Abstract
Objective:
Depressive and anxiety disorders are common in youth who are at risk for bipolar disorder (i.e., youth who have at least one parent with bipolar disorder) and antidepressants are commonly prescribed as treatment. However, there are few data regarding the safety and tolerability of antidepressants in this population. Therefore, we sought to prospectively examine the effects of these medications in children and adolescents who are diagnosed with depressive or anxiety disorders and have a parent with bipolar I disorder.
Methods:
Youth aged 9–20 years, with at least one parent with bipolar I disorder [high risk (HR)] were recruited (n = 118) and assessed using semi-structured diagnostic interviews. Participants were prospectively evaluated using a modified version of the Longitudinal Interval Follow-up Evaluation to assess changes in affective and anxiety symptoms and were treated naturalistically
Results:
Over the course of 43 to 227 weeks (mean duration of follow-up: 106 ± 55 weeks), 21% (n = 25) of youth had antidepressant exposure and, of these, 57% (n = 12) had an adverse reaction (e.g., irritability, aggression, impulsivity, hyperactivity) that led to antidepressant discontinuation. Those patients who experienced an adverse reaction were significantly younger than those who did not (p = 0.02) and discontinuation of antidepressant therapy secondary to an adverse event occurred at an average of 16.7 ± 17.4 weeks (median: 11 weeks, range: 2–57 weeks). Cox proportional hazard analyses yielded a hazard ratio of 0.725 (p = 0.03), suggesting that there is a 27% decrease in the likelihood of an antidepressant-related adverse event leading to discontinuation with each one-year increase in age.
Conclusions:
Antidepressant medications may be poorly tolerated in youth with a familial risk for developing mania. Controlled studies further assessing treatments for depression and anxiety in HR youth are urgently needed.
Keywords: anxiety disorders, bipolar disorder, major depressive disorder, mania, SSRI
Bipolar disorder is a chronic, disabling neuropsychiatric illness which frequently begins during adolescence and is highly heritable (1, 2). Offspring of parents with bipolar disorder have an elevated risk of developing mood and anxiety disorders (3–7). Moreover, when offspring of parents with bipolar disorder develop prominent mood symptoms, their risk for mania (and by definition bipolar disorder) increases. Importantly, antidepressant medications, which are commonly used to treat symptoms of depression (8, 9) and anxiety (10–12) in youth may accelerate the onset of mania or manic symptoms (13, 14).
It has been suggested that the emergence of an earlier age of onset of manic episodes may be associated with increased use of antidepressant (and stimulant) medications in patients who may already be at risk of developing bipolar disorder (15). Indeed, a recent study found that 50% of children with or at high risk for bipolar disorder had experienced antidepressant-induced mania, and 26% experienced new onset suicidal ideation within one month of starting an antidepressant (16). In a retrospective case review, 58% of adolescents with bipolar disorder who were treated with antidepressants or stimulants met criteria for treatment-emergent mania within an average of 14 days, and treatment-emergent mania was more frequently observed following treatment with antidepressants than stimulants (44% versus 18%) (17). Similarly, a retrospective study found that five out of six (83%) patients transitioning to mania were previously treated with a selective serotonin reuptake inhibitor (SSRI) antidepressants (18).
Currently, there are few data that directly or prospectively address the safety and tolerability of antidepressants in youth with a familial risk for developing bipolar disorder. In a small prospective study of nine youth (aged 7–16 years) with family histories of bipolar disorder treated with an SSRI (paroxetine) in comparison to the combination of paroxetine and divalproex, paroxetine treatment (either as monotherapy or as combination treatment with divalproex) was poorly tolerated (19). In this series, > 50% of youth experienced manic or hypomanic symptoms or developed suicidality ‘despite the use of a conservative dosing strategy’ (19, 20). Studies evaluating the efficacy, safety, and tolerability of antidepressants for depressive and anxiety symptoms in youth with a parent with bipolar disorder are necessary as an initial step toward establishing effective treatments in this population (21).
With these considerations in mind, we prospectively evaluated the effects of antidepressant treatment in a cohort of youth at-risk for developing bipolar disorder. We hypothesized that antidepressant treatment—although standard clinical practice for youth with anxiety and depressive disorders—would exacerbate illness course and would be poorly tolerated.
Methods
Subjects and assessments
Youth (n = 118), aged 9–20 years old, with at least one parent with bipolar I disorder [high risk (HR)] were recruited from the community. At baseline, patients could not have a history of alcohol or substance dependence, an IQ total score < 70, or a DSM-IV-TR diagnosis of bipolar disorder or a psychotic disorder [i.e., schizophrenia, schizoaffective disorder, psychosis not otherwise specified (NOS)]. All subjects and their legal guardians (if < 18 years) provided assent and consent, respectively.
Offspring were assessed using the Washington University Kiddie–Schedule for Affective Disorders and Schizophrenia and parents were assessed using the Structured Clinical Interview for DSM-IV administered by raters with established diagnostic reliability (kappa > 0.9). To be included in the study, each child or adolescent had to have at least one biological parent diagnosed with DSM-IV-TR bipolar I disorder. Each child or adolescent was prospectively evaluated every 1–4 months (depending on mood symptom severity) using a modified version of the Longitudinal Interval Follow-up Evaluation (22) to assess changes in mood symptoms. Treatment during the study was naturalistic and was provided either by board-certified child and adolescent psychiatrists (MPD, DHB, and JRS) who were also investigators on the current study or by community providers. Information regarding past or current antidepressant exposure was collected by the investigators. Age (at baseline), sex, race, psychiatric diagnoses, type and duration of antidepressant exposure, and any adverse events that led to antidepressant discontinuation were recorded and patients were categorized by the presence or absence of an adverse reaction to antidepressant exposure that led to discontinuation. In this regard, at each patient visit, medication adherence as well as mood and anxiety symptoms were systematically recorded and rated, respectively by the treating psychiatrist over each week since the preceding visit. Additionally, in circumstances in which antidepressant medication had been discontinued, the date of discontinuation was recorded by the treating psychiatrist.
Statistical analysis
Fisher’s Exact and Wilcoxon tests were used to assess demographic and clinical differences between high-risk youth exposed and not exposed to antidepressants as well as those with and without an adverse reaction that lead to discontinuation of antidepressants. In addition, to test the hypothesis that higher baseline scores on the Young Mania Rating Scale (YMRS) (23) would be present in the patients who experienced adverse events leading to discontinuation, Wilcoxon tests were used to compare YMRS scores between the two groups (e.g., antidepressant adverse event and no antidepressant-related adverse event). Finally, exact logistic regression [as implemented in SAS PROC LOGISTIC (v9.2)] was used to assess risk factors for having an adverse reaction to antidepressants which led to discontinuation. For model parameters and predicted probabilities, we report 95% confidence intervals (CIs). Additionally, a Cox proportional hazard analysis was utilized to evaluate the relationship between age and time to discontinuation of antidepressant secondary to an adverse event.
Results
Demographics and clinical characteristics
The mean [± standard deviation (SD)] age of the HR youth sample (n = 118) was 14 ± 3 years, 54 (46%) were girls, and 87 (74%) were White. Twenty-one (18%) of the offspring had antidepressant exposure either immediately prior to (n = 2) or during prospective follow-up (n = 19). There were no statistically significant differences in demographic characteristics between HR youth with and without antidepressant exposure (age at baseline: p = 0.83; sex: p = 0.20; race: p = 0.27). The mean (SD) age (at baseline) of the HR youth exposed to antidepressants was 14 (2) years, 7 (33%) were girls, and 18 (86%) were White. Eleven (52%) of the 21 were diagnosed with a depressive disorder, eight (38%) were diagnosed with an anxiety disorder, four (19%) were diagnosed with a disruptive behavior disorder, and nine (43%) were diagnosed with attention-deficit hyperactivity disorder (ADHD). Twelve (57%) were diagnosed with more than one disorder. The total follow-up period of the patients was ranged from 43 to 227 weeks (106 ± 55 weeks).
Antidepressant treatment
Rates of depressive disorders (p = 0.04), anxiety disorders (p = 0.02), and disruptive behavior disorders (p = 0.02) were greater in those exposed to antidepressants than in those without exposure. Fourteen (66%) of the youth had taken SSRIs, eight (38%) had taken bupropion, and one (5%) had taken duloxetine. Two youth had received more than one antidepressant trial. The mean doses (mg/day) were: fluoxetine 16 ± 5 (range: 10–20); sertraline 28 ± 32 (range: 5–50); escitalopram 20 (n = 1); bupropion 264 ± 63 (range: 150–300); citalopram 25 ± 13 (range: 10–40) and duloxetine 60 (n = 1).
Adverse events associated with antidepressant treatment
Twelve (57%) of the HR youth had an adverse reaction to antidepressant treatment that led to discontinuation and three of these had diagnoses of major depressive disorder (MDD), two had diagnoses of depressive disorder NOS, and five were diagnosed with anxiety disorders. Among patients treated with antidepressants who had an adverse reaction, the mean duration of treatment was 16.7 + 17.4 weeks (median: 11 weeks, range: 2–57 weeks). The most common adverse reaction was increased irritability (n = 7), followed by increased aggression (n = 5), psychosis (n = 2), increased impulsivity (n = 2), suicidal ideation (n = 1), insomnia (n = 1), and increased hyperactivity (n = 1). Additionally, three patients were hospitalized (two for aggression and one for suicidal ideation). There was no difference in rates of adverse reactions between those taking SSRIs and those taking bupropion (50% of those who received a SSRIs versus 63% of those who received bupropion; Fisher’s Exact p = 1.00). HR youth with an adverse reaction were significantly younger than those without an adverse reaction (13 ± 2 versus 15 ± 2 years, p < 0.03, Table 1). There were no statistically significant differences in sex, race, or the presence of an anxiety disorder, depressive disorder, disruptive behavior disorder, or ADHD between those with and without an adverse reaction to antidepressants (Table 1). Additionally, the time to discontinuation in patients who had an adverse reaction did not differ between patients with or without anxiety disorders (p = 0.2) or between patients with and without depressive disorders (p = 0.6). Of the patients who discontinued antidepressants as a result of adverse events (n = 12), 2 were subsequently treated with alpha-2 agonists, one with a second generation antipsychotic and SSRI, one with atomoxetine, three with stimulant monotherapy, one with lamotrigine, and the remainder did not resume pharmacologic treatments.
Table 1.
Comparison of demographic and clinical variables among patients at high risk for developing bipolar disorder who, following antidepressant treatment, developed adverse events (including activation and treatment-emergent manic symptoms)
| Variables | Antidepressant-related adverse events leading to discontinuation (n = 12) |
No antidepressant-related adverse events leading to discontinuation (n = 9) |
|---|---|---|
| Age, years, mean ± SD | 13 ± 2 | 15 ± 2a |
| Sex, boys, n (%) | 8 (67) | 6 (67) |
| Race, White, n (%) | 9 (75) | 9 (100) |
| Comorbid conditions, n (%) | ||
| ADHD (current) | 6 (50) | 3 (33) |
| Anxiety disorders | 5 (42) | 4 (44) |
| Depressive disorders | 4 (33) | 5 (56) |
| Tic disorders | 0 (0) | 0 (0) |
| ODD | 3 (25) | 0 (0) |
| Conduct disorder | 2 (17) | 0 (0) |
| Antidepressant treatment, n | 14b | 10c |
| SSRI | ||
| Paroxetine | 0 | 0 |
| Fluvoxamine | 0 | 0 |
| Fluoxetine | 5 | 2 |
| Citalopram | 1 | 4 |
| Escitalopram | 1 | 0 |
| Sertraline | 1 | 1 |
| SNRI | ||
| Duloxetine | 1 | 0 |
| Venlafaxine | 0 | 0 |
| Other | ||
| Bupropion | 5 | 3 |
SD = standard deviation; ADHD = attention-deficit hyperactivity disorder; ODD = oppositional defiant disorder; SSRI = selective serotonin reuptake inhibitors; SNRI = serotonin–norepinephrine reuptake inhibitor.
p < 0.05.
One patient had trials of fluoxetine and bupropion followed by a re-trial of fluoxetine.
One patient had poor response to fluoxetine then improved following a switch to citalopram.
Patients who developed adverse events (n = 12) leading to antidepressant discontinuation compared with those not experiencing adverse events (n = 6) showed no statistically significant differences in baseline YMRS (p = 0.53) or Hamilton Rating Scale for Depression total scores (p = 0.57). Logistic regression analyses revealed that for every year decrease in age the odds of an adverse reaction to antidepressants increased by a factor of 1.8 (95% CI = 1.1, 3.6, p = 0.02). Our logistic regression model predicted that for a 9-year-old, the chance of having an adverse reaction to antidepressants was 97% as compared to 4% for a 20-year-old (Fig. 1), although the probability at any particular age is necessarily rather imprecise given the small sample size. Additionally, our Cox proportional hazard analysis of the relationship between age and time to discontinuation of antidepressant secondary to an adverse event yielded a hazard ratio estimate of 0.725 (CI: 0.54–0.97, p = 0.03), suggesting that there is a 27% decrease in the likelihood of an antidepressant-related adverse event leading to discontinuation with each one-year increase in age.
Fig. 1.
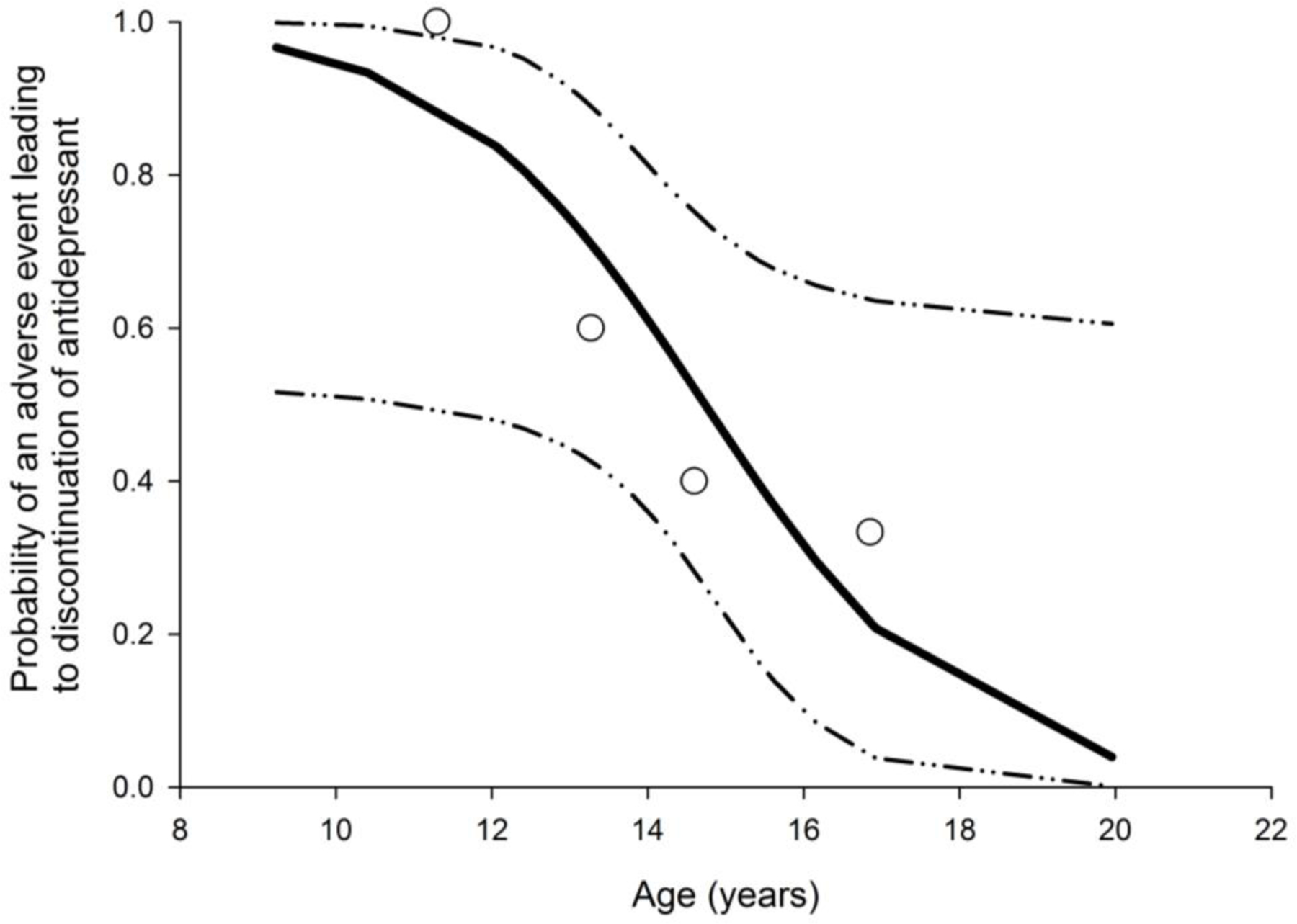
The probability of an antidepressant-related adverse event leading to discontinuation was significantly higher in younger high-risk patients (p < 0.02). Open circles represent the actual probability of an adverse event leading to medication discontinuation at each patient age quartile. The solid black line represents the predicted probability of and adverse event leading to medication discontinuation with dashed lines representing the 95% CIs for the model.
Conclusions
Our findings suggest that antidepressants are commonly used to treat depressive and anxiety disorders in HR youth. Moreover, although the current study of youth at familial risk for bipolar disorder is naturalistic and not controlled, these results suggest that younger patients are more likely to experience antidepressant-induced adverse events (including antidepressant-induced activation and manic symptoms). In this regard, Martin and colleagues (14) observed that younger and peripubertal children were at higher risk for antidepressant-associated manic symptoms than older children and young adults (14). Interestingly, the presence of baseline and treatment-emergent manic symptoms in pediatric patients with SSRI-resistant major depressive disorder was recently identified as a specific risk factor for poor outcome (24). However, in the present study, we observed trends towards higher irritability and motor hyperactivity in patients who subsequently developed adverse events with antidepressant treatment, consistent with the notion that subsyndromal manic symptoms (or behavioral activation) predict poor treatment response across diagnoses. While we lack a direct comparison group in the present study, it is of interest that, in a demographically similar group of youth (n = 8, mean age 13 ± 3 years), with MDD (and frequent comorbid anxiety disorders) who did not have any family history of bipolar disorder and who were treated with SSRI or serotonin–norepinephrine reuptake inhibitor (SNRI) monotherapy, did not experience antidepressant-induced activation or antidepressant-induced manic symptoms which resulted in discontinuation of antidepressants. Additionally, our group followed a cohort of youth (mean age 13.3 ± 3 years, n = 29) years with a diagnosis of MDD (and frequent comorbid anxiety) who were treated with placebo over the course of eight weeks and we did not observe any treatment-emergent manic symptoms nor treatment-emergent activation. Last, these data suggest that co-morbidity may contribute to the possibility of antidepressant adverse events leading to discontinuation. In this regard, the presence of ADHD was associated with an odds ratio = 2 for an antidepressant-related adverse event, however, this because of the small sample size, this odds ratio should be interpreted cautiously.
Our findings also raise the possibility that, given the poor tolerability of antidepressants in HR youth presenting with anxiety and depressive disorders, alternative interventions for these conditions might be considered prior to traditional antidepressant medications. Among potential interventions are psychotherapeutic treatments that are currently recommended for these anxiety and depressive disorders in children and adolescents (8, 10, 25). Additionally, the potential negative effects of antidepressant medications in youth at high risk for developing bipolar disorder suggest that alternative interventions are needed for the treatment of prodromal mood symptoms. Lithium was evaluated for the treatment of MDD in 30 prepubertal children with a family history of bipolar disorder (80%) or a multigenerational family history of MDD without bipolar disorder (20%) in a six-week, double-blind, placebo-controlled trial. However, in this study, lithium (mean serum level: 1 ± 0.16 mEq/L) was no more effective than placebo for treating prepubertal depression in these high-risk children (26). A 12-week open-label study of divalproex for the treatment of 24 children and adolescents with at least one biological parent with bipolar disorder and at least one of the following DSM-IV disorders: MDD, dysthymic disorder, cyclothymic disorder, or ADHD and moderate affective symptoms found that 75% of patients were responders by primary outcome criteria (very much improved or much improved on the Clinical Global Impressions–Improvement scale) (27). However, a study of 56 youth ages 5–17 years with bipolar disorder NOS or cyclothymia who also had at least one biological parent with bipolar disorder were randomly assigned to double-blind treatment with either divalproex or placebo for up to five years. The groups did not significantly differ in survival time for discontinuation for any reason (p = 0.93) or discontinuation due to a mood event (p =0.55). Additionally, changes in mood symptom ratings and psychosocial functioning from baseline to study discontinuation did not differ between groups (28). These findings suggest that conventional mood-stabilizer medications do not produce clinically meaningful improvements in this high-risk population either.
Previously, our group published a 12-week, single-blind study of quetiapine for the treatment of 20 adolescents with mood disorder diagnoses other than mania who had a first-degree relative with bipolar disorder (29). We found that 87% of patients were responders as defined by an endpoint Clinical Global Impressions–Improvement scale score of very much or much improved. However, there was a statistically significant increase in body mass index and over half (55%) of the patients experienced somnolence during the course of the study. Together, these data highlight the need to establish safe and efficacious treatments for mood symptoms in high-risk youth.
In view of evidence for a protective effect of long-chain omega-3 (LCn-3) fatty acids in youth at high risk for developing psychosis (30), it is relevant that preliminary prospective intervention trials have found that LCn-3 fatty acids administered as monotherapy or adjunctively significantly reduce depression and/or manic symptom severity in pediatric and adolescent patients with MDD (31) or bipolar disorder (32, 33). Our group is currently conducting a 12-week controlled trial to investigate the efficacy, safety, and tolerability of LCn-3 fatty acid monotherapy for the treatment of MDD symptoms in adolescents at high risk for developing mania. Moreover, Nadkarni and Fristad (34) found that multifamily psychoeducation groups exerted a protective effect on conversion to bipolar spectrum disorders among children with depressive spectrum disorders, and a second one-year, open trial found that family-focused treatment of 13 children who had a parent with bipolar disorder resulted in significant improvements in depression, hypomania, and psychosocial functioning scores (25).
Additionally, the data described herein also raise the possibility that, given the poor tolerability of antidepressants in HR youth presenting with anxiety and depressive disorders, psychotherapeutic interventions that are currently recommended for these conditions (35, 36) might be tried prior to antidepressant medications. Analogously in some populations with anxiety disorders, psychotherapy decreased the incidence of treatment-emergent adverse events (37) and in pediatric patients has led to both independent and synergistic improvement (when combined with SSRIs) in youth with anxiety disorders and MDD (38, 39).
Among the limitations of this prospective study are the lack of a control group and the lack of standardized antidepressant treatment. Additionally, the lack of diversity among antidepressant treatment may limit our ability to determine if the risk of treatment-emergent activation is related to a specific class of antidepressants, or analogously if some SNRIs or SSRIs are better tolerated in HR youth. Nonetheless, prior evaluations of treatment-emergent adverse events in youth have suggested that these effects may be more prevalent in youth treated with SSRIs than those treated with tricyclic antidepressants (14). Finally, only a minority of the HR patients was treated with antidepressants and thus the samples size is small and generalizability may be limited accordingly. Nonetheless, for clinicians, these data suggest that younger HR patients with anxiety and depression who are treated with antidepressants should be carefully monitored for treatment-emergent adverse events or manic symptoms. Moreover, the frequency of adverse events and treatment-emergent manic symptoms raise the possibility that psychiatrists treating younger HR patients with anxiety and depressive disorders might consider psychotherapeutic interventions for these conditions prior to antidepressants, particularly in young children. These findings highlight the urgent need for controlled data to assess the risk of antidepressant-induced activation or manic symptoms in youth with a familial risk for developing mania, as well as the need for controlled studies to evaluate safe and effective treatments for depressive and anxiety disorders in child and adolescent offspring of parents with bipolar disorder. Collectively, these findings endorse the adoption of a ‘clinical staging’ treatment approach for youth at high risk for developing mania (40), and additional prospective research is warranted to evaluate the efficacy of low-risk first-line interventions in youth at high risk for developing mania.
Acknowledgements
This study was supported by grants NIH P50MH077138, R01MH080973, and R34MH083924. Additional support was also received from the National Center for Research Resources and the National Center for Advancing Translational Sciences; and from the National Institutes of Health (NIH) through Grant 8 UL1 TR000077–04. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures
JRS receives research support from Eli Lilly & Co., Shire, Forest Research Laboratories, and the American Academy of Child and Adolescent Psychiatry. CMA has received research support from AstraZeneca, Amylin, Eli Lilly & Co., GlaxoSmithKline, Lundbeck, Martek, Merck, Novartis, Otsuka, Pfizer, Takeda, and Shire; and has recently been on the lecture bureau for Merck for which he has received honoraria. RKM has received research support from Martek Biosciences Inc., the Inflammation Research Foundation, Ortho-McNeil Janssen, AstraZeneca, Eli Lilly & Co., NARSAD, NIMH, NCCAM, and NIA; and is a consultant for the Inflammation Research Foundation. DHB receives research support from NIMH, the Oxley Foundation, Cincinnati Children’s Hospital Medical Center, American Academy of Psychiatry and the Law Institute for Education and Research, and the Center for Clinical and Translational Science and Training (University of Cincinnati Academic Health Center). MAC has received research support from NIMH. KDC has received research funding from GlaxoSmithKline and Merck; and has served as a consultant to GlaxoSmithKline, Merck, Eli Lilly & Co., and Bristol-Myers Squibb. SMS serves as chair of the Data-safety Monitoring Boards at Sunovion and Novartis; and is a consultant to Procter and Gamble. MPD receives research support from AstraZeneca, Amylin, Eli Lilly & Co., Pfizer, Otsuka, GlaxoSmithKline, Merck, Martek, Novartis, Lundbeck and Shire; is on the lecture bureau for Otsuka, Merck, and Bristol-Myers Squibb; and serves as a consultant to, or has received honoraria from Merck, Pfizer, Dey, Lundbeck, Sunovian, and Otsuka. JAW, SMB, and NPM have no biomedical conflicts of interest to report.
References
- 1.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry 2003; 60: 497–502. [DOI] [PubMed] [Google Scholar]
- 2.Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet 2003; 123C: 48–58. [DOI] [PubMed] [Google Scholar]
- 3.Strober M, Carlson G. Bipolar illness in adolescents with major depression: Clinical, genetic, and psychopharmacologic predictors in a three to four-year prospective follow-up investigation. Arch Gen Psychiatry 1982; 39: 549–555. [DOI] [PubMed] [Google Scholar]
- 4.Chang KD, Steiner H, Ketter TA. Psychiatric phenomenology of child and adolescent bipolar offspring. J Am Acad Child Adolesc Psychiatry 2000; 39: 453–460. [DOI] [PubMed] [Google Scholar]
- 5.Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry 2001; 158: 125–127. [DOI] [PubMed] [Google Scholar]
- 6.Singh MK, DelBello MP, Stanford KE et al. Psychopathology in children of bipolar parents. J Affect Disord 2007; 102: 131–136. [DOI] [PubMed] [Google Scholar]
- 7.Dineen Wagner K Bipolar disorder and comorbid anxiety disorders in children and adolescents. J Clin Psychiatry 2006; 67: 16–20. [PubMed] [Google Scholar]
- 8.Birmaher B, Brent D; AACAP Work Group on Quality Issues et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 2007; 46: 1503–1526. [DOI] [PubMed] [Google Scholar]
- 9.Cheung AH, Emslie GJ, Mayes TL. Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry 2005; 46: 735–754. [DOI] [PubMed] [Google Scholar]
- 10.Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2007; 46: 267–283. [DOI] [PubMed] [Google Scholar]
- 11.Strawn JR, Wehry AM, DelBello MP, Rynn MA, Strakowski SM. Establishing the neurobiologic basis of treatment in children and adolescents with generalized anxiety disorder. Depress Anxiety 2012; 29: 328–339. [DOI] [PubMed] [Google Scholar]
- 12.Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of anxiety disorders in children and adolescents. Child Adolesc Psychiatric Clin 2012; 21: 527–539. [DOI] [PubMed] [Google Scholar]
- 13.Cicero D, El-Mallakh RS, Holman J, Robertson J. Antidepressant exposure in bipolar children. Psychiatry 2003; 66: 317–322. [DOI] [PubMed] [Google Scholar]
- 14.Martin A, Young C, Leckman JF, Mukonoweshuro C, Rosenheck R, Leslie D. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adolesc Med 2004; 158: 773–780. [DOI] [PubMed] [Google Scholar]
- 15.Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord 2004; 78: 81–84. [DOI] [PubMed] [Google Scholar]
- 16.Baumer F, Howe M, Gallelli K, Simenova DI, Hallmayer J, Chang KD. A pilot study of antidepressant-induced mania in pediatric bipolar disorder: characteristics, risk factors, and the serotonin transporter gene. Biol Psychiatry 2006; 60: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 17.Faedda GL, Baldessarini RJ, Glovinsky IP, Austin NB. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord 1998; 82: 149–158. [DOI] [PubMed] [Google Scholar]
- 18.Bechdolf A, Nelson B, Cotton SM, Chanen A, Thompson A, Kettle J, Conus P, Amminger GP, Yung AR, Berk M, McGorry PD. A preliminary evaluation of the validity of at-risk criteria for bipolar disorders in help-seeking adolescents and young adults. J Affect Disord 2010; 127: 316–320. [DOI] [PubMed] [Google Scholar]
- 19.Findling RL, Lingler J, Rowles BM, McNamara NK, Calabrese JR. A pilot pharmacotherapy trial for depressed youths at high genetic risk for bipolarity. J Child Adolesc Psychopharmacol 2008; 18: 615–621. [DOI] [PubMed] [Google Scholar]
- 20.Findling RL, Reed MD, Myers C, O’Riordan MA, Fiala S, Branicky L, Waldorf B, Blumer JL. Paroxetine pharmacokinetics in depressed children and adolescents. J Am Acad Child Adolesc Psychiatry 1999; 38: 952–959. [DOI] [PubMed] [Google Scholar]
- 21.Goldsmith M, Singh M, Chang K: Antidepressants and Psychostimulants in Pediatric Populations. Is there an Association with Mania? Pediatric Drugs 2011; 225–239. [DOI] [PMC free article] [PubMed]
- 22.Leon AC, Solomon DA, Mueller TI et al. A brief assessment of psychosocial functioning of subjects with bipolar disorder: the Longitudinal Interval Follow-Up Examination-RIFT. Longitudinal Interval Follow-Up Evaluation-Range Impaired Functioning Tool. J Nerv Ment Dis 2000; 188: 805–812. [DOI] [PubMed] [Google Scholar]
- 23.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133: 429–435. [DOI] [PubMed] [Google Scholar]
- 24.Maalouf FT, Porta G, Vitiello B et al. Do sub-syndromal manic symptoms influence outcome in treatment resistant depression in adolescents? A latent class analysis from the TORDIA study. J Affect Disord 2012; 138: 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miklowitz DJ, Chang KD, Taylor DO et al. Early psychosocial intervention for youth at risk for bipolar I or II disorder: a one-year treatment development trial. Bipolar Disord 2011; 13: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geller B, Cooper TB, Zimerman B, Frazier J, Williams M, Heath J, Warner K. Lithium for prepubertal depressed children with family history predictors of future bipolarity: a double-blind, placebo-controlled study. J Affect Disord 1998; 51: 165–175. [DOI] [PubMed] [Google Scholar]
- 27.Chang KD, Dienes K, Blasey C, Adleman N, Ketter T, Steiner H. Divalproex monotherapy in the treatment of bipolar offspring with mood and behavioral disorders and at least mild affective symptoms. J Clin Psychiatry 2003; 64: 936–942. [DOI] [PubMed] [Google Scholar]
- 28.Findling RL, Frazier TW, Youngstrom EA et al. Double-blind, placebo-controlled trial of divalproex monotherapy in the treatment of symptomatic youth at high risk for developing bipolar disorder. J Clin Psychiatry 2007; 68: 781–788. [DOI] [PubMed] [Google Scholar]
- 29.DelBello MP, Adler CM, Whitsel RM, Stanford KE, Strakowski SM. A 12-week single blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J Clin Psychiatry 2007; 68: 789–795. [DOI] [PubMed] [Google Scholar]
- 30.Amminger GP, Schäfer MR, Papageorgiou K et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry 2010; 67: 146–154. [DOI] [PubMed] [Google Scholar]
- 31.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry 2006; 163: 1098–1100. [DOI] [PubMed] [Google Scholar]
- 32.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr 2009; 63: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 33.Wozniak J, Biederman J, Mick E et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol 2007; 17: 440–447. [DOI] [PubMed] [Google Scholar]
- 34.Nadkarni RB, Fristad MA. Clinical course of children with a depressive spectrum disorder and transient manic symptoms. Bipolar Disord 2010; 12: 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2007; 46: 267–283. [DOI] [PubMed] [Google Scholar]
- 36.Birmaher B, Brent D, Bernet W et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 2007; 46: 1503–1526. [DOI] [PubMed] [Google Scholar]
- 37.Marcus SM, Gorman J, Shear MK et al. A comparison of medication side effect reports by panic disorder patients with and without concomitant cognitive behavior therapy. Am J Psychiatry 2007; 164: 273–275. [DOI] [PubMed] [Google Scholar]
- 38.Walkup JT, Albano AM, Piacentini J et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 2008; 359, 2753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brent D, Emslie G, Clarke G et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA 2008; 299, 901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamara RK, Nandagopal JJ, Strakowski SM, DelBello MP. Preventative strategies for early-onset bipolar disorder: Towards a clinical staging model. CNS Drugs 2010; 24: 983–996. [DOI] [PubMed] [Google Scholar]


