Abstract
Study Objectives:
To determine whether home sleep apnea testing with peripheral arterial tonometry (HSAT-PAT) can be used in upper airway stimulation to evaluate therapy success.
Methods:
Data analysis from 50 consecutive patients receiving upper airway stimulation was performed. Baseline values were measured by polysomnography and HSAT-PAT. Follow-up was performed during and after titration (3–6 months) by polysomnography and HSAT-PAT and after 1 year by HSAT-PAT only. Primary outcome measures were reduction in the apnea-hypopnea index and oxygen desaturation index. In addition, an analysis of night-to-night variability for HSAT-PAT was performed.
Results:
All 50 patients completed their posttitration visit (3–6 months) and 41 patients completed the 1-year follow-up. In HSAT-PAT after 1 year, the mean apnea-hypopnea index (desaturation 3%) was reduced from 29.5 ± 17.1 events/h to 19.9 ± 13.1 events/h (P < .01) and the oxygen desaturation index (desaturation 4%) was reduced from 17.8 ± 12.6 events/h to 10.2 ± 8.3 events/h (P < .01). Therapy adherence after 1 year was high (6.6 ± 1.9 hours per night) and led to improvement in daytime sleepiness, meaning a reduction in the Epworth Sleepiness Scale score from 12.8 ± 5.4 to 5.9 ± 4.0 (P < .01). Analysis of night-to-night variability showed similar apnea-hypopnea index values between the 2 nights.
Conclusions:
Upper airway stimulation was able to reduce the apnea-hypopnea index and oxygen desaturation index after 1 year, as assessed by full-night efficacy studies with HSAT-PAT. In addition, improvements in self-reported outcome parameters were observed. The importance of publishing the scoring criteria is highlighted and whether data are based on full-night efficacy studies or a selected period of time from a sleep study. This is a prerequisite for comparing data with other trials in the emerging field of upper airway stimulation.
Citation:
Hinder D, Schams SC, Knaus C, Tschopp K. Home sleep apnea testing with peripheral arterial tonometry to assess outcome in upper airway stimulation. J Clin Sleep Med. 2022;18(9):2197–2205.
Keywords: obstructive sleep apnea, hypoglossal nerve stimulation, upper airway stimulation, positive airway pressure failure, home sleep apnea testing, peripheral arterial tonometry, night-to-night variability, full-night efficacy study, treatment AHI
BRIEF SUMMARY
Current Knowledge/Study Rationale: Therapy efficacy in upper airway stimulation has been mostly assessed by polysomnography even though home sleep apnea testing is an accepted alternative. Because polysomnography availability is low in many countries, we aimed to determine whether home sleep apnea testing with peripheral arterial tonometry can be used in patients receiving upper airway stimulation to evaluate outcome parameters.
Study Impact: This is the first study using home sleep apnea testing with peripheral arterial tonometry for follow-up in patients receiving upper airway stimulation. We highlight the importance of full-night efficacy studies and standardization of sleep indexes, because they allow better comparison of outcome data in upper airway stimulation patients.
INTRODUCTION
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder among middle-aged adults.1,2 Recent epidemiological studies showed a prevalence of up to 23% in women and 50% in men older than age 40 years,3 which is more than twice the prevalence reported in studies from the 1990s.4 OSA is an independent risk factor for cardiovascular disease5,6 and can complicate the treatment of arterial hypertension.7–9 Another burden of the disease is the 3- to 7-fold increased risk of traffic accidents because of excessive daytime sleepiness.10 In conclusion, OSA is a significant public health issue causing high socioeconomic costs.1,10
Current guidelines recommend positive airway pressure (PAP) as first-line treatment for OSA, because PAP is the most powerful in reducing the apnea-hypopnea index (AHI) compared to any alternative therapy.11 Despite the simplicity and safety of PAP, treatment effectiveness is limited by poor adherence. The long-term adherence is usually not better than 60%, which leads to untreated OSA in up to 40% of patients.12 Patients stop using PAP because of comfort issues, claustrophobia, or unwillingness to use this therapy for life. A recent multicenter randomized controlled trial showed that PAP did not reduce the number of cardiovascular incidents in patients with moderate to severe OSA.13 The authors explained these findings as resulting from poor therapy adherence of less than 4 hours per night in most of the patients. Low therapy adherence leads to untreated OSA and shows the need for alternative treatment options.12,14
In recent years, upper airway stimulation (UAS) therapy has been established as a second-line therapy for patients intolerant of PAP. Several trials have shown its efficacy, leading to significant AHI reductions.15–17 From the patient’s point of view, UAS can improve daytime sleepiness, snoring, and quality of sleep, which is reflected in higher adherence rates than with PAP.9,13,18
Therapy efficacy in UAS has been mostly assessed by sleep laboratory testing using polysomnography (PSG).15,18,19 As described in current guidelines, home sleep apnea testing (HSAT) is as equally accepted as PSG in diagnostic testing for OSA.20 Different methods of HSAT are available. This study aimed to determine whether HSAT with peripheral arterial tonometry (PAT) can be used in patients receiving UAS to evaluate therapy success in a 1-year follow-up.
METHODS
We analyzed prospectively collected data from 50 consecutive patients who received a unilateral UAS implant (Inspire Medical Systems, Minneapolis, MN) between July 2014 and February 2020 at our institution.
All patients fulfilled the selection criteria for implantation, as defined in the STAR trial21: 4 adult (≥ 18 years of age) patients experiencing moderate to severe OSA (AHI, 15–65 events/h) with less than 25% of central and mixed apneas in PSG, intolerance to PAP, no complete concentric collapse at the palate in drug-induced sleep endoscopy, and no severe overweight. According to the initial STAR trial recommendations, the body mass index limit was ≤ 32 kg/m2. In 2019, the body mass index limit was increased to ≤ 35 kg/m2 because of rising evidence about successful implantation in patients with a higher body mass index.19,22 Therefore, 5 patients in our cohort had a BMI between 32 and 35 kg/m2. Surgery was performed according to the latest recommendations from the manufacturer and the literature.23,24
Sleep indexes were measured by PSG and by HSAT with PAT (HSAT-PAT). Only full-night efficacy studies were conducted for both PSG and HSAT-PAT. This procedure was in contrast with that of other UAS publications, in which selected periods of a night are extracted from a sleep study to calculate the so-called treatment AHI.
In PSG, the AHI was calculated according to the recommended scoring criteria from The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (AASM Scoring Manual), version 2.6.25,26 For hypopneas, a respiratory event was scored if all of the following criteria were met: (1) the peak signal excursions dropped by ≥ 30% of pre-event baseline using nasal pressure; (2) the duration of the ≥ 30% drop in signal excursion was ≥ 10 seconds; and (3) there was a ≥ 3% oxygen desaturation from pre-event baseline, or the event was associated with an arousal. No respiratory events with ≥ 4% oxygen desaturation were scored.
HSAT-PAT was performed using the WatchPAT device (Itamar Medical, Caesarea, Israel).27,28 This device is worn around the wrist in combination with pulse oximetry and actigraphy to assess respiratory disturbances (Figure 1). By measuring obstruction-induced transient elevations of sympathetic tone, the device indirectly detects respiratory events via measuring peripheral arterial volume changes using a finger-mounted plethysmograph. No differentiation between apnea and hypopnea is possible because no flow signal is available. Therefore, classical scoring criteria as used in PSG cannot be applied. The device uses an automatic scoring algorithm. According to the company, manual scoring is possible and has been shown to improve correlation and agreement with PSG-derived sleep and breathing indexes.29 In our cohort, all HSAT-PAT measurements were scored automatically to eliminate interobserver variability. The automatic scoring for AHI with ≥ 3% oxygen desaturation limit (desaturation 3%) was used. In a subgroup of patients, data from HSAT-PAT over 2 nights was available, and the AHI was calculated as the mean of the 2 nights to decrease night-to-night variability (NNV). Regarding the oxygen desaturation index (ODI), a 4% desaturation limit (desaturation 4%) was used in HSAT-PAT. This is a default setting that could not be modified because of the technical limitations of the WatchPAT device.
Figure 1. HSAT device using PAT (WatchPAT).
HST = home sleep apnea testing, PAT = peripheral arterial tonometry.
UAS was activated 1 month after implantation, and patients started “self-titration” by increasing the stimulation amplitude with the remote control at home. After 2–3 months, the sleep laboratory titration by PSG was performed. All patients were followed systematically after 3–6 months and after 1 year. During each of these 2 follow-up visits, a 2-night HSAT-PAT was performed. In addition, all patients received a clinical exam and answered a questionnaire assessing daytime sleepiness (Epworth Sleepiness Scale [ESS]30), severity of snoring, social-work issues, and physical embarrassment (Snoring Symptoms Inventory31).
Primary endpoints were the reduction in AHI (desaturation 3%) and ODI (desaturation 4%). Secondary endpoints were therapy adherence and self-reported outcome measures including daytime sleepiness, assessed by ESS, and severity of snoring, social-work issues, and physical embarrassment, assessed by the Snoring Symptoms Inventory. Furthermore, the NNV between two nights, measured by HSAT-PAT, was analyzed preoperatively and after 1 year.
Statistical analysis
Descriptive statistics were calculated for demographic variables using the unpaired t test for continuous data. Results for outcome parameters are reported as mean ± standard deviation, median, and interquartile range. Normally distributed variables are summarized as mean (± 1 standard deviation) and were compared using the 2-tailed paired t test. Regarding NNV, the 2 nights were compared using the 2-tailed t test and data were described using Bland–Altman plots. A P value < 0.05 was considered significant. Data were analyzed using GraphPad Prism version 9.1.2 for macOS (GraphPad Software, San Diego, CA).
Ethical approval
The Ethics Committee of Northwest and Central Switzerland evaluated our study (Project 2018-01579) and concluded that it fulfilled the ethical and scientific standards for research according to the Human Research Act. Informed consent was obtained from each patient.
RESULTS
Patient characteristics
Table 1 shows the demographic characteristics and baseline sleep parameters at the time of implantation. Regarding the posttitration visit (3–6 months after implantation), 46 out of 50 patients (92%) were willing to undertake HSAT-PAT and all 50 patients could be seen in our outpatient clinic. After 1 year, 41 patients (82%) undertook HSAT-PAT, 38 patients (76%) were seen for a clinical examination and questionnaire, and the mean body mass index was 26.7 kg/m2, which did not differ significantly from the baseline value as shown in Table 1.
Table 1.
Demographic characteristics before implantation (baseline).
| Female | Male | Overall | P | |
|---|---|---|---|---|
| n (%) | 7 (14%) | 43 (86%) | 50 (100%) | |
| Age, y | 60.0 ± 7.9 | 57.2 ± 11.2 | 57.6 ± 10.8 | .43 |
| Body mass index, kg/m2 | 26.2 ± 2.5 | 27.1 ± 3.6 | 27 ± 3.4 | .44 |
| AHI (3%, PSG), events/h | 34.8 ± 18.6 | 37.5 ± 14.9 | 37.1 ± 15.3 | .72 |
| AHI (3%, HSAT-PAT), events/h | 18.5 ± 12.5 | 31.1 ± 16.6 | 29.3 ± 16.6 | .04 |
| ODI (4%, HSAT-PAT), events/h | 13.2 ± 10.8 | 18.6 ± 12.8 | 17.8 ± 12.6 | .26 |
| Epworth Sleepiness Scale | 9.3 ± 5.1 | 13.3 ± 5.3 | 12.8 ± 5.4 | .09 |
Data displayed as mean ± SD for continuous variables or n (%) for categorical variables. P values are unpaired t tests between men and women. AASM = American Academy of Sleep Medicine, AHI = apnea-hypopnea index (AASM scoring criteria for hypopnea using 3% oxygen desaturation), HSAT-PAT = home sleep apnea testing with peripheral arterial tonometry, ODI = oxygen-desaturation index (using 4% oxygen desaturation), PSG = polysomnography.
The reasons for patients lost to follow up were as follows: 1 patient died of previously unknown lung cancer, 1 patient had severe depression and asked for explantation of the device, 3 patients developed intolerance because of insomnia aggravated by the stimulation, and 2 patients had to be revised because of technical issues with the stimulation and the sensing lead. The remaining patients live far away from our clinic and are followed by their referring clinicians instead of us.
Primary and secondary outcome measures
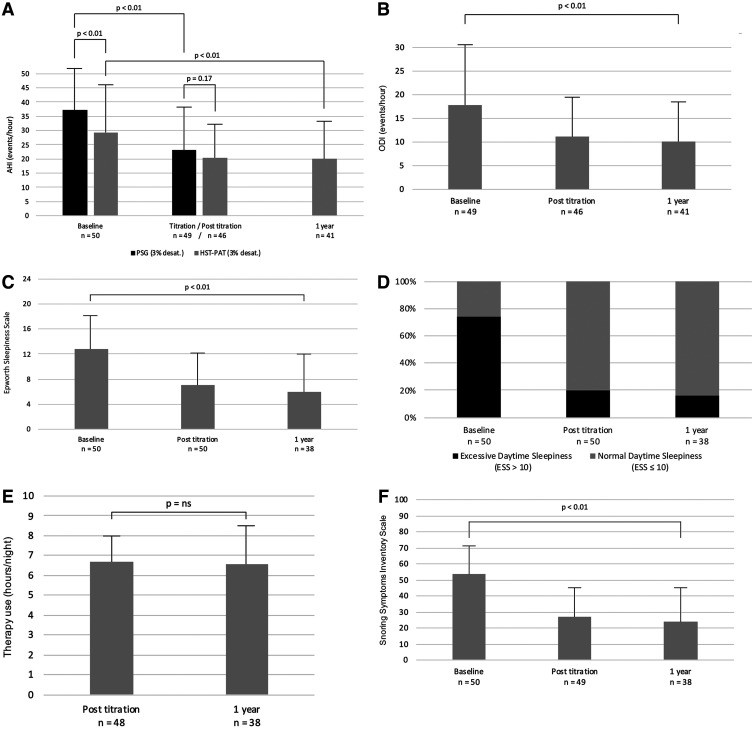
After 1 year, in 41 patients (82%) who undertook HSAT-PAT, the mean AHI (desaturation 3%) was reduced from 29.3 ± 16.6 events/h to 19.9 ± 13.1 events/h (P < .01) and ODI (desaturation 4%) was reduced from 17.8 ± 12.6 events/h to 10.2 ± 8.3 events/h (P < .01; Figure 2A, Figure 2B). AHI and ODI values during the posttitration visit (3–6 months after implantation) and after 1 year did not differ significantly from each other (Table 2). When comparing the 2 different modalities of sleep study, we found that baseline AHI was higher according to PSG than according to HSAT-PAT (37.1 vs 29.3 events/h; P < .01; Figure 2A). Regarding AHI during titration night (PSG) and posttitration (HSAT-PAT), the values were similar (23.1 vs 20.2 events/h; P = .17; Figure 2A). After 1 year, no PSG was regularly performed.
Figure 2. Primary and secondary outcome measures at baseline and during follow-up.
(A) Mean change in AHI (events/h, desaturation 3%), measured by PSG and HSAT-PAT. (B) Mean change in ODI (events/h, desaturation 4%), measured by HSAT-PAT. (C) Mean change in ESS score. (D) Normalization in daytime sleepiness. (E) Mean therapy adherence in hours per night. (F) Snoring Symptoms Inventory questionnaire; assessing severity of snoring, social-work issues, and physical embarrassment. AHI = apnea-hypopnea index, ESS = Epworth Sleepiness Scale, HSAT-PAT = home sleep apnea testing with peripheral arterial tonometry, ODI = oxygen desaturation index, PSG = polysomnography.
Table 2.
Primary and secondary outcome measures.
| Baseline | 3–6 Mo | After 1 Y | P | |
|---|---|---|---|---|
| Primary outcomes | n = 50 | n = 46 | n = 41 | |
| AHI | 29.3 ± 16.6 | 20.2 ± 11.6 | 19.9 ± 13.1 | .0007 |
| Median | 25.2 | 18.1 | 17.9 | |
| Interquartile range | 19.4–35.4 | 10.9–26.6 | 12.1–24.5 | |
| ODI | 17.8 ± 12.6 | 11.1 ± 8.4 | 10.2 ± 8.3 | .0002 |
| Median | 15.1 | 9.1 | 8.3 | |
| Interquartile range | 10.2–22 | 4.1–17.2 | 4.6–12.4 | |
| Secondary outcomes | n = 50 | n = 50 | n = 38 | P |
| ESS | 12.8 ± 5.4 | 7.1 ± 5.0 | 5.9 ± 4.0 | < .0001 |
| Median | 13.0 | 6.0 | 4.0 | |
| Interquartile range | 10.3–15.8 | 4.0–9.8 | 3.0–9.0 | |
| SSI | 53.8 ± 18.3 | 26.8 ± 19.4 | 23.7 ± 22.5 | < .0001 |
| Median | 53.5 | 22.0 | 16.0 | |
| Interquartile range | 39.3–71.8 | 9.0–44.0 | 9.0–33.0 | |
| Therapy adherence (h/ night) | 6.7 ± 1.3 | 6.6 ± 1.9 | .83 |
AHI and ODI measured by HSAT-PAT. P values are paired t test between baseline and after-1-year values. AASM = American Academy of Sleep Medicine, AHI = apnea-hypopnea index (AASM scoring criteria for hypopnea using 3% oxygen desaturation), ESS = Epworth Sleepiness Scale, HSAT-PAT = home sleep apnea testing with peripheral arterial tonometry, ODI = oxygen-desaturation index (using 4% oxygen desaturation), SSI = Snoring Symptoms Inventory.
Self-reported outcome measures could be assessed in 38 patients (76%) after 1 year: Daytime sleepiness showed a reduction in the ESS score from 12.8 ± 5.4 to 5.9 ± 4.0 (P < .01; Figure 2C). Excessive daytime sleepiness is defined as an ESS score > 10. Before implantation, 74% of the patients reported an ESS score > 10. After 1 year, 16% of the patients reported an ESS score > 10, meaning that 84% reported normal daytime sleepiness under UAS therapy (Figure 2D). Therapy adherence remained high during follow-up, with similar usage posttitration and after 1 year (6.7 ± 1.3 vs 6.6 ± 1.9 hours per night; P = .83; Figure 2E). Scores on the Snoring Symptoms Inventory questionnaire, reflecting the severity of snoring, social-work issues, and physical embarrassment, indicated significant improvement from baseline to after 1 year (53.8 ± 18.3 vs 23.7 ± 22.5; P < .01; Figure 2F). Quality of sleep assessed using the visual analog scale was reported to be higher and better after 1 year vs before implantation (5.6 ± 2.6 vs 1.9 ± 1.9; P < .01).
NNV
For the first patients implanted between 2014 and 2017, we measured only 1 night with HSAT-PAT. To decrease NNV, we began performing HSAT-PAT over 2 nights in 2017. This resulted in complete data to calculate NNV in 20 patients (40%) at baseline and 32 patients (64%) after 1 year. We performed an analysis of NNV by comparing the AHI of the first and the second night.
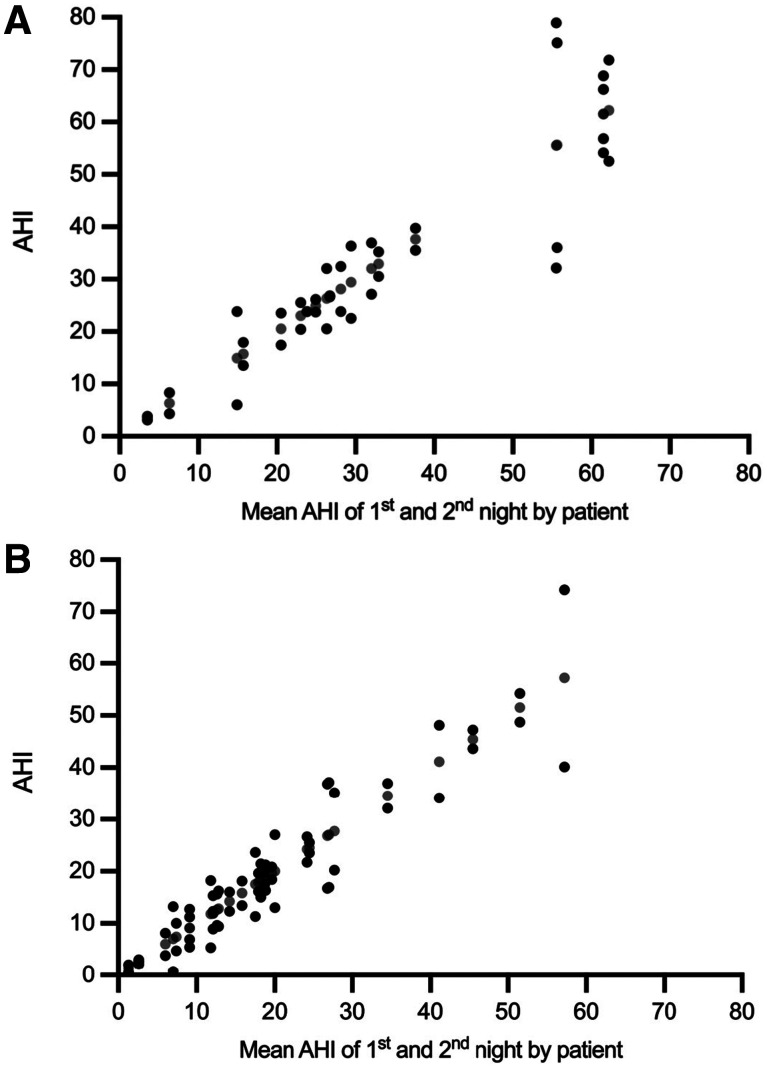
At baseline, the mean AHI for first vs second night was 29.7 ± 18.7 events/h vs 34.5 ± 20.9 events/h (n = 20, P = .19). After 1 year, the mean AHI for first vs second night was 18.8 ± 13.9 events/h vs 20.3 ± 15.7 events/h (n = 32, P = .43). Results for individual patients are shown in Figure 3 and Figure 4 at baseline and after 1 year.
Figure 3. NNV in HSAT-PAT at baseline and after 1 year per patient.
(A) NNV in HSAT-PAT at baseline. (B) NNV in HSAT-PAT after 1 year. Black dots show the AHI values during each night, and gray dots indicate the corresponding mean of the 2 nights. Data for 2 nights are available for 20 patients at baseline and 32 patients after 1 year. The residual patients had HSAT-PAT for 1 night only and NNV could not be shown. AHI = apnea-hypopnea index, HST-PAT = home sleep apnea testing with peripheral arterial tonometry, NNV = night-to-night variability.
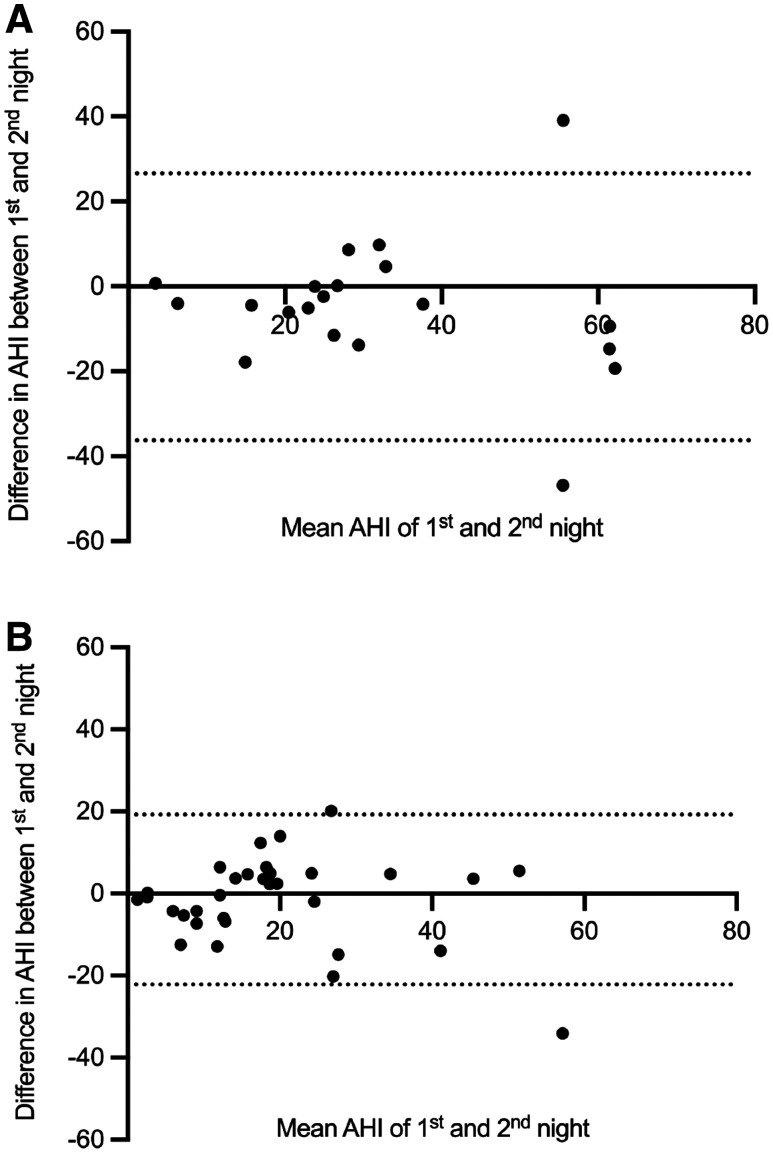
Figure 4. NNV in HSAT-PAT at baseline and after 1 year shown in Bland–Altman plots.
(A) NNV in HSAT-PAT at baseline. (B) NNV in HSAT-PAT after 1 year. The horizontal solid line is the mean value, and the dotted lines represent the limits of agreement (mean ± 1.96 × SD). Data for 2 nights are available for 20 patients at baseline and 32 patients after 1 year. The residual patients had HSAT-PAT for 1 night only and NNV could not be shown. AHI = apnea-hypopnea index, HST-PAT = home sleep apnea testing with peripheral arterial tonometry, NNV = night-to-night variability, SD = standard deviation.
DISCUSSION
This consecutive single-center series analyzed the results in 50 patients who had undergone UAS implantation for moderate to severe OSA. HSAT was performed using the WatchPAT device, which is a well-validated, commercially available type of home sleep study that utilizes the combination of oxygen desaturation and PAT to detect sleep-disordered breathing.27,28,32 PAT monitors sympathetic activation by measuring vasoconstriction in the fingertip using a sensor attached to the patient’s hand. Through an intrinsic algorithm, the device can detect arousals and desaturations. Therefore, the AHI is estimated as a surrogate marker while the oxygen desaturation is measured directly. To eliminate interobserver variability, all HSAT-PATs were scored automatically. Because UAS is based on nerval stimulation, a change in sympathetic activity could hypothetically be caused by electric stimulation of the 12th cranial nerve and its proximity to other neural structures, such as sympathetic neurons or the vagal nerve. This question has been addressed by Ikeda et al33: 8 patients receiving UAS were analyzed in a sleep laboratory using PSG in combination with PAT. The authors found that UAS did not alter the PAT signal before or during the stimulation. In addition, PAT signal attenuation was independent of UAS intensity.
Regarding the primary outcome measures assessed by HSAT-PAT, the baseline AHI of 29.3 ± 16.6 events/h was reduced to 20.2 ± 16.6 events/h after 3–6 months and remained stable at 19.9 ± 13.1 events/h after 1 year. At first sight, these values seem surprisingly high when compared to the literature, where AHI values between 9 and 15.3 events/h have been reported 1 year after UAS implantation.9,15–17,22,33–35 We have 2 explanations for the observed discrepancy. First, it is important to realize the difference between the so-called treatment AHI and the AHI from a full-night efficacy study. Treatment AHI is extracted from a full-night sleep study, including only data from a selected time frame. This information does usually not include the switch-on delay time, which can vary from 0 up to 75 minutes, and other parts of the night, when sleep stage or even titration is not ideal. The following citation is an example how the AHI is typically calculated in UAS patients.17 In 2018, Dedhia and Woodson advocated for standardized reporting for UAS outcomes.36 We are in line with their recommendation to use only full-night efficacy studies in our cohort for showing results from “real nights” and not only selected periods of a night. It is well recognized that a full-night AHI is higher than the treatment AHI, which is reported in most of the literature. Second, another explanation for the discrepancy in AHI can be found in the scoring criteria for sleep studies. We draw the attention to the fact that using different hypopnea definitions leads to marked differences in AHI.37 In patients receiving UAS, most sleep laboratories still use the 4% oxygen desaturation limit to score hypopneas. Therefore, most of the data in the literature are based on this limit for AHI and ODI.9,15–17,22,33–35 We found only 1 study, focusing on UAS therapy in children with Down syndrome, using the 3% oxygen desaturation limit.38 In another study, no definition of the scoring criteria could be found at all.19 Typically, an AHI based on the 4% oxygen desaturation limit is lower than an AHI based on the 3% limit. This is the second reason why we report higher AHI values after 1 year compared to the literature.
In accordance with current guidelines, we are following the latest American Academy of Sleep Medicine (AASM) criteria for hypopnea as defined in the Methods section. As stated by the AASM task force in 2012, there is rising evidence about the correlation between obstructive respiratory events leading to 3% desaturations and cardiometabolic disease.39 Therefore, hypopneas should be scored using ≥ 3% oxygen desaturations instead of 4%. A recent epidemiological study analyzed the impact of the 3 standard AASM hypopnea definitions (from 1999, 2007, and 2012) on the prevalence of cardiometabolic outcomes in > 2,162 patients.37 Using the AASM 2012 criteria,39 an independent association with diabetes, hypertension, and metabolic syndrome could be found in AHI > 10.7, 14.4, and 11.8 events/h, respectively. This leads us to the conclusion that OSA should be treated in AHI > 15 events/h, using scoring criteria according to AASM 2012.39 We are aware that in some countries the scoring criteria may differ due to payer policy requirements, such as Medicare in the United States, where the acceptable AASM Scoring Manual criteria for scoring hypopneas are used, which include only diminished airflow plus ≥ 4% oxygen desaturation.26 This is a regulatory requirement for eligible patients receiving UAS. In our country, we do not have these regulations. Accordingly, we only used the recommended AASM Scoring Manual criteria25 for hypopneas as defined in the Methods section.
Regarding ODI and in contrast to AHI, a 4% oxygen desaturation limit was used in our patients for HSAT-PAT. This was a limitation of the device (WatchPAT) because it cannot be modified by the user. On the other hand, it allowed comparison with the mentioned trials, where mostly a 4% oxygen desaturation limit was used. After 1 year, mean ODI was reduced to 10.2 ± 8.3 events/h, which is consistent with the literature.15,21,22,35 In the STAR trial, for example, the ODI could be reduced from 28.9 ± 12.0 events/h to 13.9 ± 15.7 events/h after 1-year.21 This corresponded to an absolute ODI reduction of 16.9 events/h. In our cohort, UAS could reduce the ODI by only 7.6 events/h, which is less than half of the reduction in the STAR trial. An explanation for the lower ODI reduction in our cohort is the lower baseline values. In our study, 62% of our patients had moderate OSA (AHI, 15–30 events/h), whereas most patients in the other trials had severe OSA (AHI, > 30 events/h). In general, the baseline AHI and ODI values of the mentioned trials were high, especially when considering the 4% oxygen desaturation limit: In the STAR trial, the baseline AHI was 32 events/h and in the ADHERE trial it was 36.3 events/h.17,21 This leads to an estimated baseline AHI of more than 45 events/h when converting into a scoring based on a 3% oxygen desaturation limit. The baseline mean AHI in our patients was only 29.3 ± 16.6 events/h and therefore considerably lower than in the mentioned trials.17,21 This result may explain the less-pronounced AHI reduction in our cohort.
Concerning self-reported outcome measures, an impressive change in daytime sleepiness was documented after implantation. Excessive daytime sleepiness is defined as an ESS score > 10, which was reported by 74% of the patients before implantation. This was reduced to 19% of patients during the first follow-up visit after 3–6 months and remained similarly low after 1 year (Figure 2D). The absolute reduction in mean ESS from baseline to after 1 year was 6.9 (Figure 2C). This amount is more than what is seen in the literature, where ESS reductions from 4.6 up to 5.8 have been reported.15,17,22 We explained this difference in our relatively high lost-to-follow-up rate of 24% after 1 year.
Focusing on NNV with HSAT-PAT, we found similar AHI values between the 2 nights: At baseline, the mean AHI of the second night was 4.8 events/h higher than during the first night. After 1 year, the mean difference between the 2 nights was only 1.5 events/h. At baseline, we explained the higher AHI during the second night with a higher percentage of patients in the supine position compared to the first night (46.0% vs 49.3%). After 1 year, the percentage of patients in the supine position for the first vs the second night was similar (43.1% vs 41.7%). Another explanation for a higher AHI during the second night may be the so-called first night effect, which was observed mainly in sleep laboratory settings, where the authors found reduced total sleep time, decreased sleep efficiency, and an increased proportion of light sleep as the main factors, leading to a lower AHI in the first of 2 nights.40–42 Therefore, measuring 2 nights in a sleep laboratory is recommended for the reduction of NNV. Another study focusing on HSAT using PAT found no first-night effect in this specific sleep laboratory modality.43 A possible explanation might be the more comfortable environment when performing sleep apnea testing at home. In general, our patients reported no complaints about HSAT-PAT with the WatchPAT device even when performing a recording of 2 nights. Although the mean AHI of the 2 nights did not differ for the cohort, considerable differences were observed in individual patients (Figure 3, Figure 4). In this respect, recording 2 nights may give a better estimation of the patient’s sleep than measuring only 1 night.
To our knowledge, this is the first published study using HSAT-PAT for follow-up in patients receiving UAS. Compared to in-laboratory testing and other HSAT modalities, HSAT-PAT offers more patient comfort (Figure 1). A meta-analysis from 2013 found correlations in the range of 0.85–0.9 between AHI values from PSG and HSAT-PAT.32 Therefore, the automatic scoring algorithm was always considered to be well validated. Nevertheless, a recent study looking at data from 500 patients using concomitant PSG and HSAT-PAT found diagnostic concordance in 42%, 41%, and 83% of mild, moderate, and severe OSA, respectively, leading to an accuracy of only 53%.44 The authors concluded that HSAT-PAT leads to high rates of diagnostic misclassification of OSA severity. In particular, patients with no or mild OSA in HSAT-PAT but high clinical suspicion of OSA should undergo PSG for definite diagnosis.
It is certainly a limitation of our study that we did not perform PSG testing after 1 year. As with every sleep testing modality, HSAT-PAT has its limitations, and the physician must be aware of potential diagnostic misclassification. We see the value of HSAT-PAT mostly for assessing pre- and postinterventional data for scientific reasons. In this setting, the automatic scoring has clear advantages because it eliminates interobserver variability. However, we consider performing PSG 1 year after UAS implantation to reduce diagnostic misclassification and obtain a better picture of the outcome of our patients.
Irrespective of the chosen sleep testing modality, we want to highlight the importance of publishing the scoring criteria. From a scientific point of view, the standardization of sleep indexes is of utmost importance because it allows the comparison of results in the emerging field of UAS. AHI and ODI should be assessed using both the 3% and 4% oxygen desaturation limits. In addition, we advocate for full-night efficacy studies because they reflect the real condition of our patients.
ACKNOWLEDGMENTS
Author contributions: D.H. and S.S. contributed to the acquisition, analysis, and interpretation of data; drafting the work; revising the work; and final approval. C.K. and K.T. contributed to the acquisition of data, revising the work, and final approval.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- ESS
Epworth Sleepiness Scale
- HSAT
home sleep apnea testing
- HSAT-PAT
home sleep apnea testing with peripheral arterial tonometry
- NNV
night-to-night variability
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PAT
peripheral arterial tonometry
- PSG
polysomnography
- UAS
upper airway stimulation
DISCLOSURE STATEMENT
All authors have seen and approved the final manuscript. The authors report no funding, financial relationships, or conflicts of interest.
REFERENCES
- 1. Punjabi NM . The epidemiology of adult obstructive sleep apnea . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 136 – 143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young T , Shahar E , Nieto FJ , et al .; for the Sleep Heart Health Study Research Group . Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study . Arch Intern Med. 2002. ; 162 ( 8 ): 893 – 900 . [DOI] [PubMed] [Google Scholar]
- 3. Heinzer R , Vat S , Marques-Vidal P , et al . Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study . Lancet Respir Med. 2015. ; 3 ( 4 ): 310 – 318 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young T , Finn L , Kim H ; The University of Wisconsin Sleep and Respiratory Research Group . Nasal obstruction as a risk factor for sleep-disordered breathing . J Allergy Clin Immunol. 1997. ; 99 ( 2 ): S757 – S762 . [DOI] [PubMed] [Google Scholar]
- 5. Kasai T , Narui K , Dohi T , et al . Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure . Chest. 2008. ; 133 ( 3 ): 690 – 696 . [DOI] [PubMed] [Google Scholar]
- 6. Pearce DC , Cadilhac DA , Pierce RJ , Thrift AG , David S , Donnan GA ; SCOPES II Study Group . Estimating the prevalence of sleep-disordered breathing in community-based, long-term stroke survivors using a validated predictive model . Cerebrovasc Dis. 2008. ; 26 ( 4 ): 441 – 446 . [DOI] [PubMed] [Google Scholar]
- 7. Haentjens P , Van Meerhaeghe A , Moscariello A , et al . The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials . Arch Intern Med. 2007. ; 167 ( 8 ): 757 – 764 . [DOI] [PubMed] [Google Scholar]
- 8. Bazzano LA , Khan Z , Reynolds K , He J . Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea . Hypertension. 2007. ; 50 ( 2 ): 417 – 423 . [DOI] [PubMed] [Google Scholar]
- 9. Walia HK , Thompson NR , Strohl KP , et al . Upper airway stimulation vs positive airway pressure impact on BP and sleepiness symptoms in OSA . Chest. 2020. ; 157 ( 1 ): 173 – 183 . [DOI] [PubMed] [Google Scholar]
- 10. Somers VK , White DP , Amin R , et al . Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing . J Am Coll Cardiol. 2008. ; 52 ( 8 ): 686 – 717 . [DOI] [PubMed] [Google Scholar]
- 11. Patil SP , Ayappa IA , Caples SM , Kimoff RJ , Patel SR , Harrod CG . Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med. 2019. ; 15 ( 2 ): 335 – 343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sawyer AM , Gooneratne NS , Marcus CL , Ofer D , Richards KC , Weaver TE . A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions . Sleep Med Rev. 2011. ; 15 ( 6 ): 343 – 356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McEvoy RD , Antic NA , Heeley E , et al . SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea . N Engl J Med. 2016. ; 375 ( 10 ): 919 – 931 . [DOI] [PubMed] [Google Scholar]
- 14. Rotenberg BW , Vicini C , Pang EB , Pang KP . Reconsidering first-line treatment for obstructive sleep apnea: a systematic review of the literature . J Otolaryngol Head Neck Surg. 2016. ; 45 ( 1 ): 23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woodson BT , Strohl KP , Soose RJ , et al . Upper airway stimulation for obstructive sleep apnea: 5-year outcomes . Otolaryngol Head Neck Surg. 2018. ; 159 ( 1 ): 194 – 202 . [DOI] [PubMed] [Google Scholar]
- 16. Steffen A , Sommer JU , Hofauer B , Maurer JT , Hasselbacher K , Heiser C . Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study . Laryngoscope. 2018. ; 128 ( 2 ): 509 – 515 . [DOI] [PubMed] [Google Scholar]
- 17. Heiser C , Steffen A , Boon M , et al .; on behalf of the ADHERE registry investigators . Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry . Eur Respir J. 2019. ; 53 ( 1 ): 1801405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thaler E , Schwab R , Maurer J , et al . Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy . Laryngoscope. 2020. ; 130 ( 5 ): 1333 – 1338 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasselbacher K , Hofauer B , Maurer JT , Heiser C , Steffen A , Sommer JU . Patient-reported outcome: results of the multicenter German post-market study . Eur Arch Otorhinolaryngol. 2018. ; 275 ( 7 ): 1913 – 1919 . [DOI] [PubMed] [Google Scholar]
- 20. Kapur VK , Auckley DH , Chowdhuri S , et al . Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med. 2017. ; 13 ( 3 ): 479 – 504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strollo PJ Jr , Soose RJ , Maurer JT , et al . STAR Trial Group . Upper-airway stimulation for obstructive sleep apnea . N Engl J Med. 2014. ; 370 ( 2 ): 139 – 149 . [DOI] [PubMed] [Google Scholar]
- 22. Steffen A , Sommer UJ , Maurer JT , Abrams N , Hofauer B , Heiser C . Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea . Sleep Breath. 2020. ; 24 ( 3 ): 979 – 984 . [DOI] [PubMed] [Google Scholar]
- 23. Heiser C , Thaler E , Soose RJ , Woodson BT , Boon M . Technical tips during implantation of selective upper airway stimulation . Laryngoscope. 2018. ; 128 ( 3 ): 756 – 762 . [DOI] [PubMed] [Google Scholar]
- 24. Heiser C , Thaler E , Boon M , Soose RJ , Woodson BT . Updates of operative techniques for upper airway stimulation . Laryngoscope. 2016. ; 126 : S12 – S16 . [DOI] [PubMed] [Google Scholar]
- 25. Berry RB , Quan SF , Abreu AR , et al .; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. Darien, IL: : American Academy of Sleep Medicine; ; 2020. . [Google Scholar]
- 26. Malhotra RK , Kirsch DB , Kristo DA , et al . American Academy of Sleep Medicine Board of Directors . Polysomnography for obstructive sleep apnea should include arousal-based scoring: an American Academy of Sleep Medicine position statement . J Clin Sleep Med. 2018. ; 14 ( 7 ): 1245 – 1247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Donnell CP , Allan L , Atkinson P , Schwartz AR . The effect of upper airway obstruction and arousal on peripheral arterial tonometry in obstructive sleep apnea . Am J Respir Crit Care Med. 2002. ; 166 ( 7 ): 965 – 971 . [DOI] [PubMed] [Google Scholar]
- 28. Pittman SD , Ayas NT , MacDonald MM , Malhotra A , Fogel RB , White DP . Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation . Sleep. 2004. ; 27 ( 5 ): 923 – 933 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Z , Sowho M , Otvos T , et al . A comparison of automated and manual sleep staging and respiratory event recognition in a portable sleep diagnostic device with in-lab sleep study . J Clin Sleep Med. 2020. ; 16 ( 4 ): 563 – 573 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johns MW . A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 31. Douglas SA , Webster S , El Badawey MR , et al . The development of a snoring symptoms inventory . Otolaryngol Head Neck Surg. 2006. ; 134 ( 1 ): 56 – 62 . [DOI] [PubMed] [Google Scholar]
- 32. Yalamanchali S , Farajian V , Hamilton C , Pott TR , Samuelson CG , Friedman M . Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis . JAMA Otolaryngol Head Neck Surg. 2013. ; 139 ( 12 ): 1343 – 1350 . [DOI] [PubMed] [Google Scholar]
- 33. Ikeda AK , Li Q , Quyuumi AA , Dedhia RC . Hypoglossal nerve stimulation therapy on peripheral arterial tonometry in obstructive sleep apnea: a pilot study . Sleep Breath. 2019. ; 23 ( 1 ): 153 – 160 . [DOI] [PubMed] [Google Scholar]
- 34. Mehra R , Steffen A , Heiser C , et al . Upper airway stimulation versus untreated comparators in positive airway pressure treatment-refractory obstructive sleep apnea . Ann Am Thorac Soc. 2020. ; 17 ( 12 ): 1610 – 1619 . [DOI] [PubMed] [Google Scholar]
- 35. Steffen A , König IR , Baptista PM , Abrams N , Jeschke S , Hasselbacher K . Home sleep testing to direct upper airway stimulation therapy optimization for sleep apnea . Laryngoscope. 2020. ; 131 ( 4 ): E1375 – E1379 . [DOI] [PubMed] [Google Scholar]
- 36. Dedhia RC , Woodson BT . Standardized reporting for hypoglossal nerve stimulation outcomes . J Clin Sleep Med. 2018. ; 14 ( 11 ): 1835 – 1836 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hirotsu C , Haba-Rubio J , Andries D , et al . Effect of three hypopnea scoring criteria on OSA prevalence and associated comorbidities in the general population . J Clin Sleep Med. 2019. ; 15 ( 2 ): 183 – 194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caloway CL , Diercks GR , Keamy D , et al . Update on hypoglossal nerve stimulation in children with Down syndrome and obstructive sleep apnea . Laryngoscope. 2020. ; 130 ( 4 ): E263 – E267 . [DOI] [PubMed] [Google Scholar]
- 39. Berry RB , Budhiraja R , Gottlieb DJ , et al . Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events . J Clin Sleep Med. 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sforza E , Roche F , Chapelle C , Pichot V . Internight variability of apnea-hypopnea index in obstructive sleep apnea using ambulatory polysomnography . Front Physiol. 2019. ; 10 : 849 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bittencourt LR , Suchecki D , Tufik S , et al . The variability of the apnoea-hypopnoea index . J Sleep Res. 2001. ; 10 ( 3 ): 245 – 251 . [DOI] [PubMed] [Google Scholar]
- 42. Newell J , Mairesse O , Verbanck P , Neu D . Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples . Psychiatry Res. 2012. ; 200 ( 2–3 ): 795 – 801 . [DOI] [PubMed] [Google Scholar]
- 43. Tschopp S , Wimmer W , Caversaccio M , Borner U , Tschopp K . Night-to-night variability in obstructive sleep apnea using peripheral arterial tonometry: a case for multiple night testing . J Clin Sleep Med. 2021. ; 17 ( 9 ): 1751 – 1758 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ioachimescu OC , Allam JS , Samarghandi A , et al . Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort . J Clin Sleep Med. 2020. ; 16 ( 10 ): 1663 – 1674 . [DOI] [PMC free article] [PubMed] [Google Scholar]