Abstract
Background
Uncontrolled seizures in patients with gliomas have a significant impact on quality of life and morbidity, yet the mechanisms through which these tumors cause seizures remain unknown. Here, we hypothesize that the active metabolite d-2-hydroxyglutarate (d-2-HG) produced by the IDH-mutant enzyme leads to metabolic disruptions in surrounding cortical neurons that consequently promote seizures.
Methods
We use a complementary study of in vitro neuron-glial cultures and electrographically sorted human cortical tissue from patients with IDH-mutant gliomas to test this hypothesis. We utilize micro-electrode arrays for in vitro electrophysiological studies in combination with pharmacological manipulations and biochemical studies to better elucidate the impact of d-2-HG on cortical metabolism and neuronal spiking activity.
Results
We demonstrate that d-2-HG leads to increased neuronal spiking activity and promotes a distinct metabolic profile in surrounding neurons, evidenced by distinct metabolomic shifts and increased LDHA expression, as well as upregulation of mTOR signaling. The increases in neuronal activity are induced by mTOR activation and reversed with mTOR inhibition.
Conclusion
Together, our data suggest that metabolic disruptions in the surrounding cortex due to d-2-HG may be a driving event for epileptogenesis in patients with IDH-mutant gliomas.
Keywords: D-2-HG, IDH-mutated gliomas, mTOR, Tumor-related epilepsy
Graphical Abstract
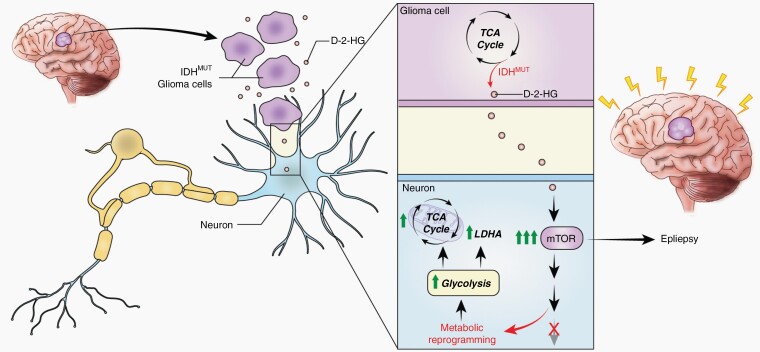
Graphical Abstract.
Key Points.
d-2-HG, metabolite produced by IDH-mutated gliomas, promotes neuronal spiking and distinct metabolic profiles in surrounding neurons.
Activation of the mTOR pathway by d-2-HG is a potential mechanism of epileptogenesis in patients with IDH-mutated gliomas.
Importance of the Study.
Recent studies have identified robust and previously unrecognized interactions between gliomas and surrounding neurons which suggest that these brain tumors can actively alter the electrical profile of the surrounding cortex. Indeed, patients with IDH-mutant gliomas often suffer from uncontrolled seizures, and such seizures can have a profound impact on their overall quality of life and morbidity. Yet, the precise mechanisms through which these tumors cause seizures still remain unknown. Here, we provide evidence that IDH-mutated gliomas, through its metabolite d-2-HG, promote epileptogenesis in surrounding neurons by disrupting baseline metabolic functions and upregulating mTOR signaling.
Gliomas are a heterogeneous group of brain tumors that commonly present with seizures. Broadly speaking, gliomas can be divided into those containing mutations of the metabolic enzyme isocitrate dehydrogenase (IDH), which comprise 80% of lower-grade gliomas and have a better prognosis than those without this mutation.1,2 Seizures, however, are far more common in patients with IDH-mutated gliomas with an incidence approaching 80%, and over half of these patients have seizures that are resistant to medical therapy.3 Uncontrolled seizures in these patients are consequently a major contributor to brain tumor morbidity and reduction in quality of life, and patients that suffer from a greater seizure burden experience more severe deterioration of cognitive function.1,3
Despite the strong association between IDH-mutated gliomas and seizures, the precise mechanisms through which epilepsy develops in these patients remain poorly understood and are likely multifactorial. Gliomas can infiltrate surrounding brain tissue, cause mass effect, or cause dysregulation of the excitatory-inhibitory balance in the peritumoral cortex, all mechanisms that could lead to seizures. Within gliomas, however, IDH mutation status is an independent risk factor for epileptogenesis, even when controlling for the grade of the tumor and the proximity to the eloquent cortex.1,4 The neomorphic enzymatic activity of the mutant IDH protein, converting alpha-ketoglutarate (α-KG) to d-2-hydroglutarate (d-2-HG), is a key feature in its pathogenesis1,5,6 (Supplementary Figure S1A). This has motivated the hypothesis that d-2-HG may be a primary oncometabolite responsible for the development of seizures in the surrounding brain.
Whether and how d-2-HG may play a role in epileptogenesis in surrounding brain tissue, however, remains unclear. One possibility is that due to its structural similarity with the excitatory neurotransmitter glutamate, d-2-HG increases overall excitability in surrounding neurons through interactions with the NMDA receptor.1 Recent evidence, however, suggests that d-2-HG may not alter glutamatergic neurotransmission nor bind NMDA receptors in a manner that mimics glutamate.1,7,8 Moreover, the elevated d-2-HG concentrations produced by IDH-mutated gliomas, which can reach up to 30 mM 5,9,10, are significantly higher than concentrations of glutamate necessary for glutamate toxicity.9,11 Therefore, although little evidence exists regarding the affinity between d-2-HG and NMDA receptors, even if this were the case, such high concentrations could lead to neurotoxicity and cell death rather than epileptogenesis.10,12 The role of d-2-HG in epileptogenesis may instead relate to its effects on different intracellular signaling pathways within surrounding brain tissue. Substantial evidence suggests that d-2-HG can lead to significant changes in a cell’s metabolic profile, including causing defects in the TCA cycle and the development of hypermetabolic phenotypes.13–15 This raises the alternative possibility that the role of d-2-HG in the development of seizures in patients with IDH-mutated gliomas may relate to the metabolic consequences of its uptake in surrounding neurons.
Here, we use an in vitro model of IDH-mutated glioma-related seizures to test the hypothesis that d-2-HG results in elevated spiking activity in surrounding neurons by changing their metabolic profile. We find that the release of d-2-HG by IDH-mutated gliomas leads to activation of the mTOR (mechanistic target of rapamycin) signaling pathway in surrounding cortical tissue. Consistent with significant previous evidence that mTOR activity is associated with epileptogenesis,16 we find that mTOR activation consequently leads to overall increases in neuronal activation. We compare the changes observed in our in vitro model to samples of human cortex resected from patients with IDH-mutated tumors and drug-resistant seizures and find similar evidence of metabolic reprogramming and activation of the mTOR pathway. Together our data suggest that d-2-HG, released by IDH-mutated gliomas, may directly promote seizures through its metabolic effects on surrounding neurons.
Materials and Methods
Neuronal Activity of Cell Cultures on Microelectrode Array Plates
To examine the effects of IDHR132H glioma cells and the oncometabolite 2-hydroxyglutarate on neuronal activity, we used an established mixed neuron-glia rat cortical cell culture model on a microelectrode array (MEA) and an established CT-2A rodent glioma cell line on trans-well inserts (see Supplementary Materials and Methods).17 We performed micro-electrode recordings using the Maestro Pro MEA system (Axion BioSystems, Atlanta, GA). Each well of the 96-well MEA plate contains 8 electrodes that record the spontaneous activity of electrically active networked cells. We analyzed neuronal data using the Neural Metrics Tool (Axion BioSystems, Atlanta, GA) (Supplementary Figure 11; Supplementary Materials and Methods).
Clinical Data and Human Tissue Acquisition
Five participants (2 females; 31.00 ± 7.969 years) with IDH-mutant gliomas and drug-resistant seizures underwent a surgical procedure in which platinum recording contacts (PMT Corporation, Chanhassen, MN) were implanted on the cortical surface as well as within the brain parenchyma. In each case, the clinical team determined the placement of contacts to localize epileptogenic regions around the tumor. A board-certified epileptologist identified electrodes overlying the seizure onset zone as well as electrodes overlying cortex not directly involved in the epileptic network but that still lay within the planned resection for removal of the tumor and/or epileptogenic zone. We collected surgical specimens using a standard surgical technique (see Supplementary Materials and Methods). Based on the clinical assessment by our epileptologist, we labeled all resected tissue as the epileptic cortex, nonepileptic cortex, and tumor. We collected all specimens and data at the Clinical Center at the National Institutes of Health (NIH; Bethesda, MD). The Institutional Review Board (IRB) approved the research protocol (ClinicalTrials.gov identifier NCT02639325), and informed consent was obtained from the participants in the study
Statistical Analysis
We report all data as mean ± SEM unless otherwise specified. We used GraphPad Prism (San Diego, CA) for all statistical analyses. We designated the level of significance for all statistical tests as P < .05 or lower. Asterisks (*), (**), (***), and (****) indicates significance P < .05, P < .01, P < .001, and P < .0001, respectively.
Results
IDH-Mutated Glioma Cells Increase Neuronal Bursting via the Oncometabolite d-2-hydroxyglutarate
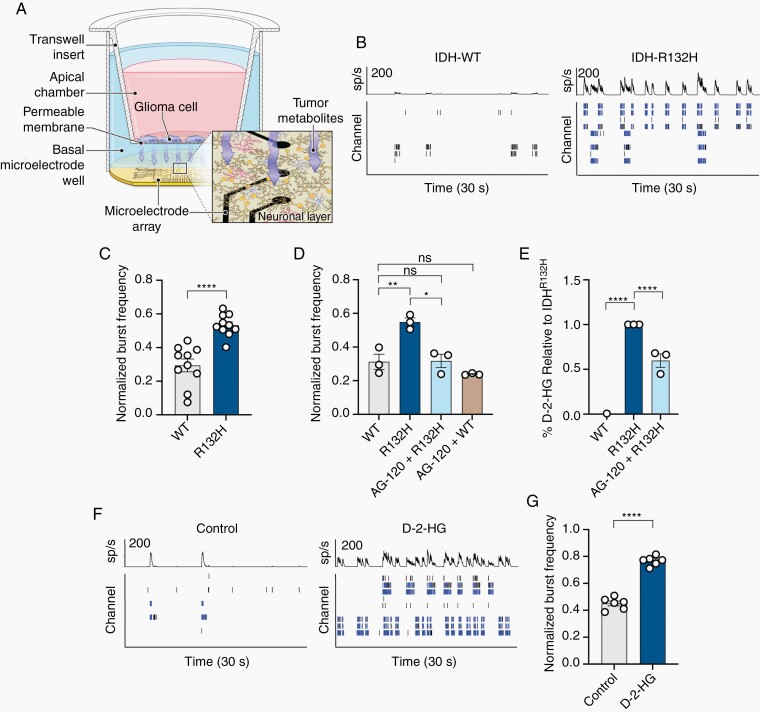
To investigate the role the peritumoral environment of IDH-mutated gliomas plays in epileptogenesis, we created an in vitro model using IDHR132H and IDHWT CT-2A murine glioma cells in trans-well inserts overlying primary rat mixed neuron-glial cultures while recording neuronal activity with microelectrode arrays (MEA; Figure 1A; see Methods). In the presence of CT-2A IDHR132H cells, neurons demonstrate overall increases in firing and the number of bursts of neuronal spiking per second compared to activity in the presence of WT cells (Figure 1B,C; Supplementary Figure S1B). Bursts were utilized as a surrogate marker for seizure liability as it correlates with known epileptogenic compounds and greater bursting activity is more likely to promote network activation.18 These increases are eliminated in the presence of AG-120 (Ivosidenib), a small molecule inhibitor of the mutant IDH enzyme12 (Figure 1D).
Fig. 1.
IDH-mutated tumors promote epileptogenesis via d-2-HG. (A) Schematic demonstrating interactions through media of glioma cell line cultured on trans-well and cortical rat neurons and astrocytes cultured on a MEA. (B) Thirty second raster plots (bottom) and spike histograms (top) of spiking activity in eight electrode channels in a single well. Right, IDHR132H induces greater number of bursts (blue bars) compared to IDHWT. (C) Normalized burst frequency demonstrating increased bursting activity of neurons interacting with IDHR132H compared to IDHWT (n = 10, t(18) = 5.524, P < .0001, paired t test). (D) Increased bursting activity following interaction with IDHR132H is corrected with inhibition of IDH mutation with AG-120 (n = 3; WT vs AG-120 + WT, t(2) = 1.631, P = .2445, paired t test; WT vs R132H, t(2) = 11.18, P = .0079, paired t test; R132H vs AG-120 + R132H, t(2) = 9.564, P = .0108, paired t test; WT vs AG-120 + R132H, t(2) = 0.1542, P = .8916, paired t test). (E) d-2-HG assay of the media demonstrating increased d-2-HG in the media in the presence of IDHR132H cells, which is reduced with AG-120 (n = 3; WT vs R132H, t(4) = 21.10, P < .0001, paired t test; WT vs AG-120 + R132H, t(4) = 19.95, P < .0001, paired t test; R132H vs AG-120 + R132H, t(4) = 5.637, P = .0049, paired t test). (F) Spike histogram and raster plot of control (left) and d-2-HG (right) treated cortical rat culture. (G) Normalized burst frequency of cortical rat neurons treated with control (PBS) and d-2-HG (n = 6, t(5) = 12.01, P < .0001, paired t test).
We examined whether the increases in neuronal activity in the presence of IDH-mutant glioma cells are related to the product of IDH mutant metabolism, d-2-HG. CT-2A IDHR132H cells in the trans-well insert cause a significant increase in the levels of d-2-HG in the supernatant compared to IDHWT cells (Figure 1E; Supplementary Figure 1C). This increase is reduced in the presence of AG-120 (Figure 1E). Elevated levels of d-2-HG in the supernatant are associated with elevated levels of intracellular d-2-HG in neurons (Supplementary Figure 1D).19 Direct treatment with d-2-HG to neuron-glial cultures in the absence of the IDH glioma cell trans-well inserts causes similar increases in firing rate and the rate of spiking bursts compared to control cultures (Figure 1F,G; Supplementary Figures S1E,F, S2A–H).
Increased d-2-HG Is Associated with Metabolic Reprogramming
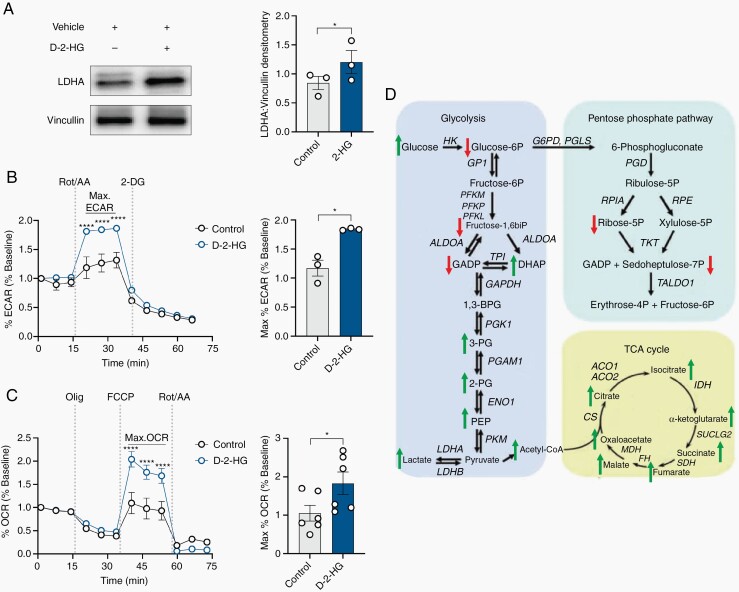
We were interested in understanding the cellular mechanisms through which d-2-HG may lead to increased neuronal spiking activity. We observed that the neuron-glial culture treated with d-2-HG demonstrates increases in LDHA expression, suggesting that d-2-HG may promote metabolic changes (Figure 2A). Following d-2-HG treatment, both maximal glycolytic rate and maximal mitochondrial respiratory activity increase compared to control cultures (Figure 2B,C). Using liquid chromatography-mass spectrometry (LC-MS), we observed distinct shifts in the neuron-glial metabolome with d-2-HG (Figure 2D; Supplementary Figure S3).
Fig. 2.
d-2-HG induces metabolic reprogramming in cortical tissue. (A) Western blot probing for LDH-A expression in control and d-2-HG-treated cortical rat neurons along with Densitometry analysis across three biological replicates (n = 3, t(2) = 4.534, P = .0454, paired t-test). (B) Maximal glycolytic rate (max ECAR) is significantly higher in d-2-HG-treated neurons compared to control (F(10,231) = 7.288, P < .0001, Two-way ANOVA with Sidak’s multiple-comparisons test). Averaged across time points exhibiting maximal glycolytic rate, ECAR is significantly higher in the d-2-HG treated neurons (n = 3, t(2) = 5.107, P = .0363, paired t-test). (C) Max oxidative consumption rate (OCR) is significantly higher in d-2-HG-treated neurons compared to control (F(11,522) = 8.430, P < .0001, Two-way ANOVA with Sidak’s multiple-comparisons test). Averaged across time points exhibiting maximal respiration, max OCR is significantly higher in d-2-HG treated neurons compared to control (n = 3, t(2) = 2.927, P = .0327, paired t-test). (D) Schematic representation of the enzymatic reactions from glycolysis, PPP, and TCA cycle highlighting the changes at the metabolite levels between control and d-2-HG treated cortical rat cultures through over-representation analysis. Up arrows (red) and down arrows (green) denote increased or decreased, respectively, enrichment comparing d-2-HG relative to control-treated cortical rat neurons.
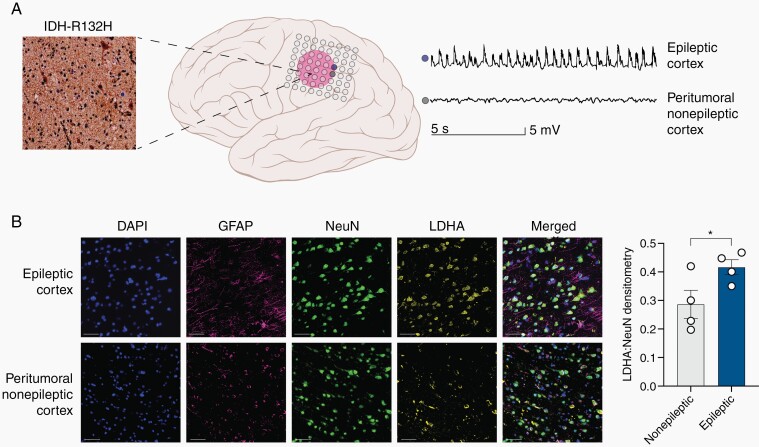
In five patients with confirmed IDH-mutated gliomas, we had the opportunity to examine resected human peritumoral cortical tissue that was identified as epileptic or nonepileptic through intracranial electrode monitoring (Figure 3A; Supplementary Figure S4A,B; see Methods). Similar to the mixed neuronal cultures, the epileptic cortex exhibits significantly elevated total LDHA expression compared to the nonepileptic cortex, which was not associated with tumor cell infiltration (Supplementary Figure 4C–F). Using multiplex immunofluorescence staining on the resected tissue, we observed clear increased expression of LDHA that co-localizes with the expression of NeuN as compared to the nonepileptic cortex (Figure 3B). LC-MS of epileptic and nonepileptic cortex in the presence of IDH-mutated tumors exhibits a metabolomic shift that is similar to the changes we observed in the neuronal-glial cultures (Supplementary Figures S5A–B, S6). These data suggest that the changes in LDHA expression observed in neuron-glial cultures treated with d-2-HG are recapitulated in the human brain tissue lying adjacent to IDH-mutated gliomas that exhibit seizures.
Fig. 3.
The epileptic human cortex demonstrates upregulation of LDHA compared to the nonepileptic cortex. (A) Diagram demonstrating epileptic cortex (blue electrode) and peritumoral nonepileptic cortex (grey electrode) in the setting of the IDH mutant glioma determined via intracranial EEG monitoring. The glioma stained positive for IDH (R132H) mutation. This patient had a WHO Grade III Astrocytoma IDH-mutant. (B) Multiplex immunofluorescence staining for DAPI (blue), NeuN (green), GFAP (purple), and LDH-A (yellow) of the peritumoral nonepileptic cortex and epileptic cortex demonstrating increased LDH-A expression primarily in neurons. DAPI is a control nuclear DNA stain, GFAP stains astrocytes, NeuN stains neurons, and LDH-A, the metabolic enzyme of interest. LDH-A co-staining with NeuN is significantly increased in the epileptic cortex compared to the peritumoral nonepileptic cortex (n = 4, t(3) = 3.799, P = .0320, paired t-test).
d-2-HG Induces Metabolic Reprogramming in an mTOR-dependent Manner
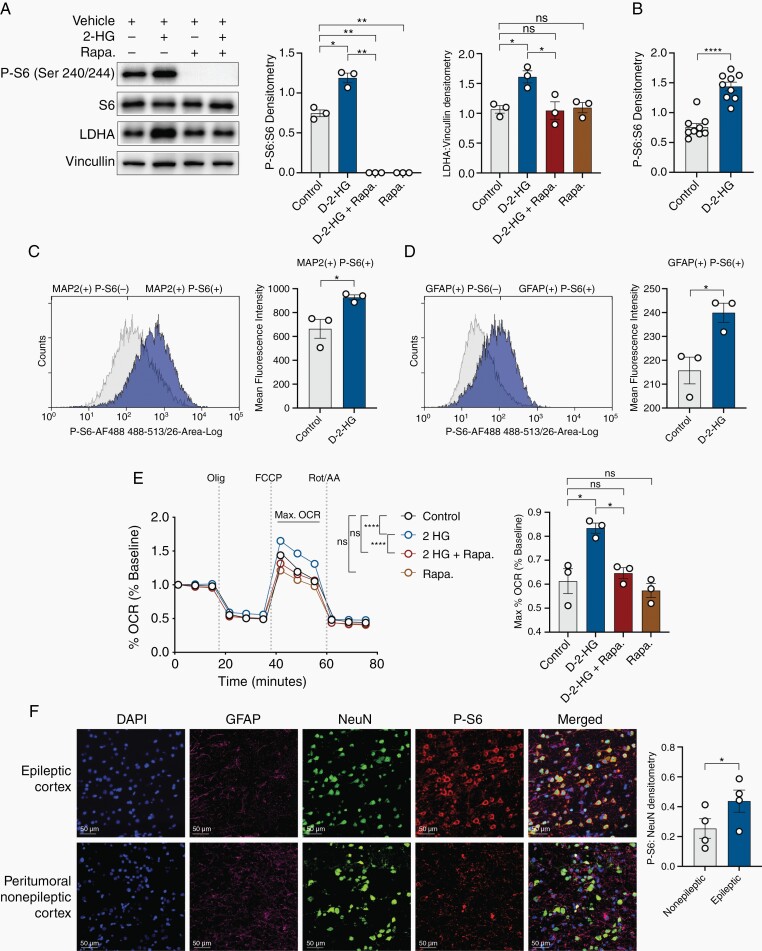
One possible link between elevated neuronal activity and metabolic reprogramming in the context of IDH-mutated gliomas may involve mTOR, which is both a major regulator of metabolism and which has also been implicated in epilepsy.16 To examine this possibility, we probed for the phosphorylation of Ribosomal Protein S6 (P-S6) (Ser 240/244), a surrogate for mTOR activation.13 The neuron-glial cultures treated with d-2-HG exhibit increased P-S6:S6 (Figure 4A,B). These increases in P-S6 are eliminated when treated with the mTOR inhibitor rapamycin. Rapamycin also eliminates the increases in LDHA expression (Figure 4A,B). Flow cytometric analysis revealed an increase in P-S6 in d-2-HG treated cultures, both in neurons (MAP2 positive) and astrocytes (GFAP positive cells), suggesting d-2-HG causes global increases in P-S6 in the cortex (Figure 4C,D). Moreover, increases in maximal mitochondrial respiration observed with d-2-HG treatment are also corrected with rapamycin (Figure 4E). mTOR inhibition in d-2-HG treated cultures partially normalized the LC-MS observed metabolic shift, suggesting metabolic reprogramming may in part be attributed to mTOR activity (Supplementary Figures S7, S8).
Fig. 4.
d-2-HG induces mTOR hyperactivation in cortical tissue and mTOR inhibition corrects aspects of metabolic reprogramming. (A) Western blot analysis demonstrates that d-2-HG upregulates P-S6:S6 (n = 3, control vs d-2-HG, t(2) = 6.193, P = .0251, paired t test). mTOR inhibition results in loss of P-S6 expression (n = 3, control vs d-2-HG + rapa., t(2) = 17.58, P = .0032, paired t test; d-2-HG vs d-2-HG + rapa., t(2) = 19.55, P = .0026, paired t test; control vs rapa., t(2) = 17.58, P = .0032, paired t test). d-2-HG increases LDHA expression (n = 3, control vs d-2-HG, t(2) = 4.886, P = .0394), which is corrected with rapamycin (n = 3, control vs d-2-HG + Rapa., t(2) = 0.2288, P = .8403, d-2-HG vs d-2-HG + rapa., Fig. 4 (Continued) t(2) = 4.467, P = .0466). Rapamycin does not decrease LDHA protein expression relative to control (n = 3, control vs Rapa., t(2) = 0.2302, P = .8393). (B) Cumulative P-S6:S6 across nine biological replicates demonstrates d-2-HG increases mTOR signaling in the cortical culture (n = 9, t(8) = 8.207, P < .0001, paired t test). (C) Flow cytometry example (left) demonstrating histogram of MAP2(+) cells and P-S6(+) and P-S6(–). Mean fluorescence intensity in MAP2(+) P-S6(+) cells increases across cultures treated with d-2-HG compared to control (n = 3; t(2) = 4.541, P = .0452, paired t-test). (D) Flow cytometry example (left) demonstrating histogram of GFAP(+) cells and P-S6(+) and P-S6(–). Mean fluorescence intensity in GFAP(+) P-S6(+) cells increases across cultures treated with d-2-HG compared to control (n = 3; t(2) = 15.05, P = .0044, paired t-test). (E) Max OCR is higher in d-2-HG treated neurons, but is corrected to control levels with co-treatment of rapamycin. Rapamycin treatment alone did not affect OCR compared to control (F(33, 528) = 10.53, P < .0001, Two-way ANOVA with Sidak’s multiple comparisons test). Averaged across time points exhibiting maximal respiration, max OCR is significantly higher in d-2-HG treated neurons compared to control, but this is corrected with treatment with rapamycin (n = 3; control vs d-2-HG, t(2) = 7.240, P = .0185, paired t test; d-2-HG vs d-2-HG + rapa., t(2) = 4.687, P = .0426, paired t test; control vs d-2-HG + rapa., t(2) = 0.5274, P = .6506, paired t test; control vs rapa., t(2) = 0.4957, P = .6692, paired t test). (F) Multiplex immunofluorescence staining for DAPI (blue), NeuN (green), GFAP (purple), and P-S6 (red) of peritumoral nonepileptic cortex and epileptic cortex demonstrating increased LDH-A expression primarily in neurons. P-S6 co-staining with NeuN is significantly increased in the epileptic cortex compared to the peritumoral nonepileptic cortex (n = 4, t(3) = 5.756, P = .0104, paired t test).
Complementing the changes in mTOR activity observed in the neuron-glial culture, we also examined human peritumoral tissue identified as epileptic and nonepileptic for evidence of mTOR activity. Using multiplex immunofluorescence, we found that the epileptic human cortex adjacent to IDH-mutated gliomas also exhibits significantly higher levels of P-S6 expression compared to the peritumoral nonepileptic cortex (Figure 4F). These changes in P-S6 expression are co-localized to cortical neurons and are consistent across all participants.
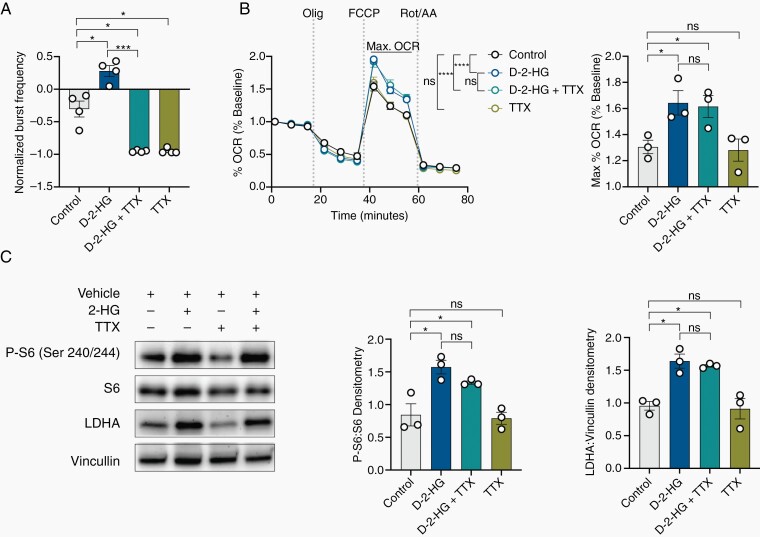
Recognizing that the increases in mTOR signaling and the changes in metabolism observed in the setting of d-2-HG may simply be a result of increased neuronal activity,20 we investigated the effects of d-2-HG on metabolism and mTOR signaling when neuronal activity is silenced using the Na+ channel inhibitor tetrodotoxin (TTX). Despite complete silencing of neuronal activity with TTX (Figure 5A, Supplementary Figure S9A), the presence of d-2-HG still increases maximal mitochondrial respiration compared to cells that were not exposed to d-2-HG (Figure 5B). Similarly, the reduction in neuronal activity with TTX does not alter the overall shift in metabolites observed in the presence of d-2-HG that complement these changes in mitochondrial respiration (Supplementary Figure S9B–E). Neuron-glial cultures treated with d-2-HG in the presence of TTX still exhibit significant upregulation of P-S6 and LDHA (Figure 5C). These data, therefore, suggest that increased mTOR activity and the associated metabolic changes do not emerge in the presence of d-2-HG solely due to increased spiking.
Fig. 5.
d-2-HG induces mTOR upregulation and metabolic reprogramming independent of neuronal bursting. (A) Tetrodotoxin (TTX) silences all neuronal firing compared to control (n = 4, t(3) = 5.792, P = .0102, paired t test) even with co-treatment of d-2-HG (n = 4; control vs d-2-HG + TTX, t(3) = 5.312, P = .0130, paired t test; d-2-HG vs d-2-HG +TTX, t(3) = 16.06, P = .0005, paired t test). d-2-HG without TTX increases normalized burst frequency compared to control (n = 4, t(3) = 4.164, P = .0252, paired t test). (B) d-2-HG increases maximal respiration compared to control and in the setting of TTX (n = 4, F(33, 356) = 5.319, P < .0001, ns: not significant, Two-way ANOVA with Sidak’s multiple comparisons test). (C) Averaged across time points exhibiting maximal respiration, d-2-HG increases max OCR compared to control (n = 3, t(2) = 5.719, P = .0292, paired t test) even with neuronal silencing in the presence of TTX (n = 3, t(2) = 0.2604, P = .8189, paired t test). TTX did not change maximal respiration compared to control (n = 3, t(2) = 0.5140, P = .6584, paired t test). C) Western blot analysis demonstrates that d-2-HG continues to upregulate P-S6:S6 (n = 3, control vs d-2-HG + TTX, t(2) = 3.413, P = .0454, paired t test) and LDHA:Vinculin (n = 3, mean ±SEM, control vs d-2-HG + TTX, t(2) = 12.56, P = .0063, paired t test) in the setting of TTX.
mTOR Activation by d-2-HG Leads to Increased Neuronal Spiking
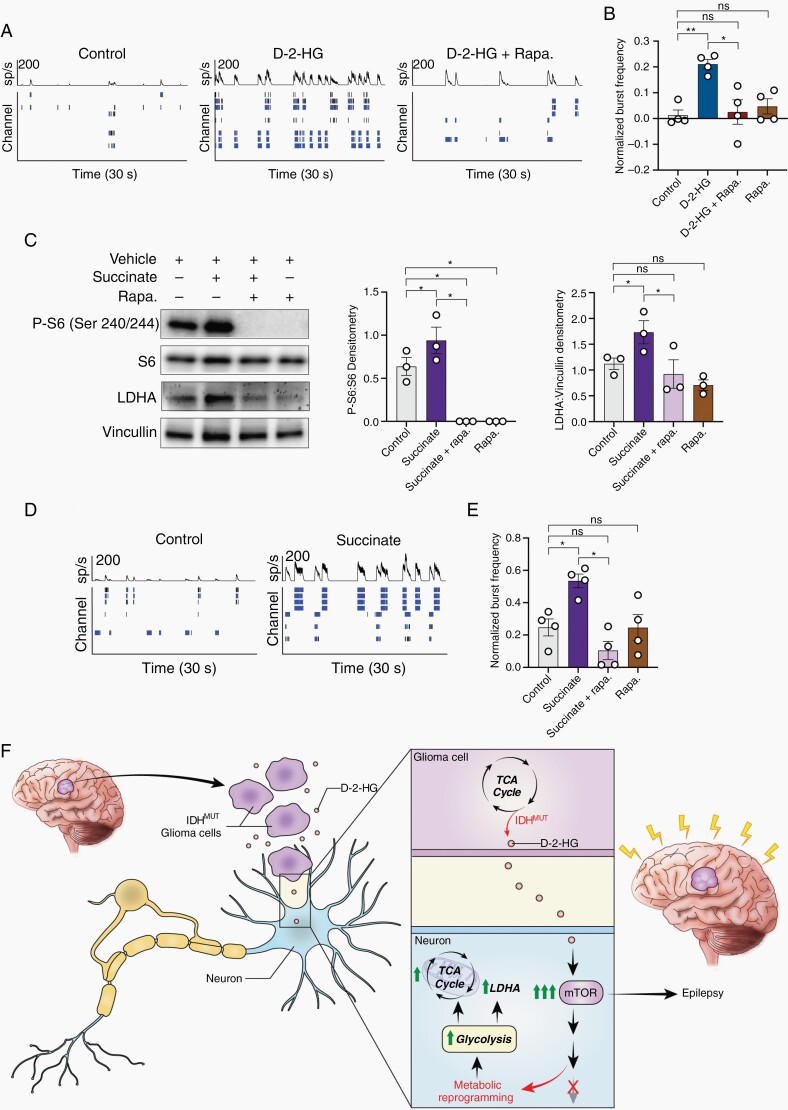
As the metabolic changes observed following exposure to d-2-HG appear to emerge independently of the increases in spiking, we hypothesized that mTOR activation and the associated metabolic shift may in fact promote greater neuronal activity. In this scenario, IDH-mutated gliomas lead to elevated spiking activity primarily through the effects of d-2-HG on the metabolic profile of the surrounding cells. To examine this, we first treated the neuron-glial culture with d-2-HG and rapamycin. The increases in bursting activity observed with d-2-HG compared to control cells are eliminated when the mTOR pathway is inactivated (Figure 6A,B).
Fig. 6.
Metabolites d-2-HG and succinate causes neuronal hyperexcitability in an mTOR-dependent manner. A) Spike histogram and raster plot of control (left), d-2-HG (middle), and d-2-HG and rapamycin (left) treated cortical rat neurons. B) Normalized burst frequency of cortical rat Fig. 6 (Continued) neurons treated with control (PBS), d-2-HG, d-2-HG and rapamycin, and rapamycin. d-2-HG treated neurons significantly increase normalized burst frequency compared to control (n = 4, t(3) = 6.942, P = .0061, paired t test) and d-2-HG + Rapa. (n = 4, t(3) = 4.574, P = .0196, paired t test). Additionally, d-2-HG and rapamycin treated neurons have similar normalized burst frequency compared to control (n = 4, t(3) = 0.3735, P = .7336, paired t test). Rapamycin alone does not significantly decrease normalized burst frequency compared to control (n = 4, t(3) = 1.249, P = .3004, paired t test). (C) Succinate significantly upregulates P-S6:S6 (n = 3, control vs Succinate, t(2) = 5.393, P = .0327, paired t test), surrogate marker for mTOR signaling. This increase is inhibited with rapamycin (n = 3; control vs Succinate + rapa., t(2) = 6.109, P = .0258, paired t test; Succinate vs Succinate + rapa., t(2) = 6.094, P = .0259; control vs rapa., t(2) = 6.109, P = .0258, paired t test). Succinate also increases LDHA protein expression (n = 3, control vs Succinate, t(2) = 4.386, P = .0482, paired t test), which is corrected with rapamycin to control levels (n = 3,control vs Succinate + rapa., t(2) = 0.8713, P = .4754, paired t test; Succinate vs Succinate + rapa., t(2) = 6.920, P = .0202, paired t test; control vs rapa., t(2) = 2.893, P = .1016, paired t test). (D) An example spike raster demonstrating that treatment with succinate results in increased bursting activity compared to control. (E) Across cultures, succinate increases normalized burst frequency compared to control following 7-day treatment (n = 4, t(3) = 3.475, P = .0402, paired t test). This increase is corrected to control levels with co-treatment of rapamycin (n = 4; control vs Succinate + rapa., t(3) = 2.633, P = .0781, paired t test; Succinate vs Succinate + rapa., t(3) = 4.755, P = .0177, paired t test; control vs rapa., t(3) = 0.01238, P = .9909, paired t test). (F) Proposed mechanism: IDH-mutated gliomas produce d-2-HG, which is released into the peritumoral environment. d-2-HG upregulates mTOR signaling in surrounding neurons, leading to metabolic reprogramming and epileptogenesis.
We then examined whether activation of the mTOR pathway through a different metabolite also leads to increased neuronal spiking activity. We utilized succinate, which is similar to d-2-HG as it increases methylation of H3K9 via inhibition of KDM4A (Supplementary Figure S10)21 and has been shown to activate mTOR signaling.22 Similar to the effects of d-2-HG, treatment with succinate alone increases P-S6 and LDHA expression in the mixed culture (Figure 6C). In the presence of succinate alone, neurons exhibit a clear increase in bursting activity (Figure 6D,E). This increase is eliminated in the presence of rapamycin, suggesting the effect of succinate on neuronal spiking activity is mediated through the mTOR signaling pathway.
Discussion
Our data demonstrate that d-2-HG, a key metabolite produced by IDH-mutated gliomas, may promote neuronal spiking by changing the metabolic profile of surrounding neurons and activating the mTOR signaling pathway both in culture and in the human peritumoral epileptic cortex. The enhanced spiking activity of cortical neurons in our cell cultures is induced by mTOR activation and reversed with rapamycin. Our data, therefore, suggest that activation of the mTOR pathway by d-2-HG may be a driving event that leads to increased neuronal spiking in patients with IDH-mutated gliomas (Figure 6F).
Although IDH-mutated gliomas portend a favorable prognosis, the IDH mutation has emerged as an independent risk factor for developing seizures.1,4 Uncontrolled seizures can directly impact the quality of life by causing discomfort, fatigue, neurological deficits, or anxiety, and can have practical and psycho-social consequences.1,3,23 Given the complexity of managing patients with epilepsy in the context of brain tumors, an important question that therefore requires resolution is precisely how IDH-mutated gliomas give rise to seizures.
Our data provide direct evidence that d-2-HG, a primary oncometabolite produced by IDH-mutated gliomas, gives rise to increased spiking in surrounding neurons. Although it is unclear whether neuronal cultures alone can exhibit seizures, such in vitro models are commonly used as a proxy for epileptic activity.18 Our analysis can only focus on electrophysiological characteristics of these neurons and their activation. Nonetheless, our results are consistent with previous studies demonstrating that d-2-HG can promote seizures.1 However, our data diverge from recent proposals suggesting that d-2-HG, due to its structural similarity with the excitatory neurotransmitter glutamate, leads to neuronal excitation simply through interactions with NMDA receptors.1 Indeed, several studies have reported that d-2-HG binding of NMDA receptors is not robust.1,7,8 Moreover, even if d-2-HG does bind the NMDA receptor well, other studies have suggested that such activation of the NMDA receptor by d-2-HG is more likely to lead to neurotoxicity rather than excitability, particularly at the high concentrations of d-2-HG present in the peritumoral environment around IDH-mutated gliomas.10–12 Our results cannot exclude, however, the role that NMDA may play in neuronal firing in the presence of d-2-HG. NMDA receptor activity, for example, is necessary for synchronization of action potential firing activity (ie burst activity).24 Moreover, NMDA receptor activation and mTOR signaling may share a close relationship. Chronic NMDA inhibition may in fact upregulate mTOR signaling, while NMDA receptor activation through inhibition of amino acid transporters dampens mTOR signaling.25
Instead, our data suggest that d-2-HG can have broad effects on the metabolic profile of surrounding cells, which can consequently lead to seizures. The intracellular impact of d-2-HG is well known. d-2-HG plays a major role in metabolic reprogramming associated with cancer, as it is a competitive inhibitor to a large family of metabolite-dependent enzymes termed α-KG dependent dioxygenases.6,14 Inhibition of this family of enzymes results in global alterations of gene expression and protein interactions, which directly impacts cellular activities and the metabolic landscape of the cell.21 In addition, α-KG itself is an important regulator of cellular metabolic status, and if metabolic intermediates such as d-2-HG inhibit binding to its catalytic site, global metabolic changes are also likely to occur.26 IDH-mutated and IDH wild-type tumor cells, therefore, have distinct metabolic profiles, largely due to the presence or absence of d-2-HG.27 The metabolic changes observed in IDH-mutant gliomas are not limited to the tumor cells alone, but also emerge in surrounding cells up the uptake of d-2-HG.15,19 Thus, the effect of d-2-HG on the metabolic state of surrounding brain tissue may be involved in neuronal excitability.
Previous studies have established the association between epilepsy, where hyperexcitable neurons are overwhelmingly synchronized through pathologic network behavior, and metabolic dysfunction, either as a result of periodic spikes of energy demand resulting in metabolic adaptation28 or through initial metabolic insults resulting in epileptic activity.29 Distinctly, epileptogenic tissue exhibits increased LDHA expression, a key marker of metabolic disruption, and LDHA may in turn feedback to play a role in regulating seizure activity.30 These metabolic alterations are accompanied by changes in mTOR signaling, an important regulator of cellular metabolism, in both our in vitro cell cultures and in human samples. The mTOR signaling pathway is known to play key roles in various forms of epilepsy, including Tuberous sclerosis complex (TSC), infantile spasms, status epilepticus, focal cortical dysplasia, and even injury associated epilepsy.16 The exact mechanism by which mTOR hyperactivation results in epileptogenesis or seizures, however, remains unknown, although it may involve disrupting the balance of excitatory and inhibitory synaptic transmission, modulation of ion channels, and neurotransmitter regulation.31 In addition, activation of mTOR signaling can induce intrinsic excitability in neurons without histological changes to brain structures.32 These studies collectively establish a strong link between metabolic dysregulation and epilepsy and suggest that mTOR activation alone can promote seizure susceptibility.
We find that the introduction of d-2-HG to surrounding neurons indeed leads to metabolic disruptions and activation of the mTOR signaling pathway. Consistent with prior work, we similarly found that activation of mTOR consequently leads to neuronal excitability. Moreover, we determined that the mTOR inhibitor, rapamycin, reduces neuronal activation, complementing previous studies that demonstrated rapamycin can suppress seizures in both genetic mTOR activation models33 as well as acquired seizure models.34 Our data, therefore, provide a direct link between the seizures observed in the context of IDH-mutated gliomas and the previous literature on the role of mTOR activation in epileptogenesis.
There are several different pathways through which the introduction of d-2-HG may lead to changes in mTOR activation and cell metabolism. There is evidence, for example, that mTOR signaling and KDM regulation are tightly linked.35 Hence, d-2-HG dependent inhibition of KDM4A may result in destabilization of DEPTOR, a negative regulator of mTORC1/2,13,15 which may be sufficient to upregulate mTOR signaling and thus increase bursting activity.36 Notably, however, d-2-HG has also been shown to inhibit mTOR activity when mTOR is chronically activated through PTEN loss or other causes,37 but not demonstrated in cell lines with intact signaling upstream of mTOR. Alternatively, our metabolomics analyses reveal a substantial number of enriched metabolites observed in the context of d-2-HG, such as succinate and S-adenosylmethionine (SAM), that are not reversed with rapamycin. This suggests these metabolites that are upregulated in the setting of elevated d-2-HG may play a role in driving mTOR activation through an indirect nutrient-sensing pathway.38 Nevertheless, the precise mechanism by which d-2-HG or similar metabolites upregulates mTOR signaling requires further study for elucidation.
Although the mechanisms through which epilepsy develops in patients with IDH-mutated gliomas are likely multi-factorial, the metabolic changes we observe here may directly complement the known structural and synaptic changes observed in the context of primary brain tumors. In the setting of neuronal excitability, gliomas, in particular, are of great interest due to their propensity to communicate with neurons directly.39,40 Gliomas have long been known to preferentially interact with surrounding neurons,41 and such microanatomical clustering appears to play an active role in glioma progression via activity-regulated neurotransmitters and growth factors42,43 released into the glioma microenvironment. The close spatial relationship between neurons and glioma cells that these studies highlight suggests that the concentrations of d-2-HG required to promote mTOR activation in surrounding neurons may be reduced. This close proximity may also facilitate direct communication between gliomas and surrounding neurons through direct glutamatergic synapses. Recent evidence has demonstrated that neuronal activity can promote glioma growth, and in turn gliomas may promote neuronal activity.40,44,45 Such interactions therefore likely also contribute to increased excitability in surrounding neurons, and molecular-specific entities within gliomas, such as IDH-mutant gliomas and PIK3CA variants, may promote these interactions.46
In addition to d-2-HG, other nonsynaptic secretions have been implicated in disrupting the excitatory and inhibitory balance in neurons. Gliomas can promote the release of glutamate by the x(c)(-) cystine-glutamate transporter independent of synaptic secretion.47 In a model of glioblastoma tumor-related epilepsy, there has been evidence of secretion of remodeling and degrading enzymes, which promote the destruction of perineuronal nets, ultimately reducing the release of GABA.48 In an additional example of a molecular-specific tumor-related epileptogenesis, PIK3CA variants can promote brain hyperactivity during gliomagenesis due to increased expression of GPC3 which promotes neuronal excitability.46
Together, our results demonstrate that d-2-HG produced by the IDH mutation can lead to mTOR activation within the cortex, thereby suggesting an additional possible mechanism of epileptogenesis in patients with IDH-mutated gliomas. Our results, therefore, raise the possibility that mTOR inhibition a pathway currently being explored for other forms of epilepsies,49 may also be a promising treatment of seizures in patients with these tumors, especially for those resistant to established antiepileptic drugs. Exploring the efficacy of mTOR for seizures could complement current studies exploring the role of mTOR inhibition in targeting tumor growth.50 Our results also suggest that it may be possible to consider the inhibition of the IDH-mutant enzyme to reduce d-2-HG, which may reduce epileptogenicity. More broadly, however, our results provide insight into the pathogenesis of seizures and how metabolic perturbations may critically regulate neuronal excitability.
Supplementary Material
Acknowledgments
We are indebted to all patients who have generously participated in this study.
Contributor Information
Armin Mortazavi, Surgical Neurology Branch, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Islam Fayed, Department of Neurosurgery, Georgetown University, Washington, District of Columbia, USA.
Muzna Bachani, NeuroTherapeutics Development Unit, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Tyrone Dowdy, NeuroOncology Branch, NCI, National Institutes of Health, Bethesda, Maryland, USA.
Jahandar Jahanipour, Flow and Cytometry Core, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Anas Khan, Surgical Neurology Branch, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Jemima Owotade, Surgical Neurology Branch, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Stuart Walbridge, Surgical Neurology Branch, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Sara K Inati, Surgical Neurology Branch, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Joseph Steiner, NeuroTherapeutics Development Unit, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Jing Wu, NeuroOncology Branch, NCI, National Institutes of Health, Bethesda, Maryland, USA.
Mark Gilbert, NeuroOncology Branch, NCI, National Institutes of Health, Bethesda, Maryland, USA.
Chun Zhang Yang, NeuroOncology Branch, NCI, National Institutes of Health, Bethesda, Maryland, USA.
Mioara Larion, NeuroOncology Branch, NCI, National Institutes of Health, Bethesda, Maryland, USA.
Dragan Maric, Flow and Cytometry Core, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Alexander Ksendzovsky, Department of Neurosurgery, University of Maryland, Baltimore, Maryland, USA.
Kareem A Zaghloul, Surgical Neurology Branch, NINDS, National Institutes of Health, Bethesda, Maryland, USA.
Funding
This work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health.
Conflict of interest
The authors declare no competing interests.
Authorship statement
Conceptualization, A.M., I.F., A.K., and K.A.Z.; Methodology, A.M., I.F., M.B., T.D., J.J., D.M., M.L., and K.A.Z; Investigation, A.M., I.F., T.D., J.J., A.K., S.W., S.K.I., C.Z.Y., and K.A.Z.; Resources, J.S., D.M., M.L., and K.A.Z.; Writing – Original Draft, A.M. and K.A.Z.; Writing – Review and Editing, A.M., I.F., M.B., T.D, J.W., M.G., C.Z.Y., D.M., A.K., and K.A.Z. Project Administration, S.W., J.S., D.MS., M.L., and K.A.Z. Supervision, K.A.Z.
References
- 1. Chen H, Judkins J, Thomas C, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duan WC, Wang L, Li K, et al. IDH mutations but not TERTp mutations are associated with seizures in lower-grade gliomas. Medicine (Baltim). 2018;97(50):e13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huberfeld G, Vecht CJ. Seizures and gliomas—towards a single therapeutic approach. Nat Rev Neurol. 2016;12(4):204–216. [DOI] [PubMed] [Google Scholar]
- 4. Stockhammer F, Misch M, Helms HJ, et al. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure 2012;21(3):194–197. [DOI] [PubMed] [Google Scholar]
- 5. Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4(116):116ra–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang H, Ye D, Guan KL, Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res. 2012;18(20):5562–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kölker S, Pawlak V, Ahlemeyer B, et al. NMDA receptor activation and respiratory chain complex V inhibition contribute to neurodegeneration in d-2-hydroxyglutaric aciduria. Eur J Neurosci. 2002;16(1):21–28. [DOI] [PubMed] [Google Scholar]
- 8. Junqueira D, Brusque AM, Porciúncula LO, et al. In vitro effects of D-2-hydroxyglutaric acid on glutamate binding, uptake and release in cerebral cortex of rats. J Neurol Sci. 2004;217(2):189–194. [DOI] [PubMed] [Google Scholar]
- 9. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009;462(7274):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi C, Raisanen JM, Ganji SK, et al. Prospective longitudinal analysis of 2-hydroxyglutarate magnetic resonance spectroscopy identifies broad clinical utility for the management of patients with IDH-mutant glioma. J Clin Oncol. 2016;34(33):4030–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaarmann A, Kovac S, Holmström KM, Gandhi S, Abramov AY. Dopamine protects neurons against glutamate-induced excitotoxicity. Cell Death Dis. 2013;4(1):e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013;340(6132):626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carbonneau M, M Gagné L, Lalonde ME, et al. The oncometabolite 2-hydroxyglutarate activates the mTOR signalling pathway. Nat Commun. 2016;7(1):12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garrett M, Sperry J, Braas D, et al. Metabolic characterization of isocitrate dehydrogenase (IDH) mutant and IDH wildtype gliomaspheres uncovers cell type-specific vulnerabilities. Cancer Metab 2018;6(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bottcher M, Renner K, Berger R, et al. D-2-hydroxyglutarate interferes with HIF-1alpha stability skewing T-cell metabolism towards oxidative phosphorylation and impairing Th17 polarization. Oncoimmunology 2018;7(7):e1445454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12(7):379–392. [DOI] [PubMed] [Google Scholar]
- 17. Seyfried TN, el-Abbadi M, Roy ML. Ganglioside distribution in murine neural tumors. Mol Chem Neuropathol 1992;17(2):147–167. [DOI] [PubMed] [Google Scholar]
- 18. Bradley JA, Luithardt HH, Metea MR, Strock CJ. In vitro screening for seizure liability using microelectrode array technology. Toxicol Sci. 2018;163(1):240–253. [DOI] [PubMed] [Google Scholar]
- 19. Bunse L, Pusch S, Bunse T, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018;24(8):1192–1203. [DOI] [PubMed] [Google Scholar]
- 20. Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29(25):8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuan HX, Xiong Y, Guan KL. Nutrient sensing, metabolism, and cell growth control. Mol Cell. 2013;49(3):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tan HWS, Sim AYL, Long YC. Glutamine metabolism regulates autophagy-dependent mTORC1 reactivation during amino acid starvation. Nat Commun. 2017;8(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Y, Mao Q, Wang X, et al. An analysis of 170 glioma patients and systematic review to investigate the association between IDH-1 mutations and preoperative glioma-related epilepsy. J Clin Neurosci. 2016;31:56–62. [DOI] [PubMed] [Google Scholar]
- 24. Molina LA, Skelin I, Gruber AJ. Acute NMDA receptor antagonism disrupts synchronization of action potential firing in rat prefrontal cortex. PLoS One. 2014;9(1):e85842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoon SC, Seo MS, Kim SH, et al. The effect of MK-801 on mTOR/p70S6K and translation-related proteins in rat frontal cortex. Neurosci Lett. 2008;434(1):23–28. [DOI] [PubMed] [Google Scholar]
- 26. Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 2011;19(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khurshed M, Molenaar RJ, Lenting K, Leenders WP, van Noorden CJF. In silico gene expression analysis reveals glycolysis and acetate anaplerosis in IDH1 wild-type glioma and lactate and glutamate anaplerosis in IDH1-mutated glioma. Oncotarget 2017;8(30): 49165–49177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Theodore WH. Cerebral blood flow and glucose metabolism in human epilepsy. Adv Neurol. 1999;79(1):873–881. [PubMed] [Google Scholar]
- 29. Rahman S. Pathophysiology of mitochondrial disease causing epilepsy and status epilepticus. Epilepsy Behav. 2015;49(1):71–75. [DOI] [PubMed] [Google Scholar]
- 30. Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 2015;347(6228):1362–1367. [DOI] [PubMed] [Google Scholar]
- 31. Meng XF, Yu JT, Song JH, Chi S, Tan L. Role of the mTOR signaling pathway in epilepsy. J Neurol Sci. 2013;332(1-2):4–15. [DOI] [PubMed] [Google Scholar]
- 32. Abs E, Goorden SM, Schreiber J, et al. TORC1-dependent epilepsy caused by acute biallelic Tsc1 deletion in adult mice. Ann Neurol. 2013;74(4):569–579. [DOI] [PubMed] [Google Scholar]
- 33. Weston MC, Chen H, Swann JW. Loss of mTOR repressors Tsc1 or Pten has divergent effects on excitatory and inhibitory synaptic transmission in single hippocampal neuron cultures. Front Mol Neurosci. 2014;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang X, Zhang H, Yang J, et al. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40(1):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Rechem C, Black JC, Boukhali M, et al. Lysine demethylase KDM4A associates with translation machinery and regulates protein synthesis. Cancer Discov 2015;5(3):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. [DOI] [PubMed] [Google Scholar]
- 37. Fu X, Chin RM, Vergnes L, et al. 2-Hydroxyglutarate inhibits ATP synthase and mTOR signaling. Cell Metab. 2015;22(3):508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gu X, Orozco JM, Saxton RA, et al. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science 2017;358(6364):813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pallud J, Capelle L, Huberfeld G. Tumoral epileptogenicity: how does it happen? Epilepsia 2013;54(Suppl 9):30–34. [DOI] [PubMed] [Google Scholar]
- 40. Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019;573(7775):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scherer HJ. Structural development in gliomas. Am J Cancer 1938;34(3):333–351. [Google Scholar]
- 42. Johung T, Monje M. Neuronal activity in the glioma microenvironment. Curr Opin Neurobiol. 2017;47(1):156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Venkatesh HS, Johung TB, Caretti V, et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 2015;161(4):803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Venkataramani V, Tanev DI, Strahle C, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019;573(7775):532–538. [DOI] [PubMed] [Google Scholar]
- 45. Pan Y, Hysinger JD, Barron T, et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature 2021;594(7862):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu K, Lin CJ, Hatcher A, et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 2020;578(7793):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tewari BP, Chaunsali L, Campbell SL, et al. Perineuronal nets decrease membrane capacitance of peritumoral fast spiking interneurons in a model of epilepsy. Nat Commun. 2018;9(1):4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Steriade C, French J, Devinsky O. Epilepsy: key experimental therapeutics in early clinical development. Expert Opin Investig Drugs. 2020;29(4):373–383. [DOI] [PubMed] [Google Scholar]
- 50. Wahl M, Chang SM, Phillips JJ, et al. Probing the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in gliomas: A phase 2 study of everolimus for recurrent adult low-grade gliomas. Cancer 2017;123(23):4631–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.