Abstract
Chromatin configuration is highly dynamic during embryonic development in animals, exerting an important point of control in transcriptional regulation. Yet there exists remarkably little information about the role of evolutionary changes in chromatin configuration to the evolution of gene expression and organismal traits. Genome-wide assays of chromatin configuration, coupled with whole-genome alignments, can help address this gap in knowledge in several ways. In this study we present a comparative analysis of regulatory element sequences and accessibility throughout embryogenesis in three sea urchin species with divergent life histories: a lecithotroph Heliocidaris erythrogramma, a closely related planktotroph H. tuberculata, and a distantly related planktotroph Lytechinus variegatus. We identified distinct epigenetic and mutational signatures of evolutionary modifications to the function of putative cis-regulatory elements in H. erythrogramma that have accumulated nonuniformly throughout the genome, suggesting selection, rather than drift, underlies many modifications associated with the derived life history. Specifically, regulatory elements composing the sea urchin developmental gene regulatory network are enriched for signatures of positive selection and accessibility changes which may function to alter binding affinity and access of developmental transcription factors to these sites. Furthermore, regulatory element changes often correlate with divergent expression patterns of genes involved in cell type specification, morphogenesis, and development of other derived traits, suggesting these evolutionary modifications have been consequential for phenotypic evolution in H. erythrogramma. Collectively, our results demonstrate that selective pressures imposed by changes in developmental life history rapidly reshape the cis-regulatory landscape of core developmental genes to generate novel traits and embryonic programs.
Keywords: Evolution, development, gene regulation, sea urchins, life history, ATAC-seq
Introduction
Cis-regulatory elements are significant contributors to phenotypic evolution and trait diversification by modulating transcriptional regulation via gene network interactions (Davidson and Erwin 2006; Wray 2007; Wittkopp and Kalay 2012). Extensive empirical evidence demonstrates that the function of cis-regulatory elements depends on distinct molecular characteristics such as the element’s sequence and epigenetic landscape that collectively provide a dynamic and heritable genomic substrate affecting interspecific and intraspecific diversification. Enabled by advances in genomic technology and reduced sequencing costs, more recent work has demonstrated the importance of cis-regulatory elements in diverse biological contexts across a variety of animal systems at a genome-wide scale; for example: origination of human-specific traits (Shibata et al. 2012; Swain-Lenz et al. 2019), convergent evolution of flight loss in birds (Sackton et al. 2019), population-level adaptation (Lewis and Reed 2019), genetic assimilation (van der Burg et al. 2020) and color identity (Livraghi et al. 2021) in butterfly wing patterning, embryonic regulation of gene expression in a crustacean (Sun and Patel 2021), limb bud development in chicken and mice (Jhanwar et al. 2021), and single-cell resolution of Drosophila cell lineages (Cusanovich et al. 2018). However, direct integration of large-scale cis-regulatory analysis and transcription in a defined evolutionary-developmental (evo-devo) context is lacking. In particular, relationships governing divergence in chromatin and gene expression, the role of such changes in trait evolution, and the action of natural selection on these processes remain largely unexplored within an interrogable study system providing resolved phylogenetic relationships, chromosome-level reference genomes, and sufficiently recent divergence times that noncoding regions can be aligned with confidence. In this study, we address these important questions by comparing the function of cis-regulatory elements in embryonic gene expression of sea urchin species with divergent developmental life history strategies to assess the extent cis-regulatory elements play in the evolution of gene expression, developmental systems, and by extension, trait variation and origination.
Among the most common and well-studied life history shifts in the animal kingdom is the evolution of feeding or nonfeeding larval development (Flatt and Heyland 2011). In marine invertebrates, planktotrophy (feeding larval development) is characterized by species that produce many, relatively small eggs and have a longer planktonic larval period, whereas lecithotrophic species (nonfeeding larval development) invest more maternal resources into a fewer number of large eggs that develop through an abbreviated larval stage (Strathmann 1985). Importantly, the ecological circumstances associated with each strategy are predictable and well-studied across diverse marine clades (Vance 1973; Strathmann 1985; Pechenik 1999; Marshall et al. 2012; Morgan 2020), providing natural systems to analyze how selection shapes often rapid phenotypic divergence in developmental regulation and physiology. For example, within echinoderms, lecithotrophy has independently evolved from the ancestral planktotrophic state more than 20 times (Raff 1987; Wray 1996; McEdward and Miner 2001; Hart 2002), implying that recurring sets of environmental conditions (e.g., limited availability of phytoplankton) and selection can enact evolutionary modifications to even highly conserved developmental processes. The consequences include changes throughout development, ranging from egg composition (Prowse et al. 2009; Falkner et al. 2015; Byrne and Sewell 2019; Davidson et al. 2019) and zygotic genome activation (Wray and Raff 1990; Israel et al. 2016; Wang et al. 2020), cell type specification and embryonic patterning (Wray and Raff 1989; Love and Raff 2006), to construction of larval structures such as the skeleton and imaginal adult rudiment (Emlet 1995; Koop et al. 2017). Still, the impact selection exerts on the evolution of cis-regulatory interactions underlying alternative developmental programs and contrasting life history strategies is not known. This gap in knowledge is manifested more broadly in our current sparse understanding of the biological relationships governing regulatory element function, evolutionary constraints inherent to development and gene network architecture, and phenotypic diversification.
Perhaps the best studied example of the life history switch from planktotrophic to lecithotrophic development is the sea urchin Heliocidaris erythrogramma. Prior work has found dramatic modifications to morphogenesis and embryonic patterning in H. erythrogramma relative to planktotrophic sea urchin species (for reviews see Raff and Byrne 2006; Wray 2022). For instance, dorso-ventral and left-right axis establishment occurs considerably earlier in this species (Henry et al. 1990), concordant with accelerated rudiment development and metamorphosis (Williams and Anderson 1975). At other embryonic domains, specification of cell lineages such as those responsible for mesenchymal tissue types have been delayed, lost, or spatially rearranged (Wray and Raff 1990; Klueg et al. 1997; Wilson, Andrews, Rudolf Turner, et al. 2005; Davidson et al. 2021). Recent transcriptomic studies have shown that expression profiles of key developmental gene regulatory network (dGRN) genes in this species have diverged from the highly conserved expression patterns in planktotrophic species (Israel et al. 2016), and many of these regulatory changes are predicted to be based in cis (Wang et al. 2020). However, the role of evolutionary changes in the sequence and configuration of cis-regulatory elements to the rapid evolution of these derived phenotype in H. erythrogramma is not known.
To address this, we compared chromatin accessibility, regulatory element sequence, and gene expression across six developmental stages in three sea urchin species spanning this life history switch: the lecithotroph H. erythrogramma, a closely-related (∼ 5 my divergence) planktotroph H. tuberculata, and a distantly-related (∼ 40 my divergence) planktotroph, Lytechinus variegatus (fig. 1A). Planktotrophic (feeding) larvae represent the ancestral life history mode within echinoderms relative to the derived lecithotrophic state (Strathmann 1978; Raff 1987; Wray 1996; McEdward and Miner 2001), so recent and dramatic changes in developmental phenotypes in H. erythrogramma provide an exceptional natural system to analyze the consequences of strong shifts in selection and life history on the evolution of gene regulatory mechanisms (Wray 2022).
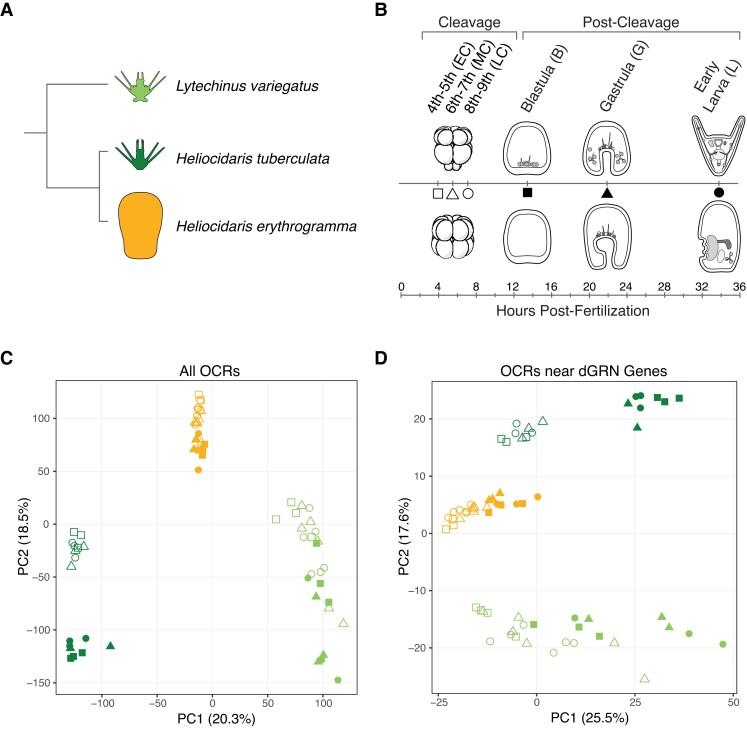
Fig. 1.
Global variation in chromatin accessibility between sea urchin embryos is largely driven by developmental stage and life history strategy. (A) Three focal sea urchin species of this study, including two planktotrophic species representing the ancestral life history (light green: Lytechinus variegatus; dark green: Heliocidaris tuberculata) and one lecithotrophic species representing the derived life history (orange: Heliocidaris erythrogramma). This color scheme is consistent throughout the study. (B) Developmental stages assayed in this study for each species. The ancestral life history is illustrated above, with corresponding developmental stages of the derived life history below. Developmental times are shown for Heliocidaris tuberculata at 21 °C. Note, however, samples were collected on the basis of developmental stage, not hours postfertilization, for all three species. (C-D) Principal component analysis of variation in normalized chromatin accessibility among (C) all orthologous OCRs and (D) only OCRs near dGRN genes through embryogenesis. OCR: open chromatin region; dGRN: developmental gene regulatory network.
In this study, we investigate the potential contribution of changes in regulatory element accessibility and sequence, as well as their interactions, on the evolution of developmental gene expression at a genome-wide scale. We consider our results in the context of two simple mechanistic models for an evolutionary change in the function of an individual regulatory element during development. (1) local mutation first: a local mutation alters the recruitment of a transcription factor, histone remodeler, or other local chromatin modifier (Clapier and Bradley 2009; Zaret and Carroll 2011), thereby altering the accessibility of the regulatory element and, in turn, possibly the transcription of a nearby gene. (2) regulatory factor first: a change elsewhere in the genome alters the expression of a pioneer transcription factor, histone remodeler, or other transcription factor that directly alters the accessibility of regulatory element in question and, in turn, possibly the transcription of a nearby gene. Both models accommodate an evolutionary increase or decrease in accessibility, and the accessibility change could alter interactions with other regulatory proteins, by masking or unmasking additional existing protein:DNA binding sites. Finally, in both models, the precipitating mutation(s) could be visible to selection through their impact on gene expression.
Within this framework, we identify distinct epigenetic and mutational signatures of evolutionary modifications in the noncoding genome of H. erythrogramma relative to species with the ancestral life history. Furthermore, we find that these changes have accumulated nonuniformly across the genome, and are notably enriched near genes with derived expression that are involved in cell type specification, morphogenesis, and development of other derived traits. The patterns of cis-regulatory modifications characterized here highlight the contribution of the epigenome to the evolution of transcriptional regulation and trait evolution more broadly.
Results and Discussion
We performed ATAC-seq on six developmental stages of H. erythrogramma (He), H. tuberculata (Ht), and L. variegatus (Lv) in order to identify open chromatin regions (OCRs) representing putative cis-regulatory elements (CREs) during embryogenesis. In this study, we use the term ‘OCR’ when reporting analysis results because ATAC-seq is an assay of open chromatin. We apply the more specific term ‘CRE’ when discussing the biological interpretation of our results because cis-regulatory elements are the operational units putatively identified within open chromatin peaks used to understand mechanisms of gene regulatory evolution. Importantly, however, while OCRs are known to be enriched for CRE sequences (Thurman et al. 2012), no strict relationship governs the presence of CREs based on occurrence of open chromatin peaks alone. Furthermore, even if an OCR is an authentic CRE, an evolutionary change in chromatin state may or may not have impact on the expression of a nearby gene. Indeed, many OCRs in sea urchin embryos open hours before any nearby gene is transcribed, including experimentally validated CREs (Shashikant et al. 2018; Arenas-Mena et al. 2021); this finding suggests that the initial opening of many OCRs during early development makes a permissive rather than determinative contribution to activation of the zygotic transcriptome.
The developmental stages analyzed here include 4th/5th, 6th/7th, and 8th/9th mixed cleavage stage embryos, as well as hatched blastula, mid-gastrula, and early larva (fig. 1B), enabling characterization of regulatory element dynamics from the onset of zygotic transcription through the specification and morphogenesis of complex tissue types. We then carried out alignments of chromosome-scale genome assemblies to find evolutionarily conserved OCRs between species to compare accessibility and sequence composition of putative CREs. These analyses provide a simultaneous comparison of gene regulatory mechanisms in two ways: (1) availability of CREs to transcription factor binding associated with differential chromatin configuration (accessibility) and (2) affinity of transcription factor binding to CREs via changes in nucleotide sequence, as evidenced by motif analysis and signatures of positive selection. Importantly, the genomes of these sea urchin species were assembled and annotated using an identical set of sequencing technology and methodology (Davidson et al. 2020, 2021), minimizing technical bias and permitting high resolution identification of changes in regulatory element activity.
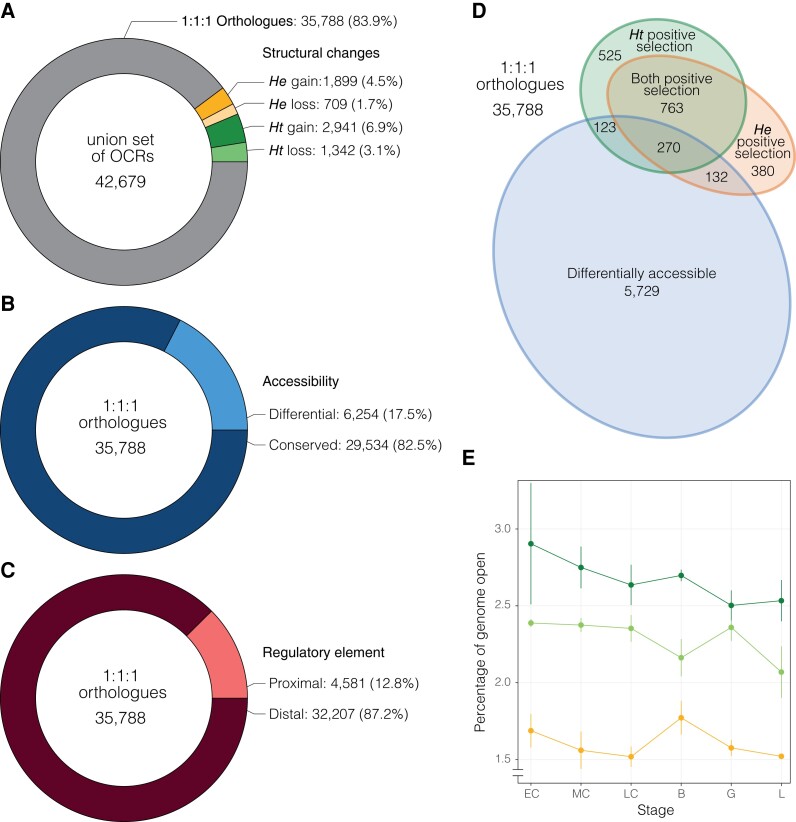
Of 42,679 OCRs identified across the three species, we found 35,788 1:1:1 orthologous, high confidence OCRs proximal to 11,679 gene models for cross-species comparison (fig. 2A). Note that inclusion in this set of 1:1:1 orthologues does not require that chromatin be open in all three species, but rather that it be open in at least one species at one time point surveyed and that the orthologous genomic sequence is present in all three species. This set of orthologous OCRs forms the basis of most analyses presented here. In addition to orthologous OCRs, we also identified sets of species-specific gains or losses of OCRs occurring on entire genomic segments present or lost in a single species (fig. 2A). Because these indel-based differences in OCRs are only present in at most two of the three sea urchin species compared in this study, they are treated as distinct sets of putative CREs. Although the changes in regulatory element function examined in this study are not applicable to species-specific OCRs, it is important to note that many insertions containing species-specific OCR gains occur nearby genes with differential expression levels in H. erythrogramma, suggesting gain or loss of entire CREs may be an important component of the evolution of the derived life history strategy in this species (supplementary fig. 1).
Fig. 2.
Summary statistics of regulatory element gains, losses, accessibility, and positive selection. (A) Union set of all OCRs, including structural differences between species such as lineage-specific gains and losses as well as 35,788 1:1:1 orthologous OCRs shared between all three species. These orthologous OCRs are the primary focus of this study. Breakdown of (B) OCR differentially accessibility between life history strategies and (C) OCR distance to gene models for these orthologous sites. (D) Overlap of OCR differential accessibility and evidence of positive selection on the He, Ht, or both branches. (E) Percentage of each species genome that is ‘open’ at each stage of development. ‘Open’ is defined as the cumulative portion of the genome represented by all OCRs (orthologous and lineage-specific) with significant levels of accessibility for a given stage and species (see supplementary fig. 3 for additional information). Error bars indicate standard deviation of % openness across biological replicates for each species and developmental stage. OCR: open chromatin region; He: Heliocidaris erythrogramma; Ht: Heliocidaris tuberculata.
Development and Life History Drive Global Variation Patterns of Accessibility
First, we assessed which biological factors may significantly contribute to variation in chromatin accessibility at a genomic scale. Principal component analysis (PCA) suggests species-level differences (PC 1: 20.3%) and the interaction of development and life history (PC 2: 18.5%) are the most important factors explaining variation in chromatin accessibility when all orthologous OCRs are considered (fig. 1C). Within each species, chromatin accessibility differences between stages roughly mirrors developmental progression as early and late stages cluster more closely to one another. Strikingly, though, there is far greater variation in accessibility through development in both species with the ancestral life history relative to He, suggesting the developmental chromatin landscape of the derived state is less dynamic during embryogenesis. This finding is consistent with delayed blastomere specification (Wray and Raff 1989) and simplified larval morphology in He (Emlet 1995), suggesting chromatin state changes normally associated with establishing cell identity and tissue differentiation have been modified during the evolution of its derived developmental life history.
Interestingly, when considering only OCRs near dGRN genes, the primary driver of variation in chromatin accessibility shifts to developmental life history rather than species-level differences (fig. 1D; PC 1: 25.5%), a finding corroborated by variance partitioning of the dataset (supplementary fig. 2). This result suggests chromatin configuration of critical developmental network genes is associated with developmental strategy in these species, as accessibility of regulatory elements controls the flow and deployment of embryonic programs, ultimately driving cell fate specification and morphogenesis. Therefore, changes in accessibility to CREs have the potential to modify the function and construction of novel developmental gene networks via altered regulatory interactions.
Loss of Regulatory Element Accessibility Within the Ancestral dGRN
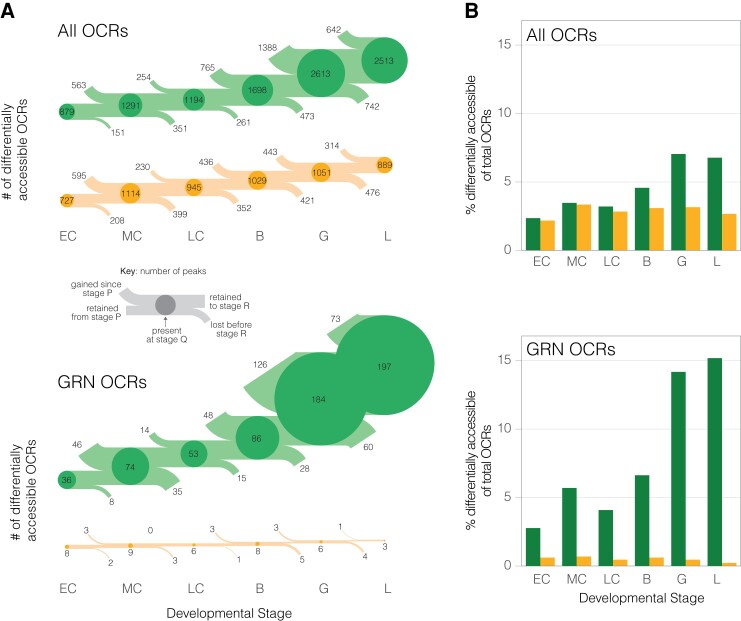
We next quantified how the accessibility of 35,788 OCRs varies between life history strategies in order to better understand how chromatin configuration at specific CREs may influence the evolution and function of alternative embryonic programs. Across each cleavage stage examined (timepoints spanning 16-cell through ∼500-cell stage embryos), we found an average of 5.7% of OCRs were differentially accessible between ancestral and derived life histories, including a modest difference in sites more accessible in Ht and Lv (3.1%) or He (2.6%) (fig. 3A,B; top). Strikingly, starting at blastula stage, the proportion of differentially accessible OCRs across the genome rises, with most sites increasing in accessibility (‘opening’) in the ancestral life history, but not in He. For example, by the larval stage, 9.5% of OCRs are differentially accessible between life histories, nearly three quarters of which (73.9%) are more accessible in Ht and Lv. Furthermore, when all OCRs are considered, including both orthologous and nonorthologous sites across species, a much greater proportion of the He genome is closed compared with Ht and Lv, suggesting that, at a genome-wide scale, He is characterized by zygotic quiescence during early development relative to ancestral life history (fig. 2E; supplementary fig. 3). Our results are consistent with the prediction that, as embryonic development proceeds, more regulatory sites open and become available to regulatory factor binding as zygotic transcription activates, cell lineages differentiate, tissue types are specified, and morphological structures are constructed. However, this genome ‘awakening’ and reconfiguration of chromatin state across the embryo unfolds at a much grander scale in Ht and Lv relative to He. This finding supports the idea that embryonic fate specification in He is delayed relative to the ancestral state (Wray and Raff 1989, 1990) and collectively, is concordant with a morphologically simplified larva (Williams and Anderson 1975) and derived gene expression patterning in this species (Wilson, Andrews, and Raff 2005; Wilson, Andrews, Rudolf Turner, et al. 2005; Smith et al. 2008; Israel et al. 2016; Koop et al. 2017).
Fig. 3.
The chromatin landscape of the derived life history is far less accessible than that of species with the ancestral life history mode, particularly near core dGRN genes. (A) Number (flow diagram) and (B) percentage (barplots) of OCRs that are significantly differentially accessible in H. erythrogramma or species with the ancestral life history strategy for all orthologous OCRs (top) and only OCRs near dGRN genes (bottom). A key for flow diagrams is provided to explain how the number of differentially accessible OCRs are gained, retained, and lost between developmental stages. Circle size is proportional to the percentage of each set of OCRs (those near all genes or only dGRN genes) that are differentially accessible at a given stage for either H. erythrogramma or species with the ancestral developmental mode. OCR: open chromatin region; dGRN: developmental gene regulatory network.
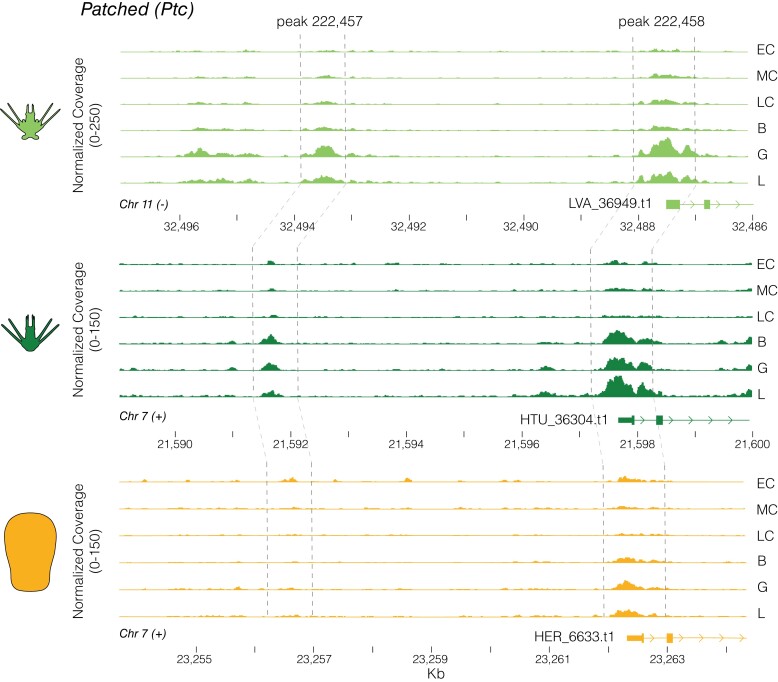
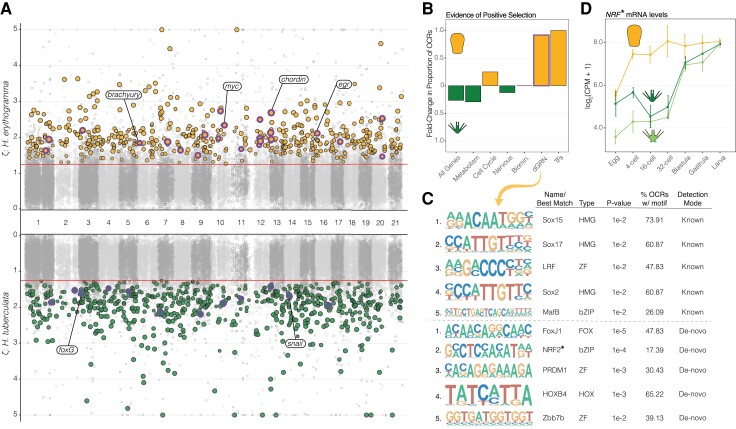
This delayed zygotic activation hypothesis is further supported when OCR accessibility of dGRN genes is compared. Near these critical developmental regulatory genes, we find a remarkable asymmetry in putative CRE accessibility between life history modes from the onset of development: on average, 0.5% of OCRs near dGRNs are more accessible in He during development, whereas this figure ranges from 2.8% (early cleavage) to 15.1% (larva) for species with the ancestral life history (fig. 3A,B; bottom). Therefore, modifications to CRE accessibility in He are not uniformly distributed across the genome, as would be expected of neutral processes such as drift, but instead are strikingly enriched near dGRN genes (Fisher’s exact test, two-sided: P < 1.31 × 10−11). An example of possible CRE activation in the ancestral state that is not found in He is the chromatin landscape proximal to the gene patched (ptc) (fig. 4). This gene encodes a receptor of the Hedgehog ligand; its misexpression results in skeletal defects and abnormal mesodermal patterning in the ancestral sea urchin developmental program (Walton et al. 2009).
Fig. 4.
Example of an evolutionary change in chromatin accessibility during early development. The embryonic chromatin landscape near the gene Patched is shown for the three study species, with gene models on the right and dashed lines indicating orthologous noncoding regions. In the two planktotrophs, the most proximal OCR (likely promoter based on location at the 5’ end of the 5’ UTR) opens substantially after cleavage, and a distal OCR ∼7 kb upstream opens by gastrula stage. In in H. erythrogramma, the proximal OCR is substantially less accessible and the distal OCR is not above background accessibility levels.
Given that certain ecological conditions likely favor the evolution of nonfeeding development (Strathmann 1978, 1985; Marshall et al. 2012), the finding that differences in chromatin accessibility are concentrated around developmental regulatory genes suggests natural selection has sculpted the epigenetic landscape nearby the sea urchin dGRN during life history evolution, generating potentially adaptive changes to transcriptional regulation and expression patterning during embryogenesis. Furthermore, our results indicate that the evolutionary changes in putative CRE accessibility have functional consequences—if they had no impact on gene expression or some other trait, natural selection would not influence their status during development.
Developmental Regulatory Elements Have Accumulated Mutational Signatures of Positive Selection
Prior studies have found enrichment of signals of positive selection in OCRs near genes that are differentially expressed between species (Shibata et al. 2012; Edsall et al. 2019; Swain-Lenz et al. 2019). To test whether a similar relationship exists in our study clade and whether it was accentuated during the evolution of lecithotrophy, we next analyzed sequence evolution of orthologous regulatory loci to identify instances of mutational pileup as a signal of positive selection within these sites. Substitution rates of 35,788 OCRs on the He and Ht branches (table S1) were compared with 10 randomly selected neutrally evolving, noncoding reference sequences, and a likelihood ratio test was implemented to fit substitution rates across the tree to a branch-specific positive selection model, using Lv as the outgroup species (see Methods and Materials for details). Our goal was to identify cases of gene regulatory evolution via nucleotide variation within CRE sequences, potentially acting to modify transcription factor binding affinity of these elements and associated changes to gene network expression and connectivity.
In total, we identified 648 OCRs with evidence of positive selection exclusively in the Ht genome, compared with 512 exclusively in the He genome (fig. 5A: green and orange points, respectively; see also fig. 2D). This result thus suggests that, whereas the life history of these two sea urchin species dramatically differs, many potential changes in gene regulation associated with other aspects of their biology have accumulated within each species. At a genome-wide scale, these putatively adaptive changes have occurred perhaps even more so in Ht, the species with ancestral developmental life history.
Fig. 5.
Sequence changes indicative of positive selection are enriched within cis-regulatory elements near dGRN genes and other transcription factors in the H. erythrogramma genome. (A) Zeta values (ratio of OCR substitution rate to that of neutrally evolving reference sequences) for H. erythrogramma (top) and H. tuberculata (bottom) across the genome. OCRs with significant support for lineage-specific evidence of positive selection are noted for H. erythrogramma and H. tuberculata, and those nearby dGRN genes are outlined in purple. (B) Fold-change in proportion of OCRs with more frequent evidence of positive selection in either H. erythrogramma or H. tuberculata, partitioned by functional categories of nearby genes (see supplementary fig. 4 for all categories). (C) Transcription factor binding motif enrichment results of OCRs near dGRN genes with evidence of positive selection in H. erythrogramma. Detection mode indicates OCR sequences were (i) searched against a ‘known’ set of optimized motif models or (ii) enriched motifs within the OCR were identified ‘de-novo’ and matched to a known set of motifs. (D) Embryonic mRNA expression levels for Nuclear factor erythroid 2-related factor (NRF) for each species. OCR: open chromatin region; dGRN: developmental gene regulatory network.
Partitioning OCR selection results by the function of the nearest gene reveals only limited enrichment of positive selection within the putative regulatory elements of most functional classes of genes (fig. 5B, supplementary fig. 4). A prominent exception to this finding involves OCRs near genes in the sea urchin dGRN or near genes encoding other transcription factors. In these cases, approximately twice as many OCRs exhibit evidence of positive selection on the He branch relative to Ht. This result is especially striking considering Ht has 26.3% more OCRs with branch-specific evidence of positive selection across the entire genome (previous paragraph and fig. 5B). Moreover, nucleotide sequences of the 23 dGRN OCRs under selection in He are highly enriched for binding motifs of critical developmental transcription factors, including three SOX, three zinc finger, and one homeobox protein (fig. 5C). The SOX binding motifs are interesting because many transcription factors in this family act as ‘pioneer factors’ that are capable of initiating chromatin opening (Zaret and Carroll 2011). Another interesting example involves NRF2: the binding motif for this transcription factor is overrepresented in OCRs under selection in He (fig. 5C) and the gene is expressed at much higher levels in He embryos relative to those in the ancestral life history (fig. 5D). This protein has gene regulatory functions in diverse biological contexts ranging from antioxidant response and cellular defense to metabolism and stem cell quiescence (Tonelli et al. 2018), which in this case, could be related to derived embryonic detoxification and physiological systems (Tsushima et al. 1995; Davidson et al. 2019) or early cell lineage specification programs (Wray and Raff 1989, 1990; Davidson et al. 2021) of He.
Taken together, putative regulatory elements tasked with wiring the ancestral sea urchin dGRN have accumulated a disproportionate number of mutations on the He branch. These likely play a significant role in regulation of divergent gene expression patterns and its highly derived developmental program. However, determining which mutations drive the signal of positive selection and their contribution to gained or lost transcription factor binding motifs remains a challenge. More broadly, our results provide strong evidence that natural selection can efficiently generate heritable changes concentrated within the cis-regulatory landscape of specific subsets of genes or gene networks to rapidly produce changes in transcriptional regulation.
Overlap of Evolutionary Changes in Regulatory Element Accessibility and Sequence
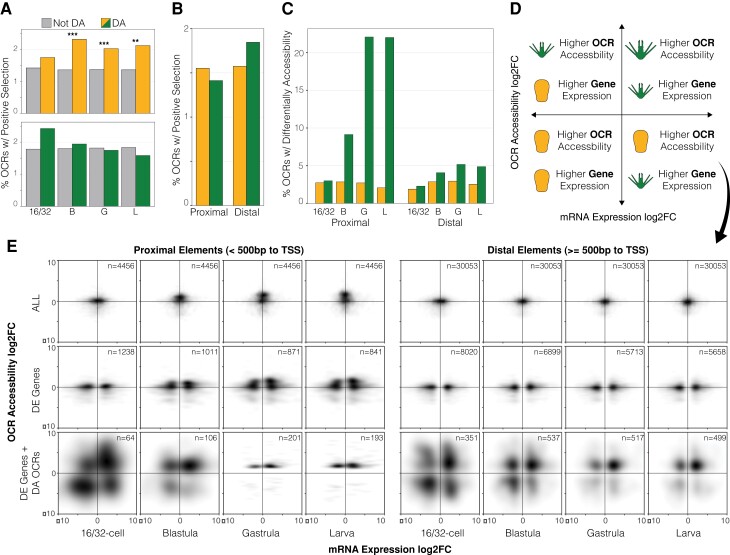
The previous two sections describe how two distinct modifications to regulatory element function, namely accessibility and sequence, have accumulated across the He genome, and particularly so near critical developmental network genes. We next asked whether there exists any interaction between these two evolutionary changes to better understand the extent to which both epigenetic and sequence changes affect the same regulatory element. At all three postcleavage stages, we found that differentially accessible OCRs contain a significantly greater proportion of sites with evidence of selection on the He branch relative to nondifferentially accessible OCRs, including as much as an ∼50% proportional increase at the blastula stage (Fisher’s exact test, two-sided: P < 5 × 10−3) (fig 6A: top). This same trend is observed at early cleavage (16/32-cell embryos), although not statistically significant. In contrast, we detected no statistical enrichment for positive selection within differentially accessible OCRs in the Ht genome; at early cleavage this relationship does approach significance, but no such trend exists at the later developmental stages (fig. 6A: bottom) (Fisher’s exact test, two-sided: P > 0.05).
Fig. 6.
Independent and synergistic changes to regulatory element function have evolved during life history evolution in H. erythrogramma. (A) Proportion of differentially accessible or nondifferentially accessible OCRs with evidence of positive selection on the H. erythrogramma branch (top) or H. tuberculata branch (bottom). Two-sided, Fisher’s exact test: **P ≤ 5 × 10−3; ***P ≤ 5 × 10−4. (B) Proportion of proximal of distal OCRs with evidence of positive selection in either Heliocidaris species. (C) Differential accessibility of proximal and distal OCRs at four stages of development. (D) Diagram describing possible correlative patterns between changes in OCR accessibility and gene expression in either life history strategy (ancestral, planktotroph larva; derived, lecithotroph larva): the layout of this panel is a key for interpreting the density plots in panel (E) below. (E) Fold-change of mRNA expression vs nearby OCR accessibility between life history strategies for proximal (left) and distal (right) OCRs. Relationships are shown for all genes and OCRs (E: top row), significantly differentially expressed genes (E: middle row), and significantly differentially expressed genes and differentially accessible OCRs (E: bottom row). OCR: open chromatin region; DE: differentially expressed (genes); DA: differentially accessible (OCRs).
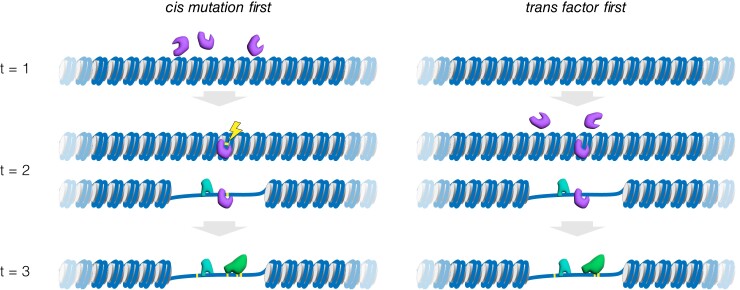
Significant overlap in differential accessibility and positive selection in the He genome suggests a possible biological interaction between the two evolutionary mechanisms that may operate in tandem to alter regulatory function. It therefore becomes useful to consider the order in which these molecular events could take place (fig. 7). Measuring chromatin accessibility in hybrids provides a way to distinguish cis from trans genetic contributions to differences in individual OCRs (Connelly et al. 2014), using an approach originally developed for investigating the genetic basis for evolutionary changes in transcription (Wittkopp et al. 2008; Crowley et al. 2015). In the present case, the disproportionate occurrence of positive selection within differentially accessible OCRs only in the He genome suggests a potentially synergistic set of gene regulatory processes may operate to drive developmental change during the evolution of its derived life history. Because the tests for positive selection identify regions containing multiple derived mutations, additional cis mutations likely accumulated after the accessibility change, regardless of which molecular mechanism originally opened the region (fig. 7, t = 3). The observation that this association is restricted to the He branch suggests that this interaction may often produce adaptive changes in transcription during development. More broadly, tandem accessibility and mutational changes may be a common gene regulatory method to efficiently modify gene expression for organismal adaptation and innovation across organismal lineages.
Fig. 7.
Mechanistic models for the evolutionary origin of an open chromatin region. Three distinct evolutionary time slices are illustrated: t = 1 prior to the appearance of the open chromatin region (OCR), t = 2 the event that precipitates local chromatin opening, and t = 3 many generations after the origin of the OCR. Two general molecular processes could transform a given region of the genome from a relatively closed to a relatively open region of chromatin, based on the location of the genetic basis: cis or local and trans or distant. We illustrate this distinction based on a pioneer transcription factor, a class of proteins capable of sequence-specific binding to DNA in closed chromatin causing a local opening, typically on the scale of 1–2 nucleosomes (Zaret and Carroll 2011). Note, however, that several other specific molecular mechanisms are also possible, involving chromatin remodeling complexes, histone acetylase/deacetylases, histone methylase/demethylases, and other proteins capable of acting to modify local chromatin configuration. (Left) ‘cis mutation first’ model. Here, the precipitating event is a local mutation (yellow; adjacent to the lightning bolt) that allows a pioneer transcription factor (purple protein) to bind to relatively closed chromatin. This pioneer factor was already present in the nucleus (see t = 1), but can only bind locally following the new mutation that creates a binding site for it (t = 2). Because pioneer factors can convert closed to open chromatin, other transcription factors also present in the nucleus (teal protein) may gain access to DNA within the newly opened region without the need for any additional mutations. (Right) ‘trans factor first’ model. Here, the pioneer factor is not originally present (t = 1) in the nucleus at the developmental stage of interest. A mutation elsewhere in the genome (t = 2) results in expression of the pioneer factor transcription factor, which can bind locally due to a preexisting recognition site and thus without the requirement for a local mutation. Once present, the pioneer factor can open the local DNA, potentially allowing other transcription factors (teal protein) to bind without the need for any local mutations. Any impact on the transcription of a local gene could be susceptible to natural selection, since it has a genetic basis and a trait consequence. Over extended evolutionary timescales, additional mutations could accumulate within the OCR (t = 3), potentially altering the suite of interacting transcription factors (for example, green protein now binds, purple protein no longer binds). In exceptional cases, natural selection may favor fixation of several new mutations within the OCR that alter gene expression. This figure illustrates the evolutionary origin of an OCR, but the evolutionary loss of an OCR could similarly occur through distinct molecular mechanisms based on the location of the genetic basis: for example, loss of a binding site for a transcription factor or loss of expression of a pioneer factor.
Relationship of Evolutionary Regulatory Mechanisms Between Proximal and Distal Elements
Next, we asked whether evolutionary changes in OCR sequence and chromatin accessibility differ between core promoters and more distal elements such as enhancers. We quantified changes in proximal (<500 bp to translational start site of a gene) and distal (≥500 bp to translational start site of a gene) OCRs to test if either change covaries with promoter or enhancer evolution between species. Although the transcription start site would be a more appropriate genomic reference for defining putative promoter regions, the reference genomes of these species currently lack annotation of 5’ untranslated regions for all genes. Thus, the translation start site was used instead to provide a consistent, albeit less sensitive, reference point of comparison of all genes for each species. Although many proximal and distal OCRs show evidence of positive selection on the Ht and He branches, we found no significant enrichment between the two categories of putative CREs (fig. 6B). This finding suggests mutations in both enhancers and promoters are likely important contributors in driving gene expression change, via altered binding and recruitment of regulatory factors and transcriptional machinery.
In contrast, accessibility changes show a much greater asymmetry between proximal and distal elements (fig. 6C). Although accessibility distributions are comparable at early cleavage (16/32-cell embryos), many more accessibility differences accumulate in proximal OCRs relative to distal ones later in development, after zygotic activation and tissue specification has commenced. For example, by the gastrula and larval stages, more than 20% of proximal elements are significantly more accessible in Lv and Ht, whereas this figure is ∼5% for distal elements.
There are several possible biological and technical explanations for this result. Unlike enhancers, whose activity is often restricted to a single tissue or stage (Thurman et al. 2012), promoters need to be accessible whenever the gene is transcribed, regardless of space and time. This has important implications for interpreting differences in chromatin configuration among species. At a biological level, greater promoter accessibility in the embryos of species with the ancestral life history (Ht and Lv) may merely reflect overall greater transcriptional activity and more changes in expression during development (fig. 1, 3; supplementary fig. 5) (Israel et al. 2016; Wang et al. 2020). Secondly, for a given gene, the accessibility of a promoter region is normally greater than that of nearby enhancers (Thurman et al. 2012), which may lend increased statistical power in detecting accessibility differences between species and contribute to an apparent enrichment of promoter accessibility changes. In addition, these chromatin configuration data were collected from whole embryos, so cell or lineage-specific differences in accessibility will be averaged within the sample, a technical factor that may be especially important for detecting accessibility changes of enhancer elements with tissue-specific dynamics. Lastly, promoter accessibility changes may simply be more often selected for when large changes in transcription are favored, at least for some genes. Adjusting enhancer accessibility can certainly induce changes in gene expression, but activating or shutting down a promoter may be a much more potent mechanism for large-scale changes in transcriptional regulation, such as those involved in rapid developmental evolution.
Relationships Between Magnitude of Gene Expression and Chromatin Accessibility Changes
Multiple lines of evidence presented above suggest that changes in the sequence or accessibility of cis-regulatory elements contributed to the evolution of development in He. The association between these changes and divergence in gene expression, however, is unclear. To understand this relationship, we compared mRNA expression fold-change of genes with accessibility fold-change of nearby OCRs at four developmental stages to measure instances of correlated changes in the magnitude of expression and chromatin configuration. A diagram of possible relationships between OCR accessibility and gene expression differences is provided in fig. 6D, to which datapoints are mapped and shown as density plots in fig. 6E. We partitioned the data into three categories: (1) all OCRs and genes (no filtering), (2) only differentially expressed genes and their nearby OCRs, and (3) differentially expressed genes and their nearby differentially accessible OCRs.
When all genes and OCRs are considered (fig. 6E: top row), there is no correlation between the magnitude and direction of accessibility and expression differences across developmental life histories. This result is not surprising at a global scale, given that expression levels and accessibility are conserved between species for most genes and OCRs, respectively. However, a notable set of proximal sites have greater accessibility in species with the ancestral life history at the blastula, gastrula, and larval stages, consistent with overall greater promoter accessibility in Ht and Lv (see previous section). A similar result occurs when only differentially expressed genes and nearby OCRs are considered (fig. 6E: middle row). One possible explanation for the lack of an obvious relationship is that, whereas only genes that are differentially expressed between life histories are plotted, each of these genes has an average of 3.1 OCRs assigned to it (based on proximity). Each of these OCRs may not be active at a given stage of development, or they may function antagonistically to one another (i.e., regulatory elements can enhance or repress transcription), thereby collectively dampening a correlative signal between gene expression and accessibility.
Lastly, we considered only differentially expressed genes and nearby OCRs with differential accessibility. This analysis reveals interesting relationships between transcription and chromatin configuration (fig. 6E: bottom row). As development progress, the relatively diffuse relationship between OCR accessibility and gene expression quickly tightens (compare 16/32-cell stage with larva). This likely reflects synchronization of zygotic genome activation with the embryo’s cis-regulatory landscape as maternal mRNA reserves are depleted and nascent transcription and cell differentiation commences. Greater accessibility at the majority of proximal sites in Ht and Lv is still found in this subset of genes and OCRs, though smaller sets of putative promoters with greater accessibility in He and corresponding expression increases are detected (faint gray spots). Among distal elements, we observed a considerably more diffuse relationship between accessibility, expression, and life history, possibly reflecting the potential activating or repressive functions of enhancers and downstream gene network interactions. At each developmental stage, the most common relationship was increased gene expression and accessibility in Ht and Lv, consistent with the preponderances of activating (as opposed to repressive) interactions within the ancestral dGRN.
Despite these relationships, our results highlight the difficulty in measuring correlations between the magnitude of cross-species changes in gene expression and regulatory element accessibility at a genomic scale. Prior studies have also reported a weak relationship between evolutionary changes in OCR accessibility and the expression of nearby genes between species (Shibata et al. 2012; Pizzollo et al. 2018). Several factors likely contribute a weak correlation, including: (1) a change in chromatin accessibility is just one of several possible molecular mechanisms that can change a gene’s expression; (2) individual CREs can have antagonistic effects on transcriptional regulation as activators or repressors, thus canceling out effects; and (3) CRE accessibility is permissive rather than directive of regulatory factor binding and downstream gene expression, such that ‘openness’ does not necessarily indicate the CRE is actively regulating transcription.
Developmental Trajectories of Chromatin Configuration and Gene Expression Have Evolved Most Frequently Along the H. erythrogramma Branch
To address the challenges associated with understanding the relationship between expression and accessibility changes of putative regulatory elements, we applied the ‘jump score’ method to measure the magnitude of evolutionary changes in developmental trajectories, which are represented as distance in principal component space (Israel et al. 2016). This metric is particularly useful because the jump score summarizes patterns of conservation or divergence in gene expression across development, rather than considering only a single stage. Here we apply this approach to evolutionary changes in the accessibility of putative CREs, as well as expression of genes, across developmental trajectories (fig. 8).
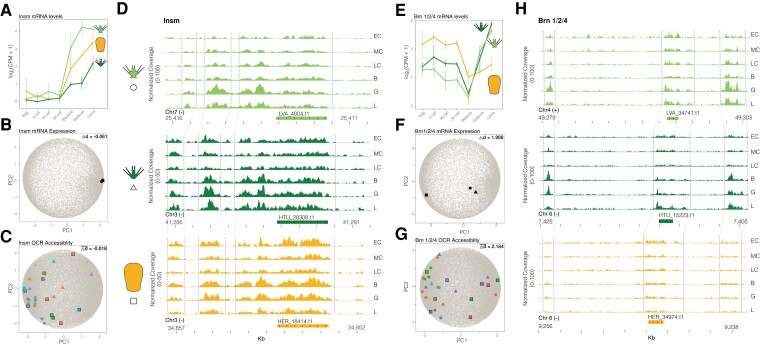
Fig. 8.
Quantifying developmental expression and accessibility changes in principal component space predicts divergent patterns of gene regulation. (A-D) An example of conserved developmental gene expression and chromatin accessibility for the gene insm. (A) Developmental mRNA expression profile of insm for each sea urchin species and (B) their coordinates in PCA space. (C) Coordinates in PCA space of developmental accessibility of orthologous OCRs near insm and (D) normalized chromatin accessibility coverage of these same OCRs through embryogenesis for each species. (E-H) An example of divergent developmental gene expression and chromatin accessibility associated with life history for the gene brn 1/2/4. (E) Developmental mRNA expression profile of brn 1/2/4 for each sea urchin species and (F) their coordinates in PCA space. (G) Coordinates in PCA space of developmental accessibility of orthologous OCRs near brn 1/2/4 and (H) normalized chromatin accessibility coverage of these same OCRs through embryogenesis for each species. See Supplementary Figure 6 for a summary figure of PCA distances for all genes and OCRs. OCR: open chromatin region; PCA: principal component analysis.
We compared the expression jump score of 9,423 genes and the mean jump score of nearby OCRs to test how developmental changes in transcriptional regulation and chromatin status are distributed along the He and Ht branches. For example, expression of insm, a transcription factor involved in neurogenesis in sea urchins (McClay et al. 2018), is conserved across He, Ht, and Lv (fig. 8A). This is reflected by a low difference in jump scores between He-Lv and Ht-Lv, and tight clustering of expression profiles in principal component space (fig. 8B). Similar expression profiles are accompanied by conserved cis-regulatory landscape dynamics through development in each species, exemplified by a near-zero mean OCR jump score difference and accessibility profile of ten nearby OCRs (fig. 8C,D). In contrast, brn 1/2/4, a regulator of midgut patterning (Yuh et al. 2005), has a divergent developmental gene expression trajectory in He relative to the ancestral state and large jump score differences in He-Lv and Ht-Lv branch comparisons (fig. 8E,F). This large expression change is accompanied by significant reduction in chromatin accessibility at the blastula through larval stages in He, represented by a large mean difference in OCR jump scores along the He branch (fig. 8G,H). This locus is especially interesting considering He larvae do not feed and have lost development of a functional through-gut.
Given the highly derived developmental program of He relative to the ancestral life history, we predicted that the most frequent changes in transcriptional regulation would occur along the He branch. Indeed, comparing jump scores of all genes and OCRs identifies concerted modifications to OCR accessibility and gene expression profiles during He development as the most frequent set of changes among genes and OCRs with divergent expression and chromatin accessibility trajectories (1,582 genes) (supplementary fig. 6; Data S7). Furthermore, the least common combination of changes includes both expression and accessibility changes occurring on the Ht branch (1,114 genes), consistent with an overall more conserved embryonic gene expression program in this species. Thus, we find that local patterns of CRE accessibility have evolved in concert with divergent developmental expression patterns during life history evolution in He. Collectively, our results suggest that modified transcription factor access to regulatory elements is associated with rapid and adaptive evolutionary changes to gene expression, and by extension, dGRN functionality. However, it remains to be determined which changes evolved as a cause or consequence (or neither) of life history evolution in He—a challenge potentially addressable via empirical validation of candidate enhancer function.
Distinct cis-regulatory Modifications Differentially Effect Evolution of Gene Expression
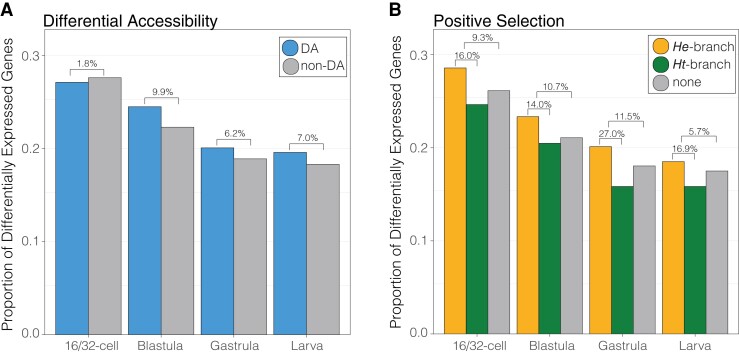
Lastly, we tested whether changes in nucleotide sequence and accessibility within OCRs are distributed near genes that are differentially expressed between developmental life histories. We found a small, though insignificant, increase in differential expression of genes with nearby differentially accessible OCRs relative to genes lacking differentially accessible OCRs at blastula, gastrula and larval stages (+6.2–9.9% change). No such increase exists during early cleavage (-1.8% change), which may be expected given the transcriptome is composed almost entirely of maternally-derived transcripts during early cleavage (Fisher’s exact test, two-sided: P > 0.05) (fig. 9A).
Fig. 9.
Evolutionary changes to cis-regulatory element function associated with life history evolution in sea urchins and expression changes of nearby genes. Proportion of genes that are differentially expressed between developmental life history strategies at four developmental stages, distinguished as having at least one nearby OCR with (A) differential accessibility or (B) evidence of lineage-specific positive selection. OCR: open chromatin region. Values above brackets indicate the percent change in proportion of genes that are differentially expressed between categories.
Similarly, we detected no significant difference in the proportion of differentially expressed genes and the presence of positive selection within OCRs of He when compared with OCRs lacking evidence of positive selection or OCRs with evidence of positive selection in Ht at each developmental stage (Fisher’s exact test, two-sided: P > 0.05) (fig. 9B). Still, we note that throughout development, incidences of differential expression are higher when there exists one or more nearby OCRs with evidence of positive selection relative to OCRs lacking evidence of positive selection (+5.7–11.5% change; fig. 9B: orange and gray bars) or OCRs with evidence of positive selection in Ht (+14.0–27.0% change; fig. 9B: orange and green bars). This finding is concordant with our predictions of evolutionary mechanisms of gene expression change, as selective changes in CRE sequences in He are most often coupled with concordant divergence in developmental gene expression possibly associated with their derived life history strategy. Positive selection within CRE sequences of Ht, on the other hand, are less often linked to changes in gene expression associated with life history, as Ht has retained the ancestral sea urchin developmental program. Lastly, whereas the absolute number of accessibility changes between developmental life histories is typically greater than detected instances of positive selection (figs. 3, 5), mutations within putative CRE sequences are more often associated with changes in gene regulation and expression relative to a no-change state (mean +9.3% change) than changes in accessibility (mean +5.3% change).
These results may reflect distinct characteristics of the molecular mechanisms that modify cis-regulatory function. In particular, an evolutionary change in the chromatin accessibility of CRE might only manifest at certain developmental stages or in certain cell types, whereas a change in the DNA sequence of a CRE is present throughout development and in every cell type. For this reason, mutations that influence chromatin status might in general have lower pleiotropy than mutations within OCRs. This possibility is an extension of the idea that mutations in CREs that drive tissue-specific expression may have lower pleiotropy than those in CREs that influence expression throughout development and in all cell types (Wray et al. 2003; Stern 2010; Singh and Yi 2021; but see Preger-Ben Noon et al. 2018). Evolutionary changes in chromatin configuration are an additional molecular mechanism with the potential to limit pleiotropy, by physically masking a mutation within a CRE such that it can only exert an effect on transcription when chromatin is open.
Conversely, opening chromatin in a larger range of conditions could expose cryptic genetic variation to natural selection. Although there is good empirical evidence that cryptic genetic variation exists within natural populations (Gibson and Dworkin 2004; Paaby and Rockman 2014), it is less clear how it becomes visible. One possibility (among others) is that mutations accumulate within a cell type-specific CRE that have no consequence because they do not influence interactions with any of the trans-acting factors in that particular cell type. An evolutionary change that opens the local chromatin in a different cell type might, however, expose those new mutations to a different suite of trans-acting factors, potentially altering their interactions with the CRE and thus transcription. Deconvolving the interplay and evolutionary consequences of epigenetic and sequence changes within CREs remains an important challenge for understanding the evolution of transcriptional regulation.
Conclusions
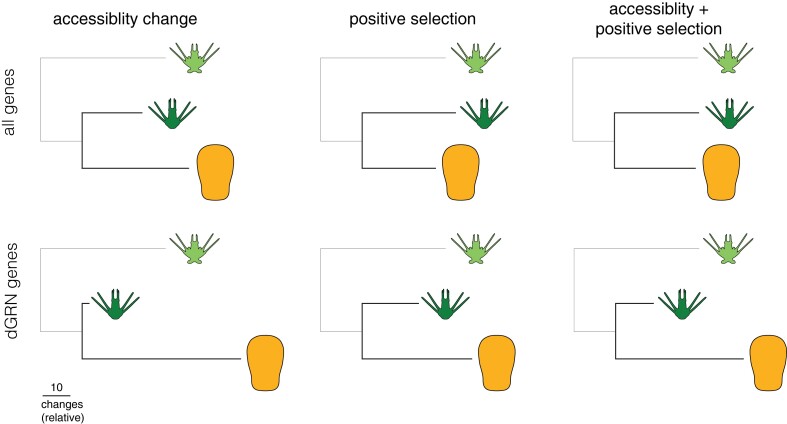
In this study, we leveraged a recent life history switch in sea urchins that was accompanied by elevated rates of adaptive changes in developmental gene expression (Israel et al. 2016; Wang et al. 2020; Wray 2022) to investigate how evolutionary mechanisms act on mechanisms of transcriptional regulation and embryonic gene expression. In particular, we distinguish two distinct molecular mechanisms within cis-regulatory elements that could alter gene expression: sequence changes that might influence transcription factor:DNA interactions and epigenetic changes that might limit access of transcription factors to DNA. We find that evolutionary changes in chromatin are common near differentially expressed genes, especially on the branch leading to the derived life history and especially so near critical developmental gene regulatory network (dGRN) genes (fig. 10, left column). Elevated accumulation of mutations within OCRs indicative of positive selection is not enriched on the branch leading to the derived life history when considering all genes, but strikingly enriched near dGRN genes specifically (fig. 10, central column). These distinct molecular mechanisms may also act synergistically, as seen by enrichment for both changes within putative dGRN CREs on the branch leading to the derived life history (fig. 10, right column). Although extensive prior evidence indicates that changes in protein:DNA binding contributes to the evolution of gene expression, much less empirical support exists regarding the contribution of epigenetic changes. Our results suggest that evolutionary changes in local chromatin configuration during early development can contribute to the evolution of embryonic gene expression, whereas branch- and dGRN-specific enrichments suggest that many of these changes are adaptive and may be tied to the evolution of the derived life history. More broadly, the comparative approaches developed in this study offer new ways to analyze multimodal functional genomic datasets to investigate the evolutionary mechanisms that operate on cis-regulatory elements and their consequences for gene expression and organismal traits.
Fig. 10.
Chromatin accessibility changes, mutational signatures of positive selection, and the coincidence of both evolutionary changes are abundant in the H. erythrogramma genome, especially nearby dGRN genes. Enrichment of regulatory modifications to OCRs near dGRN genes suggests selective pressures imposed by changes in developmental life history can rapidly modify cis-regulatory interactions underlying developmental processes. Tandem accessibility and mutational changes may act as a common gene regulatory method to efficiently modify gene expression for organismal adaptation and innovation. Branch lengths scaled to relative number of changes between Heliocidaris branches for each set of modifications.
Materials and Methods
Embryo Rearing and Sample Preparation
H. erythrogramma and H. tuberculata adult animals were collected near Sydney, NSW, Australia and maintained at 21 °C in aquaria with circulating natural seawater. L. variegatus individuals were collected near the Duke University Marine Lab in Beaufort, NC, USA and maintained at 23 °C in aquaria at Duke University, Durham, NC, USA. Each sea urchin species was induced to spawn via injection of 0.5 M KCl solution into the coelom. Eggs were washed twice in 20 µm filtered seawater (FSW). Sperm was collected dry and stored at 4 °C until usage. Washed eggs were fertilized with 1 µL of sperm, and after 10 minutes, were washed three times in FSW. For each species, three unique male-female pairs were crossed to produce three biologically-independent replicates of sea urchin embryos. Embryos were reared at 23 °C in large glass culture dishes supplied with FSW, and water was changed every six hours. Because these species exhibit different developmental rates, developmental milestones, rather than absolute developmental time, were selected to maximize developmental synchrony within cultures and between species for comparison. These stages include 1) 4th/5th (EC), 2) 6th/7th (MC), and 3) 8th/9th (LC) cleavage stage embryos, as well as 4) hatched blastula (B), 5) midgastrula (G), and 6) early larva (L) (two-armed pluteus for H. tuberculata and L. variegatus; 32 hours postfertilization for H. erythrogramma). Quickly distinguishing embryonic stages is difficult for cleavage stage embryos, so we closely monitored development of each culture and used known division rates for each species and water temperature to estimate when the culture would be most enriched for the targeted developmental stage. Once a culture reached the appropriate stage, a subset of the culture was retrieved for processing, stage confirmed by visual inspection, arrested or delayed embryos removed by hand, and the remaining embryos transferred into a 1.5 mL Eppendorf tube for ATAC-seq processing. Microscopic examination confirmed that most embryos had reached the target stage.
ATAC-seq Protocol and Sequencing
ATAC sample preparation was carried out according to the Omni-ATAC-seq protocol (Corces et al. 2017). For each replicate, embryos were washed once in 1 µm filtered seawater, lysed, and ∼50,000 nuclei were isolated for the transposition reaction using the Illumina TDE1 enzyme and tagmentation (TD) buffer (Cat. No. 20034197 and 20034198) (San Diego, CA, USA). Sequencing libraries for each replicate were amplified via PCR after separately determining the optimal number of amplification cycles for each sample using qPCR as described in (Buenrostro et al. 2015), and libraries were purified and size selected using Ampure XP Beads at a 1.8:1 bead volume:library volume (Beckman Coulter, Brea, CA, USA). Library quality and transposition efficiency was accessed using a Fragment Analyzer and PROSize 2.0 (Agilent, Santa Clara, CA) at the Duke University Center for Genomic and Computational Biology. H. erythrogramma and L. variegatus libraries were sequenced on an Illumina HiSeq 4000 instrument using 50 bp SE sequencing. H. tuberculata libraries were sequenced on an Illumina NovaSeq 6000 instrument using 50 bp PE sequencing (only SE were used for data analysis).
ATAC-seq Data Analysis
Raw ATAC-seq reads were trimmed for quality and sequencing adapters using ‘cutadapt’ (Martin 2011) v2.3 with the following parameters: -a CTGTCTCTTATACACATCT -q 20 –trim-n -m 40. Trimmed reads were then aligned to each species’ respective genome via ‘stampy’ (Lunter and Goodson 2011) v.1.0.28 using the ‘—sensitive’ set of parameters. The Lv genome was retrieved from Davidson et al. 2020 (NCBI BioProject: PRJNA657258). The Heliocidaris genome assembles were retrieved from Davidson et al. 2021 (NCBI BioProject: He- PRJNA827916; Ht- PRJNA827769). ATAC-seq alignments were filtered for mitochondrial sequences and required an alignment quality score of at least 5 using ‘samtools’ (Li et al. 2009) v.1.9. Filtered alignments are available in BAM and Bedgraph format (see ‘Data Availability’ below).
In this study, we aimed to compare evolution of orthologous regulatory elements so to accomplish this, we performed a series of liftOvers (Hinrichs et al. 2006) to convert ATAC-seq alignments between genomic coordinates of each sea urchin species. We took an iterative, reciprocal strategy described below to minimize possible reference bias associated with converting between genome assemblies, requiring a minMatch of 0.75 when lifting alignments between species pairs: 1) H. erythrogramma: He → Lv → He; 2) H. tuberculata: Ht → Lv → He; 3) L. variegatus: Lv → He → Lv → He. At the end of filtering and coordinate conversion, all ATAC-seq alignments were referenced to the H. erythrogramma genome. LiftOver files are available in (Davidson et al. 2021).
ATAC-seq peaks were called from these filtered, converted alignments using the ‘MACS2’ v2.1.2 (Zhang et al. 2008) callpeak function (parameters: –nomodel, –keep-dup = auto, –shift 100, –extsize 200) for each developmental stage separately at an FDR threshold of 5%. Peak coordinates were merged using the ‘bedtools’ (Quinlan and Hall 2010) v2.25 merge function requiring a peak overlap of at least 200 bp to be merged into a single peak. Lastly, for each sample, accessibility of each peak was measured with the ‘bedtools’ multiBamCov function and imported into R v4.0.2 for statistical analysis (Data S1). Although not a primary focus of this study, all ATAC-seq peaks and raw accessibility counts for each species, independent of sequence conservation between species, is additionally provided in Data S8, as are ATAC-seq peaks specific to Lv (not found in Heliocidaris).
Estimates of Positive Selection
To measure branch-specific signatures of positive selection in the noncoding genome, the ‘adaptiPhy’ (Berrio et al. 2020) pipeline (https://github.com/wodanaz/adaptiPhy) for global tests of natural selection was followed. First, orthologous sequences for noncoding sites of interest (ATAC-seq peaks) were extracted from each species’ genome into FASTA format. Sequences were trimmed to include only contiguous DNA sequence using the prunning.py script and filtered using the filtering.py script, requiring a minimum alignment length of 75 base pairs. These trimmed and filtered alignments serve as ‘query’ sequences of tests for selection. To generate a neutral reference for comparison, ten orthologous, neutral sites were randomly selected from a curated set of ∼88,000 putatively neutral sequences (Davidson et al. 2021) (Data S2) and concatenated into a single neutral reference sequence. A global neutral reference, rather than a local reference, was implemented to more conservatively estimate signatures of positive selection as recent work has demonstrated a local neutral reference may more likely overestimate positive selection—an especially acute issue in smaller, gene-dense genomes (Berrio et al. 2020). Then, substitution rates of both the query and randomly concatenated neutral reference were estimated using phyloFit (Siepel and Haussler 2004), and the zeta score was calculated as the ratio of the query substitution rate to the neutral reference substitution rate. In addition, P-values of likelihood ratio tests for significant levels of branch-specific positive selection were calculated with adaptiPhy (Berrio et al. 2020) pipeline using HyPhy (Pond et al. 2005). For each query site, tests for positive selection were repeated 10 times against a unique neutral reference to ensure reproducible estimates of zeta and significance values for each noncoding site of interest. P-values and substitution rates for all query and neutral sites were then imported in R v4.0.2. For each query site, the median zeta score and P-value for each test of positive selection across the ten replicate calculations were used for downstream analyses.
OCR Filtering
After accessibility and estimates of positive selection were calculated for each ATAC-seq peak, herein referred to as an ‘open chromatin region’ (OCR), a series of filtering and quality control metrics were carried out to ensure only high confidence and quality peaks were compared between species. These filtering steps are as follows: 1) each OCR is required to have at least 75 bp of contiguous, single copy sequence (see previous section) for accurate estimations of selection; 2) a local composition complexity (LCC) (https://biopython.org/docs/1.75/api/Bio.SeqUtils.lcc.html) value of 1.9 or more was required of the OCR for each species to remove repetitive or other low-complexity sequences that may generate inaccurate estimations of selection (module: biopython.org/docs/1.75/api/Bio.SeqUtils.lcc.html); 3) a CPM of 3 or more was required in at least 9 samples to remove OCRs with extremely low accessibility across samples; 4) the midpoint of the OCR must lie within 25 Kb (in either direction) of the translational start site of a gene model. The 25 Kb window for assigning OCRs to genes was selected on the basis that the median and mean intergenic distance within the He genome are 21.4 Kb and 31.9 Kb, respectively. See Supplementary Table 1 for a summary of these filtering steps and resulting number of OCRs. Given nearly no prior knowledge is known of the cis-regulatory landscape for these sea urchin species, these stringent filtering methods were carried out in order to maximize confidence in comparisons of noncoding sequence evolution and function. This resulted in a final set 35,788 high-confidence OCRs for cross-species analysis (Data S3).
ATAC-seq Statistical Analysis
Principal component analysis and variance partitioning was performed on the regularized log-transformed (rlog) data (Love et al. 2014) of filtered OCR accessibility counts via the prcomp function and the ‘variancePartition’ v1.18.3 package (Hoffman and Schadt 2016) in R using the formula: ∼ (1 | Stage) + (1 | Species) + (1 | Life History) + (1 | Genus) + (1 | Stage: Life History). Raw accessibility counts of the filtered OCRs were loaded into DESeq2 (Love et al. 2014) v1.30 to calculate differential accessibility between sample groups. Differentially accessible sites between life history strategies were classified as having a 2-fold accessibility difference and supported by an FDR of 10% in He vs Ht and well as He vs Lv comparisons (including the sign of the accessibility difference being in the same direction) (see supplementary fig. 7 for all significance cutoff combinations of differential accessibility results). For graphical purposes, the average fold-change in the He vs Ht and He vs Lv comparisons is displayed within figures. Significant levels of positive selection in either the He or Ht branch were classified as OCRs having a zeta value >1.25 and supported by a median false-discovery rate <10% in one species, and failing to meet both criteria in the other. Motif enrichment analyses were carried out with ‘Homer’ v4.11 (Heinz et al. 2010) via the findMotifsGenome.pl script using all OCRs as the background set and regions size of 200 bp. Sea urchin GRN genes are provided in Data S4, retrieved from the Biotapestry project of the Institute of Systems Biology, Seattle, WA, USA (www.biotapestry.org: accessed June 27, 2017).
RNA-seq Expression Analyses
Raw RNA-seq reads from eggs, 4-cell, 16-cell + 32-cell (collectively EE), blastula (B), gastrula (G), and early larva (L) stage embryos of He, Ht, and Lv were retrieved from (Israel et al. 2016), trimmed and filtered for low quality bases and reads with Trimmomatic (Bolger et al. 2014), and aligned to each species respective genomes and gene models with STAR (Dobin et al. 2013). From these alignments, mRNA expression was estimated with Salmon (Patro et al. 2017) and loaded to R for statistical analysis. Read counts for summed to each gene’s best match to the S. purpuratus v4.2 gene models to generate a common reference for expression comparisons between species as described in (Israel et al. 2016) (Data S5). Differentially expressed genes between life histories were called as having a fold-change in expression >2 and supported by an FDR of 10% or less in He vs Ht and well as He vs Lv comparisons (including the sign of the accessibility difference being in the same direction) (Data S6). These criteria were chosen to make RNA and ATAC-seq analyses between species comparable as possible.
Principal Component Distances of Developmental Profile
To identify OCRs and genes whose developmental regulatory profiles diverged in H. erythrogramma or H. tuberculata, we calculated principal component distances of chromatin accessibility and gene expression profile shapes between species throughout development as described in (Israel et al. 2016). In this method, developmental time course data is plotted onto principal component space for each species, and pairwise distances between species are calculated for each gene or OCR (see below). Large differences in these distances provide evidence that the shape of the developmental trajectory for a given gene or OCR has changed in one of the species (the species with the larger pairwise distance). Mean accessibility and expression profiles of regularized log-transformed (rlog) OCR and genes counts were standardized using the standardize function of the ‘mfuzz’ v2.48.0 package (Kumar and Futschik 2007). From these data, scaled distance measures (‘jump scores’) between orthologous noncoding sites or genes were calculated using loadings of the first two principal components (Data S7): ; . A significant change in the developmental trajectory of gene expression or OCR accessibility was determined to have a jump score difference of at 0.15 on one branch over the other.
Supplementary Material
Acknowledgements
We would like to thank the Sydney Institute of Marine Sciences and their staff for assistance with animal care, laboratory set up, and carrying out experiments. We also thank Allison Edgar and Hannah Devens for assistance preparing sea urchin embryo cultures associated with this work. This work was supported by the National Science Foundation Division of Integrative Organismal Systems (award no. 1929934 to GAW).
Contributor Information
Phillip L Davidson, Department of Biology, Duke University, Durham, NC, USA.
Maria Byrne, School of Life and Environmental Sciences, University of Sydney, Sydney, NSW, Australia.
Gregory A Wray, Department of Biology, Duke University, Durham, NC, USA.
Supplementary Materials
Supplementary material is available at Molecular Biology And Evolution online.
Competing Interests
The authors have no competing interests to declare.
Author Contributions
PLD and GAW conceived and designed the study. PLD and MB performed sample collection. PLD performed the data analysis. PLD and GAW wrote the manuscript. All authors revised the manuscript.
Data Availability
Raw ATAC-seq reads are available in FASTQ format on NCBI’s Short Read Archive (PRJNA828607). Filtered alignments of ATAC-seq reads after between-species liftOver files are available on Dryad (https://doi.org/10.5061/dryad.jsxksn0cp). Results files of ATAC-seq analyses are available as Supplementary Data files.
References
- Arenas-Mena C, Miljovska S, Rice EJ, Gurges J, Shashikant T, Wang Z, Ercan S, Danko CG. 2021. Identification and prediction of developmental enhancers in sea urchin embryos. BMC Genomics 22(1):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrio A, Haygood R, Wray GA. 2020. Identifying branch-specific positive selection throughout the regulatory genome using an appropriate proxy neutral. BMC Genomics 21:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. 2015. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Current Protocols in Molecular Biology 109:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M, Sewell MA. 2019. Evolution of maternal lipid provisioning strategies in echinoids with non-feeding larvae: selection for high-quality juveniles. Mar Ecol Prog Ser. 616:95–106. [Google Scholar]
- Clapier CR, Bradley RC. 2009. The biology of chromatin remodeling complexes. Annu Rev Biochem. 78:273–304. [DOI] [PubMed] [Google Scholar]
- Connelly CF, Wakefield J, Akey JM. 2014. Evolution and genetic architecture of chromatin accessibility and function in yeast. PLoS Genet. 10(7):e1004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces MR, Trevino AE, Hamilton EG, Greenside PG, Sinnott-Armstrong NA, Vesuna S, Satpathy AT, Rubin AJ, Montine KS, Wu B, et al. 2017. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods. 14:959–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Zhabotynsky V, Sun W, Huang S, Pakatci IK, Kim Y, Wang JR, Morgan AP, Calaway JD, Aylor DL, et al. 2015. Analyses of allele-specific gene expression in highly divergent mouse crosses identifies pervasive allelic imbalance. Nat Genet. 47(4):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanovich DA, Reddington JP, Garfield DA, Daza RM, Aghamirzaie D, Marco-Ferreres R, Pliner HA, Christiansen L, Qiu X, Steemers FJ, et al. 2018. The cis-regulatory dynamics of embryonic development at single-cell resolution. Nature 555:538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH, Erwin DH. 2006. Gene regulatory networks and the evolution of animal body plans. Science 311:796–800. [DOI] [PubMed] [Google Scholar]
- Davidson PL, Guo H, Swart JS, Massri AJ, Edgar A, Wang L, Berrio A, Devens HR, Koop D, Cisternas P, et al. 2021. Recent reconfiguration of an ancient developmental gene regulatory network in Heliocidaris sea urchins. bioRxiv 2022.03.03.482868. [DOI] [PubMed] [Google Scholar]
- Davidson PL, Guo H, Wang L, Berrio A, Zhang H, Chang Y, Soborowski AL, McClay DR, Fan G, Wray GA. 2020. Chromosomal-level genome assembly of the sea urchin Lytechinus variegatus substantially improves functional genomic analyses. Genome Biol Evol. 12:1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PL, Thompson JW, Foster MW, Moseley MA, Byrne M, Wray GA. 2019. A comparative analysis of egg provisioning using mass spectrometry during rapid life history evolution in sea urchins. Evol Dev. 21:188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall LE, Berrio A, Majoros WH, Swain-Lenz D, Morrow S, Shibata Y, Safi A, Wray GA, Crawford GE, Allen AS. 2019. Evaluating chromatin accessibility differences across multiple primate species using a joint modeling approach. Genome Biol Evol. 11(10):3035–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlet RB. 1995. Larval spicules, cilia, and symmetry as remnants of indirect development in the direct developing sea urchin Heliocidaris erythrogramma. Dev Biol. 167:405–415. [DOI] [PubMed] [Google Scholar]
- Falkner I, Sewell MA, Byrne M. 2015. Evolution of maternal provisioning in ophiuroid echinoderms: characterisation of egg composition in planktotrophic and lecithotrophic developers. Mar Ecol Prog Ser. 525:1–13. [Google Scholar]
- Flatt T, Heyland A. 2011. Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. Oxford: Oxford University Press. [Google Scholar]
- Gibson G, Dworkin I. 2004. Uncovering cryptic genetic variation. Nat Rev Genet 5(9):681–690. [DOI] [PubMed] [Google Scholar]
- Hart MW. 2002. Life history evolution and comparative developmental biology of echinoderms. Evol Dev 4:62–71. [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 38:576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ, Wray GA, Raff RA. 1990. The dorsoventral axis is specified prior to 1st cleavage in the direct developing sea urchin Heliocidaris erythrogramma. Development 110:875–884. [DOI] [PubMed] [Google Scholar]
- Hinrichs AS, Karolchik D, Baertsch R, Barber GP, Bejerano G, Clawson H, Diekhans M, Furey TS, Harte RA, Hsu F. 2006. The UCSC genome browser database: update 2006. Nucleic Acids Res. 34:D590–D598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Schadt EE. 2016. Variancepartition: interpreting drivers of variation in complex gene expression studies. BMC Bioinf. 17:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel JW, Martik ML, Byrne M, Raff EC, Raff RA, McClay DR, Wray GA. 2016. Comparative developmental transcriptomics reveals rewiring of a highly conserved gene regulatory network during a major life history switch in the sea urchin genus Heliocidaris. PLoS Biol. 14:e1002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhanwar S, Malkmus J, Stolte J, Romashkina O, Zuniga A, Zeller R. 2021. Conserved and species-specific chromatin remodeling and regulatory dynamics during mouse and chicken limb bud development. Nat Commun. 12:5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klueg KM, Harkey MA, Raff RA. 1997. Mechanisms of evolutionary changes in timing, spatial expression, and mRNA processing in the msp130 gene in a direct-developing sea urchin, Heliocidaris erythrogramma. Dev Biol. 182:121–133. [DOI] [PubMed] [Google Scholar]
- Koop D, Cisternas P, Morris VB, Strbenac D, Yang JY, Wray GA, Byrne M. 2017. Nodal and BMP expression during the transition to pentamery in the sea urchin Heliocidaris erythrogramma: insights into patterning the enigmatic echinoderm body plan. BMC Dev Biol. 17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar L, Futschik ME. 2007. Mfuzz: a software package for soft clustering of microarray data. Bioinformation 2:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JJ, Reed RD. 2019. Genome-wide regulatory adaptation shapes population-level genomic landscapes in Heliconius. Mol Biol Evol. 36:159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livraghi L, Hanly JJ, Van Bellghem SM, Montejo-Kovacevich G, van der Heijden ES, Loh LS, Ren A, Warren IA, Lewis JJ, Concha C, et al. 2021. Cortex cis-regulatory switches establish scale colour identity and pattern diversity in Heliconius. eLife 10:e68549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love AC, Raff RA. 2006. Larval ectoderm, organizational homology, and the origins of evolutionary novelty. J Exp Zool B Mol Dev Evol 306:18–34. [DOI] [PubMed] [Google Scholar]
- Lunter G, Goodson M. 2011. Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 21:936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DJ, Krug PJ, Kupriyanova EK, Byrne M, Emlet RB. 2012. The biogeography of marine invertebrate life histories. Annu Rev Ecol Evol Syst. 43:97–114. [Google Scholar]
- Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17:10–12. [Google Scholar]
- McClay DR, Miranda E, Feinberg SL. 2018. Neurogenesis in the sea urchin embryo is initiated uniquely in three domains. Development 145(21):dev167742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEdward LR, Miner BG. 2001. Larval and life-cycle patterns in echinoderms. Can J Zool. 79:1125–1170. [Google Scholar]
- Morgan SG. 2020. Life and death in the plankton: larval mortality and adaptation. In: McEdward LR, editor. Ecology of marine invertebrate larvae. Baca Raton: (FL: ): CRC Press. p. 279–321. [Google Scholar]
- Paaby AB, Rockman MV. 2014. Cryptic genetic variation: evolution's hidden substrate. Nat Rev Genet. 15(4):247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. 2017. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechenik JA. 1999. On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar Ecol Prog Ser. 177:269–297. [Google Scholar]
- Pizzollo J, Nielsen WJ, Shibata Y, Safi A, Crawford CE, Wray GA, Babbitt CC. 2018. Comparative serum challenges show divergent patterns of gene expression and open chromatin in human and chimpanzee. Genome Biol Evol. 10:826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond SL, Frost SD, Muse SV. 2005. Hyphy: hypothesis testing using phylogenies. Bioinformatics 21:676–679. [DOI] [PubMed] [Google Scholar]
- Preger-Ben Noon E, Sabarís G, Ortiz DM, Sager J, Liebowitz A, Stern DL, Frankel N. 2018. Comprehensive analysis of a cis-regulatory region reveals pleiotropy in enhancer function. Cell Rep. 22(11):3021–3031. [DOI] [PubMed] [Google Scholar]
- Prowse TAA, Falkner I, Sewell MA, Byrne M. 2009. Long-term storage lipids and developmental evolution in echinoderms. Evol Ecol Res. 11:1069–1083. [Google Scholar]
- Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff RA. 1987. Constraint, flexibility, and phylogenetic history in the evolution of direct development in sea urchins. Dev Biol. 119:6–19. [DOI] [PubMed] [Google Scholar]
- Raff RA, Byrne M. 2006. The active evolutionary lives of echinoderm larvae. Heredity (Edinb). 97:244–252. [DOI] [PubMed] [Google Scholar]
- Sackton TB, Grayson P, Cloutier A, Hu Z, Liu JS, Wheeler NE, Gardner PP, Clarke JA, Baker AJ, Clamp M, et al. 2019. Convergent regulatory evolution and loss of flight in paleognathous birds. Science 364:74–78. [DOI] [PubMed] [Google Scholar]
- Shashikant T, Khor JM, Ettensohn CA. 2018. Global analysis of primary mesenchyme cell cis-regulatory modules by chromatin accessibility profiling. BMC Genomics 19(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Sheffield NC, Fedrigo O, Babbitt CC, Wortham M, Tewari AK, London D, Song L, Lee B-K, Iyer VR, et al. 2012. Extensive evolutionary changes in regulatory element activity during human origins are associated with altered gene expression and positive selection. PLoS Genet. 8:e1002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepel A, Haussler D. 2004. Phylogenetic estimation of context-dependent substitution rates by maximum likelihood. Mol Biol Evol. 21:468–488. [DOI] [PubMed] [Google Scholar]
- Singh D, Yi SV. 2021. Enhancer pleiotropy, gene expression, and the architecture of human enhancer-gene interactions. Mol Biol Evol. 38(9):3898–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Turner FR, Raff RA. 2008. Nodal expression and heterochrony in the evolution of dorsal-ventral and left-right axes formation in the direct-developing sea urchin Heliocidaris erythrogramma. J Exp Zool B Mol Dev Evol. 310:609–622. [DOI] [PubMed] [Google Scholar]
- Stern DL. 2010. Evolution, development, and the predictable genome. Austin: (TX: ): Roberts and Company Publishers. [Google Scholar]
- Strathmann RR. 1978. The evolution and loss of feeding larval stages of marine invertebrates. Evolution 32:894–906. [DOI] [PubMed] [Google Scholar]
- Strathmann RR. 1985. Feeding and nonfeeding larval development and life history evolution in marine invertebrates. Annu Rev Ecol Syst. 16:339–361. [Google Scholar]
- Sun DA, Patel NH. 2021. Identification and classification of cis-regulatory elements in the amphipod crustacean Parhyale hawaiensis. bioRxiv 2021.09.16.460328. [DOI] [PubMed] [Google Scholar]
- Swain-Lenz D, Berrio A, Safi A, Crawford GE, Wray GA. 2019. Comparative analyses of chromatin landscape in white adipose tissue suggest humans may have less beigeing potential than other primates. Genome Biol Evol. 11:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, et al. 2012. The accessible chromatin landscape of the human genome. Nature 489:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli C, Chio IIC, Tuveson DA. 2018. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 29:1727–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima M, Byrne M, Amemiya S, Matsuno T. 1995. Comparative biochemical studies of carotenoids in sea urchins—III. Relationship between developmental mode and carotenoids in the Australian echinoids heliocidaris erythrogramma and H. tuberculata and a comparison with Japanese species. Comp Biochem Physiol B Biochem Mol Biol. 110:719–723. [Google Scholar]
- Vance RR. 1973. On the reproductive strategies of marine benthic invertebrates. Am Nat. 107:339–352. [DOI] [PubMed] [Google Scholar]
- van der Burg KRL, Lewis JJ, Brack BJ, Fandino RA, Mazo-Vargas A, Reed RD. 2020. Genomic architecture of a genetically assimilated seasonal color pattern. Science 370:721–725. [DOI] [PubMed] [Google Scholar]
- Walton KD, Warner J, Hertzler PH, McClay DR. 2009. Hedgehog signaling patterns mesoderm in the sea urchin. Dev Biol. 331:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Israel JW, Edgar A, Raff RA, Raff EC, Byrne M, Wray GA. 2020. Genetic basis for divergence in developmental gene expression in two closely related sea urchins. Nat Ecol Evol. 4:831–840. [DOI] [PubMed] [Google Scholar]
- Williams DHC, Anderson DT. 1975. Reproductive system, embryonic development, larval development and metamorphosis of sea urchin heliocidaris erythrogramma (val) (Echinoidea-echinometridae). Aust J Zool. 23:371–403. [Google Scholar]
- Wilson KA, Andrews ME, Raff RA. 2005. Dissociation of expression patterns of homeodomain transcription factors in the evolution of developmental mode in the sea urchins Heliocidaris tuberculata and H. erythrogramma. Evol Dev. 7:401–415. [DOI] [PubMed] [Google Scholar]
- Wilson KA, Andrews ME, Rudolf Turner F, Raff RA. 2005. Major regulatory factors in the evolution of development: the roles of goosecoid and msx in the evolution of the direct-developing sea urchin Heliocidaris erythrogramma. Evol Dev. 7:416–428. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Haerum BK, Clark AG. 2008. Independent effects of cis- and trans-regulatory variation on gene expression in Drosophila melanogaster. Genetics 178:1831–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp PJ, Kalay G. 2012. Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat Rev Genet. 13:59–69. [DOI] [PubMed] [Google Scholar]
- Wray GA. 1996. Parallel evolution of nonfeeding larvae in echinoids. Syst Biol. 45:308–322. [Google Scholar]
- Wray GA. 2007. The evolutionary significance of cis-regulatory mutations. Nat Rev Genet. 8:206–216. [DOI] [PubMed] [Google Scholar]
- Wray GA. 2022. Extreme phenotypic divergence and the evolution of development. Curr Top Dev Biol. 146:79–112. [DOI] [PubMed] [Google Scholar]
- Wray GA, Hahn MW, Abouheif E, Balhoff JP, Pizer M, Rockman MV, Romano LA. 2003. The evolution of transcriptional regulation in eukaryotes. Mol Biol Evol. 20(9):1377–1419. [DOI] [PubMed] [Google Scholar]
- Wray GA, Raff RA. 1989. Evolutionary modification of cell lineage in the direct-developing sea urchin Heliocidaris erythrogramma. Dev Biol. 132:458–470. [DOI] [PubMed] [Google Scholar]
- Wray GA, Raff RA. 1990. Novel origins of lineage founder cells in the direct-developing sea urchin Heliocidaris erythrogramma. Dev Biol. 141:41–54. [DOI] [PubMed] [Google Scholar]
- Yuh CH, Dorman ER, Davidson EH. 2005. Brn1/2/4, the predicted midgut regulator of the endo16 gene of the sea urchin embryo. Dev Biol. 281:286–298. [DOI] [PubMed] [Google Scholar]
- Zaret KS, Carroll JS. 2011. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25:2227–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W. 2008. Model-based analysis of ChIP-seq (MACS). Genome Biol. 9:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw ATAC-seq reads are available in FASTQ format on NCBI’s Short Read Archive (PRJNA828607). Filtered alignments of ATAC-seq reads after between-species liftOver files are available on Dryad (https://doi.org/10.5061/dryad.jsxksn0cp). Results files of ATAC-seq analyses are available as Supplementary Data files.