Abstract
Background
We carried out a meta-analysis since there is not enough evidence to recommend for or against therapeutic-dose anticoagulation compared with thromboprophylaxis in noncritically ill patients hospitalized with Covid-19.
Methods
We performed a systematic literature search using PubMed, Embase, Cochrane Library, and MedRxiv for randomized trials that included therapeutic-dose with low-molecular-weight heparin (LMW) or thromboprophylaxis with LMW heparin in noncritically ill patients admitted to the hospital with Covid-19. We identified five open-label studies for analysis with a total of 3220 patients. Two independent researchers selected, assessed, and extracted the data in duplicate. The outcomes evaluated were all-cause mortality, progression to invasive mechanical ventilation, incidence of venous thromboembolism, and major bleeding. The studies did not show risk for selection, detection, attrition, or reporting bias.
Results
Therapeutic-dose anticoagulation with LMW heparin compared with thromboprophylaxis with LMW heparin had no significant effect of all-cause death (risk ratio [RR] 0.85; 95% confidence interval [CI] 0.67–1.07; P = 0.16; I2 = 48%), or progression to invasive mechanical ventilation (RR 0.89; CI 0.73–1.08; P = 0.24; I2: 0%). Therapeutic-dose anticoagulation significantly reduced the risk of venous thromboembolic disease (RR 0.42; 95% CI 0.28–0.62; P = 0.0001; I2 = 0%) [Number needed to treat = 37]. Major bleeding occurred in 1.79% of the patients receiving therapeutic-dose anticoagulation and in 0.97% of those receiving thromboprophylaxis [Number needed to harm 125].
Conclusion
Therapeutic-dose anticoagulation in noncritically ill patients with Covid-19 could be indicated for patients at high risk of venous thromboembolic disease and low risk of bleeding.
Keywords: Low-molecular-weight heparin, Anticoagulation, Prophylaxis, Covid-19, Meta-analysis
Introduction
Severe coronavirus disease 2019 (Covid-19) caused by SARS-CoV-2 causes hypercoagulability and increases the risk of thromboembolic disease [1, 2].
The autopsy studies in 12 patients with severe Covid-19 confirmed that venous thrombosis occurred in 58% of patients, being pulmonary embolism the direct cause of death in 33% [3]. D-dimer is a biomarker for coagulation disorders that, in patients with Covid-19, can predict severity, mortality, and thromboembolic disease. A D-dimer level greater than four times the upper limit of the normal range [UNR] (standard reference laboratory values ≤ 0.50 mcg/mL fibrinogen equivalent units) predicted hospital mortality with fair performance in patients with Covid-19 [4].
Based on these findings, current guidelines endorse low-molecular-weight prophylaxis for all patients with severe Covid-19 [5]. Nevertheless, some studies have documented significant thromboembolic disease in patients with heparin prophylaxis [6, 7]. Therefore, the optimal dose of anticoagulation is still a matter of debate. An observational study with propensity score matching suggested benefits of full-dose anticoagulation compared with heparin prophylaxis in terms of lower mortality [8].
Given the uncertainty of the optimal intensity of anticoagulation in patients with moderate Covid-19 in several outcomes, we carried out the present meta-analysis of randomized controlled trials. We aimed to assess the efficacy in terms of mortality, progression to invasive mechanical ventilation, incidence of venous thromboembolism, and safety regarding major bleeding events of full-dose anticoagulation compared with prophylaxis with low-molecular-weight heparin.
Methods
Data sources and searches
We performed a systematic literature search using electronic datasets (i.e., PubMed, Embase, Cochrane Central, MedRxiv). The search strategy used was low-molecular-weight heparin AND Covid-19 AND Clinical Trial—no language restriction. We also screened for references from original articles and previous systematic review trials until May 2022.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA Guidelines) [9]. The protocol for this systematic review is registered with PROSPERO (number CRD42022331950).
Eligibility criteria
We included studies involving patients with Covid-19 hospitalized in noncritically ill areas with random allocation to receive therapeutic-dose or thromboprophylaxis with low-molecular-weight heparin. Studies should report outcomes in terms of death during hospitalization, progression to invasive mechanical ventilation, incidence of venous thromboembolism, and development of a major hemorrhagic complication.
We excluded observational cohort studies, study protocols, duplicates, studies without enough data, studies on critically ill patients, patients´ data regarding intermediate dose of low-molecular-weight heparin.
Data extraction and quality assessment
We assessed the trials' risk of bias using the Cochrane Handbook for Systematic Reviews of Interventions Tool [10]. Two independent reviewers appraised all eligible citations. The data extracted from the original trial report were first author, publication year, geographic regions, study design, sample size, participant characteristics, and the outcomes of interest. We double-checked the data to reduce typing or entry errors.
Data synthesis and analysis
We followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions for data analyses [10]. The I-squared (I2) statistical tests were used to explore the statistical heterogeneity among studies. We used the pooled risk ratio (RR) with 95% confidence interval to estimate (CI) the effect size. When significant heterogeneity was present (I2 > 50%) a random-effects model was used to estimate the pooled risk ratio and 95% CI; otherwise, a fixed-effects model was adopted. Subgroup analysis according to D-dimer values was also conducted when related data were available [11]. Egger’s test was to evaluate the possible publication bias, and a P value of < 0.05 indicates potential publication bias [12].
Results
Characteristics of the studies
The search strategy carried out on the electronic databases identified the following potential studies for analysis: PubMed (N = 28), EMBASE (N = 200), Cochrane library (N = 38), and MedRxiv (N = 330). After applying eligibility criteria, we identified five complete studies for analysis (N = 3220 patients) [13–17]. The studies were carried out multicenter in the United States and Canada (n = 2), Spain (n = 2), and multicenter in 6 countries (Brazil, Canada, Ireland, Saudi Arabia, United Arab Emirates, and the United States) (n = 1). All studies were open-label (Table 1).
Table 1.
Study characteristics
| Study | Population | D-dimer | Intervention | Outcome: all-cause death | Outcome: progression to IMV | Outcome: VTE | Outcome: major hemorrhage |
|---|---|---|---|---|---|---|---|
| ATTACC, ACTIV-4ª, REMAP-CAP [13] | Patients admitted without the need for ICU-level care | Patients were stratified in 3 groups according to their baseline D-dimer level: high D-dimer (≥ 2 times the ULN) [N = 630 patients], low D-dimer (< 2 times the ULN) [N = 1075 patients], and unknown D-dimer level [N = 514 patients] |
Therapeutic-dose: Any one of enoxaparin, dalteparin, tinzaparin, fondaparinux, or heparin dosed according to patient weight Prophylaxis dose: Dose of chosen agent should not be sufficient to result in therapeutic anticoagulation |
Therapeutic-dose group: 7.29% 86/1180 Prophylaxis dose group: 8.22% 86/1046 |
Therapeutic-dose group: 10.93% 129/1180 Prophylaxis dose group: 2.14% 127/1046 |
Therapeutic-dose group: 1.35% 16/1180 Prophylaxis dose group:2.68% 28/1046 |
Therapeutic-dose group: 1.86% 22/1180 Prophylaxis dose group: 0.86% 9/1046 |
| Marcos-Jubilar et al. [14] | Patients admitted to non-critical ward (CURB65 ≤ 2 points) and SpO2 ≥ 90%, D-dimer > 500 ng/mL |
D-dimer median (Q1-Q3) Therapeutic-dose group: 780 ng/mL (600–1125) Prophylaxis Dose group: 770 ng/mL (590–1030) |
Therapeutic-dose: Bemiparin 115 IU/Kg QD Prophylaxis dose: Bemiparin 3500 IU/ QD |
Therapeutic-dose group: 6.25% 2/32 Prophylaxis dose group: 3.03% 1/33 |
Therapeutic-dose group: 28.12% 9/32 Prophylaxis dose group:24.24% 8/33 |
Therapeutic-dose group: 0% 0/32 Prophylaxis dose group:9.09% 3/33 |
Therapeutic-dose group: 0% 0/32 Prophylaxis dose group:0% 0/33 |
| Muñoz-Rivas et al. [15] | Patients admitted to non-critical Ward and SpO2 ≤ 94%, D-Dimer > 1000 µg/L, C reactive protein > 150 mg/dL or IL6 > 40 pg/mL |
Peak D-dimer median (Q1-Q3) Therapeutic-dose group: 620 µg/dL (363–1200) Prophylaxis dose group: 618 µg/dL (375–1100) |
Therapeutic-dose group: Tinzaparin 175 IU/Kg QD Prophylaxis dose: Tinzaparin 4500 IU QD |
Therapeutic-dose group: 2.91% 3/103 Prophylaxis dose group: 1.89% 2/106 |
Therapeutic-dose group: 2.91% 3/103 Prophylaxis dose group: 0.95% 1/106 |
Therapeutic-dose group: 1.94% 2/103 Prophylaxis dose group: 3.77% 4/106 |
Therapeutic-dose group: 2.91% 3/103 Prophylaxis dose group: 3.77% 4/106 |
| Sholzberg et al. [16] | Patients admitted to noncritical Ward and SpO2 < 93%, D-dimer above the upper limit of normal |
Geometric mean (SD) D-dimer ratio Therapeutic-dose: 2.1 (0.7) Prophylaxis dose: 2.5 (0.9) |
Therapeutic-dose: LMW heparin or unfractionated heparin as used for the treatment of VTE Prophylaxis dose: LMW heparin or unfractionated heparin adjusted for BMI and creatinine clearance |
Therapeutic-dose group: 1.75% 4/228 Prophylaxis dose group: 7.59% 18/237 |
Therapeutic-dose group: 4.82% 11/228 Prophylaxis dose group: 6.75% 16/237 |
Therapeutic-dose group: 0.9% 2/228 Prophylaxis dose group: 2.5% 6/237 |
Therapeutic-dose group: 0.87% 2/228 Prophylaxis dose group:1.68% 4/237 |
| Spyropoulos et al. [17] | D-dimer level greater than 4 times the upper limit of normal or a sepsis-induced coagulopathy score of 4 or greater. A total of 83 (33%) patients stratified as ICU-level | D-dimer median (Q1-Q3) Therapeutic-dose: 1451 ng/mL (1045–3393) Prophylaxis dose: 1700 ng/mL (1072–2942) |
Therapeutic-dose: Enoxaparin 1 mg/kg BID or 0.5 mg/kg BID if Creatinine clearance 15–29 mL/min Prophylaxis dose: Enoxaparin 30–40 mg QD |
Therapeutic-dose group: 19.37% 25/129 Prophylaxis dose group: 25.0% 31/124 |
Therapeutic-dose group: 13.07% 17/130 Prophylaxis dose group: 16.53% 21/127 |
Therapeutic-dose group: 10.9% 14/129 Prophylaxis dose group: 29% 36/124 |
Therapeutic-dose group: 4.65% 6/129 Prophylaxis dose group: 1.57% 2/127 |
IL6, interleukin 6; IMV, Invasive mechanical ventilation; LMW, low-molecular-weight; ULN, Upper normal limit of the normal range
All studies included patients admitted to hospital wards for Covid-19 without the need for ICU-level care [13–16], except for the study by Spyropoulos et al., where 33% of patients were stratified as ICU-level of care [17]. Patients´outcomes were adjudicated in a blinded fashion or by an independent committee in three studies [13, 16, 17]. Studies carried out in Spain had outcomes objectively adjudicated by the investigators [14, 15]. All studies excluded patients with substantial bleeding risk at entry.
The studies showed a weighted average mean age of 61 years of patients. A total of 58% of patients were male. The control group's weighted average mean incidence of thromboembolic disease was 7.55% (from 2.1 to 29%). The weighted average mean of D-dimer was 2.33 (range from 1.24 to 4.0) times the ULN. There was one study with data stratified according to patients´ D-dimer values (high, low, unknown) [13], two studies including patients with low average D-dimer values [D-dimer low] [14, 15], and two studies, including patients with high average D-dimer values (D-dimer high] [16, 17].
Major hemorrhage was defined according to the International Society on Thrombosis and Hemostasis (ISTH)/Scientific and Standardization Committee (SSC) definitions and bleeding assessment tool in non-surgical patients as in all studies [18]. Major hemorrhage included fatal bleeding; and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome; and/or bleeding causing a fall in hemoglobin level of ≥ 20 g/L, or leading to transfusion of 2 or more units of whole blood or red cells.
The quality of trials assessed by the Cochrane Handbook for Systematic Reviews of Interventions did not show risk for selection, detection, attrition, or reporting bias. Since all trials were open-label, there was a risk of performance bias. The funnel plot did not show evidence of small-study bias.
Efficacy of therapeutic-dose low-molecular-weight heparin
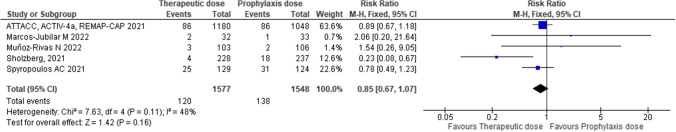
For the efficacy outcome, the fixed effects model showed a trend for lower all-cause death during hospitalization in patients with therapeutic-dose anticoagulation compared with thromboprophylaxis (RR 0.85; 95% confidence interval [CI] 0.67–1.07; P = 0.16; I2 = 48%) (Fig. 1). In studies including patients with high D-dimer values (N = 1348 patients), therapeutic-dose anticoagulation showed a trend for benefit on all-cause death compared with thromboprophylaxis, although with significant heterogeneity (RR 0.66; 95% CI 0.37–1.19: P = 0.17; I2 = 65%). However, in studies including patients with low D-dimer values (N = 1349 patients), therapeutic-dose anticoagulation was associated with an increased risk of all-cause death (RR 2.05; 95% CI 1.28–3.27; P = 0.003; I2 = 0%).
Fig. 1.
Efficacy of therapeutic-dose of low-molecular-weight heparin compared with heparin thromboprophylaxis on all-cause death during hospitalization
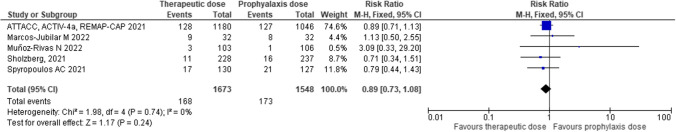
Regarding progression to invasive mechanical ventilation, the meta-analysis showed not significant benefit of therapeutic-dose anticoagulation compared with thromboprophylaxis (RR 0.89; CI 0.73–1.08; P = 0.24; I2 0%). (Fig. 2) In studies including patients with high D-dimer values (RR 0.82; 95% CI 0.62–1.09; P = 0.18; I2 = 0%), or patients with low D-dimer values (RR 1.05; 95% CI 0.76–1.47; P = 0.75; I2 = 0%) therapeutic-dose anticoagulation had no significant effect compared with thromboprophylaxis for progression to mechanical ventilation.
Fig. 2.
Efficacy of therapeutic-dose of low-molecular-weight heparin compared with heparin thromboprophylaxis to reduce progression to invasive mechanical ventilation
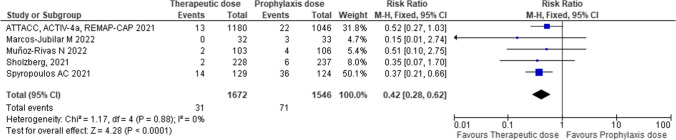
Therapeutic-dose anticoagulation reduced the incidence of venous thromboembolism compared with thromboprophylaxis (RR 0.42; 95% CI 0.28–0.62; P = 0.0001; I2 = 0%) [Number needed to treat = 37] (Fig. 3). These figures translated into a number needed to treat of 37. Since the study ATTAC, ACTIV-4a, REMAP-CAP did not provide data for subgroup analysis on this outcome, it was not possible to assess differences in the incidence of venous thromboembolism between patients with high and low D-dimer values according to the anticoagulation dose.
Fig. 3.
Efficacy of therapeutic-dose of low-molecular-weight heparin compared with heparin thromboprophylaxis to reduce the incidence of venous thromboembolism
Safety of therapeutic-dose low-molecular-weight heparin
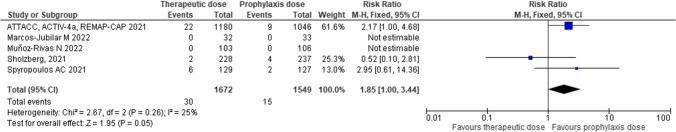
Major bleeding occurred in 1.79% of the patients receiving therapeutic-dose anticoagulation and in 0.97% of those receiving thromboprophylaxis. (Fig. 4). These figures translated into a number needed to harm of 125.
Fig. 4.
Major bleeding with therapeutic-dose of low-molecular-weight heparin compared with heparin thromboprophylaxis
Discussion
Our meta-analysis shows that in noncritically ill patients hospitalized with Covid-19 and high D-dimer values, therapeutic-dose anticoagulation with low-molecular-weight heparin had no significant effect on all-cause death at 30 days or progression to mechanical ventilation. However, therapeutic-dose anticoagulation significantly reduced the risk of venous thromboembolism but it was associated with an increased risk of major hemorrhage compared with thromboprophylaxis.
We did not evaluate the efficacy of intermediate doses of low-molecular-weight heparin since subgroup analyses in published trials showed a neutral effect compared with thromboprophylaxis [15, 17–20]. We excluded trials in critically ill patients admitted to intensive care since therapeutic anticoagulation did not improve major thrombotic events or deaths; we included for analysis one trial in which 33% (N = 83 patients) of the population was admitted to ICU-level care [17]. It might be that the overwhelming inflammatory reaction and accompanying thrombotic complications in critically ill patients are too pronounced to be restored [21].
Five previously published meta-analyses studied the effect of anticoagulant regimens with low-molecular-weight heparin in hospitalized patients with Covid-19 [22–26]. Neither of the meta-analysis demonstrated benefits in terms of mortality in patients receiving full-dose anticoagulation compared with thromboprophylaxis. All of them showed that therapeutic dose of low-molecular-weight heparin reduced around 50% the risk of thrombotic events compared with thromboprophylaxis. Three of the meta-analysis concluded that full-dose anticoagulation was associated with an increased risk of bleeding [22–24]. Expert consensus and guidance suggest therapeutic intensity anticoagulation for moderately ill hospitalized patients at risk of disease progression defined by supplemental oxygen requirement and an elevated D-dimer (> 2–4 times the upper limit of normal range) who are not at risk for anticoagulant-related bleeding [27, 28].
Our meta-analysis including five randomized clinical trials analyzed the importance of D-dimer values as a surrogate marker of disease severity in Covid-19. Subgroup analyses based on a D-dimer cut-off value ≥ 2 times the upper limit normal failed to discriminate patients in whom therapeutic-dose anticoagulation could show a benefit on survival, or progression to mechanical ventilation. Nevertheless, in accordance with other meta-analysis we also found that therapeutic-dose anticoagulation reduced the risk of thrombotic events compared with thromboprophylaxis with a number needed to treat of 37, and a number needed to harm of 125. Understanding of pathophysiologic mechanisms of thrombosis in COVID-19 has evolved, with recognition that patients may be at risk for both macrothrombotic events (e.g., deep venous thrombosis, pulmonary embolism) and immunothrombosis in situ) [29]. However, biomarkers of coagulation (e.g., d-dimer, fibrinogen level, and activated partial thromboplastin time) or inflammation (Leukocyte count, C-reactive protein) failed to discriminate between patients with or without thrombotic complications [30]. Therapeutic-dose anticoagulation should be reserved for patients with a documented venous thrombotic event or those showing high probability of thromboembolism defined by a Wells score of 3 in suspected deep vein thrombosis and a Wells score of 4 in suspected pulmonary embolism and D-dimers of 500 ng/mL and above [31].
The strength of the present meta-analysis is that we included its conduct and analysis according to PRISMA guidelines. We only analyzed randomized controlled trials to reduce the risk of bias. The search of the literature was complete, including non-published information. Two studies showed potential for performance bias due to not using independent end-point adjudication committee. However, the outcomes were objective and predefined minimizing the possibility of bias. Also, there could be a potential for ascertainment bias due to different criteria for screening for venous thromboembolism or major hemorrhage across the studies.
In conclusion, therapeutic-dose anticoagulation with low-molecular-weight heparin compared with thromboprophylaxis reduced the risk of venous thromboembolism in non-critically ill patients with Covid-19 albeit with an increased risk of major hemorrhagic events. Therapeutic-dose anticoagulation had no effect on overall mortality or progression to mechanical ventilation.
Acknowledgements
Carmen Sanchez Ardila, a librarian, helped with the search in databases.
Authors' contributions
This manuscript is co-authored by JE and VV (conception and design, analysis and interpretation of data, and manuscript writing). All authors read and approved the final manuscript.
Funding
The authors did not receive financial support for the research, authorship and/or publication of this article.
Availability of data and materials
Not applicable.
Declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Ethics approval and consent waived. All the data presented in this review is from previously published studies.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 2.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, Morrison RB, Leiva O, Fanikos J, Nauffal V, Almarzooq Z, Goldhaber SZ. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan H, Chen H, Liu C, Cheng L, Yan S, Li H, Li Y. Diagnostic value of D-dimer in COVID-19: a meta-analysis and meta-regression. Clin Appl Thromb Hemost. 2021;27:10760296211010976. doi: 10.1177/10760296211010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jirjees F, Saad AK, Al Hano Z, Hatahet T, Al Obaidi H, Dallal Bashi YH. COVID-19 treatment guidelines: do they really reflect best medical practices to manage the pandemic? Infect Dis Rep. 2021;13(2):259–284. doi: 10.3390/idr13020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoliquido A, Porfidia A, Nesci A, De Matteis G, Marrone G, Porceddu E, et al. Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J Thromb Haemost. 2020;18(9):2358–2363. doi: 10.1111/jth.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franco-Moreno A, Muñoz-Rivas N, Mestre-Gómez B, Torres-Macho J. Tromboembolismo pulmonar y COVID-19: un cambio de paradigma [Pulmonary embolism and COVID-19: a paradigm change] Rev Clin Esp. 2020;220(7):459–461. doi: 10.1016/j.rce.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ionescu F, Jaiyesimi I, Petrescu I, Lawler PR, Castillo E, Munoz-Maldonado Y, et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: a retrospective propensity score-weighted analysis. Eur J Haematol. 2021;106(2):165–174. doi: 10.1111/ejh.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutton B, Salanti G, Caldwell DM, Chairman A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;18(343):d5928. doi: 10.1136/BMJ.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 12.Sterne JA, Jüni P, Schulz KF, Altman DG, Bartlett C, Egger M. Statistical methods for assessing the influence of study characteristics on treatment effects in 'meta-epidemiological' research. Stat Med. 2002;21(11):1513–1524. doi: 10.1002/sim.1184. [DOI] [PubMed] [Google Scholar]
- 13.ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically Ill patients with Covid-19. N Engl J Med. 2021;385(9):790–802. 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed]
- 14.Marcos-Jubilar M, Carmona-Torre F, Vidal R, Ruiz-Artacho P, Filella D, Carbonell C, et al. Therapeutic versus prophylactic Bemiparin in hospitalized patients with nonsevere COVID-19 pneumonia (BEMICOP Study): an open-label, multicenter, randomized. Controlled Trial Thromb Haemost. 2022;122(2):295–299. doi: 10.1055/a-1667-7534. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz-Rivas N, Aibar J, Gabara-Xanco C, Trueba-Vicente A, Urbelz-Perez A, Gomez-del Olmo V, et al. Optimal thromboprophylaxis in noncritically ill patients with COVID-19 pneumonia, the PROTHROMCOVID randomized controlled trial. medRxiv. 2022 doi: 10.1101/2022.05.03.22274594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sholzberg M, Tang GH, Rahhal H, AlHamzah M, Kreuziger LB, Áinle FN, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;14(375):n2400. doi: 10.1136/BMJ.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spyropoulos AC, Goldin M, Giannis D, Diab W, Wang J, Khanijo S, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612–1620. doi: 10.1001/jamainternmed.2021.6203.Erratum.In:JAMAInternMed.2022Feb1;182(2):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C. Subcommittee on control of anticoagulation of the scientific and standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 19.Perepu US, Chambers I, Wahab A, Ten Eyck P, Wu C, Dayal S, et al. Standard prophylactic versus intermediate-dose enoxaparin in adults with severe COVID-19: a multicenter, open-label, randomized controlled trial. J Thromb Haemost. 2021;19(9):2225–2234. doi: 10.1111/jth.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bikdeli B, Talasaz AH, Rashidi F, Bakhshandeh H, Rafiee F, Rezaeifar P, et al. Intermediate-dose versus standard-dose prophylactic anticoagulation in patients with COVID-19 Admitted to the intensive care unit: 90-day results from the INSPIRATION randomized trial. Thromb Haemost. 2022;122(1):131–141. doi: 10.1055/a-1485-2372. [DOI] [PubMed] [Google Scholar]
- 21.REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACK Investigators, Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically Ill patients with Covid-19. N Engl J Med. 2021;385(9):777–789. 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed]
- 22.Giossi R, Menichelli D, Pani A, Tratta E, Romanini A, Roncato R, Nani A, et al. A systematic review and a meta-analysis comparing prophylactic and therapeutic low molecular weight heparins for mortality reduction in 32,688 COVID-19 patients. Front Pharmacol. 2021;2(12):698008. doi: 10.3389/fphar.2021.698008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorda A, Siller-Matula JM, Zeitlinger M, Jilma B, Gelbenegger G. Anticoagulant treatment regimens in patients with covid-19: a meta-analysis. Clin Pharmacol Ther. 2022;111(3):614–623. doi: 10.1002/cpt.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kow CS, Ramachandram DS, Hasan SS. The effect of higher-intensity dosing of anticoagulation on the clinical outcomes in hospitalized patients with COVID-19: a meta-analysis of randomized controlled trials. J Infect Chemother. 2022;28(2):257–265. doi: 10.1016/j.jiac.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sholzberg M, da Costa BR, Tang GH, Rahhal H, AlHamzah M, Baumann KL. Randomized trials of therapeutic heparin for COVID-19: a meta-analysis. Res Pract Thromb Haemost. 2021;5(8):e12638. doi: 10.1002/rth2.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilia E, Belletti A, Fresilli S, Finco G, Landoni G. Efficacy and safety of heparin full-dose anticoagulation in hospitalized non-critically ill COVID-19 patients: a meta-analysis of multicenter randomized controlled trials. J Thromb Thrombolysis. 2022 doi: 10.1007/s11239-022-02681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes GD, Burnett A, Allen A, Ansell J, Blumenstein M, Clark NP, et al. Thromboembolic prevention and anticoagulant therapy during the COVID-19 pandemic: updated clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2022;17:1–14. doi: 10.1007/s11239-022-02643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 29.Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirsadraee S, Gorog DA, Mahon CF, Rawal B, Semple TR, Nicol ED, et al. Prevalence of thrombotic complications in ICU-treated patients with coronavirus disease 2019 detected with systematic CT scanning. Crit Care Med. 2021;49(5):804–815. doi: 10.1097/CCM.0000000000004890. [DOI] [PubMed] [Google Scholar]
- 31.Raj K, Chandna S, Doukas SG, Watts A, Jyotheeswara Pillai K, Anandam A, et al. Combined use of wells scores and D-dimer levels for the diagnosis of deep vein thrombosis and pulmonary embolism in COVID-19: a retrospective cohort study. Cureus. 2021;13(9):e17687. doi: 10.7759/cureus.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.