Abstract
Study Objectives:
As sleep latency is an important factor in the diagnosis of many disorders, it is important to know whether the patient’s self-reported evaluation of sleep latency corresponds with an objectively measured evaluation. Some studies indicate that patients usually overestimate their sleep latency. We sought to determine how comorbidities affect the patient’s ability to assess their sleep latency.
Methods:
This was a retrospective study of 240 patients who had a polysomnography recorded at our sleep unit or at home in 2017–2020. Data on comorbidities were collected from hospital records.
Results:
Mean objective sleep latency (29.5 minutes, standard deviation [SD] 35.5) was significantly lower than self-reported sleep (37.4 minutes, SD 41.6) (P < .001). The patients who overestimated their sleep latency had higher mean apnea-hypopnea index (18.8 events/h, SD 21.6, vs 13.4 events/h, SD 12.8; P = .04) and higher mean sleep efficiency (81.7%, SD 13.6%, vs 75.2%, SD 13.9%; P = .004) than those who underestimated their sleep latency. There were significantly more patients with migraine in the overestimation group than in the underestimation group (20/159 patients vs 3/81 patients; P = .035). This difference was not observed in patients with headache without migraine (P = 1.000).
Conclusions:
We hypothesize that migraine is markedly associated with overestimation of sleep latency. This overestimation was not observed in patients with other headache types. Further studies are needed to explore the relation between migraine and sleep onset misperception.
Citation:
Rantanen O, Hollmen M, Bachour A. Migraine may disturb sleep perception during sleep onset: a retrospective data analysis. J Clin Sleep Med. 2022;18(9):2113–2117.
Keywords: sleep latency, polysomnography, migraine, obstructive sleep apnea, sleeping disorders, sleep study
BRIEF SUMMARY
Current Knowledge/Study Rationale: Some studies have reported differences between objective and self-reported sleep latency, but no rational explanation for this difference was reported related to comorbidity. We reviewed overnight polysomnography studies and collected data on comorbidities.
Study Impact: Our study confirmed previously reported results; patients tend to overestimate their sleep latency. Patients with migraine (as opposed to other headache types) were generally unable to estimate their actual sleep latency time, raising the question on whether migraine has a pathophysiological pathway unrelated to pain that affects sleep-onset misperception.
INTRODUCTION
The prevalence of the sleeping disorders is increasing, mostly due to overweight and obesity.1 Polysomnography (PSG) is the gold-standard method for diagnosing obstructive sleep apnea (OSA)2 and can be performed either at home or in a sleep laboratory.
Sleep latency (SL) is defined as the time from the beginning of the measurement to the first epoch of any stage of sleep.3 Many factors, such as age, total sleep time (TST), and medical conditions (ie, narcolepsy, depression) affect the length of SL.2,4,5 PSG measures SL objectively. The patient’s self-reported estimation on SL and TST is often inaccurate, particularly for patients with OSA, patients with excessive daytime sleepiness, patients with insomnia, and patients with psychosomatic disorders. These patients tend to overestimate their SL.6–11 The reason why some patients are unable to accurately estimate their SL is not completely understood.
SL is important in the diagnosis of many disorders. Hence, it is imperative to know whether self-reported SL corresponds to objective SL and whether comorbidity has a role in sleep-onset misperception. Two studies (Castillo et al6 and Bianchi et al11) reported that patients with predominantly insomnia symptoms and mild-to-moderate sleep apnea tend to overestimate their SL,6 and continuous positive airway pressure treatment corrected this overestimation.11 These studies did not report data on comorbidity or other risk factors for sleep-onset misperception.
The purpose of this study was to evaluate self-reported SL in relation to PSG results and comorbidities and how comorbidities affect under- or overestimation of SL.
METHODS
Study design and patients
This retrospective study was conducted at the Sleep Unit, Department of Pulmonology. Our sleep unit is considered a tertiary referral center for sleep apnea and serves as a part of the Helsinki University Hospital. The study population consists of adult patients undergoing diagnostic or treatment PSG during 2017–2020. The main indication for PSG was sleep apnea. Insomnia alone was not an indication for PSG in our sleep laboratory. The study protocol was approved by the Helsinki University Hospital Ethics Committee on January 13, 2020 (code HUS/144/2020, number §3).
The sleep study was performed with PSG Nox A1 (Nox Medical Global, Reykjavik, Iceland). PSGs were recorded either at home or in our sleep laboratory. The PSG is recorded at the sleep laboratory when it is expected that the patient may need help with the PSG, the patient had a previous unsuccessful sleep study at home, a transcutaneous CO2 recording was indicated, or when videorecording of the PSG was necessary.
SL was calculated from the time of lights off to the first episode of sleep. The morning after the PSG, patients were asked to report self-reported SL and to assess whether they fell asleep faster, slower, or as usual.
The following demographic data were collected: age, sex, body mass index (BMI), and comorbidities. We grouped the comorbidities as thyroid diseases, psychiatric disorders (including depression, panic disorder, other psychiatric disease), headaches (migraine or other headache type), neurological disorders (including stroke, brain injury, memory impairment, epilepsy), metabolic/cardiac disorders (diabetes, high blood pressure, coronary disease, heart failure, arrhythmia), and respiratory diseases (asthma, allergy, allergic rhinitis, sleep apnea, chronic sinusitis).
We gathered the following variables from the PSG report: apnea-hypopnea index (AHI), objective SL, TST, and sleep efficiency (SE). Patients without information on SL or complete PSG data were excluded.
The PSG recording started at the sleep laboratory when the patients said they were going to sleep and the lights were turned off. At home, patients started the PSG when they were going to sleep and the lights were turned off.
None of the patients had acute symptoms or disease during the sleep study (eg, infection or migraine attack). Patients with OSA did not have treatment for OSA at the time of the sleep study.
The difference between objective SL and self-reported SL was assessed. Patients were divided into the following 2 groups: those who overestimated their SL (self-reported SL > objective SL) and those who underestimated it (objective SL > self-reported SL).
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics version 25 (SPSS, Inc, Chicago, IL). All data are expressed either as means with standard deviation (SD) or as absolute numbers with percentages. All analyzed variables were tested for distribution. The t test and analysis of variance were used for variables with normal distribution and Mann-Whitney U test for parameters with skewed distribution. The correlations were analyzed using the Pearson’s correlation coefficient for normally distributed parameters and the Spearman correlation coefficient for parameters with skewed distribution. Significant factors in univariate analysis were included in multivariate analysis. A P value < .05 (2-sided) was considered significant.
RESULTS
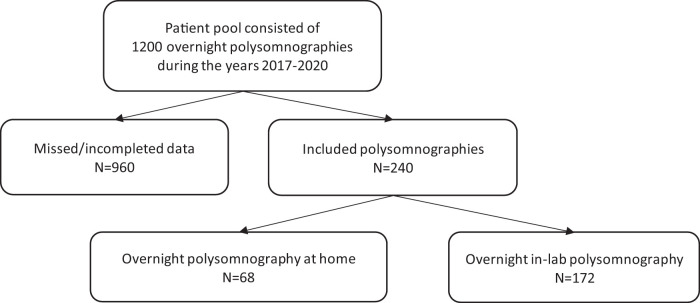
We reviewed 1,200 PSGs recorded between 2017 and 2020. Data were incomplete in 960 cases; the 240 cases with complete data were included in this study (Figure 1).
Figure 1. Flowchart of the study population.
The PSG was recorded in the sleep laboratory in 68 (28.3%) cases and at home in 172 (71.7%) cases. Mean AHI was 15.9 events/h (SD 17.9), mean TST was 365 minutes (SD 73.8), and mean SE was 81.3% (SD 13.6%). One-hundred fifty-six (65%) patients were male. Mean age was 45.9 years (SD 12.6) and mean BMI was 29.0 kg/m2 (SD 5.9).
There were no significant differences regarding age, BMI, sex, AHI, TST, SE, objective SL, and self-reported SL between the patients who had their PSG recorded in the sleep laboratory compared with those who recorded it at home (data not shown).
Sleep latency
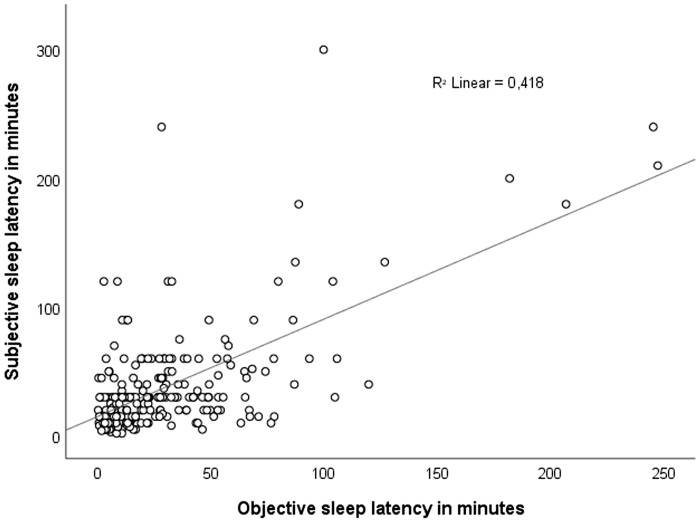
Mean objective SL was significantly lower than self-reported SL (29.5 minutes, SD 35.5, vs 37.4 minutes, SD 41.6) (F[1, n = 240] = 4.981; P = .026) (Figure 2). The objective SL correlated significantly with self-reported SL (r = .65, P < .001).
Figure 2. Scatterplot showing the relationship between the objective and self-reported sleep latency in the entire study population (n = 240).
The patients were divided into 2 groups (overestimation and underestimation) according to their SL estimation. The overestimation group had 159 patients and the underestimation group had 81 patients. There were no significant differences in age or in BMI values between the groups.
The mean self-reported SL was 17.7 minutes higher in the overestimation group than in the underestimation group (P = .003). The mean objective SL was 35.1 minutes lower in the overestimation group than in the underestimation group (P < .001). The patients who overestimated their SL had a higher mean AHI (18.8 events/h, SD 21.6, vs 13.4 events/h, SD 12.8; P = .04) and higher mean SE (81.7%, SD 13.6%, vs 75.2%, SD 13.9%; P = .004) than those who underestimated their SL.
Comorbidities
Metabolic disorders, respiratory disease, and psychiatric diseases were the most common comorbidities (Table 1).
Table 1.
Sleep latency estimation by comorbidity group.
| Comorbidities | Overestimated (n) | Underestimated (n) | P |
|---|---|---|---|
| Thyroid diseases | 18 | 6 | NS |
| Metabolic disorders | 53 | 21 | NS |
| Pulmonary diseases | 85 | 41 | NS |
| Psychiatric disorders | 32 | 26 | NS |
| Neurological disorders | 8 | 5 | NS |
| Nonmigraine headache | 7 | 3 | NS |
| Migraine | 20 | 3 | .035 |
NS = not significant.
There were significantly more patients with migraine in the overestimation group than in the underestimation group (χ2 [1, n = 240] = 4.878 [P = .035]). This difference was not observed in patients with headache without migraine (χ2 [1, n = 240] = 0.066 [P = 1.000]). No significant differences in other comorbidities were observed (Table 1).
In total, 176 patients had OSA and 64 did not. Among patients with OSA, 17 had migraine and 159 did not. Among patients without OSA, 6 had migraine and 58 did not. There were no significant differences in the prevalence of migraine and SL in OSA and non-OSA groups (data not shown).
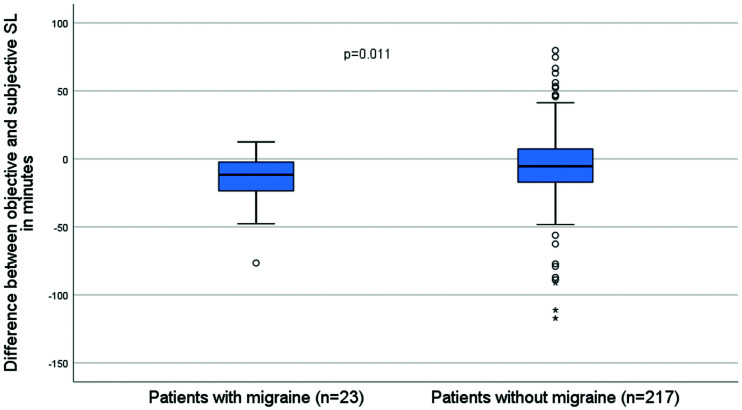
The difference between objective and self-reported SL was significantly higher in patients with migraine than in patients without migraine (P = .011; Figure 3).
Figure 3. Difference between objective and self-reported SL in patients with migraine compared with patients without migraine.
Points and asterisks show outlier and extreme outlier values, respectively. SL = sleep latency.
In contrast with the overall patient population (84/240, 35.0%), patients with migraine were more often women (15/23, 65.2%) (χ2 [1, n = 240] = 10.210 [P = .002]). However, there were no significant differences between men and women in evaluating their SL in the whole study population (χ2 [1, n = 240] = 0.149 [P = .775]). Moreover, there were no significant differences regarding age, BMI, or AHI values between patients with or without migraine (Table 2).
Table 2.
Clinical characteristics of patients with migraine compared with patients without migraine.
| Clinical Characteristics | Patients with Migraine (23) | Patients without Migraine (217) | P |
|---|---|---|---|
| Age, years (± SD) | 44.2 (11.1) | 46.2 (12.6) | NS |
| BMI, kg/m2 (± SD) | 31.2 (8.6) | 28.8 (5.6) | NS |
| Sleep latency, min (± SD) | |||
| Self-reported sleep latency | 52.8 (55.9) | 35.8 (39.6) | NS |
| Objective sleep latency | 28.4 (32.2) | 29.7 (35.9) | NS |
| AHI, events/h (± SD) | 17.1 (25.5) | 15.7 (16.9) | NS |
| Total sleep time, min (± SD) | 367 (74.5) | 365 (73.9) | NS |
| Sleep efficiency, % (± SD) | 80.4 (16.8) | 81.4 (13.2) | NS |
| Number of comorbidities (± SD) | 3.83 (1.7) | 1.72 (1.7) | < .001 |
| Study location, n (%) | |||
| Home | 16 (69.6%) | 156 (71.9%) | NS |
| Sleep laboratory | 7 (30.4%) | 61 (28.1%) | NS |
AHI = apnea-hypopnea index, BMI = body mass index, NS = not significant, SD = standard deviation.
Patients with multiple (≥ 3) comorbidities had higher mean objective SL (37.8 vs 30.1 minutes; P = .031), higher mean self-reported SL (55.1 vs 37.3; P = .002), lower mean TST (338 vs 366 minutes; P = .012), and lower mean SE (75.6% vs 81.3%; P = .005) compared with those with fewer comorbidities. No statistically significant differences were found between patients with multiple comorbidities compared with a few comorbidities regarding their estimation of SL (χ2 [1, n = 240] = 0.083 [P = .884]).
DISCUSSION
We examined the differences between self-reported and objective SL. We observed that patients tend to overestimate their SL, especially patients with migraine. This difference was not observed in patients with nonmigraine headache. Our results confirmed previous reports that patients with sleep apnea overestimate their SL.6,11
To our knowledge, this is the first report on the relation between migraine and sleep-onset misperception. Migraine seems to affect a specific perception of sleep onset that is not shared with other types of headaches. It is known that the pathophysiology of migraine differs from that of common headache, although pain is a common symptom. Sleep-onset misperception was observed mainly in patients with migraine when compared with patients with common headache, suggesting that pain is not the main pathophysiologic pathway for sleep-onset misperception.
A recent Finnish study showed that The International Classification of Headache Disorders, version 3, diagnostic criteria correlated consistently with the polygenic risk score (PRS) of a genomic biomarker of a common variant burden of migraine. Moreover, the more typical and complex the migraine, the higher PRS we observed.12 This emphasizes the complexity of migraine pathogenesis. We are uncertain whether undiagnosed OSA may participate in the chronification of migraine.
Our patients with migraine were predominantly women, which is consistent with previous reports on migraine.13 Our study population was representative of the usual patient population at our sleep unit (such as age and male predominance). The sex difference in patients with migraine did not explain the sleep-onset misperception in our study, as there was no difference in SL assessment between sexes in the entire study population.
We observed that patients with metabolic disorders were older and had a higher BMI, which was expected. Their TST was shorter, which is consistent with previous studies showing that TST decreases with age.14,15 Objective SL was longer in patients with psychiatric disorders, consistent with previous studies.16 Patients with multiple comorbidities had longer SL and shorter TST. This may be explained by the observation that chronic medical conditions have a positive correlation with the prevalence of sleep disorders.17
Recently, Sagaspe et al18 reported that misjudgment in self-perceived sleep during the maintenance of wakefulness test is associated with the occurrence of self-reported sleepiness-related traffic near-misses and accidents in the past year in patients with sleep disorders. We do not have data suggesting that patients with migraine have more sleepiness-related traffic near-misses and accidents than the general population.
This study has some limitations. This was a single-center and observational study, so a cause-effect relationship could not be assessed. Data are missing from a large number of patients due to 2 different questionnaires used during the time of this study (the patients were asked to score their self-reported SL numerically only in 1 questionnaire). We had no data on migraine treatment. We should consider our results as the consequence of a hypothesis generated by a retrospective data analysis study.
Our study also has several strengths. We reviewed a large number of overnight PSGs that were scored by 1 sleep specialist, thus eliminating interindividual variability. Our sleep unit is the largest sleep unit in Finland and provides services to a third of the Finnish population. Moreover, we collected data from the national health care registry; therefore, our data on comorbidities are accurate.
In conclusion, patients tend to overestimate their SL. This overestimation was marked in patients with migraine but was not observed in patients with other headache types without migraine. Our results should be interpreted with caution due to the nature of the study. Further studies are needed to explore the relation between migraine and sleep-onset misperception.
ACKNOWLEDGMENTS
Author contributions: Olli Rantanen participated in study design, data collection, data analysis, and writing. Maria Hollmen participated in study design, data collection, data analysis, and writing. Adel Bachour participated in study design, data collection, data analysis, and writing.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SD
standard deviation
- SE
sleep efficiency
- SL
sleep latency
- TST
total sleep time
DISCLOSURE STATEMENT
All authors have approved the final version of the manuscript. Work for this study was performed at the Helsinki University Hospital, Helsinki, Finland. Adel Bachour has received a grant from the Research Fund at the Helsinki University Hospital (number 1169006, code Y2020SK003). The authors report no conflicts of interest.
REFERENCES
- 1. Peppard PE , Young T , Barnet JH , Palta M , Hagen EW , Hla KM . Increased prevalence of sleep-disordered breathing in adults . Am J Epidemiol. 2013. ; 177 ( 9 ): 1006 – 1014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rundo J , Downey R . Polysomnography . In: Levin K , Chauvel P , eds. Handbook of Clinical Neurology. Vol. 160 . Elsevier; ; 2019. ; 381 – 392 . [DOI] [PubMed] [Google Scholar]
- 3. Kryger M , Roth T , Dement W . Principles and Practice of Sleep Medicine. Philadelphia, PA: : Elsevier; ; 2017. . [Google Scholar]
- 4. Kushida C . Encyclopedia of Sleep. Stanford, CA: : Academic Press; ; 2013. . [Google Scholar]
- 5. Berry R , Wagner M . Sleep Medicine Pearls. Gainesville, FL: : Saunders; ; 2014. . [Google Scholar]
- 6. Castillo J , Goparaju B , Bianchi MT . Sleep-wake misperception in sleep apnea patients undergoing diagnostic versus titration polysomnography . J Psychosom Res. 2014. ; 76 ( 5 ): 361 – 367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chervin RD , Guilleminault C . Overestimation of sleep latency by patients with suspected hypersomnolence . Sleep. 1996. ; 19 ( 2 ): 94 – 100 . [DOI] [PubMed] [Google Scholar]
- 8. Vanable PA , Aikens JE , Tadimeti L , Caruana-Montaldo B , Mendelson WB . Sleep latency and duration estimates among sleep disorder patients: variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics . Sleep. 2000. ; 23 ( 1 ): 71 – 79 . [PubMed] [Google Scholar]
- 9. Carskadon MA , Dement WC , Mitler MM , Guilleminault C , Zarcone VP , Spiegel R . Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia . Am J Psychiatry. 1976. ; 133 ( 12 ): 1382 – 1388 . [DOI] [PubMed] [Google Scholar]
- 10. Wilson DL , Fung A , Walker SP , Barnes M . Subjective reports versus objective measurement of sleep latency and sleep duration in pregnancy . Behav Sleep Med. 2013. ; 11 ( 3 ): 207 – 221 . [DOI] [PubMed] [Google Scholar]
- 11. Bianchi MT , Williams KL , McKinney S , Ellenbogen JM . The subjective-objective mismatch in sleep perception among those with insomnia and sleep apnea . J Sleep Res. 2013. ; 22 ( 5 ): 557 – 568 . [DOI] [PubMed] [Google Scholar]
- 12. Häppölä P , Gormley P , Nuottamo ME , et al. International Headache Genetics Consortium (IHGC) . Polygenic risk provides biological validity for the ICHD-3 criteria among Finnish migraine families . Cephalalgia. 2022. ; 42 ( 4-5 ): 345 – 356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robbins MS . Diagnosis and management of headache: a review . JAMA. 2021. ; 325 ( 18 ): 1874 – 1885 . [DOI] [PubMed] [Google Scholar]
- 14. Floyd JA , Janisse JJ , Jenuwine ES , Ager JW . Changes in REM-sleep percentage over the adult lifespan . Sleep. 2007. ; 30 ( 7 ): 829 – 836 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorffner G , Vitr M , Anderer P . The effects of aging on sleep architecture in healthy subjects . Adv Exp Med Biol. 2015. ; 821 : 93 – 100 . [DOI] [PubMed] [Google Scholar]
- 16. Baglioni C , Nanovska S , Regen W , et al . Sleep and mental disorders: a meta-analysis of polysomnographic research . Psychol Bull. 2016. ; 142 ( 9 ): 969 – 990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barczi S . Sleep and Medical Comorbidities . In: Avidan A , Alessi C , eds. Geriatric Sleep Medicine. 1st ed. Boca Raton, FL: : Informa Healthcare; ; 2008. . [Google Scholar]
- 18. Sagaspe P , Micoulaud-Franchi JA , Bioulac S , et al . Self-perceived sleep during the Maintenance of Wakefulness Test: how does it predict accidental risk in patients with sleep disorders? Sleep. 2021. ; 44 ( 11 ): zsab159 . [DOI] [PubMed] [Google Scholar]