Abstract
Study Objectives:
Numerous types of mandibular advancement devices (MADs) are available to treat patients with obstructive sleep apnea, varying from noncustom to custom devices. Only a limited number of studies have been performed to determine whether a noncustom MAD could be used to predict treatment success of a custom MAD. In this study, we investigated the potential of a new-generation noncustom MAD, by comparing its effectiveness with a custom MAD. We hypothesized that the effectiveness of the devices is similar with regard to both objective (polysomnography) and self-reported (questionnaires, adherence, and patient satisfaction) outcomes.
Methods:
This was a single-center prospective randomized crossover study including a consecutive series of patients with obstructive sleep apnea. Patients were randomized to start either with the noncustom or custom MAD. Both MADs were applied for 12 weeks, followed by polysomnography with MAD in situ and questionnaires. After the first 12 weeks of follow-up, a washout period of 1 week was applied. Equal effectiveness was defined as no significant differences in both objective and self-reported outcomes between both devices.
Results:
Fifty-eight patients were included; 40 completed the full follow-up. The median apnea-hypopnea index significantly decreased from 16.3 (7.7, 24.8) events/h to 10.7 (5.6, 16.6) events/h with the custom MAD (P = .010) and to 7.8 (2.9, 16.1) events/h with the noncustom MAD (P < .001). Self-reported outcomes significantly improved in both groups. No significant differences were found between both devices.
Conclusions:
The effectiveness of a noncustom and custom MAD is comparable, which suggests that a noncustom MAD can be used as a selection tool for MAD treatment eligibility to improve MAD treatment outcome.
Clinical Trial Registration:
Registry: Netherlands Trial Register; Name: The Use of a Boil and Bite Mandibular Advancement Device vs a Custom Mandibular Advancement Device in Obstructive Sleep Apnea Management; URL: https://www.trialregister.nl/trial/7249; Identifier: NL64738.100.18.
Citation:
Bosschieter PFN, Uniken Venema JAM, Vonk PE, et al. Equal effect of a noncustom vs a custom mandibular advancement device in treatment of obstructive sleep apnea. J Clin Sleep Med. 2022;18(9):2155–2165.
Keywords: obstructive sleep apnea, sleep-disordered breathing, mandibular advancement device, treatment success, drug-induced sleep endoscopy
BRIEF SUMMARY
Current Knowledge/Study Rationale: The designs of mandibular advancement devices (MADs) have improved over the years. This study investigated the effectiveness of a new generation of noncustom MADs compared with that of custom MADs in the treatment of patients with obstructive sleep apnea.
Study Impact: The effectiveness of a noncustom and custom MAD with regard to objective (polysomnography) and self-reported outcomes is comparable, which suggests that a noncustom MAD can be used as a selection tool for MAD treatment eligibility to improve MAD treatment outcome.
INTRODUCTION
A mandibular advancement device (MAD) is one of the treatment options for patients with obstructive sleep apnea (OSA),1,2 especially in patients with mild to moderate OSA. Other OSA treatments include continuous positive airway pressure, upper-airway surgery, upper-airway stimulation, maxillomandibular advancement surgery, and positional therapy.3 MADs are designed to advance the mandible, preventing upper-airway collapse. Advancement of the tongue base, epiglottis, and soft palate improves upper-airway patency.4 Depending on OSA severity and the criteria used to define treatment success, the efficacy of MADs ranges from 40% to 92%.5
Numerous types of MADs are available, varying from noncustom (thermoplastic “boil-and-bite” or new-generation devices) to custom MADs, each consisting of a single part (monobloc) or 2 separate parts (bibloc).4,6
Some studies have been performed to determine whether a noncustom (“boil-and-bite”) MAD could be used to predict the treatment success of a subsequently produced custom MAD.7,8 Vanderveken et al8 compared the efficacy of a noncustom thermoplastic monobloc MAD (hereafter, noncustom MAD) with that of a custom MAD. They concluded that a custom MAD was more effective than a noncustom MAD and, in addition, that the effect of a noncustom MAD does not predict the effect of the subsequently produced custom MAD.8 In 69% of the patients, treatment failed (no treatment success or not compliant) with the noncustom MAD compared with 40% with the custom MAD.8 In addition, the poor adherence to the noncustom MAD mainly resulted from poor retention of the device, suggesting the need for a noncustom device with better retention.
The oral device industry has improved MAD designs significantly over the last few decades. Historically, most thermoplastic “boil-and-bite” devices are monoblocs, which are set to a predetermined, arbitrary position. A new generation of noncustom thermoplastic MADs are titratable, utilizing trays for both the upper and lower dentition, joined by a mechanism that moves the mandible forward in relationship to the maxilla. All oral appliances are classified as custom and noncustom based on whether an impression is used to construct the trays. This new generation of noncustom MADs is more comfortable and has better retentive features. They can be fitted chairside within 15 minutes, so therapy can be initiated immediately. In addition, noncustom MADs are potentially cheaper than a custom MAD. These features allow the patients to experience the benefits and possible disadvantages of MAD therapy before a more expensive custom MAD is applied, providing an efficient and cost-effective way of screening MAD treatment eligibility.
So far, the effectiveness of this new generation of noncustom MADs has not been compared with custom MADs. Bearing this in mind, we tested the potential of a noncustom (new-generation) MAD to its antecedent device that is of similar design mechanically but that differs in terms of the material. We hypothesized that the devices are similar with regard to objective and self-reported outcomes in treating patients with OSA and therefore a noncustom MAD might be used as a screening tool for MAD treatment eligibility.
METHODS
Study participants
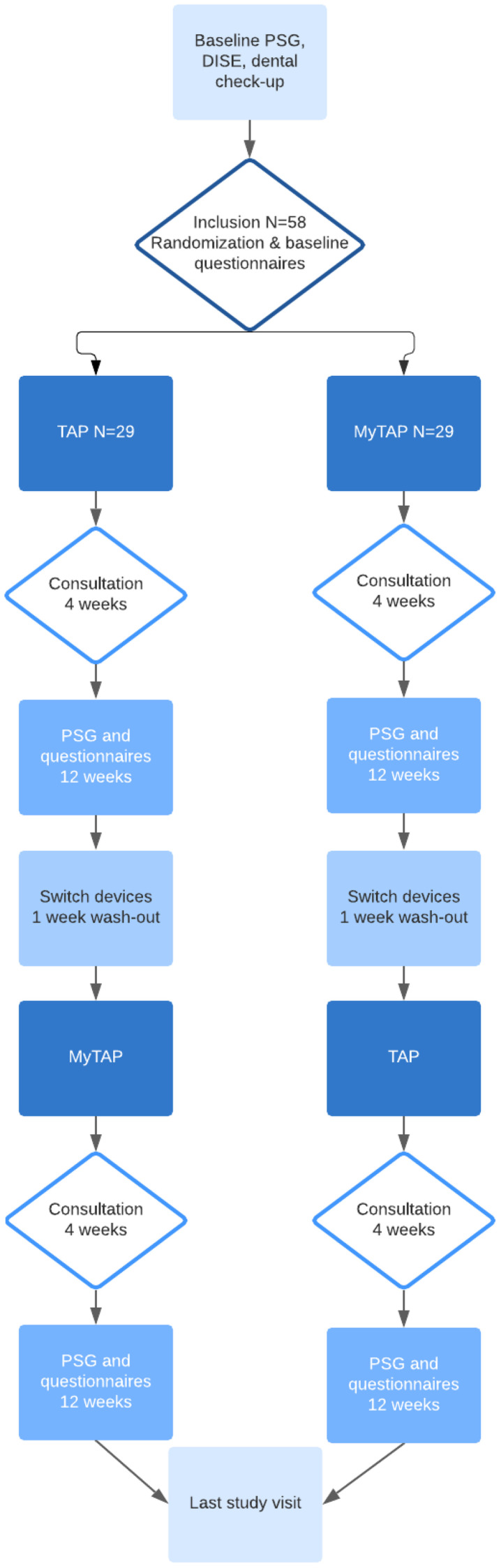
We performed a single-center, prospective, randomized crossover study with a follow-up of 12 weeks in each allocation group in a consecutive series of patients with OSA. Patients were diagnosed through an overnight polysomnography (PSG). Patients were included if they were 18 years or older and had an apnea-hypopnea index (AHI) ≥5 events/h. Written informed consent was obtained for all patients prior to inclusion. Patients were excluded if they had reversible morphological upper-airway abnormalities (eg, enlarged tonsils), clear failure or nonacceptance of previous MAD therapy, central sleep apnea syndrome (> 50% of central apneas confirmed by PSG), (extensive) periodontal disease or tooth decay (confirmed by orthopantomogram X-ray), active temporomandibular joint disease (including severe bruxism), restrictions in maximal mouth opening (< 25 mm) or in protrusion of the mandible (< 5 mm), or partial or complete edentulous (< 8 teeth in the upper or lower jaw). Patients were subsequently randomized to start with either the noncustom MAD or the custom MAD. We used a validated variable block randomization model, which was constructed in such a way that randomized inclusions are divided across groups (without stratification) in variable block sizes (4, 6, 8) to ensure true randomness during the allocation. Allocation was automatically reported in the online case report forms prior to informing the clinical staff. During all baseline measurements, MAD allocation was not yet determined. Thereafter, both the participants and the practitioners were informed about which MAD was applied. Scoring of the PSGs by the sleep technicians was performed blinded. Both MADs were applied during a follow-up of 12 weeks with a consultation after 4 weeks. After 12 weeks of follow-up, treatment effect was measured by PSG and self-reported outcomes were obtained. Therapy was discontinued during a washout period of 1 week. Subsequently, patients used the noncustom or custom MAD for 12 weeks, depending on randomization, with the same clinical analyses and self-reported outcomes after 12 weeks (Figure 1).
Figure 1. Study procedures flow diagram.
DISE = drug-induced sleep endoscopy, MAD = mandibular advancement device, MyTAP = My Thornton Adjustable Positioner, noncustom MAD, PSG = polysomnography, TAP = Thornton Adjustable Positioner type 1, custom MAD.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (medical ethical committee) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the national medical ethical committee and by the Board of Directors (Institutional Review Board) of OLVG hospital (WO18.168).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Outcome measures
Primary outcome measures were respiratory PSG outcomes, including total AHI, apnea index (AI), supine AHI, nonsupine AHI, percentage of total sleep time (TST) in a supine position, oxygen desaturation index (ODI; 3%), mean saturation, and lowest saturation.
Secondary outcome measures included self-reported outcomes using 3 different questionnaires: the Epworth Sleepiness Scale (ESS),9 the Functional Outcomes of Sleep Questionnaire,10 and the Research and Development-36 Survey (RAND-36).11 Data on adherence were based on self-reported therapy usage. Patient satisfaction and complaints were evaluated by collecting side-effects mentioned during follow-up visits. Secondary outcome measures were collected at baseline and after 12 weeks of follow-up for both devices.
Both primary and secondary outcome measures were analyzed comparing baseline and results after 12 weeks of follow-up for both devices and comparing the results between the noncustom and custom MADs.
Definitions
We defined equal effectiveness of the custom and noncustom MAD as no significant differences between the 2 devices regarding the primary outcome variables, which were the PSG outcomes.
Complete MAD treatment success was achieved when the posttreatment AHI was < 5 events/h. Partial success was defined as a reduction in the AHI of more than 50% and an AHI > 5 events/h. Patients were considered nonresponders when not meeting the criteria for complete or partial treatment success.
Thornton Adjustable Positioner (TAP) type 1
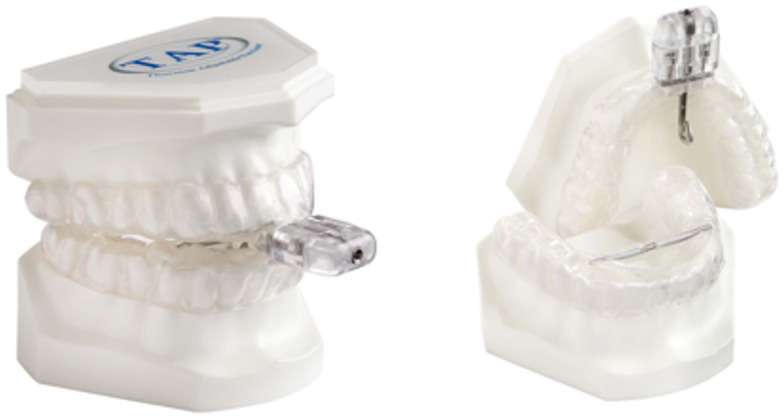
As a custom MAD we used the TAP1 (Airway Management Inc, Dallas, TX, USA), consisting of 2 separate trays covering the upper and lower dental arches (Figure 2). Custom MAD fabrication requires either a digital or physical impression and a cast or milled technology. Thin, resilient trays are made from an impression, which has 2 main features: retention and protrusion. Retention prevents the tray from dislodging from the teeth and protrusion by a mechanism moves the mandible forward in increments of 1 mm or less. The protrusive mechanism used in custom TAP appliances is single-point midline traction. The TAP moves the mandible in a forward position by a screw mechanism incorporated in the frontal area of the appliance and has a protrusive range of over 20 mm. The TAP1 has an AccuTherm (Airway Management Inc, Dallas, TX, USA) thermoplastic lining to achieve maximum retention and comfort.
Figure 2. TAP custom mandibular advancement device.
TAP = Thornton Adjustable Positioner type 1 (Airway Management Inc, Dallas, TX, USA).
My Thornton Adjustable Positioner (myTAP)
As a new generation noncustom MAD we used the myTAP (Airway Management Inc, Dallas, TX, USA), which utilizes all of the features of a custom oral appliance (Figure 3). This novel thermoplastic MAD consists of 2 separate trays of hard-plastic framework overmolded with ThermAcryl (Airway Management Inc, Dallas, TX, USA) material, which fully covers the upper and lower dental arches. This combination of materials provides accurate molding capabilities, using a thin, durable tray system that can be reheated and remolded as many times as necessary to achieve maximum retention and comfort. The thermoplastic material virtually takes an impression of the teeth, similar to a custom thermoplastic impression material. Upon cooling, the material retains its shape and is as resilient as a custom appliance. The protrusive mechanism used in myTAP is single-point midline traction. The myTAP moves the mandible forward by a single screw (covered in plastic) with a protrusive range of over 20 mm. Titration, both protrusive and vertical (up to 12 mm with adjustment stops), can be easily adjusted by the clinician or patient. After fitting both trays chairside, the patients can immediately start wearing the device.
Figure 3. myTAP noncustom mandibular advancement device.
myTAP = My Thornton Adjustable Positioner (Airway Management Inc., Dallas, TX, USA).
Titration of the devices
Before initiation of the trial, maximum voluntary mandibular protrusion was determined. The maximum voluntary mandibular protrusion was achieved by twisting the screw in the frontal area of the myTAP until patients started to experience pain or discomfort in their teeth, jaw, or muscles. From that point, the device was set to retrude the mandible for 1 mm. When initiating, both the custom and noncustom MAD were positioned at 50% of the maximum protrusion, as determined by a George Gauge bite registration.12 Patients were instructed to advance the devices during the first 4 weeks of follow-up until the maximum voluntary protrusion was reached. This procedure was the same for both devices. In both study arms, at the first follow-up visit (4 weeks), the amount of protrusion was determined. At the 3-month follow-up visit, it was verified whether the amount of protrusion had changed since the 4-week follow-up visit.
Polysomnography
A standard PSG (Somnoscreen; SOMNOmedics GmbH, Randersacker, Germany) was performed in all patients. To determine the stages of sleep, an electroencephalogram (F3, F4, C3, C4, M1, M2, O1, O2), electro-oculogram, and electromyogram of the submental muscle were obtained. Nasal airflow was measured by a nasal cannula/pressure transducer inserted in the opening of the nostrils. An oronasal thermal flow sensor was used to determine the difference between the temperature of exhaled and ambient air to estimate airflow and detect mouth breathing. Arterial blood oxyhemoglobin was recorded with the use of a finger pulse oximeter. Thoracoabdominal excursions were measured qualitatively by respiratory effort belts placed over the ribcage and abdomen. Body position was determined by a position sensor, which differentiates between the upright, left side, right side, prone, and supine position. Limb movements were detected with an anterior tibial electromyogram with surface electrodes. Electrocardiography was performed to score cardiac events and a snore sensor was applied for occurrence of snoring.
Statistical analysis
Statistical analysis was performed using SPSS (version 26; SPSS Inc, Chicago, IL, USA). Quantitative data are reported as mean and standard deviation (SD) or as median and (quartile 1, quartile 3) when not normally distributed. To determine whether continuous variables were normally distributed, the Shapiro-Wilk test was used. A P value of < .05 was considered to indicate statistical significance. Primary outcomes, adherence, and ESS are reported with both per-protocol and intention-to-treat analysis. For the latter, missing follow-up data were replaced by baseline data. In the assessment of therapy effect, the follow-up AHI values were used as the primary outcome measure.
To compare the outcomes of the baseline PSG and follow-up PSG (both custom and noncustom) a paired t test was used in the case of normally distributed data and the Wilcoxon signed-rank test in the case of non–normally distributed data. The same analysis was used for the evaluation of differences in PSG outcomes comparing the 2 devices. To evaluate differences in self-reported outcomes between baseline and follow-up visit at 12 weeks (both devices), the Wilcoxon signed-rank test was used. The Wilcoxon signed-rank test was also used for the evaluation of the self-reported outcomes and treatment outcomes between the 2 devices. A paired-samples t test was used to analyze differences in titration of the devices. To investigate the role of potential predictors for treatment success, such as age, body mass index (BMI), AHI, and adherence, a Mann-Whitney U test was performed comparing responders and nonresponders for each device.
Sample size
Sample size calculation was performed with the statistical program G*Power-3.1 Heinrich Heine Universiteit, Dusseldorf. The sample size estimation was based on the primary outcome measure, the AHI. For the calculation, we used results of the study performed by Vanderveken et al.8 Based on an estimated AHI reduction of 9 events/h with an SD of 9 events/h with the custom MAD and 5 events ± 8 events/h with the noncustom MAD an alpha of 0.05 and power of 0.85, 43 patients were required per treatment group. It was expected that 25% of patients would drop out.8 Therefore, the estimated number for this randomized controlled trial was 58 patients in a total.
RESULTS
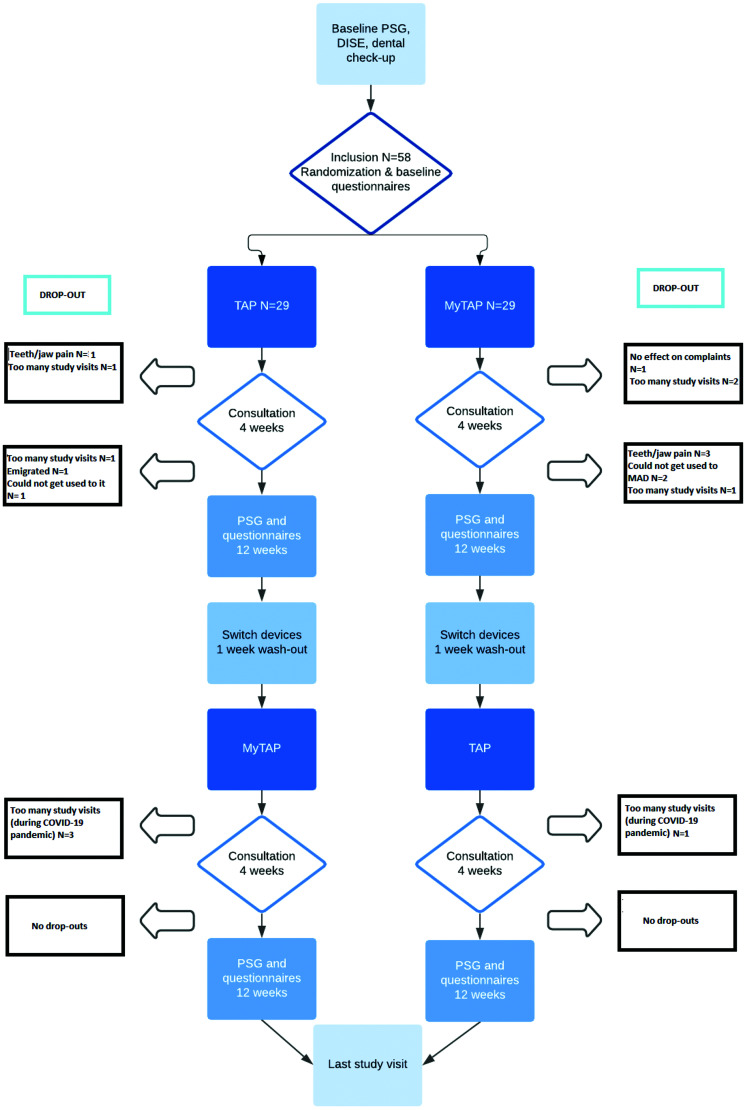
In total, 58 patients were included, of whom 18 did not complete follow-up. Figure 4 shows the specific time points and reasons for dropping out. The majority of the patients (n = 58) were male (n = 45, 78%), the median age was 51 (38, 56) years, and the median BMI was 26.2 (24.2, 29.2) kg/m2. The median AHI at baseline was 16.2 (7.4, 21.9) events/h (Table 1). The maximum voluntary protrusion at baseline was 70.9 ± 33.7% of maximum protrusion. At the 4-week follow-up, the percentage of maximal protrusion for the custom MAD and noncustom MAD was 71.1 ± 27.9% and 97.4 ± 43.4%, respectively. The amount of protrusion did not significantly differ between baseline and follow-up values or between the 2 MADs.
Figure 4. Dropouts.
COVID-19 = coronavirus disease 2019, DISE = drug-induced sleep endoscopy, MAD = mandibular advancement device, MyTAP = My Thornton Adjustable Positioner, noncustom MAD, PSG = polysomnography, TAP = Thornton Adjustable Positioner, custom MAD.
Table 1.
Patient characteristics.
| Total | |
|---|---|
| Number of patients (n) | 58 |
| Sex (male:female) (n) | 45:13 |
| Age (years) | 51 [38, 56] |
| BMI (kg/m2) | 26.2 [24.2, 29.2] |
| Pretreatment AHI (events/h) | 16.2 [7.4, 21.9] |
| Set protrusion (% of maximum protrusion) | 70.9 ± 33.7 |
Data are presented as mean ± standard deviation or median [Q1, Q3]. AHI = apnea-hypopnea index, BMI = body mass index, Q = quartile.
Primary outcome measures
Comparison of a custom MAD and a noncustom MAD regarding respiratory PSG outcomes
The median AHI at baseline of the 40 patients who completed follow-up was 16.3 (7.7, 24.8) events/h (Table 2). The AHI was significantly reduced to 10.7 (5.6, 16.6) events/h with the custom MAD (P = .010) and to 7.8 (2.9, 16.1) events/h with the noncustom MAD (P < .001). The small difference between both devices in follow-up AHI was not statistically significant (P = .346). The median ODI 3% at baseline was 17.1 (8.4, 28.1) events/h. The ODI was significantly reduced to 9.6 (4.6, 15.5) events/h with the custom MAD (P = .011) and to 6.9 (2.4, 13.3) events/h with the noncustom MAD (P = .003). In addition, the AI and the AHI in the supine and nonsupine position significantly decreased using both devices, without significant differences between both. The percentage of TST in the supine position did not significantly change. The mean lowest measured saturation at baseline was 85.5% (82.0, 90.0%), which increased to 88% with both devices; this improvement was not significant. Intention-to-treat analysis of the data showed significant improvement in the AHI, AI, supine AHI, ODI, and mean saturation for both devices, without significant differences between the 2 devices (Table 3). Complete treatment success with the custom MAD was achieved in 20% (Table 2). With the noncustom MAD, this outcome was 35%, demonstrating a significant difference (P < .001). With the custom MAD, the AHI did not decrease sufficiently in 60% of the patients. With the noncustom MAD, the AHI was significantly (P = .003) less (ie, 47.5%). Additional analysis showed no impact of potential predictors (age, BMI, baseline AHI, and adherence) on treatment outcome. No significant carry-over and period effects were found.
Table 2.
PSG outcomes, custom vs noncustom MAD (per-protocol analysis).
| P Value | ||||||
|---|---|---|---|---|---|---|
| PSG Baseline | TAP PSG 12 Weeks | myTAP PSG 12 Weeks | Baseline vs TAP | Baseline vs myTAP | TAP vs myTAP | |
| Total AHI (events/h) | 16.3 [7.7, 24.8] | 10.7 [5.6, 16.6] | 7.8 [2.9, 16.1] | .010* | < .001* | .346 |
| Total AI (events/h) | 5.2 [2.2, 11.2] | 1.9 [0.4, 4.4] | 1.2 [0.5, 5.7] | .010* | .002* | .821 |
| Supine AHI (events/h) | 25.2 [11.0, 39.8] | 14.3 [7.3, 27.7] | 10.7 [3.9, 24.2] | .004* | < .001* | .179 |
| Nonsupine AHI (events/h) | 6.6 [2.3, 15.8] | 5.4 [1.2, 9.2] | 4.4 [1.4, 10.8] | .245 | .197 | .896 |
| % of TST in supine position | 38.9 [24.5, 51.2] | 42.4 [21.6, 61.3] | 43.6 [27.5, 53.7] | .308 | .398 | .676 |
| ODI (3%) (events/h) | 17.1 [8.4, 28.1] | 9.6 [4.6, 15.5] | 6.9 [2.4, 13.3] | .011* | .003* | .637 |
| Mean saturation (%) | 95.0 [94.0, 96.0] | 95.0 [93.0, 96.0] | 95.0 [93.3, 96.0] | .105 | .030* | .652 |
| Lowest saturation (%) | 85.5 [82.0, 90.0] | 88.0 [82.0, 91.0] | 88.0 [84.3, 90.8] | .367 | .192 | .908 |
| Treatment outcome, n (%) | ||||||
| Complete success | NA | 8 (20%) | 14 (35%) | NA | NA | .001* |
| Partial success | NA | 8 (20%) | 7 (17.5%) | NA | NA | .030* |
| Nonresponder | NA | 24 (60%) | 19 (47.5%) | NA | NA | .003* |
Data are presented as median [Q1, Q3] unless otherwise indicated. n = 40. *P < .05. AHI = apnea-hypopnea index, AI = apnea index, MAD = mandibular advancement device, myTAP = My Thornton Adjustable Positioner, noncustom MAD, NA = not applicable, ODI = oxygen desaturation index, PSG = polysomnography, Q = quartile, TAP = Thornton Adjustable Positioner custom MAD, TST = total sleep time.
Table 3.
PSG outcomes, custom vs noncustom MAD (intention-to-treat analysis).
| P Value | ||||||
|---|---|---|---|---|---|---|
| PSG Baseline | TAP PSG 12 Weeks | myTAP PSG 12 Weeks | Baseline vs TAP | Baseline vs myTAP | TAP vs myTAP | |
| Total AHI (events/h) | 16.2 [7.4, 20.9] | 11.0 [5.7, 16.8] | 10.6 [4.9, 16.9] | .002* | .001* | .950 |
| Total AI (events/h) | 4.7 [2.8, 11.5] | 2.5 [0.6, 6.1] | 2.5 [0.6, 7.9] | .003* | .002* | .302 |
| Supine AHI (events/h) | 25.2 [10.6, 40.4] | 14.3 [7.8, 31.8] | 13.3 [6.8, 34.4] | .002* | < .001* | .476 |
| Nonsupine AHI (events/h) | 6.3 [2.4, 14.5] | 5.3 [1.6, 9.5] | 4.4 [1.8, 10.6] | .123 | .162 | .638 |
| % of TST in supine position | 39.3 [24.1, 59.4] | 42.3 [20.1, 65.7] | 41.3 [27.1, 54.7] | .372 | .401 | .614 |
| ODI (3%, events/h) | 16.4 [10.3, 26.6] | 10.9 [4.9, 16.0] | 10.6 [3.8, 16.6] | < .001* | < .001* | .669 |
| Mean saturation (%) | 95.0 [94.0, 96.0] | 95.0 [94.0, 96.0] | 95.0 [94.0, 96.0] | .013* | .002* | .557 |
| Lowest saturation (%) | 87.0 [82.0, 90.0] | 88.0 [82.0, 90.5] | 88.0 [85.0, 90.0] | .740 | .461 | .951 |
Data are presented as mean ± standard deviation or median [Q1, Q3]. n = 58. *P < .05. AHI = apnea-hypopnea index, AI = apnea index, MAD = mandibular advancement device, myTAP = My Thornton Adjustable Positioner, noncustom MAD, ODI = oxygen desaturation index, PSG = polysomnography, Q = quartile, TAP = Thornton Adjustable Positioner custom MAD, TST = total sleep time.
Secondary outcome measures
Adherence
The mean self-reported therapy usage was 7 hours per night, 7 nights per week, which equals 49 hours per week for both devices without significant differences between the 2 MADs (Table 4 and Table 5).
Table 4.
Self-reported outcomes (questionnaires), custom vs noncustom MAD (per-protocol analysis).
| P Value | ||||||
|---|---|---|---|---|---|---|
| Baseline (n = 36) | TAP 12 Weeks (n = 39) | myTAP 12 Weeks (n = 39) | Baseline vs TAP | Baseline vs myTAP | TAP vs myTAP | |
| Adherence (h/wk) | NA | 49 | 49 | NA | NA | .519 |
| ESS | 5 [0, 20] | 5.0 [0, 17] | 5 [0, 16] | .005* | .005* | .890 |
| FOSQ | ||||||
| General productivity | 2.6 [1.1, 3.0] | 2.9 [0.9, 3.0] | 2.9 [1.0, 3.0] | .035* | .001* | .038* |
| Social outcome | 4.0 [1.0, 4.0] | 4.0 [0.0, 4.0] | 4.0 [0.0, 4.0] | .088 | .058 | .204 |
| Activity level | 3.4 [1.3, 4.0] | 3.8 [0.3, 4.0] | 3.7 [0.3, 4.0] | .016* | .006* | .977 |
| Vigilance | 3.5 [1.1, 4] | 3.7 [0.6, 4.0] | 3.7 [0.6, 4.0] | .101 | .044* | .831 |
| Intimate relationships and sexual activity | 4.0 [0.0, 4.0] | 4.0 [0.0, 4.0] | 4.0 [0.3, 4.0] | .097 | .198 | .335 |
| RAND-36 | ||||||
| Physical functioning | 100 [45.0, 100] | 100 [40.0, 100] | 100 [40, 100] | .301 | .482 | .208 |
| Social functioning | 100 [12.5, 100] | 88.0 [0.0, 100] | 88.0 [37.5, 100] | .190 | .183 | .742 |
| Role limitations due to physical health | 100 [0.0, 100] | 100 [0.0, 100] | 100 [0.0, 100] | .080 | .061 | .566 |
| Role limitations due to emotional problems | 100 [0.0, 100] | 100 [0.0, 100] | 100 [0.0, 100] | .592 | .784 | .502 |
| Emotional well-being | 78.0 [28.0, 100] | 76.0 [44.0, 96.0] | 76.0 [28.0, 100] | .437 | .779 | .992 |
| Energy, fatigue | 60.0 [20.0, 100] | 65.0 [8.00, 100] | 65.0 [30.0, 100] | .104 | .156 | .531 |
| General health | 77.0 [40.0, 92.0] | 72.0 [35, 97] | 72.0 [30.0, 100] | .619 | .569 | .634 |
| Change in health | 50 [0.0, 100] | 50 [25, 100] | 50.0 [25.0, 100] | .013* | .045* | .868 |
| Pain | 100 [10.0, 100] | 100 [41, 100] | 100 [30.0, 100] | .022* | .256 | .133 |
Data are presented as median [Q1, Q3] unless otherwise indicated. Four patients did not complete the questionnaires at baseline. *P < .05. ESS = Epworth Sleepiness Scale, FOSQ = Functional Outcomes of Sleep Questionnaire, MAD = mandibular advancement device, myTAP = My Thornton Adjustable Positioner noncustom MAD, NA = not applicable, Q = quartile, RAND-36 = Research and Development-36 Survey, TAP = Thornton Adjustable Positioner, custom MAD.
Table 5.
Self-reported outcomes, custom vs noncustom MAD (intention-to-treat analysis).
| P Value | ||||||
|---|---|---|---|---|---|---|
| Baseline | TAP 12 Weeks | myTAP 12 Weeks | Baseline vs TAP | Baseline vs myTAP | TAP vs myTAP | |
| Adherence (h/wk) (n = 58) | NA | 49 | 49 | NA | NA | .776 |
| ESS (n = 49) | 6 [2, 11] | 5 [2, 8] | 5 [2, 8] | .017 | .005* | .893 |
Data are presented as median [Q1, Q3] unless otherwise indicated. *P < .05. ESS = Epworth Sleepiness Scale, MAD = mandibular advancement device, myTAP = My Thornton Adjustable Positioner, noncustom MAD, NA = not applicable, Q = quartile, TAP = Thornton Adjustable Positioner, custom MAD.
Questionnaires
Daytime sleepiness using the ESS was significantly reduced with both devices (P = .005), without significant differences between the 2 groups (P = .890). Self-reported outcomes from questionnaires are displayed in Table 4. As part of the Functional Outcomes of Sleep Questionnaire, activity and general productivity level significantly improved with both devices. However, the latter significantly differed between the 2 devices. General productivity increased significantly more with the noncustom MAD than with the custom MAD (P = .038). Vigilance increased with both devices. This increase was only significant with the noncustom MAD (P = .044). With regard to the RAND-36 questionnaire, no significant differences between the 2 MADs were found.
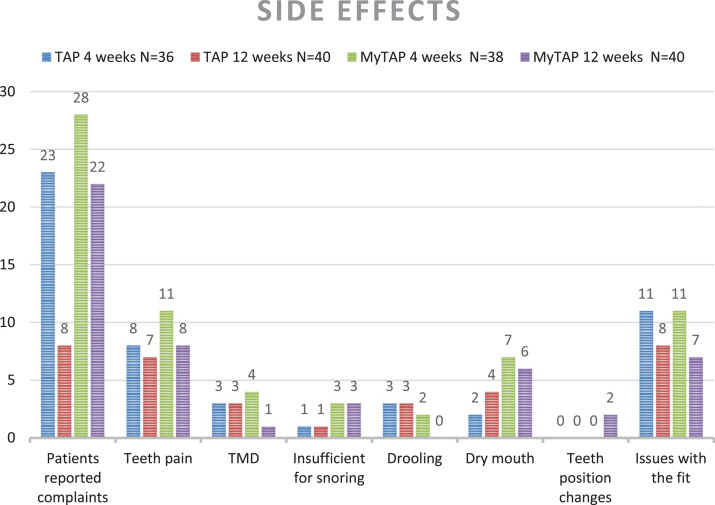
Side effects
After 4 and 12 weeks of follow-up, patients were interviewed with respect to side effects while wearing the devices (Figure 5). Overall, more complaints were reported for the noncustom device; n = 28 patients had complaints after 4 weeks and n = 22 after 12 weeks of follow-up. For the custom MAD, n = 23 patients had complaints at 4 weeks and n = 8 after 12 weeks of follow-up. The most reported issue concerned the fit of the device, which was described as uncomfortable, painful, too tight, or too loose. Two patients reported changes in teeth position at 12 weeks wearing the custom MAD; for the noncustom MAD, this phenomenon was not reported. The majority of complaints decreased over time; at 12 weeks’ follow-up, most reported complaints were less than at the consultation after 1 month. Only complaints of a dry mouth increased over time, for both devices. At the end of the study, n = 19 patients preferred the custom MAD, n = 16 patients preferred the noncustom MAD, and n = 5 patients had no preference; this difference was not significant.
Figure 5. Reported side effects, custom vs noncustom MAD.
“Patients reported complaints” is the total number of patients who had at least one of the complaints as described in the figure. MAD = mandibular advancement device, MyTAP = My Thornton Adjustable Positioner, noncustom MAD, TAP = Thornton Adjustable Positioner, custom MAD, TMD = temporomandibular disorders.
DISCUSSION
This prospective, randomized, controlled crossover trial evaluated the effectiveness of a noncustom (eg, myTAP) and custom MAD (eg, TAP) and the potential of a noncustom MAD in providing an efficient and cost-effective way of screening MAD treatment eligibility for patients with OSA. Intuitively, one would expect that the more precise the design (ie, custom), the better the results. This does not apply to the devices evaluated in the present study. Our findings suggest that the effect of a noncustom MAD is comparable to that of a custom MAD, in terms of respiratory PSG outcomes and self-reported outcomes. AHI, AI, and ODI significantly improved for both devices, with no significant differences between the 2 MADs. In addition, the ESS, general productivity, activity level, and changes in health significantly improved with both devices. General productivity, as measured by Functional Outcomes of Sleep Questionnaire, did significantly differ between the 2 subgroups. Since the mean outcome was the same for both subgroups and significantly improved, we do not regard this as a clinically important difference.
To prevent a reporting bias, given the relatively high drop-out rate, an intention-to-treat analysis was performed. The outcomes at 3 months’ follow-up were slightly less favorable, but the AHI, AI, and ODI significantly improved for both devices, with no significant differences between them.
The fact that we did not find significant differences between the effectiveness of both devices suggests the possibility of using the myTAP as a temporary MAD in screening patients with OSA for treatment eligibility for a custom MAD. Advantages of using a noncustom device prior to a custom one include that it is cheaper, directly ready for use, and patients can experience potential benefits and complaints of (sleeping with) the device before making a custom MAD, which is overall more expensive.
The results of this study are not in line with the previously published studies of Johal et al7 and Vanderveken et al.8 Their results showed that a custom MAD was more efficacious than a noncustom MAD. This might be explained by the fact that different noncustom devices were used than in our study.13,14 The study by Vanderveken et al8 and of Johal et al7 were performed with a noncustom thermoplastic monobloc MAD, for which the amount of protrusion was not adjustable. In the study by Vanderveken et al8 the protrusion of the custom MAD was set at 65%, against 50% for the noncustom MAD. Therefore, the treatment effect of the custom MAD might have been stronger. Johal et al7 also reported a significant difference in the amount of protrusion in favor of the custom MAD. These differences in amount of protrusion and differences in patient comfort and retention (and therefore adherence) could have caused a better outcome of the custom MAD found in these studies. The oral device industry has improved MAD designs significantly by adding the possibility for titration, which could result in more comparable effectiveness using either a noncustom or custom MAD. In our study, the titration and therefore amount of protrusion of the noncustom and the custom MAD was set to the same amount of protrusion. In both study arms the amount of protrusion did not significantly differ from maximum voluntary protrusion at baseline, which means the devices were optimally titrated. In addition, the amount of protrusion during PSG did not significantly differ between the 2 MADs. Therefore, in our study, this variable could not affect outcome differences between the devices.
Overall, treatment success was not as high as expected for both devices. A potential explanation for this outcome could derive from the included population. We also included 8 patients with an AHI > 30 events/h and 5 patients with a BMI > 30 kg/m2. For such patients, standard of care is continuous positive airway pressure therapy. If continuous positive airway pressure is not tolerated, other treatment options, such as an MAD, are considered. These inclusions might have contributed to less favorable MAD treatment outcomes, since BMI > 32 kg/m2 and baseline AHI > 30 events/h negatively influence treatment outcome.15–17 To determine the influence of these inclusions, we performed additional analysis. We did not find a relation between these parameters and treatment outcome. This could be explained by the small sample size of patients with a high BMI or high baseline AHI. A higher baseline AHI and BMI are often related to a larger degree of mandibular advancement but also induce a greater degree of short-term muscle and dental discomfort.14,18,19 This could explain the lower adherence to the noncustom MAD compared with the custom MAD in the study of Johal et al.7
Another interesting finding is that adherence in our study was relatively high, which, in the end, can lead to better therapeutic efficacy. During the past 15 years, thermoplastic MADs have also been improved regarding comfort and retention. In the study of Vanderveken et al8 adherence to the thermoplastic MAD was lower than to the custom MAD, which had a negative influence on treatment outcome of the noncustom device. In our study, however, the adherence to both MADs was similar and therefore had no influence on treatment outcomes.
A recent systematic review by Uniken Venema et al5 compared several types of MADs. They found that a custom MAD is more comfortable, yields better objective and self-reported treatment outcomes, and is associated with a more favorable adherence when compared with a thermoplastic MAD. However, the quality of thermoplastic devices can vary. In their systematic review, different thermoplastic devices were evaluated, with none of them being the myTAP. In our study, patients had also more complaints wearing the noncustom MAD than the custom MAD: 6 patients ended their participation prematurely because of complaints with the device compared with two dropouts wearing the custom MAD. Interestingly, these complaints did not influence adherence, which was similar for both conditions. In contrast to the results of this systematic review, treatment success was even higher with the noncustom MAD in our study, while objective and self-reported outcomes were similar.
Limitations
This study has some limitations. Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, the outcomes of the questionnaires are less reliable. At baseline, the pandemic was not yet present, but at 12 weeks at least half of the patients completed the questionnaires in the middle of the pandemic. This obviously had an impact on their daytime routine, social activities, stress level, and maybe health. Therefore, the pandemic probably influenced the outcomes of the quality-of-life and sleep questionnaires. In addition, all nonurgent consultations were converted into telephone consultations. This has influenced our titration protocol. We aimed to have a consultation at 4 and 12 weeks after starting therapy. The consultation at 12 weeks included a dental check-up, completion of questionnaires, evaluating (side) effects of the MAD, and checking the amount of protrusion of the device before the final PSG. Due to the reduction in consultations, we postponed this consultation until after the PSG to be able to directly discuss the PSG results and provide the second MAD. This protocol deviation influenced the titration process. Instead of checking the amount of protrusion prior to the PSG, we checked the used setting afterwards by asking the patient if changes were made and by checking the set protrusion on the used MAD. Since this was done after performing the PSG, we are not certain about the exact amount of protrusion in 10% of the PSGs with the noncustom MAD and in 15% of the PSGs with the custom MAD. Having fewer consultations to titrate patients could explain the low treatment success rates for both MADs, since we know that titration is crucial for a positive MAD treatment outcome. Our drop-out rate was 31%, which is quite high; 50% of the dropouts were related to the number of study visits. This could partially be explained by the fact that, during the pandemic, patients were less willing to come to the hospital for nonurgent care. Two comparable studies of Johal et al7 and Vanderveken et al8 reported similar drop-out rates of 29% and 34%, respectively. Due to the high drop-out rate, the sample size for this trial was too small to accurately declare equivalence for all patients at randomization, especially for those who have stopped using the MAD for whatever reason.
Clinical relevance and future perspectives
The clinical consequences of this study can be substantial. The durability of a custom MAD is at least 5 years, which is in line with the guarantee period of TAP. For this type of noncustom MAD (the myTAP), the durability is 1 year and the guarantee period is 90 days. This means that patients have 1 year to explore if an MAD works for their complaints (objectively and by self-report) and if they tolerate wearing the device. When satisfied with the noncustom MAD, the custom MAD can be provided. If not satisfied or when patients are not compliant, other therapy options can be considered. While prices of MADs differ greatly per country, in general, custom MADs are more expensive than thermoplastic designs. With an overall success rate of approximately 65%, 1 out of 3 of these expensive MADs will not be effective or tolerated, representing a considerable waste of time and money, since these products cannot be returned or used by other patients.
CONCLUSIONS
In the present study, the effectiveness of a noncustom MAD was similar to that of a custom MAD in the treatment of patients with mild to severe OSA, The PSG outcomes did not differ significantly between the 2 devices, nor did the self-reported outcomes. These outcomes open the avenue to the possibility of using a noncustom MAD as a selection tool for MAD treatment eligibility to improve MAD treatment outcome. An advantage of using a noncustom MAD prior to a custom MAD is that therapy can be initiated directly. This allows the patient to experience the benefits and possible disadvantages of MAD therapy before a more expensive custom MAD is applied, thus providing an efficient and potentially cost-effective way of screening MAD treatment eligibility.
ACKNOWLEDGMENTS
The authors thank Anja van de Velde and Karlijn Beers for their help in preparing the study materials, organizing the inclusion of patients, and their study consultations. Availability of data and material: The data that support the finding of this study are available upon reasonable request.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AI
apnea index
- BMI
body mass index
- ESS
Epworth Sleepiness Scale
- MAD
mandibular advancement device
- MyTAP
my Thornton Adjustable Positioner
- NA
not applicable
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- Q
quartile
- TAP
Thornton Adjustable Positioner
- TST
total sleep time
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was supported by Airway Management Inc. The funding was distributed by the Academic Center Dentistry Amsterdam (ACTA) as an independent party over several cost items. Dr. A. Hoekema is medical advisor for Airway Management Inc, SomnoMed, and Zephyr Sleep Technologies. Prof. Dr. F. Lobbezoo receives research grants from Sunstar Suisse SA, SomnoMed, and Vivisol-ResMed and is an unpaid member of the academic advisory boards for GrindCare and for Oral Function (Sunstar Suisse SA). Prof. Dr. N. de Vries is a member of the Medical Advisory Board of NightBalance and consultant for Philips Healthcare, Inspire Medical Systems, and Nyxoah. The other authors report no conflicts of interest.
REFERENCES
- 1. Ramar K , Dort LC , Katz SG , et al . Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015 . J Clin Sleep Med. 2015. ; 11 ( 7 ): 773 – 827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rotenberg BW , Vicini C , Pang EB , Pang KP . Reconsidering first-line treatment for obstructive sleep apnea: a systematic review of the literature . J Otolaryngol Head Neck Surg. 2016. ; 45 : 23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Epstein LJ , Kristo D , Strollo PJ Jr , et al. ; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine . Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults . J Clin Sleep Med. 2009. ; 5 ( 3 ): 263 – 276 . [PMC free article] [PubMed] [Google Scholar]
- 4. Dieltjens M , Vanderveken O . Oral appliances in obstructive sleep apnea . Healthcare (Basel). 2019. ; 7 ( 4 ): 141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uniken Venema JAM , Rosenmöller BRAM , de Vries N , et al . Mandibular advancement device design: a systematic review on outcomes in obstructive sleep apnea treatment . Sleep Med Rev. 2021. ; 60 : 101557 . [DOI] [PubMed] [Google Scholar]
- 6. Almeida FR , Lowe AA . Principles of oral appliance therapy for the management of snoring and sleep disordered breathing . Oral Maxillofac Surg Clin North Am. 2009. ; 21 ( 4 ): 413 – 420 . [DOI] [PubMed] [Google Scholar]
- 7. Johal A , Haria P , Manek S , Joury E , Riha R . Ready-made versus custom-made mandibular repositioning devices in sleep apnea: a randomized clinical trial . J Clin Sleep Med. 2017. ; 13 ( 2 ): 175 – 182 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vanderveken OM , Devolder A , Marklund M , et al . Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea . Am J Respir Crit Care Med. 2008. ; 178 ( 2 ): 197 – 202 . [DOI] [PubMed] [Google Scholar]
- 9. Johns MW . A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 10. Weaver TE , Laizner AM , Evans LK , et al . An instrument to measure functional status outcomes for disorders of excessive sleepiness . Sleep. 1997. ; 20 ( 10 ): 835 – 843 . [PubMed] [Google Scholar]
- 11. Hays RD , Sherbourne CD , Mazel RM . The RAND 36-Item Health Survey 1.0 . Health Econ. 1993. ; 2 ( 3 ): 217 – 227 . [DOI] [PubMed] [Google Scholar]
- 12. George PT . A new instrument for functional appliance bite registration . J Clin Orthod. 1992. ; 26 ( 11 ): 721 – 723 . [PubMed] [Google Scholar]
- 13. Quinnell TG , Bennett M , Jordan J , et al . A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO) . Thorax. 2014. ; 69 ( 10 ): 938 – 945 . [DOI] [PubMed] [Google Scholar]
- 14. Johnston CD , Gleadhill IC , Cinnamond MJ , Gabbey J , Burden DJ . Mandibular advancement appliances and obstructive sleep apnoea: a randomized clinical trial . Eur J Orthod. 2002. ; 24 ( 3 ): 251 – 262 . [DOI] [PubMed] [Google Scholar]
- 15. Petri N , Christensen IJ , Svanholt P , Sonnesen L , Wildschiødtz G , Berg S . Mandibular advancement device therapy for obstructive sleep apnea: a prospective study on predictors of treatment success . Sleep Med. 2019. ; 54 : 187 – 194 . [DOI] [PubMed] [Google Scholar]
- 16. Marklund M , Stenlund H , Franklin KA . Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success . Chest. 2004. ; 125 ( 4 ): 1270 – 1278 . [DOI] [PubMed] [Google Scholar]
- 17. Cunha TCA , Guimarães TM , Schultz TCB , et al . Predictors of success for mandibular repositioning appliance in obstructive sleep apnea syndrome . Braz Oral Res. 2017. ; 31 : e37 . [DOI] [PubMed] [Google Scholar]
- 18. Dieltjens M , Braem MJ , Vroegop AVMT , et al . Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing . Chest. 2013. ; 144 ( 5 ): 1495 – 1502 . [DOI] [PubMed] [Google Scholar]
- 19. Gao X , Otsuka R , Ono T , Honda E , Sasaki T , Kuroda T . Effect of titrated mandibular advancement and jaw opening on the upper airway in nonapneic men: a magnetic resonance imaging and cephalometric study . Am J Orthod Dentofacial Orthop. 2004. ; 125 ( 2 ): 191 – 199 . [DOI] [PubMed] [Google Scholar]