Abstract
The circulating precursor cells that give rise to human resident memory T cells (TRM) are poorly characterized. We utilized an in vitro differentiation system and human skin-grafted mice to study TRM generation from circulating human memory T cell subsets. In vitro TRM differentiation was associated with functional changes, including enhanced IL-17A production and FOXP3 expression in CD4+ T cells and granzyme B production in CD8+ T cells, changes that mirrored the phenotype of T cells in healthy human skin. Effector memory T cells (TEM) had the highest conversion rate to TRM in vitro and in vivo but central memory T cells (TCM) persisted longer in the circulation, entered the skin in larger numbers and generated increased numbers of TRM. In summary, TCM are highly efficient precursors of human skin TRM, a feature that may underlie their known association with effective long-term immunity.
One sentence summary:
Central memory T cells are the most efficient precursors for human skin resident memory T cells.
Introduction
Resident memory T cells (TRM) accumulate in epithelial barrier tissues following infection, undergo a distinct differentiation program, have strong effector functions and provide potent and front-line protection against known pathogens (1–3). It is not known which circulating human T cell subsets have the ability to differentiate into TRM in peripheral tissues and what if any functional differences exist in TRM generated from distinct memory cell types. A better understanding of human TRM precursor cells is needed to develop vaccination strategies that optimally populate peripheral tissues with TRM. We utilized an in vitro differentiation system incorporating human keratinocytes and an in vivo differentiation system using immunodeficient mice grafted with human skin to study the ability of human circulating precursor cells to give rise to TRM. We report here our findings that CCR7+ L-selectin+ central memory T cells are the most efficient precursors for human skin TRM.
Results
Multiple circulating memory T cell subsets generate TRM in vitro
We tested three major circulating CD45RA− memory T cell subsets for their ability to generate TRM (Fig. 1A). Central memory T cells (TCM) co-express L-selectin and CCR7, actively recirculate between the blood and lymph nodes, and a subset of these cells also recirculate through skin and other healthy human peripheral tissues (4, 5). Effector memory T cells (TEM) lack expression of L-selectin and CCR7, are tropic for peripheral tissues and can differentiate into highly protective effector cells. Migratory memory cells (TMM) are a subset recently described in humans that express CCR7 but lack L-selectin, have effector functions intermediate between TCM and TEM, and recirculate between the blood and the skin but appear to be excluded from central lymph nodes (6).
Figure 1. Multiple human circulating memory T cell subsets can generate TRM in vitro.
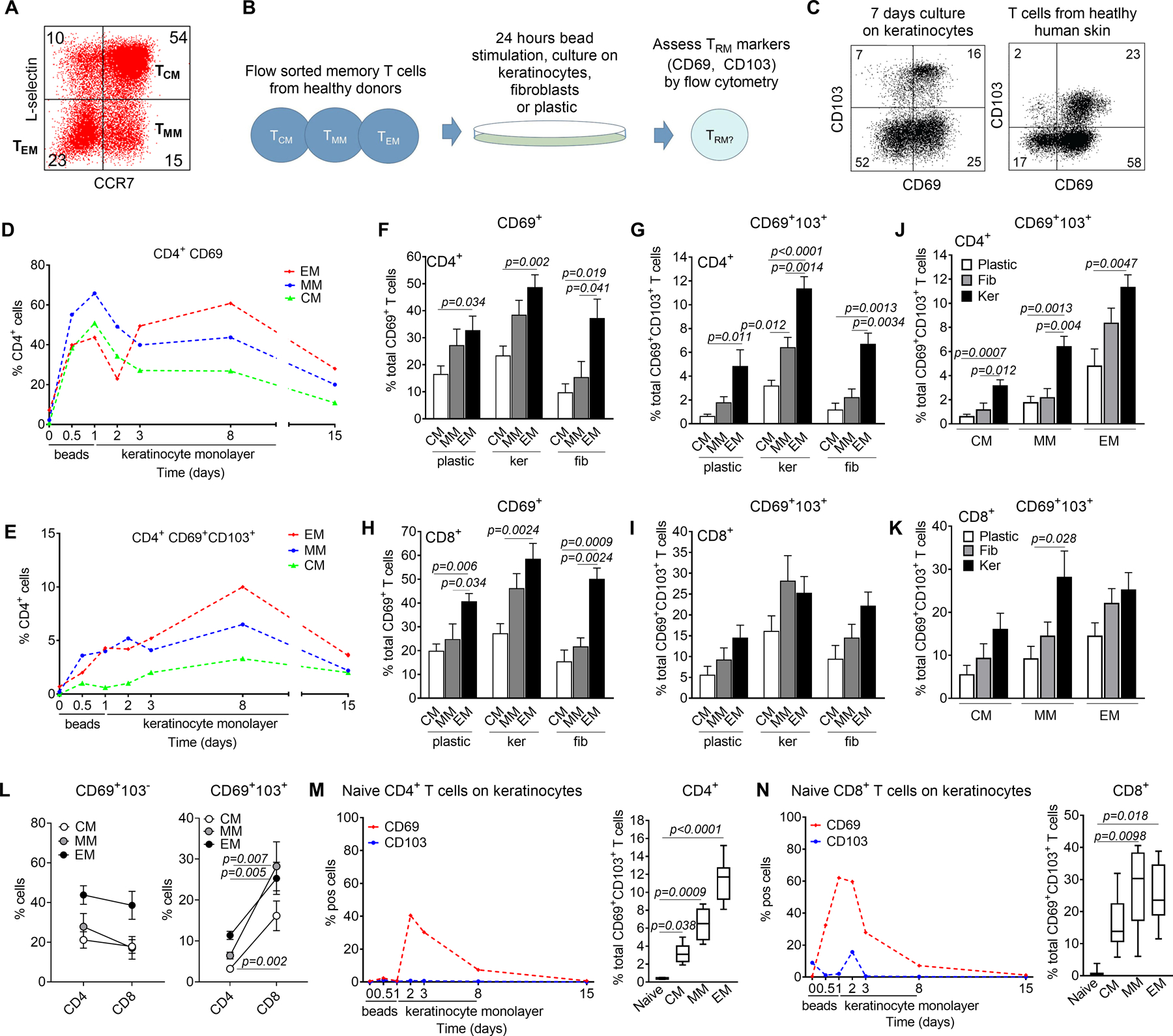
(A) The gating strategy used for sorting of human peripheral blood CD45RA− memory T cells into purified populations of CCR7−L-selectin− effector memory T cells (TEM), CCR7+L-selectin− migratory memory T cells (TMM) and CCR7+L-selectin+ central memory T cells (TCM) is shown. (B) Experimental strategy for the study of TRM generation in vitro. Sorted purified T cell subsets from human blood were stimulated for 24 hours with an anti-CD3/CD2/CD28 beads, cultured on monolayers of keratinocytes, fibroblasts or on tissue culture plastic and analyzed by flow cytometry. (C) Seven days of culture on keratinocyte monolayers generated single positive CD69+CD103− and double positive CD69+CD103+ T cells (left panel), similar in phenotype to those observed in healthy human skin (right panel). (D, E) Time course of CD69 and CD103 acquisition in vitro after culture on keratinocyte monolayers. A representative culture is shown; similar results were obtained in a total of four donors. (F-I) All memory subsets tested gave rise to CD69+ T cells, some of which also upregulated CD103. Results for CD4 (top) and CD8 (bottom) T cells are shown after culture on tissue culture plastic (plastic), keratinocyte monolayers (ker) or fibroblast monolayers (fib). The mean and SEM of at least six donors are shown. (J, K) Culture on keratinocyte monolayers generated increased numbers of CD4 (J) and CD8 (K) T cells expressing TRM markers. The mean and SEM of a minimum of four donors are shown. (L) CD4+ and CD8+ T cells were equally efficient at up regulating CD69 (left panel) but CD8+ T cells were more efficient at generating double positive CD69+CD103+ T cells. The mean and SEM of at least four donors cultured on keratinocyte monolayers are shown. (M,N) Naïve T cells did not upregulate TRM markers in vitro. CD4+ (M) and CD8+ (N) CD45RO−CD45RA+ naïve T cells were bead stimulated and cultured on keratinocyte monolayers then analyzed by flow cytometry. Similar results were obtained in a total of three naïve donors. Naïve T cells produced significantly fewer CD69+ CD103+ T cells (right panels); the mean and SEM of three naïve donors and six memory donors are shown. Significance was determined by a one-way ANOVA and Tukey’s post-hoc test for multiple comparisons (F-K, M,N) or by two-tailed T-tests (L).
We stimulated flow sorted human memory T cell subsets for 24 h with anti-CD2/CD3/CD28 beads, then cultured them on human keratinocytes, fibroblasts or tissue culture plastic and subsequently assayed for the expression of the TRM markers CD69 and CD103 by flow cytometry (Fig. 1B). In humans, CD69 is expressed at high and constant levels by all TRM and CD103 is additionally expressed by a subset of TRM enriched for epithelial localization (2, 6). Culture of CD45RA− memory T cells from peripheral blood on keratinocyte monolayers for seven days generated a population of CD69+ T cells, a subset of which also expressed CD103, similar to the TRM populations observed in human skin (Fig. 1C). When purified populations of CD4+ TCM, TMM and TEM were bead stimulated and added to keratinocyte monolayers, CD69 was transiently upregulated at 24 hours, consistent with its known role as an early activation marker (Fig. 1D). After declining at 48 hours, CD69 was subsequently stably expressed on a subset of T cells, some of which also expressed CD103 (Fig. 1D,E). T cell expression of CD69 and CD103 remained stable for as long as the feeder keratinocyte monolayer was intact. After one week in culture, the keratinocyte monolayer degenerated and the number of CD69 and CD103-expressing T cells subsequently declined (Fig. 1D,E). All three circulating subsets tested gave rise to populations of CD69+ and CD103+ expressing T cells in vitro (Fig. 1F–K). CD4+ and CD8+ TEM memory subpopulations generated the most CD69+ T cells (Fig. 1F,H). CD4+ TEM also gave rise to the largest number of CD69+CD103+ co-expressing T cells (Fig. 1G), whereas CD8 TMM and TEM were equally effective at generating CD69+CD103+ double positive cells (Fig 1I). Culture on keratinocyte monolayers was the most effective stimulus for inducing CD69 and CD103 expression on T cells (Fig. 1J,K). CD4+ and CD8+ T cells gave rise to similar numbers of CD69+ T cells whereas CD8+ T cells generated more CD69 and CD103 double expressing cells (Fig. 1L). In contrast to memory T cell subsets, naïve T cells only transiently upregulated CD69 and did not upregulate CD103 (Fig. 1M,N). Given the short time course, these experiments do not evaluate the ability of effector T cells generated from naïve T cell to develop into TRM,
CXCR6, a molecule necessary for TRM generation, was also upregulated on in vitro CD69-expressing T cells and inclusion of the mTOR inhibitor rapamycin blocked induction of CD69 and CD103 in both CD4+ and CD8+ T cells (Fig. S1). Expression of CCR4 and L-selectin were lost on the majority of T cells that acquired TRM markers (Fig. S2).
TRM generated from distinct circulating T cell subsets differ in their functional characteristics
All three memory circulating T cell subsets tested (TCM, TMM, TEM) gave rise in vitro to T cells that expressed TRM markers. We next used mass cytometry to determine if there were functional differences between TRM generated in vitro from distinct circulating precursor T cell populations. T cells expressing TRM markers were generated in vitro from each memory subset; CD69+ CD103− and CD103+ cells from each subset were then pooled and analyzed by mass cytometry (Fig. 2A). Clustering suggested functional differences existed between TRM generated from distinct precursors (Fig. 2B, individual tSNE plots shown in Fig. S3, antibodies used included in Table S1). In-depth analyses demonstrated that CD4+ CD69+ CD103− T cells generated from TEM expressed significantly more IFNγ, those derived from TMM expressed significantly more IL-17A and those derived from TCM had significantly higher numbers of FOXP3+ T cells (Fig. 2C–E). Similar differences were observed in the CD4+ CD103+ populations, although only FOXP3 findings reached statistical significance. There was also a nonsignificant trend towards higher IL-13 production by TEM -derived CD103+ CD4+ TRM (Fig. 2F).
Figure 2. TRM generated from distinct circulating T cell subsets differ in their functional characteristics.
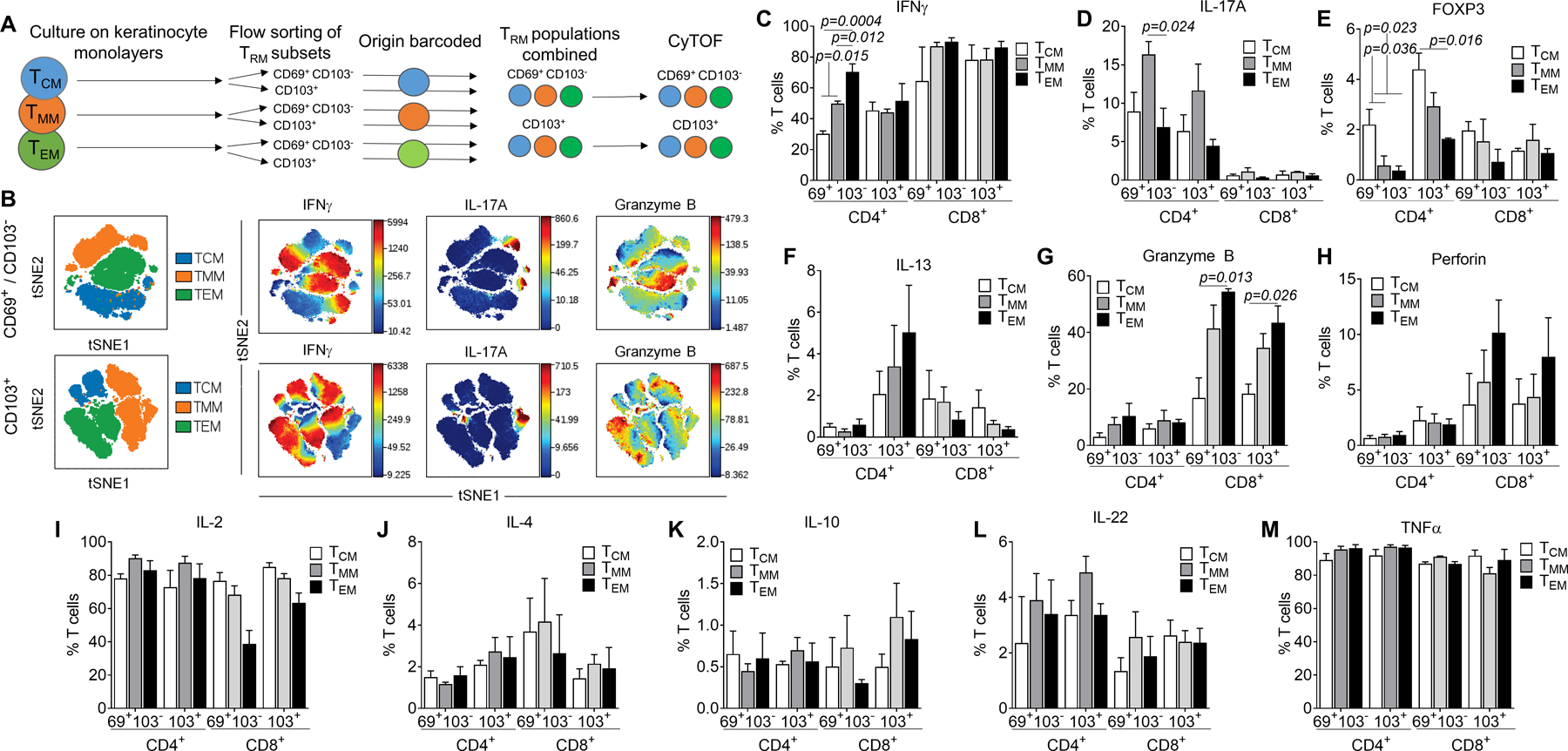
(A) Mass cytometry was used to compare the functional characteristics of TRM generated in vitro from circulating precursor T cell populations. T cells expressing TRM markers were generated in vitro from each memory subset; CD69+ CD103− and CD103+ cells from each subset were pooled and analyzed together by mass cytometry. (B) Clustering suggested functional differences existed between TRM generated from distinct precursors. (C-M) The expression of each cytokine in the CD69+ CD103−− and CD103+ T cell populations for both CD4+ and CD8+ T cells are shown. The mean and SEM of experiments from three separate donors are shown. Individual tSNE plots and a list of antibodies used are included in Fig. S3 and Table SI. Significance was determined by a one-way ANOVA and Tukey’s post-hoc test for multiple comparisons.
Among CD8+ TRM, there was significantly higher granzyme B production and a trend towards higher perforin production by TEM-derived TRM (Fig. 2G,H). There were no significant differences in the production of IL-2, IL-4, IL-10, IL-22 or TNFα (Fig. 2I–M).
Acquisition of TRM markers is accompanied by changes in T cell function
The distinct functional characteristics of TRM derived from different circulating T cell subsets could reflect pre-existing differences between the precursor populations or differences induced by TRM differentiation. We compared expression of each cytokine or polarization marker by the precursor population (input cells) with that of the post culture cells, comparing it to: i) the cells that did not upregulate TRM markers, ii) CD69+ CD103− TRM and iii) CD103+ TRM (Fig. 3). Surprisingly, IL-17A production and FOXP3 expression were significantly increased, and IL-4 production was significantly decreased in CD4+ T cells that acquired TRM markers, compared to the input population and cells that did not upregulate TRM markers (Fig. 3A–C). There was also a trend towards higher IL-22 production in T cells that acquired TRM markers (Fig. 3D). There were no significant differences in the expression of IL-17A, FOXP3, IL-4 or IL-22 among the different input memory cell populations. The observed changes with TRM marker acquisition occurred in all of the precursor cell types but were most significant in TMM (for IL-17A) and TCM (for FOXP3). Interestingly, these cytokine shifts mirrored differences in cytokine production and FOXP3 expression between T cells isolated from blood and healthy human skin T (Fig. 3A–D third panels). CD8+ T cells underwent a marked induction of granzyme B expression with TRM marker acquisition; this change was observed in all precursor populations but was most significant for TEM-derived TRM (Fig. 3E). There was also a trend towards enhanced perforin production with TRM marker acquisition (Fig. 3F). Again, these shifts mirrored the increased levels of granzyme B and perforin expressed by healthy skin T cells as compared to circulating T cells (Fig. 3E,F, third panel). In contrast, there were no significant changes in the production of IL-13, IFNγ, IL-2 or IL-10 with TRM marker acquisition (Fig. 3G–J). The increased IFNγ production we observed in TRM derived from TEM resulted from a higher level of production by TEM precursor cells that was maintained after TRM transition (Fig. 3G).
Figure 3. Acquisition of TRM markers is accompanied by changes in T cell function.
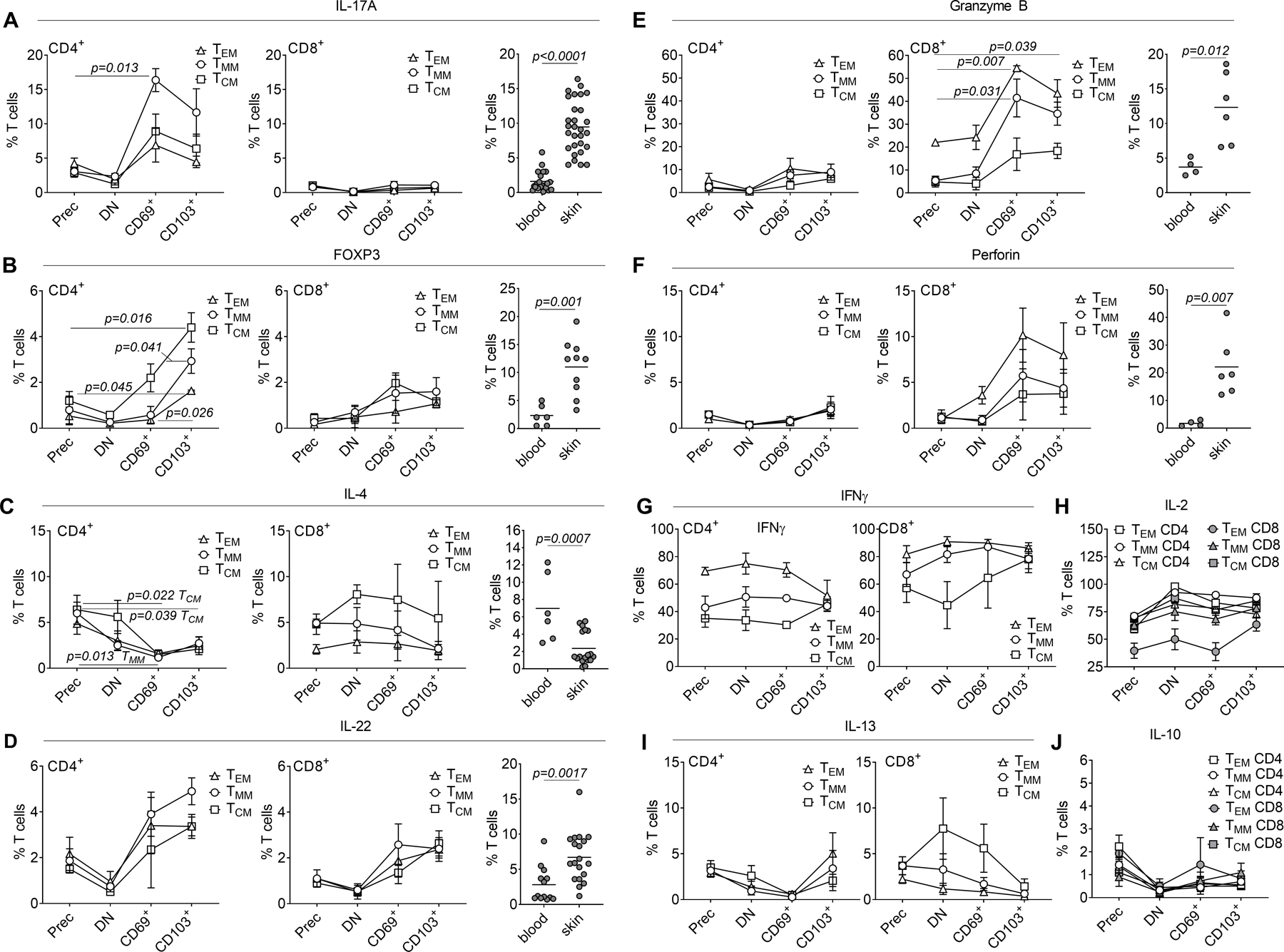
Mass cytometry was utilized to compare production of cytokines and expression of FOXP3 in freshly isolated precursor T cell populations (Prec), vs. double negative (DN) CD69− CD103− T cells, single positive CD69+ CD103− (CD69+) and CD103+ (CD103+) T cells isolated after 1 day bead stimulation and 7 days of culture on keratinocyte monolayers. (A-J) Expression of the indicated cytokines is shown in each population, with comparisons to expression in blood CLA+ skin tropic T cells and T cells from healthy skin (A-F). The mean and SEM of mass cytometry results from three separate donors are shown. Significance was determined by a one-way ANOVA and Tukey’s post-hoc test for multiple comparisons (mass cytometry) or by two-tailed T-tests (flow cytometry studies of blood vs. skin).
Central memory T cells are the most efficient TRM precursors in vivo
To evaluate the ability of human T cell subsets to give rise to TRM in vivo, we grafted NSG mice with human neonatal foreskin, a tissue that lacks T cells (6), and infused them i.v. with allogeneic PMBC or sorted PBMC T cell subsets from a second human donor (Fig. 4A)(6). Human T cells that migrate into the human skin graft proliferate in response to allogeneic antigen-presenting cells, and a subset of these T cells differentiate into T cells expressing CD69 and/or CD103 (6). The two defining characteristics of resident memory T cells are that they persist within peripheral tissues and are nonrecirculating (2). We isolated T cells from the human skin graft of PBMC-injected mice three weeks and six weeks after PBMC infusion and studied the cells by flow cytometry. Both CD4+ and CD8+ CD69+CD103− and CD69+CD103+ T cells were present within the graft at three weeks and persisted at similar numbers six weeks after PBMC infusion, demonstrating persistence within the skin (Fig. 4B). To demonstrate a lack of recirculation, we treated skin-grafted mice intraperitoneally with alemtuzumab, an anti-CD52 antibody that depletes T cells in the blood but spares tissue resident memory T cells (5, 6). Alemtuzumab treated mice retained CD69 and CD103 expressing T cells within the graft but cells lacking CD69, including CCR7+L-selectin+ cells, were depleted (Fig. 4C,D). These data confirm that the cells expressing TRM markers in our model are true TRM. We then utilized skin-grafted mice to determine which circulating precursor populations were the most effective at generating TRM in vivo in human skin. Foreskin-grafted mice were injected intravenously with populations of TCM, TMM and TEM purified by flow sorting and T cells were harvested from the skin graft and studied by flow cytometry at three weeks and six weeks after grafting (Fig. 4E–M). At three weeks after grafting, populations of CD69+CD103− and CD69+CD103+ T cells were present within the graft (Fig. 4E). TEM had the highest percentage of conversion to CD69+ TRM, analogous to our in vitro study finding (Fig. 4F,G). However, there were significantly more T cells in the skin grafts of TCM- and TMM-injected mice, as compared to TEM-injected mice, as measured by quantitative flow cytometry (Fig. 4H) and histologic counts of T cells in immunostained sections (Fig. 4I). Because of this difference in T cell density in skin, 3 and 6 week grafts from TCM-injected mice contained significantly more CD4 and CD8 TRM than mice injected with other precursor populations (Fig. 4J–M).
Figure 4. Central memory T cells are the most efficient TRM precursors in vivo.
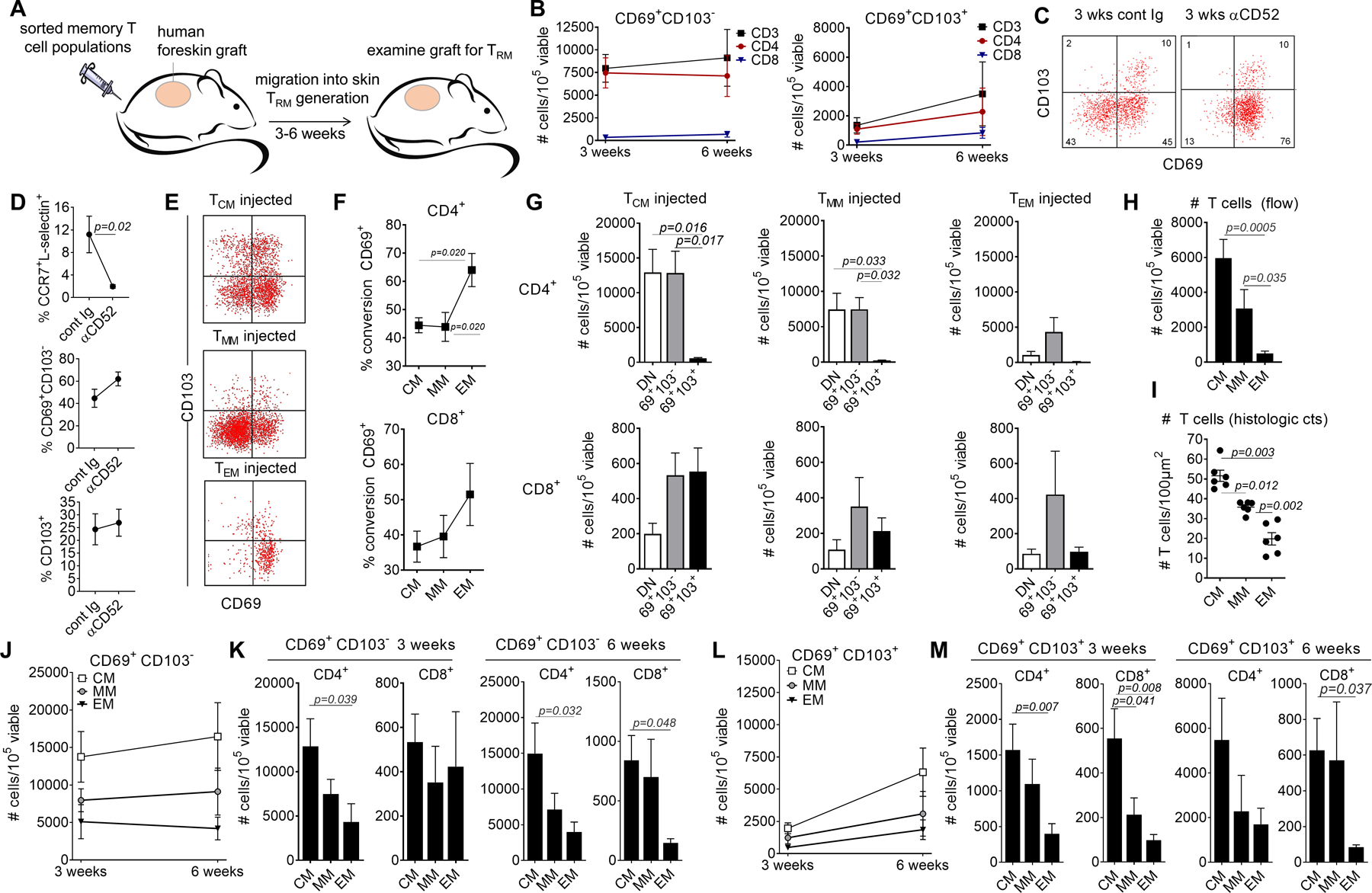
(A) An in vivo model using NSG mice grafted with human foreskin and infused with allogeneic human PBMC was utilized to study TRM formation in vivo in human skin. (B) TRM generated in this model were persistent. T cells were isolated from skin grafts three and six weeks after allogeneic PBMC infusion. The numbers of single positive CD69+ CD103− TRM (left panel) and double positive CD69+ CD103+ TRM (right panel) are shown for total CD3+, CD4+ and CD8+ T cells. The mean and SEM of 8 (3 weeks) and at least 4 (6 weeks) experiments are shown. (C,D) TRM generated in this model do not recirculate. Skin-grafted mice were treated i.p. with alemtuzumab or control Ig. (C) Example histograms from control Ig treated (cont Ig, left panel) and alemtuzumab treated mice (αCD52, right panel) are shown. Similar results were obtained in a total of four experiments, using four different blood donors. (D) The number of recirculating (CCR7+/L-selectin+) and TRM populations in mice treated with control Ig (cont Ig) and alemtuzumab (αCD52) are shown. The mean and SEM of four experiments using four different blood donors are shown. (E-G) All three memory subsets generated TRM in vivo. Foreskin-grafted mice were injected i.v. (1 mouse per precursor population) with flow purified populations of TCM, TMM and TEM and T cells were harvested from the skin graft and studied by flow cytometry after three weeks. A representative histogram is shown (E); similar results were obtained in a total of eight experiments, using eight different blood donor and a total of 24 mice. TEM had the highest percentage of conversion to CD69+ TRM (F,G). Cells were quantified as the number of T cells per 105 total viable cells (of all types) isolated from collagenase digested skin grafts. The mean and SEM of eight experiments are shown, using eight blood donors and 24 mice. (H, I) The total number of T cells in the skin grafts of injected mice are shown, as measured by quantitative flow cytometry (H) and histologic counts of T cells in CD3+ immunostained sections (I). The mean and SEM of 8 (H) and 6 (I) experiments are shown. (J-M) TCM generated the most TRM in vivo. The numbers of CD69+ CD103− (J,K) and CD69+ CD103+ TRM (L,M) among CD4+ (J,K) and CD8+(L,M) T cells are shown. The mean and SEM of experiments from eight donors (3 weeks) and at least four donors (6 weeks) are shown. Significance was determined by a one-way ANOVA and Tukey’s post-hoc test for multiple comparisons.
Diversity and functional characteristics of in vivo generated TRM
Grafts from TCM-injected mice contained dense infiltrates of T cells localized to both the lower parts of the epidermis and the dermis six weeks after T cell infusion (Fig. 5A). We carried out high-throughput TCR sequencing to track T cell clones within the grafts. Individual T cell clones identified in three week skin grafts were also identifiable at six weeks, demonstrating that TCM-injected mice generated a diverse population of persistent TRM in skin (Fig. 5B,C). TCM-injected mice had an increased number of unique T cell clones in skin, although this was not statistically significant (Fig. 5D). T cell clonality, a measure of the diversity of the overall T cell repertoire, was similar between groups, demonstrating that the increased number of TRM in TCM-injected mice was not the result of clonal expansion of a small number of founder TRM (Fig. 5E). We carried out NanoString-based gene expression profiling of the skin grafts to determine if the functional differences identified by our in vitro studies were also present in in vivo generated TRM. T cell normalized expression of TBX21 (T-bet) was significantly higher in TEM-injected mice (Fig. 5F), analogous to the higher production of IFNγ observed in TRM generated from TEM in vitro. FOXP3 expression was highest in skin grafts from TCM-injected mice, and three IL-17 pathway associated genes (IL17A, RORC and IL23R) were most highly expressed in grafts from TMM-injected mice, although only IL23R was statistically significant (Fig. 5G,H). There were no significant differences in expression of IL4 and TNF (Fig. 5I,J). In summary, the functional TH1/TC1 dominance of TEM, TH17/TC17 dominance of TMM and FOXP3/Treg dominance of TCM observed in in vitro generated TRM were detectable to some extent in vivo. Expression of CCR4 and L-selectin were lost on the majority of T cells that acquired TRM markers (Fig. S2).
Figure 5. Diversity and functional characteristics of in vivo generated TRM.
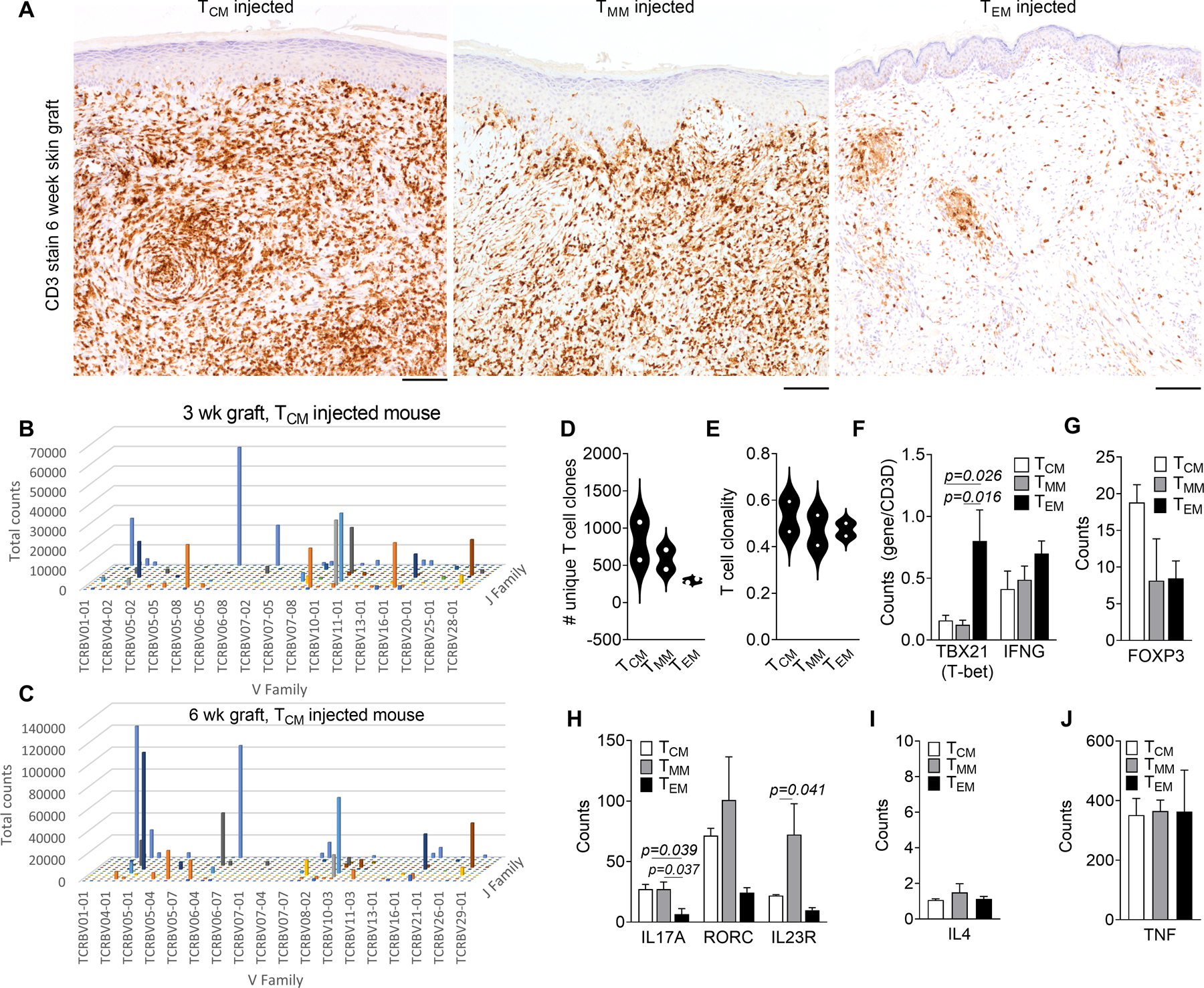
(A) T cell infiltration into skin as assessed by CD3 immunostaining of grafts harvested six weeks after T cell injection is shown. Representative images from a total of eight experiments using eight different blood donors are shown. Scale bars = 100μm. (B,C) TCM-injected mice generated a diverse population of persistent TRM in skin. High-throughput TCR sequencing (HTS) results from 3 week (B) and 6 week (C) post infusion grafts from a single blood donor are shown. T cell clones identified in three weeks skin grafts were also identifiable at six weeks. (D, E) The number of unique T cell clones (D) and T cell clonality (E) in skin 6 weeks after infusion in TCM-injected mice is shown. The mean of experiments from two different blood donors are shown. (F-J) NanoString-based gene expression profiling was utilized to study functional characteristics of TRM generated from each memory subset. Results for type I (F), regulatory T cell (G), type 17 (H), type 2 (I) and TNFα (J) associated genes are shown. The mean and SEM from three experiments using three different blood donors, are shown. Significance was determined by a one-way ANOVA and Tukey’s post-hoc test for multiple comparisons.
TCM are the most efficient TRM precursors because they persist longer in the circulation, are skin tropic and replenish other memory T cell subsets
We analyzed memory T cell precursor populations by mass cytometry to determine if the TEM fraction had characteristics that would make them less likely to enter or survive in skin (Fig. 6A–G). TCM and TMM had significantly higher expression levels of cutaneous lymphocyte antigen (CLA), the homing addressin that mediates rolling on E-selectin expressing cutaneous endothelial cells, a necessary step for entry into skin (7) (Fig. 6A). CCR4, a second homing addressin necessary for entry into skin, was expressed at similar levels in all subsets (Fig. 6B). Expression of CCR5, a chemokine receptor important for migration into affected organs in both mouse and human graft versus host disease (GVHD), was actually significantly higher on TEM than on TCM (8–10). Levels of CXCR3, a receptor associated with migration into inflamed tissues, initial recruitment of T cells into GVHD sites, and required for differentiation into CD103+ TRM in mice were also higher on TEM and TMM than on TCM (11, 12). These findings agree with prior studies showing that TEM express higher levels of CXCR3 and CCR5 than TCM (13–15).
Figure 6. TCM persist longer in the circulation, are skin tropic and replenish other memory T cell subsets.

(A-G) Circulating memory T cell precursor populations were analyzed by mass cytometry for the expression of cutaneous lymphocyte antigen (A), chemokine receptors (B), HLA-DR (C), BCL-2 (D), exhaustion markers PD-1 and Tim-3 (E,F) and Ki-67 (G). The mean and SEM of results from six different blood donors are shown. (H,I) TCM persist longer in the circulation. T cells from the blood and spleen of injected mice three weeks after infusion were analyzed by flow cytometry. The mean and SEM of six experiments using six different blood donors are shown. (I) TCM replenished other T cell subsets. The phenotype of T cells in blood three weeks after T cell infusion is shown. The mean and SEM of experiments using three different blood donors are shown. Significance was determined by a one-way ANOVA and Tukey’s post-hoc test for multiple comparisons.
There was also no significant difference between memory T cell populations in activation status (HLA-DR) or apoptotic marker expression (BCL-2) (Fig. 6C,D). TEM expressed significantly higher levels of the exhaustion marker PD-1 but there were no differences in Tim-3 or in recent proliferation history (Ki-67, Fig. 6E–G). Taken together, these findings demonstrate that TCM are more skin tropic and less exhausted than TEM.
Analyses of the blood and spleen at the end of the three-week experiment demonstrated that TCM and TMM also persisted longer in the periphery than TEM (Fig. 6H). There were 19-fold more human T cells persisting in the circulation of TCM-injected mice three weeks after injection than in TEM-injected mice. Analysis of the phenotype of circulating T cells blood three weeks after infusion demonstrated that TCM were capable of replenishing the circulation with all three memory T cell subsets, TCM, TMM and TEM (Fig. 6I). In contrast, TEM did not replenish other T cell subsets.
Discussion
The circulating precursor cells that give rise to human resident memory T cells (TRM) are poorly characterized in humans and only partially characterized in mice (11, 16, 17). We utilized an in vitro differentiation system and human skin-grafted mice to study TRM generation from circulating human memory T cell subsets.
Our in vitro and in vivo studies demonstrate that all circulating memory T cell subsets tested can differentiate into TRM after entry into the skin. These results suggest that there is not a single unique precursor cell type for TRM. Instead, the TRM differentiation program can be induced in any memory cell that has encountered its cognate antigen and has entered a peripheral tissue such as the skin. However, there were functional biases in TRM derived from different memory T cell subsets; TEM, TMM and TCM were particularly effective at generating IFNγ, IL-17A and FOXP3+ TRM, respectively. We also observed changes in T cells as they underwent TRM differentiation and our results suggest that peripheral tissues may have a role in tuning these responses. IL-17A and granzyme B production and FOXP3 expression were increased, and IL-4 production was decreased in skin-generated TRM in our models; these shifts match the differences observed between circulating and skin T cells from healthy individuals.
Our studies in human skin-grafted mice found that TCM were the most effective precursor cells of skin resident memory T cells in vivo. Although TEM had a higher TRM conversion rate, suggesting, they may represent a state closer to TRM, TCM generated vastly increased numbers of TRM in skin. Circulating TCM were more skin tropic, less exhausted, persisted longer in the circulation and had the ability to differentiate into TCM, TMM and TEM in vivo. Prior studies in mice and macaques have found that TCM persist in the circulation and can replenish other cell types, confirming this characteristic is not unique to humans (5, 18). Earlier in vitro studies have also shown that human TCM can differentiate into other memory T cell types (19).
TCM have been given little historical credit for their ability to protect peripheral tissues. TCM were first characterized as recirculating only through the blood and lymph nodes and as having poor effector functions (4). However, more recent studies have demonstrated that TCM are in fact tropic for multiple peripheral tissues, have impressive effector functions and are found in healthy human peripheral tissues including skin, lung, colon and cervix (20). In healthy human skin, TCM comprise 10–20% of the total T cell population (6). In addition, the protective role of TCM is now being acknowledged in mouse models of infection. CD8+ TCM entered skin and generated functional CD69+CD103+ TRM following vaccinia infection in mice (21). The ability of TCM to seed peripheral tissues with highly protective TRM may explain their known association with long-term immunity to pathogens in both humans and animal models (22).
Most studies of TRM generation in mice have focused on CD8+ T cells, likely because the most common experimental infections used, including vaccinia, HSV and LCMV are viral in nature and tend to generate CD8+ centric immune responses. However, the majority of TRM in healthy human skin are CD4+ (6). To our knowledge, these are the first studies of human CD4+ TRM generation. We find that CD4+ and CD8+ T cells have similar abilities to generate TRM, although CD8+ T cells are more likely to co-express CD103. This is consistent with the role of anti-viral CD8+ TRM, such as those recognizing HSV, to reside in or near the epidermis (23).
In summary, our studies demonstrate that multiple human memory T cell subsets can give rise to TRM and that functional changes accompany TRM differentiation. TCM generate more TRM than other memory T cell subsets because of their ability to persist and populate the skin in large numbers. These studies demonstrate that TCM can provide long lasting immunity in both the circulating and tissue compartments, and that generation of TCM should be a goal of vaccination strategies for infections and cancer.
Materials and Methods:
Study design:
The study was designed to evaluate the ability of human memory T cell subsets to differentiate into TRM. In vitro cultures and injection into NSG mice grafted with human neonatal foreskin was used. Data was analyzed by flow cytometry, mass cytometry, immunohistochemistry, high-throughput TCR sequencing and measurement of cytokine associated genes by NanoString. The studies were unblinded and not randomized.
Human samples:
All studies were performed in accordance with the Declaration of Helsinki. Blood from healthy individuals was obtained after leukapheresis. Neonatal foreskins were obtained from infants undergoing circumcision at the Brigham and Women’s Hospital. All tissues were collected with previous approval from the Institutional Review Board of the Mass General Brigham Human Research Committee (Mass General Brigham Research Management, IRB protocol numbers: 2018P002169 and 2012P000406).
Flow sorting of purified memory T cell subsets from human peripheral blood:
PBMC were stained with antibodies to CD3 (BioLegend, San Diego, CA), CD62L (BD Biosciences, San Jose, CA), CCR7 (R&D Systems, Minneapolis, MN), and CD45RA (BioLegend) then sorted into TCM, TMM and TEM fractions on a FACS Aria cell sorter (BD Biosciences).
In vitro culture on tissue culture plastic, fibroblasts or keratinocytes:
Primary human keratinocytes and fibroblasts were cultured to confluence in 96-well flat-bottomed plates. T cells from the three sorted memory subtypes were stimulated with αCD3/CD2/CD28 T cell activation beads (Miltenyi Biotec, San Diego, CA) at a 1:10 ratio for 24 hr. Stimulated memory T cells from each subtype were added to designated wells of confluent layers of keratinocytes, fibroblasts, or tissue culture wells without keratinocytes or fibroblasts (plastic) in Iscove’s Modified Dulbecco’s Medium supplemented with 10% FBS and L-glutamine. At indicated time points, T cells were collected and immunostained with directly conjugated anti-human monoclonal antibodies from BioLegend and Life Technologies. Acquisition of flow cytometry samples was performed on a FACS Canto instrument and data were analyzed using FACS Diva software V8.0 (BD Biosciences).
mTOR inhibition:
Primary human keratinocytes were cultured to confluence in 96-well flat-bottomed plates. PBMC were stimulated with αCD3/CD2/CD28 T cell activation beads (Miltenyi Biotec, San Diego, CA) at a 1:10 bead:T cell ratio for 24 hours. PBMC were added to wells of keratinocytes in Iscove’s Modified Dulbecco’s Medium supplemented with 10% FBS and L-glutamine (control medium) or in control medium plus rapamycin (Selleck Chemicals, Houston, TX) at indicated concentrations. Some wells cultures were started in control medium and switched to rapamycin containing medium on day 4 or 8. Cultures were fed with control or rapamycin containing medium every other day for 7 or 14 days. At the end of the culture period, T cells were collected and stained with directly conjugated anti-human monoclonal antibodies from BioLegend and Life Technologies.
Human engrafted mouse model:
Human neonatal foreskins were grafted onto the backs of 6- to 8-week old nonobese diabetic /severe combined immunodeficient/IL-2 receptor γ chain null mice (NSG, Jackson Laboratory, Bar Harbor, ME). One week later, 3 × 106 allogeneic PBMCs, TCM, TMM or TEM were injected intravenously. In indicated experiments, alemtuzumab (2.5 μg) or isotype control (human IgG1, BioLegend) was injected i.p. three times per week for 3 weeks. Three weeks after cell injection, blood, spleen and skin grafts were harvested for analysis. All experiments were approved by the Brigham and Women’s Hospital Institutional Animal Care and Use Committee (IACUC protocol # 2016N000591).
Flow cytometric analysis of T cells from blood, spleen, and human skin grafts:
Human skin grafts were removed from mice and T cells were isolated by collagenase digestion as previously described (24). Spleens were manually dissociated. Splenic cell suspensions and blood were subjected to red blood cell lysis (Red Blood Cell Lysing Buffer, Sigma-Aldrich), filtered through a 40-µm cell strainer, then stained with directly conjugated antibodies and analyzed as described above.
Immunohistochemical studies:
Human CD3 was detected in formalin-fixed, paraffin-embedded (FFPE) tissue sections (5 µm) by standard immunohistochemical techniques with anti-human CD3 (rabbit polyclonal, Dako # A0452, Santa Clara, CA) at a 1:250 dilution and 3,3’-diaminobenzidine (DAB) as the substrate.
CyTOF analyses of memory T cells subsets:
Human PBMC were immunostained with rare earth metal-tagged monoclonal antibodies. Available pre-conjugated antibodies were purchased from Fluidigm (San Francisco, CA) and others were conjugated to metal isotopes using the Maxpar antibody conjugation kit (Fluidigm) according to manufacturer’s instructions. Cells were stained as previously described (6) and analyzed on a CyTOF 2 mass cytometer (Fluidigm) at an event rate of approximately 500 cells/second. To normalize CyTOF data over different days, EQ Four Element Calibration Beads (Fluidigm) were added in all samples. Data were analyzed with Cytobank software (Beckman Coulter, Indianapolis, IN). To remove debris and doublets, single cells were gated based on cell length and DNA content as described by Bendall et al. (25). High dimensional single-cell data produced by mass cytometry were interpreted using the Cytobank viSNE package, allowing visualization of high-dimensional cytometry data on a 2-dimensional map at single-cell resolution preserving the nonlinearity (26). The antibodies used for mass cytometry studies are included in Table S1 and tSNE plots for each marker are included in Figure S3.
High-throughput TCR sequencing:
DNA was extracted from human skin grafts using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA) as per manufacturer’s instructions with overnight tissue digestion. TCRβ CDR3 regions were amplified and sequenced by ImmunoSEQ (Adaptive Biotechnologies, Seattle, WA) from 300 ng DNA template as previously described (27).
Statistics:
Information on the statistical tests used for each set of experiments is included in the figure legends. For comparison of two groups, two-tailed T-tests were used. For comparison of more than two groups, significance was determined by a one-way ANOVA and Tukey’s post-hoc test for multiple comparisons. In all comparisons, α= 0.05.
Supplementary Material
Acknowledgments.
The authors thank the Lubin Family Foundation for providing funds to support purchase of the Mantra Imaging microscope. Mass cytometry studies were carried out at the Longwood Medical Area CyTOF Mass Cytometry Core using antibodies obtained from the Brigham and Women’s CyTOF Antibody Resource and Core.
Funding.
This work was supported by R01 AR063962 NIH/NIAMS (to R.A.C.), R01 AR056720 NIH/NIAMS (to R.A.C.), P30 AR069625 NIH/NIAMS (to R.A.C.), R01 AI097128 NIAID (to T.S.K./R.A.C.), R01 CA203721 (to R.A.C./T.S.K.), T32 AR-07098–36 (to T.S.K., supplied salary for J.T.O.), AstraZeneca Foundation/Faculty of Medicine of the University of Lisbon Research Grant (to T.R.M.), NNF15OC0014092 Novo Nordisk Foundation (B.D-A), and R182–2014-3641 Lundbeck Foundation (B.D-A), the Austrian Science Fund FWF (W1241) and the Medical University of Graz through the Ph.D. Program DK- MOLIN (supplied salary for T.B.), the Austrian Marshall Plan Foundation (to T.B.) and the Austrian Society for Dermatology and Venereology (Klaus Wolff fellowship to T.B.).
Footnotes
List of Supplementary Materials:
Figure S1. In vitro generated TRM express CXCR6 and TRM generation is inhibited by rapamycin
Figure S2. CCR7 and L-selectin expression decreased after acquisition of TRM markers in vitro and in vivo
Figure S3. Individual tSNE plots for data summarized in Fig. 2B
Table S1. Antibodies used in mass cytometry experiments described in Fig. 2
Data file S1. Raw data file (Excel spreadsheet)
MDAR Reproducibility Checklist
Competing interests. JTO is currently a full-time employee at Sanofi and holds company stock and/or stock options; there is no relevant conflict of interest with the work presented here. The other authors also report no competing interests.
Data and materials availability:
All data are included in the manuscript or the Supplementary Materials.
References and Notes:
- 1.Park CO, Kupper TS, The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nature medicine 21, 688–697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark RA, Resident memory T cells in human health and disease. Sci Transl Med 7, 269rv261 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller SN, Mackay LK, Tissue-resident memory T cells: local specialists in immune defence. Nature reviews Immunology 16, 79–89 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Geginat J, Lanzavecchia A, Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annual review of immunology 22, 745–763 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Clark RA et al. , Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Science Translational Medicine 4, 117ra117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe R et al. , Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Science Translational Medicine 7, 279ra239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong BF, Murphy J-E, Kupper TS, Fuhlbrigge RC, E-Selectin, Thymus- and Activation-Regulated Chemokine/CCL17, and Intercellular Adhesion Molecule-1 Are Constitutively Coexpressed in Dermal Microvessels: A Foundation for a Cutaneous Immunosurveillance System. J Immunol 172, 1575–1581 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Reshef R et al. , Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. The New England journal of medicine 367, 135–145 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.C. A. Wysocki et al. , Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J Immunol 173, 845–854 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Murai M et al. , Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nature immunology 4, 154–160 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Mackay LK et al. , The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nature immunology 14, 1294–1301 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Groom JR, Luster AD, CXCR3 in T cell function. Experimental cell research 317, 620–631 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strazza M, Mor A, Consider the chemokines: a review of the interplay between chemokines and T cell subset function. Discovery medicine 24, 31–39 (2017). [PMC free article] [PubMed] [Google Scholar]
- 14.Griffith JW, Sokol CL, Luster AD, Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annual review of immunology 32, 659–702 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Mackay CR, CXCR3(+)CCR5(+) T cells and autoimmune diseases: guilty as charged? The Journal of clinical investigation 124, 3682–3684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheridan BS et al. , Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity 40, 747–757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henson SM et al. , KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood 113, 6619–6628 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Wherry EJ et al. , Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature immunology 4, 225–234 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Geginat J, Lanzavecchia A, Sallusto F, Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 101, 4260–4266 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Gehad A et al. , A primary role for human central memory cells in tissue immunosurveillance. Blood Adv 2, 292–298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborn JF et al. , Central memory CD8+ T cells become CD69+ tissue-residents during viral skin infection independent of CD62L-mediated lymph node surveillance. PLoS Pathog 15, e1007633 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaph C, Uzonna J, Beverley SM, Scott P, Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nature medicine 10, 1104–1110 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Zhu J et al. , Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature 497, 494–497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlapbach C et al. , Human TH9 Cells Are Skin-Tropic and Have Autocrine and Paracrine Proinflammatory Capacity. Science Translational Medicine 6, 219ra218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bendall SC et al. , Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amir el AD et al. , viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nature biotechnology 31, 545–552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirsch IR et al. , TCR sequencing facilitates diagnosis and identifies mature T cells as the cell of origin in CTCL. Sci Transl Med 7, 308ra158 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript or the Supplementary Materials.


