Abstract
Background
Myelosuppression is the major toxicity encountered during temozolomide chemoradiotherapy for newly diagnosed glioblastoma.
Methods
We assessed the association of myelosuppression (neutropenia, thrombocytopenia, anemia, and lymphopenia) during temozolomide chemoradiotherapy alone or in combination with experimental agents with progression-free survival (PFS) or overall survival (OS) in 2073 patients with newly diagnosed glioblastoma enrolled into five clinical trials: CENTRIC, CORE, EORTC 26082, AVAglio, and EORTC 26981. A landmark Cox model was used. For each primary association analysis, a significance level of 1.7% was used.
Results
Lower neutrophil counts at baseline were associated with better PFS (P = .011) and OS (P < .001), independently of steroid intake. Females experienced uniformly more myelotoxicity than males. Lymphopenia during concomitant chemoradiotherapy was associated with OS (P = .009): low-grade (1-2) lymphopenia might be associated with superior OS (HR 0.78, 98.3% CI 0.58–1.06), whereas high-grade (3-4) lymphopenia might be associated with inferior OS (HR 1.08, 98.3% CI 0.75–1.54). There were no associations of altered hematological parameters during concomitant chemoradiotherapy with PFS. During maintenance chemoradiotherapy, no significant association was found between any parameter of myelosuppression and PFS or OS, although exploratory analysis at 5% significance level indicated that either mild-to-moderate (HR 0.76, 95% CI 0.62–0.93) or high-grade lymphopenia (HR 0.65, 95% CI 0.46–0.92) was associated with superior OS (P = .013), but not PFS.
Conclusions
The association of higher neutrophil counts at baseline with inferior PFS and OS requires further prospective evaluation. The link of therapy-induced lymphopenia to better outcome may guide the design for immunotherapy trials in newly diagnosed glioblastoma.
Keywords: anemia, chemoradiotherapy, lymphopenia, neutropenia, progression, temozolomide, thrombocytopenia, survival
Key Points.
Lower neutrophil counts at baseline were associated with better PFS and OS, independently of steroid intake.
Females experienced uniformly more myelotoxicity than males.
Lymphopenia during concomitant chemoradiotherapy was associated with OS.
Importance of the Study.
In this cohort of 2073 patients from five clinical trials, lower neutrophil counts at baseline were associated with better PFS (P = .011) and OS (P < .001), independently of steroid intake. Females experienced uniformly more myelotoxicity than males. Lymphopenia during concomitant chemoradiotherapy was associated with OS (P = .009). During maintenance chemoradiotherapy, no significant association was found between any parameter of myelosuppression and PFS or OS. The observation of higher neutrophil counts at baseline with inferior PFS and OS requires further prospective evaluation.
The standard of care for patients with newly diagnosed glioblastoma consists of maximum neurosurgical resection as safely feasible followed by concomitant temozolomide chemotherapy with radiotherapy and six cycles of maintenance chemotherapy with temozolomide.1,2 Myelosuppression has been defined as the major dose-limiting toxicity of temozolomide and may be seen more often in females and in patients with tumors which exhibit O6-methylguanine DNA methyltransferase (MGMT) promoter methylation who as a group receive more cycles of temozolomide.1,3–7
The major prognostic factors in newly diagnosed glioblastoma have been defined as age, performance status, extent of resection, and MGMT promotor methylation status in the tumor tissue.8 Beyond these established prognostic factors, there has recently been a lot of interest in the question whether constitutive or therapy-associated myelosuppression may be associated with outcome. Thus, it is conceivable that patients who are more susceptible to side effects are also more likely to derive benefit from the intervention.9,10 Conversely, high-grade myelosuppression affecting any cell type may cause delays or even cessation of chemotherapy resulting in undertreatment. Anemia might compromise physical fitness and contribute to tumor hypoxia and radioresistance, while neutropenia- and lymphopenia-induced immunosuppression results in impaired anti-tumor-immunity and susceptibility to infection. In fact, severe therapy-induced lymphopenia (<500 cells/mm3) has been associated with inferior outcome in newly diagnosed glioblastoma.11,12 Here we interrogated the EORTC Brain Tumor Group`s clinical trial database to conclusively address the prognostic significance of chemoradiotherapy-associated myelosuppression in patients enrolled into clinical trials for newly diagnosed glioblastoma.
Material and Methods
Patients and Methods
We explored the association of neutropenia, thrombocytopenia, anemia, or lymphopenia with outcome in patients with newly diagnosed glioblastoma treated with temozolomide chemoradiotherapy. We performed a pooled retrospective analysis using individual patient data from five clinical trials—CENTRIC,13 CORE,14 EORTC 26082,15 AVAglio,16 and EORTC 26081.1 All patients enrolled into CENTRIC (n = 545), CORE (n = 265), and AVAglio (n = 921) were included in the analysis since they were all treated with temozolomide chemoradiotherapy. Although bevacizumab prolonged PFS, no interaction of myelosuppression, treatment arm, and PFS or OS was found in a sensitivity analysis of the AVAglio trial (data not shown). Further, we also included patients treated with temozolomide in the experimental arm of EORTC 26981 (n = 287) and in the standard arm of EORTC 26082 (n = 55) (Supplementary Table S1). Data on lymphocyte counts were not available in AVAglio and EORTC 26981. Thus, lymphopenia was explored in dataset S (small), including CENTRIC, CORE, and EORTC 26082, whereas neutropenia, thrombocytopenia, and anemia were explored in dataset L (large), including the five trials.
All sites had obtained approval for trial participation by their institutional review boards. Informed consent was obtained from all patients. For each trial, datasets were received with coded individual patient information including date of randomization, PFS, OS and the baseline covariates age, sex, WHO performance status, extent of resection, steroid use, Mini-Mental State Examination (MMSE), MGMT promoter methylation status, and detailed laboratory values.
Data on neutropenia, thrombocytopenia, anemia, and lymphopenia were collected at baseline, defined as values obtained within 14 days prior to the initiation of study treatment, from the start of concomitant temozolomide chemoradiotherapy until the last day prior to maintenance temozolomide and during maintenance temozolomide defined as the start of maintenance until day 28 of the sixth cycle of maintenance. All data were used and worst grade over the period was used for the analysis. Myelosuppression raw data were re-graded retrospectively using Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. PFS was defined as the number of days from the date of randomization to the date of progression or death from any cause, whichever came first. In case a patient was alive and without progression, PFS was censored at the date last known to be alive. OS was calculated as the number of days from the date of randomization to the date of death from any cause. For patients alive or lost to follow-up, OS was censored at the date last known to be alive (Supplementary Figure S1).
Statistical analysis
Supplementary Table S1 illustrates how many patient data sets were available for each analysis.
For the descriptive analysis of patients and hematological laboratory parameters, categorical variables were described by frequencies and percentages, whereas continuous variables were described by their first quartile, median and third quartile. The association between hematology values (absolute neutrophil count, platelet count, hemoglobin concentration, and lymphocyte count) and PFS or OS were assessed during three periods: at baseline, during concomitant treatment, and during maintenance treatment. Given the time-dependent nature of myelosuppression, a landmark analysis approach was used for the concomitant and maintenance phase analyses (Supplementary Figure S2).17,18 Patients with progression prior to the predefined landmark timepoint were not considered in the PFS landmark analysis and the history of myelosuppression before the landmark only was considered for the OS analysis. This approach was selected to avoid confounding effects related to early progression during treatment. Sensitivity analyses for the concomitant and maintenance phase OS landmark analyses were performed by excluding patients with disease progression prior to the landmark.
For the association of myelosuppression during concomitant temozolomide with radiotherapy with PFS or OS, the end of concomitant treatment plus a 4-week break, i.e., end of week 10 since start of radiotherapy and before the start of maintenance temozolomide, was used as the landmark time. At the landmark time, patients who were still free of progression and alive for PFS, and alive for OS and not censored were included in the analysis.
A Cox proportional hazard model was used to assess the association of myelosuppression during concomitant treatment with PFS or OS. Each model was stratified by trial (pooling data from CENTRIC and CORE as one for their complementary setup), and included neutropenia, thrombocytopenia, anemia, and lymphopenia, and was adjusted for the following prognostic factors (primary analysis): age (<55 years vs. ≥ 55 years), sex (male or female), baseline WHO performance status (0 or > 0), baseline steroid use (yes or no), MGMT promotor methylation status (unmethylated, methylated or unknown), MMSE score (< 27 or > 27), and extent of surgery (partial resection or biopsy, or gross total resection). A similar method was used for the maintenance phase analyses where three landmark times were specified: the end of the first, the third, and the sixth maintenance temozolomide cycle. All three-landmark datasets (separate datasets for PFS and OS) were stacked. Cox models on the stacked dataset were stratified by the three landmark times and trial. Robust standard errors were computed to account for repeated use of patients’ data.19
As the association of myelosuppression with PFS or OS was assessed at three periods, the overall significance level of 5% was split into three, i.e., for each primary analysis, a significance level of 1.7% was used. The corresponding 98.3% confidence intervals were reported for all variables.
The primary adjusted analyses included all pre-selected prognostic variables whether significant or not. In exploratory analyses, variable selection with the Collett approach was used to select the variables which are significantly associated with PFS and OS at a 5% significance.20 The Collett variable selection approach has several advantages including selection of a set of equally good subsets of variables rather than identification of only one particular subset as obtained by the automated selection methods. The subset selected is the one with the best goodness of fit. Also, in the exploratory analyses, using the neutrophil-to-lymphocyte ratio (NLR) assessed at baseline, end of concomitant treatment and start of maintenance treatment, the association of NLR with PFS and OS was explored.
Interaction tests from the Cox models were also used to assess whether the association of myelosuppression and PFS or OS was different at 1% significance between sex and MGMT strata. SAS version 9.4 (© 2002-2012 per SAS Institute Inc., Cary, NC, USA) was used for all the analyses.
Results
Patient characteristics at baseline
Sex and age distributions were similar across trials (Supplementary Table S2). The percentage of patients with a performance status of 0 was lowest in EORTC 26981 (39.4%) and highest in EORTC 26082 (72.7%). CENTRIC enrolled only patients with MGMT promoter-methylated glioblastoma, whereas CORE and EORTC 26082 enrolled only patients with MGMT promoter-unmethylated glioblastoma. The percentage of patients with gross total resection was lowest in EORTC 26082 (27.3%). Steroid use at baseline was highest in EORTC 26981 (67.2%). Baseline values were similar between trials for platelets, hemoglobin, and lymphocytes. In EORTC 26981, baseline neutrophil counts were higher (Supplementary Figure S3) and more patients used steroids at baseline (Supplementary Table S2). Patients treated with steroids at baseline had higher neutrophil counts (median: 7.1 × 109/L vs. 4.1 × 109/L) (Supplementary Figure S4).
Prognostic associations of baseline hematology parameters
Primary analysis.
—In the unadjusted analysis of dataset L, lower values of neutrophils and hemoglobin were associated with better PFS and OS (Table 1). After adjustment, lower neutrophil counts (PFS P = .011, OS P < .001), age below 55 years (PFS P = .002, OS P < .001), MGMT promoter methylation, gross total resection, and MMSE above 27 (all PFS and OS P < .001) were associated with better PFS and OS. WHO performance status of 0 was also significantly associated with better OS (P < .001), but not PFS (P = .032) (Table 1). In dataset S, with lymphocyte data available, no significant association was found between lymphocyte count at baseline and PFS or OS (Supplementary Table S3).
Table 1.
Association between baseline hematology values and PFS or OS in the unadjusted and adjusted analysis in dataset L
| Unadjusted analysis | PFS | OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 98.3% CI | P | HR | 98.3% CI | P | ||
| Neutrophil count | N = 2012 | 1.05 | 1.03–1.07 | <0.001 | 1.07 | 1.05–1.09 | <0.001 |
| Platelet count | N = 2047 | 1.00 | 1.00–1.00 | 0.236 | 1.00 | 1.00–1.00 | 0.877 |
| Hemoglobin | N = 2052 | 1.07 | 1.00–1.15 | 0.016 | 1.08 | 1.01–1.17 | 0.011 |
| Adjusted analysis | PFS | OS | |||||
| N = 2002 | HR | 98.3% CI | P | HR | 98.3% CI | P | |
| Baseline neutrophils | 2002 (100.0) | 1.02 | 1.00–1.05 | 0.011 | 1.05 | 1.02–1.07 | <0.001 |
| Baseline platelets | 2002 (100.0) | 1.00 | 1.00–1.00 | 0.040 | 1.00 | 1.00–1.00 | 0.838 |
| Baseline hemoglobin | 2002 (100.0) | 1.01 | 0.94–1.10 | 0.670 | 1.02 | 0.93–1.11 | 0.588 |
| Sex | |||||||
| Male | 1206 (60.2) | 1 | 1 | 0.036 | |||
| Female | 796 (39.8) | 0.88 | 0.76–1.00 | 0.018 | 0.88 | 0.76–1.02 | |
| Age group | |||||||
| < 55 years | 850 (42.5) | 1 | 1 | <0.001 | |||
| ≥ 55 years | 1152 (57.5) | 1.17 | 1.04–1.32 | 0.002 | 1.45 | 1.27–1.66 | |
| WHO performance status | |||||||
| 0 | 1024 (51.1) | 1 | 1 | <0.001 | |||
| >0 | 978 (48.9) | 1.11 | 0.99–1.26 | 0.032 | 1.24 | 1.09–1.42 | |
| MGMT promoter | |||||||
| Unmethylated | 802 (40.1) | 1 | <0.001 | <0.001 | |||
| Methylated | 807 (40.3) | 0.54 | 0.46–0.62 | 0.44 | 0.37–0.52 | ||
| Unknown | 393 (19.6) | 0.80 | 0.67–0.95 | 0.81 | 0.68–0.98 | ||
| Extent of surgery | |||||||
| Partial resection or biopsy | 1120 (55.9) | 1 | <0.001 | 1 | <0.001 | ||
| Gross total resection | 882 (44.1) | 0.78 | 0.69–0.88 | 0.74 | 0.65–0.84 | ||
| MMSE | |||||||
| < 27 | 449 (22.4) | 1 | <0.001 | 1 | <0.001 | ||
| ≥ 27 | 1475 (73.7) | 0.78 | 0.68–0.91 | 0.76 | 0.65–0.88 | ||
| Unknown | 78 (3.9) | 0.99 | 0.58–1.69 | 1.14 | 0.65–2.01 | ||
| Steroid use at baseline | |||||||
| No | 1116 (55.7) | 1 | 0.172 | 1 | 0.033 | ||
| Yes | 886 (44.3) | 1.08 | 0.94–1.24 | 1.14 | 0.98–1.32 |
MGMT, O6-methylguanine DNA methyltransferase; MMSE, Mini-Mental State Examination; N, number of patients; OS, overall survival; PFS, progression-free survival; WHO, World Health Organization
Exploratory analyses.
—Lower neutrophil counts (PFS and OS P < .001), female sex (PFS P = .017, OS P = .011), age below 55 years (PFS P = .002, OS P < .001), WHO performance status of 0 (PFS P = .031, OS P < .001), MGMT promoter methylation, gross total resection, and MMSE (all PFS and OS P < .001) were associated with better PFS and OS. Steroid use at baseline was associated with worse OS (P = .031) in datasets L and S (Supplementary Tables S4 and S5). None of the interaction tests of myelosuppression by sex and MGMT status were significant for either PFS or OS (data not shown). Exploratory analyses of the single studies showed similar results by trends (Supplementary Tables S6–S9).
Myelosuppression during concomitant chemoradiotherapy
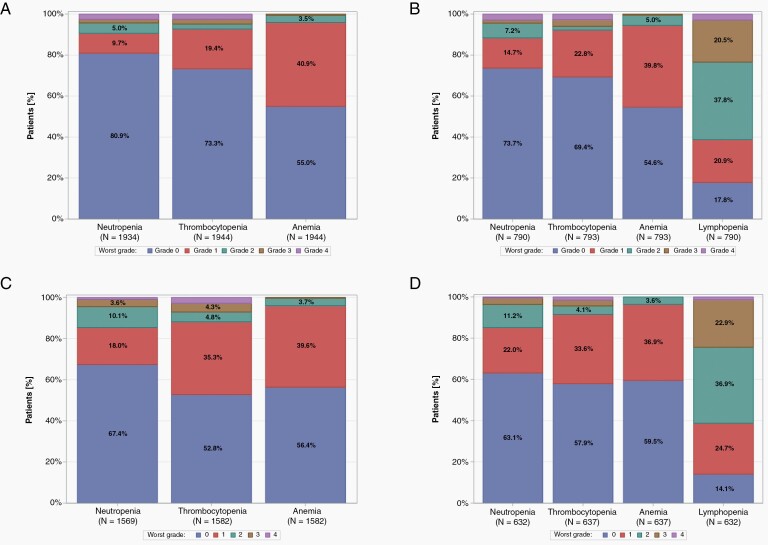
Only 56 patients (2.7%) did not receive concomitant TMZ with radiotherapy (Supplementary Table S10). Myelosuppression by grade is depicted in Table 2 and Figure 1A and B. Any grade toxicity was documented in 369 patients (18.9%) for neutrophils, in 519 patients (26.5%) for platelets, in 875 patients (44.7%) for hemoglobin, and in 649 patients (81.2%) for lymphocytes. High-grade toxicity was documented in 84 patients (4.3%) for neutrophils, in 95 patients (4.9%) for platelets, in 11 patients (0.6%) for hemoglobin, and in 185 patients (23.2%) for lymphocytes. High-grade toxicities were uniformly more common in females than in males (Supplementary Table S11). As high-grade anemia was documented in 11 patients (0.6%) only, anemia of any grade was explored for correlation with outcome.
Table 2.
Worst grade hematologic toxicity by trial, for patients alive after period 1 (concomitant treatment) and after period 2 (maintenance treatment)
| During concomitant treatment | During maintenance temozolomide | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CENTRIC N = 504 N (%) |
CORE N = 242 N (%) |
EORTC 26082 N = 53 N (%) |
AVAglio N=882 N (%) |
EORTC 26981 N = 275 N (%) |
TOTAL N = 1956 N (%) |
CENTRIC N = 427 N (%) |
CORE N = 213 N (%) |
EORTC 26082 N = 39 N (%) |
AVAglio N = 761 N (%) |
EORTC 26981 N = 221 N (%) |
TOTAL N = 1661 N (%) |
|
| Neutropenia | ||||||||||||
| Any grade | 131 (26.0) | 70 (28.9) | 7 (13.2) | 109 (12.4) | 52 (18.9) | 369 (18.9) | 166 (38.9) | 54 (25.4) | 13 (33.3) | 218 (28.6) | 60 (27.1) | 511 (30.8) |
| Grade | ||||||||||||
| None | 365 (72.4) | 171 (70.7) | 46 (86.8) | 768 (87.1) | 215 (78.2) | 1565 (80.0) | 232 (54.3) | 141 (66.2) | 26 (66.7) | 511 (67.1) | 148 (67.0) | 1058 (63.7) |
| Grade 1–2 | 108 (21.4) | 60 (24.8) | 5 (9.4) | 72 (8.2) | 40 (14.5) | 285 (14.6) | 147 (34.4) | 50 (23.5) | 13 (33.3) | 180 (23.7) | 51 (23.1) | 441 (26.6) |
| Grade 3–4 | 23 (4.6) | 10 (4.1) | 2 (3.8) | 37 (4.2) | 12 (4.4) | 84 (4.3) | 19 (4.4) | 4 (1.9) | 0 (0.0) | 38 (5.0) | 9 (4.1) | 70 (4.2) |
| Missing | 8 (1.6) | 1 (0.4) | 0 (0.0) | 5 (0.6) | 8 (2.9) | 22 (1.1) | 29 (6.8) | 18 (8.5) | 0 (0.0) | 32 (4.2) | 13 (5.9) | 92 (5.5) |
| Thrombocytopenia | ||||||||||||
| Any grade | 164 (32.5) | 74 (30.6) | 5 (9.4) | 246 (27.9) | 30 (10.9) | 519 (26.5) | 185 (43.3) | 78 (36.6) | 5 (12.8) | 420 (55.2) | 59 (26.7) | 747 (45.0) |
| Grade | ||||||||||||
| None | 335 (66.5) | 167 (69.0) | 48 (90.6) | 632 (71.7) | 243 (88.4) | 1425 (72.9) | 216 (50.6) | 119 (55.9) | 34 (87.2) | 310 (40.7) | 156 (70.6) | 835 (50.3) |
| Grade 1–2 | 129 (25.6) | 64 (26.4) | 3 (5.7) | 206 (23.4) | 22 (8.0) | 424 (21.7) | 164 (38.4) | 71 (33.3) | 5 (12.8) | 362 (47.6) | 33 (14.9) | 635 (38.2) |
| Grade 3–4 | 35 (6.9) | 10 (4.1) | 2 (3.8) | 40 (4.5) | 8 (2.9) | 95 (4.9) | 21 (4.9) | 7 (3.3) | 0 (0.0) | 58 (7.6) | 26 (11.8) | 112 (6.7) |
| Missing | 5 (1.0) | 1 (0.4) | 0 (0.0) | 4 (0.5) | 2 (0.7) | 12 (0.6) | 26 (6.1) | 16 (7.5) | 0 (0.0) | 31 (4.1) | 6 (2.7) | 79 (4.8) |
| Anemia | ||||||||||||
| Any grade | 227 (45.0) | 104 (43.0) | 29 (54.7) | 338 (38.3) | 177 (64.4) | 875 (44.7) | 169 (39.6) | 70 (32.9) | 19 (48.7) | 302 (39.7) | 130 (58.8) | 690 (41.5) |
| Grade | ||||||||||||
| None | 272 (54.0) | 137 (56.6) | 24 (45.3) | 540 (61.2) | 96 (34.9) | 1069 (54.7) | 232 (54.3) | 127 (59.6) | 20 (51.3) | 428 (56.2) | 85 (38.5) | 892 (53.7) |
| Grade 1–2 | 223 (44.2) | 104 (43.0) | 29 (54.7) | 332 (37.6) | 176 (64.0) | 864 (44.2) | 169 (39.6) | 70 (32.9) | 19 (48.7) | 299 (39.3) | 128 (57.9) | 685 (41.2) |
| Grade 3–4 | 4 (0.8) | 0 (0.0) | 0 (0.0) | 6 (0.7) | 1 (0.4) | 11 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.4) | 2 (0.9) | 5 (0.3) |
| Missing | 5 (1.0) | 1 (0.4) | 0 (0.0) | 4 (0.5) | 2 (0.7) | 12 (0.6) | 26 (6.1) | 16 (7.5) | 0 (0.0) | 31 (4.1) | 6 (2.7) | 79 (4.8) |
| Lymphopenia * | ||||||||||||
| Any grade | 412 (81.7) | 198 (81.8) | 39 (73.6) | – | – | 649 (81.2) | 354 (82.9) | 161 (75.6) | 28 (71.8) | – | – | 543 (80.0) |
| Grade | ||||||||||||
| None | 84 (16.7) | 43 (17.8) | 14 (26.4) | – | – | 141 (17.6) | 44 (10.3) | 34 (16.0) | 11 (28.2) | – | – | 89 (13.1) |
| Grade 1–2 | 289 (57.3) | 144 (59.5) | 31 (58.5) | – | – | 464 (58.1) | 247 (57.8) | 125 (58.7) | 17 (43.6) | – | – | 389 (57.3) |
| Grade 3–4 | 123 (24.4) | 54 (22.3) | 8 (15.1) | – | – | 185 (23.2) | 107 (25.1) | 36 (16.9) | 11 (28.2) | – | – | 154 (22.7) |
| missing | 8 (1.6) | 1 (0.4) | 0 (0.0) | –– | – | 9 (1.1) | 29 (6.8) | 18 (8.5) | 0 (0.0) | – | – | 47 (6.9) |
*Lymphocyte counts were not available in AVAglio and EORTC 26082
Fig. 1.
Worst grade of neutropenia, thrombocytopenia, anemia, and lymphopenia for patients during concomitant treatment (A, dataset L; B, dataset S) and maintenance treatment (C, dataset L; D, dataset S). Patients who were alive at the end of concomitant treatment were considered for the analysis during concomitant treatment. Patients who were alive after the first maintenance cycle were considered for the analysis during the maintenance treatment. Neutropenia, thrombocytopenia, and anemia were analyzed in dataset L, lymphopenia in dataset S.
Primary analysis.
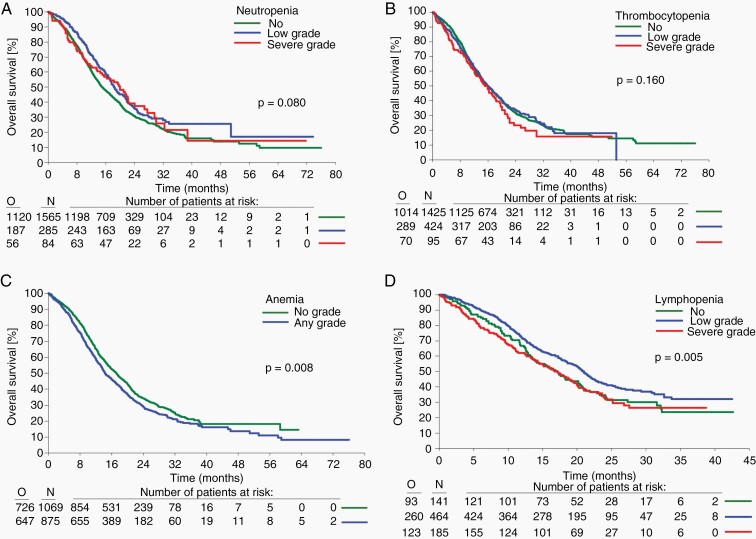
—The unadjusted analysis in dataset L showed no association between any hematological toxicity and PFS. In contrast, patients who had anemia of any grade had an inferior OS than those who experienced no anemia (P = .008) (Table 3, Supplementary Figure S5, Figure 2). Adjusted analysis showed no association between any hematological toxicity and PFS or OS. Female sex (PFS P = .003, OS P = .004), age below 55 years (PFS P = .004, OS P < .001), MGMT promoter methylation, gross total resection, MMSE above 27 (all PFS and OS P < .001), and absence of steroid use at baseline (PFS P = .008, OS P < .001) were associated with longer PFS and OS. WHO performance status of 0 was associated with longer OS (P < .001) but was not associated with PFS (P = .034).
Table 3.
Association between hematology values during concomitant treatment and PFS and OS, unadjusted and adjusted analysis in dataset L
| Unadjusted analysis | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 98.3% CI | P | HR | 98.3% CI | P | |||
| Neutropenia | N = 1662 | N = 1934 | ||||||
| None | 1323 (79.6) | 1 | 0.119 | 1565 (80.9) | 1 | 0.080 | ||
| Grade 1–2 | 259 (15.6) | 0.92 | 0.77–1.11 | 285 (14.7) | 0.86 | 0.71–1.04 | ||
| Grade 3–4 | 80 (4.8) | 0.78 | 0.57–1.07 | 84 (4.3) | 0.83 | 0.60–1.15 | ||
| Thrombocytopenia | N = 1668 | N = 1944 | ||||||
| None | 1219 (73.1) | 1 | 0.426 | 1425 (73.3) | 1 | 0.160 | ||
| Grade 1–2 | 365 (21.9) | 0.92 | 0.78–1.08 | 424 (21.8) | 1.05 | 0.89–1.23 | ||
| Grade 3–4 | 84 (5.0) | 1.00 | 0.73–1.37 | 95 (4.9) | 1.26 | 0.94–1.69 | ||
| Anemia | N = 1668 | N = 1944 | ||||||
| No | 922 (55.3) | 1 | 0.116 | 1069 (55.0) | 1 | 0.008 | ||
| Any grade | 746 (44.7) | 1.09 | 0.96–1.24 | 875 (45.0) | 1.16 | 1.01–1.32 | ||
| Adjusted analysis | PFS | OS | ||||||
| N = 1656 | HR | 98.3% CI | P | N = 1927 | HR | 98.3% CI | P | |
| Neutropenia | ||||||||
| None | 1317 (79.5) | 1 | 0.591 | 1559 (80.9) | 1 | 0.161 | ||
| Grade 1-2 | 259 (15.6) | 0.97 | 0.80–1.17 | 284 (14.7) | 0.89 | 0.73–1.08 | ||
| Grade 3-4 | 80 (4.8) | 0.86 | 0.61–1.22 | 84 (4.4) | 0.80 | 0.56–1.14 | ||
| Thrombocytopenia | ||||||||
| None | 1208 (72.9) | 1 | 0.334 | 1411 (73.2) | 1 | 0.111 | ||
| Grade 1–2 | 364 (22.0) | 0.91 | 0.77–1.07 | 422 (21.9) | 1.05 | 0.89–1.23 | ||
| Grade 3–4 | 84 (5.1) | 1.05 | 0.74–1.47 | 94 (4.9) | 1.32 | 0.96–1.83 | ||
| Anemia | ||||||||
| None | 917 (55.4) | 1 | 0.216 | 1062 (55.1) | 1 | 0.107 | ||
| Any grade | 739 (44.6) | 1.07 | 0.94–1.23 | 865 (44.9) | 1.10 | 0.96–1.26 | ||
| Sex | ||||||||
| Male | 1007 (60.8) | 1 | 0.003 | 1167 (60.6) | 1 | 0.004 | ||
| Female | 649 (39.2) | 0.84 | 0.74–0.97 | 760 (39.4) | 0.85 | 0.74–0.97 | ||
| Age group | ||||||||
| < 55 years | 723 (43.7) | 1 | 0.004 | 829 (43.0) | 1 | <0.001 | ||
| ≥ 55 years | 933 (56.3) | 1.18 | 1.03–1.35 | 1098 (57.0) | 1.42 | 1.23–1.62 | ||
| WHO performance status | ||||||||
| 0 | 873 (52.7) | 1 | 0.034 | 1012 (52.5) | 1 | <0.001 | ||
| > 0 | 783 (47.3) | 1.13 | 0.98–1.29 | 915 (47.5) | 1.22 | 1.07–1.40 | ||
| MGMT promoter | ||||||||
| Unmethylated | 653 (39.4) | 1 | <0.001 | 778 (40.4) | 1 | <0.001 | ||
| Methylated | 675 (40.8) | 0.49 | 0.42–0.58 | 777 (40.3) | 0.43 | 0.36–0.51 | ||
| Unknown | 328 (19.8) | 0.78 | 0.65–0.95 | 372 (19.3) | 0.78 | 0.65–0.94 | ||
| Extent of surgery | ||||||||
| Partial resection or biopsy | 899 (54.3) | 1 | <0.001 | 1065 (55.3) | 1 | <0.001 | ||
| Gross total resection | 757 (45.7) | 0.81 | 0.71–0.92 | 862 (44.7) | 0.75 | 0.66–0.86 | ||
| MMSE | ||||||||
| < 27 | 348 (21.0) | 1 | <0.001 | 413 (21.4) | 1 | <0.001 | ||
| ≥ 27 | 1251 (75.5) | 0.76 | 0.65–0.90 | 1437 (74.6) | 0.74 | 0.63–0.87 | ||
| Unknown | 57 (3.4) | 1.02 | 0.59–1.75 | 77 (4.0) | 1.07 | 0.60–1.91 | ||
| Steroid use at baseline | ||||||||
| No | 932 (56.3) | 1 | 0.008 | 1076 (55.8) | 1 | <0.001 | ||
| Yes | 724 (43.7) | 1.16 | 1.02–1.34 | 851 (44.2) | 1.27 | 1.11–1.46 |
MGMT, O6-methylguanine DNA methyltransferase; MMSE, Mini-Mental State Examination; N, number of patients; OS, overall survival; PFS, progression-free survival; WHO, World Health Organization.
Fig. 2.
Association between myelosuppression during concomitant treatment and overall survival. Neutropenia (A), thrombocytopenia (B), anemia (C): dataset L, lymphopenia (D): dataset S; O observed events of death, N number of patients at risk.
Using dataset S, unadjusted analysis confirmed the absence of a significant association between hematological toxicity and PFS, but showed that lymphopenia was significantly associated with OS (P = .005) (Supplementary Figure S5D, Supplementary Table S12, Figure 2D). Adjusted analysis confirmed that lymphopenia during concomitant treatment was associated with OS (HR for low-grade versus no grade was 0.78 (98.3% CI 0.58–1.06) and HR for high-grade grade versus no grade was 1.08 (98.3% CI 0.75–1.54, P = .009).
Exploratory analysis.
—In dataset L, female sex (PFS and OS P = .001), age (PFS P = .003, OS P < .001), WHO performance status (PFS P = .038, OS P < .001), MGMT promoter methylation status, extent of surgery, MMSE (all PFS and OS P < .001), and steroid use at baseline (PFS P = .006, OS P < .001) were all associated with PFS and OS, but none of the parameters of myelosuppression (Supplementary Table S13). In dataset S, lymphopenia was significantly associated with OS (P = .008) but not PFS (Supplementary Table S14). None of the interaction tests of myelosuppression by sex and MGMT were significant for either PFS or OS (data not shown). The exploratory analyses of the single studies showed the same trends (Supplementary Tables S15–S18). Further, sensitivity analyses were performed excluding patients with disease progression prior to the landmark which might confound the OS analyses. The results were overall similar to those of the primary analyses except that the complex association of lymphopenia by grade with OS was no longer significant (Supplementary Table S19).
Myelosuppression during maintenance chemotherapy
More than 70% of patients in each trial and overall 1676 patients (80.8%) started maintenance TMZ; 1024 patients (49.4%) received at least 6 cycles of TMZ (Supplementary Table S10). Baseline characteristics of patients alive for the maintenance analysis (N = 1661, 80.1%) were similar to the distribution of the baseline characteristics of all randomized patients (Supplementary Tables S2 and S20). Any grade toxicity was documented in 511 patients (30.8%) for neutrophils, in 747 patients (45.0%) for platelets, in 690 patients (41.5%) for haemoglobin, and in 543 patients (80.0%) for lymphocytes. Grade 3 or 4 toxicity was documented in 70 patients (4.2%) for neutrophils, in 112 patients (6.7%) for platelets, in 5 patients (0.3%) for hemoglobin, and in 154 patients (22.7%) for lymphocytes (Table 2, Figure 1C and D). Again, high-grade toxicities were uniformly more common in females than in males (Supplementary Table S21).
For patients who were alive and free of progression (for PFS) or alive (for OS), by construction, the percentage of patients who had myelosuppression at each landmark increased from the end of the first to the third and to the sixth maintenance cycle. Supplementary Table S22 shows data for the pooled cohort, and Supplementary Tables 23–26 show data for each single trial. All grades of anemia were again pooled for outcome correlation.
Primary analysis.
—Unadjusted analysis did not show significant association of myelotoxicity with PFS. In contrast, neutropenia was associated with better OS (P = .002), whereas anemia was associated with poorer OS (P = .011) (Table 4). Adjusted analysis did not confirm the significant association of neutropenia and anemia with OS. Female sex (PFS P = .006, OS P = .002), MGMT promoter methylation status, complete resection (both PFS and OS P < .001), and MMSE above 27 (PFS P < .001, OS P = .005) were significantly associated with longer PFS and longer OS. Age below 55 years (P < .001), performance status of 0 at baseline (P = .011), and absence of steroid use at baseline (P = .003) were also associated with longer OS.
Table 4.
Association between hematology values during maintenance treatment and PFS and OS, in dataset L
| Unadjusted analysis | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 98.3% CI | P | HR | 98.3% CI | P | |||
| Neutropenia | N = 1467 | |||||||
| No | 1 | 0.038 | N = 1569 | 1 | 0.002 | |||
| Grade 1–2 | 0.88 | 0.76–1.04 | 0.77 | 0.64–0.91 | ||||
| Grade 3–4 | 1.17 | 0.89–1.55 | 0.91 | 0.64–1.31 | ||||
| Thrombocytopenia | N = 1478 | N = 1582 | ||||||
| No | 1 | 0.661 | 1 | 0.253 | ||||
| Grade 1–2 | 1.04 | 0.90–1.20 | 1.11 | 0.95–1.30 | ||||
| Grade 3–4 | 0.94 | 0.69–1.28 | 1.05 | 0.76–1.45 | ||||
| Anemia | ||||||||
| No | N = 1478 | 1 | 0.346 | N = 1582 | 1 | 0.011 | ||
| Any grade | 1.06 | 0.92–1.23 | 1.18 | 1.01–1.28 | ||||
| Adjusted Analysis | PFS | OS | ||||||
| N = 1461 | HR | 98.3% CI | P | N = 1563 | HR | 98.3% CI | P | |
| Neutropenia | ||||||||
| No | 1 | 0.077 | 1 | 0.037 | ||||
| Grade 1-2 | 0.97 | 0.82–1.15 | 0.82 | 0.68–0.99 | ||||
| Grade 3-4 | 1.31 | 0.96–1.80 | 0.87 | 0.57–1.32 | ||||
| Thrombocytopenia | ||||||||
| No | 1 | 0.759 | 1 | 0.137 | ||||
| Grade 1-2 | 1.02 | 0.88–1.19 | 1.13 | 0.96–1.33 | ||||
| Grade 3-4 | 0.93 | 0.67–1.29 | 1.20 | 0.85–1.69 | ||||
| Anemia | ||||||||
| No | 1 | 0.706 | 1 | 0.054 | ||||
| Grade 1–2 | 1.03 | 0.87–1.20 | 1.14 | 0.97–1.35 | ||||
| Sex | ||||||||
| Male | 902 (61.7) | 1 | 0.006 | 972 (62.2) | 1 | 0.002 | ||
| Female | 559 (38.3) | 0.84 | 0.72–0.98 | 591 (37.8) | 0.80 | 0.68–0.95 | ||
| Age group | ||||||||
| < 55 years | 666 (45.6) | 1 | 0.024 | 713 (45.6) | 1 | <0.001 | ||
| ≥ 55 years | 795 (54.4) | 1.15 | 0.99–1.34 | 850 (54.4) | 1.30 | 1.11–1.54 | ||
| WHO performance status | ||||||||
| 0 | 795 (54.4) | 1 | 0.195 | 849 (54.3) | 1 | 0.011 | ||
| > 0 | 666 (45.6) | 1.08 | 0.93–1.26 | 714 (45.7) | 1.19 | 1.01–1.40 | ||
| MGMT promoter | ||||||||
| Unmethylated | 593 (40.6) | 1 | <0.001 | 639 (40.9) | 1 | <0.001 | ||
| Methylated | 587 (40.2) | 0.49 | 0.40–0.59 | 627 (40.1) | 0.38 | 0.31–0.47 | ||
| Unknown | 281 (19.2) | 0.75 | 0.61–0.94 | 297 (19.0) | 0.79 | 0.62–1.00 | ||
| Extent of surgery | ||||||||
| Partial resection or biopsy | 784 (53.7) | 1 | <0.001 | 848 (54.3) | 1 | <0.001 | ||
| Gross total resection | 677 (46.3) | 0.80 | 0.68–0.93 | 715 (45.7) | 0.77 | 0.65–0.91 | ||
| MMSE | ||||||||
| < 27 | 300 (20.5) | 1 | <0.001 | 321 (20.5) | 1 | 0.005 | ||
| ≥ 27 | 1119 (76.6) | 0.73 | 0.61–0.88 | 1189 (76.1) | 0.78 | 0.64–0.96 | ||
| Unknown | 42 (2.9) | 1.02 | 0.55–1.87 | 53 (3.4) | 1.38 | 0.62–3.06 | ||
| Steroid use at baseline | ||||||||
| No | 838 (57.4) | 1 | 0.115 | 893 (57.1) | 1 | 0.003 | ||
| Yes | 623 (42.6) | 1.11 | 0.95–1.30 | 670 (42.9) | 1.23 | 1.04–1.46 |
MGMT O6-methylguanine DNA methyltransferase; MMSE, Mini-Mental State Examination; N, number of patients; OS, overall survival; PFS, progression-free survival; WHO, World Health Organization.
Unadjusted analysis of dataset S showed no significant association between lymphopenia and PFS or OS, but here, OS was significantly longer in patients with neutropenia (P = 0.001). In the adjusted analysis, lymphopenia was not significantly associated with PFS (P = .105) or OS (P = .042, low-grade HR = 0.77, 98.3% CI 0.59–1.01, high-grade grade HR = 0.66, 98.3% CI 0.42–1.05). No significant association was found between the other parameters of myelosuppression and PFS or OS (Supplementary Table S27).
Exploratory analysis.
—In dataset L, female sex (PFS P = .010, OS P = .001), age below 55 years (PFS P = .013, OS P < .001), MGMT promoter methylation status, complete resection (both PFS and OS P < .001), and MMSE above 27 (PFS P < .001, OS P = .003) were associated with longer PFS and longer OS. Anemia (P = .042) and steroid use at baseline (P = .003) were also identified as important factors for worse OS (Supplementary Table S28). Lymphopenia (P = .013) was part of the significant variables for OS in dataset S (Supplementary Table S29). For both PFS and OS, none of the interaction tests of myelosuppression by sex and MGMT were significant (data not shown).
Since severe lymphopenia appeared to be linked to inferior outcome in the concomitant phase, but to superior outcome in the maintenance phase, we explored changes in the respective patient populations under study. Indeed, of the 799 patients included in the analysis of OS and lymphopenia in the concomitant phase, 679 patients were also included in the maintenance phase analysis. Thus, 120 of 799 patients included in the concomitant phase analysis died prior to the end of the first maintenance treatment cycle and were not included in the maintenance phase analysis. Patients who died prior to the end of the first maintenance treatment cycle were more likely to have severe lymphopenia compared to those patients who were alive and included in the maintenance phase analysis (32.5% vs. 21.5%. P = .28, OR = 1.37, 95% CI = 0.78–2.42) (Supplementary Table S30). The exploratory analyses of the single studies showed the same trends (Supplementary Tables S31–S34). Further, sensitivity analyses were performed excluding patients with disease progression prior to the landmark which might confound the OS analyses, but the results were similar to those of the primary analyses (Supplementary Table S35).
Outcome associations of the neutrophil lymphocyte ratio (NLR)
A higher NLR at baseline was associated with inferior OS (P < .001, HR = 1.05, 95% CI 1.02–1.07), but not PFS in the unadjusted analysis, but not in the adjusted analysis (data not shown). An analysis of the last NLR per patient documented during concomitant treatment revealed again an association with inferior OS (P < .001, HR = 1.04, 95% CI 1.02–1.05), but not PFS in the unadjusted analysis, but this association was confirmed in the adjusted analysis (Supplementary Table S36). Finally, an analysis of the first NLR during maintenance treatment revealed a trend towards inferior OS (P = .049, HR = 1.03, 95% CI 1.00–1.07), but not PFS in the unadjusted analysis, but no association in the adjusted analysis (data not shown).
Discussion
The present study used the EORTC Brain Tumor Group clinical trial database to explore a current controversial issue in the prognostic assessment and therapeutic management of patients with newly diagnosed glioblastoma, the associations of myelosuppression with outcome. We confirm that myelosuppression is frequent in this setting. The most frequent toxicity during concomitant and maintenance treatment was lymphopenia, occurring in 82% of the patients during concomitant treatment and in 80% during maintenance treatment, with more than 20% of high-grade lymphopenia during both concomitant and maintenance treatment. All grade thrombocytopenia, which may necessitate treatment delays or discontinuation, was observed in more than 25% of the patients during concomitant treatment and in up to 45% during maintenance treatment, with almost 7% of severe high-grade thrombocytopenia during maintenance therapy. All grade neutropenia was noted in almost 20% of patients during concomitant treatment and in 31% during maintenance. Anemia, which can enhance fatigue, was also frequent (Figure 1).
The present analysis of more than 2000 patients enrolled into clinical trials addressed several contemporary issues:
First, we find that neutrophil counts at study entry were prognostic in that higher neutrophil counts were associated with inferior PFS and OS (Table 1). This effect persisted when controlled for corticosteroid medication which per se has been identified as a negative prognostic factor in various similar datasets.21 One might speculate that higher neutrophil counts may signify proinflammatory state associated with gliomas that itself may have pro-tumorigenic properties.22 While the effect may not be strong enough to justify stratification by neutrophil counts in clinical trials, the biological basis for this observation warrants further study.
Second, we do not confirm an overall association between chemotherapy-induced myelotoxicity and improved outcome. The induction of any level of neutropenia during concomitant or maintenance chemoradiotherapy was not significantly associated with PFS and OS (Figure 2A). This contrasts with some previous observations in smaller cohorts,9,23 but results from this largest cohort of patients ever studied suggests that individualized dosing of temozolomide to achieve a certain degree of neutropenia may not improve outcome. Similarly, thrombocytopenia was prognostic neither in the concomitant nor in the maintenance phase (Figure 2B). This was somewhat unexpected since one might assume that induced thrombocytopenia causes dose delays and dose reductions of temozolomide, which might compromise its therapeutic efficacy. In fact, induced thrombocytopenia has been the rational to develop therapeutic interventions such as the thrombopoetin receptor agonist romiplostim which is a powerful drug to enable completion of maintenance chemotherapy.24
Compared with myelosuppression in other compartments, anemia was not a leading toxicity by grade in this pooled cohort of clinical trial patients (Figure 1). Overall anemia was not significantly associated with PFS and OS when observed in the concomitant and maintenance treatment phase (Figure 2C).
Complex associations with survival were observed for lymphopenia. Lymphopenia is associated with decreased cellular immunity and should thus be associated with inferior outcome of cancer patients in general. Interrelations of temozolomide-induced lymphopenia and efficacy of immunotherapy have recently attracted a lot of interest.25 Early reports on prolonged exposure to temozolomide in melanoma patients had indicated that profound lymphopenia particularly affected the regulatory T cell compartment,26,27 whereas in newly diagnosed glioblastoma, lymphopenia induction with relative preservation of regulatory T cells was observed,28 potentially also related to different definitions of regulatory T cells. We observed that low, but not high-grade lymphopenia in the concomitant treatment phase might be associated with favorable OS; furthermore, in the maintenance phase, either low- or high-grade lymphopenia was linked with better OS. In principle, mild lymphopenia might be associated with favorable outcome because some immunosuppressive T-cell populations like T regulatory T-cells are depleted.28 However, when lymphopenia becomes more severe, the overall immune response is severely impaired, potentially rendering outcome less favorable because of impaired tumor immune surveillance or infection or both. Yet, this separation by grade of lymphopenia was only apparent in the concomitant treatment phase. There was a non-significant trend for higher risk of death prior to the start of maintenance therapy in patients with severe lymphopenia. Altogether, our observations rather lend support to the notion that temozolomide may be able to deplete T cell populations that promote rather than inhibit the state of immunosuppression characteristic of glioblastoma. Conversely, our data also indicate that an increased neutrophil lymphocyte ratio (NLR) during chemoradiotherapy may be associated with inferior outcome.29
We present here the largest database to date of over 2000 patients treated with the current standard of care of temozolomide chemoradiotherapy as the backbone. All data were collected prospectively within 5 interventional clinical trials. Yet, our study has some major limitations. The analysis was retrospective in nature, albeit on prospectively assembled data with a prospectively planned and defined statistical analysis plan. Potential confounding effects of comedications such as cotrimoxazole or anticonvulsants were not consistently captured with sufficient detail to be integrated in the analyses. Patients progressing within the set time intervals for this analysis might have an inherently inferior outcome, but have also experienced less exposure to chemotherapy and therefore lower likelihood of experiencing myelosuppression, thus introducing bias towards finding an apparent link between myelosuppression and superior outcome. However, sensitivity analyses excluding patients with progressive disease confirmed the key results of this study (Supplementary Tables S19 and S35).
Furthermore, patients enrolled into clinical trials necessarily represent a selection of patients which may not reflect the full spectrum of the disease. Conclusions may thus not be fully applicable to glioblastoma patients with less favorable disease characteristics. Validation of the major observations reported here in an adequately sized independent patient cohort with comparable data quality would be welcome. Finally, all clinical trial protocols have predetermined rules how to dose-modify, delay or interrupt chemotherapy in response to myelosuppression, but it is impossible to determine to what extent reduced exposure to treatment actually caused inferior outcome in this patient population.
In conclusion, we report that higher neutrophil counts at baseline are associated with inferior PFS and OS, suggesting that this parameter could be explored as a stratification factor in clinical trials and that a proinflammatory state could be protumorigenic in glioblastoma. The association of therapy-induced lymphopenia with superior outcome suggests that monitoring lymphocyte subsets during the disease course and correlating such changes with outcome might provide clinically relevant information in future efforts to develop more effective immunotherapies for patients with glioblastoma.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Acknowledgments
We are grateful to Merck KGaA and Pfizer for supporting the independent EORTC studies 26071 and 26082.
Contributor Information
Emilie Le Rhun, Departments of Neurosurgery and Neurology & Brain Tumor Center, University Hospital and University of Zurich, Zurich, Switzerland.
Felix Boakye Oppong, EORTC Headquarters, Brussels, Belgium.
Maureen Vanlancker, EORTC Headquarters, Brussels, Belgium.
Roger Stupp, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Lausanne, Switzerland; Malnati Brain Tumor Center of the Lurie Comprehensive Cancer Center and Departments of Neursurgery and Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL 60611, USA.
Burt Nabors, Department of Neurology, Division of Neuro-Oncology, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
Olivier Chinot, Aix-Marseille University, AP-HM, Service de Neuro-Oncologie, CHU Timone, Marseille, France.
Wolfgang Wick, Department of Neurology and Neuro-oncology Program at the National Center for Tumor Diseases, University Hospital Heidelberg and German Cancer Research Center, Heidelberg, Germany.
Matthias Preusser, Department of Medicine I, Division of Oncology, Medical University of Vienna, Vienna, Austria.
Thierry Gorlia, EORTC Headquarters, Brussels, Belgium.
Michael Weller, Department of Neurology and Brain Tumor Center, University Hospital and University of Zurich, Zurich, Switzerland.
Funding
The study was supported by the European Organization for Research and Treatment of Cancer (EORTC) Brain Tumor Group. The work of Felix B. Oppong as Fellow at EORTC Headquarters was supported by a grant from the EORTC Brain Tumor Group.
Conflict of interest statement. E.L.R. has received honoraria for lectures or advisory board from Adastra, Bayer, Leo Pharma, Seattle Genetics. F.B.O. has nothing to disclose. M.V. has nothing to disclose. R.S. has nothing to disclose. B.N. serves on Scientific Advisory Board for Karyopharm. O.C. has received honoraria for consultation or advisory board participation from Bristol-Myer Squibb and AbbVie. W.W. has nothing to disclose. M.P. has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen. The following for-profit companies have supported clinical trials and contracted research conducted by MP with payments made to his institution: Böhringer-Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, AbbVie. T.G. has nothing to disclose. M.W. has received research grants from Adastra, Merck, Sharp & Dohme (MSD), Merck (EMD) and Novocure, and honoraria for lectures or advisory board participation or consulting from Bristol Meyer Squibb (BMS), Medac, Merck, Sharp & Dohme (MSD), Merck (EMD), Nerviano Medical Sciences, Orbus, Philogen, Roche, and y-Mabs.
Authors’ Contributions
Experimental design and its implementation: E.L.R. and F.B.O. Acquisition, analysis, or interpretation of data: all authors. Statistical analysis: F.B.O. and T.G. Writing of the manuscript: all authors.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Weller M, van den Bent M, Preusser M, et al. . EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 4. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 5. Gerber DE, Grossman SA, Zeltzman M, Parisi MA, Kleinberg L. The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro-oncology 2007;9(1):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong TS, Cao Y, Scheurer ME, et al. . Risk analysis of severe myelotoxicity with temozolomide: the effects of clinical and genetic factors. Neuro-oncology 2009;11(6):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lombardi G, Rumiato E, Bertorelle R, et al. . Clinical and genetic factors associated with severe hematological toxicity in glioblastoma patients during radiation plus temozolomide treatment: a prospective study. Am J Clin Oncol. 2015;38(5):514–519. [DOI] [PubMed] [Google Scholar]
- 8. Wen PY, Weller M, Lee EQ, et al. . Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saito T, Sugiyama K, Hama S, et al. . Prognostic importance of temozolomide-induced neutropenia in glioblastoma, IDH-wildtype patients. Neurosurg Rev. 2018;41(2):621–628. [DOI] [PubMed] [Google Scholar]
- 10. Vaios EJ, Nahed BV, Muzikansky A, Fathi AT, Dietrich J. Bone marrow response as a potential biomarker of outcomes in glioblastoma patients. J Neurosurg. 2017;127(1):132–138. [DOI] [PubMed] [Google Scholar]
- 11. Grossman SA, Ellsworth S, Campian J, et al. . Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13(10):1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grossman SA, Ye X, Lesser G, et al. . Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stupp R, Hegi ME, Gorlia T, et al. . Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 14. Nabors LB, Fink KL, Mikkelsen T, et al. . Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro-oncology 2015;17(5):708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wick W, Gorlia T, Bady P, et al. . Phase II study of radiotherapy and temsirolimus versus radiochemotherapy with temozolomide in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation (EORTC 26082). Clin Cancer Res. 2016;22(19):4797–4806. [DOI] [PubMed] [Google Scholar]
- 16. Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 17. Morgan CJ. Landmark analysis: a primer. J Nucl Cardiol. 2019;26(2):391–393. [DOI] [PubMed] [Google Scholar]
- 18. Putter H, van Houwelingen HC. Understanding landmarking and its relation with time-dependent Cox regression. Stat Biosci. 2017;9(2):489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klein JP, van Houwelingen HC, Ibrahim JG, Scheike TH.. Handbook of Survival Analysis. New York: CRC Press; 2016. [Google Scholar]
- 20. Collett D. Modelling Survival Data in Medical Research. New York: CRC Press; 2015. [Google Scholar]
- 21. Pitter KL, Tamagno I, Alikhanyan K, et al. . Corticosteroids compromise survival in glioblastoma. Brain 2016;139(Pt 5):1458–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang C, Wen HB, Zhao YH, et al. . Systemic inflammatory indicators as prognosticators in glioblastoma patients: a comprehensive meta-analysis. Front Neurol. 2020;11:580101. doi: 10.3389/fneur.2020.580101. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schernberg A, Nivet A, Dhermain F, et al. . Neutrophilia as a biomarker for overall survival in newly diagnosed high-grade glioma patients undergoing chemoradiation. Clin Transl Radiat Oncol. 2018;10:47–52. doi: 10.1016/j.ctro.2018.04.002. eCollection 2018 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Rhun E, Devos P, Houillier C, et al. . Romiplostim for temozolomide-induced thrombocytopenia in glioblastoma: the PLATUM trial. Neurology 2019;93(19):e1799–e1806. [DOI] [PubMed] [Google Scholar]
- 25. Hotchkiss KM, Sampson JH. Temozolomide treatment outcomes and immunotherapy efficacy in brain tumor. J Neurooncol. 2021;151(1): 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su YB, Sohn S, Krown SE, et al. . Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22(4): 610–616. [DOI] [PubMed] [Google Scholar]
- 27. Iversen TZ, Brimnes MK, Nikolajsen K, et al. . Depletion of T lymphocytes is correlated with response to temozolomide in melanoma patients. Oncoimmunology 2013;2(2):e23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sampson JH, Aldape KD, Archer GE, et al. . Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mason M, Maurice C, McNamara MG, et al. . Neutrophil-lymphocyte ratio dynamics during concurrent chemo-radiotherapy for glioblastoma is an independent predictor for overall survival. J Neurooncol. 2017;132(3):463–471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.