Abstract
Study Objectives:
Continuous positive airway pressure (CPAP) improves sleepiness in patients with obstructive sleep apnea, but some patients remain sleepy. The objective of this study was to identify determinants that are associated with improvements in self-reported sleepiness in patients with obstructive sleep apnea on CPAP therapy.
Methods:
A retrospective cohort study was performed in a clinic-based population to determine which variables contributed to the improvement in the Epworth Sleepiness Scale (ESS) in patients on CPAP therapy for OSA, stratified by baseline ESS score (< 11 or ≥ 11). Variables associated with ESS scores normalizing with CPAP were also assessed.
Results:
Patients with a baseline high ESS score showed greater improvements in the ESS with CPAP. When looking at interactions between baseline ESS classification and changes in ESS, we found that a higher apnea-hypopnea index was only associated with improvement in the ESS among patients with a high baseline ESS. Other assessed factors or covariates were not significantly different. When looking at ESS normalization, we found that female sex and lower body mass index were associated with a lower likelihood of ESS normalization. The difference in the rate of ESS normalization between females and males was higher with more days on CPAP.
Conclusions:
Of all the assessed factors and covariates, only the apnea-hypopnea index was associated with the change in the ESS differently in patients with a high or normal baseline ESS score. ESS normalization rates were lower in females than in males, and this disparity was amplified by more days on CPAP.
Citation:
Scharf MT, Zhang P, Walker NA, et al. Sex differences in Epworth Sleepiness Scale normalization with continuous positive airway pressure. J Clin Sleep Med. 2022;18(9):2273–2279.
Keywords: OSA, CPAP, Epworth Sleepiness Scale, sex
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleepiness remains a problem in many patients with obstructive sleep apnea despite adherence to continuous positive airway pressure therapy. The purpose of this study was to identify determinants of improvement in self-reported sleepiness with continuous positive airway pressure therapy.
Study Impact: This study showed that higher baseline Epworth Sleepiness Scale scores were associated with greater improvement in the Epworth Sleepiness Scale and that female sex was associated with lower rates of Epworth Sleepiness Scale normalization. This suggests that sex may be a determinant of persistent sleepiness in patients with obstructive sleep apnea on continuous positive airway pressure.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disease in the United States and worldwide.1 OSA is caused by intermittent collapse of the upper airway during sleep and can be treated by continuous positive airway pressure (CPAP), which serves as a pneumatic splint to maintain patency of the upper airway. Although other treatments are available, CPAP remains the mainstay of therapy for OSA.
Excessive daytime sleepiness (EDS) is a common complaint of patients with OSA. The Epworth Sleepiness Scale (ESS) is an 8-item questionnaire asking patients their likelihood of falling asleep in common situations. The ESS is commonly used in both clinical and research settings. An ESS ≥ 11 is commonly considered to indicate EDS.2–10
CPAP improves the ESS in patients with OSA, particularly in those with a high baseline ESS (≥ 11) and severe OSA.11 In patients with a normal baseline ESS, studies have shown contradictory results, with some studies showing no improvement in the ESS after treatment11 and other studies showing an improvement in the ESS.6,12 The reasons for this disparity in the literature are unknown. Furthermore, in patients with a high baseline ESS, the ESS often does not improve to the normal range with CPAP therapy. Even with a relatively high amount of nightly CPAP use, some patients continue to have an abnormally high ESS.3–5,7 Although the literature has yielded somewhat conflicting results, variables implicated in the persistence of an abnormally high ESS with CPAP therapy include depression, diabetes mellitus, heart disease,7 male sex, chronic pain,5 and younger age.6
The objectives of the present study were to (1) identify variables independently associated with an improvement in the ESS in patients with OSA on CPAP therapy, stratified by low or high ESS score, and (2) in those in the high–ESS score group, determine which characteristics are associated with a persistently high ESS score.
METHODS
Study population
A retrospective cohort study was performed on the population served by the Comprehensive Sleep Disorders Center at Rutgers Robert Wood Johnson Medical School (New Brunswick, NJ). Records on patients seen between July 1, 2016 and December 31, 2019 were reviewed. Inclusion criteria included the following: (1) age ≥ 18 years, (2) apnea-hypopnea index (AHI) ≥ 5 events/h, (3) CPAP therapy initiated within 1 year after OSA diagnosis, (4) CPAP adherence data available for at least the first 30 days after initiation of CPAP therapy, and (5) baseline and follow-up ESS data available within 90 days of starting CPAP therapy (for baseline) and 30–120 days after starting CPAP (follow-up). Because this study was done on a clinical population, in keeping with insurance requirements for CPAP therapy, patients with an AHI ≥ 15 events/h or those with an AHI ≥ 5 events/h and a recognized comorbid condition were offered CPAP therapy. Data were retrieved from the electronic medical record (Centricity, General Electric, Chicago, IL), and CPAP therapy data were obtained from either the Airview (Resmed, San Diego, CA) or Encoreanywhere/CareOrchestrator (Philips Respironics, Murrysville, PA) websites. Variables included age, sex, race, body mass index (BMI), presence of diabetes or hypertension, AHI on diagnostic sleep study, type of diagnostic sleep study, average hours of CPAP use per night for the initial 30 days of CPAP use, total days of CPAP use between initiation of CPAP and administration of the second ESS, and ESS scores. CPAP adherence was defined as use for ≥ 4 hours per night for ≥ 70% of nights within the first 30 days. Average CPAP use over the first 30 days was assessed because this point was common to all patients in this study and there were no differences in average CPAP use hours in the initial 30 days compared to the initial 90 days of CPAP use in either males or females. For patients with a high baseline ESS, the average 30-day CPAP use was 5.0 ± 2.2 hours and the average 90-day CPAP use was 5.0 ± 2.0 hours. For patients with a normal baseline ESS, the average 30-day CPAP use was 4.7 ± 2.4 hours and the average 90-day CPAP use was 4.7 ± 2.5 hours. The institutional review board at Rutgers Robert Wood Johnson Medical School approved this study (New Brunswick Health Sciences IRB—Pro20170000820), and ethical standards were observed during the study.
Self-reported sleep measure
The ESS is an 8-item questionnaire asking patients to rate their likelihood of falling asleep during common situations. Each item is scored on a scale of 0–3 with a maximum score of 24. Patients were given an ESS with written instructions on how to fill it out. If a patient asked for further assistance or clarification, it was provided by clinical staff. Higher scores indicate a higher likelihood of falling asleep. An ESS < 11 is commonly considered to be normal, while an ESS ≥ 11 indicates EDS.2–10 An improvement in a baseline ESS ≥ 11 to < 11 with CPAP therapy is considered normalization of the ESS.3,4
Sleep studies
Sleep studies were conducted by an accredited sleep laboratory. Patients had either an in-laboratory polysomnogram or a home sleep apnea test (HSAT). HSATs were done using the Apnea Risk Evaluation System device.13 The device is worn on the forehead and measures airflow from a nasal cannula connected to a pressure transducer, oxygen saturation and pulse rate from reflectance oximetry, snoring with a microphone, and head movement and position from accelerometers. The Apnea Risk Evaluation System device also provides audible alerts if the air flow or oxygen saturation signals are of poor quality to enable the patient to reposition the device. For HSATs, apneas and hypopneas associated with a desaturation of ≥ 4% were scored. In-laboratory polysomnograms were conducted according to the guidelines of The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications.14 Scoring was done according to American Academy of Sleep Medicine criteria, which recognize apneas, hypopneas associated with a desaturation of ≥ 3%, and hypopneas associated with an electrophysiologic arousal. When required by Medicare or Medicaid, the alternate rule was used. The alternate rule recognizes apneas and hypopneas associated with a desaturation of ≥ 4%. All studies were scored by a board-certified sleep physician.
Statistical methods
Patient baseline characteristics are presented using summary statistics (mean ± standard deviation, or proportions). We classified the baseline ESS scores into normal vs high (ESS < 11 vs ESS ≥ 11) and used linear regression analysis to assess the association between the change in ESS scores after CPAP and the baseline ESS classification (normal vs high), controlling for one or more of the following covariates in the statistical models: age, sex, race, BMI, presence of diabetes, presence of hypertension, AHI, average hours of CPAP use over the initial 30 days, type of sleep study on initial diagnosis (HSAT vs polysomnogram), and total days of CPAP use between initiation of CPAP and administration of the second ESS. Interactions between each covariate and ESS classification were also examined. Regression coefficient estimates, representing the mean change of the ESS associated with one unit increase in the continuous covariates or the difference between classes of a categorical variable, were presented with the 95% confidence intervals (CIs) and P values. We next defined ESS normalization as a baseline ESS ≥ 11 decreasing to < 11 after CPAP therapy. Logistic regression analysis was applied to a subset of patients whose baseline was ESS ≥ 11 to study the association of ESS normalization (yes/no) with average hours of CPAP use over the initial 30 days, sex, and the following covariates: age, race, BMI, presence of diabetes, presence of hypertension, AHI, type of sleep study on initial diagnosis (HSAT vs polysomnogram), and total days of CPAP use between initiation of CPAP and administration of the second ESS. Interactions of ESS normalization with sex and each covariate were also examined. Odds ratio (OR) estimates associated with per-unit change in a continuous covariate or a between-class comparison with respect to a categorical variable were presented with 95% CIs and P values. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Two hundred six patients were included in this study. Of these, 203 patients were started on auto-CPAP, 1 on fixed-pressure CPAP, and 2 on fixed-pressure bilevel positive airway pressure therapy. For ease of discussion, all positive airway pressure therapy in this study is referred to as CPAP. The study cohort had an age of 54.9 ± 11.7 (mean ± standard deviation) years and a BMI of 35.3 ± 7.7 kg/m2, and 56.3% of the patients were male. Ninety-seven patients (47.1%) had a baseline ESS score ≥ 11 and 109 (52.9%) had a baseline ESS score < 11. The level of OSA severity was moderate, with an AHI of 29.4 ± 23.5 events/h. The average number of days on CPAP until administration of the post-CPAP ESS was 59.4 ± 19.6. Average CPAP use was 4.8 ± 2.3 hours per day, and 62.6% of the patients met the definition for CPAP adherence within the initial 30 days of use. Baseline demographics and information are presented in Table 1.
Table 1.
Baseline characteristics.
| Variable | Overall | Female | Male |
|---|---|---|---|
| n | 206 | 90 | 116 |
| Age, y | 54.9 ± 11.7 | 57.2 ± 12.2 | 53.1 ± 11.0 |
| BMI, kg/m2 | 35.3 ± 7.7 | 37.3 ± 8.0 | 33.9 ± 7.1 |
| Diabetes, % | 28.6 | 32.2 | 25.9 |
| Hypertension, % | 51.9 | 60.0 | 45.7 |
| AHI, events/h | 29.4 ± 23.5 | 25.1 ± 18.9 | 32.7 ± 26.1 |
| HSAT, % | 64.1 | 58.9 | 68.1 |
| Average 30-day CPAP usage (h/d) | 4.8 ± 2.3 | 4.7 ± 2.5 | 5.0 ± 2.2 |
| Adherent (30-day, > 4 h/d > 70%), % | 62.1 | 55.6 | 67.2 |
| Days on CPAP | 59.4 ± 19.6 | 60.0 ± 20.8 | 58.9 ± 18.7 |
| Baseline ESS | 10.2 ± 5.4 | 10.5 ± 5.9 | 10.0 ± 5.0 |
| Post-CPAP ESS | 7.7 ± 4.4 | 8.2 ± 4.7 | 7.4 ± 4.2 |
| High baseline ESS, % | 47.1 | 51.1 | 44.0 |
Adherent means those patients meeting Medicare requirements (> 4 hours of use per day, for > 70% of days, within the initial 30 days of CPAP use). Data are presented as mean ± SD or percentages. AHI = apnea-hypopnea index, BMI = body mass index, CPAP = continuous positive airway pressure, ESS = Epworth Sleepiness Scale, HSAT = home sleep apnea test, SD = standard deviation.
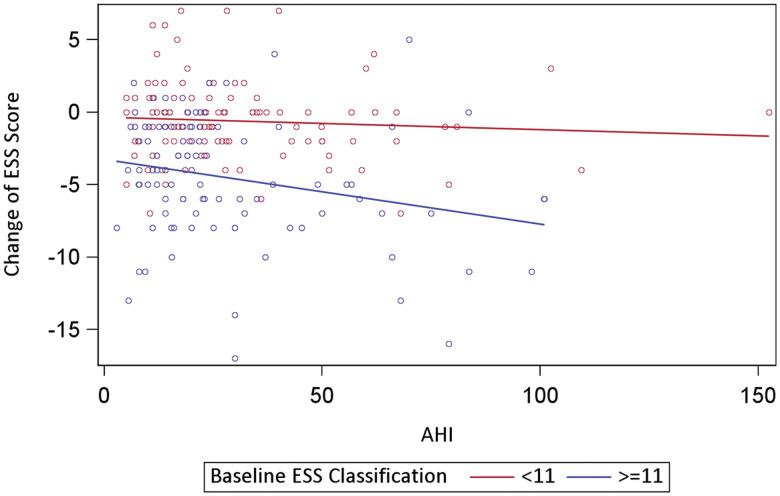
To elucidate the relationship between the change in the ESS score with CPAP and the baseline ESS classification (normal vs high), a linear regression analysis was conducted. In a model considering all included covariates (Table 2), only baseline ESS classification and days on CPAP were significant. Specifically, the results showed that the change of ESS with CPAP was statistically lower in those whose baseline ESS was classified as normal, compared to those whose baseline ESS was classified as high (β = 3.78; 95% CI, 2.79–4.77; P ≤ .001). ESS scores after CPAP decreased with number of days on CPAP (β = –0.04; 95% CI, –0.06 to –0.01; P = .003). In separate linear regression analyses with each covariate in addition to baseline ESS classification, only AHI and days on CPAP were significant. Specifically, the mean change in ESS scores after CPAP decreased by 0.03 if the AHI score increased by 1 unit (β = –0.03; 95% CI, –0.046 to –0.004; P = .018) and decreased by 0.03 if CPAP use increased by 1 day (β = –0.03; 95% CI, –0.051 to –0.002; P = .037). No statistically significant interaction between baseline ESS classification and any covariate was found. However, for those with a baseline high ESS score (≥ 11), their ESS scores significantly decreased with AHI (β = –0.05; 95% CI, –0.075 to –0.014; P = .004); for those with a normal baseline ESS score (< 11), there was no significant correlation between changes in ESS and AHI (Figure 1). These data showed that there was a greater decrease in the ESS with a higher baseline AHI only in patients with a high baseline ESS score.
Table 2.
Linear regression analysis with change in the ESS with CPAP as the dependent variable.
| Variable | β Estimatea (95% CI) | P |
|---|---|---|
| Baseline ESS (< 11 vs ≥ 11) | 3.78 (2.79 to 4.77) | < .001 |
| Average CPAP use/d (h) | –0.14 (–0.36 to 0.08) | .212 |
| Age (y) | –0.01 (–0.06 to 0.03) | .546 |
| Sex (female vs male) | 0.30 (–0.76 to 1.35) | .580 |
| Race (Black vs others) | 1.48 (–0.37 to 3.33) | .116 |
| Race (Hispanic vs others) | –0.38 (–2.42 to 1.65) | .709 |
| Race (White vs others) | 0.37 (–1.22 to 1.96) | .645 |
| BMI (kg/m2) | –0.01 (–0.08 to 0.05) | .664 |
| Hypertension (yes vs no) | 0.19 (–0.94 to 1.31) | .746 |
| AHI | –0.02 (–0.05 to 0.00) | .056 |
| CPAP use (d) | –0.04 (–0.06 to –0.01) | .003 |
| Diabetes (yes vs no) | –0.98 (–2.13 to 0.17) | .095 |
| HSAT vs polysomnogram | –0.37 (–1.42 to 0.69) | .493 |
aRegression coefficient estimates represent the mean change of ESS associated with 1-unit increase in the continuous covariates or the difference between classes of a categorical variable. High baseline ESS and increasing numbers of days on CPAP were associated with a greater decrease in the ESS. AHI = apnea-hypopnea index, BMI = body mass index, CI = confidence interval, CPAP = continuous positive airway pressure, ESS = Epworth Sleepiness Scale, HSAT = home sleep apnea test.
Figure 1. Baseline ESS was a determinant of the change in the ESS with increasing AHI.
There was a greater decrease in the ESS with a higher baseline AHI only in patients with a high baseline ESS score. AHI = apnea-hypopnea index, ESS = Epworth Sleepiness Scale.
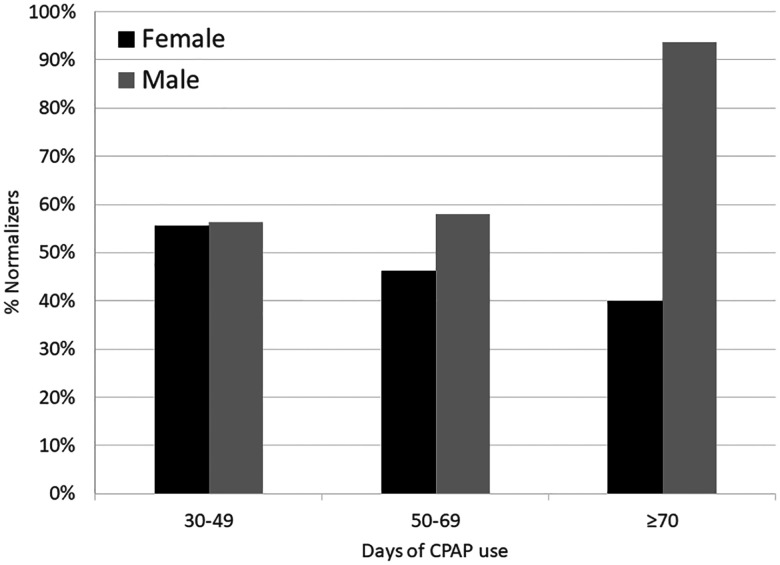
Of the 97 patients with a high baseline ESS, 40 patients had a persistently high ESS after CPAP therapy, while 57 patients developed a normal ESS. To determine which covariates were associated with normalization of the ESS with CPAP use, a logistic regression analysis was conducted with normalization of the ESS (yes vs no) as the dependent variable. Considering all included covariates, only sex and BMI were significant (Table 3). Specifically, female sex was associated with a decreased likelihood of ESS normalization compared to males (OR, 0.34; 95% CI, 0.12–0.99; P = .048) and higher BMI was associated with an increased likelihood of ESS normalization (OR of ESS normalization associated with per-unit increase in BMI was 1.08; 95% CI, 1.01–1.16; P =.031). To further explore the relationship between sex and ESS normalization, a logistic regression analysis was carried out with each covariate and sex. Only BMI was significant (OR, 1.06; 95% CI, 1.00–1.13; P = .037). Moreover, in separate logistic regression analyses that included the main effects and interaction between each covariate and sex, there was a significant interaction of days of CPAP use and sex, suggesting that the OR of normalization associated with days of CPAP use was significantly higher in males than in females (OR, 1.05; 95% CI, 1.01–1.10; P = .014 in males; OR, 0.99; 95% CI, 0.97–1.02; P = .650 in females; the ratio between the OR of males and females was 1.06; 95% CI, 1.01–1.12; P = .021). To enable visual presentation of these sex differences with longer durations of CPAP use, days of CPAP use were grouped into 30–49 days, 50–69 days, and ≥ 70 days (Figure 2). The data show that ESS normalization rates were higher in males with longer duration of CPAP use compared to females.
Table 3.
Logistic regression analysis with normalization of the ESS with CPAP (yes vs no) as the dependent variable.
| Variable | ORa (95% CI) | P |
|---|---|---|
| Average CPAP use/d (h) | 1.18 (0.941.49) | .151 |
| Age (y) | 1.01 (0.971.06) | .560 |
| Sex (female vs male) | 0.34 (0.120.99) | .048 |
| Race (Black vs others) | 0.64 (0.123.55) | .613 |
| Race (Hispanic vs others) | 1.59 (0.298.66) | .593 |
| Race (White vs others) | 2.45 (0.639.51) | .195 |
| BMI (kg/m2) | 1.08 (1.011.16) | .031 |
| Hypertension (yes vs no) | 1.85 (0.605.75) | .286 |
| AHI | 1.00 (0.981.02) | .816 |
| CPAP use (d) | 1.01 (0.991.04) | .273 |
| Diabetes (yes vs no) | 0.43 (0.141.37) | .155 |
| HSAT vs polysomnogram | 0.72 (0.26-2.00) | .524 |
aOR estimates associated with per-unit increase in a continuous covariate or between-class comparison of a categorical variable. Female sex and lower BMI were associated with lower rates of ESS normalization. AHI = apnea-hypopnea index, BMI = body mass index, CI = confidence interval, CPAP = continuous positive airway pressure, ESS = Epworth Sleepiness Scale, HSAT = home sleep apnea test, OR = odds ratio.
Figure 2. ESS normalization rates with greater duration of CPAP use.
ESS normalization rates were higher in males with longer duration of CPAP use. CPAP = continuous positive airway pressure, ESS = Epworth Sleepiness Scale.
The present analysis did not show a change in ESS normalization with increasing hours of CPAP use per night. This finding indicates that the lower rates of ESS normalization in females were not due to lower hours of CPAP use per night. To directly compare these results to previously published studies assessing the relationship between ESS normalization and hours of CPAP use,3,4 Table 4 presents the raw numbers in females and males. ESS normalization rates were generally lower in females than in males across different hours of use categories.
Table 4.
ESS normalization stratified by hours of use category and sex.
| Percentage ESS Normalization | CPAP Use (h/night) | |||||
|---|---|---|---|---|---|---|
| < 2 | 2 to < 4 | 4 to < 5 | 5 to < 6 | 6 to < 7 | ≥ 7 | |
| Female | 14.3% (1/7) | 50.0% (5/10) | 40.0% (2/5) | 81.8% (9/11) | 0.0% (0/2) | 45.5% (5/11) |
| Male | 25.0% (1/4) | 62.5% (5/8) | 83.3% (5/6) | 81.3% (13/16) | 50.0% (5/10) | 85.7% (6/7) |
ESS normalization rates were generally lower in females than in males across different hours of use categories. CPAP = continuous positive airway pressure, ESS = Epworth Sleepiness Scale.
DISCUSSION
The purpose of the present study was to assess whether the variables associated with improvement in the ESS in patients with OSA on CPAP were different if the baseline ESS was high or normal and, in patients with a high baseline ESS, to assess which variables were associated with improvement of the ESS to a normal value. Perhaps the most striking finding was that when looking at improvement of the ESS from high to normal with CPAP (ie, normalization of the ESS), female sex was associated with lower rates of normalization. Furthermore, there was a significant interaction between ESS normalization, sex, and days of CPAP use. The longer that patients were on CPAP within the prespecified limits of this study (≤ 120 days), the greater this divergence in normalization rates between females and males. For example, if we compare the use of CPAP for 90 days vs 30 days (a difference of 60 days of use), the odds of normalization in females were only 3% of the odds in males. This finding has not previously been reported.
There are a number of possible explanations for the difference in normalization rates between males and females. It could be simply that females need more time than males to normalize the ESS. Indeed, in one very large study where ESS was assessed at baseline and 6 months later, female sex was associated with a higher, not lower, likelihood of ESS normalization.5 There were some differences in the variables assessed between that study and the present one: Depression in that study and diabetes and hypertension in the present study, and that the other study was a clinical trial (the APPLES trial) and the present study was done in a routine clinical setting. Given the fact that the proportion of females to males who normalized the ESS diverged over the limited time frame of the present study (30 to ≤ 120 days), it seems unlikely that in the study cohort there is an inflection point beyond 120 days where the rates of ESS normalization between females and males become similar. The aforementioned study evaluated the post-CPAP ESS at a single time point (6 months) and therefore could not assess how these changes evolved over time. Why one study showed female sex associated with higher rates of ESS normalization and the present study showed lower rates of ESS normalization clearly requires further elucidation.
It is possible that in females, high ESS is due to different reasons than in males. In a mouse model of OSA using intermittent hypoxia with assessment of sleepiness with a murine multiple sleep latency test, young female mice were resistant to the effects of intermittent hypoxia compared to male mice but showed greater sleepiness following sleep deprivation.15 These data suggest that young female mice do not develop sleepiness with the intermittent hypoxia model of OSA but are more sensitive to sleep deprivation compared to male mice. If sleepiness in females with OSA is not as directly linked to the OSA itself as compared to sleepiness in males, then one could argue that since CPAP treatment is addressing the direct deleterious consequences of OSA such as intermittent hypoxia, males are more likely to benefit than females. Interestingly, the present study does not suggest that females just need a higher number of hours of CPAP use to normalize the ESS at rates similar to those in males. The present study also did not directly assess sleep quality, which may have improved differently in females and males. Studies looking at other factors that may contribute to sleepiness in females vs males may be illuminating.
A higher BMI was associated with a higher likelihood of ESS normalization with CPAP therapy. In the APPLES study, there was no relationship between BMI and likelihood of ESS normalization with CPAP therapy.5 It is unclear why these results are different (see discussion, 2 paragraphs above). Since different categories of BMI (eg, normal, overweight, obese, morbidly obese) were not prespecified to ensure adequate numbers of patients in each group, a BMI-stratified analysis was beyond the scope of this study. A study with adequate representation of different categories of BMI may yield more insights.
Consistent with most earlier studies,11 high baseline ESS scores were associated with greater improvements in the ESS in the present study. When looking at the interaction between baseline ESS scores and the other assessed covariates and factors, we found that there were greater improvements in the ESS in patients with a higher AHI only in patients with a high baseline ESS score. Baseline AHI has been demonstrated to be one of many variables associated with the ESS,16 and the AHI was not associated with the improvement in the ESS with CPAP therapy in a large clinical trial.5 The results from the present study suggest that OSA severity, as assessed by the AHI, is associated with the improvement in the ESS with CPAP therapy predominantly in patients with a high baseline ESS.
One surprising result of the present study was that a relationship between hours of CPAP use and the improvement in the ESS was not observed. This may have been due, at least in part, to the relatively adherent cohort in this study. Nearly two-thirds of the patients met Medicare criteria for CPAP adherence within the first 30 days of use (≥ 4 hours per day for ≥ 70% of nights). The high adherence rates in this study are likely due to the inclusion criteria requiring patients to return for a follow-up visit after starting CPAP to fill out the second ESS. Indeed, in a clinical population at a Veterans Affairs medical center, those patients who returned to the clinic for a follow-up visit after starting CPAP had more than double the average hours of nightly CPAP use.17 It is likely that if the study cohort had a wider range of hours of nightly CPAP use, then there would have been more improvement in the ESS with higher amounts of CPAP use. Therefore, one limitation of the present study is an underrepresentation of a less CPAP–adherent cohort. Whether the observed differences in ESS normalization rates between females and males will hold true with a less-adherent cohort is unknown.
The common convention of using a threshold of ESS ≥ 11 to define sleepiness was used in the present study. This number was first suggested in the original ESS validation study of Johns in 1991.2 Since then, many studies have followed this convention.3–7,9,10 Interestingly, in a study looking at ESS normalization using a threshold of ESS ≥ 11 in relation to daily hours of CPAP use, the authors found a threshold of CPAP use beyond which there was relatively little improvement in the rate of ESS normalization. Similar results were obtained with use of other ESS thresholds (8 or 12).3 This finding suggests that the use of a particular threshold within this narrow range of ESS values is unlikely to make a large difference.
One limitation of the present study is the use of a self-reported measure of sleepiness, the ESS. The optimal way to assess sleepiness remains an ongoing issue. The correlation between self-reported sleepiness measures and deleterious outcomes including mortality18–20 suggests that there is biological significance to self-reported measures of sleepiness. In the APPLES study,5 sleepiness was assessed with the self-reported ESS and objectively with the Maintenance of Wakefulness Test. Female sex, absence of chronic pain, and CPAP use of > 4 hours per night were associated with a lower chance of a high ESS (≥ 11) at 6 months, but only CPAP use of > 4 hours per night was associated with a lower chance of an abnormally sleepy Maintenance of Wakefulness Test score (< 20 minutes).5 This finding suggests that there is likely some overlap in what is being measured by self-reported and objective testing, but there are likely some differences as well.
Another limitation of the present study is the use of HSATs as the predominant form of OSA testing where measures of baseline sleep quality or sleep fragmentation cannot be assessed. It is possible that the sleep disturbance caused by OSA was different between females and males in this study, which may have affected the response to CPAP therapy. An additional limitation is a lack of menopausal status in females. Menopause increases OSA risk,21 and it is possible that changes in sleepiness with CPAP may be different before or after menopause. Finally, this was a retrospective study that could be subject to unknown confounders or biases. Prospective studies, particularly those investigating the role of sex in changes in the ESS over time with CPAP, would be very valuable.
One strength of the present study is that nearly 45% of the study cohort was female. Historically, most studies assessing OSA and CPAP have had a predominantly male population. For example, in 2 studies assessing the relationship between ESS normalization and hours of CPAP use, the cohort was 86% male in 1 study3 and 75% male in another study.4 These studies did not address the contribution of sex either directly3 or at all.4 A secondary analysis of 1 of these studies3 showed similar improvements in the ESS in males and females overall (as did our study; see Table 2) but did not assess ESS normalization rates.22 Interestingly, in a recent study comparing the patient populations seen in a sleep clinic vs randomized controlled trials for cardiovascular outcomes for patients with OSA on CPAP, the authors suggested that the underrepresentation of females in randomized controlled trials is a factor limiting the generalizability of these studies to real-world patient populations.23 The underrepresentation of females in much of the literature on OSA and CPAP is a significant limitation of the understanding of OSA in females.
EDS is important not only because it is an unpleasant feeling for patients, but also because it is associated with deleterious consequences such as an increased risk for motor vehicle crashes,24 occupational injury,25 and cognitive impairment in older adults26 and is independently associated with increased mortality.18,20 EDS in patients with OSA on CPAP is a common clinical problem. The reasons for the lower rates of ESS normalization in females in the present study are unknown. Future studies assessing other variables including psychological factors, sleep duration, sleep quality, circadian factors, and longer durations of CPAP use would likely be informative. Sex differences are likely to have substantial impacts on the benefits of CPAP therapy, and the elucidation of the various contributing elements is likely to impact the treatment of OSA in females.
ACKNOWLEDGMENTS
The authors thank the staff of the Comprehensive Sleep Disorders Center at Rutgers Robert Wood Johnson Medical School for assistance in data collection.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- HSAT
home sleep apnea test
- OR
odds ratio
- OSA
obstructive sleep apnea
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
REFERENCES
- 1. Senaratna CV , Perret JL , Lodge CJ , et al . Prevalence of obstructive sleep apnea in the general population: a systematic review . Sleep Med Rev. 2017. ; 34 : 70 – 81 . [DOI] [PubMed] [Google Scholar]
- 2. Johns MW . A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 3. Weaver TE , Maislin G , Dinges DF , et al . Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning . Sleep. 2007. ; 30 ( 6 ): 711 – 719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antic NA , Catcheside P , Buchan C , et al . The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA . Sleep. 2011. ; 34 ( 1 ): 111 – 119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Budhiraja R , Kushida CA , Nichols DA , et al . Predictors of sleepiness in obstructive sleep apnoea at baseline and after 6 months of continuous positive airway pressure therapy . Eur Respir J. 2017. ; 50 ( 5 ): 1700348 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pépin JL , Viot-Blanc V , Escourrou P , et al . Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study . Eur Respir J. 2009. ; 33 ( 5 ): 1062 – 1067 . [DOI] [PubMed] [Google Scholar]
- 7. Koutsourelakis I , Perraki E , Economou NT , et al . Predictors of residual sleepiness in adequately treated obstructive sleep apnoea patients . Eur Respir J. 2009. ; 34 ( 3 ): 687 – 693 . [DOI] [PubMed] [Google Scholar]
- 8. Berger M , Hirotsu C , Haba-Rubio J , et al . Risk factors of excessive daytime sleepiness in a prospective population-based cohort . J Sleep Res. 2021. ; 30 ( 2 ): e13069 . [DOI] [PubMed] [Google Scholar]
- 9. Kapur VK , Baldwin CM , Resnick HE , Gottlieb DJ , Nieto FJ . Sleepiness in patients with moderate to severe sleep-disordered breathing . Sleep. 2005. ; 28 ( 4 ): 472 – 477 . [DOI] [PubMed] [Google Scholar]
- 10. Gottlieb DJ , Whitney CW , Bonekat WH , et al . Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study . Am J Respir Crit Care Med. 1999. ; 159 ( 2 ): 502 – 507 . [DOI] [PubMed] [Google Scholar]
- 11. Patel SR , White DP , Malhotra A , Stanchina ML , Ayas NT . Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis . Arch Intern Med. 2003. ; 163 ( 5 ): 565 – 571 . [DOI] [PubMed] [Google Scholar]
- 12. Craig SE , Kohler M , Nicoll D , et al . Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial . Thorax. 2012. ; 67 ( 12 ): 1090 – 1096 . [DOI] [PubMed] [Google Scholar]
- 13. Ayappa I , Norman RG , Seelall V , Rapoport DM . Validation of a self-applied unattended monitor for sleep disordered breathing . J Clin Sleep Med. 2008. ; 4 ( 1 ): 26 – 37 . [PMC free article] [PubMed] [Google Scholar]
- 14.Berry RB, Brooks R, Gamaldo CE, et al; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0. Darien, IL: American Academy of Sleep Medicine; 2012. [Google Scholar]
- 15. Sanfilippo-Cohn B , Lai S , Zhan G , et al . Sex differences in susceptibility to oxidative injury and sleepiness from intermittent hypoxia . Sleep. 2006. ; 29 ( 2 ): 152 – 159 . [DOI] [PubMed] [Google Scholar]
- 16. Prasad B , Steffen AD , Van Dongen HPA , et al . Determinants of sleepiness in obstructive sleep apnea . Sleep. 2018. ; 41 ( 2 ): zsx199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scharf MT , Keenan BT , Pack AI , Kuna ST . Mask refills as a measure of PAP adherence . J Clin Sleep Med. 2017. ; 13 ( 11 ): 1337 – 1344 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Empana JP , Dauvilliers Y , Dartigues JF , et al . Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study . Stroke. 2009. ; 40 ( 4 ): 1219 – 1224 . [DOI] [PubMed] [Google Scholar]
- 19. Gooneratne NS , Richards KC , Joffe M , et al . Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults . Sleep. 2011. ; 34 ( 4 ): 435 – 442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newman AB , Spiekerman CF , Enright P , et al .; The Cardiovascular Health Study Research Group . Daytime sleepiness predicts mortality and cardiovascular disease in older adults . J Am Geriatr Soc. 2000. ; 48 ( 2 ): 115 – 123 . [DOI] [PubMed] [Google Scholar]
- 21. Mirer AG , Young T , Palta M , Benca RM , Rasmuson A , Peppard PE . Sleep-disordered breathing and the menopausal transition among participants in the Sleep in Midlife Women Study . Menopause. 2017. ; 24 ( 2 ): 157 – 162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ye L , Pien GW , Ratcliffe SJ , Weaver TE . Gender differences in obstructive sleep apnea and treatment response to continuous positive airway pressure . J Clin Sleep Med. 2009. ; 5 ( 6 ): 512 – 518 . [PMC free article] [PubMed] [Google Scholar]
- 23. Reynor A , McArdle N , Shenoy B , et al . Continuous positive airway pressure and adverse cardiovascular events in obstructive sleep apnea: are participants of randomized trials representative of sleep clinic patients? Sleep. 2022. ; 45 ( 4 ): zsab264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ward KL , Hillman DR , James A , et al . Excessive daytime sleepiness increases the risk of motor vehicle crash in obstructive sleep apnea . J Clin Sleep Med. 2013. ; 9 ( 10 ): 1013 – 1021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melamed S , Oksenberg A . Excessive daytime sleepiness and risk of occupational injuries in non-shift daytime workers . Sleep. 2002. ; 25 ( 3 ): 315 – 322 . [DOI] [PubMed] [Google Scholar]
- 26. Ohayon MM , Vecchierini MF . Daytime sleepiness and cognitive impairment in the elderly population . Arch Intern Med. 2002. ; 162 ( 2 ): 201 – 208 . [DOI] [PubMed] [Google Scholar]