Abstract
Skilled reading is important in daily life. While the understanding of the neurofunctional organization of this uniquely human skill has advanced significantly, it does not take into consideration the common bilingual experiences around the world. To examine the role of early bilingualism on the neural substrates supporting English word processing, we compared brain activity, as well as functional connectivity, in Spanish‐English early bilingual adults (N = 25) and English monolingual adults (N = 33) during single‐word processing. Activation analysis revealed no significant differences between the two groups. A seed‐to‐voxel analysis using eight a priori selected seed‐regions (placed in regions known to be involved in reading) revealed relatively stronger functional connectivity in bilinguals between two sets of regions: left superior temporal gyrus seed positively with left lingual gyrus and left middle frontal gyrus seed negatively with left anterior cingulate cortex. Together these results suggest that an early Spanish‐English bilingual experience does not modulate local brain activity for English word reading. It does, however, have some influence on the functional intercommunication between brain regions during reading, specifically in two regions associated with reading, which are functionally connected to those inside and outside of the reading network. We conclude that brain regions involved in processing English words are not that different in Spanish‐English early bilingual adults relative to monolingual adult users of English.
Keywords: bilingualism, biliteracy, brain activity, English word processing, functional connectivity, reading
To examine the role of early bilingualism on the neural substrates supporting English word processing we compared brain activity, as well as functional connectivity, in Spanish‐English early bilingual adults (N = 25) and English monolingual adults (N = 33) during single‐word processing. We found that an early Spanish‐English bilingual experience did not influence local brain activity for English word reading. It did, however, have some influence on the functional intercommunication between brain regions during reading, specifically in two regions associated with reading, which are functionally connected to those inside and outside of the reading network.
1. INTRODUCTION
Reading is central to educational, vocational, and other daily activities. There have been substantial advances in characterizing the brain regions involved in reading (Martin et al., 2015; Price, 2012). This work, however, has largely been limited to participants who are monolinguals or whose language background was not described. More than half of the world's population has been reported to use two or more languages every day (European Commission Special Eurobarometer, Europeans and Their Languages, 2012; Grosjean, 2010). Indeed, dual‐language instruction is becoming more prevalent worldwide, even in countries that cite only one language as their official language. As such, it is important understand the functional anatomy of reading in bilinguals and it is possible that current brain‐based models of reading may not be representative of the world's large bilingual population.
A widely accepted brain‐based model of reading describes three prominent areas in the left hemisphere: occipito‐temporal cortex (OTC) associated with memory‐based visual word form recognition, temporo‐parietal cortex (TPC) involved in grapheme‐to‐phoneme conversion and phonological processing, and inferior frontal cortex (IFC) considered to underlie semantic processing, phonological decoding, and articulatory recoding of print (Dehaene & Cohen, 2007; Price, 2012; Pugh et al., 2001; Sandak et al., 2004). In the most comprehensive meta‐analysis to date, Martin and colleagues describe these left hemisphere regions emerging from multiple studies examining brain function underlying reading or reading‐related tasks in users of alphabetic languages (Martin et al., 2015). When considering the 20 original studies that went into the meta‐analysis of reading in adults (mostly in native users of English, with some native users of German or French), only one of them noted that all their participants were monolinguals, while the other 19 studies did not mention language background (e.g., “monolingual,” “bilingual,” or “dual language”) in the description of their participants. If most participants were monolingual, which is likely, the question arises to what degree does the current knowledge of the reading brain apply to those with a dual language experience?
There has been a strong interest in the impact of experiential factors on the brain signature for reading. For example, there have been investigations to establish whether activation during reading is modulated as a function of orthographic depth, that is whether the sound‐to‐print correspondence is deep (e.g., in English) versus shallow (e.g., in Spanish) (Brignoni‐Perez et al., 2020; Jamal et al., 2012); or, as a function of the writing system, that is whether the language uses an alphabetic (e.g., English) versus logographic (e.g., Chinese) writing system (Bolger et al., 2005; Mei et al., 2015). Also, of interest is whether activity during the processing of print is influenced by pedagogical approaches to reading and writing (James & Engelhardt, 2012), participants' age (Schlaggar et al., 2002; Turkeltaub et al., 2003), or the presence of reading disability (Eden et al., 2016; Gabrieli, 2009). Here, we asked whether brain function during the reading of single English words is different for those who speak and read proficiently in two languages. Importantly, our focus is on early, cultural bilinguals who acquired both languages at an early age from their primary caregivers and their environment. For these cultural bilinguals, the dual language experience is a product of their upbringing and distinguishes them from individuals born into monolingual families who then went on to excel at learning a second language outside of their home environment. Knowing whether the neurofunctional bases of reading in adults is influenced by their experience of early bilingualism and biliteracy has important implications for our understanding of the brain bases of reading development. If the reading brain operates differently in bilinguals (relative to monolinguals), this observation would raise questions about the best instructional approaches to the teaching of reading to bilinguals. It would also require differentiated research into developmental (dyslexia) and acquired (alexia) disorders of reading in bilinguals.
A multilingual brain‐based model of reading may be different than a monolingual model when considering findings in bilinguals in the domain of language. For example, bilinguals have an overall larger vocabulary (both languages combined), although their vocabulary size for each language may be smaller than that reported for monolinguals (Allman, 2005). There has been some suggestion that phonological processing, a foundational skill required for learning to read (Wagner & Torgesen, 1987), is better in bilinguals (Eviatar & Ibrahim, 2000; Kovelman et al., 2008b; Kuo & Anderson, 2012), although not all studies support this observation (Martin, 2011). Brain imaging studies comparing both early and late bilinguals have shown that both languages are mapped to the same brain regions in bilinguals with early age of acquisition and high proficiency, but to divergent brain regions in those with late second language acquisition and lower proficiency (for review, see Połczyńska & Bookheimer, 2021). One might expect that the processing of two languages in the same brain region in early bilinguals would represent differently from that in monolinguals, where that same brain region is dedicated to only a single language. Studies examining activation during linguistic tasks such as syntactic judgment or picture and word naming, have shown that bilinguals engage regions of the TPC and the IFC to a greater extent than monolinguals (Jones et al., 2012; Kovelman et al., 2008a).
Another mechanism by which bilingualism may have a modulatory role on the neural bases of reading is via executive function. Executive function is important for reading, primarily being associated with reading comprehension and sometimes with decoding (Arrington et al., 2014; Locascio et al., 2010). It has been argued that bilinguals have better executive function than monolinguals, specifically in conflict resolution, task switching, and inhibitory control (Bialystok, 2006; Bialystok et al., 2004; Costa et al., 2008; Luk, Sa, et al., 2011). This heightened executive function is thought to be driven by the constant management of a bilingual's two languages (both languages are “active,” requiring the brain to suppress the nontarget language and attend to the target language), ultimately leading to better cognitive control. Neuroimaging investigations have shown that brain structure, activity, and functional connectivity in brain areas associated with executive function differ between bilinguals and monolinguals (Hayakawa & Marian, 2019; Kroll & Bialystok, 2013; Mechelli et al., 2004; Olulade et al., 2016; Wong et al., 2016). However, findings on heightened language and nonlanguage skills in bilinguals (Kroll et al., 2012, 2014; Kroll & Bialystok, 2013) have been challenged (see Dick, 2018; Lehtonen et al., 2018; Nichols et al., 2020; Paap et al., 2015) and the complexities of these studies have been discussed (DeLuca et al., 2020; Połczyńska & Bookheimer, 2021).
This evidence from studies on language and on executive function in bilinguals raises the question whether an early bilingual experience influences the brain bases of reading. Learning to read involves a process of neuronal recycling where areas originally devoted to other, yet related functions are repurposed for reading (Dehaene & Cohen, 2007). There have been two neuroimaging studies on single‐word processing in alphabetic languages in early bilingual adults, to test whether this process results in a different pattern for reading in bilinguals. Using functional magnetic resonance imaging (fMRI), Rodriguez‐Fornells et al. (2002) found greater engagement of regions in the left TPC and IFC during Spanish single‐word reading in Spanish‐Catalan early bilingual compared to Spanish‐speaking monolingual adults. A functional near‐infrared spectroscopy (fNIRS) study examined English single‐word reading in bilingual (English together with one of several languages using various writing systems) and English‐speaking monolingual adults and children (Jasińska & Petitto, 2014). These investigators also found greater activation in regions of left (and right) TPC and the left IFC during English word reading in bilingual compared to monolingual adults. Taken together, these two studies show that early experience with two languages leads to greater use of brain regions in the TPC and the IFC during word processing in adults. These studies were, however, limited in that they used small sample sizes (Rodriguez‐Fornells et al., 2002 had N = 7 per group and Jasińska & Petitto 2014 had N = 8 per group) and language or reading proficiency for these participants was not reported. The present study sought to extend this work by examining brain activity during English word processing in a larger group of bilinguals with early dual language experience, high levels of language and reading proficiency in their two languages, as well as reading proficiency in the language of the task (English) equal to the monolinguals. Further, we also investigated functional connectivity as a way to gauge the nature of the interaction between distributed brain regions and to shed further light on the mechanisms that are at play during reading in early bilinguals.
Functional connectivity represents correlations between measures of neuronal activity, more specifically statistical dependencies between distant neurophysiological events (Biswal et al., 1995; Friston, 1994, 2011). Prior studies have identified those brain regions positively correlated with each other during reading, including the angular gyrus (in TPC) and the inferior frontal gyrus pars triangularis and pars opercularis (in IFC) (Bitan et al., 2006; Hampson et al., 2006; Horwitz et al., 1998; Mechelli et al., 2005; Pugh et al., 2000). There have been no studies assessing whether these functional connections during reading differ in bilinguals relative to monolinguals. It has been reported that bilingual adults have stronger functional connectivity in fronto‐parietal control, salience, and default mode networks compared to monolinguals (Costumero et al., 2015; Grady et al., 2015), supporting the notion that early acquisition of two languages influences the brain's connectivity. One might, therefore, expect that the bilingual experience would impact the interactions between the components of the reading network, especially the left inferior frontal gyrus, middle frontal gyrus, and inferior parietal lobule, regions known to be involved in reading (Martin et al., 2015) and executive function (Ardila et al., 2018; Cieslik et al., 2015).
In the present study, we tested whether the Spanish‐English early bilingual experience influences adults' brain function during English word processing at the local level (activity); and, for the first time, at the network level (connectivity). Our participants were early, balanced cultural bilingual adults. Based on prior studies (Jasińska & Petitto, 2014; Rodriguez‐Fornells et al., 2002), we predicted different patterns of engagement in the TPC and the IFC in bilinguals, relative to monolingual adult users of English, possibly as a consequence of adaptations in the domain of language or executive control. Further, we predicted differences in the communication of brain regions that are known to subserve reading with other regions involved in reading and possibly those subserving executive function (TPC and IFC), in early bilinguals compared to monolinguals.
2. MATERIALS AND METHODS
2.1. Participants
Sixty‐six healthy, young adults participated in the study. Eight participants' data were excluded from the analyses (one because of not having a structural image, another due to irreparable distortion of the functional images, and six because of excessive head movement during the fMRI scanning). The final groups consisted of 25 Spanish‐English early bilinguals (17 females and 8 males with an average age of 22.1 years) and 33 English‐speaking monolinguals (13 females and 20 males with an average age of 22.9 years) matched on sex and age (see Table 1).
TABLE 1.
Description of all bilingual and monolingual participants and in‐scanner task performance
| Bilinguals | Monolinguals | t | p | |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| N | 25 | 33 | ||
| Sex (female/male) | 17/8 | 13/20 | ||
| Age (years) | 22.1 (2.8) | 22.9 (3.3) | −0.99 | .33 |
| Age range (years) | 18.4–28.5 | 18.7–29.2 | ||
| English single‐word reading ability (SS) a | 104.7 (6) | 107.1 | −1.26 | .21 |
| Socioeconomic status b | 14.5 (1.3) | 14.6 (1.0) | −0.27 | .79 |
| In‐scanner implicit reading task | ||||
| RW‐FF accuracy difference (%) c | 0.8 (1.0) | 0.2 (1.0) | 0.75 | .45 |
| RW‐FF response time difference (ms) c | −36.3 (83.9) | −32.0 (54.3) | −0.33 | .74 |
Abbreviations: FF, false fonts; M, mean; SD, standard deviation; RW, real words.
Note: There were no significant differences between the groups (independent samples t‐test).
Average of Word Identification and Word Attack subtests standard scores from Woodcock et al. (2001).
SES scoring adapted from Noble et al. (2015).
Difference of mean scores for real words versus false fonts.
All participants from either group lived in the greater metropolitan Washington, D.C. area, had normal or corrected‐to‐normal vision at the time of testing, and no history of neurological disease or learning disabilities. To ensure that none of the participants had a reading disability and that the two groups performed equally well in the reading of English words (the language of the reading task in the scanner), we assessed English single‐word reading using a combined score of the Word Identification (real words) and Word Attack (pseudowords) subtests from the Woodcock–Johnson III: Tests of Achievement (Woodcock et al., 2001). All participants had an average standard score of 85 or greater, thereby performing in the “normal” range or above. Importantly, when directly comparing the bilingual and monolingual groups, there were no differences in single‐word reading performance of English (Table 1). Education level is associated with socioeconomic status (SES; Coleman, 1968; Conger & Donnellan, 2007; Liu et al., 2020; Sirin, 2005), and it is important to match groups on SES in studies comparing bilinguals and monolinguals. At the time of testing, all participants in the bilingual group as well as in the monolingual group reported having completed high school, with most participants in both groups being in the process of attaining a bachelor's degree, and a few participants having completed a bachelor's degree. For making a comparison, we used the scoring system described by Noble and colleagues (Noble et al., 2015), where “High school graduate” = 12, “Some college (1‐3 years, AA, business schools)” = 14, “Four‐year college graduate (BA, BS, BM)” = 16, and “Professional degree (MA, MS, ME, MD, PhD, LLD, JD, etc.)” = 18. The groups' average scores were 14.5 for the bilingual group and 14.6 for the monolingual group (calculated based on 23 participants, as this information was not available for the remainder of this group), with no differences between the two groups (see Table 1).
Turning to the bilingual participants specifically, they were Spanish‐English balanced bilinguals and biliterates of Hispanic/Latino ethnicity. Ten were born in the United States and the other 15 in a country where Spanish is the official language. As detailed in Table 2, all learned both languages early (by or at Age 6) in their home environment and learned English on average at Age 3. They went on to use both languages in formal studies, on average about 12 years in Spanish and about 15 years in English and had no major exposure to other languages. They reported using both languages at the time of testing (using English more than Spanish on average on a daily basis). All bilingual participants completed a self‐assessment questionnaire by Meschyan and Hernandez (Meschyan & Hernandez, 2006). This questionnaire uses a scale of 1–7, with 1 representing low competence and 7 representing native‐like competence. All participants scored 6 or 7 for “Listening Comprehension” and for “Speaking Proficiency” in English, and group averages revealed overall high proficiency. The same was true for Spanish, and there were no significant differences between the two languages for either measure (see Table 2). Further, we measured single‐word reading in Spanish with the “Identificación de letras y palabras” and “Analisis de palabras” subtests from the “Batería III Woodcock‐Muñoz: Pruebas de aprovechamiento” (Munoz‐Sandoval, Woodcock, McGrew, & Mather, 2005), which are the Spanish equivalent of the English Word Identification and Word Attack subtest described above. Just as for the English version, all bilingual participants had a standard score of 85 or above on the Spanish average reading score, indicating that they were proficient readers in Spanish (Spanish reading was stronger than English reading, see Table 2).
TABLE 2.
Language background of the bilingual participants
| English language | Spanish language | t | p | |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| Age of first exposure (years)* | 3.3 (2.0) | 0.3 (0.6) | 7.29 | <.001 |
| Formal study (years) | 14.9 (5.2) | 12.0 (6.7) | 2.05 | .05 |
| Currently spoken per day (%)* | 72.2 (21.9) | 27.8 (21.9) | 5.06 | <.001 |
| Listening comprehension score (1–7) | 6.7 (0.54) | 6.7 (0.54) | <0.001 | 1.00 |
| Speaking proficiency score (1–7) | 6.6 (0.60) | 6.4 (0.80) | 0.55 | .59 |
| Single‐word reading ability (SS)* , a | 104.7 (6) | 120.5 (12) | −5.20 | <.001 |
The study protocol was approved by the Georgetown University Institutional Review Board. All participants gave written informed consent before starting the experimental procedures. Some of the bilingual participants' data have been reported previously for studies comparing English and Spanish word reading in bilinguals (Brignoni‐Perez et al., 2020; Jamal et al., 2012).
2.2. fMRI word reading task
We used an implicit reading task adapted from the original work of Price et al. (1996) and shown to activate brain regions involved in orthographic, phonological, and semantic processing. While inside the MRI scanner, participants looked at English single real words in the middle of the screen and pressed the button in their right thumb, if the word had a “tall letter” (such as “b” and “t” in the word “beast”), and the button in their left thumb, if the word did not have a “tall letter” (such as the word “sauce”). Participants were also presented with false‐font strings, which served as the active control condition. These false‐font strings were made up of symbols with no resemblance to real words. Participants had to perform the same action as that while viewing real words (i.e., press the button in their right thumb if the false‐font string had an ascender, and the button in their left thumb if the string did not have an ascender). While both tasks involve visual processing, attention, motor responses, and response selection, the false‐font task does not involve the orthographic, phonological, or semantic processing associated with real words.
The tasks consisted of 40 real words and 40 false‐font strings, divided between two experimental runs (20 items of each stimulus type in each run). Real words were either monosyllabic or disyllabic, composed of five letters, and categorized as less frequently encountered in a text corpus (Kucera‐Francis 8.05, SD 6.03, MRC Psycholinguistic Database; Coltheart, 1981). False‐font string equivalents were matched in length and in position of ascenders (e.g., d, h, k), as well as of descenders (e.g., c, o, v) or hanging letters (e.g., j, q, y).
Before the MRI scan, all participants learned how to perform the task, with a different set of stimuli than those used during the scanning session for both the real word and the false font conditions. Once in the scanner, participants performed the task during two separate runs. Each run had two blocks of the real word condition and two blocks of the false font condition. Within each block, there were 10 stimuli (1.2 s) interspersed with a fixation crosshair (3.0 s), five with ascenders and five without ascenders. Words and false‐font strings were individually shown in black Arial font on a white screen. Additionally, real word and false font blocks were interspersed with five fixation blocks (18 s) within each run. The two runs were counterbalanced across participants.
2.3. In‐scanner task performance and postscanning stimuli recognition test
To ensure that participants performed the tasks, we recorded accuracy and response time inside the MRI scanner. Following the scan, participants also completed a forced‐choice pencil‐and‐paper recognition test (Turkeltaub et al., 2003), which assessed whether participants recognized the real words presented during the scan at greater than chance levels. Participants were provided with a list of 80 real words and 80 false‐font strings, half of which had been presented in the scanner, and had to indicate which stimuli they remembered seeing during the MRI session.
2.4. fMRI data acquisition and preprocessing
We collected MRI images using a Siemens Vision Magnetom 3.0‐Tesla scanner with a circularly polarized head coil (at Georgetown University's Center for Functional and Molecular Imaging). To align functional images with brain anatomy, we acquired one high‐resolution three‐dimensional T1‐weighted image from each participant. Eighty‐nine whole‐head echo planar imaging (EPI) volumes were collected for each of the two experimental runs under the following acquisition parameters: 3.0 s TR, 30 ms TE, 50 contiguous axial slices, 2.8 mm slice thickness (0.2 mm interslice gap), 192 mm FOV, 64 × 64 matrix (3.0 mm cubic voxels). The first five EPI volumes were not included in the analyses, leaving a final number of 84 volumes per run.
Using the CONN toolbox v.18.a (https://www.nitrc.org/projects/conn) (Whitfield‐Gabrieli & Nieto‐Castanon, 2012), we preprocessed the imaging data following a standard pipeline of slice‐time correction, realignment, coregistration to structural image, normalization, and smoothing. We corrected for differences in timing of slice acquisition and realigned the functional images to the first volume of each experimental run under a rigid‐body motion transformation approach. During normalization, structural images were segmented into gray matter, white matter, and cerebrospinal fluid, and functional images were sampled to 3‐mm cubic voxels, all based on the Montreal Neurological Institute (MNI) stereotaxic space templates (Cocosco et al., 1997). To detect and control for global mean intensity and motion outliers (scan‐to‐scan motion greater than a threshold of 0.75 mm, 25% of the voxel size) in the fMRI signal, we used the Artifact Detection Tools implemented through CONN (ART; https://www.nitrc.org/projects/artifact_detect/). We regressed out all the identified “bad scans” (outliers) from the fMRI model and did not include participants that had 30% or more of outliers in at least one of their two experimental runs in any analyses. EPI volumes were spatially smoothed using an 8‐mm full width at half‐maximum isotropic Gaussian kernel.
2.5. Brain activity during English word reading in bilinguals and monolinguals
To examine whole‐brain activity associated with reading within each group, we used the Statistical Parametric Mapping (SPM)12 toolbox (https://www.fil.ion.ucl.ac.uk/spm/), constructing and testing the fit of the imaging data to a general linear model (Friston et al., 1994). For each group, we created statistical parametric maps that corresponded to the time‐courses of the contrast of real word reading (RW) greater than false fonts (FF), that is (RW > FF). At the first level analysis, voxel‐wise t‐maps were constructed for each of the participants. At the second level, we tested for group effects, using the amplitude maps. A one‐sample t‐test was performed to test for whole‐brain random effects for this contrast of interest, (RW > FF), within each group (bilinguals and monolinguals).
We then compared mean brain activity during word reading between bilinguals and monolinguals. For this second‐level analysis, we examined between‐group differences using the activation maps of each group (bilinguals [RW > FF], and monolinguals [RW > FF]) in two‐sample t‐tests, resulting in the following maps: Bilinguals (RW > FF) > monolinguals (RW > FF), and monolinguals (RW > FF) > bilinguals (RW > FF). The results are reported at a cluster‐size threshold of p < .05 with false discovery rate (p‐FDR) and a height threshold of p < .005. Using the SPM12 brain template, we present all group‐level activation maps surface‐rendered in MNI space. We used the SPM Anatomy Toolbox (Eickhoff et al., 2005) v2.1 (https://www.fz‐juelich.de/SharedDocs/Downloads/INM/INM‐1/DE/Toolbox/Toolbox_18.html?nn=1090980) to identify the anatomical labels of the resulting clusters and the MNI ← → Talairach Converter (1.3) (https://bioimagesuiteweb.github.io/webapp/mni2tal.html) from the BioImage Suite Web to identify the Brodmann Area (BA).
2.6. Brain functional connectivity during English word reading in bilinguals and monolinguals
To test for a potential effect of the bilingual experience on functional connectivity, we performed a seed‐to‐voxel analysis testing whether bilinguals show functional connectivity during word reading that is different from that observed in monolinguals. This seed‐based analysis examines the temporal correlation between a given region of interest (ROI) and all the other voxels across the experimental session (Friston et al., 1997). Instead of using an ROI‐to‐ROI approach, we chose a seed‐to‐voxel analysis to capture brain regions that are outside the seed ROIs, yet are relevant to the neurobiology of reading or executive control.
We defined eight seed ROIs in the left hemisphere based on the main coordinates reported in two meta‐analyses of fMRI studies of reading in adults (Martin et al., 2015: German, French, English; Bolger et al., 2005: Italian, German, French, English) and as reported by (Brignoni‐Perez et al., 2020). From the Martin et al. (2015) meta‐analysis of 20 studies in adults, we used seven coordinates to create the following regions in the left hemisphere: inferior temporal gyrus (L‐ITG) (x = −48, y = −62, z = −20), inferior occipital gyrus (L‐IOG) (x = −44, y = −74, z = −4), and middle occipital gyrus (L‐MOG) (x = −42, y = −86, z = −2) in the OTC; intraparietal sulcus (L‐IPS) (x = −42, y = −48, z = 48) in the TPC; and, inferior frontal gyrus pars opercularis (L‐IFG, oper) (x = −52, y = 18, z = 14), inferior frontal gyrus pars triangularis (L‐IFG, tri) (x = −52, y = 20, z = 18), and middle frontal gyrus (L‐MFG) (x = −42, y = 4, z = 48) in the IFC. Additionally, from the Bolger et al. (2005) meta‐analysis of 25 studies in adults, we identified an eighth coordinate in the left superior temporal gyrus (L‐STG) (MNI transformed: x = −54, y = −29, z = 10) in the TPC. We used the MarsBaR toolbox 0.44 (http://marsbar.sourceforge.net/) to create a spherical seed with a 6‐mm radius for each ROI, similar to other studies of reading or bilingualism, or both (Li et al., 2015; Oliver et al., 2016; Stevens et al., 2017).
To perform our functional connectivity analysis, we used the CONN toolbox v.18.a. We did not high band‐pass filter (i.e., 0.008‐Inf) the functional data, so that we could detect task‐derived correlations. White matter and cerebrospinal fluid signals, as well as their derivatives, were modeled as covariates, in order to capture signals coming only from gray matter (CompCor in CONN: Behzadi et al., 2007). Since our research question focuses on task‐derived functional connectivity (i.e., reading), we carried out the psycho‐physiological interaction (gPPI) analysis. As described earlier, we used eight ROIs (L‐ITG; L‐IOG; L‐MOG; L‐IPS; L‐STG; L‐IFG, oper; L‐IFG, tri; L‐MFG) as seeds to compare their signals with that of all the voxels in the cerebrum and the cerebellum, testing for changes in regional interhemispheric correlation within and between the two groups. At the single‐participant level, we defined a psychological variable representing our two conditions of interest (i.e., main task, RW, and active control task, FF), a physiological variable (i.e., the time course within each seed), and a PPI term (i.e., the interaction between these two regressors).
We examined functional connectivity during English word reading within each group (bilinguals [RW > FF]; monolinguals [RW > FF]) and between the groups (bilinguals [RW > FF] > monolinguals [RW > FF]; monolinguals [RW > FF] > bilinguals [RW > FF]). All the results are reported using a cluster‐size threshold of p‐FDR < .05 and a height threshold of p < .005. In addition, we corrected for multiple comparisons (Bonferroni; p‐FDR .05/8 ROIs), establishing a more stringent threshold of p < .006 (results surviving this threshold are marked with * in the reporting of the results). Group‐based, functional correlation maps were surface‐rendered in MNI space, using the brain template available in the CONN toolbox. We used the same tools for anatomical labeling and BA identification as for the brain activation results (Section 2.6) and the CONN anatomy labeling approach when the SPM Anatomy Toolbox was not sufficiently specific.
3. RESULTS
3.1. In‐scanner task performance and postscanning stimuli recognition test
For both accuracy and response time, we compared the bilingual and the monolingual groups using the difference scores between the two active task conditions (RW‐FF), to parallel the imaging data analyses and found them not to differ (independent samples t‐test, see Table 1; one bilingual participant's data were not available for analysis). The findings from the pencil‐and‐paper recognition test, administered following the MRI scan, indicated that participants from both groups implicitly processed the real‐word stimuli: participants in both groups recognized real words at levels greater than chance (60% accuracy in bilinguals [N = 23; 2 participants' data were not available for analysis] and 64% accuracy in monolinguals) and did not differ from each other.
3.2. Brain activity for English word reading within bilinguals and monolinguals
3.2.1. Bilinguals
English word reading yielded activation in a large cluster in the left inferior frontal gyrus pars opercularis, extending to pars triangularis (Figure 1; Table 3), as previously reported by Brignoni‐Perez et al. (2020).
FIGURE 1.
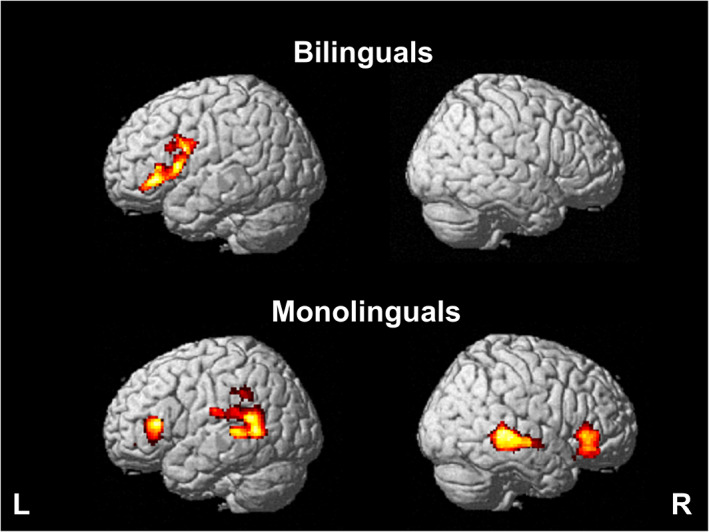
Brain activity within groups. English real word reading relative to false fonts (RW > FF) in bilinguals (top) and monolinguals (bottom). Cluster size pFDR <0.05, height threshold p < .005
TABLE 3.
Results of mean brain activity within and between groups
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Group | Cluster location | x y z | Peak Z | Voxels | BA |
| Bilinguals | |||||
| L inferior frontal gyrus pars opercularis extending to pars triangularis | −38 −6 28 | 3.90 | 1124 | N/A | |
| Monolinguals | |||||
| L middle temporal gyrus | −54 −50 16 | 3.91 | 1252 | 39 | |
| L inferior frontal gyrus pars triangularis | −48 30 10 | 5.18 | 408 | 45 | |
| R middle temporal gyrus | 70 –36 2 | 4.01 | 1265 | 21 | |
| R inferior frontal gyrus pars triangularis extending to pars orbitalis | 54 32 0 | 4.89 | 579 | 45 | |
| Bilinguals > Monolinguals | |||||
| n.s. | |||||
| Monolinguals > Bilinguals | |||||
| n.s. | |||||
Abbreviations: BA, Brodmann's area; L, left hemisphere; MNI, Montreal Neurological Institute; n.s., nonsignificant (pFDR = or >.05); N/A, outside defined BAs; R, right hemisphere.
3.2.2. Monolinguals
English word reading yielded activation in four clusters: left middle temporal gyrus and inferior frontal gyrus pars triangularis and right middle temporal gyrus and inferior frontal gyrus pars triangularis extending to pars orbitalis (Figure 1; Table 3).
3.3. Brain activity for English word reading compared between bilinguals and monolinguals
3.3.1. Bilinguals > Monolinguals
Bilinguals had no activity that was significantly greater than that observed in monolinguals during English word reading.
3.3.2. Monolinguals > Bilinguals
Relative to bilinguals, monolinguals had no areas of significantly greater brain activity.
3.4. Brain functional connectivity during English word Reading within bilinguals and monolinguals
3.4.1. Bilinguals
Positive functional connectivity was observed in the bilingual group between the seed in the left superior temporal gyrus (L‐STG) and a cluster in the left lingual gyrus extending to right cerebellar lobule VI.
Negative functional connectivity was found between the seed in the left middle occipital gyrus (L‐MOG) and a cluster in the left postcentral gyrus extending to the left precentral gyrus; and, between the seed in the left middle frontal gyrus (L‐MFG) and a cluster spanning the right and the left anterior cingulate cortices extending to left and right paracingulate gyri (Figure 2a; Table 4).
FIGURE 2.
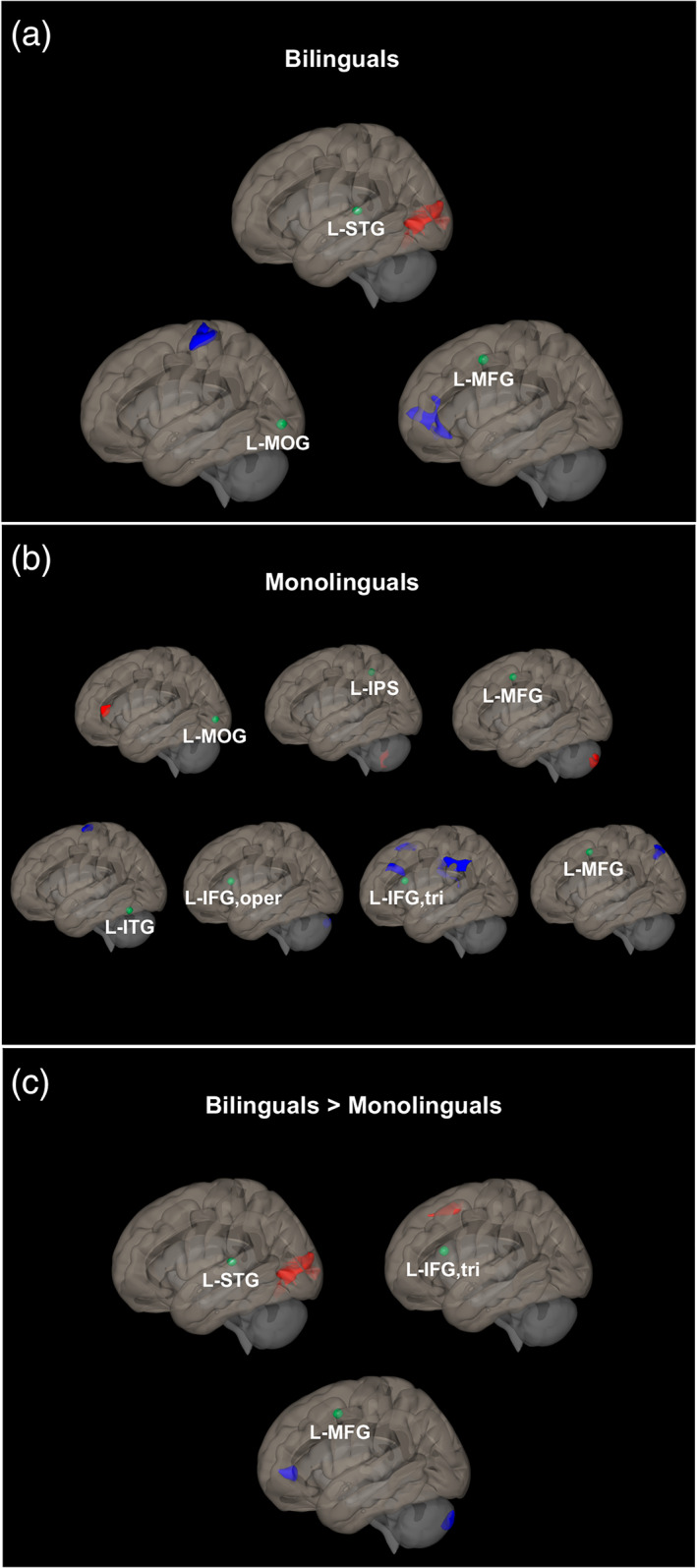
Brain functional connectivity within and between groups. (a) Positive (top/red clusters) and negative (bottom/blue clusters) functional connectivity during English real word reading relative to false fonts (RW > FF) in bilinguals. (b) Positive (top/red clusters) and negative (bottom/blue clusters) functional connectivity during English real word reading relative to false fonts (RW > FF) in monolinguals. (c) Positive (top/red clusters) and negative (bottom/blue clusters) functional connectivity differences during English real word reading in bilinguals compared to monolinguals (bilinguals [RW > FF] > monolinguals [RW > FF]). Cluster size pFDR <.05, height threshold p < .005
TABLE 4.
Results of brain functional connectivity within and between groups
| MNI Coordinates | |||||
|---|---|---|---|---|---|
| Group | Seed ROIs | Cluster location | x y z | Voxels | BA |
| Bilinguals | |||||
| L‐STG | (+) L lingual gyrus extending to R cerebellar lobule VI* | −12 −74 0 | 1425 | 18 | |
| L‐MOG | (−) L postcentral gyrus extending to L precentral gyrus* | −46 −20 64 | 480 | N/A | |
| L‐MFG | (−) R and L anterior cingulate cortices extending to L and R paracingulate gyri* | 12 40 –12 | 647 | 11 | |
| Monolinguals | |||||
| L‐MOG | (+) L inferior frontal gyrus pars triangularis | −38 34 6 | 243 | 45 | |
| L‐IPS | (+) R cerebellar crus II extending to crus I | 48 –62 −46 | 227 | N/A | |
| L‐MFG | (+) L cerebellar crus II* | −26 −86 −38 | 330 | N/A | |
| L‐ITG | (−) L and R precentral gyri | 0 –20 72 | 261 | 6 | |
| L‐IFG, oper | (−) R cerebellar crus I extending to crus II | 30 –86 −28 | 241 | N/A | |
| L‐IFG, tri | (−) R supramarginal gyrus extending to postcentral gyrus* | 68 –16 28 | 462 | N/A | |
| (−) L and R medial frontal cortices extending to L and R paracingulate gyri* | −2 16 54 | 393 | N/A | ||
| (−) L supramarginal gyrus extending to postcentral gyrus* | −66 −42 34 | 342 | N/A | ||
| (−) L middle frontal gyrus* | −38 26 28 | 282 | 9 | ||
| L‐MFG | (−) L superior parietal lobule | −24 −74 52 | 255 | 7 | |
| Bilinguals > Monolinguals | |||||
| L‐STG | (+) L lingual gyrus extending to R lingual gyrus | −12 −72 0 | 367 | 18 | |
| L‐IFG, tri | (+) L and R medial frontal cortices extending to L and R paracingulate gyri* | −4 12 52 | 502 | 6 | |
| L‐MFG | (−) L anterior cingulate cortex extending to L and R paracingulate gyri | −4 40 –2 | 343 | 32 | |
| (−) L cerebellar crus II | −14 −86 −42 | 301 | N/A | ||
| Monolinguals > Bilinguals | n.s. |
Abbreviations: BA, Brodmann's area; MNI, Montreal Neurological Institute; N/A, outside defined BAs; L, left hemisphere; R, right hemisphere; (+), positive FC; (−), negative FC.
Survived Bonferroni correction p < .006.
3.4.2. Monolinguals
Positive connectivity was found in the monolingual group between the seed in the L‐MOG and a cluster in the left inferior frontal gyrus pars triangularis; the seed in the left intraparietal sulcus (L‐IPS) and a cluster in right cerebellar crus II extending to crus I; and, the seed in the L‐MFG and a cluster in left cerebellar crus II.
Negative connectivity was found between the seed in the left inferior temporal gyrus (L‐ITG) and a cluster spanning left and right precentral gyri; the seed in the left inferior frontal gyrus pars opercularis (L‐IFG, oper) and a cluster in right cerebellar crus I extending to crus II; the seed in the left inferior frontal gyrus pars triangularis (L‐IFG, tri) and a cluster in the right supramarginal gyrus extending to the postcentral gyrus, a cluster in bilateral medial frontal cortex extending into bilateral paracingulate gyrus, a cluster in the left supramarginal gyrus extending to the postcentral gyrus, and a cluster in the left middle frontal gyrus; and, lastly, between the seed in the left middle frontal gyrus (L‐MFG) and a cluster in the left superior parietal lobule (Figure 2b; Table 4).
3.5. Brain functional connectivity during English word Reading compared between bilinguals and monolinguals
3.5.1. Bilinguals > Monolinguals
Bilinguals compared to monolinguals had stronger positive functional connectivity in two seed regions: between the seed in the left superior temporal gyrus (L‐STG) and a cluster in the left lingual gyrus (also found in the bilingual within‐group functional connectivity map) extending to the right lingual gyrus; and, between the seed in the left inferior frontal gyrus pars triangularis (L‐IFG, tri) and a cluster spanning the left and the right medial frontal cortices extending to the left and the right paracingulate gyri.
Bilinguals also had stronger negative connectivity (i.e., stronger anticorrelation) between one seed region, left middle frontal gyrus (L‐MFG), and a cluster in the left anterior cingulate cortex (similar to the connection in the bilinguals within‐group map) extending to the left and the right paracingulate gyri, and another cluster in left cerebellar crus II (Figure 2c; Table 4).
3.5.2. Monolinguals > Bilinguals
Relative to bilinguals, monolinguals had no significantly stronger functional connectivity.
4. DISCUSSION
Reading is an important learned skill that predicts academic and vocational outcome. Brain‐based models of reading have been derived primarily from monolinguals, even though bilingualism is prevalent across the world. As such, the brain bases of reading need to be investigated specifically in bilinguals. To date, no study has compared early bilingual adults to monolingual adults during English word reading using fMRI, even though there are many fMRI studies of reading in adult English speakers. Here, we compared brain function in 25 early, balanced Spanish‐English bilingual adults to that of 33 English‐speaking monolingual adults during an English word processing task. At the local activation level, we found that our groups engaged regions typically associated with reading, specifically the left inferior frontal gyrus pars opercularis extending to pars triangularis (IFC) in the bilinguals, and the left inferior frontal gyrus pars triangularis (IFC), as well as the left middle temporal gyrus (OTC) in the monolinguals. When directly comparing the two groups, however, we did not observe any statistically significant differences. When we expanded this comparison to the network level, we observed functional connections for both groups between some of the preselected seed regions (based on their known involvement in reading) and the rest of the brain. Some of these regions are associated with reading, whereas others are not. A between‐group comparison revealed relatively stronger functional connectivity between two pairs of regions in bilinguals compared to monolinguals. Specifically, between the left superior temporal gyrus (TPC) and the left lingual gyrus (positive), and between the left middle frontal gyrus (IFC) and the left anterior cingulate cortex (negative). The monolingual group did not show any relatively stronger functional connectivity during reading compared to the bilingual group.
Our result of no differences in activity during word processing between bilinguals and monolinguals, combined with few differences in functional connectivity, suggests that the influence of a Spanish‐English bilingual experience on the brain bases of reading is relatively subtle. This finding alleviates concerns that current models of reading cannot be applied to Spanish‐English early bilingual adults. It implies that other areas of reading research, such as reading development, reading instruction, and reading disabilities, may be very similar for Spanish‐English early bilinguals and English‐speaking monolinguals. Our results also suggest that there may be no need to distinguish between Spanish‐English early bilinguals and monolinguals during the selection of participants in studies of reading. The observed results will be discussed in the context of prior work next.
4.1. Brain activity during word reading in bilinguals and monolinguals
In an fMRI study of Spanish word reading in seven Spanish‐Catalan bilingual adults compared to seven Spanish‐speaking monolingual adults, Rodriguez‐Fornells et al. (2002) found relatively more activity in the left planum temporale/superior temporal gyrus and the left inferior frontal gyrus pars opercularis in the bilinguals. The authors suggested that this difference reflected the neurobiological bases of inhibition of the nontarget language (at the level of the indirect/sublexical route), while processing the target language. They concluded that having a bilingual background affects the brain bases of reading, because of the cognitive advantages afforded by the dual language experience. However, as it will be discussed in more detail below, their reading task heavily depended on cognitive control, the sample size was small, and the existence of such cognitive adaptation in bilinguals has been debated. In the context of English word reading, Jasińska and Petitto (2014) conducted an fNIRS study in children and adults who were bilingual in English and various other languages (compared to English‐speaking monolinguals). These authors found more activity in bilateral superior temporal gyrus and the left inferior frontal gyrus in bilingual adults (N = 8) compared to monolingual adults (N = 8). Despite the similarity to the results from Rodriguez‐Fornells et al. (2002), Jasińska and Petitto (2014) offered a different interpretation to theirs. They proposed that the observed difference reflected the bilinguals' need to engage their phonological system to a greater extent, given that they have access to two languages (more load/information) instead of one. Both studies concluded that the bilingual experience modifies the language‐related brain systems that support reading.
In the present study, however, we did not observe differences in activity between the two groups. The earlier two studies differed from the current study in their experimental design in ways that may explain the lack of convergence across the results. Rodriguez‐Fornells et al. (2002) employed a single‐word processing task based on Go/No‐go actions. Specifically, participants were presented with Spanish words, Catalan words, and pseudowords (half derived from Spanish and half derived from Catalan). They had to make a discriminative response for Spanish words (press a button on the left or right hand, if the first letter is a vowel or consonant, respectively), and withhold any responses for Catalan words and pseudowords. fMRI data derived from the Spanish word task (which effectively represented a Go condition) were contrasted to those from a consonant strings‐based baseline control condition. Therefore, their word processing task likely involved some level of executive function that was not controlled for with the baseline condition, making it possible that their between‐group differences have more to do with inhibitory control and task switching than with reading. Jasińska and Petitto (2014) used an aloud English single‐word reading task. Our reading task involved making a decision based on silent single‐word processing (compared to another active control task), like many tasks used in the field of reading neuroimaging research (Martin et al., 2015). Thus, these two prior studies and the current one all used fairly different reading tasks.
Also, it is worth considering the languages involved in these studies and how well participants read in the language in which words were presented during brain imaging. Rodriguez‐Fornells et al. (2002) examined Spanish word reading, in bilinguals who also spoke and read in Catalan, both of which are alphabetic languages. Jasińska and Petitto (2014) examined English word reading in bilinguals whose other language for speaking and reading was another alphabetic (e.g., French, Spanish, Russian) or nonalphabetic (e.g., Cantonese) language. Despite these differences in the languages, these two prior studies had results that were similar to one another and, surprisingly, dissimilar to the results of the present study. A potential explanation for the discrepancy between the current study and these prior studies could be reading proficiency of the bilinguals relative to the monolinguals for the language in which the reading task was performed during the scan. Critically, our bilinguals and monolinguals had reading abilities in the typical (or above) range for English word reading on a standardized, objective measure, and their performance was matched. This avoids the concern that any differences in activation in bilinguals and monolinguals during English word processing can be attributed to a discrepancy in the two groups' reading abilities. The two prior studies did not report reading ability for their bilinguals and monolinguals in this way, and thus it is unknown if their brain‐based findings could be attributed to differences in reading ability. It has been reported in studies of developmental dyslexia that adults with lower reading abilities show relatively lower brain activity in the left superior temporal and inferior frontal gyri (Maisog et al., 2008; Richlan et al., 2011), the same regions reported to be different in bilinguals in these two prior investigations. At the same time, it is not likely that our lack of a difference in activity during English word reading between our bilinguals and monolinguals is due to the bilinguals not having a strong representation of Spanish. The bilinguals had 12 years on average of formal study in Spanish, reported high levels of spoken Spanish proficiency, and performed very well on standardized measures of Spanish word reading.
In terms of statistical approaches, there are also differences between these studies and the present study. Rodriguez‐Fornells et al. (2002) used a lenient threshold (uncorrected p < .001, and clusters of 10 contiguous voxels). Jasińska and Petitto (2014) used fNIRS, and hence the analyses are different from those performed with fMRI data. Importantly, group sizes were considerably smaller in both studies, with Rodriguez‐Fornells et al. (2002) having seven participants per group, and Jasińska and Petitto (2014) having eight per group. By comparison, our study overall had quadruple the sample size, with 58 participants (25 bilinguals and 33 monolinguals), and thus significantly more power. We also used a more stringent statistical threshold, one that reflects current practices in the field. Given our null result, we wondered whether there would be between‐group differences, if we applied a more focused approach, such as an ROI‐based analysis. While not described in Sections 2 and 3 because it was not our a priori analysis approach, we used the eight spheres that are known to be involved in reading (those used as seed regions for the functional connectivity analysis) to compare activity between the groups. We did not find any between‐group differences with this ROI‐based approach, either.
Taken together, the most likely explanations for why our fMRI results do not agree with those from Rodriguez‐Fornells et al. (2002) are due to our methodology: ensuring good reading proficiency and matching the two groups on reading ability for the language used during the task; using a simple reading task that is consistent with those used in the field of reading and not involving an executive function paradigm; and, applying a more robust statistical approach (larger sample sizes and rigorous statistical thresholds). These factors will be critical for future studies in early bilinguals. Further, such studies should be conducted in languages other than Spanish and English, to assess whether the lack of a difference in brain activity seen in the current study generalizes to early bilingual users of other languages.
The goal of our study was to address the fact that the large corpus of reading studies has been limited to monolingual participants (or participants whose language background was not described) and to assess whether the early bilingual experience, like other early experiences, influences brain function during reading. Such a finding would motivate the need for a model describing the brain bases of reading in multilingual populations. There would also be practical consequences to reading instruction; and, reading disability (e.g., developmental dyslexia) would need to be given separate consideration in bilinguals. In the field of brain imaging, participant selection for studies of reading would need to take language experience into consideration, knowing that the results would be dependent on the proportion of bilingual versus monolingual participants. As outlined in the Introduction, prior behavioral and neuroimaging studies have shown differences in language and executive function in bilinguals relative to monolinguals, raising the possibility that such adaptations could infiltrate the reading process. While our research question had to be considered in the context of theories that being bilingual may come with adaptations relative to and beyond language, our study did not test the “bilingual advantage” previously reported by others. While it has been shown that bilinguals activate regions involved in executive function more than monolinguals (Costa & Sebastián‐Gallés, 2014; Grundy et al., 2017; Pliatsikas & Luk, 2016) and also activate these regions when switching between their languages (Luk, Green, et al., 2011; Luk, Sa, & Bialystok, 2011) as a mechanism for bilingual control (Abutalebi & Green, 2008), it is not clear whether these processes permeate reading. As already noted above, it is possible that the task used by Rodriguez‐Fornells et al. (2002) demanded executive function, thereby testing executive function more than reading per se. Our own result for local activation suggests that the influence of bilingualism on brain activity, if it exists, does not impact English single‐word reading in Spanish‐English early bilingual adults.
4.2. Brain functional connectivity during English word reading in bilinguals and monolinguals
In bilinguals, when testing for functional connectivity during English word reading using the eight seed regions, we found positive functional connectivity between the left superior temporal gyrus seed and the left lingual gyrus. This group also had negative functional connectivity between the left middle occipital gyrus seed and the left post‐ and precentral gyri, as well as between the left middle frontal gyrus seed and bilateral anterior cingulate cortex. Monolinguals had positive functional connectivity between regions of the left middle occipital gyrus seed and the left inferior frontal gyrus pars triangularis, as reported by others (Bokde et al., 2001; Mechelli et al., 2005; Perrone‐Bertolotti et al., 2017). Additionally, in this group, there was positive functional connectivity between the left intraparietal sulcus seed and the right cerebellum, as well as between the left middle frontal gyrus seed and the left cerebellum. The monolingual group also showed several negative functional connections. These connections were between the left inferior temporal gyrus seed and bilateral precentral gyrus, as well as between the left inferior frontal gyrus pars opercularis seed and the right cerebellum. The left inferior frontal gyrus pars triangularis seed had four negative functional connections with regions of the TPC (left and right supramarginal gyri) and the IFC (bilateral medial frontal cortex and left middle frontal gyrus). Lastly, the left middle frontal gyrus seed had a negative functional connection with the left superior parietal lobule. Thus, both groups displayed seed‐to‐voxel functional connections between a subset of the eight seed regions selected from studies of reading and other brain regions. These other brain regions have largely been associated with reading, but in some cases with executive function (i.e., anterior cingulate cortex, medial frontal cortex, and superior parietal lobule).
During reading‐related tasks (Bitan et al., 2006; Hampson et al., 2006; Horwitz et al., 1998; Mechelli et al., 2005; Pugh et al., 2000), as well as during rest (Koyama et al., 2010, 2011), it has been shown that the OTC, TPC, and IFC are functionally correlated. For example, Horwitz et al. (1998) and Pugh et al. (2000) showed correlations of activity between regions of the OTC and the IFC during word reading; similar findings have been reported by Bokde et al. (2001), Mechelli et al. (2005), and Perrone‐Bertolotti et al. (2017). Even at rest, Koyama et al. (2010) showed that brain regions involved in reading are functionally connected, reflecting their strong cohesiveness as a function of long‐term reading experience. In the present study, we observed positive functional connectivity between cortical regions of the OTC and the IFC during reading within the monolingual group that is consistent with these reports from prior studies of reading.
When comparing the two groups to test whether there are differences in functional connectivity during English word reading between bilinguals and monolinguals, we found between‐group differences for three of our eight seeds. The bilinguals showed stronger positive functional connectivity between the superior temporal gyrus seed and the left lingual gyrus relative to monolinguals. This functional connection was also observed in the bilingual within‐group functional connectivity map, suggesting it to be a connection used in bilinguals, but not in monolinguals. Both regions are associated with reading (but not executive function), suggesting that this heightened functional connection in bilinguals may indicate a stronger need in readers of two languages to connect between regions that represent the sound and the visual form of a word (although it should be noted that the location of the connection is not in the actual functional “visual word form area”) (Mechelli et al., 2000). We also found that bilinguals had stronger positive functional connectivity between the left inferior frontal gyrus seed and bilateral medial frontal cortex and paracingulate gyrus. This connection was not, however, observed in the within‐group analysis, making it difficult to gauge how robust this finding is, but noting that medial frontal cortex is known to be involved in executive function (Chen et al., 2009; Hsu et al., 2011; Öngür et al., 2003; Ridderinkhof et al., 2007).
We also observed stronger negative functional connectivity between the left middle frontal gyrus seed and the left cerebellum, as well as the left anterior cingulate cortex, a functional connection that manifested also in the bilingual within‐group map (which was not the case for the cerebellum). The anterior cingulate cortex has been shown to be involved in executive function, but not in word reading, suggesting that this functional connection is not associated with reading specifically, but perhaps with aspects of executive control (Fellows & Farah, 2005). Of note, the origin, interpretation, association with structural connectivity, and neurophysiological role of negative functional connectivity, or anticorrelation, has been a matter of discussion (Chen et al., 2011). However, various studies have shown it to be as neurobiologically relevant as positive functional connectivity is (Chen et al., 2011; Fox et al., 2009; Schölvinck et al., 2010; Schwarz & McGonigle, 2011), including fronto‐parietal and occipital networks (Zhan et al., 2017). Monolinguals had no stronger functional connectivity in comparison to bilinguals.
Given these findings at the network level in the absence of differences in local activity between the groups, our results suggest that bilinguals have some but not many differences when compared to monolinguals. It is important to note that our eight left‐hemisphere seed regions were chosen based on prior studies of reading and we did not specifically place seeds in regions associated with executive control. We tested, however, for functional connections between these seed regions and the rest of the brain; and, several of the seed regions are in brain areas associated with executive control, yet most of them did not display differences between the two groups. Thus, while it has been suggested that an advantage in executive function in bilinguals permeates written language processing (Rodriguez‐Fornells et al., 2002), our results do not speak to a strong effect. Such influence would have been demonstrated by functional connections between seed regions in areas that subserve reading and brain regions known to be involved in executive function, such as dorsolateral prefrontal and parietal cortices. We did find stronger functional intercommunication between one of the frontal seed regions, the left middle frontal gyrus, and a brain region known to be involved in executive function, the left anterior cingulate cortex. This result does conform to the prediction and raises the possibility that executive function does play a role during English word reading in Spanish‐English bilinguals relative to monolinguals, but it is a relatively isolated finding. The other main result was between the superior temporal gyrus seed and the lingual gyrus, a region associated with reading, which had positive functional connectivity, even though this latter region was not sufficiently robust in the literature (Bolger et al., 2005; Martin et al., 2015) to include it as one of our eight ROIs.
Our results raise the question whether our findings would be similar if our monolinguals had been Spanish speakers rather than English speakers and the brain imaging task had involved Spanish word reading instead of English word reading. Prior studies have compared reading of English versus reading of Spanish words in Spanish‐English participants. Meschyan and Hernandez (2006) and Jamal et al. (2012) reported differences in activation between English word reading and Spanish word reading. However, when more studies were conducted in larger groups of Spanish‐English bilinguals, they reported no differences in activation between English versus Spanish word reading (Brignoni‐Perez et al., 2020; Hernandez et al., 2015). Based on these more recent results, one would expect similar results as those reported here, that is independent of the orthographic depth of the language.
Taken together, these observations at the local level and the network level do not provide strong support for the notion that early dual language experience with Spanish and English influences the neurofunctional bases of reading words in English extensively. While we report some differences in functional connections, they are limited, and they do not bear the signature of being under the influence of multiple regions known to be involved in executive function.
4.3. Future studies
Since our study focused on Spanish‐English early bilingual adults, the question arises whether our findings can be generalized to bilinguals using other languages and other writing systems and of different ages. Future studies need to address this question, specifically focusing on a range of languages and writing systems, ideally using the same study designs and protocols. The current study focused on adults. There have been some investigations into reading in bilingual children (Jasińska et al., 2017; Jasińska & Petitto, 2014), and it will be important to include children in future studies. These studies will benefit from large samples of participants matched on reading ability and the use of stringent statistical thresholds. Lastly, studies could be done with the inclusion of both early and late bilinguals as a way to assess the more profound impact of experience‐dependent plasticity typically reported in late bilinguals (Połczyńska & Bookheimer, 2021).
5. CONCLUSIONS
Prior studies on the brain bases of reading have focused almost exclusively on monolinguals. The goal of this study was to test whether an early bilingual experience drives differences in brain activity and functional connectivity during English word reading. We found no differences in local brain activity between Spanish‐English early bilingual and English‐speaking monolingual adults during English word reading. While bilinguals differed from monolinguals in the strength of some of their functional connections between seed regions chosen based on their involvement in reading and the rest of the brain, the results overall did not indicate many differences. These findings counter an earlier fMRI study of reading in Spanish, which found differences in local activation between bilinguals and monolinguals and attributed these to executive control (Rodriguez‐Fornells et al., 2002). Based on our task and larger sample size, and the fact that our groups were equated for reading abilities, we interpret our findings of no differences in local activity and small differences in functional connectivity to mean that brain‐based models of English word reading developed from monolinguals likely extend to Spanish‐English early bilingual adults.
CONFLICT OF INTEREST
The authors declare that they have no competing financial interests or commercial considerations and that this material has not been published (nor is it under consideration for publication) elsewhere.
ACKNOWLEDGMENTS
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P50 HD040095 and R01 HD081078), the National Science Foundation (SBE 0541953), and a supplement from the National Institutes of Health and the National Science Foundation to the SBE 0541953. The authors thank the Georgetown University's Biomedical Graduate Education and the Office of the Dean for Research, as well as the Center for Functional and Molecular Imaging under the support of the Intellectual and Development Disorders Research Center grant (P30HD040677). The authors would like to acknowledge Lynn Flowers, Melanie Lozano, Eileen Napoliello, Emma Cole, and Jenni Rosenberg, for their assistance. The authors thank K. Breana Downey for contributing to the data collection and for providing comments on the manuscript. The authors also thank all our participants for their time.
Brignoni‐Pérez, E. , Jamal, N. I. , & Eden, G. F. (2022). Functional neuroanatomy of English word reading in early bilingual and monolingual adults. Human Brain Mapping, 43(14), 4310–4325. 10.1002/hbm.25955
Funding information Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: P50 HD40095, R01 HD081078; National Science Foundation, Grant/Award Number: SBE 0541953
DATA AVAILABILITY STATEMENT
Data used in this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abutalebi, D. J. , & Green, D. W. (2008). Control mechanisms in bilingual language production: Neural evidence from language switching studies. Language and Cognitive Processes, 23(4), 557–582. 10.1080/01690960801920602 [DOI] [Google Scholar]
- Allman, B. (2005). Vocabulary size and accuracy of monolingual and bilingual preschool. ISB4: Proceedings of the 4th International Symposium on Bilingualism, ed. JamesCohen, Kara T. McAlister, Kellie Rolstad, and Jeff MacSwan, Somerville, MA: Cascadilla Press Children, 21, 58–77. [Google Scholar]
- Ardila, A. , Bernal, B. , & Rosselli, M. (2018). Executive functions brain system: An activation likelihood estimation meta‐analytic study. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 33(4), 379–405. 10.1093/arclin/acx066 [DOI] [PubMed] [Google Scholar]
- Arrington, C. N. , Kulesz, P. A. , Francis, D. J. , Fletcher, J. M. , & Barnes, M. A. (2014). The contribution of attentional control and working memory to reading comprehension and decoding. Scientific Studies of Reading, 18(5), 325–346. 10.1080/10888438.2014.902461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi, Y. , Restom, K. , Liau, J. , & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok, E. (2006). Effect of bilingualism and computer video game experience on the Simon task. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Expérimentale, 60(1), 68–79. 10.1037/cjep2006008 [DOI] [PubMed] [Google Scholar]
- Bialystok, E. , Craik, F. I. M. , Klein, R. , & Viswanathan, M. (2004). Bilingualism, aging, and cognitive control: Evidence from the Simon task. Psychology and Aging, 19(2), 290–303. 10.1037/0882-7974.19.2.290 [DOI] [PubMed] [Google Scholar]
- Biswal, B. , Zerrin Yetkin, F. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar mri. Magnetic Resonance in Medicine, 34(4), 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Bitan, T. , Burman, D. D. , Lu, D. , Cone, N. E. , Gitelman, D. R. , Mesulam, M.‐M. , & Booth, J. R. (2006). Weaker top‐down modulation from the left inferior frontal gyrus in children. NeuroImage, 33(3), 991–998. 10.1016/j.neuroimage.2006.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde, A. L. W. , Tagamets, M.‐A. , Friedman, R. B. , & Horwitz, B. (2001). Functional interactions of the inferior frontal cortex during the processing of words and word‐like stimuli. Neuron, 30(2), 609–617. 10.1016/S0896-6273(01)00288-4 [DOI] [PubMed] [Google Scholar]
- Bolger, D. J. , Perfetti, C. A. , & Schneider, W. (2005). Cross‐cultural effect on the brain revisited: Universal structures plus writing system variation. Human Brain Mapping, 25(1), 92–104. 10.1002/hbm.20124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignoni‐Perez, E. , Jamal, N. I. , & Eden, G. F. (2020). An fMRI study of English and Spanish word reading in bilingual adults. Brain and Language, 202, 104725. 10.1016/j.bandl.2019.104725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.‐Y. , Muggleton, N. G. , Tzeng, O. J. L. , Hung, D. L. , & Juan, C.‐H. (2009). Control of prepotent responses by the superior medial frontal cortex. NeuroImage, 44(2), 537–545. 10.1016/j.neuroimage.2008.09.005 [DOI] [PubMed] [Google Scholar]
- Chen, G. , Chen, G. , Xie, C. , & Li, S.‐J. (2011). Negative functional connectivity and its dependence on the shortest path length of positive network in the resting‐state human brain. Brain Connectivity, 1(3), 195–206. 10.1089/brain.2011.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik, E. C. , Mueller, V. I. , Eickhoff, C. R. , Langner, R. , & Eickhoff, S. B. (2015). Three key regions for supervisory attentional control: Evidence from neuroimaging meta‐analyses. Neuroscience & Biobehavioral Reviews, 48, 22–34. 10.1016/j.neubiorev.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocosco, C. A. , Kollokian, V. , Kwan, R. K.‐S. , Pike, G. B. , & Evans, A. C. (1997). BrainWeb: Online Interface to a 3D MRI simulated brain database. NeuroImage, 5, 425. [Google Scholar]
- Coleman, J. S. (1968). Equality of educational opportunity. Equity & Excellence in Education, 6(5), 19–28. 10.1080/0020486680060504 [DOI] [Google Scholar]
- Coltheart, M. (1981). The MRC psycholinguistic database. The Quarterly Journal of Experimental Psychology Section A, 33(4), 497–505. 10.1080/14640748108400805 [DOI] [Google Scholar]
- Conger, R. D. , & Donnellan, M. B. (2007). An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology, 58(1), 175–199. 10.1146/annurev.psych.58.110405.085551 [DOI] [PubMed] [Google Scholar]
- Costa, A. , Hernández, M. , & Sebastián‐Gallés, N. (2008). Bilingualism aids conflict resolution: Evidence from the ANT task. Cognition, 106(1), 59–86. 10.1016/j.cognition.2006.12.013 [DOI] [PubMed] [Google Scholar]
- Costa, A. , & Sebastián‐Gallés, N. (2014). How does the bilingual experience sculpt the brain? Nature Reviews Neuroscience, 15, 336–345. 10.1038/nrn3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costumero, V. , Rodríguez‐Pujadas, A. , Fuentes‐Claramonte, P. , & Ávila, C. (2015). How bilingualism shapes the functional architecture of the brain: A study on executive control in early bilinguals and monolinguals. Human Brain Mapping, 36(12), 5101–5112. 10.1002/hbm.22996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene, S. , & Cohen, L. (2007). Cultural recycling of cortical maps. Neuron, 56(2), 384–398. 10.1016/j.neuron.2007.10.004 [DOI] [PubMed] [Google Scholar]
- DeLuca, V. , Segaert, K. , Mazaheri, A. , & Krott, A. (2020). Understanding bilingual brain function and structure changes? U bet! A unified bilingual experience trajectory model. Journal of Neurolinguistics, 56, 100930. 10.1016/j.jneuroling.2020.100930 [DOI] [Google Scholar]
- Dick, A. (2018). No bilingual advantage for executive function: Evidence from a large sample of children in the Adolescent Brain and Cognitive Development (ABCD) Study. 10.31234/osf.io/gjh95 [DOI]
- Eden, G. F. , Olulade, O. A. , Evans, T. M. , Krafnick, A. J. , & Alkire, D. R. (2016). Developmental dyslexia. In Hickok I. G. & Small S. (Eds.), Neurobiology of language. Elsevier. [Google Scholar]
- Eickhoff, S. B. , Stephan, K. E. , Mohlberg, H. , Grefkes, C. , Fink, G. R. , Amunts, K. , & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25(4), 1325–1335. 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- European Commission Special Eurobarometer, Europeans and their languages . (2012). http://ec.europa.eu/publicopinion/archives/ebs/ebs243en.pdf
- Eviatar, Z. , & Ibrahim, R. (2000). Bilingual is as bilingual does: Metalinguistic abilities of Arabic‐speaking children. Applied PsychoLinguistics, 21(4), 451–471. 10.1017/S0142716400004021 [DOI] [Google Scholar]
- Fellows, L. K. , & Farah, M. J. (2005). Is anterior cingulate cortex necessary for cognitive control? Brain, 128(4), 788–796. 10.1093/brain/awh405 [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Zhang, D. , Snyder, A. Z. , & Raichle, M. E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101(6), 3270–3283. 10.1152/jn.90777.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. (1994). Functional and effective connectivity in neuroimaging: A synthesis. Human Brain Mapping, 2(1–2), 56–78. 10.1002/hbm.460020107 [DOI] [Google Scholar]
- Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity, 1(1), 13–36. 10.1089/brain.2011.0008 [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Buechel, C. , Fink, G. R. , Morris, J. , Rolls, E. , & Dolan, R. J. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6(3), 218–229. 10.1006/nimg.1997.0291 [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Holmes, A. P. , Worsley, K. J. , Poline, J.‐P. , Frith, C. D. , & Frackowiak, R. S. J. (1994). Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping, 2(4), 189–210. 10.1002/hbm.460020402 [DOI] [Google Scholar]
- Gabrieli, J. D. E. (2009). Dyslexia: A new synergy between education and cognitive neuroscience. Science (New York, N.Y.), 325(5938), 280–283. 10.1126/science.1171999 [DOI] [PubMed] [Google Scholar]
- Grady, C. L. , Luk, G. , Craik, F. I. M. , & Bialystok, E. (2015). Brain network activity in monolingual and bilingual older adults. Neuropsychologia, 66, 170–181. 10.1016/j.neuropsychologia.2014.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean, F. (2010). Bilingual: Life and reality. Harvard University Press. 10.4159/9780674056459 [DOI] [Google Scholar]
- Grundy, J. G. , Anderson, J. A. E. , & Bialystok, E. (2017). Neural correlates of cognitive processing in monolinguals and bilinguals. Annals of the New York Academy of Sciences, 1396, 183–201. 10.1111/nyas.13333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson, M. , Tokoglu, F. , Sun, Z. , Schafer, R. J. , Skudlarski, P. , Gore, J. C. , & Constable, R. T. (2006). Connectivity‐behavior analysis reveals that functional connectivity between left BA39 and Broca's area varies with reading ability. NeuroImage, 31(2), 513–519. 10.1016/j.neuroimage.2005.12.040 [DOI] [PubMed] [Google Scholar]
- Hayakawa, S. , & Marian, V. (2019). Consequences of multilingualism for neural architecture. Behavioral and Brain Functions, 15(1), 6. 10.1186/s12993-019-0157-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, A. E. , Woods, E. A. , & Bradley, K. A. L. (2015). Neural correlates of single word reading in bilingual children and adults. Brain and Language, 143, 11–19. 10.1016/j.bandl.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, B. , Rumsey, J. M. , & Donohue, B. C. (1998). Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences of the United States of America, 95(15), 8939–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, T.‐Y. , Tseng, L.‐Y. , Yu, J.‐X. , Kuo, W.‐J. , Hung, D. L. , Tzeng, O. J. L. , Walsh, V. , Muggleton, N. G. , & Juan, C.‐H. (2011). Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. NeuroImage, 56(4), 2249–2257. 10.1016/j.neuroimage.2011.03.059 [DOI] [PubMed] [Google Scholar]
- Jamal, N. I. , Piche, A. W. , Napoliello, E. M. , Perfetti, C. A. , & Eden, G. F. (2012). Neural basis of single‐word reading in Spanish‐English bilinguals. Human Brain Mapping, 33(1), 235–245. 10.1002/hbm.21208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, K. H. , & Engelhardt, L. (2012). The effects of handwriting experience on functional brain development in pre‐literate children. Trends in Neuroscience and Education, 1(1), 32–42. 10.1016/j.tine.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasińska, K. K. , Berens, M. S. , Kovelman, I. , & Petitto, L. A. (2017). Bilingualism yields language‐specific plasticity in left hemisphere's circuitry for learning to read in young children. Neuropsychologia, 98, 34–45. 10.1016/j.neuropsychologia.2016.11.018 [DOI] [PubMed] [Google Scholar]
- Jasińska, K. K. , & Petitto, L. A. (2014). Development of neural Systems for Reading in the monolingual and bilingual brain: New insights from functional near infrared spectroscopy neuroimaging. Developmental Neuropsychology, 39(6), 421–439. 10.1080/87565641.2014.939180 [DOI] [PubMed] [Google Scholar]
- Jones, O. P. , Green, D. W. , Grogan, A. , Pliatsikas, C. , Filippopolitis, K. , Ali, N. , Lee, H. L. , Ramsden, S. , Gazarian, K. , Prejawa, S. , Seghier, M. L. , & Price, C. J. (2012). Where, when and why brain activation differs for bilinguals and monolinguals during picture naming and reading aloud. Cerebral Cortex, 22(4), 892–902. 10.1093/cercor/bhr161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman, I. , Baker, S. A. , & Petitto, L.‐A. (2008a). Bilingual and monolingual brains compared: A functional magnetic resonance imaging investigation of syntactic processing and a possible “neural signature” of bilingualism. Journal of Cognitive Neuroscience, 20(1), 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman, I. , Baker, S. A. , & Petitto, L.‐A. (2008b). Age of first bilingual language exposure as a new window into bilingual reading development*. Bilingualism: Language and Cognition, 11(2), 203–223. 10.1017/S1366728908003386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, M. S. , Di Martino, A. , Zuo, X.‐N. , Kelly, C. , Mennes, M. , Jutagir, D. R. , Castellanos, F. X. , & Milham, M. P. (2011). Resting‐state functional connectivity indexes Reading competence in children and adults. The Journal of Neuroscience, 31(23), 8617–8624. 10.1523/JNEUROSCI.4865-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, M. S. , Kelly, C. , Shehzad, Z. , Penesetti, D. , Castellanos, F. X. , & Milham, M. P. (2010). Reading networks at rest. Cerebral Cortex, 20(11), 2549–2559. 10.1093/cercor/bhq005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll, J. F. , & Bialystok, E. (2013). Understanding the consequences of bilingualism for language processing and cognition. Journal of Cognitive Psychology, 25(5), 497–514. 10.1080/20445911.2013.799170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll, J. F. , Bobb, S. C. , & Hoshino, N. (2014). Two languages in mind: Bilingualism as a tool to investigate language, cognition, and the brain. Current Directions in Psychological Science, 23(3), 159–163. 10.1177/0963721414528511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll, J. F. , Bogulski, C. A. , & McClain, R. (2012). Psycholinguistic perspectives on second language learning and bilingualism: The course and consequence of cross‐language competition. Linguistic Approaches to Bilingualism, 2(1), 1–24. 10.1075/lab.2.1.01kro [DOI] [Google Scholar]
- Kuo, L. J. , & Anderson, R. C. (2012). Effects of early bilingualism on learning phonological regularities in a new language. Journal of Experimental Child Psychology, 111(3), 455–467. 10.1016/j.jecp.2011.08.013 [DOI] [PubMed] [Google Scholar]
- Lehtonen, M. , Soveri, A. , Laine, A. , Järvenpää, J. , de Bruin, A. , & Antfolk, J. (2018). Is bilingualism associated with enhanced executive functioning in adults? A meta‐analytic review. Psychological Bulletin, 144(4), 394–425. 10.1037/bul0000142 [DOI] [PubMed] [Google Scholar]
- Li, L. , Abutalebi, J. , Zou, L. , Yan, X. , Liu, L. , Feng, X. , Wang, R. , Guo, T. , & Ding, G. (2015). Bilingualism alters brain functional connectivity between “control” regions and “language” regions: Evidence from bimodal bilinguals. Neuropsychologia, 71, 236–247. 10.1016/j.neuropsychologia.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Peng, P. , & Luo, L. (2020). The relation between family socioeconomic status and academic achievement in China: A meta‐analysis. Educational Psychology Review, 32(1), 49–76. 10.1007/s10648-019-09494-0 [DOI] [Google Scholar]
- Locascio, G. , Mahone, E. M. , Eason, S. H. , & Cutting, L. E. (2010). Executive dysfunction among children with reading comprehension deficits. Journal of Learning Disabilities, 43(5), 441–454. 10.1177/0022219409355476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk, G. , Green, D. W. , Abutalebi, J. , & Grady, C. (2011). Cognitive control for language switching in bilinguals: A quantitative meta‐analysis of functional neuroimaging studies. Language and Cognitive Processes, 27(10), 1479–1488. 10.1080/01690965.2011.613209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk, G. , Sa, E. D. , & Bialystok, E. (2011). Is there a relation between onset age of bilingualism and enhancement of cognitive control?*. Bilingualism: Language and Cognition, 14(4), 588–595. 10.1017/S1366728911000010 [DOI] [Google Scholar]
- Maisog, J. M. , Einbinder, E. R. , Flowers, D. L. , Turkeltaub, P. E. , & Eden, G. F. (2008). A meta‐analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences, 1145, 237–259. 10.1196/annals.1416.024 [DOI] [PubMed] [Google Scholar]
- Martin, A. (2011). Critical review: Comparison of phonological awareness abilities of bilingual and monolingual children. https://www.semanticscholar.org/paper/Critical‐Review%3A‐Comparison‐of‐Phonological‐of‐and‐Martin/7c32482a3e9bd72312985a66fe70dcecc00a820f
- Martin, A. , Schurz, M. , Kronbichler, M. , & Richlan, F. (2015). Reading in the brain of children and adults: A meta‐analysis of 40 functional magnetic resonance imaging studies. Human Brain Mapping, 36(5), 1963–1981. 10.1002/hbm.22749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli, A. , Crinion, J. T. , Long, S. , Friston, K. J. , Lambon Ralph, M. A. , Patterson, K. , McClelland, J. L. , & Price, C. J. (2005). Dissociating reading processes on the basis of neuronal interactions. Journal of Cognitive Neuroscience, 17(11), 1753–1765. 10.1162/089892905774589190 [DOI] [PubMed] [Google Scholar]
- Mechelli, A. , Crinion, J. T. , Noppeney, U. , O'Doherty, J. , Ashburner, J. , Frackowiak, R. S. , & Price, C. J. (2004). Neurolinguistics: Structural plasticity in the bilingual brain. Nature, 431(7010), 757. 10.1038/431757a [DOI] [PubMed] [Google Scholar]
- Mechelli, A. , Humphreys, G. W. , Mayall, K. , Olson, A. , & Price, C. J. (2000). Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proceedings of the Royal Society B: Biological Sciences, 267(1455), 1909–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, L. , Xue, G. , Lu, Z.‐L. , Chen, C. , Wei, M. , He, Q. , & Dong, Q. (2015). Long‐term experience with Chinese language shapes the fusiform asymmetry of English Reading. NeuroImage, 110, 3–10. 10.1016/j.neuroimage.2015.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschyan, G. , & Hernandez, A. E. (2006). Impact of language proficiency and orthographic transparency on bilingual word reading: An fMRI investigation. NeuroImage, 29(4), 1135–1140. 10.1016/j.neuroimage.2005.08.055 [DOI] [PubMed] [Google Scholar]
- Munoz‐Sandoval, A. F. , Woodcock, R. W. , McGrew, K. S. , & Mather, N. (2005). Bateria III Woodcock‐Munoz: Pruebas de aprovechamiento. The Riverside Publishing Company. [Google Scholar]
- Nichols, E. S. , Wild, C. J. , Stojanoski, B. , Battista, M. E. , & Owen, A. M. (2020). Bilingualism affords no general cognitive advantages: A population study of executive function in 11,000 people. Psychological Science, 31(5), 548–567. 10.1177/0956797620903113 [DOI] [PubMed] [Google Scholar]
- Noble, K. G. , Houston, S. M. , Brito, N. H. , Bartsch, H. , Kan, E. , Kuperman, J. M. , Akshoomoff, N. , Amaral, D. G. , Bloss, C. S. , Libiger, O. , Schork, N. J. , Murray, S. S. , Casey, B. J. , Chang, L. , Ernst, T. M. , Frazier, J. A. , Gruen, J. R. , Kennedy, D. N. , Van Zijl, P. , & Sowell, E. R. (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, M. , Carreiras, M. , & Paz‐Alonso, P. M. (2016). Functional dynamics of dorsal and ventral reading networks in bilinguals. Cerebral Cortex., 27, 5431–5443. 10.1093/cercor/bhw310 [DOI] [PubMed] [Google Scholar]
- Olulade, O. A. , Jamal, N. I. , Koo, D. S. , Perfetti, C. A. , LaSasso, C. , & Eden, G. F. (2016). Neuroanatomical evidence in support of the bilingual advantage theory. Cerebral Cortex, 26(7), 3196–3204. 10.1093/cercor/bhv152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür, D. , Ferry, A. T. , & Price, J. L. (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology, 460(3), 425–449. 10.1002/cne.10609 [DOI] [PubMed] [Google Scholar]
- Paap, K. R. , Johnson, H. A. , & Sawi, O. (2015). Bilingual advantages in executive functioning either do not exist or are restricted to very specific and undetermined circumstances. Cortex, 69, 265–278. 10.1016/j.cortex.2015.04.014 [DOI] [PubMed] [Google Scholar]
- Perrone‐Bertolotti, M. , Kauffmann, L. , Pichat, C. , Vidal, J. R. , & Baciu, M. (2017). Effective connectivity between ventral occipito‐temporal and ventral inferior frontal cortex during Lexico‐semantic processing. A dynamic causal modeling study. Frontiers in Human Neuroscience, 11, 325. 10.3389/fnhum.2017.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliatsikas, C. , & Luk, G. (2016). Executive control in bilinguals: A concise review on fMRI studies. Bilingualism, 19(4), 699–705. 10.1017/S1366728916000249 [DOI] [Google Scholar]
- Połczyńska, M. M. , & Bookheimer, S. Y. (2021). General principles governing the amount of neuroanatomical overlap between languages in bilinguals. Neuroscience & Biobehavioral Reviews, 130, 1–14. 10.1016/j.neubiorev.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. (2012). A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. , Wise, R. J. S. , & Frackowiak, R. S. J. (1996). Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex, 6(1), 62–70. 10.1093/cercor/6.1.62 [DOI] [PubMed] [Google Scholar]
- Pugh, K. R. , Mencl, W. E. , Jenner, A. R. , Katz, L. , Frost, S. J. , Lee, J. R. , Shaywitz, S. E. , & Shaywitz, B. A. (2001). Neurobiological studies of reading and reading disability. Journal of Communication Disorders, 34(6), 479–492. 10.1016/S0021-9924(01)00060-0 [DOI] [PubMed] [Google Scholar]
- Pugh, K. R. , Mencl, W. E. , Shaywitz, B. A. , Shaywitz, S. E. , Fulbright, R. K. , Constable, R. T. , Skudlarski, P. , Marchione, K. E. , Jenner, A. R. , Fletcher, J. M. , Liberman, A. M. , Shankweiler, D. P. , Katz, L. , Lacadie, C. , & Gore, J. C. (2000). The angular gyrus in developmental dyslexia: Task‐specific differences in functional connectivity within posterior cortex. Psychological Science, 11(1), 51–56. 10.1111/1467-9280.00214 [DOI] [PubMed] [Google Scholar]
- Richlan, F. , Kronbichler, M. , & Wimmer, H. (2011). Meta‐analyzing brain dysfunctions in dyslexic children and adults. NeuroImage, 56(3), 1735–1742. 10.1016/j.neuroimage.2011.02.040 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof, K. R. , Nieuwenhuis, S. , & Braver, T. S. (2007). Medial frontal cortex function: An introduction and overview. Cognitive, Affective, & Behavioral Neuroscience, 7(4), 261–265. 10.3758/CABN.7.4.261 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Fornells, A. , Rotte, M. , Heinze, H.‐J. , Nösselt, T. , & Münte, T. F. (2002). Brain potential and functional MRI evidence for how to handle two languages with one brain. Nature, 415(6875), 1026–1029. 10.1038/4151026a [DOI] [PubMed] [Google Scholar]
- Sandak, R. , Mencl, W. E. , Frost, S. J. , & Pugh, K. R. (2004). The neurobiological basis of skilled and impaired Reading: Recent findings and new directions. Scientific Studies of Reading, 8(3), 273–292. 10.1207/s1532799xssr0803_6 [DOI] [Google Scholar]
- Schlaggar, B. L. , Brown, T. T. , Lugar, H. M. , Visscher, K. M. , Miezin, F. M. , & Petersen, S. E. (2002). Functional neuroanatomical differences between adults and school‐age children in the processing of single words. Science, 296(5572), 1476–1479. [DOI] [PubMed] [Google Scholar]
- Schölvinck, M. L. , Maier, A. , Ye, F. Q. , Duyn, J. H. , & Leopold, D. A. (2010). Neural basis of global resting‐state fMRI activity. Proceedings of the National Academy of Sciences of the United States of America, 107(22), 10238–10243. 10.1073/pnas.0913110107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, A. J. , & McGonigle, J. (2011). Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. NeuroImage, 55(3), 1132–1146. 10.1016/j.neuroimage.2010.12.047 [DOI] [PubMed] [Google Scholar]
- Sirin, S. R. (2005). Socioeconomic status and academic achievement: A meta‐analytic review of research. Review of Educational Research, 75(3), 417–453. 10.3102/00346543075003417 [DOI] [Google Scholar]
- Stevens, W. D. , Kravitz, D. J. , Peng, C. S. , Tessler, M. H. , & Martin, A. (2017). Privileged functional connectivity between the visual word form area and the language system. Journal of Neuroscience, 37(21), 5288–5297. 10.1523/JNEUROSCI.0138-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Gareau, L. , Flowers, D. L. , Zeffiro, T. A. , & Eden, G. F. (2003). Development of neural mechanisms for reading. Nature Neuroscience, 6(7), 767–773. 10.1038/nn1065 [DOI] [PubMed] [Google Scholar]
- Wagner, R. K. , & Torgesen, J. K. (1987). The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin, 101(2), 192–212. 10.1037/0033-2909.101.2.192 [DOI] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wong, B. , Yin, B. , & O'Brien, B. (2016). Neurolinguistics: Structure, function, and connectivity in the bilingual brain. BioMed Research International, 2016, 7069274. 10.1155/2016/7069274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock, R. W. , McGrew, K. S. , & Mather, N. (2001). Woodcock‐Johnson III: Tests of achievement. The Riverside Publishing Company. [Google Scholar]
- Zhan, L. , Jenkins, L. M. , Wolfson, O. E. , GadElkarim, J. J. , Nocito, K. , Thompson, P. M. , Ajilore, O. A. , Chung, M. K. , & Leow, A. D. (2017). The significance of negative correlations in brain connectivity. Journal of Comparative Neurology, 525(15), 3251–3265. 10.1002/cne.24274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study are available from the corresponding author upon reasonable request.


