Abstract
Astaxanthin is a lipid-soluble carotenoid produced by various microorganisms and marine animals, including bacteria, yeast, fungi, microalgae, shrimps and lobsters. Astaxanthin has antioxidant, anti-inflammatory and anti-apoptotic properties. These characteristics suggest that astaxanthin has health benefits and protects against various diseases. Owing to its ability to cross the blood-brain barrier, astaxanthin has received attention for its protective effects against neurological disorders, including Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, cerebral ischemia/reperfusion, subarachnoid hemorrhage, traumatic brain injury, spinal cord injury, cognitive impairment and neuropathic pain. Previous studies on the neurological effects of astaxanthin are mostly based on animal models and cellular experiments. Thus, the biological effects of astaxanthin on humans and its underlying mechanisms are still not fully understood. The present review summarizes the neuroprotective effects of astaxanthin, explores its mechanisms of action and draws attention to its potential clinical implications as a therapeutic agent.
Keywords: astaxanthin, Alzheimer's disease, Parkinson disease, amyotrophic lateral sclerosis, cerebral ischemia/reperfusion, subarachnoid hemorrhage
1. Introduction
Astaxanthin (AST) is a lipid-soluble, red-orange pigment (1) that belongs to a group of carotenoids called xanthophylls, which includes β-cryptoxanthin, canthaxanthin, lutein and zeaxanthin (Fig. 1) (2). Since the antioxidant, anti-inflammatory and anti-apoptotic properties of AST have been demonstrated in several studies, leading to its approval as a dietary supplement (3).
Figure 1.
Classification of carotenoids.
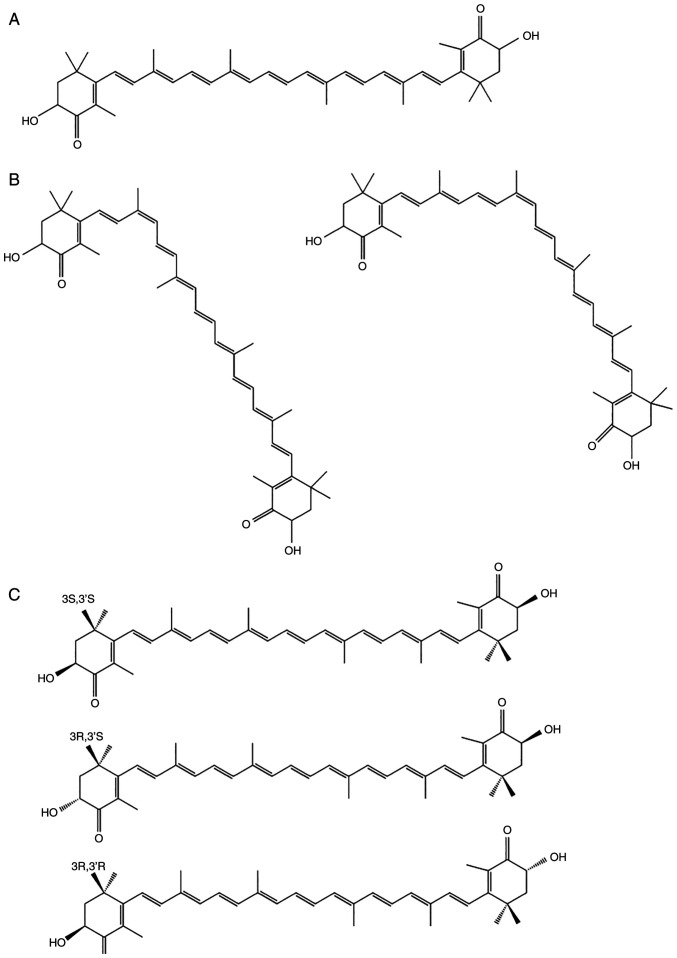
AST has a molecular structure similar to those of β-carotene and other carotenoids (4). However, the oxygen groups in its molecular structure distinguish AST from other carotenoid subtypes (5). AST has a polar region at each end of the molecule's ionone rings that neutralizes free radicals. In contrast to the 11 carbon-carbon double polyunsaturated bonds in β-carotene, the central nonpolar zone of AST is made up of 13 bonds, which allow AST to remove high-energy electrons (6). The hydroxyl and ketone moieties on both rings increase the polarity of AST and greatly enhance its capacity to cross the cell membrane (7,8). These unique chemical properties bestow AST with some bioactivity-related advantages, including a higher antioxidant effect than other carotenoids (9). Owing to its carbon-carbon double polyunsaturated bonds, AST has two isomeric forms; trans and cis (Fig. 2A) (10). The cis isomer, cis-AST, includes 9-cis and 13-cis configurations (Fig. 2B). Due to the two stereogenic carbon atoms at the C-3 and C-3′ positions, all-trans-AST has three stereoisomers: (3S, 3′S), (3R, 3′R) and (3R, 3′S) (Fig. 2C). The structure of all-trans-AST is more stable than that of cis-AST, indicating that all-trans-AST is the predominant form of AST in nature (11). In addition, 3S, 3-S-AST is a more powerful antioxidant than the other stereoisomers (12). Owing to the stability of all-trans-AST, it has been used as an experimental material in several studies. Therefore, the aim of the present review was to explore the biological activities and neurological functions of all-trans-AST.
Figure 2.
Structures of all-trans-astaxanthin (A), 9-cisastaxanthin, 13-cisastaxanthin (B) and (3S, 3′S), (3R, 3′R) and (3R, 3′S) all-trans-astaxanthin subtypes (C).
AST is extracted from microorganisms; phytoplankton; bacteria; yeast; and marine animals, such as shrimps, lobster, asteroidean, algae, crustaceans, trout, krill, red sea bream and salmon (13,14). In nature, AST is initially synthesized by microalgae and phytoplankton, accumulating in zooplankton and crustaceans and reaching higher level marine animals through the food chain (14). Haematococcus pluvialis (H. pluvialis) produces the largest quantity of natural AST (15). However, large-scale cultivation of H. pluvialis is considered costly (16); thus, synthetic production is currently the predominant source of AST. However, synthetic AST shows only 50% of the biological activity of natural AST (17).
Previous studies have shown that AST can mediate the processes of various diseases through antioxidant, anti-inflammatory and anti-apoptotic activities (Fig. 3). In a study of a diabetic retinopathy model, AST treatment increased the levels of the antioxidant enzyme heme oxygenase-1 (HO-1) and maintained the homeostasis of retinal ganglion cells (18). Yoshihisa et al (19) found that AST can protect keratinocytes from ultraviolet-related damage by decreasing the expression of oxidative factors [inducible nitric oxide, (iNOS)] and the inflammatory factors IL-1β and TNF-α. In addition, a clinical trial demonstrated that oral AST protects the skin from ultraviolet injury (20). Furthermore, AST maintains the homeostasis of lipid metabolism (21) and controls the courses of cardiovascular diseases and cancer by regulating apoptosis factors and cell proliferation (22,23).
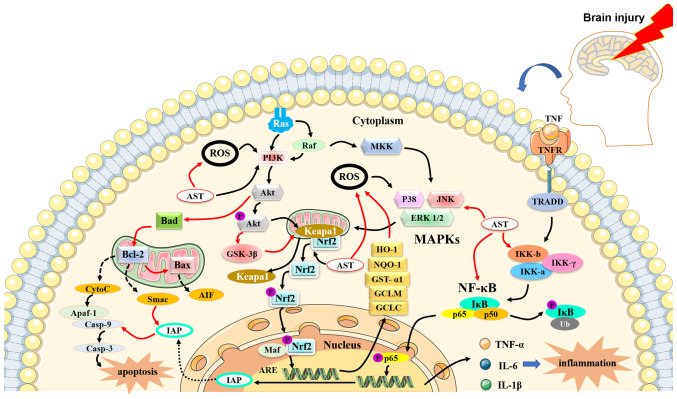
Figure 3.
The antioxidant, anti-inflammatory and anti-apoptotic properties of AST. AST can activate phosphoinositide 3-kinase/protein kinase B and nuclear factor erythroid 2-related factor 2 signaling pathways, leading to the production of heme oxygenase-1, NAD(P)H quinone oxidoreductase-1, glutathione-S-transferase-α1, glutamate-cysteine ligase modifier subunit and glutamate-cysteine ligase catalytic subunit for the attenuation of oxidant effects. The NF-κB signaling pathway is initially activated by tumor necrosis factor and produces pro-inflammatory cytokines, chemokines and growth factors, such as IL-1β, IL-6 and tumor necrosis factor α. AST can block excessive NF-κB signaling and downregulate the expression of pro-inflammatory cytokines. AST reduces the expression of inflammatory factors and suppresses the activation of caspases and Bax, while increasing the level of Bcl-2. Red arrows indicate inhibitory action and black arrows denote enhancement action. AST, astaxanthin; Casp, caspase; MDA, malondialdehyde; IL-1β, interleukin-1 β; TNF-α, tumor necrosis factor α; IL-6, interleukin-6; JNK, c-Jun N-terminal kinase; AD, Alzheimer's disease; p-IKKα, p-IκB kinase α; Bcl-2, B-cell lymphoma-2; HO-1, heme oxygenase-1; Nrf2, nuclear factor erythroid 2-related factor 2; Akt, Akt, protein kinase B; GSK-3β, glycogen synthase kinase 3β; PARP, poly (ADP-ribose) polymerase; AIF, apoptosis inducing factor; ROS, reactive oxygen species; Bax, BCL2-Associated X; Cyt-c, cytochrome c; NMDA, N-methyl-D-aspartate; PD, Parkinson's disease; NOX2, nitrogen oxide 2; SOD, superoxide dismutase; GSH, glutathione; IR, ischemic reperfusion; NQO1, NAD(P)H quinone oxidoreductase-1; GFAP, glial fibrillary acidic protein;MAP-2, microtubule-associated protein-2; BDNF, brain-derived neurotrophic factor; GAP-43, growth-associated protein 43; NO, nitric oxide; iNOS, inducible nitric oxide; MDA, malondialdehyde; CAT, catalase; GPX, Glutathione peroxidase; GST-α1, glutathione-s-transferase-α1;SAH, subarachnoid hemorrhage; ICAM-1a, intercellular cell adhesion molecule-1 a; Apaf-1, apoptotic protease activating factor-1.
Previous studies have identified the health benefits of AST against neurological disorders, including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), cerebral ischemia/reperfusion (IR), subarachnoid hemorrhage (SAH) and cognitive disorders (24,25). Therefore, the present review focused on the biological activities and neurological functions of AST.
2. Biological activities
Antioxidant activities of astaxanthin
Molecular oxygen (O2) is the most significant radical in living systems. O2 is a reactive oxygen species (ROS) that is generated through a series of metabolic and physical processes. Overproduction of ROS can result in oxidative stress (OS) (26). ROS and OS can have deleterious effects on the structures of cells, including lipids, membranes, proteins and DNA (27). Due to its unique features, AST can maintain the integrity of the cell membrane and mediate immune system function and gene expression by neutralizing O2, scavenging radicals and managing lipid peroxidation (LPO) (28,29). In addition, some studies have indicated that the antioxidant activity of AST is more powerful than that of other carotenoids (30). Nakajima et al (31) found that AST exerts neuroprotective functions in a N-methyl-D-aspartate (NMDA)-induced excitotoxicity model by decreasing LPO and oxidative DNA damage. Another study shows that AST antagonizes OS in a 1-methyl-4-phenylpyridinium (MPP+)-induced PC12 cell model by decreasing the expression of NMDA receptor subunit 1 (NR1), which has previously been linked to neurodegenerative disorders (32).
AST activates the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway (33) and the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated protein kinase (ERK) pathway. The two pathways facilitate the dissociation of nuclear factor erythroid 2-related factor 2 (Nrf2) from Kelch-like ECH-associated protein 1. Nrf2 is translocated to the nucleus and activates the Nrf2 antioxidant response element (ARE) signaling pathway (33). The PI3K/Akt pathway upregulates the expression of HO-1, NAD(P)H quinone oxidoreductase-1 (NQO-1), glutathione-S-transferase-α1, the glutamate-cysteine ligase modifier subunit and the glutamate-cysteine ligase catalytic subunit, which provide protection against OS both in vitro and in vivo (24,34–36). In addition, it has been reported that rats fed AST show elevated levels of other antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT) (37,38), thiobarbituric acid reactive substances and peroxidase, in the liver and plasma (24,39).
Anti-inflammatory activities of astaxanthin
Inflammation is a complex host defense response to infection, injury, ischemia, toxins and radiation. Inflammation also facilitates the tissue repair process through the actions of immune cells and inflammatory mediators. However, excessive or inappropriate inflammatory activity is deleterious to the host and can cause or aggravate numerous diseases (40). The NF-κB signaling pathway is an important and ubiquitous nuclear transcription pathway that serves important roles in inflammatory and immune responses (41). Excessive activation of the NF-κB signaling pathway is related to inflammatory changes in rheumatoid arthritis and heart and brain diseases. In unstimulated conditions, NF-κB (p50-p65) remains inactive in the cytoplasm and interacts with the inhibitory (IκB) family (IκB-α) (42). Under stimulation by extracellular agents, NF-κB is activated through dissociation of IκB, which is phosphorylated by the IκB kinase complex (IKK, including IKKα and IKKβ). Dissociated NF-κB enters the nucleus and binds to κB regulatory elements that produce the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α (41,43). Therefore, blocking the NF-κB signaling pathway is important for the mediation of inflammatory diseases. A recent study showed that AST can block excessive NF-κB signaling by downregulating the phosphorylation of IκB-α or increasing the cellular expression of IκB-α mRNA and protein (44). AST also exhibits anti-inflammatory effects by inhibiting cyclooxygenase-1 and nitric oxide in lipopolysaccharide-stimulated BV2 microglial cell biogenesis through the regulation of multiple genes (39).
Anti-apoptotic activities of astaxanthin
Apoptosis, a process of cell suicide, is a common mechanism in living systems. This process is vital for tissue development, maintenance of homeostasis and defense against a variety of extracellular and intracellular insults and mutations (45). However, excessive apoptosis disrupts homeostasis and leads to numerous diseases. The apoptotic pathway is regulated by the Bcl-2 family, including the pro-apoptotic cytokines Bad and Bax and the anti-apoptotic cytokines Bcl-2 and Bcl-xL (46,47). Under apoptotic stimulation, Bad and Bax promote the release of cytochrome c (Cyt c) from the mitochondria into the cytoplasm. A complex comprising Cyt c, apoptotic protease activator-1 and caspase-9 then activates caspase-3, which triggers apoptosis. Bcl-2 and Bcl-xL inhibit the release of Cyt c and induce apoptosis (48). In addition, the PI3K/Akt pathway inhibits Bad and Bax and the JAK/STAT pathway or the SCR/STAT pathway promotes the expression of Bcl-2 and Bcl-xL, contributing to anti-apoptosis.
AST can regulate some key apoptotic proteins and prevent related diseases (49). In addition, studies have shown that AST serves an important role in the activation of the PI3K/Akt signaling pathway, mediation of the phosphorylation of Bad and downregulation of the activation of Cyt c and caspase-3 (24,50–52). Fan et al (22) found that AST supplementation protects rats from homocysteine-related apoptosis through the regulation of Bcl-2 levels. In a study of a steatotic liver model, AST treatment reduced the expression of inflammatory factors and suppressed the activation of caspases and Bax while increasing the level of Bcl-2 (53).
3. Neuroprotective activities of astaxanthin
The central nervous system (CNS) is one of the most important systems in the human body and it contains billions of neuronal and glial cells. The blood-brain barrier (BBB) is a selectively permeable barrier between capillaries and the brain that isolates the CNS from other systems of the body. This barrier is crucial for maintaining brain homeostasis and protecting the neuronal environment from harmful materials (54). However, the BBB occasionally prevents the transportation of therapeutic agents to the CNS for the treatment of neurological disorders. As mentioned previously, AST is a lipid-soluble pigment that can cross the BBB, a feature that is crucial for the treatment of neurological diseases. Manabe et al (55) found that AST accumulates in the hippocampi and cerebral cortexes of rat brains after single and repeated dietary ingestion. The accumulation of AST in the cerebral cortex may maintain and improve cognitive function. Some studies have shown that treatment using AST can promote nerve cell regeneration and increase gene expression of proteins important for brain recovery, such as glial fibrillary acidic protein (GFAP), microtubule associated protein 2 (MAP-2), brain-derived neurotrophic factor (BDNF) and growth-associated protein 43 (GAP-43) (56–59). GFAP serves significant roles in the repair of CNS injury, promotion of cell communication and alleviation of BBB damage (60). MAP-2 can regulate microtubule growth and neuronal regeneration. BDNF is responsible for neuronal survival and growth and the differentiation of new neurons (61), whereas upregulation of GAP-43 stimulates the protein kinase pathway and promotes neurite formation, regeneration and plasticity (61). The biological activities of AST in the courses of neurological diseases are summarized in Table I.
Table I.
Biological effects of astaxanthin in Alzheimer's Disease, Parkinson Disease, Cerebral Ischemia/Reperfusion, Subarachnoid Hemorrhage and Amyotrophic lateral sclerosis.
| Author, year | Target, cell line | Effect | Concentration | Intervention | Duration | Outcome | Disease | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Ito, 2019 | Human | Neuroprotective | 3 mg AST + 5 mg sesamin, oral | Post-treatment | 12 weeks | Psychomotor speed,↑ processing speed | MCI | (71) |
| Sekikawa, 2020 | Human | Neuroprotective | 9 mg AST + 50 mg tocotrienol, oral | Post-treatment | 12 weeks | Composite memory↑ and verbal memory | Cognition | (72) |
| Taksima, 2019 | Male Wistar rats, | Anti-oxidant | 10 mg/kg/day, oral | Post-treatment | 30 days | Learning and memory↑ ability, glutathione peroxidase, neuronal survival; MDA, protein carbonyl | AD | (73) |
| Kim, 2020 | BV-2 microglial cells | Anti-inflammatory | 1-10 µg/ml | Pre-treatment | 4 h | IL-1β, TNF-α,↑ IL-6, pJNK activation, neuronal cell death; IL-10 and ↓ arginase-1, Akt phosphorylation | AD | (77) |
| Kim, 2010 | BV-2 microglial cells | Anti-inflammatory | 25 µM | Pre-treatment | 24 h | IL-6, p-IKKα,↓ | AD | (78) |
| p-IκBα, p-NF-κBp65 | AD | (79) | ||||||
| Wen, 2015 | Hippocampal HT22 cells anti-oxidant, | Anti-apoptosis | 1.25-5 µM | Pre-treatment | 2 h | cell viability, Bcl-2, HO-1, Nrf2, p-Akt,p-GSK-3β (Ser9) caspase-3/8/9 activity,↓ PARP, AIF, ROS, Bax, Cyt-c | ||
| Ye, 2013 | PC12 cells | Anti-oxidant | 10 µmol/l | Pre-treatment | 2 h | PC12 cell viability;↑ activated transcription factor, NMDA receptor↓ subunit 1 protein and mRNA | PD | (32) |
| Ye, 2012 | PC12 cells | Anti-oxidant | 10 µM | Pre-treatment | 2 h | ROS, NOX2↑ HO-1, Nrf2↓ | PD | (84) |
| Brasil, 2021 | Human neuroblastoma SH-SY5Y cells | Anti-oxidant | 20 µM | Pre-treatment | 24 h | H2O2-induced cytotoxicity↓ cytochrome c, caspase-9 and caspase-3, IL-1β and TNF-α; HO-1, Nrf2 ↑ | PD | (85) |
| Lee, 2011 | Human neuroblastoma SH-SY5Y cells, C57BL/6 mice | Anti-oxidant | 50 µM 10, 30 mg/kg/day/(animal model) | Pre-treatment | 24 h (cell), 28 days (animal model) | ROS, cytotoxicity,↓ a-synuclein Bax, caspase-3, argyrophilic neurons; Bcl-2, SOD,↑ catalase, tyrosine hydroxylase neurons | PD | (86) |
| Xue, 2017 | Male mice | ICR anti-oxidant | 10 mg/kg/day, intragastric | Post-treatment | 28 days | Learning and memory↑ ability, GSH, SOD, Bcl-2; Cyt c, Bax↓ | IR | (93) |
| Pan, 2017 | Male (Sprague Dawley) rat | SD anti-oxidant | 5 mg/kg, 10 mg/kg, intragastrical | Pre-treatment | 7 days | Nrf2, HO-1, NQO1,↑ Bcl-2, GFAP, MAP-2, BDNF, GAP-43; Infarction volume, Bax↓ | IR | (94) |
| Lee, 2010 | Human SY5Y neuroblastoma cells male Wistar rats | SH-anti-oxidant | 10, 25, 50, 100 µM 30 mg/kg, intra-peritoneally (animal model) | Pre-treatment | 90 min (cells), 0 and 90 min of cerebral reperfusion (animal model) | Neuronal cell↑ density, HO-1; NO, iNOS↓ | IR | (95) |
| Lu, 2010 | Male Sprague-Dawley rats | Anti-oxidant | 50, 80 mg/kg, oral | Pre-treatment | 5 and 1 h before ischemia | Infarct volume↓ cell viability↑ | IR | (96) |
| Yang, 2021 | SD rats | Anti-oxidant anti-inflammatory anti-apoptosis | 100 mg/kg, gavage | Pre-treatment | 3 days | Brain edema,↓ cerebral infarct area, TNF-α; IL-1β, IL-6, MDA,↑ Bax Nrf-2, HO-1, Bcl-2, CAT, SOD, GPX | IR | (97) |
| Wu, 2014 | Male SD rats | Anti-oxidant | 0.1 mM, left ventricle injection | Post-treatment | 24 h after SAH | Brain edema,↓ BBB disruption; BBB disruption; neurological scores, Nrf2, HO-1, NQO1, ↑ GST-α1 | SAH | (34) |
| Zhang, 2014 | Male SD rats, male New Zealand rabbits | Anti-oxidant | 0.01, 0.1 mmol/l intracerebroven-tricular injection 25, 75 mg/kg oral | Post-treatment | 30 min after SAH, 3 h after SAH | Brain edema, caspase-3,↓ MDA; BBB permeability,↑ GSH, SOD | SAH | (103) |
| Zhang, 2019 | Male SD rats, C57BL/6 mice, TLR4 gene KO mice | Anti-inflammatory | 01, 0.1 and 0.2 mM 20ml, left lateral ventricle injection (rat); 2.0 ml, right lateral ventricle injection (mice) | Post-treatment | 30 min, 4 h, or 8 h after SAH (rats), 30 min after SAH (mice) | IL-1b,TNF-a,↓ ICAM-1a, CD68 (+) microglia, NF-кB p65, p-IκB, Toll-like receptor 4 activation; Cell viability↑ | SAH | (102) |
| Wang, 2019 | Male SD rats | Anti-apoptosis | 75 mg/kg, gavage | Post-treatment | 3 h after SAH | Mitochondrial↑ membrane potential, synaptic protein, nerve growth and neuronal differentiation factors; Bax/Bcl-2 ratio,↓ Cyt c, caspase-3 | SAH | (104) |
| Isonaka, 2011 | Wistar rats | Antioxidant | 100 nM | Pretreatment | 24 h pretreatment + 72 h treatment period | Neurite lengths↑ | ALS | (109) |
Up arrows denote enhancement action and down arrows indicate inhibitory action. AST, astaxanthin; MCI, mild cognitive impairment; MDA, malondialdehyde; IL-1β, interleukin-1 β; TNF-α, tumor necrosis factor α; IL-6, interleukin-6; JNK, c-Jun N-terminal kinase; AD, Alzheimer's disease; p-IKKα, p-IκB kinase α; Bcl-2, B-cell lymphoma-2; HO-1, heme oxygenase-1; Nrf2, nuclear factor erythroid 2-related factor 2; Akt, Akt, protein kinase B; GSK-3β, glycogen synthase kinase 3β; PARP, poly (ADP-ribose) polymerase; AIF, apoptosis inducing factor; ROS, reactive oxygen species; Bax, BCL2-Associated X; Cyt-c, cytochrome c; NMDA, N-methyl-D-aspartate; PD, Parkinson's disease; NOX2, nitrogen oxide 2; SOD, superoxide dismutase; GSH, glutathione; IR, ischemic reperfusion; NQO1, NAD(P)H quinone oxidoreductase-1; GFAP, glial fibrillary acidic protein;MAP-2, microtubule-associated protein-2; BDNF, brain-derived neurotrophic factor; GAP-43, growth-associated protein 43; NO, nitric oxide; iNOS, inducible nitric oxide; MDA, malondialdehyde; CAT, catalase; GPX, Glutathione peroxidase; BBB, blood-brain barrier; GST-α1, glutathione-s-transferase-α1;SAH, subarachnoid hemorrhage; ICAM-1a, intercellular cell adhesion molecule-1 a; ALS, amyotrophic lateral sclerosis.
Protective activities of astaxanthin: AD
Neurodegenerative disorders are difficult to prevent and treat. In addition, improving their prognoses is quite challenging. AD is the most common neurodegenerative disorder among older individuals (62). Extensive research has demonstrated that the number of people with AD is steadily increasing. AD tends to have a long course, various comorbidities and medical requirements for long-term care. These data suggest that AD places a heavy socioeconomic burden on the families of patients and the society at large (63,64).
Patients with AD show a significant degree of oxidative damage in the brain. This oxidate damage is associated with the accumulation of amyloid-β peptide (Aβ) (65). Aβ is the main component of senile plaques, neurofibrillary tangles and neutrophil threads in the brain (66). In addition to Aβ, mitochondrial abnormalities and hyperphosphorylated tau also induce oxidative and inflammatory reactions that contribute to the pathology of AD (67). Furthermore, metal ions (68,69), LPO (66) and DNA abnormalities (70) are implicated in the oxidative process of AD.
AST, with its antioxidant and anti-inflammatory effects, is recommended for the prevention or reduction of the progression of AD and the improvement of its prognosis (37). Indeed, two double-blind placebo-controlled studies conducted in Japan demonstrate that AST supplementation could effectively improve cognitive ability, which enables individuals to accomplish tasks more precisely and rapidly (71,72). In their study, Taksima et al (73) found that Wistar rats treated with AST decreased their escape latency time and increased the time spent in the target quadrant in the Morris water maze test. AST intake has been found to reduce brain oxidative indices, such as the LPO product malondialdehyde (MDA) and the percentage of superoxide anion and increase glutathione peroxidase activity. A previous study demonstrated that neurodegenerative disorders may be related to insulin resistance, which could lead to the accumulation of Aβ, mitochondrial dysfunction and increased levels of inflammatory cytokines (74). In a previous animal study, Rahman et al (75) found that AST not only improved the cognitive assessment results of rats, but also attenuated central insulin resistance indicators, Aβ level and TNF-α level in the hippocampi of Wistar rats (76).
In a study of a PM2.5-induced neuroinflammation model, AST treatment decreased the expression of M1 pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) and increased the expression of M2 anti-inflammatory cytokines (IL-10 and arginase-1) (77). Similar anti-inflammatory activities of AST have been reported in other studies. For example, a previous study showed that AST (50 µM) significantly reduces the release of inflammatory mediators in activated microglial cells through the modulation of factors involved in the NF-κB cascade (e.g., IKKα/β, IκBα, NF-κB p65, IL-6 and MAPK) (78). HT12 cells and PC12 cells are rat-derived neuronal cells that are used to imitate the nervous system in vivo for studying neurodegeneration. Another study demonstrated that after treatment using 1.25-5 µM AST, these neuronal cells are protected from neurotoxicity stimulated by glutamate-induced cytotoxicity and reduced lactate dehydrogenase (LDH) release. This protective effect is attributable to decreased caspase-3/8/9 expression, poly (ADP-ribose) polymerase (PARP), suppressed ROS accumulation, increased nuclear Nrf2 and HO-1 expression and modulated Akt/glycogen synthase kinase 3β (GSK-3β) signaling (79). It has been reported that AST improves the behavioral scores of rats in hippocampal-dependent tasks; however, the underlying molecular process is not fully understood. These results show that administration of AST can serve as an augmentative treatment for AD.
Protective activities of astaxanthin: PD
PD is the second most common neurodegenerative disease globally. As with AD, the proportion of the global population with PD is increasing (80). James Parkinson first described this disease as a ‘shaking palsy’ 200 years ago (81). It is now recognized as a complex and heterogeneous disorder characterized by classic motor symptoms (bradykinesia, rigidity and tremor) induced by the loss of dopaminergic neurons and non-motor manifestations (altered posture, balance and gait) (81). Numerous studies have shown that neuronal loss and formation of Lewy bodies are the pathological hallmarks of PD. These pathological features, which have been identified in the basal forebrain, anterior thalamus, hypothalamus, amygdala and cerebral cortex, disrupt the normal function of the brain and interrupt the actions of important chemical messengers, such as acetylcholine and dopamine (82). Although the pathogenesis of PD is not completely understood, increasing evidence from human and animal studies suggest that OS and mitochondrial and calcium dysfunction are important mediators in its pathogenesis (83). The antioxidant and anti-inflammatory properties of AST make it a promising therapeutic agent for PD. In a previous study of a PD model, AST was found to increase PC12 cell viability, decrease mRNA production and decrease the expression of proteins linked to neurodegenerative disorders, such as activated transcription factor Sp1 and NR1 (32). AST also suppresses NADPH oxidase 2 levels, generates ROS and considerably increases Nrf2 and HO-1 levels (84). Studies have demonstrated that AST induces mitochondrial protection and reduces oxidative injury through the ERK1/2 and PI3K/Akt/Nrf2/HO-1 pathways (36,85). In a study of MPP+/1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced apoptosis in SH-SY5Y cells and a PD model, Lee et al (86) found that AST shows anti-apoptotic and neuroprotective effects through the upregulation of Bcl-2 protein expression and the inhibition of Bax and α-synuclein expression and caspase-3 activation. However, there are currently no clinical trials on AST for the treatment of PD. Considering the findings of previous trials on PD and carotene (87), it is possible that AST could serve an important role in improving the progression of PD in the future.
Protective activities of astaxanthin: cerebral ischemia/reperfusion
Ischemic stroke (IS) has a high incidence rate and is one of the most common causes of serious morbidity and mortality worldwide (88). Blockage of cerebral blood flow results in pathophysiological responses, including excitotoxicity, mitochondrial disorders, ROS release, inflammatory changes, apoptosis, calcium imbalance and DNA damage (88,89). In a study of a rat model, IR was found to activate the redox-sensitive transcription factors NF-κB, AP-1, MAPKs, JNK and p38 under minimal activation of ERK (90). Superoxide dismutase (SOD) and glutathione (GSH), which are free radical scavengers found in the brain tissue after cerebral IR, can suppress the harmful effects of IS (91,92). Previous studies have shown the neuroprotective effect of AST in reducing adverse reactions related to cerebral IR injury during brain recovery (93–97).
Xue et al (93) investigated the potential neuroprotective effects of AST in a mouse model of vascular cognitive impairment induced by repeated IR injury. The results of the study demonstrated that AST improves learning and memory and ameliorates the loss of and ultrastructural changes in the hippocampal pyramidal neurons of mice with repeated IR injury. The strong antioxidant and anti-apoptotic effects of AST on hippocampal neurons may be attributed to the downregulation of MDA, Bax, Cyt c and cleaved caspase-3, as well as the upregulation of GSH, Bcl-2 and SOD.
Pan et al (94) found that administration of AST protects rats from cerebral ischemia damage induced by middle cerebral artery occlusion. These results demonstrate that pre-treatment with AST reduces cerebral infarction volume and cell death through upregulation of Bcl-2 and inhibition of Bax. Furthermore, AST increases the expression of Nrf2, HO-1 and NQO-1 through the ARE signaling pathway. It has been reported that expressions of GFAP, MAP-2, BDNF and GAP-43 are significantly upregulated in rats with high AST levels. Lee et al (95) also found that AST reduces the level of iNOS and increases the levels of HO-1 and heat shock protein 70 after oxygen glucose deprivation injury. Further research has shown that AST has protective and anti-apoptotic effects against IR injuries (96,97).
Protective activities of astaxanthin: subarachnoid hemorrhage and amyotrophic lateral sclerosis
SAH is a severe disease with high morbidity and mortality rates worldwide. The clinical symptoms of SAH include coma and varying degrees of neurological disorders, such as aphasia, hemiplegia, hemianopia, paresthesia, headache and dysphrenia (98). The pathological course of SAH within the first 72 h is defined as early brain injury, which includes destruction of the BBB, cerebral edema, inflammation, increased intracranial pressure and neuronal apoptosis (99–101). These changes suggest an unfavorable prognosis and create significant individual and social burden. A series of studies conducted by Zhang et al (102) showed that AST ameliorates inflammation and OS and improves neuronal survival in SAH by modifying the Nrf2-ARE and Akt/Bad pathways and the toll-like receptor 4 signaling pathway (34). As mentioned previously, these pathways can inhibit the expression of inflammatory cytokines (IL-1β, TNF-α and NF-кB p65) and apoptotic cytokines (Bax, Cyt c and caspase-3) while rescuing mitochondrial function and BBB integrity (34,52,102,103). Wang et al (104) show that AST inhibits mitochondria-associated neuronal apoptosis after SAH by stabilizing the mitochondrial membrane potential, decreasing the Bax/Bcl-2 ratio, inhibiting Cyt c and suppressing caspase-3 enzyme activity. In addition, AST has been found to restore the expression of synapsin-1, postsynaptic density-95, GAP-4, BDNF and purine-rich binding protein-α associated with nerve growth and neuronal differentiation.
ALS is a progressive and lethal neurological disease characterized by irreversible loss of the upper and lower spinal or bulbar motor neurons (105). Most patients with ALS experience muscle paralysis until death, which is caused by respiratory failure within 3–5 years of the onset of symptoms. The number of patients with ALS has been rapidly increasing as a result of increasing aging of the global population. It has been reported that approximately 400,000 people worldwide will have ALS by 2040 (106). Although the underlying mechanisms of ALS are not fully understood, the most common cause may be related to a mutation in the gene encoding Cu/Zn SOD1. SOD1 is a significant cytosolic metalloenzyme that catalyzes the dismutation of the superoxide anion radical (O2−) into H2O2 and O2. In addition, mitochondrial dysfunction, neuroinflammation and calcium flux-related excitotoxicity serve important roles in the progression of ALS (105,107). The unstable structure of mutant SOD1 leads to the accumulation free radicals from OS. OS causes oxidative damage to lipids, proteins and nucleic acids, resulting in neuronal death. Free radicals can be produced by antioxidants, such as vitamin C, vitamin E and AST. Bond et al (108) showed that antioxidants are promising as therapeutic agents for increasing the quality of life of patients with ALS. Moreover, Isonaka et al (109) demonstrate that spinal motor neurons treated with antioxidants and the SOD1 inhibitor diethyldithiocarbamate (DDC) have longer neurite lengths than neurons treated with DDC alone, indicating that antioxidants may improve pathological changes in ALS. In addition, clinical dietary studies have shown that carotenoid intake improves respiratory function in patients with ALS and reduces the risk for the disease (110,111).
4. Conclusion
AST, with its antioxidant, anti-inflammatory and anti-apoptotic properties, has various health benefits for humans. The present review highlighted the mechanisms of action and benefits of AST in neurological diseases. In addition, owing to its lipid-soluble characteristics, AST may serve an important role in improving neurological diseases. However, previous studies on AST are mainly focused on animal models. Thus, further in vivo and in vitro studies on AST are warranted to clarify the specific signaling pathways involved in its effects and to elucidate its benefits for effective therapy. More research is needed to explore the potential applications of AST in the prevention, management and treatment of neurological diseases.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AD
Alzheimer's disease
- Akt
protein kinase B
- ALS
amyotrophic lateral sclerosis
- AST
astaxanthin
- Aβ
amyloid-β peptide
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- Cyt c
cytochrome c
- DDC
diethyldithiocarbamate
- ERK
extracellular signal-regulated protein kinase
- GAP-43
growth-associated protein 43
- GFAP
glial fibrillary acidic protein
- GSK-3β
glycogen synthase kinase 3β
- HD
Huntington's disease
- IKK
IκB kinase
- iNOS
inducible nitric oxide
- IR
ischemia/reperfusion
- IS
ischemic stroke
- LPO
lipid peroxidation
- MAP-2
microtubule associated protein 2
- MAPK
mitogen-activated protein kinase
- MDA
malondialdehyde
- MPP
1-methyl-4-phenylpyridinium
- NMDA
N-methyl-D-aspartate
- NQO-1
NAD(P)H quinone oxidoreductase-1
- NR1
NMDA receptor subunit 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- OS
oxidative stress
- PARP
poly (ADP-ribose) polymerase
- PD
Parkinson's disease
- PI3K
phosphoinositide 3-kinase
- ROS
reactive oxygen species
- SAH
subarachnoid hemorrhage
- SOD
superoxide dismutase
Funding Statement
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as no data sets were generated or analyzed during the current study.
Authors' contributions
PS was a major contributor to writing the manuscript and prepared the figures. CZ performed the literature search and selection and was responsible for editing the references. The two authors read and approved the final version of the manuscript, were responsible for all aspects of the work and approved the submission in its current form. Data authentication is not applicable.
Ethics approval and consent to participate
No applicable.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hu J, Nagarajan D, Zhang Q, Chang JS, Lee DJ. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol Adv. 2018;36:54–67. doi: 10.1016/j.biotechadv.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Zheng YF, Bae SH, Kwon MJ, Park JB, Choi HD, Shin WG, Bae SK. Inhibitory effects of astaxanthin, β-cryptoxanthin, canthaxanthin, lutein, and zeaxanthin on cytochrome P450 enzyme activities. Food Chem Toxicol. 2013;59:78–85. doi: 10.1016/j.fct.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 3.Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Boussiba S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol Plant. 2010;108:111–117. doi: 10.1034/j.1399-3054.2000.108002111.x. [DOI] [Google Scholar]
- 5.Higuera-Ciapara I, Félix-Valenzuela L, Goycoolea FM. Astaxanthin: A review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 6.Tume RK, Sikes AL, Tabrett S, Smith DM. Effect of background colour on the distribution of astaxanthin in black tiger prawn (Penaeus monodon): Effective method for improvement of cooked colour. Aquaculture. 2009;296:129–135. doi: 10.1016/j.aquaculture.2009.08.006. [DOI] [Google Scholar]
- 7.Mosaad YO, Gobba NA, Hussein MA. Astaxanthin; a promising protector against gentamicin-induced nephrotoxicity in rats. Curr Pharm Biotechnol. 2016;17:1189–1197. doi: 10.2174/1389201017666160922110740. [DOI] [PubMed] [Google Scholar]
- 8.Curek GD, Cort A, Yucel G, Demir N, Ozturk S, Elpek GO, Savas B, Aslan M. Effect of astaxanthin on hepatocellular injury following ischemia/reperfusion. Toxicology. 2010;267:147–153. doi: 10.1016/j.tox.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto Y, Yoshida H, Kondo K. Potential anti-atherosclerotic properties of astaxanthin. Mar Drugs. 2016;14:35. doi: 10.3390/md14020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zajac G, Machalska E, Kaczor A, Kessler J, Bouř P, Baranska M. Structure of supramolecular astaxanthin aggregates revealed by molecular dynamics and electronic circular dichroism spectroscopy. Phys Chem Chem Phys. 2018;20:18038–18046. doi: 10.1039/C8CP01742E. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Armstrong DW, Chang CD. Rapid baseline separation of enantiomers and a mesoform of all-trans-astaxanthin, 13-cis-astaxanthin, adonirubin, and adonixanthin in standards and commercial supplements. J Chromatogr A. 2008;1194:172–177. doi: 10.1016/j.chroma.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Luo Q, Cao Y, Goulette T, Liu X, Xiao H. Mechanism of different stereoisomeric astaxanthin in resistance to oxidative stress in caenorhabditis elegans. J Food Sci. 2016;81:H2280–H2287. doi: 10.1111/1750-3841.13417. [DOI] [PubMed] [Google Scholar]
- 13.Yuan JP, Peng J, Yin K, Wang JH. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol Nutr Food Res. 2011;55:150–165. doi: 10.1002/mnfr.201000414. [DOI] [PubMed] [Google Scholar]
- 14.Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications-a review. Mar Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakhri S, Abbaszadeh F, Dargahi L, Jorjani M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol Res. 2018;136:1–20. doi: 10.1016/j.phrs.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R. A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol. 2008;34:77–88. doi: 10.1080/10408410802086783. [DOI] [PubMed] [Google Scholar]
- 17.Capelli B, Bagchi D, Cysewski GR. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods. 2013;12:145–152. doi: 10.1007/s13749-013-0051-5. [DOI] [Google Scholar]
- 18.Baccouche B, Benlarbi M, Barber AJ, Ben Chaouacha-Chekir R. Short-term administration of astaxanthin attenuates retinal changes in diet-induced diabetic psammomys obesus. Curr Eye Res. 2018;43:1177–1189. doi: 10.1080/02713683.2018.1484143. [DOI] [PubMed] [Google Scholar]
- 19.Yoshihisa Y, Rehman MU, Shimizu T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp Dermatol. 2014;23:178–183. doi: 10.1111/exd.12347. [DOI] [PubMed] [Google Scholar]
- 20.Ito N, Seki S, Ueda F. The protective role of astaxanthin for UV-induced skin deterioration in healthy people-a randomized, double-blind, placebo-controlled trial. Nutrients. 2018;10:817. doi: 10.3390/nu10070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhuvaneswari S, Arunkumar E, Viswanathan P, Anuradha CV. Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet. Process Biochem. 2010;45:1406–1414. doi: 10.1016/j.procbio.2010.05.016. [DOI] [Google Scholar]
- 22.Fan CD, Sun JY, Fu XT, Hou YJ, Li Y, Yang MF, Fu XY, Sun BL. Astaxanthin attenuates homocysteine-induced cardiotoxicity in vitro and in vivo by inhibiting mitochondrial dysfunction and oxidative damage. Front Physiol. 2017;8:1041. doi: 10.3389/fphys.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JH, Park JJ, Lee BJ, Joo MK, Chun HJ, Lee SW, Bak YT. Astaxanthin inhibits proliferation of human gastric cancer cell lines by Interrupting cell cycle progression. Gut Liver. 2016;10:369–374. doi: 10.5009/gnl15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H, Niu H, Shao A, Wu C, Dixon BJ, Zhang J, Yang S, Wang Y. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar Drugs. 2015;13:5750–5766. doi: 10.3390/md13095750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimmig B, Kim SH, Nash K, Bickford PC, Douglas Shytle R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience. 2017;39:19–32. doi: 10.1007/s11357-017-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khademian M, Imlay JA. How microbes evolved to tolerate oxygen. Trends Microbiol. 2021;29:428–440. doi: 10.1016/j.tim.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammarlund EU, Flashman E, Mohlin S, Licausi F. Oxygen-sensing mechanisms across eukaryotic kingdoms and their roles in complex multicellularity. Science. 2020;370:eaba3512. doi: 10.1126/science.aba3512. [DOI] [PubMed] [Google Scholar]
- 28.Kamath BS, Srikanta BM, Dharmesh SM, Sarada R, Ravishankar GA. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur J Pharmacol. 2008;590:387–395. doi: 10.1016/j.ejphar.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 29.Rao AR, Sindhuja HN, Dharmesh SM, Sankar KU, Sarada R, Ravishankar GA. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J Agric Food Chem. 2013;61:3842–3851. doi: 10.1021/jf304609j. [DOI] [PubMed] [Google Scholar]
- 30.Naguib YM. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem. 2000;48:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima Y, Inokuchi Y, Shimazawa M, Otsubo K, Ishibashi T, Hara H. Astaxanthin, a dietary carotenoid, protects retinal cells against oxidative stress in-vitro and in mice in-vivo. J Pharm Pharmacol. 2008;60:1365–1374. doi: 10.1211/jpp.60.10.0013. [DOI] [PubMed] [Google Scholar]
- 32.Ye Q, Zhang X, Huang B, Zhu Y, Chen X. Astaxanthin suppresses MPP(+)-induced oxidative damage in PC12 cells through a Sp1/NR1 signaling pathway. Mar Drugs. 2013;11:1019–1034. doi: 10.3390/md11041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarneshan SN, Fakhri S, Farzaei MH, Khan H, Saso L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem Toxicol. 2020;145:111714. doi: 10.1016/j.fct.2020.111714. [DOI] [PubMed] [Google Scholar]
- 34.Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li W, Zhou ML, Wang XL. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar Drugs. 2014;12:6125–6141. doi: 10.3390/md12126125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y, Hou D, Zhang X. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis. 2013;19:1656–1666. [PMC free article] [PubMed] [Google Scholar]
- 36.Wang HQ, Sun XB, Xu YX, Zhao H, Zhu QY, Zhu CQ. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010;1360:159–167. doi: 10.1016/j.brainres.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 37.Al-Amin MM, Mahmud W, Pervin MS, Ridwanul Islam SM, Ashikur Rahman M, Zinchenko A. Astaxanthin ameliorates scopolamine-induced spatial memory deficit via reduced cortical-striato-hippocampal oxidative stress. Brain Res. 2019;1710:74–81. doi: 10.1016/j.brainres.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Kim SH, Lim JW, Kim H. Astaxanthin inhibits mitochondrial dysfunction and interleukin-8 expression in helicobacter pylori-infected gastric epithelial cells. Nutrients. 2018;10:1320. doi: 10.3390/nu10091320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranga Rao A, Raghunath Reddy RL, Baskaran V, Sarada R, Ravishankar GA. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J Agric Food Chem. 2010;58:8553–8559. doi: 10.1021/jf101187k. [DOI] [PubMed] [Google Scholar]
- 40.Netea MG, Balkwill F, Chonchol M, Cominelli F, Donath MY, Giamarellos-Bourboulis EJ, Golenbock D, Gresnigt MS, Heneka MT, Hoffman HM, et al. A guiding map for inflammation. Nat Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat Rev Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 43.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang C, Hassan YI, Liu R, Zhang H, Chen Y, Zhang L, Tsao R. Anti-inflammatory effects of different astaxanthin isomers and the roles of lipid transporters in the cellular transport of astaxanthin isomers in Caco-2 cell monolayers. J Agric Food Chem. 2019;67:6222–6231. doi: 10.1021/acs.jafc.9b02102. [DOI] [PubMed] [Google Scholar]
- 45.Grilo AL, Mantalaris A. Apoptosis: A mammalian cell bioprocessing perspective. Biotechnol Adv. 2019;37:459–475. doi: 10.1016/j.biotechadv.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25:27–36. doi: 10.1038/cdd.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Wang H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar Drugs. 2015;13:4310–4330. doi: 10.3390/md13074310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong LY, Jin J, Lu G, Kang XL. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar Drugs. 2013;11:960–974. doi: 10.3390/md11030960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo SX, Zhou HL, Huang CL, You CG, Fang Q, Wu P, Wang XG, Han CM. Astaxanthin attenuates early acute kidney injury following severe burns in rats by ameliorating oxidative stress and mitochondrial-related apoptosis. Mar Drugs. 2015;13:2105–2123. doi: 10.3390/md13042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang XS, Zhang X, Wu Q, Li W, Zhang QR, Wang CX, Zhou XM, Li H, Shi JX, Zhou ML. Astaxanthin alleviates early brain injury following subarachnoid hemorrhage in rats: Possible involvement of Akt/bad signaling. Mar Drugs. 2014;12:4291–4310. doi: 10.3390/md12084291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Takahara T, Fujino M, Fukuhara Y, Sugiyama T, Li XK, Takahara S. Astaxanthin prevents ischemia-reperfusion injury of the steatotic liver in mice. PLoS One. 2017;12:e0187810. doi: 10.1371/journal.pone.0187810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein RS, Hunter CA. Protective and pathological immunity during central nervous system infections. Immunity. 2017;46:891–909. doi: 10.1016/j.immuni.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manabe Y, Komatsu T, Seki S, Sugawara T. Dietary astaxanthin can accumulate in the brain of rats. Biosci Biotechnol Biochem. 2018;82:1433–1436. doi: 10.1080/09168451.2018.1459467. [DOI] [PubMed] [Google Scholar]
- 56.El-Agamy SE, Abdel-Aziz AK, Wahdan S, Esmat A, Azab SS. Astaxanthin ameliorates doxorubicin-induced cognitive impairment (Chemobrain) in experimental rat model: Impact on oxidative, inflammatory, and apoptotic machineries. Mol Neurobiol. 2018;55:5727–5740. doi: 10.1007/s12035-017-0797-7. [DOI] [PubMed] [Google Scholar]
- 57.Lee H, Lim JW, Kim H. Effect of astaxanthin on activation of autophagy and inhibition of apoptosis in helicobacter pylori-infected gastric epithelial cell line AGS. Nutrients. 2020;12:1750. doi: 10.3390/nu12061750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Damodara Gowda KM, Suchetha Kumari N, Ullal H. Role of astaxanthin in the modulation of brain-derived neurotrophic factor and spatial learning behavior in perinatally undernourished Wistar rats. Nutr Neurosci. 2020;23:422–431. doi: 10.1080/1028415X.2018.1515301. [DOI] [PubMed] [Google Scholar]
- 59.Wang YL, Zhu XL, Sun MH, Dang YK. Effects of astaxanthin onaxonal regeneration via cAMP/PKA signaling pathway in mice with focal cerebral infarction. Eur Rev Med Pharmacol Sci. 2019;23((3 Suppl)):S135–S143. doi: 10.26355/eurrev_201908_18640. [DOI] [PubMed] [Google Scholar]
- 60.Cullen DK, Simon CM, LaPlaca MC. Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal-astrocytic co-cultures. Brain Res. 2007;1158:103–115. doi: 10.1016/j.brainres.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J Neurosci. 1995;15:5765–5778. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tublin JM, Adelstein JM, Del Monte F, Combs CK, Wold LE. Getting to the heart of Alzheimer disease. Circ Res. 2019;124:142–149. doi: 10.1161/CIRCRESAHA.118.313563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Jia J, Wang F, Wei C, Zhou A, Jia X, Li F, Tang M, Chu L, Zhou Y, Zhou C, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. 2014;10:1–9. doi: 10.1016/j.jalz.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, Fowler C, Li QX, Martins R, Rowe C, et al. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature. 2018;554:249–254. doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- 66.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. doi: 10.1016/S0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 67.Pradeepkiran JA, Reddy PH. Defective mitophagy in Alzheimer's disease. Ageing Res Rev. 2020;64:101191. doi: 10.1016/j.arr.2020.101191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Squitti R, Mendez A, Ricordi C, Siotto M, Goldberg R. Copper in glucose intolerance, cognitive decline, and Alzheimer disease. Alzheimer Dis Assoc Disord. 2019;33:77–85. doi: 10.1097/WAD.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 69.Bjørklund G, Dadar M, Peana M, Rahaman MS, Aaseth J. Interactions between iron and manganese in neurotoxicity. Arch Toxicol. 2020;94:725–734. doi: 10.1007/s00204-020-02652-2. [DOI] [PubMed] [Google Scholar]
- 70.Khan MM, Xiao J, Patel D, LeDoux MS. DNA damage and neurodegenerative phenotypes in aged Ciz1 null mice. Neurobiol Aging. 2018;62:180–190. doi: 10.1016/j.neurobiolaging.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito N, Saito H, Seki S, Ueda F, Asada T. Effects of composite supplement containing astaxanthin and sesamin on cognitive functions in people with mild cognitive impairment: A randomized, double-blind, placebo-controlled trial: Erratum. J Alzheimers Dis. 2019;68:839. doi: 10.3233/JAD-189016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sekikawa T, Kizawa Y, Li Y, Takara T. Cognitive function improvement with astaxanthin and tocotrienol intake: A randomized, double-blind, placebo-controlled study. J Clin Biochem Nutr. 2020;67:307–316. doi: 10.3164/jcbn.19-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taksima T, Chonpathompikunlert P, Sroyraya M, Hutamekalin P, Limpawattana M, Klaypradit W. Effects of astaxanthin from shrimp shell on oxidative stress and behavior in animal model of Alzheimer's disease. Mar Drugs. 2019;17:628. doi: 10.3390/md17110628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020;19:758–766. doi: 10.1016/S1474-4422(20)30231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rahman SO, Panda BP, Parvez S, Kaundal M, Hussain S, Akhtar M, Najmi AK. Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by Aβ peptides in animal model of Alzheimer's disease. Biomed Pharmacother. 2019;110:47–58. doi: 10.1016/j.biopha.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 76.Craft S, Watson GS. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 77.Kim RE, Shin CY, Han SH, Kwon KJ. Astaxanthin suppresses PM2.5-induced neuroinflammation by regulating Akt phosphorylation in BV-2 microglial cells. Int J Mol Sci. 2020;21:7227. doi: 10.3390/ijms21197227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim YH, Koh HK, Kim DS. Down-regulation of IL-6 production by astaxanthin via ERK-, MSK-, and NF-κB-mediated signals in activated microglia. Int Immunopharmacol. 2010;10:1560–1572. doi: 10.1016/j.intimp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Wen X, Huang A, Hu J, Zhong Z, Liu Y, Li Z, Pan X, Liu Z. Neuroprotective effect of astaxanthin against glutamate-induced cytotoxicity in HT22 cells: Involvement of the Akt/GSK-3β pathway. Neuroscience. 2015;303:558–568. doi: 10.1016/j.neuroscience.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 80.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: Risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 81.Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 82.Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr Med Chem. 2001;8:721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- 83.Issa AR, Sun J, Petitgas C, Mesquita A, Dulac A, Robin M, Mollereau B, Jenny A, Chérif-Zahar B, Birman S. The lysosomal membrane protein LAMP2A promotes autophagic flux and prevents SNCA-induced Parkinson disease-like symptoms in the Drosophila brain. Autophagy. 2018;14:1898–1910. doi: 10.1080/15548627.2018.1491489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ye Q, Huang B, Zhang X, Zhu Y, Chen X. Astaxanthin protects against MPP(+)-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 2012;13:156. doi: 10.1186/1471-2202-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brasil FB, Bertolini Gobbo RC, Souza de Almeida FJ, Luckachaki MD, Dall'Oglio EL, de Oliveira MR. The signaling pathway PI3K/Akt/Nrf2/HO-1 plays a role in the mitochondrial protection promoted by astaxanthin in the SH-SY5Y cells exposed to hydrogen peroxide. Neurochem Int. 2021;146:105024. doi: 10.1016/j.neuint.2021.105024. [DOI] [PubMed] [Google Scholar]
- 86.Lee DH, Kim CS, Lee YJ. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem Toxicol. 2011;49:271–280. doi: 10.1016/j.fct.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim JH, Hwang J, Shim E, Chung EJ, Jang SH, Koh SB. Association of serum carotenoid, retinol, and tocopherol concentrations with the progression of Parkinson's disease. Nutr Res Pract. 2017;11:114–120. doi: 10.4162/nrp.2017.11.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 89.George PM, Steinberg GK. Novel stroke therapeutics: Unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87:297–309. doi: 10.1016/j.neuron.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lazou A, Bogoyevitch MA, Clerk A, Fuller SJ, Marshall CJ, Sugden PH. Regulation of mitogen-activated protein kinase cascade in adult rat heart preparations in vitro. Circ Res. 1994;75:932–941. doi: 10.1161/01.RES.75.5.932. [DOI] [PubMed] [Google Scholar]
- 91.Zhang R, Liu C, Liu X, Guo Y. Protective effect of spatholobus suberectus on brain tissues in cerebral ischemia. Am J Transl Res. 2016;8:3963–3969. [PMC free article] [PubMed] [Google Scholar]
- 92.Vani JR, Mohammadi MT, Foroshani MS, Jafari M. Polyhydroxylated fullerene nanoparticles attenuate brain infarction and oxidative stress in rat model of ischemic stroke. EXCLI J. 2016;15:378–390. doi: 10.17179/excli2016-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xue Y, Qu Z, Fu J, Zhen J, Wang W, Cai Y, Wang W. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res Bull. 2017;131:221–228. doi: 10.1016/j.brainresbull.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 94.Pan L, Zhou Y, Li XF, Wan QJ, Yu LH. Preventive treatment of astaxanthin provides neuroprotection through suppression of reactive oxygen species and activation of antioxidant defense pathway after stroke in rats. Brain Res Bull. 2017;130:211–220. doi: 10.1016/j.brainresbull.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 95.Lee DH, Lee YJ, Kwon KH. Neuroprotective effects of astaxanthin in oxygen-glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. J Clin Biochem Nutr. 2010;47:121–129. doi: 10.3164/jcbn.10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu YP, Liu SY, Sun H, Wu XM, Li JJ, Zhu L. Neuroprotective effect of astaxanthin on H(2)O(2)-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010;1360:40–48. doi: 10.1016/j.brainres.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 97.Yang BB, Zou M, Zhao L, Zhang YK. Astaxanthin attenuates acute cerebral infarction via Nrf-2/HO-1 pathway in rats. Curr Res Transl Med. 2021;69:103271. doi: 10.1016/j.retram.2020.103271. [DOI] [PubMed] [Google Scholar]
- 98.Budohoski KP, Guilfoyle M, Helmy A, Huuskonen T, Czosnyka M, Kirollos R, Menon DK, Pickard JD, Kirkpatrick PJ. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2014;85:1343–1353. doi: 10.1136/jnnp-2014-307711. [DOI] [PubMed] [Google Scholar]
- 99.Vergouwen MD, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42:924–929. doi: 10.1161/STROKEAHA.110.597914. [DOI] [PubMed] [Google Scholar]
- 100.Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J, Zhang JH. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol. 2014;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serrone JC, Maekawa H, Tjahjadi M, Hernesniemi J. Aneurysmal subarachnoid hemorrhage: Pathobiology, current treatment and future directions. Expert Rev Neurother. 2015;15:367–380. doi: 10.1586/14737175.2015.1018892. [DOI] [PubMed] [Google Scholar]
- 102.Zhang X, Lu Y, Wu Q, Dai H, Li W, Lv S, Zhou X, Zhang X, Hang C, Wang J. Astaxanthin mitigates subarachnoid hemorrhage injury primarily by increasing sirtuin 1 and inhibiting the Toll-like receptor 4 signaling pathway. FASEB J. 2019;33:722–737. doi: 10.1096/fj.201800642RR. [DOI] [PubMed] [Google Scholar]
- 103.Zhang XS, Zhang X, Zhou ML, Zhou XM, Li N, Li W, Cong ZX, Sun Q, Zhuang Z, Wang CX, Shi JX. Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. J Neurosurg. 2014;121:42–54. doi: 10.3171/2014.2.JNS13730. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, Liu Y, Li Y, Liu B, Wu P, Xu S, Shi H. Protective effects of astaxanthin on subarachnoid hemorrhage-induced early brain injury: Reduction of cerebral vasospasm and improvement of neuron survival and mitochondrial function. Acta Histochem. 2019;121:56–63. doi: 10.1016/j.acthis.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 105.Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17071. doi: 10.1038/nrdp.2017.85. [DOI] [PubMed] [Google Scholar]
- 106.Arthur KC, Calvo A, Price TR, Geiger JT, Chiò A, Traynor BJ. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat Commun. 2016;7:12408. doi: 10.1038/ncomms12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nuevo Ordoñez Y, Montes-Bayón M, Blanco-González E, Sanz-Medel A. Quantitative analysis and simultaneous activity measurements of Cu, Zn-superoxide dismutase in red blood cells by HPLC-ICPMS. Anal Chem. 2010;82:2387–2394. doi: 10.1021/ac902624b. [DOI] [PubMed] [Google Scholar]
- 108.Bond L, Bernhardt K, Madria P, Sorrentino K, Scelsi H, Mitchell CS. A metadata analysis of oxidative stress etiology in preclinical amyotrophic lateral sclerosis: Benefits of antioxidant therapy. Front Neurosci. 2018;12:10. doi: 10.3389/fnins.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Isonaka R, Hiruma H, Katakura T, Kawakami T. Inhibition of superoxide dismutase selectively suppresses growth of rat spinal motor neurons: Comparison with phosphorylated neurofilament-containing spinal neurons. Brain Res. 2011;1425:13–19. doi: 10.1016/j.brainres.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 110.Fitzgerald KC, O'Reilly ÉJ, Fondell E, Falcone GJ, McCullough ML, Park Y, Kolonel LN, Ascherio A. Intakes of vitamin C and carotenoids and risk of amyotrophic lateral sclerosis: Pooled results from 5 cohort studies. Ann Neurol. 2013;73:236–245. doi: 10.1002/ana.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nieves JW, Gennings C, Factor-Litvak P, Hupf J, Singleton J, Sharf V, Oskarsson B, Fernandes Filho JA, Sorenson EJ, D'Amico E, et al. Association between dietary intake and function in amyotrophic lateral sclerosis. JAMA Neurol. 2016;73:1425–1432. doi: 10.1001/jamaneurol.2016.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no data sets were generated or analyzed during the current study.