Abstract
Study Objectives:
Patients with obstructive sleep apnea (OSA) are at increased risk of cardiovascular and cerebrovascular disease, but predicting those at greatest risk is challenging. Using latent class analysis, patients with OSA can be placed into discrete symptom subtypes. The aim of this study was to determine whether symptom subtypes are associated with future cerebrovascular disease in patients with OSA in a clinic-based cohort.
Methods:
Patients with suspected OSA referred for a polysomnogram at an academic sleep center completed a comprehensive symptom survey. Patients with OSA (apnea-hypopnea index ≥ 5 events/h) were then placed into symptom subtypes based on responses to survey questions using latent class analysis. Cardiovascular events (stroke, myocardial infarction, unstable angina, bypass grafting, percutaneous coronary intervention, cardiac resynchronization therapy, defibrillation) occurring within 8 years of polysomnogram were identified by linkage to provincial health databases.
Results:
1,607 patients were studied, of whom 1,292 had OSA. One hundred forty first events occurred within 8 years of polysomnogram. Patients in the excessively sleepy with disturbed sleep subtype had a significantly increased rate of events compared to the minimally symptomatic subtype (hazard ratio = 2.25, 95% confidence interval: 1.02–4.94; P = .04). Two symptoms (restless legs and dozing off or sleeping while talking to someone) were significantly associated with future risk of cerebrovascular disease (hazard ratio = 1.68, 1.12–2.49 and 4.23, 1.61–11.16, respectively).
Conclusions:
Patients with OSA in the clinic who are in the excessively sleepy with disturbed sleep subtype are significantly more likely to have a future cardiovascular event. This underscores the importance of understanding clinical heterogeneity and incorporating symptom subtype definitions into routine clinical care.
Citation:
Allen AJH, Jen R, Mazzotti DR, et al. Symptom subtypes and risk of incident cardiovascular and cerebrovascular disease in a clinic-based obstructive sleep apnea cohort. J Clin Sleep Med. 2022;18(9):2093–2102.
Keywords: obstructive sleep apnea, OSA, symptoms, sleepiness, cardiovascular events
BRIEF SUMMARY
Current Knowledge/Study Rationale: Patients with sleep apnea are at increased risk of cardiovascular and cerebrovascular disease, but predicting those at greatest risk is challenging. Symptom subtypes based on latent class analysis may be associated with future cardiovascular events, and the aim of this study was to determine whether symptom subtypes are associated with future cerebrovascular events in patients with obstructive sleep apnea in a clinic-based cohort.
Study Impact: Patients with sleep apnea from the clinic who are in the excessively sleepy with disturbed sleep symptom subtype are significantly more likely to have a future cardiovascular event. Incorporating symptom subtype definitions may eventually be useful in helping to risk stratify patients for adverse health outcomes.
INTRODUCTION
Obstructive sleep apnea (OSA) is the most common respiratory sleep disorder, with close to 1 billion people affected.1 OSA is associated with a significantly increased risk of cardiovascular (CV) and cerebrovascular disease (CVD)2; however, identifying patients at particularly high risk is challenging. Standard OSA severity metrics (eg, apnea-hypopnea index [AHI]) are not particularly discriminative.3 The ability to identify high-risk groups could help direct more aggressive treatment of OSA and other CV risk factors (precision care) and facilitate patient selection for recruitment into clinical trials of CV prevention. Toward this goal, recent efforts utilizing clinical and polysomnographic characteristics have been successful at identifying subgroups of patients with OSA with high CVD risk.4–6
Distinct subtypes based on symptoms are observed in OSA.7–11 Latent class analysis, a statistical procedure for grouping individuals into a set of mutually exclusive clusters based on a collection of categorical measurements,12–14 has identified between 3 and 5 primary OSA symptom subtypes.8–11 These subtypes have been identified in clinical8,9 and population-based cohorts10,11 and consistently include subtypes defined by the severity of sleepiness, presence of disturbed sleep (eg, insomnia), or a lack of traditional symptoms (eg, minimally symptomatic). These symptom subtypes are associated with adverse outcomes and a differential response to treatment.13
Symptom subtypes may be useful in helping to stratify patients with OSA for CVD risk. In the community-based Sleep Heart Health Study,11 the excessively sleepy subtype was at greater risk of incident CVD and heart failure (hazard ratio [HR], 1.7–2.4) controlling for other known risk factors. These results are consistent with other studies demonstrating the role of excessive daytime sleepiness and OSA on hypertension,15 diabetes,16 secondary events following myocardial infarction,17 and CV mortality.18
However, the association between symptom subtypes of OSA and CVD in clinic-based cohorts has not been well evaluated. This is important to assess, as these are the patients in whom clinical decisions routinely need to be made by health care practitioners. Moreover, the relationship between subtypes and CVD might be different, as OSA severity and symptoms may be greater in clinic-based cohorts. The purpose of this study was to determine the relationship between symptom subtypes and incident CVD in a cohort of patients with OSA assessed in a sleep clinic.
METHODS
Study design, setting, and participants
The study cohort has been previously described.19 Briefly, consenting adults (≥ 19 years old) referred for suspected OSA to the University of British Columbia Hospital Sleep Disorder Laboratory for inpatient polysomnography (PSG) were recruited from 2003 to 2008. Patients who were unable to speak English or were being treated for OSA were excluded. On the night of PSG, patients completed a detailed questionnaire. The study was approved by the University of British Columbia Research Ethics Board (H13-00346) and Vancouver Coastal Health Research Institutes (V11-80199).
Study protocol
Questionnaire
A survey was given to participants that included questions related to demographics, medical history, lifestyle comorbidities, medications, family history of medical disease, sleep schedule, sleep-related symptoms, and coexisting sleep disorders (including restless legs syndrome and insomnia). Sleep-specific questionnaires included the Epworth Sleepiness Scale (ESS)20 and the Pittsburgh Sleep Quality Index.21 Comorbidities and potential confounders were determined based on self-reports from questionnaires. Prior history of CVD was determined based on previous self-reported doctor diagnoses of myocardial infarction, cardiac arrhythmias, angina, and congestive heart failure. Smoking status (currently smoking vs not currently smoking) and presence of diabetes were also self-reported. Body mass index was ascertained by direct measurement (with the patient wearing light clothing) the night of PSG.
Polysomnography
Sleep and its stages were documented using standard electroencephalographic, electrooculographic and electromyographic criteria (Sandman 10.1 software, Natus Inc., Middleton, WI). Electroencephalogram was recorded with electrodes applied to central, occipital, and frontal areas. Electromyographic activity was recorded from the submental and anterior tibialis muscles. Airflow was detected by nasal pressure and a thermocouple. A single electrocardiogram (modified V2) was monitored to detect cardiac arrhythmias. Arterial oxygen saturation was monitored continuously with a pulse oximeter attached to the index finger. Chest wall and abdominal movements were monitored by bands placed around the chest and abdomen. The entire record was manually scored for sleep stage, apnea type, and duration by a registered polysomnographic technologist and reviewed by sleep medicine physicians blinded to symptom clusters. Respiratory events were scored using standard American Academy of Sleep Medicine criteria. Apneas were defined as cessation of airflow for > 10 seconds, and hypopneas were defined as a 30% decrease in airflow for > 10 seconds associated with arousal or a 3% desaturation.22 Patients were diagnosed as having OSA based on an AHI of ≥ 5 events/h. An AHI between 5 and 15 events/h was considered mild, ≥ 15–30 events/h was considered moderate, and ≥ 30 events/h was considered severe OSA.22
Ascertainment of cardiovascular events
The major outcome was a composite of incident fatal or nonfatal CV and cerebrovascular events; this composite outcome included 7 events: myocardial infarction, stroke, unstable angina, coronary artery bypass graft, percutaneous coronary intervention, cardiac resynchronization therapy, and defibrillation. Follow-up time was 8 years from the PSG date for all patients, unless an event occurred (in which case patients were censored at that date). The supplemental material includes clinical codes indicating deaths from cardiovascular-related causes (summarized in Table S1 (340.8KB, pdf) in the supplemental material), and hospitalizations, procedures, and events codes and definitions are in Table S2 (340.8KB, pdf) in the supplemental material. These events were identified by deterministic linkage of consenting patients to different provincial health databases through Population Data BC (PopdataBC), as in previous studies.23 Coding for the databases was previously validated (see https://www.popdata.bc.ca/data/listings).24,25 Only residents of British Columbia were included in the analysis; to be considered a resident, we required continuous provincial health registration with no larger than a 93-day gap in registration following the PSG date.
Continuous positive airway pressure adherence
Continuous positive airway pressure (CPAP) adherence was determined by chart review. Two authors (B.P., A.J.H.A., or M.M.) independently reviewed each medical chart for objective and subjective data on CPAP adherence. CPAP providers’ reports and patient reports dictated in physicians’ notes were used to determine adherence. Adherence was defined as a minimum of 4 h/night for at least 70% of nights.26 In the absence of objective measures, physician notes indicating a clear positive response to CPAP prescription and usage were considered as adherent to treatment. Nonadherence was defined as: reported use below 4 h/night for at least 70% of the nights, clear intolerance to CPAP, and failure to return for a follow-up consultation after being prescribed. Interrater reliability between chart reviewers was excellent (kappa value of 0.99).
Statistical analysis
A latent class analysis was performed among patients with OSA using 8 symptom questions plus the ESS, reflecting questions similar to prior publications8–11,27 (Table 1). The optimal number of clusters was defined based on the Bayesian information criterion (BIC) value and the clinical relevance of the resulting symptom subtypes. BIC is a criterion for model selection based, in part, on the likelihood function and including a penalty term related to the number of model parameters to avoid overfitting. Lower BIC values indicate better model fit, and, thus, the optimal cluster solution was defined as that with the minimum BIC value. We examined the symptom characteristics of the resulting subtypes. If reasonably distinct clinical interpretations were observed for the optimal solution based on BIC, this was chosen as the final number of subtypes. Otherwise, we examined the symptom characteristics for 1 fewer and 1 additional subtype to determine if more precise clinical interpretations emerged.
Table 1.
Questions used to produce the symptom subtypes using latent class analysis.
| Over the last 2 weeks, how often have you been bothered by any of the following problems? [Feeling tired or having little energy] |
| Over the past month, how likely are you to doze off or fall asleep when sitting down and talking to someone? |
| Over the past month, how likely are you to doze off or fall asleep in the following situation(s), in contrast to feeling just tired? [When watching TV] |
| On average how many hours do you spend napping during the daytime on weekdays/wends? |
| Over the past month, how likely are you to doze off or fall asleep in the following situation(s), in contrast to feeling just tired? [In a car while stopped for a few minutes in traffic] |
| Do you have difficulty falling or staying asleep? |
| On average, how many days/nights during the last month have you snored or been told you snored? |
| When falling asleep, how often do you have “restless legs” (a feeling of crawling, aching, or inability to keep your legs still)? |
| Epworth Sleepiness Scale score (in quartiles) |
After identifying the optimal number of subtypes, we evaluated differences among subtypes and patients without OSA for incident CV outcomes. Kaplan Meier curves were used to depict survival probabilities according to symptom subtypes. Associations of baseline variables and incidence of CV events were modeled using Cox proportional hazards models to estimate HR with 95% confidence intervals (CIs); only first events were used in the analysis. We assessed univariate associations of covariates with CV events and then constructed a final adjusted model based on a priori variables felt to be important. The adjusted model included age, sex, AHI, body mass index (BMI), hypertension, smoking, diabetes, and prior heart disease. Sensitivity analysis was also done including only patients with an AHI ≥ 15 events/h.
A supplemental analysis added CPAP adherence to the final model as a covariate. A 3-level categorization was created: Patients were classified as prescribed and adherent, prescribed and nonadherent, and not prescribed. We used similar descriptive and inferential statistics to investigate the association between CPAP adherence and CV events in this cohort. For this analysis, only patients prescribed CPAP were included.
Statistical analyses were performed using Statistical Analysis System (SAS) software (version 9.4; SAS Institute, Cary, NC).
RESULTS
A total of 1,795 patients were recruited. Of these, 70 did not have adequate follow up due to British Columbia nonresidence or lack of consent for data linkage. An additional 118 patients were excluded because they were missing at least 5 of the symptom questions that were necessary to be grouped into an OSA symptom subtype. The final number of patients included in the analysis was 1,607 (Table 2). The majority were men (66.1%), with a mean (standard deviation) age of 49.5 (11.8) years, BMI of 31.8 (9.0) kg/m2, and AHI of 22.3 (21.8) events/h.
Table 2.
Characteristics according to symptom subtype.
| Total (n = 1,607) | Non-OSA (n = 315) | Minimally Symptomatic Subtype (n = 128) | Disturbed Sleep without Excessive Sleepiness Subtype (n = 457) | Moderate Sleepiness with Disturbed Sleep Subtype (n = 515) | Excessively Sleepiness with Disturbed Sleep Subtype (n = 192) | P | |
|---|---|---|---|---|---|---|---|
| AHI (events/h) | 22.27 (2.82) | 2.39 (1.61) | 28.14 (19.65) | 24.58 (18.91) | 26.84 (20.90) | 33.26 (29.10) | < .001 |
| Age (years) | 49.51 (11.82) | 45.23 (12.00) | 53.47 (12.24) | 50.00 (12.10) | 50.00 (11.26) | 51.43 (10.12) | < .001 |
| BMI (kg/m2) | 31.75 (9.02) | 29.86 (6.89) | 33.33 (6.98) | 31.59 (6.74) | 31.95 (6.74) | 33.60 (8.42) | < .001 |
| ESS score* | 8.84 (5.82) | 8.10 (5.80) | 5.68 (3.58) | 3.76 (3.13) | 11.02 (2.68) | 18.40 (2.06) | < .001 |
| Categorical variables, n (%) | |||||||
| Sex, male | 1,062 (66.09) | 181 (57.46) | 100 (78.13) | 295 (64.55) | 349 (67.77) | 137 (71.35) | < .01 |
| Hypertension, yes | 265 (16.49) | 33 (10.48) | 25 (19.53) | 78 (17.07) | 91 (17.67) | 38 (19.79) | .024 |
| Diabetes, yes | 165 (10.21) | 22 (6.98) | 15 (11.72) | 37 (8.10) | 64 (12.43) | 26 (13.54) | .024 |
| Prior heart disease, yes | 60 (3.73) | 7 (2.22) | 6 (4.69) | 15 (3.28) | 24 (4.66) | 8 (4.17) | 0.42 |
| Current smoker, yes | 157 (12.04) | 43 (13.65) | 12 (9.30) | 37 (8.01) | 80 (15.41) | 28 (14.43) | < .01 |
Continuous variables are presented as means and standard deviation, P value from analysis of variance, chi-square test comparing variables across subtypes. *ESS scores ranged from 0 to 24. AHI = apnea-hypopnea index, BMI = body mass index, ESS = Epworth Sleepiness Scale, OSA = obstructive sleep apnea.
OSA symptom clusters
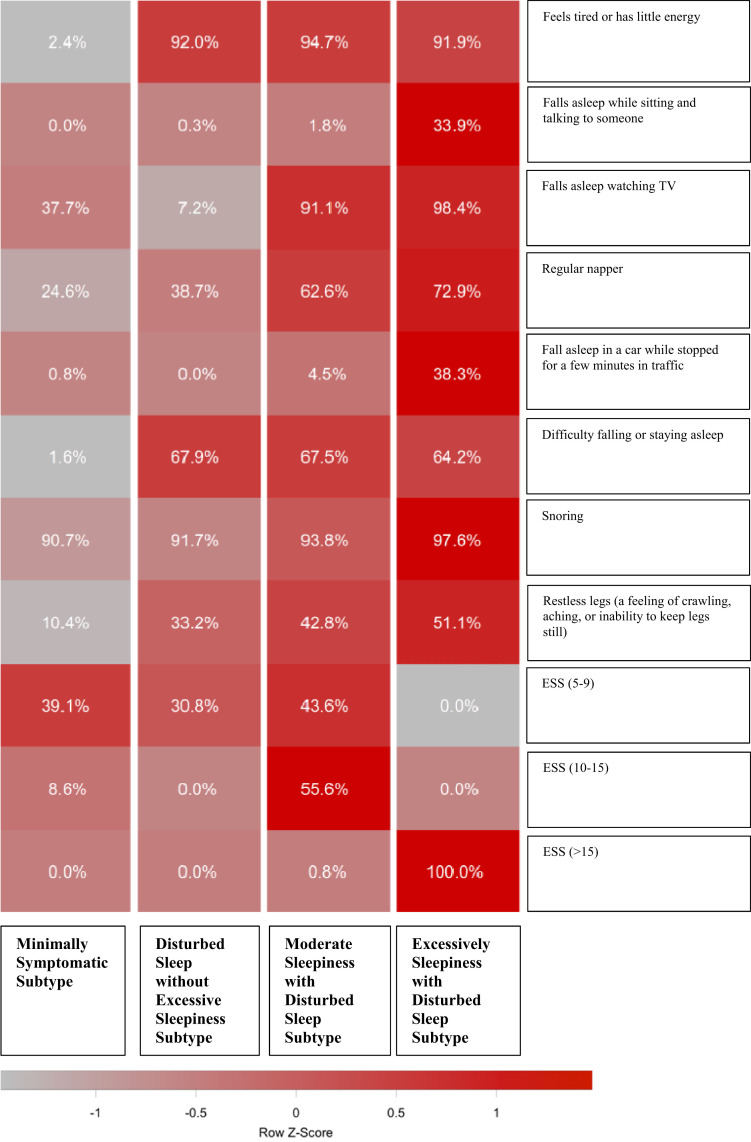
Clustering analysis among patients with AHI ≥ 5 (n = 1,292) identified 4 optimal symptom subtypes. The relative proportion of each symptom and the ESS severity group across the symptom subtypes are in Figure 1. We identified 3 subtypes that were similar to those observed in the previous literature:8–11,18,26 the disturbed sleep without excessive sleepiness (n = 457; 35.4%), the excessively sleepy with disturbed sleep (n = 192; 14.9%), and the minimally symptomatic (n = 128; 9.9%). There was also an additional subtype characterized as moderately sleepy with disturbed sleep (n = 515; 39.9%). Disturbed sleep was more prevalent in our cohort than it was in the Sleep Heart Health Study cohort. This could be the result of the nature of our cohort (clinic based) or subtle differences in the questions that were included.
Figure 1. Symptom profile of the identified obstructive sleep apnea symptom subtypes in the UBC Sleep Clinic Cohort.
The relative differences in symptom burden among subtypes are shown by the color scale, which represents the standardized (z-score) symptom proportion or ESS severity category across groups. Brighter red indicates higher relative symptom burden. ESS = Epworth Sleepiness Scale, UBC = University of British Columbia.
Table 2 summarizes the clinical characteristics of these symptom subtypes. All 4 subtypes had a mean age significantly higher than that of the non-OSA group. The minimally symptomatic subtype had a higher BMI and age than the other subtypes and a higher percentage of men; however, these differences were relatively small from a clinical standpoint. There were significant differences in AHI among the subgroups, but the magnitude of the differences was small. When compared to the non-OSA group, all 4 subtypes had higher proportions of hypertension, diabetes, and prior heart disease.
Association of CV events with symptom clusters
There were 252 events in patients with OSA; of these, 140 were first events and were used in the analysis (the supplemental material includes a breakdown of the types of events in Table S3 (340.8KB, pdf) in the supplemental material). The majority of first events were myocardial infarction, unstable angina, or percutaneous coronary intervention. In univariate Cox proportional hazards analyses, older age (P < .001), more severe AHI (P = .002), hypertension (P < .001), diabetes (P < .001), and prior heart disease (P = <0.001) were significantly associated with higher event rates.
The non-OSA controls had the lowest incidence rate of first events within 8 years of PSG (0.64 events/100 person years). In patients with OSA, the minimally symptomatic subtype (0.97 events/100 person years had the lowest incidence followed by the disturbed sleep without excessive sleepiness subtype and the moderately sleepy with disturbed sleep subtypes (both with 1.31 events/100 person years) and finally the excessively sleepy with disturbed sleep subtype (1.60 events/100 person years).
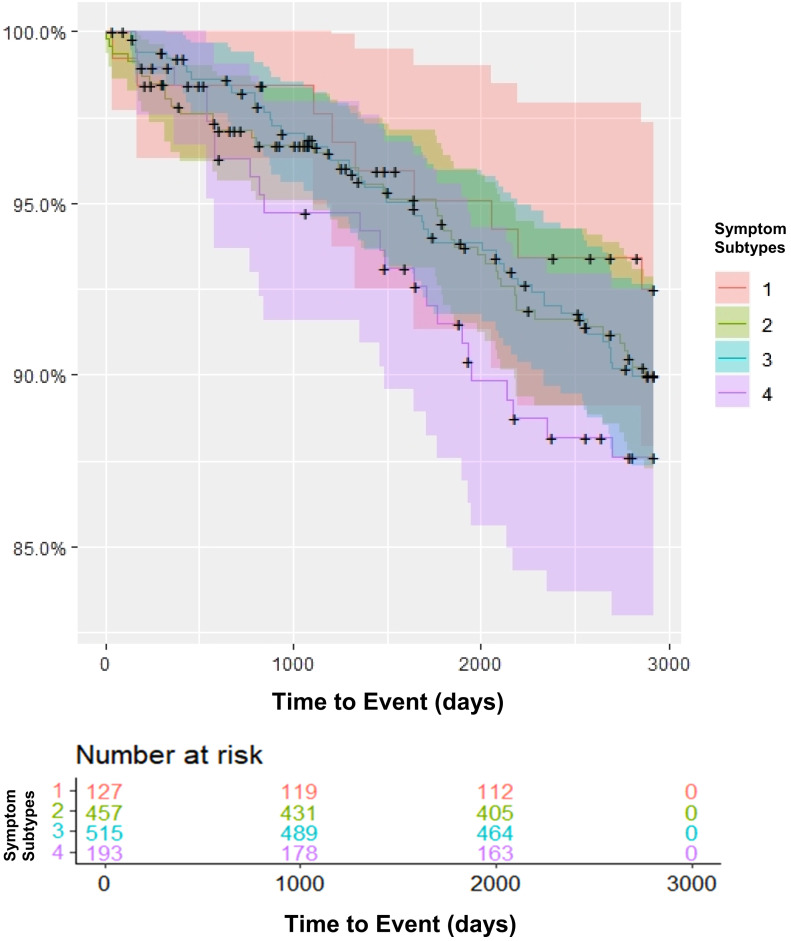
Kaplan Meier curves (Figure 2) and Cox proportional hazards models were used to compare first occurrence of CV events over time between symptom subtypes in patients with OSA. HR of the association between symptom subtypes and incident CVD, after adjusting for age, BMI, sex, AHI, hypertension, smoking status, diabetes, and prior heart disease are shown in Table 3. In the adjusted models, we observed an HR > 1 when comparing each of the symptomatic subtypes to the minimally symptomatic group; however, only the excessively sleepy with disturbed sleep subtype had a significantly higher risk (HR [95% CI] = 2.25 [1.02, 4.94]; P = .04).
Figure 2. Kaplan-Meier curve of incident cardiovascular/cerebrovascular events by symptom subtype.
Symptom subtypes: 1 = Minimally symptomatic, 2 = Disturbed sleep without excessive sleepiness, 3 = Moderate sleepy with disturbed sleep, 4 = Excessively sleepy with disturbed sleep.
Table 3.
Cox proportional hazards model with incident cardiovascular/cerebrovascular events as the outcome.
| Estimate | Standard Error | P | Hazard Ratio (95% CI) | |
|---|---|---|---|---|
| Disturbed sleep without excessive sleepiness subtype* | 0.64 | 0.39 | .10 | 1.90 (0.88, 4.12) |
| Moderate sleepiness with disturbed sleep subtype* | 0.58 | 0.38 | .12 | 1.80 (0.85, 3.78) |
| Excessively sleepiness with disturbed sleep subtype* | 0.81 | 0.40 | .04 | 2.25 (1.02, 4.94) |
| Male sex | 0.42 | 0.24 | .08 | 1.52 (0.96, 2.42) |
| Age | 0.07 | 0.01 | < .001 | 1.08 (1.06, 1.10) |
| BMI | 0.02 | 0.01 | .01 | 1.02 (1.01, 1.04) |
| AHI | 0.001 | 0.005 | .81 | 1.01 (0.99, 1.01) |
| Hypertension | 0.30 | 0.22 | .17 | 1.35 (0.88, 2.09) |
| Diabetes | 0.50 | 0.24 | .04 | 1.64 (1.03, 2.63) |
| Prior heart disease | 0.94 | 0.29 | .001 | 2.60 (1.45, 4.53) |
| Current smoker | 0.61 | 0.28 | .03 | 1.85 (1.06, 3.22) |
*Minimally symptomatic group as the reference. AHI = apnea-hypopnea index, BMI = body mass index, CI = confidence interval.
As a sensitivity analysis, we determined whether results were similar in the subset of patients with moderate to severe OSA (AHI ≥ 15 events/h). The HR of events between the excessively sleepy with disturbed sleep and the minimally symptomatic subtype was very similar to the main analysis (HR = 2.53 [0.95, 6.76]; P = .06) when a higher AHI threshold was used.
We also assessed which questions (Table 1) were driving the association of symptoms with CV events (Table 4). We individually assessed univariate and multivariate relationships between individual question responses and incident first events. In the univariate and multivariate analyses, 3 symptoms were suggestively associated with CV risk (P < .1); these included dozing while talking, the presence of restless legs, and daytime napping. ESS score per se was not significantly associated with events. These 3 questions were combined in a multivariate model; dozing while talking (HR [95% CI] = 4.23 [1.61, 11.16]; P = < 0.01) and restless legs remained significantly associated with incident events (HR [95% CI] = 1.68 [1.12, 2.49]; P = .01).
Table 4.
Cox proportional hazards models describing associations between symptom questions and events.
| Model | Estimate | Standard Error | P | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| Unadjusted | ||||
| Feeling tired or having little energy | 0.22 | 0.27 | .40 | 1.25 (0.74, 2.11) |
| Doze while talking to someone | 1.76 | 0.39 | < .001 | 5.83 (2.72, 12.50) |
| Fall asleep watching TV | 0.72 | 0.41 | .10 | 2.05 (0.92, 4.54) |
| Napping | 0.34 | 0.19 | .07 | 1.41 (0.97, 2.04) |
| Doze while stopped for a few minutes in traffic | −0.35 | 0.72 | .62 | 0.70 (0.17, 2.79) |
| Difficulty falling or staying asleep | 0.20 | 0.19 | .29 | 1.23 (0.84, 1.79) |
| Snoring | 0.40 | 0.32 | .21 | 1.49 (0.80, 2.80) |
| Restless legs | 0.49 | 0.18 | .0073 | 1.64 (1.14, 2.35) |
| ESS quartile | 0.26 | 0.27 | .35 | 1.30 (0.76, 2.22) |
| Adjusted* | ||||
| Feeling tired or having little energy | 0.38 | 0.27 | .15 | 1.47 (0.87, 2.48) |
| Doze while talking to someone | 1.58 | 0.42 | < .001 | 4.88 (2.14, 11.10) |
| Fall asleep watching TV | 0.74 | 0.46 | .11 | 2.09 (0.85, 5.15) |
| Napping | 0.33 | 0.20 | .09 | 1.40 (0.95, 2.07) |
| Doze while stopped for a few minutes in traffic | 0.023 | 0.66 | .97 | 1.02 (0.28, 3.75) |
| Difficulty falling or staying asleep | 0.32 | 0.21 | .13 | 1.38 (0.90, 2.11) |
| Snoring | 0.49 | 0.32 | .12 | 1.63 (0.87, 3.04) |
| Restless legs | 0.43 | 0.19 | .003 | 1.54 (1.05, 2.25) |
| ESS quartile | 0.38 | 0.30 | .21 | 1.46 (0.81, 2.65) |
| Adjusted plus** | ||||
| Doze while talking to someone | 1.48 | 0.41 | < .001 | 4.40 (1.97, 9.87) |
| Napping | 0.27 | 0.20 | .17 | 1.32 (0.89, 1.95) |
| Restless legs | 0.41 | 0.20 | .008 | 1.51 (1.02, 2.24) |
*Models adjusted for sex, age, body mass index, apnea-hypopnea index, hypertension, diabetes, prior cardiovascular disease, and current smoking status (each question in a separate model). **Adjusted for covariates above with all symptom questions that had a P value below .1 in the univariate model added. CI = confidence interval, ESS = Epworth Sleepiness Scale.
CPAP adherence and CV events
Of the 1,292 patients with OSA, 902 had CPAP data available; in the remainder (n = 390), either the medical record was unavailable or no data were available in the record about CPAP use/prescription. Of the 902 patients, 365 patients were not prescribed CPAP, 180 were nonadherent, and the remaining 357 were adherent. Patients were not prescribed CPAP because of many reasons, including mild OSA in the absence of symptoms, alternative therapies (eg, dental appliance), or refusal. Of note, CPAP adherence data were only available at 1 time point (usually between 30 and 60 days after PSG).
Patients who were CPAP adherent were older, had more severe OSA, were more likely to be male, were more obese, and were more likely to have other CV risk factors (hypertension, diabetes) than patients who were not prescribed CPAP or who were nonadherent ( Table S4 (340.8KB, pdf) in the supplemental material). In addition, adherence varied considerably by symptom subtypes, with the lowest adherence found in the minimally symptomatic group ( Table S5 (340.8KB, pdf) in the supplemental material). The excessively sleepy with disturbed sleep subtype (57.28%) and the moderately sleepy with disturbed sleep subtype (50.51%) had significantly greater adherence compared to the minimally symptomatic subtype (41.67%) and the disturbed sleep without excessive sleepiness subtype (42.86%).
As an exploratory analysis, we calculated the proportion of patients with any CV event stratified by CPAP adherence (Table 5). In patients with OSA adherent to CPAP, rate of events was similar regardless of symptom subclass; HR of the excessively sleepy with disturbed sleep compared to the minimally symptomatic subtype in adherent patients was 1.26 [0.33, 4.86]; P = .73. However, in those nonadherent to CPAP, patients in the excessively sleepy with disturbed sleep subtype had an increased rate of events, although this was not significant (HR [95% CI] = 2.07 [0.21, 20.79]; P = .54) likely due to small numbers of events. CPAP adherence was not associated with incident CV events (HR = 1.05 [0.52, 2.13]; P = .89) after controlling for confounders ( Table S6 (340.8KB, pdf) in the supplemental material).
Table 5.
Number and percentage of patients with CV events by CPAP adherence.
| Adherent to CPAP (n = 333) | Not Adherent (n = 168) | |
|---|---|---|
| Minimally symptomatic subtype | 3 (10.0%) | 1 (6.67%) |
| Disturbed sleep without excessive sleepiness | 10 (10.31%) | 3 (5.56%) |
| Moderate sleepiness with disturbed sleep | 17 (11.49%) | 6 (8.11%) |
| Excessively sleepiness with disturbed sleep | 7 (12.07%) | 3 (12.00%) |
CPAP = continuous positive airway pressure, CV = cardiovascular.
DISCUSSION
There were 3 major findings of our study. First, we identified 4 symptom subtypes based on a combination of disturbed sleep and excessive sleepiness. Second, the excessively sleepy with disturbed sleep subtype was associated with a significantly increased risk of a first CV event compared to the minimally symptomatic subtype (HR = 2.25 [1.02, 4.94]). Finally, 2 particular symptoms (restless legs and dozing off or sleeping while talking to someone) were strongly associated with incident risk of CVD.
Our study confirms and extends previous work with respect to symptom subtypes. Similar to previous studies, 3 of the 4 subtypes identified in this cohort included patients characterized by disturbed sleep, minimal symptoms, and excessive sleepiness with disturbed sleep.8–11 In addition, we identified a fourth subtype characterized by moderate sleepiness together with disturbed sleep. Additional subtypes have also been identified in other cohorts. For example, the study by Keenan and colleagues,9 using an international clinic-based sample, identified 5 optimal subtypes, including the core 3 (labeled disturbed sleep, minimal symptoms, and upper airway symptoms with sleepiness) and 2 additional subtypes characterized as less symptomatic (labeled upper airway symptoms dominant and sleepiness dominant). Similarly, the study by Mazzotti and colleagues,11 identified the 3 subtypes of disturbed sleep, minimally symptomatic, and excessively sleepy, and a fourth subtype labeled moderately sleepy, which has similarities to the sleepiness dominant OSA subtype identified by Keenan et al.9 Thus, our observation of 4 OSA subtypes, including the 3 identified in a prior study at our clinic and 1 additional subtype defined by both moderate sleepiness and disturbed sleep, is consistent with prior studies.
The second major finding was the association of symptom subtypes with incident first CV events. Specifically, the excessively sleepy with disturbed sleep subtype had a significantly increased rate of first CV events compared to the minimally symptomatic subtype, and the other subtypes trended in a similar direction. This confirms similar findings from the Sleep Heart Health Study community-based cohort, but, importantly, increases generalizability to a clinic-based cohort. Confirming the association between symptom subtypes and incident CV events in patients presenting for evaluation has important implications for clinical care. Specifically, these patients may be targeted for more aggressive management of CV risk factors and OSA. These findings are also consistent with a recently published study that assessed the relationship between symptom clusters and cardiovascular mortality in a clinic-based cohort of 780 patients from Chile with moderate to severe OSA.18 They showed that patients in the excessively sleepy cluster were significantly more likely to experience incident mortality (HR = 5.47, 1.74–8.29, P < .01), with a trend found in patients in the disturbed sleep cluster (HR = 2.87, 0.76–3.82, P = .11). Our study confirms and extends these findings by examining not only CV mortality, but also nonlethal CV events. In our study, we also showed that commonly appreciated CV risk factors were also risk factors in our cohort (eg increased age, male sex, diabetes). Therefore, in addition to symptoms, these should also be considered when considering future CV risk.
We also showed that 2 symptoms may be particularly relevant in CV prediction. Specifically, the presence of restless legs symptoms was significantly associated with incident events. These extend the findings of others who have also shown a significant relationship between restless legs and CVD.28 Furthermore, these findings are consistent with the study by Zinchuck and colleagues7 who showed that periodic limb movements, which are commonly found in patients with restless legs, were significantly more likely to experience an incident CV event. However, in our study, when periodic limb movement frequency (both with and without arousal) was added to the models, they were neither significantly associated with events nor did they affect the HR of the restless legs syndrome question responses (data not shown), suggesting that it was restless legs syndrome symptoms rather than limb movements per se driving this relationship.
In our exploratory CPAP analysis, we showed that both the excessively sleepy with disturbed sleep subtype and the moderately sleepy with disturbed sleep subtype were more likely to be prescribed and adhere to CPAP. The association between subtypes and CV events may be more robust in patients who were nonadherent vs those who were adherent, suggesting that CPAP adherence may confound the effect of symptoms on CV mortality and morbidity. However, we believe that the results of our CPAP analysis need to be viewed cautiously. First, we only had CPAP data on a portion of our patients. Second, our CPAP adherence data was largely based on chart review as opposed to objective CPAP downloads. Third, we only had CPAP information at 1 time point (soon after CPAP prescription) and not over a prolonged time period. Although early CPAP adherence is a strong predictor of later adherence,29 some of the patients who were “adherent” may have stopped CPAP over 8 years and others who were “not adherent” might have restarted CPAP at a later date.
Our study has many strengths. These include the use of objective outcomes for incident CV events, use of PSG to assess OSA severity, and the ability to adjust for a variety of confounders. In addition, this is 1 of the first studies of symptom subtypes and CVD prognosis in a clinical OSA population, which is of substantial clinical relevance. However, we also acknowledge that the study has many limitations. First, we used a clinic-based cohort, and referral bias is a potential issue. For example, truly asymptomatic patients would be less likely to be referred than patients who were either symptomatic or had clinical features predicting more severe disease (ie, high BMI or comorbidities). On the other hand, the relatively greater severity of OSA and symptoms could also be viewed as advantages, especially given that these are the patients in whom health care providers routinely need to be made. Second, as with any observational study, there is a risk for residual confounding from unmeasured confounders that were not included in our data collection. Third, our cohort was from 1 clinic, and the generalizability of the study could be questioned. However, our results are consistent with those of Mazzotti et al11 and Labarca et al,18 who analyzed a community-based and clinic-based cohort, respectively, and found increased CV risk among the excessively sleepy. Fourth, we did not have information on whether patients with restless legs syndrome were treated or taking medications for their symptoms.
CONCLUSIONS
This is one of the first studies to demonstrate that OSA patients seen in the clinic who are in the excessively sleepy with disturbed sleep subtype are significantly more likely to have a CV event in the 8 years after PSG compared to the minimally symptomatic subtype. Two symptoms (restless legs and dozing off or sleeping while talking to someone) may be particularly informative of CV risk. These findings further underscore the importance of understanding clinical heterogeneity and incorporating symptom subtype definitions into routine clinical care.
ABREVIATTIONS
- AHI
apnea-hypopnea index
- BIC
Bayesian Information Criterion
- BMI
body mass index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- CV
cardiovascular
- CVD
cerebrovascular disease
- ESS
Epworth Sleepiness Scale
- HR
hazard ratio
- OSA
obstructive sleep apnea
- PSG
polysomnography
DISCLOSURE STATEMENT
All the authors have seen and approved this manuscript. Work for this study was performed at the University of British Columbia. This work was funded by the Canadian Sleep and Circadian Network (CSCN), BC Lung Association Operating Grant, CIHR (Sleep Disordered Breathing Team Grant), Vancouver Coastal Health Research Institutes Investigator Award for (Dr. Rachel Jen). The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. All inferences, opinions and conclusions drawn in this paper are those of the authors, and do not reflect the opinions or policies of the Data Stewards(s). Data extracts were provided by the British Columbia Ministry of Health. These extracts are cited below in compliance with Population Data BC protocols. The authors report no conflicts of interest. Canadian Institute for Health Information [creator] (2018): Discharge Abstract Database (Hospital Separations). Population Data BC [publisher]. Data Extract. MOH (2018). http://www.popdatabc.ca/data BC Vital Statistics Agency [creator] (2018): Vital Statistics Deaths. Population Data BC [publisher]. Data Extract BC Vital Statistics Agency (2018). http://www.popdatabc.ca/data Cardiac Services BC Consolidation Registry [creator] (2018): Cardiac Services BC (CSBC). Population Data BC [publisher]. Data Extract Cardiac Services BC (2018). http://www.popdatabc.ca/data
REFERENCES
- 1. Benjafield AV , Ayas NT , Eastwood PR , et al . Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis . Lancet Respir Med. 2019. ; 7 ( 8 ): 687 – 698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marin JM , Carrizo SJ , Vicente E , Agusti AG . Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study . Lancet. 2005. ; 365 ( 9464 ): 1046 – 1053 . [DOI] [PubMed] [Google Scholar]
- 3. Malhotra A , Ayappa I , Ayas N , et al . Metrics of sleep apnea severity: beyond the apnea-hypopnea index . Sleep. 2021. ; 44 ( 7 ): zsab030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez-Garcia MA , Campos-Rodriguez F , Barbé F , Gozal D , Agustí A . Precision medicine in obstructive sleep apnoea . Lancet Respir Med. 2019. ; 7 ( 5 ): 456 – 464 . [DOI] [PubMed] [Google Scholar]
- 5. Kendzerska T , Leung RS , Gershon AS , Tomlinson G , Ayas N . The Interaction of obesity and nocturnal hypoxemia on cardiovascular consequences in adults with suspected obstructive sleep apnea. A historical observational study . Ann Am Thorac Soc. 2016. ; 13 ( 12 ): 2234 – 2241 . [DOI] [PubMed] [Google Scholar]
- 6. Azarbarzin A , Sands SA , Stone KL , et al . The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study . Eur Heart J. 2019. ; 40 ( 14 ): 1149 – 1157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zinchuk AV , Jeon S , Koo BB , et al . Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea . Thorax. 2018. ; 73 ( 5 ): 472 – 480 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ye L , Pien GW , Ratcliffe SJ , et al . The different clinical faces of obstructive sleep apnoea: a cluster analysis . Eur Respir J. 2014. ; 44 ( 6 ): 1600 – 1607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keenan BT , Kim J , Singh B , et al . Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis . Sleep. 2018. ; 41 ( 3 ): zsx214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim J , Keenan BT , Lim DC , Lee SK , Pack AI , Shin C . Symptom-based subgroups of Koreans with obstructive sleep apnea . J Clin Sleep Med. 2018. ; 14 ( 3 ): 437 – 443 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazzotti DR , Keenan BT , Lim DC , Gottlieb DJ , Kim J , Pack AI . Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes . Am J Respir Crit Care Med. 2019. ; 200 ( 4 ): 493 – 506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McLachlan G , Peel D . Finite Mixture Models. New York, NY: : John Wiley & Sons, Inc; .; 2000. . [Google Scholar]
- 13. Hagenaars J , McCutcheon A , eds. Applied Latent Class Analysis. Cambridge, UK: : Cambridge University Press; ; 2002. . [Google Scholar]
- 14. Pien GW , Ye L , Keenan BT , et al . Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic sleep apnea cohort . Sleep. 2018. ; 41 ( 3 ): zsx201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kapur VK , Resnick HE , Gottlieb DJ ; Sleep Heart Health Study Group . Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep. 2008. ; 31 ( 8 ): 1127 – 1132 . [PMC free article] [PubMed] [Google Scholar]
- 16. Lindberg E , Berne C , Franklin KA , Svensson M , Janson C . Snoring and daytime sleepiness as risk factors for hypertension and diabetes in women—a population-based study . Respir Med. 2007. ; 101 ( 6 ): 1283 – 1290 . [DOI] [PubMed] [Google Scholar]
- 17. Xie J , Sert Kuniyoshi FH , Covassin N , et al . Excessive daytime sleepiness independently predicts increased cardiovascular risk after myocardial infarction . J Am Heart Assoc. 2018. ; 7 ( 2 ): e007221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Labarca G , Dreyse J , Salas C , Letelier F , Jorquera J . A validation study of four different cluster analyses of obstructive sleep apnea and the incidence of cardiovascular mortality in an Hispanic population . Chest. 2021. ; 160 ( 6 ): 2266 – 2274 . [DOI] [PubMed] [Google Scholar]
- 19. Sandford AJ , Ha A , Ngan DA , et al . Adhesion molecule gene variants and plasma protein levels in patients with suspected obstructive sleep apnea . PLoS One. 2019. ; 14 ( 1 ): e0210732 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johns MW . A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 21. Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 22. Berry RB , Brooks R , Gamaldo C , et al. ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.4 . Darien, IL: : American Academy of Sleep Medicine; ; 2017. . [Google Scholar]
- 23. Ayas NT , Hirsch Allen AJ , Fox N , et al . C-reactive protein levels and the risk of incident cardiovascular and cerebrovascular events in patients with obstructive sleep apnea . Lung. 2019. ; 197 ( 4 ): 459 – 464 . [DOI] [PubMed] [Google Scholar]
- 24. Humphries KH , Rankin JM , Carere RG , Buller CE , Kiely FM , Spinelli JJ . Co-morbidity data in outcomes research: Are clinical data derived from administrative databases a reliable alternative to chart review? J Clin Epidemiol. 2000. ; 53 ( 4 ): 343 – 349 . [DOI] [PubMed] [Google Scholar]
- 25. Lee DS , Stitt A , Wang X , et al . Administrative hospitalization database validation of cardiac procedure codes . Med Care. 2013. ; 51 ( 4 ): e22 – e26 . [DOI] [PubMed] [Google Scholar]
- 26. Kribbs NB , Pack AI , Kline LR , et al . Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea . Am Rev Respir Dis. 1993. ; 147 ( 4 ): 887 – 895 . [DOI] [PubMed] [Google Scholar]
- 27. Morris JL , Mazzotti DR , Gottlieb DJ , Hall MH . Sex differences within symptom subtypes of mild obstructive sleep apnea . Sleep Med. 2021. ; 84 : 253 – 258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gottlieb DJ , Somers VK , Punjabi NM , Winkelman JW . Restless legs syndrome and cardiovascular disease: A research roadmap . Sleep Med. 2017. ; 31 : 10 – 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehrtash M , Bakker JP , Ayas N . Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea . Lung. 2019. ; 197 ( 2 ): 115 – 121 . [DOI] [PubMed] [Google Scholar]