Abstract
Pyrethroid insecticides are widely used throughout agriculture and household products. Recent studies suggest that prenatal exposure to these insecticides may adversely affect fetal development; however, little is known about the distribution of these chemicals in pregnant animals. The present study aimed to address this gap in knowledge by investigating the distribution of two commonly used pyrethroid insecticides, permethrin and α-cypermethrin, in maternal and fetal tissues of pregnant CD-1 mice. Dams were dosed from gestational days 6 to 16 via oral gavage with permethrin (1.5, 15, and 50 mg/kg), α-cypermethrin (0.3, 3, and 10 mg/kg), or corn oil vehicle. Pyrethroid levels were determined in gestational day 16 tissues collected 90 min after the final dose was administered. Across maternal tissues, levels of both pyrethroids were the highest in maternal ovaries, followed by liver and brain, respectively. In addition, levels of both pyrethroids in maternal tissues and placenta were significantly higher than those in the fetal body and amniotic fluid, suggesting that these compounds may exhibit low transfer across the mouse placenta. While additional toxicokinetic studies are needed to verify the time course of pyrethroids in the fetal compartment, these findings support investigation into indirect modes of action relevant to the effects of pyrethroids on mammalian fetal development.
Keywords: Pyrethroid, Tissue distribution, Fetal, Pregnancy, Placenta
Introduction
Pyrethroids represent a broad class of synthetic insecticides originating from naturally compounds in the Chrysanthemum cinerariaefolium plant (Matsuo, 2019). They are widely used in agriculture and household insect control, with increased use reported following the phasing out of organophosphate alternatives (Barr et al., 2010). Pyrethroids are frequently detected within the household and throughout the environment, with the highest environmental concentrations reported on crops, followed by sediments, soils, and water, respectively (Tang et al., 2018). As a result of environmental and household contamination, the pyrethroid metabolite 3-PBA is detectable in 46–80% of the U.S. population (Tang et al., 2018; Lehmler et al., 2020). While indoor use of pyrethroids is a significant contributor to pyrethroid exposure in humans, the main route of exposure in urban areas is the dietary intake of fruits and vegetables (Tang et al., 2018; Schettgen et al., 2002).
In mammals, pyrethroids significantly affect a range of molecular targets, including voltage-gated sodium channels, voltage-gated calcium channels, chloride channels, and ATPases (Field et al., 2017; Kakko et al., 2003). Pyrethroids are classified as either type I or type II depending on the presence of an alpha-cyano group attachment, which significantly alters the degree to which they interact with several molecular targets (Breckenridge et al., 2009; Forshaw et al., 2000). The type I pyrethroid permethrin is the most frequently used; however, the type II cypermethrin is the most frequently detected in food (Tang et al., 2018). Although pyrethroids are thought to be relatively safe in comparison to other insecticides, exposure in pregnant women is of concern, as the developing fetus may be particularly susceptible to exposures (Shafer et al., 2005). Urinary pyrethroid levels increased in U.S. pregnant women after the 2001 EPA decision to restrict the residential use of chlorpyrifos and diazinon (Williams et al., 2008). Several recent epidemiological studies have found significant correlations between pyrethroid exposure in pregnant mothers and adverse health outcomes in their children, such as low birth weight and neurodevelopmental delay (Coker et al., 2018; Dewailly et al., 2014; Ding et al., 2015; Fiedler et al., 2015; Furlong et al., 2017; Shelton et al., 2014; Viel et al., 2017; Viel et al., 2015; Watkins et al., 2016). Pyrethroid metabolites in maternal urine are negatively correlated with a range of neurodevelopmental endpoints in their children, such as motor control, social adaptation, and intelligence (Xue et al., 2013). In addition, maternal pyrethroid exposure is linked to lower cognitive, social-emotional, language composite, and expressive communication scores in children (Coker et al., 2018; Watkins et al., 2016). Children exposed prenatally to pyrethroids also have increased rates of adverse behavioral outcomes (Furlong et al., 2017).
While there are concerns regarding the strength of associations reported in humans, some animal studies have also reported adverse effects of pyrethroids on fetal growth and neurodevelopment (Shafer et al., 2005; Burns and Pastoor, 2018). However, there is a significant gap in knowledge regarding the degree to which pyrethroids are present in the fetus, a necessary first step in understanding mechanisms responsible for these adverse effects, especially following environmental pyrethroid exposure. Understanding fetal exposure is critical for evaluating the relevance of direct modes of action. In this respect, human studies have produced mixed results regarding the degree to which pyrethroids distribute in fetal tissues, with many reporting low detection rates in fetal samples (Kaneko et al., 1984; Whyatt et al., 2003; Bradman et al., 2003; Silver et al., 2015; Wren et al., 2021; Neta et al., 2010). In addition, our understanding of the degree of placental transfer from human studies is limited, in that few studies have included direct comparisons between maternal and fetal tissue levels.
In regard to the toxicokinetics of pyrethroids during pregnancy, during which tissue, blood volume, and plasma protein changes occur, few studies have characterized tissue distribution in pregnant mammals, particularly in pregnant mice (Kaneko et al., 1984; Bossi et al., 2013; Shiba et al., 1990; Liu et al., 2019; Personne et al., 2019; Bossi et al., 2013). Given the utility of mouse models in developmental neurotoxicology studies, assessment of pyrethroid tissue distribution is critical for determining the relevance of direct modes of action on fetal neurodevelopment (Wise et al., 2009). In humans, pyrethroids such as cypermethrin have been shown to have elimination half-lives ranging from 13 to 24 h following oral exposure (Woollen et al., 1992; Eadsforth et al., 1988). In comparison, previous work suggests that pyrethroids such as permethrin and cypermethrin reach maximum concentrations in the plasma of rats around 1–3 h following exposure, with elimination half-lives of approximately 3–4 h in blood and 4–7 h in brain tissue (Willemin et al., 2016). In pregnant rats, levels of permethrin were shown to reach steady state in pregnant rats after approximately 15 days of dosing (Personne et al., 2019).
The current study was undertaken to characterize the tissue distribution of two commonly used pyrethroids in pregnant mice. Pregnant CD-1 mice were dosed from gestational days 6 to 16 with either the type I pyrethroid permethrin or the type II pyrethroid α-cypermethrin via oral gavage. Levels of each pyrethroid were assessed in maternal and fetal tissues across a range of doses to investigate the distribution of each insecticide.
Materials and methods
Chemicals.
Analytical grade Permethrin (white solid; > 99% purity; 58.1% Trans-Isomer, 41.7% Cis-Isomer; PESTANAL, Sigma Aldrich; InChI Key: RLLPVAHGXHCWKJ-UHFFFAOYSA-N) was used for the animal studies. Neat α-cypermethrin (white solid; 98% purity; Toronto Research Chemicals, North York, Canada; InChI Key: KAATUXNTWXVJKI-NSHGMRRFSA-N) for the animal studies was purified by recrystallization in isopropanol to > 99% purity. Corn oil (vehicle for the animal studies) was purchased from Sigma Aldrich (Catalog No. C8267). The following analytical standards were used for the chemical extractions: α-cypermethrin (Catalog No. P-548S-CN, AccuStandard; purity > 99%), cis-permethrin (Catalog No. P288560, Toronto Research Chemicals; purity > 99%), trans-permethrin (Catalog No. P288550, Toronto Research Chemicals; purity > 99%), and deltamethrin (Catalog No. C1212000; Crescent Chemical, Islandia, New York; purity > 99%). Pesticide grade solvents (ethyl acetate and acetonitrile) were purchased from Fisher Scientific (Pittsburg, Pennsylvania).
Preparation of dosing solutions.
α-Cypermethrin and permethrin dosing solutions were prepared by first dissolving each neat compound in acetone (Fisher Chemical, Certified ACS) (volume of acetone equivalent to 2% of the total volume of corn oil added), followed by evaporation of the solvent to leave a film coating the tube. Compounds were then dissolved in corn oil, and solutions were stored at −20 °C overnight in-between dosing. A similar process was followed for the preparation of vehicle solutions to control for the effects of residual acetone. Solutions were brought to room temperature and vortexed before dosing. Solutions were remade every 2 to 3 days to ensure minimal degradation of the compounds.
Animal exposures.
All experiments were conducted with the approval of the Institutional Animal Care and Use Committee of the University of Iowa. CD-1-IGS females (7–9 weeks old) were purchased from Charles River and mated with GAD67-GFP+/− knock-in males bred on a CD-1 background. GAD67-GFP+/− knock-in males were used for breeding to visualize GABAergic cells within the embryonic brain as part of an ongoing neurotoxicology study. All mice were housed in cages on a 12 h light/dark cycle with free access to food (Labdiet 7913; Envigo, Indianapolis) and water. Gestational day 0 (GD0) was determined upon detection of a vaginal plug. Pregnant dams were singly housed and gavaged from GD0 to GD16.5 to capture critical periods of organogenesis and placental growth with a collection time point of GD16.5 to avoid changes in placenta that occur late in pregnancy prior to delivery.
A total of 70 pregnant CD-1 dams were randomly assigned to exposure groups and exposed to either permethrin (1.5, 15, or 50 mg/kg), α-cypermethrin (0.3, 3, or 10 mg/kg), or corn oil vehicle (n = 10 per group) once daily from gestational days 6 to 16 via oral gavage. The low doses for α-cypermethrin and permethrin were chosen to be roughly equivalent to the human acceptable daily intake (ADI) doses (0.02 mg/kg for α-cypermethrin and 0.05 mg/kg for permethrin), multiplied by a conversion factor of 12.3 to accommodate differences in human and mouse body surface area (Nair and Jacob, 2016). Maternal weight gain was unaffected by any exposure used in the study. However, three out of ten mice exposed to 10 mg/kg α-cypermethrin exhibited symptoms of choreoathetosis (involuntary writhing and movements) on the final day of dosing (GD16), suggestive of maternal toxicity at this dose.
On gestational day 16, all mice received a final dose 90 min before euthanasia. Previous studies have demonstrated maximum blood concentrations of pyrethroids in rats approximately 1–2 h after oral administration (Singh et al., 2016; Chata et al., 2019). Pregnant dams were euthanized by ketamine/xylazine anesthesia followed by rapid decapitation. Maternal trunk blood was collected following decapitation, allowed to clot for 30 min, and then centrifuged to collect serum. The maternal liver, ovaries, and brain were removed, rinsed with saline, and snap-frozen. Amniotic fluid was collected via puncture of the amniotic sac with a 25-gauge needle, pooled across the litter, and centrifuged before snap freezing to remove any contaminating blood. Fetuses and placentas were removed from the uterus and rinsed with saline before snap freezing.
Tissue extraction procedure.
α-Cypermethrin and permethrin were extracted from the following tissues: maternal serum (0.1–0.2 g), maternal brain (0.2–0.4 g), maternal liver (0.2–0.6 g), maternal ovary (0.1–0.2 g), amniotic fluid (0.2–0.4 g), fetal body (0.9–1.1 g), and placenta (0.15–0.25 g). Placentas and fetal bodies were pooled from two fetuses per litter to improve detection of pyrethroids at low doses. Due to the small amount of tissue, maternal ovaries were pooled across all ten dams within a group, and measurements are reported as a single value.
A modified QuEChERS (“quick, easy, cheap, effective, rugged, and safe”) extraction procedure was used to extract α-cypermethrin and permethrin from maternal and fetal samples, following a protocol similar to that used in our previous study with modifications to accommodate additional sample types (Elser et al., 2020). For maternal serum and amniotic fluid measurements, the samples were added along with 2 mL acetonitrile to a 15 mL QuEChERS tube containing 800 mg MgSO4 and 200 mg NaCl (Catalog No. ECQUUS115CT, United Chemical Technologies). Samples from permethrin-exposed animals were spiked with 100 ng α-cypermethrin in 100 μL acetonitrile as a recovery standard. In contrast, samples from α-cypermethrin-exposed animals were spiked with cis- and trans- permethrin as recovery standards (100 ng each in 100 μL acetonitrile). MilliQ water (1 mL) was then added to each tube, and samples were vortexed for 1 min before centrifuging at 3000 rpm for 5 min to facilitate phase separation. The acetonitrile phase was then transferred to a QuEChERS SpinFiltr tube containing 150 mg MgSO4 and 50 mg C18 (Catalog No. ECQUSF74CT, United Chemical Technologies) and then vortexed (30 s) and centrifuged (3000 rpm, 5 min). An additional 2 mL of acetonitrile was added to the original QuEChERS tube, and samples were re-extracted. Samples in the Spin-Filter tubes (in a total of 4 mL acetonitrile) were then evaporated under a gentle stream of nitrogen to dryness. Samples were dissolved in ethyl acetate (800 μL), and 200 μL of a deltamethrin solution (25 ng in ethyl acetate) was added as an internal standard before measurement.
For the tissue samples, each sample was placed in a 15 mL falcon tube and homogenized in 2 mL acetonitrile for 30 s at high speed using a tissue-rotor handheld homogenizer. Recovery standards (100 ng α-cypermethrin for permethrin-exposed samples, or 100 ng each of cis- and trans-permethrin for α-cypermethrin-exposed samples) were then added to the solutions along with 100 μL of 10% formic acid. Tubes were then vortexed and centrifuged at 3000 rpm for 5 min to pellet tissue debris. The acetonitrile layer was removed from each tube and added to a 15 mL QuEChERS tube along with 1 mL water. The remaining steps were consistent with those outlined in the serum and amniotic fluid extractions, including a re-extraction step with 2 mL of acetonitrile.
Water blanks, matrix blanks (from three additional unexposed mice), vehicle-exposed samples, and ongoing precision and recovery (OPR) standards (unexposed matrix spiked with 100 ng α-cypermethrin, 100 ng of cis-permethrin, and 100 ng trans-permethrin) were extracted in parallel with samples.
Pyrethroid analysis.
Pyrethroid analysis was performed using an Agilent 7890A Gas Chromatograph with an Agilent 7693 autosampler and a 63Ni-micro electron capture detector (μECD). The chromatograph was equipped with a Supelco SLB-5ms column (30 m x 0.25 mm x 0.25 μm film thickness) (Supelco, St. Louis, Missouri). Temperatures of the inlet and detector were set at 240 °C and 250 °C, respectively. The temperature program was set to the following parameters: hold at 50 °C for 1 min, 15 °C /min to 220 °C, 1 °C /min to 240 °C, hold for 12 min, 15 °C /min to 280 °C, and hold for 10 min. Helium flow was 1.0 ml/min, and makeup flow was 60 ml/min. The detector response was linear ( R2 ≥ .995) for all analytes (cis-permethrin, trans-permethrin, α-cypermethrin, and deltamethrin) over the entire concentration range encountered in this study (0.1–4000 ng).
Quality control
The limits of detection (LOD) were calculated using blank samples as LOD = average of blank samples + k * standard deviation of blank samples (k = Student’s t value for n-1 degrees of freedom, 99% confidence level, n = number of blank samples) (Kania-Korwel et al., 2007). The limit of quantification (LOQ) was calculated accordingly: LOQ = LOD * 10. LOD and LOQ for tissue and fluid samples can be found in the supporting information (Table S1). Background levels of α-cypermethrin and permethrin in tissues and fluids taken from unexposed and vehicle-exposed animals are reported in the supporting information (Table S2). Levels of α-cypermethrin and permethrin in all unexposed and vehicle-exposed samples were below the LOQ.
Average recoveries of α-cypermethrin, cis-permethrin, and trans-permethrin across all OPR samples were 86% ± 9%, 83% ± 11%, and 89% ± 11%, respectively (Ranges: 65–112%, 63–110%, 65–113%; n = 60). For α-cypermethrin-exposed samples and controls, the average recovery was 87% ± 11% (Range: 59–131%, n = 255). For extractions of permethrin-exposed samples and controls, the average recovery was 85% ± 12% (Range: 49–127%, n = 255). Recovery data for individual tissues can be found in the supporting information (Table S3). Corrections were made for recoveries below 100%.
Statistical analysis.
Statistical analyses were performed using Graph-Pad Prism version 9.1 (GraphPad Software, LLC, San Diego, California). All data are presented as mean ± standard deviation. Due to non-normal data distributions, non-parametric statistical approaches were used and all data in the range were included. Correlations between serum and tissue concentrations of both pyrethroids were assessed using Spearman correlations for values above LOQ. Differences in the ratio of cis- and trans-permethrin isomers between tissues were analyzed by Kolmogorov-Smirnov comparisons to the ratio of cis- and trans-permethrin in maternal serum. Tissue to serum ratios of α-cypermethrin and permethrin were analyzed by Kolmogorov-Smirnov comparison tests to assess differences between the pyrethroids for each tissue. For all statistical tests, the overall significance level was set at 0.05.
Results and discussion
Maternal tissue distribution of α-cypermethrin.
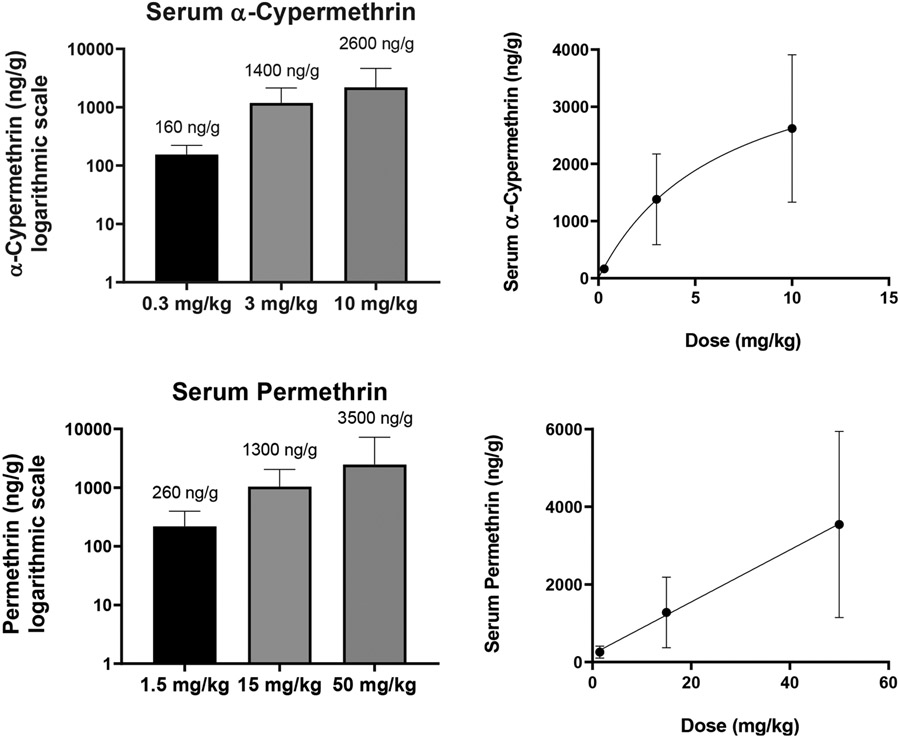
Levels of α-cypermethrin in maternal serum ranged from an average value of 160 ng/g at the 0.3 mg/kg dose to 2600 ng/g at the 10 mg/kg dose (Fig. 1, Table S4). For comparison, a previous study in male Wistar rats noted peak cypermethrin levels in the plasma of 2690 ng/mL one hour after 5 mg/kg oral cypermethrin (Singh et al., 2016). In terms of the human relevance of these levels, a previous study found that pregnant Chinese women had an average blood concentration of cypermethrin of 151 ng/mL (Simaremare et al., 2019). In addition, a study investigating occupational exposure of pesticide factory workers in Pakistan demonstrated plasma levels of cypermethrin as high as 400 ng/mL (Khan et al., 2010). As such, blood and tissue levels reported in our study at the 0.3 mg/kg α-cypermethrin dose appear to be within the range of environmental and occupational exposures in humans.
Fig. 1.
Levels of α-cypermethrin and permethrin in maternal serum of pregnant mice after exposure GD6-16. Concentrations were measured in samples taken 90 min after the final dose was administered on GD16. Means and standard deviations of each pyrethroid are represented on a logarithmic scale (left) and proportional to the dose administered (right) (n = 10 samples per group).
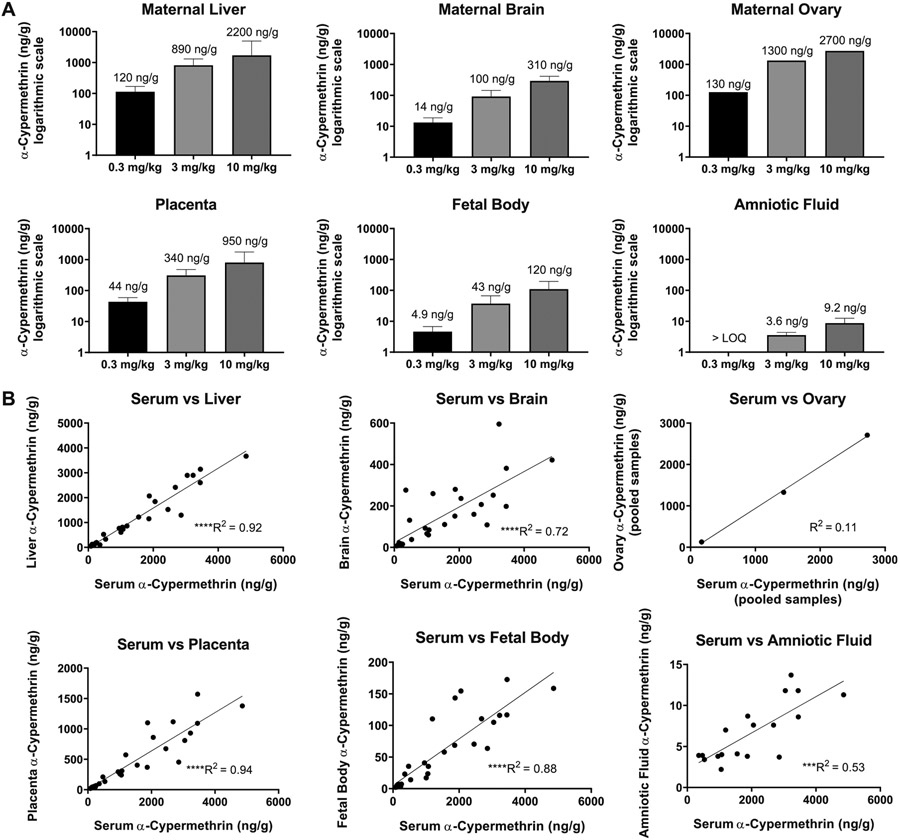
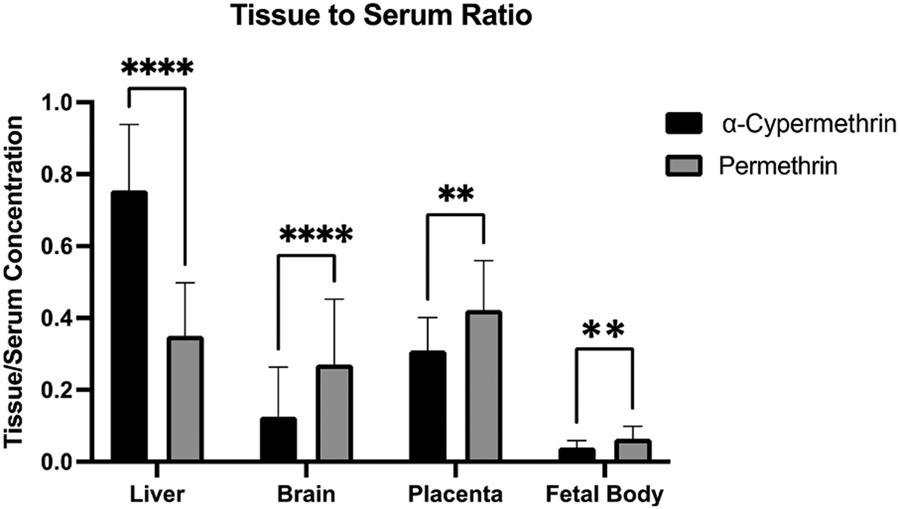
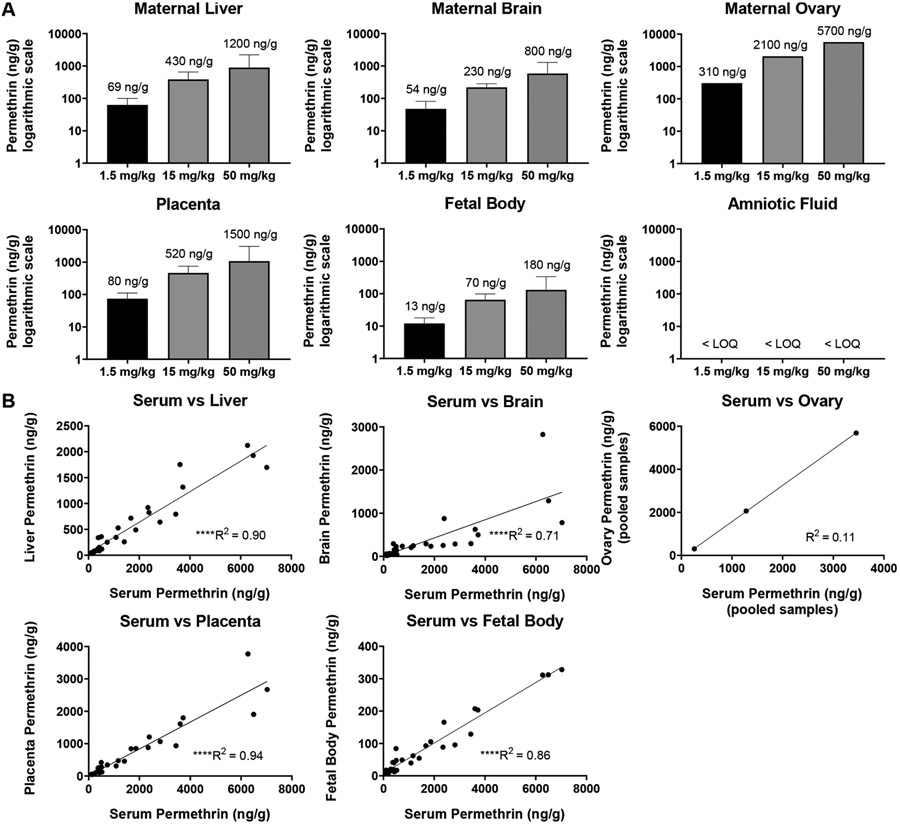
Across maternal tissues, maternal pooled ovary concentrations were the highest, followed by liver and then brain (Fig. 2A, Table S4). Maternal ovary concentrations should be interpreted with caution, as these values were obtained by pooling samples within each treatment group, and therefore each reported concentration is equivalent to n = 1. α-Cypermethrin levels in the maternal liver were highly correlated to those in maternal serum (R2 = 0.92) (Fig. 2B) likely due to oral administration which results in similar, rapid distribution to liver and blood. Compared to other maternal tissues, maternal brain levels of α-cypermethrin were relatively low (Fig. 2A). On average, maternal brain levels were approximately ten times lower than serum levels, suggesting that α-cypermethrin may exhibit low transfer across the blood:brain barrier (Fig. 4, Table S5). These results are in line with previous work demonstrating that pyrethroid penetration into brain tissue is limited by their high degree of protein binding in serum (Amaraneni et al., 2016).
Fig. 2.
Levels of α-cypermethrin in maternal and fetal tissues following exposure GD6-16. Concentrations reported as mean ± standard deviation (n = 9–10 samples per group). Ovary samples were pooled within each group and are reported as n = 1. Levels are represented on (A) a logarithmic scale and (B) plotted against maternal serum concentrations. Maternal serum samples were averaged and plotted against pooled ovary samples for correlation assessment. (****p < 0.0001, ***p < 0.001 for correlations).
Fig. 4.
Levels of α-cypermethrin and permethrin in tissues relative to maternal serum concentration following exposure GD6-16. Data represent mean ± standard deviation (n = 29–30 dams per pyrethroid for liver, brain, placenta, and fetal body). (**p < 0.01, ****p < 0.0001).
The 0.08–0.19 brain:serum ratios of α-cypermethrin found here are relatively small, compared to previous findings in rats (0.2–0.5) (Hughes et al., 2016; Starr et al., 2012). This difference in blood:brain ratio may be explained either by differences in species (rat vs. mouse) or timing of tissue collection. In this respect, previous studies collected samples 2.5–3.5 h following dosing, at which point lower serum levels would be expected due to the short blood half-life of cypermethrin (1.5 h in blood vs 2.5 h in brain) (Starr et al., 2012). It is also possible that the current study may underestimate maximum levels in maternal brain; pyrethroids may reach maximum concentrations in the rat brain approximately 2–4 h following oral exposure (Kim et al., 2010). Interestingly, we found that α-cypermethrin concentrations in the brain demonstrated lower correlation to maternal serum concentrations (R2 = 0.72; Fig. 2B). This suggests that levels in the brain may not reflect the final dose given and may instead reflect accumulated levels due to repeated dosing. Accumulation of pyrethroids within the brain has been shown in previous work demonstrating a longer half-life in brain than blood (Kim et al., 2010).
Fetal and placental tissue distribution of α-cypermethrin.
Levels of α-cypermethrin in the fetal body were found to range from an average of 4.9 ng/g at the 0.3 mg/kg dose to 120 ng/g at the 10 mg/kg dose (Fig. 2, Table S4). In comparison to maternal tissues, levels of α-cypermethrin in fetal body were relatively low, on the order of 20–30 times lower than maternal serum concentrations (Fig. 4, Table S5). In general, α-cypermethrin levels in the fetal body showed a more moderate correlation to maternal serum levels at the time of collection (R2 = 0.88) (Fig. 2B), suggesting that levels in the fetal body may primarily reflect the final dose given 90 min before sacrifice. However, low rate of metabolism and accumulation in the fetal body following repeated dosing cannot be ruled out. A previous study in pregnant rats with the type II pyrethroid fenvalerate demonstrated fetal tissue accumulation following repeated, daily dosing (Shiba et al., 1990). Fetal body levels of α-cypermethrin at the 0.3 mg/kg dose in our study are within the range of those reported in cord blood samples from Chinese newborns at the 75th percentile of exposures (Q3: 7.58 ng/mL, Range: ND-390.27 ng/mL) (Silver et al., 2015). However, levels of cypermethrin were below the LOD (0.056 ng/mL or 28 ng in a 200 μl sample in that study) in all fetal cord blood samples from a population in Baltimore, MD. While our LOD (0.06 ng) was lower than that in the Baltimore study, it is also possible the doses used in our study may not be as applicable to cypermethrin exposures in urban U.S. populations (Neta et al., 2010).
Levels in amniotic fluid were the lowest of all tissues examined in our study (Fig. 2, Table S4). Concentrations of α-cypermethrin in the amniotic fluid were below the LOQ at the 0.3 mg/kg dose and at levels approximately 300 times lower than those found in maternal serum at the 3 mg/kg and 10 mg/kg doses. Amniotic fluid levels reported here (3.6 ng/mL at the 3 mg/kg dose and 9.7 ng/mL at the 10 mg/kg dose) are consistent with levels reported in the amniotic fluid following oral cypermethrin at similar dose levels in pregnant Wistar rats (4.9 ng/mL with a 3 mg/kg dose and 9.7 mg/mL with a 9 mg/kg dose) (Bossi et al., 2013). Interestingly, α-cypermethrin levels in fetal bodies were approximately 12 times higher than those in amniotic fluid, suggesting that α-cypermethrin is more easily distributed to fetal tissue than amniotic fluid. Poor distribution of the parent compound to amniotic fluid is consistent with low rates of detection of pyrethroid metabolites in human amniotic fluid and a previous mouse study in our lab (Elser et al., 2020; Bradman et al., 2003). When considering the high lipophilicity of cypermethrin, it is expected that levels would be low in an aqueous matrix such as amniotic fluid. Protein content in the amniotic fluid is markedly lower than that in maternal and fetal serum, making amniotic fluid a relatively poor matrix for accumulation of hydrophobic pyrethroids, which rely on protein binding to persist in aqueous media (Amaraneni et al., 2016; Renfree et al., 1975).
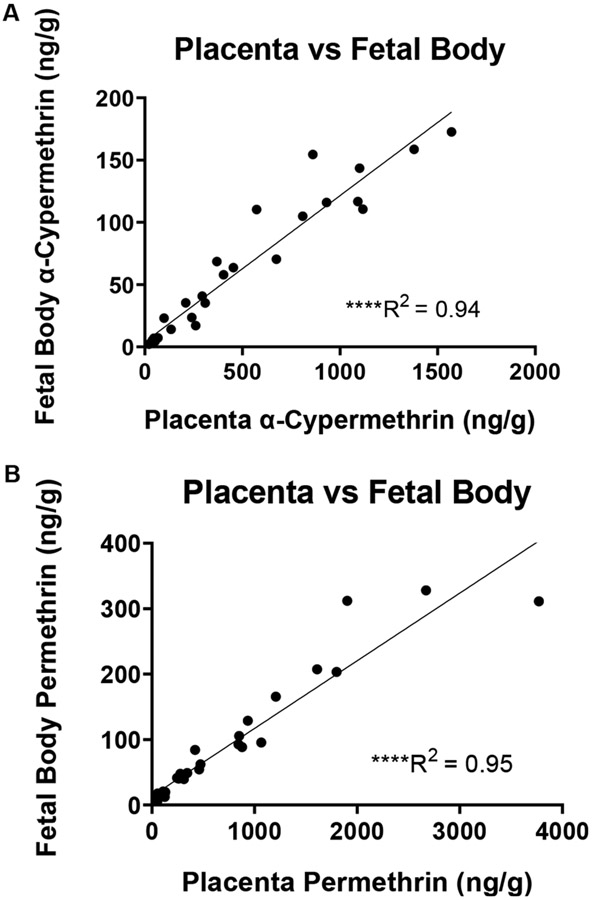
Placental tissue levels of α-cypermethrin ranged from an average of 45 ng/g at the 0.3 mg/kg dose to an average of 950 ng/g at the 10 mg/kg dose (Fig. 2A, Table S4). Placental levels were approximately three times lower than those in maternal serum, with a high correlation to serum values (R2 = 0.94) (Fig. 2B). Compared to maternal tissues such as ovaries and liver, it is interesting to note the relatively lower levels of α-cypermethrin in placental tissue. Given that the placenta comprises both maternal and fetal blood vessels, lower levels in placental tissue may be due primarily to the low level of α-cypermethrin within the fetal compartment. In this respect, there is a significant correlation of placental and fetal body levels (Fig. 6). Similar to our findings, a previous study investigating tissue distribution in pregnant rats of the type II pyrethroid fenvalerate also found that levels in the placenta were approximately three times lower than those in maternal serum (Shiba et al., 1990). Interestingly, placenta levels of α-cypermethrin reported in our study at the 0.3 mg/kg dose (21–68 ng/g tissue weight) are within the range of those reported in placentas from pregnant dolphins on the Brazilian coast (1105 ng/g lipid weight from a placenta with 3% lipid content, corresponding to 33.15 ng/g tissue weight) (Alonso et al., 2015).
Fig. 6.
Correlation between placenta and fetal body levels of (A) α-cypermethrin and (B) permethrin after exposure GD6-16 (****p < 0.0001).
Overall, our data suggest a relatively low distribution of α-cypermethrin in fetal tissues. However, relative maternal:fetal concentration data from a single timepoint of collection should be interpreted with caution, as previous work has demonstrated that some compounds show a delayed crossover from maternal to fetal circulation (Waddell and Marlowe, 1981). As such, additional toxicokinetic studies are needed to confirm these findings across a range of timepoints. However, results from this study generally align with conclusions from previous work. For example, low levels of placental transfer for zeta-cypermethrin were reported following exposure from GD16-GD20 in pregnant rats (Liu et al., 2019). However, there is concern regarding the quality of the zeta-cypermethrin study due to significant background levels of cypermethrin (80–110 ng/mL) reported in maternal and fetal blood from vehicle-exposed animals (Liu et al., 2019). In addition, recent work using a human placental perfusion model found that cypermethrin levels in the fetal circulation reached concentrations equivalent to 1/5 of the initial concentration in maternal circulation after 6 h of perfusion, a rate lower than expected for passive diffusion (Mathiesen et al., 2020).
Maternal tissue distribution of permethrin.
Levels of permethrin in maternal serum ranged from 260 ng/g at the 1.5 mg/kg dose to 3500 ng/g at the 50 mg/kg dose (Fig. 1, Table S4). In comparison to our results, a previous study investigating tissue distribution of 50 mg/kg permethrin (40:60 cis:trans) in pregnant rats found maximum blood levels of approximately 1500 ng/mL at GD20 (Personne et al., 2019). In terms of the human relevance of these levels, a previous study demonstrated an average permethrin concentration of 94 ng/mL in the blood of pregnant Chinese women (Simaremare et al., 2019).
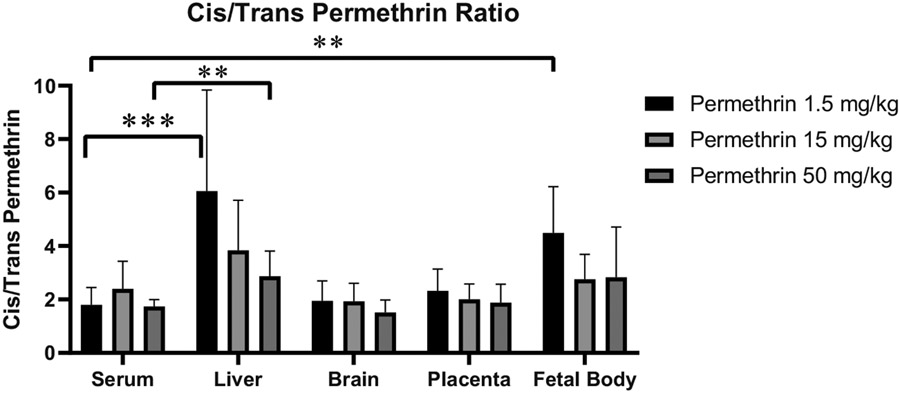
Compared to α-cypermethrin, permethrin levels demonstrated a more linear increase in serum concentrations in proportion to the dose administered (Fig. 1). However, serum and tissue concentrations of permethrin were notably lower relative to dose than those for α-cypermethrin, consistent with a higher rate of the first-pass metabolism for cis-permethrin as compared to α-cypermethrin (Crow et al., 2007). In addition, previous reports have indicated a generally higher rate of metabolism in rats for the trans- versus cis- isomer of pyrethroids in general (Tornero-Velez et al., 2012). Therefore, it is not surprising that a 40:60 cis:trans mixture of permethrin is metabolized at a faster rate than α-cypermethrin, which consists entirely of the cis-isomer. Consistent with a faster rate of metabolism of trans-permethrin, our study found significantly higher levels of the cis isomer across all tissues (e.g. maternal serum cis:trans ratio 2:1; Fig. 5).
Fig. 5.
Ratio of cis to trans permethrin isomers in maternal and fetal tissues after exposure GD6-16. Data represent mean ± standard deviation (n = 9–10 dams per dose) (**p < 0.01, ***p < 0.001).
As seen for α-cypermethrin, levels of permethrin in the maternal ovaries were the highest of all maternal tissues examined, followed by maternal liver and brain, respectively (Fig. 3). Unlike α-cypermethrin, levels of permethrin in the maternal ovaries were higher than serum concentrations across all doses examined, suggesting accumulation in the ovaries following repeated dosing (Fig. 4, Table S5); interpretation of these single measures from pooled tissue should be made with caution. Interestingly, levels of permethrin in the maternal liver were highly correlated with those in serum ( R2 = 0.90) but at a much lower concentration (Fig. 3). Compared to α-cypermethrin, the tissue to serum ratio of permethrin in the maternal liver was significantly lower, likely due to the higher rate of hepatic metabolism of permethrin (Fig. 4). Interestingly, the cis:trans ratio of permethrin isomers was significantly elevated in liver samples at a ratio of 4:1, suggesting selective hepatic clearance of the trans isomer (Fig. 5). A higher ratio of cis:trans permethrin isomers in the liver, compared to serum, is consistent with a previous study investigating tissue distribution in male rats (Tornero-Velez et al., 2012).
Fig. 3.
Levels of permethrin in maternal and fetal tissues following exposure GD6-16. Concentrations reported as mean ± standard deviation (n = 9–10 samples per group). Ovary samples were pooled within each group and are reported as n = 1. Levels are represented on (A) a logarithmic scale and (B) plotted against maternal serum concentrations. Maternal serum samples were averaged and plotted against pooled ovary samples for correlation assessment. (****p < 0.0001 for correlations).
Levels of permethrin in the maternal brain were significantly lower than other maternal tissues (Fig. 3, Table S4); correlation of brain to serum permethrin concentrations was similar to α-cypermethrin (R2 = 0.71). However, brain levels of permethrin were higher relative to serum than for α-cypermethrin, suggesting increased brain penetration by permethrin (Fig. 4, Table S5). Increased blood-brain barrier penetration of permethrin, compared to α-cypermethrin, is consistent with non-pregnant rat findings (Hughes et al., 2016). In addition, the ratio of cis: trans isomers of permethrin in the maternal brain was similar to that found in serum, suggesting that brain penetration of each isomer is approximately equivalent as previously reported in rats (Mortuza et al., 2019).
Fetal and placental tissue distribution of permethrin.
Similar to what was observed with α-cypermethrin, permethrin levels in fetal tissues were approximately 20 times lower than maternal serum (Fig. 4, Table S5). Permethrin levels in the fetal bodies ranged from 13 ng/g at the 1.5 mg/kg dose to 180 ng/g at the 50 mg/kg dose. These findings suggest a low placental transfer rate similar to that seen with α-cypermethrin. Despite these low levels, a particularly interesting finding was that fetal bodies had a significantly higher proportion of cis isomer (3:1 cis:trans) than maternal serum at low doses (2:1 cis:trans) (Fig. 5, p = 0.001). This may reflect increased placental permeability of permethrin’s cis isomer. Previous work has demonstrated a higher degree of protein binding in rat and human serum for the trans isomer of permethrin, as compared to the cis isomer, which may contribute to a lower rate of placental transfer for trans-permethrin (Sethi et al., 2019). In comparison to fetal bodies, amniotic fluid levels of permethrin were below the LOQ for all doses used here, further suggesting that amniotic fluid is a poor matrix for assessing fetal pyrethroid exposure.
Findings across results of both pyrethroids and implications.
Overall, results from our study suggest that the distribution of permethrin into fetal mouse tissue is relatively low. In comparison, a previous study of 50 mg/kg permethrin in pregnant rats noted a higher concentration of permethrin in fetal tissue, particularly when samples were collected 6 h post-dose (>0.7 μg/g in fetal and maternal liver-correlation not reported) (Personne et al., 2019). While mouse and rat species differences may explain our conflicting findings, it is also possible that our early timepoint of collection (90 min post final dose) may not reflect maximum fetal tissue levels. For example, fetal compartment levels were highest 6 h after oral exposure of the type-II pyrethroid fenvalerate in pregnant rats (Shiba et al., 1990). However, results from this study were generally consistent with our findings of low fetal tissue distribution, with a fetal:maternal blood ratio of approximately 0.1 for fenvalerate (Shiba et al., 1990).
The results from this study add to our knowledge of the potential health effects of pyrethroid exposure during mammalian pregnancy and aid in the further development of physiologically based pharmacokinetic modeling, particularly given the dynamic physiological changes across pregnancy, for this class of compounds. A significant finding of our study was the low degree of direct fetal exposure to both α-cypermethrin and permethrin following oral exposure in pregnant mice. Our data suggest that direct fetal exposure to pyrethroids may be relatively low in the context of environmentally relevant exposures. However, additional toxicokinetic studies are needed to verify the time course of pyrethroid levels in the fetal compartment, as the single timepoint of collection employed in this study (90 min post-dose) may not capture delayed crossover of the compounds from maternal to fetal circulation (Waddell and Marlowe, 1981). With respect to a low degree of placental transfer, it remains unclear whether relatively low concentrations of pyrethroids can induce direct effects on fetal development or if these effects may be better explained through an indirect mode of action. In addition, comparisons made between tissue concentrations in our study and those reported in humans and dolphins support the relevance of a 0.3 mg/kg dose in mice for studies investigating links between cypermethrin exposure and mammalian health outcomes.
In light of the reported effects of pyrethroids on fetal growth, it is interesting to note that levels of pyrethroids in our study are relatively high in the placenta as compared to the fetal body (Garnica and Chan, 1996). Fetal growth depends on the placental capacity to transfer oxygen and nutrients to fetal circulation; thus, fetal growth restriction is highly associated with placental dysfunction (Cetin and Antonazzo, 2009). Considering these findings, it is worth noting that several relevant molecular targets for pyrethroids in the placenta are critical for the active transport of nutrients and regulation of growth factor/hormone synthesis (Johansson et al., 2003; Vallejos and Riquelme, 2007). Such targets include voltage-gated calcium channels, maxi chloride channels, and plasma membrane ATPases (Vallejos and Riquelme, 2007; Bernucci et al., 2006). Given the relatively low levels that we report in fetal tissues, it may be beneficial to investigate indirect placenta-mediated modes of action to explain associations of pyrethroid exposure with impaired fetal growth and neurodevelopment (Bronson and Bale, 2016; Kundu et al., 2021).
This study also suggests that current methods for assessing fetal exposure to pyrethroids may be somewhat limited in their utility. For example, previous attempts to monitor fetal pyrethroid exposure using fetal cord blood and amniotic fluid measurements have resulted in low detection rates, consistent with the low levels reported in fetal tissues within our study. As such, there is a need for better testing methods capable of detecting and quantifying these low-level exposures. This need is particularly relevant if previous reports of adverse effects are due to low levels of direct exposure to the fetus. In this respect, our unique analysis examining correlation across tissues suggest that concentrations of either pyrethroid in the placenta and fetal body are highly correlated (R2 > 0.94 for either pyrethroid, Fig. 6), with placental levels approximately ten times higher than fetal levels (Figs. 2 and 3, Table S4). Levels of α-cypermethrin in placental tissue appear to be a stronger predictor of fetal exposure than maternal serum levels, as levels in maternal serum showed a more moderate correlation to fetal levels (R2 = 0.88, Fig. 2). As such, pyrethroid levels in human placental tissue collected at delivery may help extrapolate fetal exposure levels. It is expected that placental tissue measurements may improve detection rates due to the higher concentrations relative to fetal tissues and a large amount of available tissue at delivery. In support of the utility of placental pyrethroid measurements, a recent study demonstrated that within the same cohort, pyrethroids were detectable in 90% of placental samples as compared to only 26% of meconium samples (Fernández-Cruz et al., 2020).
Conclusions
In summary, results from our study lead us to conclude that the placental transfer of both these pyrethroids is relatively low in mice. These findings about the distribution of pyrethroids have important implications for future developmental toxicology studies focused on understanding mechanisms of action by which pyrethroids alter embryonic and fetal development in mammals. In addition, results from our study compared to tissue concentrations reported in both humans and pregnant dolphins lead us to conclude that a 0.3 mg/kg dose of α-cypermethrin for use in mouse toxicology studies has environmental relevance. Furthermore, results from our study showing differences in maternal tissue levels of each pyrethroid lead us to conclude that permethrin and α-cypermethrin have distinctive aspects of distribution in pregnant mice, as previously shown in non-pregnant rats.
Supplementary Material
Acknowledgments
Graphical Abstract art was created using BioRender.com.
Funding
This research was funded by the National Institute for Environmental Health Sciences through the University of Iowa Environmental Health Sciences Research Center (EHSRC), NIEHS/NIH P30 ES005605, the University of Iowa’s Center for Health Effects of Environmental Contamination (CHEEC), and a Microfinance Research Grant from the Pappajohn Biomedical Institute. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Footnotes
Supporting information
Limits of detection and quantification for pyrethroid-exposed samples, background levels of α-cypermethrin and permethrin in unexposed and vehicle-exposed samples, recovery data for α-cypermethrin and permethrin, concentrations of α-cypermethrin and permethrin in maternal and fetal tissues, and serum to tissue ratios of α-cypermethrin and permethrin. This material is available free of charge at http://pubs.acs.org.
CRediT authorship contribution statement
Benjamin A. Elser: Conceptualization, Data curation, Funding acquisition, Methodology, Investigation, Software, Writing – original draft. Derek Simonsen: Data curation, Formal analysis. Hans-Joachim Lehmler: Conceptualization, Funding acquisition, Supervision. Hanna E. Stevens: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare no competing financial interest.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.envadv.2022.100239.
References
- Alonso MB, et al. , 2015. Toxic heritage: maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Environ. Pollut 207, 391–402. [DOI] [PubMed] [Google Scholar]
- Amaraneni M, et al. , 2016. Plasma protein binding limits the blood brain barrier permeation of the pyrethroid insecticide, deltamethrin. Toxicol. Lett 250-251, 21–28. [DOI] [PubMed] [Google Scholar]
- Barr DB, et al. , 2010. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: national health and nutrition examination survey 1999–2002. Environ. Health Perspect 118 (6), 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernucci L, et al. , 2006. Diverse calcium channel types are present in the human placental syncytiotrophoblast basal membrane. Placenta 27 (11-12), 1082–1095. [DOI] [PubMed] [Google Scholar]
- Bossi R, et al. , 2013. Levels of pesticides and their metabolites in wistar rat amniotic fluids and maternal urine upon gestational exposure. Int. J. Environ. Res. Public Health 10 (6), 2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, et al. , 2003. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ. Health Perspect 111 (14), 1779–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge CB, et al. , 2009. Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology 30, S17–S31. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Bale TL, 2016. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41 (1), 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CJ, Pastoor TP, 2018. Pyrethroid epidemiology: a quality-based review. Crit. Rev. Toxicol 48 (4), 297–311. [DOI] [PubMed] [Google Scholar]
- Cetin I, Antonazzo P, 2009. The role of the placenta in intrauterine growth restriction (IUGR). Z. Geburtshilfe. Neonatol 213 (3), 84–88. [DOI] [PubMed] [Google Scholar]
- Chata C, et al. , 2019. Blood pharmacokinetic of 17 common pesticides in mixture following a single oral exposure in rats: implications for human biomonitoring and exposure assessment. Arch. Toxicol 93 (10), 2849–2862. [DOI] [PubMed] [Google Scholar]
- Coker E, et al. , 2018. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ. Int 113, 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JA, et al. , 2007. Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver and serum carboxylesterases. Toxicol. Appl. Pharmacol 221 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, et al. , 2014. Evaluation of pyrethroid exposures in pregnant women from 10 Caribbean countries. Environ. Int 63, 201–206. [DOI] [PubMed] [Google Scholar]
- Ding G, et al. , 2015. Prenatal exposure to pyrethroid insecticides and birth outcomes in Rural Northern China. J. Expo Sci. Environ. Epidemiol 25 (3), 264–270. [DOI] [PubMed] [Google Scholar]
- Eadsforth CV, Bragt PC, Van Sittert NJ, 1988. Human dose-excretion studies with pyrethroid insecticides cypermethrin and alphacypermethrin: relevance for biological monitoring. Xenobiotica 18 (5), 603–614. [DOI] [PubMed] [Google Scholar]
- Elser BA, et al. , 2020. Combined maternal exposure to cypermethrin and stress affect embryonic brain and placental outcomes in mice. Toxicol. Sci 175 (2), 182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Cruz T, et al. , 2020. Prenatal exposure to organic pollutants in northwestern Spain using non-invasive matrices (placenta and meconium). Sci. Total Environ 731, 138341. [DOI] [PubMed] [Google Scholar]
- Fiedler N, et al. , 2015. Neurobehavioral effects of exposure to organophosphates and pyrethroid pesticides among Thai children. Neurotoxicology 48, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field LM, et al. , 2017. Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur. Biophys. J 46 (7), 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshaw PJ, Lister T, Ray DE, 2000. The role of voltage-gated chloride channels in Type II pyrethroid insecticide poisoning. Toxicol. Appl. Pharmacol 163 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- Furlong MA, et al. , 2017. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 62, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnica AD, Chan WY, 1996. The role of the placenta in fetal nutrition and growth. J. Am. Coll. Nutr 15 (3), 206–222. [DOI] [PubMed] [Google Scholar]
- Hughes MF, et al. , 2016. Environmentally relevant pyrethroid mixtures: a study on the correlation of blood and brain concentrations of a mixture of pyrethroid insecticides to motor activity in the rat. Toxicology 359-360, 19–28. [DOI] [PubMed] [Google Scholar]
- Johansson M, et al. , 2003. Activity and protein expression of Na+/K+ ATPase are reduced in microvillous syncytiotrophoblast plasma membranes isolated from pregnancies complicated by intrauterine growth restriction. J. Clin. Endocrinol. Metab 88 (6), 2831–2837. [DOI] [PubMed] [Google Scholar]
- Kakko I, Toimela T, Tahti H, 2003. The synaptosomal membrane bound ATPase as a target for the neurotoxic effects of pyrethroids, permethrin and cypermethrin. Chemosphere 51 (6), 475–480. [DOI] [PubMed] [Google Scholar]
- Kaneko H, et al. , 1984. Metabolism and placental transfer of stereoisomers of tetramethrin isomers in pregnant rats. J. Pestic. Sci 9 (2), 249–258. [Google Scholar]
- Kania-Korwel I, et al. , 2007. Enantioselective disposition of PCB 136 (2,2′,3,3′,6,6′-hexachlorobiphenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality 19 (1), 56–66. [DOI] [PubMed] [Google Scholar]
- Khan DA, et al. , 2010. Monitoring health implications of pesticide exposure in factory workers in Pakistan. Environ. Monit. Assess 168 (1), 231–240. [DOI] [PubMed] [Google Scholar]
- Kim KB, et al. , 2010. Age, dose, and time-dependency of plasma and tissue distribution of deltamethrin in immature rats. Toxicol. Sci 115 (2), 354–368. [DOI] [PubMed] [Google Scholar]
- Kundu S, Maurer SV, Stevens HE, 2021. Future horizons for neurodevelopmental disorders: placental mechanisms. Front. Pediatr 9:653230 (243). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler HJ, et al. , 2020. Environmental exposure to pyrethroid pesticides in a nationally representative sample of U.S. adults and children: the national health and nutrition examination survey 2007–2012. Environ. Pollut 267, 115489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chandrasekaran A, Becker JM, 2019. Determination of offspring NOAEL for zeta-cypermethrin using internal exposure data from rat developmental neurotoxicity studies. Regul. Toxicol. Pharm 108, 104425. [DOI] [PubMed] [Google Scholar]
- Mathiesen L, et al. , 2020. Placental transfer of pesticides studied in human placental perfusion. Basic Clin. Pharmacol. Toxicol 127 (6), 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, 2019. Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 95 (7), 378–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortuza TB, et al. , 2019. Age dependency of blood-brain barrier penetration by cis- and trans-permethrin in the rat. Drug Metab. Dispos 47 (3), 234. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm 7 (2), 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta G, et al. , 2010. Distribution and determinants of pesticide mixtures in cord serum using principal component analysis. Environ. Sci. Technol 44 (14), 5641–5648. [DOI] [PubMed] [Google Scholar]
- Personne S, et al. , 2019. Determination of maternal and foetal distribution of cis- and trans-permethrin isomers and their metabolites in pregnant rats by liquid chromatography tandem mass spectrometry (LC-MS/MS). Anal. Bioanal.Chem 411 (30), 8043–8052. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Hensleigh HC, McLaren A, 1975. Developmental changes in the composition and amount of mouse fetal fluids. J. Embryol. Exp. Morphol 33 (2), 435. [PubMed] [Google Scholar]
- Schettgen T, et al. , 2002. Pyrethroid exposure of the general population-is this due to diet. Toxicol. Lett 134 (1-3), 141–145. [DOI] [PubMed] [Google Scholar]
- Sethi P, et al. , 2019. Plasma protein and lipoprotein binding of cis- and trans-permethrin and deltamethrin in adult humans and rats. Drug Metab. Dispos 47 (9), 941. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA, Crofton KM, 2005. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ. Health Perspect 113 (2), 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, et al. , 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ. Health Perspect 122 (10), 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K, et al. , 1990. Placental transfer of esfenvalerate and fenvalerate in pregnant rats. J. Pestic. Sci 15 (2), 169–174. [Google Scholar]
- Silver MK, et al. , 2015. Distribution and predictors of pesticides in the umbilical cord blood of Chinese newborns. Int. J. Environ. Res. Public Health 13 (1), 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simaremare SRS, et al. , 2019. Relationship between organophosphate and pyrethroid insecticides in blood and their metabolites in urine: a pilot study. Int. J. Environ. Res. Public Health 17 (1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, et al. , 2016. Validation of a rapid and sensitive UPLC-MS-MS method coupled with protein precipitation for the simultaneous determination of seven pyrethroids in 100 μL of rat plasma by using ammonium adduct as precursor ion. J. Anal. Toxicol 40 (3), 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr JM, et al. , 2012. Environmentally relevant mixtures in cumulative assessments: an acute study of toxicokinetics and effects on motor activity in rats exposed to a mixture of pyrethroids. Toxicol. Sci 130 (2), 309–318. [DOI] [PubMed] [Google Scholar]
- Tang W, et al. , 2018. Pyrethroid pesticide residues in the global environment: an overview. Chemosphere 191, 990–1007. [DOI] [PubMed] [Google Scholar]
- Tornero-Velez R, et al. , 2012. A pharmacokinetic model of cis- and trans-permethrin disposition in rats and humans with aggregate exposure application. Toxicol. Sci 130 (1), 33–47. [DOI] [PubMed] [Google Scholar]
- Vallejos C, Riquelme G, 2007. The maxi-chloride channel in human syncytiotrophoblast: a pathway for taurine efflux in placental volume regulation? Placenta 28 (11-12), 1182–1191. [DOI] [PubMed] [Google Scholar]
- Viel JF, et al. , 2015. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: the PELAGIE mother-child cohort. Environ. Int 82, 69–75. [DOI] [PubMed] [Google Scholar]
- Viel JF, et al. , 2017. Behavioural disorders in 6-year-old children and pyrethroid insecticide exposure: the PELAGIE mother-child cohort. Occup. Environ. Med 74 (4), 275–281. [DOI] [PubMed] [Google Scholar]
- Waddell WJ, Marlowe C, 1981. Transfer of drugs across the placenta. Pharmacol. Ther 14 (3), 375–390. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, et al. , 2016. Urinary 3-phenoxybenzoic acid (3-PBA) levels among pregnant women in Mexico City: distribution and relationships with child neurodevelopment. Environ. Res 147, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, et al. , 2003. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ. Health Perspect 111 (5), 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemin ME, et al. , 2016. PBPK modeling of the cis- and trans-permethrin isomers and their major urinary metabolites in rats. Toxicol. Appl. Pharmacol 294, 65–77. [DOI] [PubMed] [Google Scholar]
- Williams MK, et al. , 2008. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U.S. environmental protection agency restriction of organophosphates. Environ. Health Perspect 116 (12), 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LD, et al. , 2009. Embryo-fetal developmental toxicity study design for pharmaceuticals. Birth Defects Res. Part B Dev. Reprod. Toxicol 86 (6), 418–428. [DOI] [PubMed] [Google Scholar]
- Woollen BH, et al. , 1992. The metabolism of cypermethrin in man: differences in urinary metabolite profiles following oral and dermal administration. Xenobiotica 22 (8), 983–991. [DOI] [PubMed] [Google Scholar]
- Wren M, et al. , 2021. Analysis of six pyrethroid insecticide metabolites in cord serum using a novel gas chromatography-ion trap mass spectrometry method. J. Chromatogr. B 1173, 122656. [DOI] [PubMed] [Google Scholar]
- Xue Z, et al. , 2013. Effect of synthetic pyrethroid pesticide exposure during pregnancy on the growth and development of infants. Asia Pac. J. Public Health 25 (4 Suppl), 72S–79S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.