Abstract
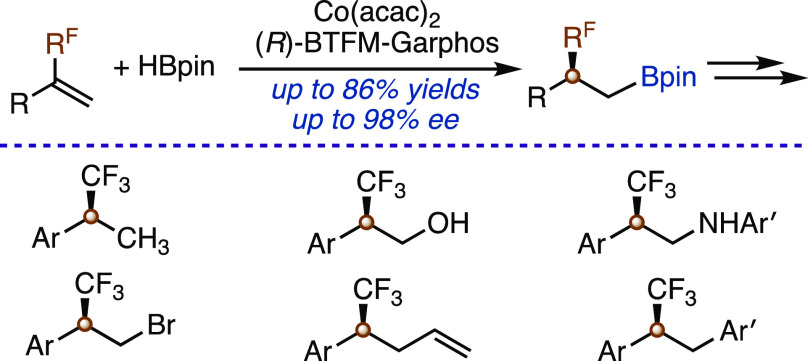
Selective defluoroborylation and asymmetric hydroboration reactions of fluoroalkyl-substituted terminal alkenes with pinacolborane (HBpin) have been developed with cobalt catalysts generated from Co(acac)2 and bisphosphine ligands. A variety of fluoroalkyl-substituted terminal alkenes undergo this enantioselective hydroboration, affording the corresponding chiral alkylboronates containing fluoroalkyl-substituted stereogenic carbon centers with high enantioselectivity (up to 98% ee). This asymmetric hydroboration provides a versatile foundation for the synthesis of a variety of chiral organofluorine compounds containing fluoroalkyl-substituted stereogenic carbon centers.
Introduction
Fluorine-containing molecules have found broad applications in pharmaceutical, agrochemical, and materials sciences due to their unique physicochemical and biological properties.1 For example, organic compounds with trifluoromethylated stereogenic carbon motifs are widespread in various biologically active compounds.2 Therefore, the development of effective catalytic protocols to access chiral molecules containing fluoroalkyl substituents, such as a trifluoromethyl or polyfluoroalkyl group, is a research field of ever-growing interest.3 In particular, it is highly desirable to achieve asymmetric syntheses of chiral fluoroalkyl compounds containing a functional group with versatile reaction chemistry such as organoboronates, because their subsequent transformations will allow convenient access to a variety of other chiral fluoroalkyl compounds.
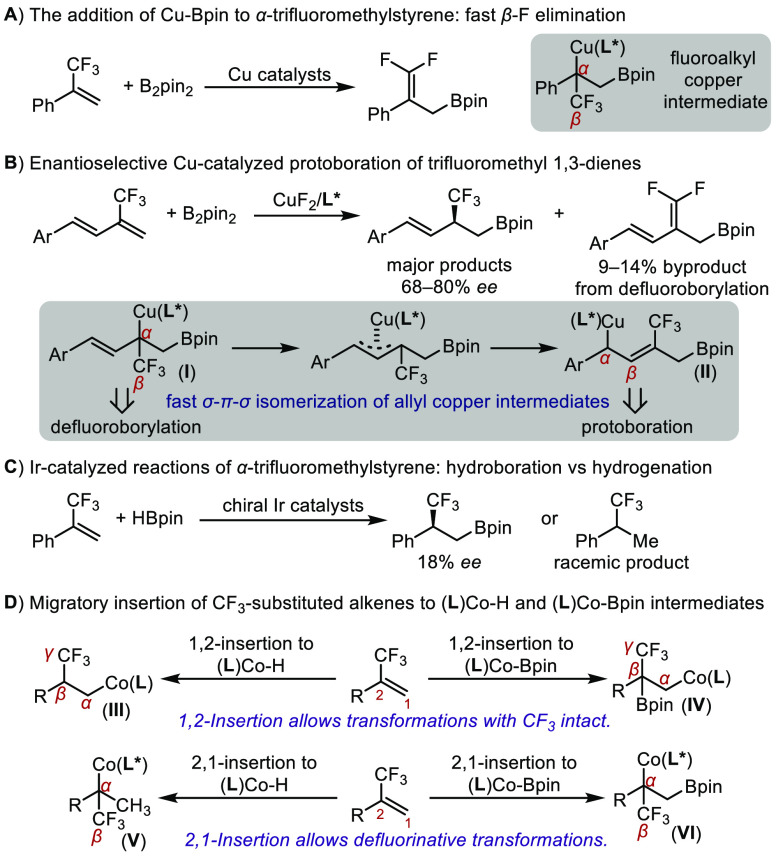
Enantioenriched organoboronates are versatile reagents for asymmetric synthesis as they can undergo various stereospecific transformations with no or minimum loss of their enantiopurity.4 The installation of boronate functionality into chiral fluoroalkyl compounds will improve their manipulability and enrich their synthetic utility.5 Catalytic hydroboration or protoboration of fluoroalkyl-substituted alkenes is one straightforward approach to prepare fluoroalkylated organoboronates.6 Asymmetric copper-catalyzed protoboration of α-trifluoromethyl styrene with B2pin2 has been attempted to synthesize chiral β-trifluoromethylated alkylboronates,6c but β-fluorine elimination occurs instead to yield gem-difluoroallylic boronates as the major product (Figure 1A).7 Therefore, the effective suppression of β-fluorine elimination is the key to developing enantioselective addition of Cu-Bpin to CF3-substituted C=C double bonds. For example, Zhang’s group recently developed a Cu-catalyzed asymmetric protoboration of CF3-containing 1,3-dienes by taking advantage of fast σ–π–σ isomerization of an allylcopper intermediate (I) with β-fluorine atoms to another allylcopper species (II) that does not have β-fluorine atoms (Figure 1B).8 Alternatively, hydroboration of α-trifluoromethyl styrene with HBpin has also been attempted with chiral iridium catalysts,6d but this reaction suffers from either low chemoselectivity or poor enantioselectivity (Figure 1C). Therefore, it still remains a challenge to develop atom-economical and enantioselective approaches to access chiral fluoroalkylated organoboronates from fluoroalkyl-substituted monoalkenes.
Figure 1.
Metal-catalyzed enantioselective protoboration and hydroboration of CF3-substituted alkenes.
In recent years, base metal catalysts, such as chiral cobalt,9 iron,10 and copper complexes,11 have been employed to catalyze enantioselective hydroboration of 1,1-disubstituted alkenes with HBpin to prepare chiral alkylboronates. During our continuous efforts in developing cobalt-catalyzed asymmetric synthesis of alkylboronates,12 we became interested in identifying chiral cobalt catalysts for asymmetric hydroboration of simple fluoroalkyl-substituted alkenes to access organoboronates containing fluoroalkyl-substituted stereogenic carbon centers. Migratory insertion of fluoroalkyl-substituted alkenes into metal-hydride and metal-boryl intermediates is a fundamental step in metal-catalyzed hydroboration reactions, and possible ways of migratory insertion of a CF3-substituted alkene into Co-H and Co-Bpin bonds are depicted in Figure 1D. 1,2-Insertion of a CF3-substituted alkene into a (L)Co-H or (L)Co-Bpin species would generate alkylcobalt intermediates III and IV with fluorine atoms located on their γ-carbon atoms. This helps to suppress fluorine elimination and thus keeps the CF3 group intact. Alternatively, 2,1-insertion of this alkene into (L)Co-H or (L)Co-Bpin species forms alkylcobalt intermediates V and VI with fluorine atoms located on their β-carbon atoms, and β-fluorine elimination from these alkylcobalt species would allow defluorinative transformations of CF3-substituted alkenes. Therefore, identifying suitable ligands that can promote the formation of alkylcobalt intermediate III or IV is crucial to develop cobalt-catalyzed hydroboration of fluoroalkyl-substituted alkenes. Herein, we report cobalt-intermediate-dependent defluoroborylation and hydroboration reactions of fluoroalkyl-substituted alkenes, with an emphasis on developing enantioselective hydroboration to access chiral alkylboronates that have fluoroalkylated stereogenic carbon centers. In addition, we also show that the chiral β-CF3-substituted alkylboronate products can be readily converted, in a stereospecific manner, to various chiral compounds containing CF3-substituted stereogenic carbon centers.
Results and Discussion
Evaluation of the Reaction of α-Trifluoromethyl Styrene with HBpin with Chiral Cobalt and Iron Catalysts Previously Reported for Asymmetric Hydroboration of 1,1-Disubstituted Vinylarenes
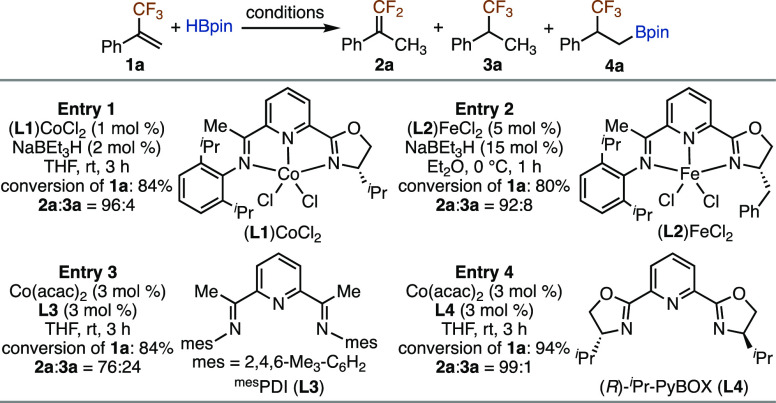
Cobalt and iron complexes containing iminopyridine oxazoline ligands have been reported to catalyze asymmetric hydroboration of 1,1-disubstituted vinylarenes—for example, α-methyl styrene, with high enantioselectivity.9,10 We first evaluated these cobalt and iron catalysts for hydroboration of α-trifluoromethyl styrene 1a under the reported conditions to determine whether it is necessary to identify new catalysts for asymmetric hydroboration of fluoroalkyl-substituted alkenes. However, the reaction of 1a with HBpin catalyzed by (L1)CoCl2 or (L2)FeCl2 afforded gem-difluoroalkene 2a as the major product, together with (1,1,1-trifluoropropan-2-yl)benzene 3a as a minor product (entries 1 and 2 in Scheme 1). These results unequivocally show the uniqueness of a CF3 group, and the replacement of the methyl group on α-methyl styrene with a CF3 group completely alters the chemoselectivity of its reaction with HBpin under identical conditions.
Scheme 1. Reaction of α-Trifluoromethyl Styrene 1a with HBpin Catalyzed by N3-Pincer-Ligated Iron and Cobalt Catalysts: Hydrodefluorination and Hydrogenation of 1a.
In addition, we also attempted this hydroboration reaction with catalysts generated from Co(acac)2 and other N3-pincer ligands mesPDI and (R)-iPr-Pybox, but these two reactions also yielded 2a as the major product (entries 3 and 4 in Scheme 1). The hydroboration product, fluoroalkylboronate 4a, was not detected by GC–MS analysis on the crude mixtures of these reactions. The predominant formation of gem-difluoroalkene 2a for the reactions in Scheme 1 suggests that α-trifluoromethyl styrene 1a tends to undergo 2,1-insertion into an N3-pincer-ligated cobalt-hydride complex to form a cobalt intermediate of type V, as shown in Figure 1D. Therefore, it remains necessary to identify new chiral catalysts for asymmetric hydroboration of fluoroalkyl-substituted alkenes.
Cobalt-Catalyzed Hydroboration and Defluoroborylation of α-Trifluoromethyl Styrene with HBpin
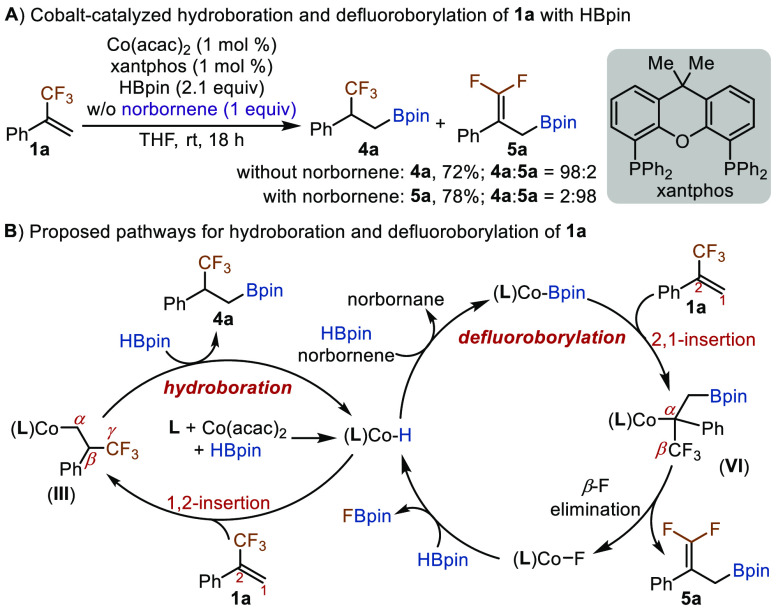
It is known that both the nature of alkenes and metal complexes influences the regioselectivity of insertion of alkenes, 1,2- or 2,1-insertion, into reactive organometallic intermediates. Therefore, we decided to evaluate phosphine-ligated cobalt catalysts for the reaction of 1a with HBpin. Recently, we have identified the conditions for selective formation of Co-H and Co-Bpin species from Co(acac)2 and bisphosphine ligands. For example, Co(acac)2 reacts with HBpin in the presence of xantphos to form a cobalt hydride species, which can be subsequently converted to a cobalt boryl intermediate in the presence of hydrogen acceptors, such as norbornene, cyclooctene, and dicyclopentadiene.9b,13 This provides us with an opportunity to test both Co-H and Co-Bpin species for the reaction of fluoroalkyl-substituted alkenes with HBpin. To our delight, we found that the reaction of 1a with HBpin catalyzed by Co(acac)2 and xantphos occurred selectively to produce β-trifluoromethyl alkylboronate 4a in 72% isolated yield, while the corresponding reaction in the presence of norbornene as a hydrogen acceptor selectively afforded gem-difluoroallylic boronate 5a in 78% yield (Scheme 2A).
Scheme 2. Cobalt-Catalyzed Hydroboration and Defluoroborylation of α-Trifluoromethyl Styrene 1a.
As rationalized in Figure 1D, cobalt species III and VI are key intermediates for the formation of 4a and 5a, respectively. A complete depiction of the proposed pathways for cobalt-catalyzed hydroboration and defluoroborylation reactions is shown in Scheme 2B. Compared with N3-pincer ligands in Scheme 1, the xantphos ligand is more sterically demanding due to the two phenyl groups on each of its phosphorus atoms, and the steric repulsion between xantphos and phenyl/CF3 groups of 1a makes 1,2-insertion of 1a into xantphos-ligated Co-H species more favorable. However, 2,1-insertion of 1a into xantphos-ligated Co-Bpin species is likely due to the more prominent steric repulsion between the Bpin group and the Ph/CF3 groups of 1a, compared with the steric repulsion between xantphos and the Ph/CF3 groups. For defluoroborylation of 1a with HBpin in the presence of norboronene, FBpin and norbornane were detected by 19F NMR and GC–MS analysis, respectively. As defluoroborylation of CF3-substituted alkenes with B2pin2 has been studied with various metal catalysts,7 we include the scope of CF3-substituted alkenes for defluoroborylation reactions with HBpin in the Supporting Information.
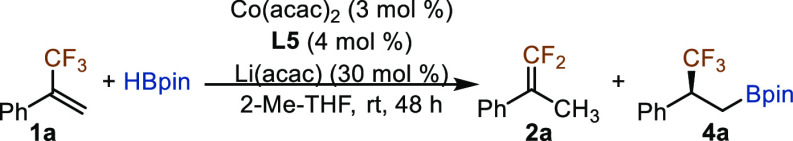
Evaluation of Chiral Phosphine Ligands and Conditions for Enantioselective Cobalt-Catalyzed Hydroboration of α-Trifluoromethyl Styrene with HBpin
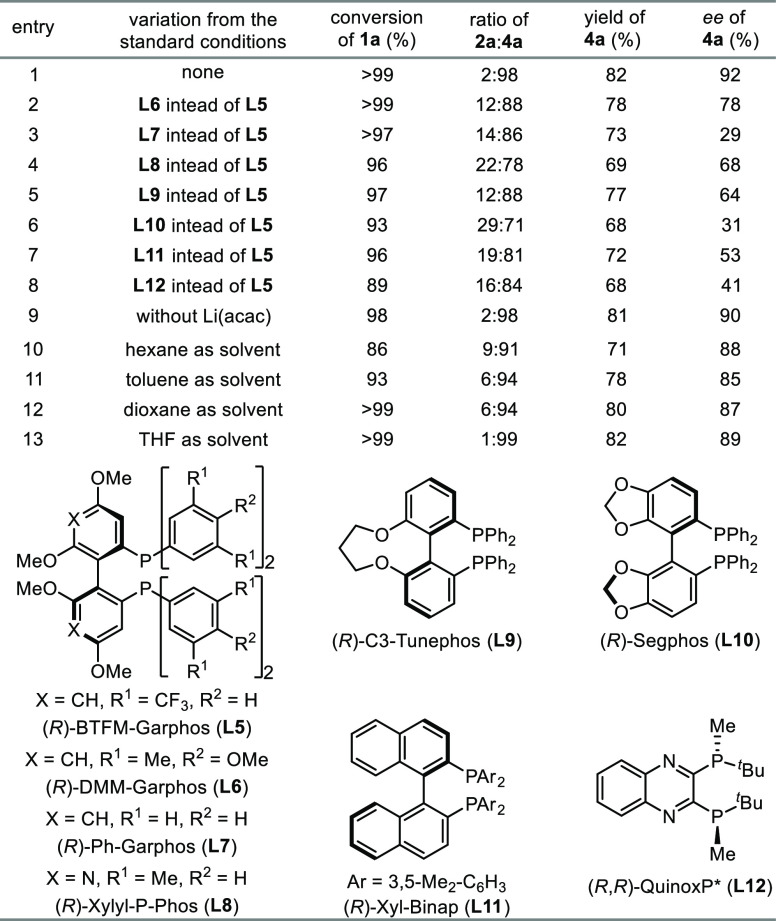
After establishing conditions for selective hydroboration of α-trifluoromethyl styrene 1a, we then aimed to identify chiral cobalt catalysts for asymmetric hydroboration of 1a. After evaluating various chiral phosphine ligands and reaction parameters (Table 1), we found that alkene 1a reacted smoothly with 1.2 equiv of HBpin in the presence of 3 mol % Co(acac)2, 4 mol % (R)-BTFM-Garphos (L5), and 30 mol % Li(acac) in 2-Me-THF at room temperature, affording chiral alkylboronate 4a in 82% isolated yield with 98% chemoselectivity and 92% ee (entry 1). The reactions employing less sterically demanding Garphos ligands L6 or L7 afforded 4a with lower enantioselectivity (entries 2 and 3). The reactions conducted with cobalt catalysts generated in situ from Co(acac)2 and other chiral biaryl phosphine ligands, such as (R)-Xylyl-P-Phos (L8, entry 4), (R)-C3-Tunephos (L9, entry 5), (R)-Segphos (L10, entry 6), and (R)-Xylyl-Binap (L11, entry 7), also occurred to form the desired product 4a in good yields (68–77%) but with modest enantioselectivity (39–68% ee). The reaction catalyzed by the combination of Co(acac)2 and (R,R)-QuinoxP* (L12) afforded 4a in 68% yield with 41% ee (entry 8). The removal of Li(acac) from the reaction conditions led to a slightly decreased enantioselectivity (90% ee, entry 9). In general, the reactions catalyzed by Co(acac)2 and phosphophine ligands L6–L12 also generated significant amounts of gem-difluoroalkene 2a (entries 2–8). In addition, we also tested various solvents, such as hexane, toluene, dioxane, and THF, for this asymmetric hydroboration reaction, and these reactions occurred with slightly lower enantioselectivity (85–89% ee, entries 10–13) compared with the reaction conducted in 2-Me-THF (entry 1).
Table 1. Evaluation of Conditions for Cobalt-Catalyzed Asymmetric Hydroboration of α-Trifluoromethyl Styrene 1aa.
Conditions: α-trifluoromethyl styrene 1a (0.100 mmol), HBpin (0.120 mmol), Co(acac)2 (3.0 μmol), ligand (4.0 μmol), Li(acac) (30 μmol), 2-Me-THF (0.1 mL) at rt. for 48 h, conversions of 1a, and ratios of 2a:4a were determined by GC analysis with tridecane as the internal standard, isolated yields were given, and ee values of 4a were determined by chiral HPLC analysis.
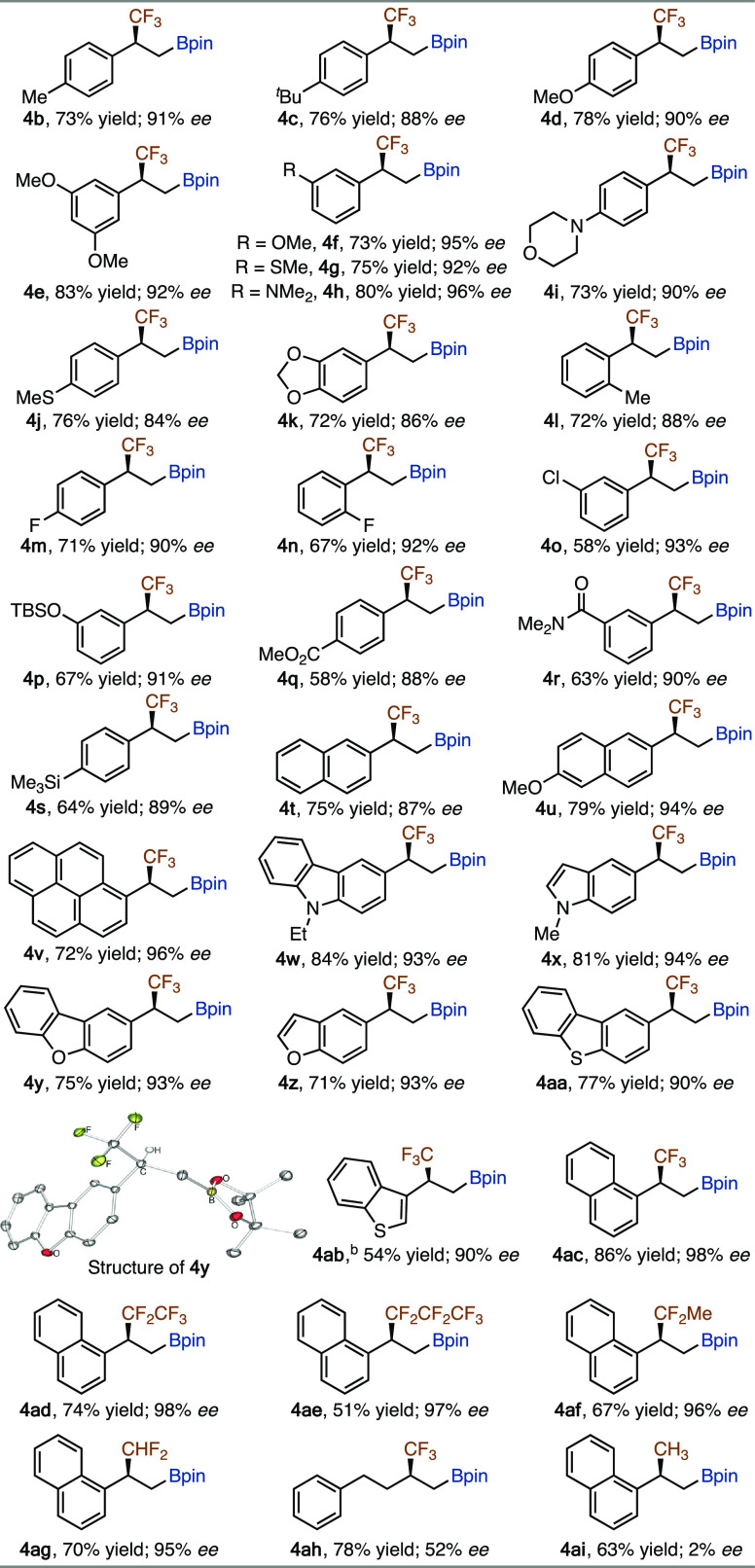
Substrate Scope of Fluoroalkyl-Substituted Alkenes
With the identified chiral catalyst and conditions (entry 1 in Table 1), we explored the scope of fluoroalkylated alkenes for this cobalt-catalyzed asymmetric hydroboration, and the results are summarized in Table 2. In general, a wide range of α-CF3-substituted vinylarenes (1b–1ac) reacted smoothly with HBpin in the presence of 3 mol % Co(acac)2 and 4 mol % (R)-BTFM-Garphos, affording the corresponding chiral β-trifluoromethylated alkylboronates (4b–4ac) in modest to high yields (54–86%) with high enantioselectivity (84–98% ee). Fluoroalkylated alkenes containing other fluoroalkyl groups, such as perfluoroethyl (1ad), perfluoropropyl (1ae), difluoroethyl (1af), and difluoromethyl (1ag), also underwent this asymmetric hydroboration reaction to produce β-fluoroalkyl-substituted alkylboronates (4ae–4ag) in high yields (51–74%) with excellent enantioselectivity (95–98% ee). In addition, 1ah, a terminal alkene containing both alkyl and trifluoromethyl substituents, could also react to afford the desired product 4ah in 78% yield, albeit with modest enantioselectivity. Methyl-substituted vinylarene 1ai underwent this cobalt-catalyzed hydroboration as well under the standard conditions, but the alkylboronate product 4ai was nearly racemic (2% ee).14 The absolute configuration of alkylboronate 4y was assigned as (S) by single-crystal X-ray analysis.
Table 2. Scope of Fluoroalkylated Alkenes for Asymmetric Cobalt-Catalyzed Hydroborationa.
Conditions: alkene (0.200 mmol), HBpin (0.240 mmol), Co(acac)2 (6.0 μmol), (R)-BTFM-Garphos (8.0 μmol), Li(acac) (60 μmol), 2-Me-THF (0.1 mL), rt., 48 h, yields of isolated products, ee values were determined by chiral HPLC analysis.
This reaction was conducted with 5 mol % Co(acac)2 and 6 mol % (R)-BTFM-Garphos.
The data in Table 2 show that the substitution pattern on the aryl groups does not have significant influence on the enantioselectivity of this asymmetric hydroboration reaction. For example, the alkenes containing para- (4b, 4c, 4d, 4i, and 4j), meta- (4e-4 h, 4o, 4p, and 4r), and ortho-substituted (4l and 4n) aryl groups reacted with similarly high enantioselectivity. CF3-substituted alkenes containing polyaryl groups, such as naphthyl (4t, 4u, and 4ac) and pyrenyl (4v), reacted to afford the corresponding β-trifluoromethylated alkylboronates in high yields (72–86%) with high enantioselectivity (87–98% ee). In addition, five-membered nitrogen-, oxygen-, and sulfur-heterocyclic CF3-substituted alkenes also underwent this asymmetric hydroboration to afford the desired products (4w–4ab) with high enantioselectivity (90–94% ee). However, hydroboration of 3-(3,3,3-trifluoroprop-1-en-2-yl)pyridine, a Py,CF3-substituted alkene, proceeded only to less than 5% conversion under the identified conditions. Furthermore, this cobalt-catalyzed asymmetric hydroboration tolerates various functional groups, such as sulfide (4g and 4j), tertiary amine (4h and 4i), fluoro (4m and 4n), chloro (4o), siloxy (4p), carboxylic ester (4q), carboxylic amide (4r), and trimethylsilyl (4s) moieties.
Synthetic Utility
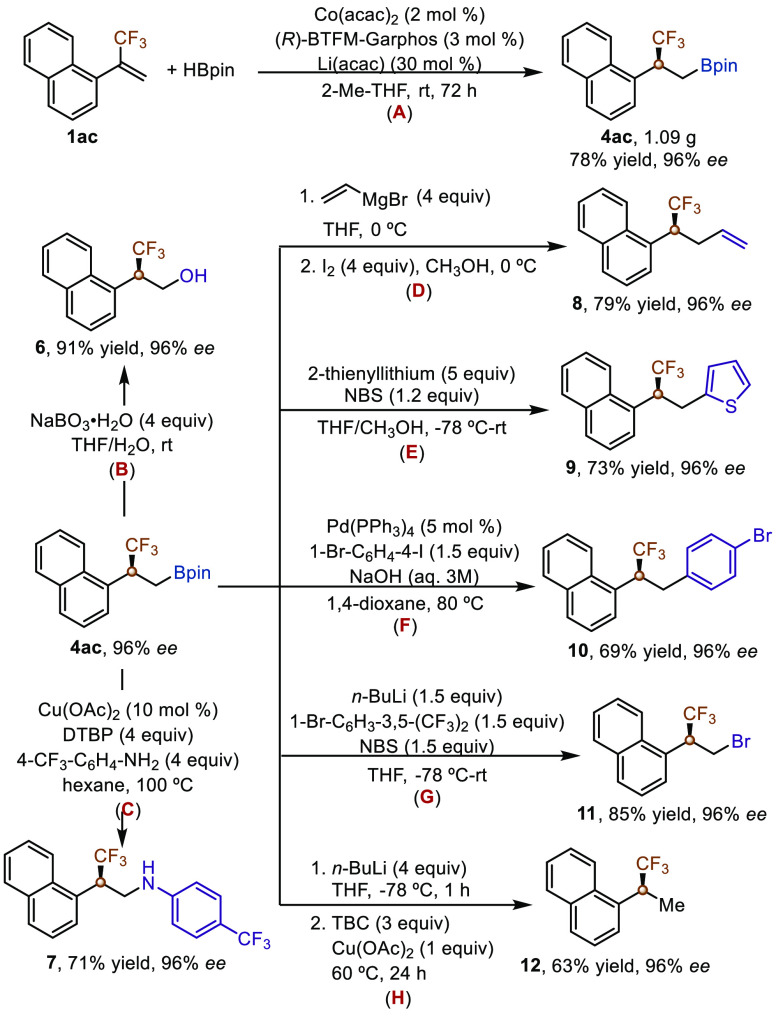
To show the synthetic utility of this enantioselective protocol, we conducted a gram-scale asymmetric hydroboration reaction of trifluoromethylated alkene 1ac with a reduced catalyst loading (2 mol %), and this reaction occurred smoothly to produce chiral alkylboronate 4ac in 78% yield with 96% ee, although a longer reaction time was required (Scheme 3A). In addition, we showed that trifluoromethylated β-stereogenic alkylboronate 4ac could undergo various stereospecific transformations without loss of enantiopurity to afford various enantioenriched trifluoromethylated compounds, which are otherwise difficult to access from readily available materials. For example, 4ac could be oxidized by NaBO3 to form chiral β-trifluoromethyl alcohol 6 in 91% yield with 96% ee (Scheme 3B).15 Compound 4ac could also underwent Chan–Lam coupling with 4-(trifluoromethyl)aniline to form β-stereogenic trifluoromethylated amine 7 in 71% yield with 96% ee (Scheme 3C).16 Chiral alkylboronate 4ac could also be employed to construct carbon–carbon bonds. For example, the vinylation of 4ac with vinylmagnesium bromide in the presence of I2 afforded chiral alkene 8 in 79% yield with 96% ee (Scheme 3D).17 The reaction of 4ac with 2-thienyllithium in the presence of NBS generated compound 9 in 73% yield with 96% ee (Scheme 3E).18 The Suzuki–Miyaura cross-coupling reaction between 4ac and 1-bromo-4-iodobenzene occurred smoothly in the presence of 5 mol % Pd(PPh3)4 at 80 °C, affording compound 10 in 69% isolated yield with 96% ee (Scheme 3F).19 Alkylboronate 4ac could also be converted to chiral alkylbromide 11 in 85% yield with 96% ee (Scheme 3G).17 Furthermore, protodeborylation of alkylboronate 4ac could afford compound 12, which contains a CF3,CH3-substituted tertiary stereogenic carbon center, in 63% isolated yield with 96% ee (Scheme 3H).20
Scheme 3. Gram-Scale Reaction and Transformations of Chiral Alkylboronate 4ac.
Conclusions
In summary, we have developed cobalt-catalyzed selective defluoroborylation and hydroboration of fluoroalkylated terminal alkenes with HBpin. These reactions proceed with different catalytically active cobalt species, with a cobalt-boryl intermediate for the defluoroborylation reaction and a cobalt-hydride species for the hydroboration reaction. Furthermore, we have identified a chiral cobalt catalyst for the asymmetric hydroboration of fluoroalkylated terminal alkenes. A variety of fluoroalkylated alkenes react with HBpin in the presence of Co(acac)2 and (R)-BTFM-Garphos, forming the corresponding chiral alkylboronates containing fluoroalkylated tertiary stereogenic carbons in high yields with high enantioselectivity. The chiral alkylboronate products from this asymmetric hydroboration reaction can be readily converted, in a stereospecific manner, to various chiral molecules by functional group manipulations of their C–B bonds. Therefore, this enantioselective hydroboration reaction provides a general and versatile foundation for the preparation of various chiral compounds containing fluoroalkylated stereogenic carbon centers.
Acknowledgments
This work was supported by the Ministry of Education (MOE) of Singapore (No. A-0004102-00-00) and A*Star under its AME IRG grant (A20E5c0097).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c06488.
Experimental details, characterization data, and copies of NMR spectra of all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]; b Ojima I.Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell, 2009, 10.1002/9781444312096. [DOI] [Google Scholar]; c Gouverneur V.; Müller K.. Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications; Imperial College Press: London, 2012, 10.1142/p746. [DOI] [Google Scholar]; d Zhu W.; Wang J.; Wang S.; Gu Z.; Aceña J. L.; Izawa K.; Liu H.; Soloshonok V. A. Recent Advances in the Trifluoromethylation Methodology and New CF3-containing Drugs. J. Fluorine Chem. 2014, 167, 37–54. 10.1016/j.jfluchem.2014.06.026. [DOI] [Google Scholar]
- For selected reviews, see:; a Wang J.; Sánchez-Roselló M.; Aceña J. L.; del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]; b Inoue M.; Sumii Y.; Shibata N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. 10.1021/acsomega.0c00830. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Hawker C. J.; Whittaker A. K. Biological Utility of Fluorinated Compounds: from Materials Design to Molecular Imaging, Therapeutics and Environmental Remediation. Chem. Rev. 2022, 122, 167–208. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see:; a Nie J.; Guo H.-C.; Cahard D.; Ma J.-A. Asymmetric Construction of Stereogenic Carbon Centers Featuring a Trifluoromethyl Group from Prochiral Trifluoromethylated Substrates. Chem. Rev. 2011, 111, 455–529. 10.1021/cr100166a. [DOI] [PubMed] [Google Scholar]; b Yang X.; Wu T.; Phipps R. J.; Toste F. D. Advances in Catalytic Enantioselective Fluorination, Mono-, Di-, and Trifluoromethylation, and Trifluoromethylthiolation Reactions. Chem. Rev. 2015, 115, 826–870. 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Huang Y.-Y.; Yang X.; Chen Z.; Verpoort F.; Shibata N. Catalytic Asymmetric Synthesis of Enantioenriched Heterocycles Bearing a C–CF3 Stereogenic Center. Chem. – Eur. J. 2015, 21, 8664–8684. 10.1002/chem.201500361. [DOI] [PubMed] [Google Scholar]; d He X.-H.; Ji Y.-L.; Peng C.; Han B. Organocatalytic Asymmetric Synthesis of Cyclic Compounds Bearing a Trifluoromethylated Stereogenic Center: Recent Developments. Adv. Synth. Catal. 2019, 361, 1923–1957. 10.1002/adsc.201801647. [DOI] [Google Scholar]; e Onyeagusi C. I.; Malcolmson S. J. Strategies for the Catalytic Enantioselective Synthesis of α-Trifluoromethyl Amines. ACS Catal. 2020, 10, 12507–12536. 10.1021/acscatal.0c03569. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Deng Y.; He J.; Cao S.; Qian X. Advances in cycloaddition and hydroaddition reaction of α-(trifluoromethyl)styrenes without defluorination: An alternative approach to CF3-containing compounds. Chin. Chem. Lett. 2022, 33, 2363–2371. 10.1016/j.cclet.2021.11.049. [DOI] [Google Scholar]
- For selected reviews, see:; a Collins B. S. L.; Wilson C. M.; Myers E. L.; Aggarwal V. K. Asymmetric Synthesis of Secondary and Tertiary Boronic Esters. Angew. Chem., Int. Ed. 2017, 56, 11700–11733. 10.1002/anie.201701963. [DOI] [PubMed] [Google Scholar]; b Sandford C.; Aggarwal V. K. Stereospecific Functionalizations and Transformations of Secondary and Tertiary Boronic Esters. Chem. Commun. 2017, 53, 5481–5494. 10.1039/C7CC01254C. [DOI] [PubMed] [Google Scholar]; c Namirembe S.; Morken J. P. Reactions of Organoboron Compounds Enabled by Catalyst-Promoted Metalate Shifts. Chem. Soc. Rev. 2019, 48, 3464–3474. 10.1039/C9CS00180H. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Yang X.; Ge S. Recent Progress in Cobalt-Catalyzed Enantioselective Hydrogenation and Hydroboration Reactions of Alkenes. Curr. Opin. Green Sustainable Chem. 2021, 31, 100542 10.1016/j.cogsc.2021.100542. [DOI] [Google Scholar]
- For selected examples, see:; a Jiang Q.; Guo T.; Yu Z. Copper-Catalyzed Asymmetric Borylation: Construction of a Stereogenic Carbon Center Bearing Both CF3 and Organoboron Functional Groups. J. Org. Chem. 2017, 82, 1951–1960. 10.1021/acs.joc.6b02772. [DOI] [PubMed] [Google Scholar]; b Liu B.; Wu H.-H.; Zhang J. Cu(II)-Catalyzed Enantioselective β-Boration of β-Trifluoromethyl, β,β-Disubstituted Enones and Esters: Construction of a CF3- and Boron-Containing Quaternary Stereocenter. ACS Catal. 2018, 8, 8318–8323. 10.1021/acscatal.8b02543. [DOI] [Google Scholar]; c Yang C.; Liu Z.-L.; Dai D.-T.; Li Q.; Ma W.-W.; Zhao M.; Xu Y.-H. Catalytic Asymmetric Conjugate Protosilylation and Protoborylation of 2-Trifluoromethyl Enynes for Synthesis of Functionalized Allenes. Org. Lett. 2020, 22, 1360–1367. 10.1021/acs.orglett.9b04647. [DOI] [PubMed] [Google Scholar]; d Huang X.; Garcia-Borrás M.; Miao K.; Kan S. B. J.; Zutshi A.; Houk K. N.; Arnold F. H. A Biocatalytic Platform for Synthesis of Chiral α-Trifluoromethylated Organoborons. ACS Cent. Sci. 2019, 5, 270–276. 10.1021/acscentsci.8b00679. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Nandakumar M.; Rubial B.; Noble A.; Myers E. L.; Aggarwal V. K. Ring-Opening Lithiation–Borylation of 2-Trifluoromethyl Oxirane: A Route to Versatile Tertiary Trifluoromethyl Boronic Esters. Angew. Chem., Int. Ed. 2020, 59, 1187–1191. 10.1002/anie.201912797. [DOI] [PubMed] [Google Scholar]
- a Brown H. C.; Chen G.-M.; Jennings M. P.; Ramachandran P. V. Markovnikov Hydroboration of Perfluoroalkylethylenes. Angew. Chem., Int. Ed. 1999, 38, 2052–2054. . [DOI] [PubMed] [Google Scholar]; b Braun T.; Ahijado Salomon M.; Altenhöner K.; Teltewskoi M.; Hinze S. C–F Activation at Rhodium Boryl Complexes: Formation of 2-Fluoroalkyl-1,3,2-Dioxaborolanes by Catalytic Functionalization of Hexafluoropropene. Angew. Chem., Int. Ed. 2009, 48, 1818–1822. 10.1002/anie.200805041. [DOI] [PubMed] [Google Scholar]; c Corberán R.; Mszar N. W.; Hoveyda A. H. NHC-Cu-Catalyzed Enantioselective Hydroboration of Acyclic and Exocyclic 1,1-Disubstituted Aryl Alkenes. Angew. Chem., Int. Ed. 2011, 50, 7079–7082. 10.1002/anie.201102398. [DOI] [PubMed] [Google Scholar]; d Magre M.; Biosca M.; Pámies O.; Diéguez M. Filling the Gaps in the Challenging Asymmetric Hydroboration of 1,1-Disubstituted Alkenes with Simple Phosphite-Based Phosphinooxazoline Iridium Catalysts. ChemCatChem 2015, 7, 114–120. 10.1002/cctc.201402822. [DOI] [Google Scholar]
- a Liu Y.; Zhou Y.; Zhao Y.; Qu J. Synthesis of gem-Difluoroallylboronates via FeCl2-Catalyzed Boration/β-Fluorine Elimination of Trifluoromethyl Alkenes. Org. Lett. 2017, 19, 946–949. 10.1021/acs.orglett.7b00168. [DOI] [PubMed] [Google Scholar]; b Kojima R.; Akiyama S.; Ito H. A Copper(I)-Catalyzed Enantioselective γ-Boryl Substitution of Trifluoromethyl-Substituted Alkenes: Synthesis of Enantioenriched γ,γ-gem-Difluoroallylboronates. Angew. Chem., Int. Ed. 2018, 57, 7196–7199. 10.1002/anie.201803663. [DOI] [PubMed] [Google Scholar]; c Zhao X.; Li C.; Wang B.; Cao S. Copper-Catalyzed Synthesis of gem-Difluoroallylboronates from α-Trifluoromethyl Alkenes and B2pin2. Tetrahedron Lett. 2019, 60, 129–132. 10.1016/j.tetlet.2018.11.073. [DOI] [Google Scholar]; d Liu Y.; Li C.; Liu C.; He J.; Zhao X.; Cao S. Cobalt- or Copper-Catalyzed Synthesis of gem-Difluoroallyl MIDA Boronates from α-Trifluoromethyl Alkenes. Tetrahedron Lett. 2020, 61, 151940 10.1016/j.tetlet.2020.151940. [DOI] [Google Scholar]
- Wu J.; Wu H.; Li X.; Liu X.; Zhao Q.; Huang G.; Zhang C. Copper-Catalyzed Highly Selective Protoboration of CF3-Containing 1,3-Dienes. Angew. Chem., Int. Ed. 2021, 60, 20376–20382. 10.1002/anie.202105896. [DOI] [PubMed] [Google Scholar]
- a Zhang L.; Zuo Z.; Wan X.; Huang Z. Cobalt-Catalyzed Enantioselective Hydroboration of 1,1-Disubstituted Aryl Alkenes. J. Am. Chem. Soc. 2014, 136, 15501–15504. 10.1021/ja5093908. [DOI] [PubMed] [Google Scholar]; b Chen J.; Xi T.; Ren X.; Cheng B.; Guo J.; Lu Z. Asymmetric Cobalt Catalysts for Hydroboration of 1,1-Disubstituted Alkenes. Org. Chem. Front. 2014, 1, 1306–1309. 10.1039/C4QO00295D. [DOI] [Google Scholar]
- Chen J.; Xi T.; Lu Z. Iminopyridine Oxazoline Iron Catalyst for Asymmetric Hydroboration of 1,1-Disubtituted Aryl Alkenes. Org. Lett. 2014, 16, 6452–6455. 10.1021/ol503282r. [DOI] [PubMed] [Google Scholar]
- a Jang W. J.; Song S. M.; Moon J. H.; Lee J. Y.; Yun J. Copper-Catalyzed Enantioselective Hydroboration of Unactivated 1,1-Disubstituted Alkenes. J. Am. Chem. Soc. 2017, 139, 13660–13663. 10.1021/jacs.7b08379. [DOI] [PubMed] [Google Scholar]; b Xi Y.; Su B.; Qi X.; Pedram S.; Liu P.; Hartwig J. F. Application of Trimethylgermanyl-Substituted Bisphosphine Ligands with Enhanced Dispersion Interactions to Copper-Catalyzed Hydroboration of Disubstituted Alkenes. J. Am. Chem. Soc. 2020, 142, 18213–18222. 10.1021/jacs.0c08746. [DOI] [PubMed] [Google Scholar]
- a Yu S.; Wu C.; Ge S. Cobalt-Catalyzed Asymmetric Hydroboration/Cyclization of 1,6-Enynes with Pinacolborane. J. Am. Chem. Soc. 2017, 139, 6526–6529. 10.1021/jacs.7b01708. [DOI] [PubMed] [Google Scholar]; b Teo W. J.; Ge S. Cobalt-Catalyzed Enantioselective Synthesis of Chiral gem-Bis(boryl)alkanes. Angew. Chem., Int. Ed. 2018, 57, 12935–12939. 10.1002/anie.201805705. [DOI] [PubMed] [Google Scholar]; c Wang C.; Ge S. Versatile Cobalt-Catalyzed Enantioselective Entry to Boryl-Functionalized All-Carbon Quaternary Stereogenic Centers. J. Am. Chem. Soc. 2018, 140, 10687–10690. 10.1021/jacs.8b06814. [DOI] [PubMed] [Google Scholar]; d Wu C.; Liao J.; Ge S. Cobalt-Catalyzed Enantioselective Hydroboration/Cyclization of 1,7-Enynes: Asymmetric Synthesis of Chiral Quinolinones Containing Quaternary Stereogenic Centers. Angew. Chem., Int. Ed. 2019, 58, 8882–8886. 10.1002/anie.201903377. [DOI] [PubMed] [Google Scholar]; e You Y.; Ge S. Cobalt-Catalyzed One-Pot Asymmetric Difunctionalization of Alkynes to Access Chiral gem-(Borylsilyl)alkanes. Angew. Chem., Int. Ed. 2021, 60, 20684–20688. 10.1002/anie.202107405. [DOI] [PubMed] [Google Scholar]
- a Teo W. J.; Ge S. Cobalt-Catalyzed Diborylation of 1,1-disubstituted Vinylarenes: A Practical Route to Branched gem-Bis(boryl)alkanes. Angew. Chem., Int. Ed. 2018, 57, 1654–1658. 10.1002/anie.201710389. [DOI] [PubMed] [Google Scholar]; b Teo W. J.; Yang X.; Poon Y. Y.; Ge S. Cobalt-Catalyzed Deoxygenative Triborylation of Allylic Ethers to Access 1,1,3-Triborylalkanes. Nat. Commun. 2020, 11, 5193. 10.1038/s41467-020-19039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhao Y.; Ge S. Synergistic Hydrocobaltation and Borylcobaltation Enable Regioselective Migratory Triborylation of Unactivated Alkenes. Angew. Chem., Int. Ed. 2022, 61, e202116133. [DOI] [PubMed] [Google Scholar]
- Similarly, low ee values were previously reported for alkylboronate product from hydroboration of α-methyl styrene with HBpin in the presence of Co(acac)2 and a chiral bisphosphine ligand. For possible explanations, see ref (12b).
- Kabalka G. W.; Shoup T. M.; Goudgaon N. M. Sodium perborate: A mild and convenient reagent for efficiently oxidizing trialkylboranes. Tetrahedron Lett. 1989, 30, 1483–1486. 10.1016/S0040-4039(00)99497-8. [DOI] [Google Scholar]
- Sueki S.; Kuninobu Y. Copper-Catalyzed N- and O-Alkylation of Amines and Phenols using Alkylborane Reagents. Org. Lett. 2013, 15, 1544–1547. 10.1021/ol400323z. [DOI] [PubMed] [Google Scholar]
- Wu N.-Y.; Xu X.-H.; Qing F.-L. Catalyzed Regioselective Borylfluoromethylation of Alkenes. ACS Catal. 2019, 9, 5726–5731. 10.1021/acscatal.9b01530. [DOI] [Google Scholar]
- Odachowski M.; Bonet A.; Essafi S.; Conti-Ramsden P.; Harvey J. N.; Leonori D.; Aggarwal V. K. Development of Enantiospecific Coupling of Secondary and Tertiary Boronic Esters with Aromatic Compounds. J. Am. Chem. Soc. 2016, 138, 9521–9532. 10.1021/jacs.6b03963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Miyaura N.; Yamada K.; Suzuki A. A New Stereospecific Cross-Coupling by the Palladium-Catalyzed Reaction of 1-Alkenylboranes with 1-Alkenyl or 1-Alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. 10.1016/S0040-4039(01)95429-2. [DOI] [Google Scholar]
- Rasappan R.; Aggarwal V. K. Synthesis of Hydroxyphthioceranic Acid Using a Traceless Lithiation–Borylation–Protodeboronation Strategy. Nat. Chem. 2014, 6, 810–814. 10.1038/nchem.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.