table S2.
The associations between regular use of PPIs and all-cause dementia, AD, and VaD outcomes were explored using Cox proportional hazard models with hazard ratios (HRs) and 95% confidence intervals (CIs). The assumption for proportional hazards was evaluated by tests based on Schoenfeld residuals [57], and violation of this assumption was not observed in our analyses. Three sets of models were performed. Model 1 was only adjusted for age and sex. Model 2 was adjusted for additional variables, including ethnicity, education, household income, Townsend deprivation index, smoking status, alcohol consumption, physical activity, BMI, occupational exposure, hypertension, coronary heart disease, diabetes, high cholesterol, stroke, traumatic brain injury, depression, anxiety, sleep apnea, cancer, and regular use of medications (statin, antihypertensive drugs, anticholinergic drugs, benzodiazepines, z-hypnotics, aspirin, non-aspirin NSAIDs, and multivitamin). To address the possible confounding effect of PPI use clinical indications, we additionally adjusted for GERD, Barrett’s esophagus, gastroduodenal ulcer, and regular H2RAs used in model 3.
To investigate potential effect modifiers, we conducted subgroup analyses according to APOE genotype (ε4 −/−, ε4 +/−, or ε4 +/+), sex (female or male), age (<65 or ≥65 years), obesity (BMI ≥ 30 kg/m2, yes or no), ever smoking (yes or no), ever drinking (alcohol consumption > 0, yes or no), hypertension (yes or no), stroke (yes or no), depression (yes or no), statin use (yes or no), and aspirin use (yes or no). The potential modifying effect was evaluated using the cross-product term of the stratifying variable with PPI use in the fully adjusted model.
We performed a series of sensitivity analyses. First, to reduce the influence of early-onset dementia, we conducted a sensitivity analysis of the associations among each APOE genotype after excluding participants under 65 years old at the baseline. Then, we performed sensitivity analyses by excluding participants who developed outcomes within 2 years to reduce potential reverse causations and excluding participants with missing values of covariates to validate the robustness of the results. Finally, we conducted a propensity score matching analysis to adjust the confounding factors with the matching ratio of 2:1. Propensity scores were estimated based on the multivariable logistic regression model by including all the covariates. All statistical analyses were performed using R v4.1.0 (R Center for Statistical Computing, Vienna, Austria), and statistical significance was determined at P-value < 0.05 (two-sided).
Supplementary Information
Additional file 1: Table S1. Disease definitions used in the UK Biobank study. Table S2. The numbers (percentage) of the missing variables. Table S3. Subgroup analysis: associations of regular use of each PPI with the risk of incident dementia. Table S4. Sensitivity analysis: associations of regular PPI Use with the risk of incident dementia after excluding participants with missing covariate data. Table S5. Sensitivity analysis: associations of regular PPI use with the risk of incident dementia after excluding participants who developed outcomes during the first two years of follow-up. Table S6. Sensitivity analysis: associations of regular PPI use with the risk of incident dementia after excluding participants who developed outcomes only recorded on death register data. Figure S1. The cumulative risk of incident all-cause dementia (A), Alzheimer’s disease (B), and vascular dementia (C) according to regular PPI use for each APOE genotype subgroup. Figure S2. Association of regular PPI use and APOE genotype with incident dementia. Figure S3. Association of regular PPI use and modifying factors with incident all-cause dementia. Figure S4. Associations of regular PPI use with incident Alzheimer’s disease stratified by potential risk factors. Figure S5. Associations of regular PPI use with incident vascular dementia stratified by potential risk factors. Figure S6. Associations of regular PPI use with incident dementia stratified by APOE genotype among participants older than 65 years at baseline.
Acknowledgements
We are grateful to the UK Biobank participants. This research has been conducted using the UK Biobank resource under application number 43795.
Authors’ contributions
All authors read and approved the final manuscript. Conceptualization: PDZ, ZHL. Data curation: PDZ, ZHL, PLC, AZ. Formal analysis: PDZ. Investigation: PDZ, ZHL, PLC, YZ, XRZ, QMH, DL. Methodology: PDZ, ZHL. Project administration: PDZ, STQ, CM. Software: PDZ, ZHL, PLC. Supervision: STQ, CM. Writing — original draft: PDZ, AZ. Writing — review and editing: PDZ, ZHL, PLC, AZ, YZ, XRZ, QMH, DL, STQ, CM.
Funding
This work was supported by the National Natural Science Foundation of China (82103931 and 82003443), the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2019), the Guangdong Basic and Applied Basic Research Foundation (2022A1515012085 and 2021A1515110230), the Science and Technology Project in Guangzhou (202002030255 and 201902020017), the China Postdoctoral Science Foundation Funded Project (2021M701633), and the Young Elite Scientists Sponsorship Program by CAST (2019QNRC001). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Data are available in a public, open access repository. Data from the UK Biobank (https://www.ukbiobank.ac.uk/) are available to researchers on application.
Declarations
Ethics approval and consent to participate
All participants provided written informed consent before enrolment in the UK Biobank, which was conducted in accordance with the Declaration of Helsinki. The UK Biobank study, and the sharing of anonymized data with the research community, was approved by the North West Multi-center Research Ethics Committee (REC reference: 12/NW/03820).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peidong Zhang, Zhihao Li, and Peiliang Chen contributed to the work equally.
Contributor Information
Songtao Qi, Email: qisongtaonfyy@126.com.
Chen Mao, Email: maochen9@smu.edu.cn.
References
- 1.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 2.Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203–1209. doi: 10.1111/j.1365-2036.2005.02454.x. [DOI] [PubMed] [Google Scholar]
- 3.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2–3. doi: 10.1136/bmj.39406.449456.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Sehested TSG, Gerds TA, Fosbøl EL, Hansen PW, Charlot MG, Carlson N, Hlatky MA, Torp-Pedersen C, Gislason GH. Long-term use of proton pump inhibitors, dose-response relationship and associated risk of ischemic stroke and myocardial infarction. J Intern Med. 2018;283(3):268–281. doi: 10.1111/joim.12698. [DOI] [PubMed] [Google Scholar]
- 7.Charlot M, Ahlehoff O, Norgaard ML, Jørgensen CH, Sørensen R, Abildstrøm SZ, Hansen PR, Madsen JK, Køber L, Torp-Pedersen C, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med. 2010;153(6):378–386. doi: 10.7326/0003-4819-153-6-201009210-00005. [DOI] [PubMed] [Google Scholar]
- 8.Poly TN, Islam MM, Yang HC, Wu CC, Li YJ. Proton pump inhibitors and risk of hip fracture: a meta-analysis of observational studies. Osteoporos Int. 2019;30(1):103–114. doi: 10.1007/s00198-018-4788-y. [DOI] [PubMed] [Google Scholar]
- 9.Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, Grams ME. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238–246. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34(11-12):1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 11.Yuan J, He Q, Nguyen LH, Wong MCS, Huang J, Yu Y, Xia B, Tang Y, He Y, Zhang C. Regular use of proton pump inhibitors and risk of type 2 diabetes: results from three prospective cohort studies. Gut. 2021;70(6):1070–1077. doi: 10.1136/gutjnl-2020-322557. [DOI] [PubMed] [Google Scholar]
- 12.Thunell J, Chen Y, Joyce G, Barthold D, Shekelle PG, Brinton RD, Zissimopoulos J. Drug therapies for chronic conditions and risk of Alzheimer’s disease and related dementias: a scoping review. Alzheimers Dement. 2021;17(1):41–48. doi: 10.1002/alz.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, Jönsson L, Liu Z, Prince M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haenisch B, von Holt K, Wiese B, Prokein J, Lange C, Ernst A, Brettschneider C, König H-H, Werle J, Weyerer S, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419–428. doi: 10.1007/s00406-014-0554-0. [DOI] [PubMed] [Google Scholar]
- 16.Gomm W, von Holt K, Thomé F, Broich K, Maier W, Fink A, Doblhammer G, Haenisch B. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410–416. doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 17.Booker A, Jacob LE, Rapp M, Bohlken J, Kostev K. Risk factors for dementia diagnosis in German primary care practices. Int Psychogeriatr. 2016;28(7):1059–1065. doi: 10.1017/S1041610215002082. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969–1974. doi: 10.1111/jgs.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taipale H, Tolppanen A-M, Tiihonen M, Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1802–1808. doi: 10.1038/ajg.2017.196. [DOI] [PubMed] [Google Scholar]
- 20.Gray SL, Walker RL, Dublin S, Yu O, Aiello Bowles EJ, Anderson ML, Crane PK, Larson EB. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc. 2018;66(2):247–253. doi: 10.1111/jgs.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang IC, Chang J, Park SM. A nationwide population-based cohort study of dementia risk among acid suppressant users. Am J Geriatr Psychiatry. 2018;26(11):1175–1183. doi: 10.1016/j.jagp.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Imfeld P, Bodmer M, Jick SS, Meier CR. Proton pump inhibitor use and risk of developing Alzheimer’s disease or vascular dementia: a case-control analysis. Drug Saf. 2018;41(12):1387–1396. doi: 10.1007/s40264-018-0704-9. [DOI] [PubMed] [Google Scholar]
- 23.Shin JM, Cho YM, Sachs G. Chemistry of covalent inhibition of the gastric (H+, K+)-ATPase by proton pump inhibitors. J Am Chem Soc. 2004;126(25):7800–7811. doi: 10.1021/ja049607w. [DOI] [PubMed] [Google Scholar]
- 24.Peri F, Nüsslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133(5):916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Yang DS, Goulbourne CN, Im E, Stavrides P, Pensalfini A, Chan H, Bouchet-Marquis C, Bleiwas C, Berg MJ, et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat Neurosci. 2022;25(6):688–701. doi: 10.1038/s41593-022-01084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badiola N, Alcalde V, Pujol A, Münter LM, Multhaup G, Lleó A, Coma M, Soler-López M, Aloy P. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837. doi: 10.1371/journal.pone.0058837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindner K, Beckenbauer K, van Ek LC, Titeca K, de Leeuw SM, Awwad K, Hanke F, Korepanova AV, Rybin V, van der Kam EL, et al. Isoform- and cell-state-specific lipidation of ApoE in astrocytes. Cell Rep. 2022;38(9):110435. doi: 10.1016/j.celrep.2022.110435. [DOI] [PubMed] [Google Scholar]
- 29.Tai S-Y, Chien C-Y, Wu D-C, Lin K-D, Ho B-L, Chang Y-H, Chang Y-P. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006. doi: 10.1371/journal.pone.0171006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan MA, Yuan Y, Iqbal U, Kamal S, Khan M, Khan Z, Lee WM, Howden CW. No association linking short-term proton pump inhibitor use to dementia: systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2020;115(5):671–678. doi: 10.14309/ajg.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 31.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 32.Cheng FC, Ho YF, Hung LC, Chen CF, Tsai TH. Determination and pharmacokinetic profile of omeprazole in rat blood, brain and bile by microdialysis and high-performance liquid chromatography. J Chromatogr A. 2002;949(1-2):35–42. doi: 10.1016/S0021-9673(01)01225-0. [DOI] [PubMed] [Google Scholar]
- 33.Rojo LE, Alzate-Morales J, Saavedra IN, Davies P, Maccioni RB. Selective interaction of lansoprazole and astemizole with tau polymers: potential new clinical use in diagnosis of Alzheimer’s disease. J Alzheimers Dis. 2010;19(2):573–589. doi: 10.3233/JAD-2010-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, Maxfield FR. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell. 2007;18(4):1490–1496. doi: 10.1091/mbc.e06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattsson JP, Väänänen K, Wallmark B, Lorentzon P. Omeprazole and bafilomycin, two proton pump inhibitors: differentiation of their effects on gastric, kidney and bone H(+)-translocating ATPases. Biochim Biophys Acta. 1991;1065(2):261–268. doi: 10.1016/0005-2736(91)90238-4. [DOI] [PubMed] [Google Scholar]
- 36.Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- 37.Fontecha-Barriuso M, Martín-Sanchez D, Martinez-Moreno JM, Cardenas-Villacres D, Carrasco S, Sanchez-Niño MD, Ruiz-Ortega M, Ortiz A, Sanz AB. Molecular pathways driving omeprazole nephrotoxicity. Redox Biol. 2020;32:101464. doi: 10.1016/j.redox.2020.101464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips PC. Epistasis--the essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet. 2008;9(11):855–867. doi: 10.1038/nrg2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 40.Kumar R, Kumar A, Nordberg A, Långström B, Darreh-Shori T. Proton pump inhibitors act with unprecedented potencies as inhibitors of the acetylcholine biosynthesizing enzyme-a plausible missing link for their association with incidence of dementia. Alzheimers Dement. 2020;16(7):1031–1042. doi: 10.1002/alz.12113. [DOI] [PubMed] [Google Scholar]
- 41.Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang LS, Romero K, Arneric SP, Redolfi A, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74(10):1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, Papp KV, Jacobs HIL, Burnham S, Hanseeuw BJ, et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimers Dement. 2018;14(9):1193–1203. doi: 10.1016/j.jalz.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durazzo TC, Mattsson N, Weiner MW. Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement. 2014;10(3 Suppl):S122–S145. doi: 10.1016/j.jalz.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onda K, Tong S, Beard S, Binder N, Muto M, Senadheera SN, Parry L, Dilworth M, Renshall L, Brownfoot F, et al. Proton pump inhibitors decrease soluble fms-like tyrosine kinase-1 and soluble endoglin secretion, decrease hypertension, and rescue endothelial dysfunction. Hypertension. 2017;69(3):457–468. doi: 10.1161/HYPERTENSIONAHA.116.08408. [DOI] [PubMed] [Google Scholar]
- 45.Akter S, Hassan MR, Shahriar M, Akter N, Abbas MG, Bhuiyan MA. Cognitive impact after short-term exposure to different proton pump inhibitors: assessment using CANTAB software. Alzheimers Res Ther. 2015;7:79. doi: 10.1186/s13195-015-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang JF, Chen YT, Fuh JL, Li SY, Chen TJ, Tang CH, Wang SJ. Proton pump inhibitor-related headaches: a nationwide population-based case-crossover study in Taiwan. Cephalalgia. 2015;35(3):203–210. doi: 10.1177/0333102414535114. [DOI] [PubMed] [Google Scholar]
- 47.Atri A, Gerson LB. Continued questions about whether avoidance of proton pump inhibitors can reduce risk of dementia. Gastroenterology. 2016;151(3):555–558. doi: 10.1053/j.gastro.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 48.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan X, Liu Z, Miyata T, Dasarathy S, Rotroff DM, Wu X, Poulsen KL, Nagy LE. Effect of acid suppressants on the risk of COVID-19: a propensity score-matched study using UK Biobank. Gastroenterology. 2021;160(1):455–458. doi: 10.1053/j.gastro.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O'Connell J, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattsson N, Groot C, Jansen WJ, Landau SM, Villemagne VL, Engelborghs S, Mintun MM, Lleo A, Molinuevo JL, Jagust WJ, et al. Prevalence of the apolipoprotein E ε4 allele in amyloid β positive subjects across the spectrum of Alzheimer’s disease. Alzheimers Dement. 2018;14(7):913–924. doi: 10.1016/j.jalz.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Calvin CM, Wilkinson T, Starr JM, Sudlow C, Hagenaars SP, Harris SE, Schnier C, Davies G, Fawns-Ritchie C, Gale CR, et al. Predicting incident dementia 3-8 years after brief cognitive tests in the UK Biobank prospective study of 500,000 people. Alzheimers Dement. 2019;15(12):1546–1557. doi: 10.1016/j.jalz.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Kendall KM, Bracher-Smith M, Fitzpatrick H, Lynham A, Rees E, Escott-Price V, Owen MJ, O'Donovan MC, Walters JTR, Kirov G. Cognitive performance and functional outcomes of carriers of pathogenic copy number variants: analysis of the UK Biobank. Br J Psychiatry. 2019;214(5):297–304. doi: 10.1192/bjp.2018.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. 2015. Available at http://health.gov/dietaryguidelines/2015/guidelines/.
- 55.Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank Study. JAMA Cardiol. 2018;3(8):693–702. doi: 10.1001/jamacardio.2018.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, Gao S, Boustani M, Crane PK, Petersen RC, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721–732. doi: 10.1001/jamaneurol.2016.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. doi: 10.1093/biomet/69.1.239. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Disease definitions used in the UK Biobank study. Table S2. The numbers (percentage) of the missing variables. Table S3. Subgroup analysis: associations of regular use of each PPI with the risk of incident dementia. Table S4. Sensitivity analysis: associations of regular PPI Use with the risk of incident dementia after excluding participants with missing covariate data. Table S5. Sensitivity analysis: associations of regular PPI use with the risk of incident dementia after excluding participants who developed outcomes during the first two years of follow-up. Table S6. Sensitivity analysis: associations of regular PPI use with the risk of incident dementia after excluding participants who developed outcomes only recorded on death register data. Figure S1. The cumulative risk of incident all-cause dementia (A), Alzheimer’s disease (B), and vascular dementia (C) according to regular PPI use for each APOE genotype subgroup. Figure S2. Association of regular PPI use and APOE genotype with incident dementia. Figure S3. Association of regular PPI use and modifying factors with incident all-cause dementia. Figure S4. Associations of regular PPI use with incident Alzheimer’s disease stratified by potential risk factors. Figure S5. Associations of regular PPI use with incident vascular dementia stratified by potential risk factors. Figure S6. Associations of regular PPI use with incident dementia stratified by APOE genotype among participants older than 65 years at baseline.
Data Availability Statement
Data are available in a public, open access repository. Data from the UK Biobank (https://www.ukbiobank.ac.uk/) are available to researchers on application.