Abstract
The Notch signaling pathway is a highly versatile and evolutionarily conserved mechanism with an important role in cell fate determination. Notch signaling plays a vital role in vascular development, regulating several fundamental processes such as angiogenesis, arterial/venous differentiation, and mural cell investment. Aberrant Notch signaling can result in severe vascular phenotypes as observed in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and Alagille syndrome. It is known that vascular endothelial cells and mural cells interact to regulate vessel formation, cell maturation, and stability of the vascular network. Defective endothelial–mural cell interactions are a common phenotype in diseases characterized by impaired vascular integrity. Further refinement of the role of Notch signaling in the vascular junctions will be critical to attempts to modulate Notch in the context of human vascular disease. In this review, we aim to consolidate and summarize our current understanding of Notch signaling in the vascular endothelial and mural cells during development and in the adult vasculature.
OVERVIEW OF NOTCH SIGNALING
The Notch pathway is a highly conserved signaling mechanism that has been studied for over a century, beginning with the identification of Notch as the gene responsible for wing margin development within Drosophila melanogaster (Dexter 1914; Morgan 1916; Mohr 1919). The subsequent cloning of the gene for the Notch receptor paved the way for the identification of components of the Notch signaling pathway (Kidd et al. 1983; Wharton et al. 1985). The Notch signaling pathway is one of a relatively small number of conserved mechanisms that mediate several fundamental physiological processes including cell proliferation, differentiation, and apoptosis during development (Bray 2006). In recent years, studies have identified critical roles for Notch signaling in the processes of vasculogenesis and angiogenesis during development and in maintenance of vascular homeostasis in the adult (Akil et al. 2021). Vascular endothelial cells [VSMCs] and mural cells (vascular smooth muscle cells and pericytes) work in unison to promote the development of durable blood vessels that can support blood flow (Sweeney and Foldes 2018). Notch signaling plays an important role in regulating cell fate determination, cell proliferation, and maturation during these processes (Baeten and Lilly 2017).
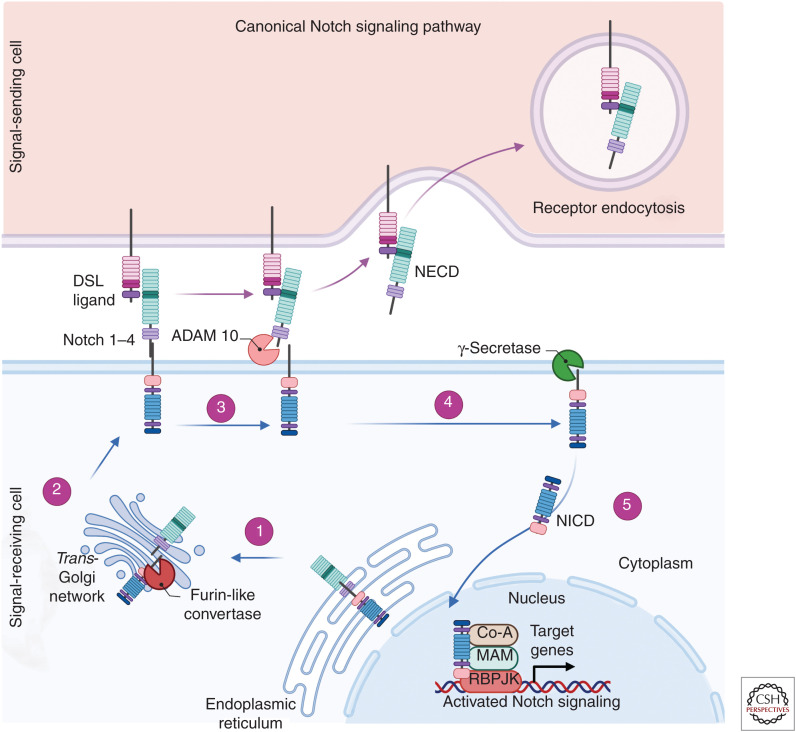
Notch receptors are transmembrane proteins that interact with membrane-bound ligands. In mammals, the canonical signaling pathway involves the interaction of one of the four Notch receptors (Notch 1, Notch 2, Notch 3, and Notch 4) with five known Delta/Serrate/Lag (DSL)-2 ligands, including Jagged 1, Jagged 2, Delta-like 1 (Dll1), Delta-like 3 (Dll3), or Delta-like 4 (Dll4) (Alabi et al. 2018). Initially formed as single-chain precursors, the Notch receptors undergo cleavage by a furin-like convertase in the Golgi apparatus to form extracellular and transmembrane regions that are held together at the cell membrane by noncovalent bonds (Tian et al. 2017). Activation of the canonical Notch signaling pathway is initiated with the extracellular interaction of a Notch receptor with a Notch ligand, resulting in two sequential proteolytic cleavage events by disintegrin and metalloproteinase domain-containing protein 10 (ADAM 10) and γ-secretase, giving rise to the release of the Notch intracellular domain (NICD) from the cell membrane (Fig. 1). The NICD subsequently translocates to the nucleus where it exerts its function in cooperation with recombination signal-binding protein for immunoglobulin κJ (RBPJK) and coactivators including Mastermind. The NICD-RBPJK-activated transcriptional complex drives the up-regulation of Notch target genes including, but not limited to, hairy and enhancer and split (HES) 1, 5, and 7 and HES-related repressor protein (HERP) 1 to 3 (Hofmann and Iruela-Arispe 2007). The response to Notch receptor activation is exquisitely sensitive to dosage. Initially subtle differences in Notch expression and signaling can distinguish single cells from a group of seemingly equivalent neighboring cells and therefore drive those cells to an alternate differentiation fate (Lai 2004; Schweisguth 2004; Fouillade et al. 2012).
Figure 1.
Schematic of the canonical Notch signaling pathway. (1) Notch receptors (1–4) are initially synthesized as single-chain precursors within the endoplasmic reticulum and subsequently transported to the Golgi apparatus. (2) The Notch precursor then undergoes cleavage by furin forming an extracellular and transmembrane domain and is modified by the glycotransferases fringe. These modified proteins are transported and inserted into the cell membrane. The Notch receptor interacts with Delta/Serrate/Lag (DSL)-2 ligands Jagged 1, Jagged 2, Delta-like 1 (Dll1), Delta-like 3 (Dll3), or Delta-like 4 (Dll4). (3) Upon binding, the Notch receptor is cleaved by ADAM 10 releasing the Notch extracellular domain (NECD) that is then degraded by receptor endocytosis. (4) There is then a subsequent proteolytic cleavage by γ-secretase releasing the Notch intracellular domain (NICD) into the cytoplasm. (5) The NICD is then translocated to the nucleus and forms a coactivator complex with factors including recombination signal-binding protein for immunoglobulin κJ (RBPJK) and Mastermind-inducing transcription of Hes and Hey genes. (Created in BioRender.com.)
Importantly, regulation of Notch signaling can occur at several steps; for example, post-translational modifications can occur within the Golgi apparatus. Fringe glycosyltransferases modify the sugar moieties within the epidermal growth factor (EGF)-like repeats through the extension of O-linked glycans on the Notch receptors and associated ligand extracellular domains (Moloney et al. 2000). In vertebrates, three fringe orthologs have been extensively characterized including lunatic fringe, manic fringe, and radical fringe. Knockout mouse models of lunatic fringe bear resemblance and display key pathological features seen in mouse models deficient in components of the Notch signaling pathway (Shen et al. 1997; Zhang and Gridley 1998; Barrantes et al. 1999). Modifications of Notch by fringe leads to differential modulation of the interaction of Notch with the DSL ligands. Lunatic fringe inhibits Jagged 1–mediated signaling and conversely increases the strength of Dll1-mediated signaling through Notch 1. In contrast, lunatic fringe potentiates both Jagged 1 and Dll1 signaling through Notch 2 (Hicks et al. 2000). These modifications have the ability to modify the strength of response of Notch receptors to their ligands and may help to better understand the context-dependent functions of Notch signaling (Hicks et al. 2000; del Álamo et al. 2011; Shen et al. 2021).
Clinical and experimental evidence has shown that Notch signaling plays a key role in both development and maintenance of the vasculature, whereas disrupted Notch signaling can have dramatic effects on vascular development and stability (Table 1). Abnormal Notch signaling components have been implicated in many developmental human pathologies including Alagille syndrome (AGS), tetralogy of Fallot, syndactyly, spondylocostal dysostoisis, and aortic valve disease (Gridley 2003; Garg et al. 2005). The disorders, however, are not limited to development, as mutations in Notch 3 lead to cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a progressive neurovascular disorder that is characterized by ischemic stroke of adult onset (Joutel et al. 1996).
Table 1.
Vascular expression of key Notch signaling components and their role in vascular smooth muscle cell (VSMC) function and associated pathologies
| Notch signaling component | Vascular expression | Function in VSMCs | Associated vascular disease |
|---|---|---|---|
| Notch 1 | Endothelial cells (Takeshita et al. 2007), pericytes (Kofler et al. 2015), VSMCs (low basal expression) (Li et al. 2009) | Promotes proliferation + cell survival (Sweeney et al. 2004) | Adams–Oliver syndrome (Stittrich et al. 2014) |
| Aortic valve disease (Garg et al. 2005) | |||
| Notch 2 | VSMCs (large caliber vessels) (High et al. 2007; Varadkar et al. 2008) | Promotes differentiation (Baeten and Lilly 2015) + Jagged 1–mediated proliferation inhibition (Boucher et al. 2013) | Alagille syndrome (McDaniell et al. 2006) |
| Hajdu–Cheney (Simpson et al. 2011) | |||
| Notch 3 | Pericytes (Joutel et al. 2000), VSMCs (Domenga et al. 2004) | Promotes differentiation + mural cell investment (Domenga et al. 2004), promotes proliferation + cell survival (Liu et al. 2010) | Cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (Joutel et al. 1996) |
| Infantile myofibromatosis (Wu et al. 2021) | |||
| Notch 4 | Endothelial cells (Wu et al. 2005) | - | - |
| Jagged 1 | VSMCs (High et al. 2007), endothelial cells (Lindner et al. 2001), pericytes (Kofler et al. 2015) | Promotes differentiation (Manderfield et al. 2012) | Alagille syndrome (Kamath et al. 2004) |
| Tetralogy of Fallot (Eldadah et al. 2001) | |||
| Dll1 | Endothelial cells (Sörensen et al. 2009) | - | - |
| Dll4 | Endothelial cells (Liu et al. 2003) | - | Adams–Oliver syndrome (Meester Josephina et al. 2015) |
Supportive references are included in the table.
The intricate and dynamic expression of Notch receptors and ligands in vascular cells further highlights the importance of Notch signaling. Notch 1 and Notch 4 are strongly expressed by the endothelium and Notch 3 expression is largely localized to VSMCs and pericytes within the brain (Joutel et al. 2000; Domenga et al. 2004). Interestingly, activated Notch 1 has been identified in the arterial vasculature during pulmonary development. Notch 2 is found at high levels within mesenchymal tissue and smooth muscle cells that surround the pulmonary arteries and aorta (High et al. 2007). Vascular endothelial cells also express the ligands Dll1, Dll4, and Jagged 1. The Notch ligand Jagged 2, Notch 1–4, Dll4, Jagged 1, and Jagged 2 are largely expressed in arteries but not veins (Villa et al. 2001). We will review the role of the Notch signaling in endothelial cells and mural cells individually during development and in the adult vasculature, then assess their cumulative interactions.
THE ROLE OF NOTCH SIGNALING IN ENDOTHELIAL CELLS DURING DEVELOPMENT
Blood vessel formation is a dynamic and complex process that has a critical role in homeostasis to ensure adequate and appropriate supply of oxygen, nutrients, and trophic factors as well as the removal of waste products in disease processes (Sweeney and Foldes 2018). Endothelial cell proliferation and sprouting are tightly controlled, organized processes involved in the development of new blood vessels, which takes place by two distinct processes: vasculogenesis or angiogenesis. During development of an embryo, the initial growth of blood vessels in an avascular environment (e.g., de novo) is referred to as vasculogenesis (Potente et al. 2011).
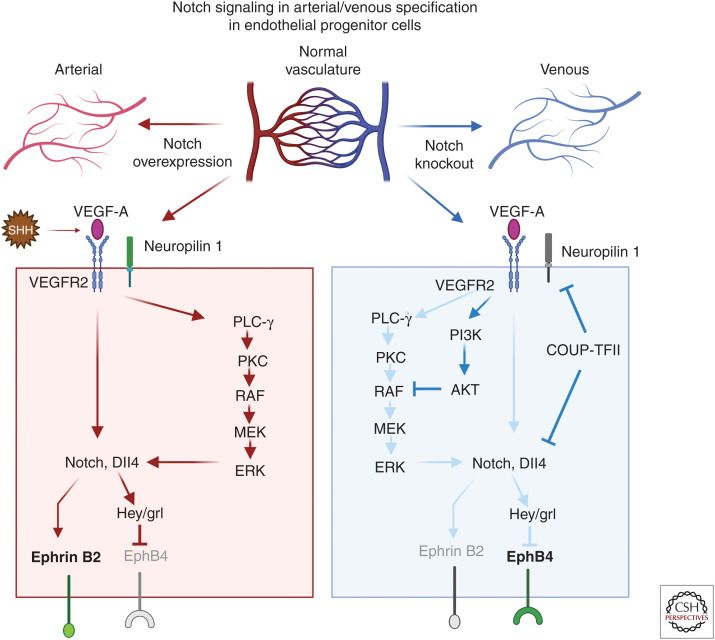
During vessel development, Notch signaling is important for vascular remodeling and in the process of arterial specification through its interactions with Notch ligands and association with other pathways such as vascular endothelial growth factor (VEGF) signaling (Krebs et al. 2000; Shawber et al. 2003). The embryonic vasculature is formed from vascular endothelial cells derived from mesodermal precursor cells (Goldie et al. 2008; Dyer and Patterson 2010). Early endothelial progenitor cells known as angioblasts arise at embryonic day 7.5 and coalesce to form a vascular plexus (Vokes and Krieg 2002). Mesodermal cells expressing sonic hedgehog produce VEGF that interacts with VEGF receptor 2 (VEGFR2) and neuropilin 1 in arterial precursor cells leading to the expression of Dll4 and Notch (Morrow et al. 2009). Once an endothelial precursor cell reaches a critical threshold level of Dll4, it induces strong Notch signaling in an adjacent cell and establishes a cell fate decision selecting an arterial over venous endothelial cell fate (Gridley 2010). Conversely, venous endothelial progenitor cells lack neuropilin 1, and low amounts of VEGF trigger expression of chicken ovalbumin upstream promoter transcription factor II (COUP-TFII). COUP-TFII represses neuropilin 1 and Notch signaling promoting a venous cell fate and EphB4 expression (Fig. 2). During development of the yolk sac, the vasculature is formed by de novo vasculogenesis. Vascular endothelial cells are produced from hemangioblasts that give rise to blood islands containing hematopoietic cells and endothelial progenitor cells (Caolo et al. 2012).
Figure 2.
The role of Notch signaling in arterial specification during development. The top panel is a schematic showing how changes in Notch expression impact arterial/venous specification in the arterial/venous network. The two major signaling pathways operate downstream of vascular endothelial growth factor (VEGF)-A, induce arterial differentiation, the Notch pathway, and the PLC/MAPK pathway. In the arterial specification pathway (red arrows), sonic hedgehog–expressing mesodermal cells produce VEGF-A and stimulate endothelial precursor cells. Mesodermal cells expressing sonic hedgehog produce VEGF that interacts with VEGFR2 and neuropilin 1 in arterial precursor cells leading to the expression of Dll4 and Notch. Once an endothelial precursor cell reaches a critical threshold level of Dll4, it causes strong Notch signaling in an adjacent cell and establishes a cell fate decision selecting an arterial over venous endothelial cell fate. There are two distinct mechanisms that drive vein differentiation (blue arrows). The venous endothelial progenitor cells lack neuropilin 1 and low amounts of VEGF trigger expression of chicken ovalbumin upstream promoter transcription factor II (COUP-TFII). COUP-TFII represses VEGFR2, neuropilin 1, and Notch signaling and promotes a venous cell fate and promotes EphB4 expression; additionally, the activation of the PI3K/AKT pathway blocks arterial specification by preventing ERK activation. (Created in BioRender.com.)
The vascular endothelium primarily expresses Notch 1 and Notch 4 receptors. The initial Notch 1 knockout model clarified its critical role in development as the knockout mice died during embryogenesis on day 11.5 (Swiatek et al. 1994). Vascular endothelial cell–specific Notch 1 knockout mice were then developed and displayed early embryonic lethality and robust vascular defects within the embryonic and yolk sac vasculature, similar to the global Notch 1 knockout phenotype suggesting that Notch 1 is critical for embryonic vascular development (Swiatek et al. 1994; Limbourg et al. 2005). Conversely, Notch 4 knockout mice do not display any obvious vascular defects. Interestingly, a double-knockout of Notch 1 and Notch 4 produces a more severe vascular phenotype than the Notch 1 knockout alone, suggesting possible compensatory mechanisms (Krebs et al. 2000). Vascular network modification and expansion occurs through the process of angiogenesis, whereby endothelial cells proliferate and sprout from the existing vasculature generating a network that remodels into arteries and veins (Potente et al. 2011). The earliest genes identified to differentiate arterial and venous endothelium were EphrinB2 and EphB4 that belong to the Eph-ephrin subclass of receptor tyrosine kinases (Pasquale 2008). During development, factors such as Notch, Hedgehog, and Coup-TFII have been identified to play important roles in arterial specification (Lawson et al. 2001; You et al. 2005). Notch signaling promotes an increase in EphrinB2 expression in arterial endothelial cells while repressing the expression of EphB4 in venous endothelial cells (Chen et al. 2021). An overexpression of Notch 4 in adult mice results in arteriovenous malformation and expression of EphrinB2 in veins that was reversible upon repression of Notch 4 expression. Dll4 knockout mice exhibit severe vascular defects and are associated with a reduction in EphrinB2 expression and an increase in EphB4 expression that indicate a failure in arterial differentiation (Duarte et al. 2004). These data show that Notch signaling is required for the proper development of arterial and venous blood vessels, and is involved in the specification of arterial endothelial cells and in the suppression of venous cell fate (Lawson et al. 2001; Zhang et al. 2014).
NOTCH SIGNALING DURING ANGIOGENESIS AND ENDOTHELIAL CELL FATE DETERMINATION
Angiogenesis is an essential process that is involved in development, tissue growth, and wound healing processes and broadly refers to the formation of new blood vessels from the existing vasculature (Takeshita et al. 2007; Akil et al. 2021). Notch signaling has been identified as an important mediator of angiogenesis through interaction with Notch ligands. Dll4 has been identified as an important ligand for stimulating angiogenesis (Benedito et al. 2009; Naito et al. 2020). Conversely, Jagged signaling has been found to compete with Dll4 to negatively regulate angiogenesis (Benedito et al. 2009). The Notch signaling pathway has been proposed as a therapeutic target for pathological angiogenesis. Inhibition of the Notch ligand Dll4 in the retinal ischemia model promoted the formation of normal vasculature that was partially able to reverse the ischemic environment (Lobov and Mikhailova 2018). Additionally, targeting Dll4 has become a focus in cancer research as Dll4 inhibition leads to excessive, nonproductive angiogenesis and consequently results in impaired tumor growth and may reduce metastasis (Kuhnert et al. 2011; Mendonça et al. 2019; Yang et al. 2020).
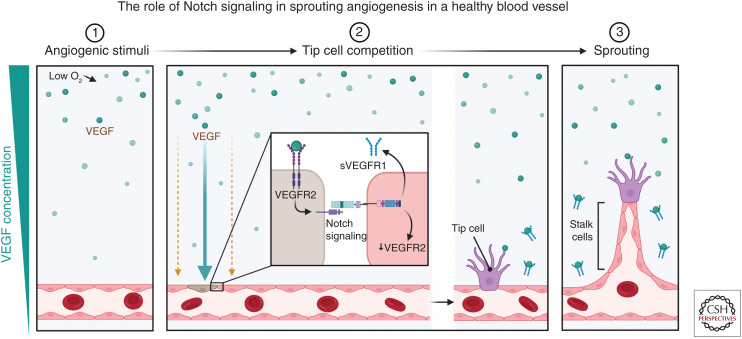
A hallmark of Notch signaling is regulation of cell fate decisions. A clear example of this can be observed in the vasculature during the specification of stalk/tip cells phenotype in the growing blood vessel sprout (Fig. 3). During angiogenesis, expression of Dll4 at the cells at the end of the sprout (tip cell) activates Notch 1 in the stalk cell (Blanco and Gerhardt 2013). Vascular sprout formation therefore relies on a coordinated temporal and spatial localization of Notch signaling. This is shown through Notch signaling inactivation at the onset of angiogenesis resulting in an increase in the number of tip cells at the expense of stalk cells. Tip cells alone are insufficient to form tubes or stable junctions (Mack and Iruela-Arispe 2018). Notch is required for stabilization of the vasculature and differentiation and suppression of endothelial cell proliferation (Ehling et al. 2013; Mack et al. 2017). Notch-mediated inhibition of proliferation requires tumor suppressor phosphatase and tensin homolog (PTEN) (Serra et al. 2015), which blocks stalk cell proliferation downstream of Notch signaling. Notch signaling suppresses excessive sprouting via lateral inhibition, a process by which Notch signaling inhibits its neighboring cells from assuming a similar differentiated adult cell state (Sjöqvist and Andersson 2019).
Figure 3.
Schematic of Notch signaling in sprouting angiogenesis. (1) Angiogenesis is initiated by a stimulus such as hypoxia, which leads to an increase in vascular endothelial growth factor (VEGF) expression in tissues. (2) The presence of VEGF (green circles) leads to the binding of VEGF receptor 2 (VEGFR2) on the surface of the endothelial cells. A VEGF/Notch-regulated mechanism ensures a limited number of tip cells are formed through a process known as lateral inhibition. After VEGF binds to the VEGFR2 receptor, it promotes the formation of a tip cell (brown cell) and promotes an increase in the Notch ligand Dll4 expression, while simultaneously inhibiting the formation of tip cells by its neighbors through Notch signaling. Activating Notch signaling results in the down-regulation of VEGFR2 and promotes the production of soluble VEGFR (sVEGFR) that can then act to scavenge extracellular VEGF and hence prevent overvascularization. (3) These cells will then become stalk cells that form the body of the sprouting vessel. (Created in BioRender.com.)
VEGF signaling promotes the up-regulation of Dll4 in the tip cells that then activates Notch 1 in the neighboring stalk cells. The formation of vascular sprouts requires a highly organized and spatial localization of Notch signaling. Ultimately, during the process of angiogenesis, the activation of Notch signaling reduces endothelial cell proliferation through blunting the cell's responsiveness to VEGF (Siekmann and Lawson 2007; Eelen et al. 2020). Additionally, stalk cells express Jagged 1 that can compete with Dll4 for cis binding of Notch receptors within the tip cells. Jagged binds but does not activate the Notch receptor in the tip cell and therefore prevents Notch signaling in the tip cell (Blanco and Gerhardt 2013). Interestingly, the ability of ligands to activate Notch is dependent on the glycosyltransferase fringe. Without fringe, the nonglycosylated Notch receptor can interact with Jagged 1 resulting in inhibition, but in the presence of fringe, a glycosylated Notch receptor is strongly activated by binding with Dll4 (Benedito et al. 2009). Dysregulation of Notch ligand levels has consequences for the vascular network; for example, mice that are heterozygous for Dll4 have hypervascularized retinas (Lobov and Mikhailova 2018; Suchting et al. 2007).
THE ROLE OF NOTCH SIGNALING IN MATURE VASCULAR ENDOTHELIAL CELLS
Local artery obstruction of blood flow to the periphery can be restored through expansion of arterioles to form collateral vessels in a process known as arteriogenesis (Heil et al. 2006). This process may not be limited to tissue repair but may also have a role in physiological vascular expansion during tissue growth in exercise (Roca and Adams 2007). It has been reported that Dll1, a Notch ligand that is largely localized to the vascular endothelium, has a pivotal role in postnatal angiogenesis and is up-regulated during ischemia-induced arteriogenesis (Limbourg et al. 2007). However, this has been difficult to study due to the embryonic lethality of the Dll1 knockout. Dll1 heterozygous mice display reduced formation of collateral arteries and, in an ischemic hind limb model, fail to restore blood flow (Limbourg et al. 2007).
In addition to the known canonical interactions of Notch with its ligands, there are also noncanonical roles for Notch in the vasculature. It is known that shear stress plays an important role in the maintenance of the blood–brain barrier via a noncanonical Notch 1 activation driving the assembly of adherens junctions through the regulation of Rac1. The process is initiated when shear stress causes the activation of Notch 1 via the Dll4 ligand, leading to the exposure of the transmembrane domain of Notch 1. The Notch 1 transmembrane domain affects barrier function by promoting the formation of a receptor complex at the membrane that is comprised by VE-cadherin, tyrosine phosphatase LAR, and a Rac guanine–nucleotide exchange factor (GEF) trio that promotes the activation of Rac1 (Polacheck et al. 2017).
Other fundamental mechanisms that are regulated by Notch signaling include apoptosis and cell survival, key events during the process of vascular remodeling (Akil et al. 2021). Notch 2 sensitizes endothelial cells by inhibiting the expression of survivin, a key antiapoptotic regulator. During the process of vascular remodeling, activation of Notch signaling can trigger apoptosis (Quillard et al. 2009).
NOTCH SIGNALING IN MURAL CELL DEVELOPMENT
Vessels formed in the early stages of vasculogenesis are composed primarily of endothelial cell tubes; to support blood flow and withstand pressure changes, they recruit mural cells through the release of PDGFB (Hellström et al. 1999). Mural cells act as support cells in vascular function. The most studied types of mural cells include VSMCs and pericytes that have distinct roles within the vasculature. VSMCs provide support and are important for contractility of large vessels, whereas pericytes are associated with smaller caliber capillaries and enhance endothelial cell stability and vessel maturation during sprouting angiogenesis (Armulik et al. 2011; Kofler et al. 2015). Different mural cell types have distinct expression of Notch receptors; VSMCs have been reported to express primarily Notch 2 and Notch 3 and may also express Notch 1 (Fouillade et al. 2012). Pericytes have been reported to express Notch 1 and Notch 3 as well as low levels of Notch 2 (Kofler et al. 2015; Diéguez-Hurtado et al. 2019). Together, mural cells play numerous roles to maintain vessel homeostasis including regulating vasopermeability and controlling blood flow. Notch receptor expression is heterogenous and has unique roles in different vascular beds. Notch 2 expression is highly expressed in large caliber vessels; within these vessels, Notch 3 is not sufficient to completely compensate for loss of Notch 2 and, additionally, loss of Notch 3 results in an exaggerated phenotype (McCright et al. 2001; Wang et al. 2012).
Haploinsufficiency of Notch signaling is causative of several genetic conditions (Table 1) including AGS, an autosomal-dominant disease caused by heterozygous mutations in either Notch 2 (McDaniell et al. 2006), Jagged 1 (Warthen et al. 2006), or, in rare instances, both (Brennan and Kesavan 2017). It is commonly characterized by developmental defects affecting the heart, skeleton, liver, and eyes (Li et al. 1997). Dysfunctional Notch signaling in VSMCs during AGS is associated with vascular phenotypes affecting the cardiac outflow tract and great vessels, and in certain circumstances mutations in Jagged 1 can result in congenital heart defects known as tetralogy of Fallot that can occur during AGS or in the absence of an AGS diagnosis (Eldadah et al. 2001; McElhinney et al. 2002; Bauer et al. 2010). Adams–Oliver syndrome (AOS) is another congenital condition that is caused by Notch 1 haploinsufficiency leading to scalp defects and limb defects and is often associated with cardiovascular abnormalities (Southgate et al. 2015; Suarez et al. 2021).
It is important to highlight that VSMCs have been shown to display a high degree of plasticity and are therefore not regarded as terminally differentiated. In response to molecular stimuli, VSMCs have been found to adapt their responses, a fact that has been implicated in vascular disease (Wu and Zhang 2009). It has been reported that deficiency in Notch signaling in pericytes leads to arteriovenous malformations in a murine model (Nadeem et al. 2020). Arteriovenous malformations are focal vascular lesions in arteries that shunt into veins with no intervening capillary bed. These vascular lesions have been reported to occur in several organ systems; however, they are of particular seriousness in the brain where they are known to result in stroke and seizures (Solomon and Connolly 2017). Additionally, the loss of Notch signaling in pericytes causes a reduction of PDGFRβ levels and increased pericyte apoptosis (Nadeem et al. 2020). Therefore, tight regulation of Notch signaling is vital to pericyte survival (Arboleda-Velasquez et al. 2014). Further supporting this, Notch3 signaling has been implicated in the expansion of brain pericyte populations in zebrafish, whereby it promotes pericyte proliferation and vascular integrity (Wang et al. 2014). Recent studies have shown that pericytes play an important role in the promoting of endothelial sprouting in postnatal vasculature. The expression of VEGFR1 by pericytes leads to a spatial restriction of VEGF signaling (Eilken et al. 2017).
NOTCH SIGNALING IN ADULT MURAL CELLS
Notch 3 is the predominant Notch receptor expressed by mural cells and has been proposed as an important mediator of maturation of VSMCs (Domenga et al. 2004; Liu et al. 2010; Kofler et al. 2015). Notch 3 knockout mice have an increase susceptibility to ischemic stroke upon challenge (Arboleda-Velasquez et al. 2008). The importance of Notch 3 expression within adult mural cells is highlighted by the hereditary disorder CADASIL, a progressive small vessel disease that is caused by mutations within the Notch 3 gene, which is often debilitating and leads to dementia and an early mortality (Schoemaker and Arboleda-Velasquez 2021). CADASIL is characterized by VSMC pathology and ischemic stroke (Joutel et al. 1996, 2000; Arboleda-Velasquez et al. 2011). A major hallmark of CADASIL is the accumulation of granular osmophilic material deposits within the basement membrane of the vasculature (Joutel et al. 2000).
CADASIL is caused by mutations within the Notch 3 gene that commonly lead to an odd number of cystine residues in the EGF-like repeats in the Notch 3 extracellular domain (Notch 3ECD) (Joutel et al. 1997), which compromises the stability of the Notch 3ECD due to an improper formation of disulfide bridges. This has been proposed to result in protein misfolding and accumulation of the Notch 3ECD (Spinner 2000). Key pathological features of CADASIL include the progressive loss of mural cells. Murine models of Notch 3 knockout do not accurately recapitulate the CADASIL phenotype despite reduced VSMC coverage and impaired VSMC maturation (Domenga et al. 2004; Liu et al. 2010; Henshall et al. 2015). Interestingly, a double transgenic murine model of Notch 3 knockout and heterozygous expression of Notch 1 leads to pericyte dysfunction and arteriovenous malformations that mimic some of the hallmarks of CADASIL (Kofler et al. 2015). Notch 3 is highly expressed in mural cells when mural cells are cocultured with endothelial cells. Notch 3 has been reported to promote its own expression and increases the expression of Jagged 1 (Liu et al. 2009). Modulation of Notch signaling has been proposed as a therapeutic target in CADASIL using a Notch 3 agonist antisera that was effective at preventing SVD phenotypes in Notch 3 knockout and CADASIL mice (Li et al. 2008; Machuca-Parra et al. 2017).
Notch signaling in mural cells also has a role in regulating cell survival and apoptosis. It has been reported in a variety of cell types that Notch signaling prevents apoptosis (Sweeney et al. 2004; Dror et al. 2007; Arboleda-Velasquez et al. 2014), and it has been proposed that noncanonical interactions of Notch and MAPK/ERK promote cell survival (Wang et al. 2002; Baeten and Lilly 2017).
NOTCH SIGNALING BETWEEN ENDOTHELIAL AND MURAL CELLS
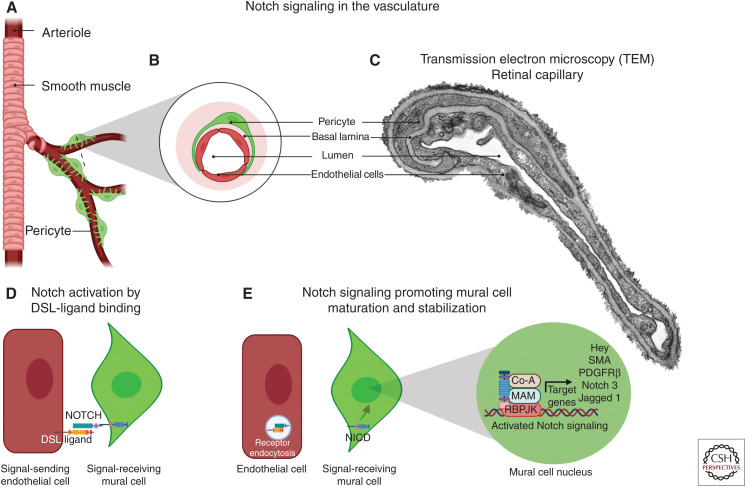
It is widely known that vascular endothelial and mural cells have regions of direct communication through myoendothelial gap junctions and microprojections (Sandow et al. 2009; Nagaraja et al. 2013; Tian et al. 2017). At least three types of endothelial cell–pericyte contacts have been reported: (1) peg and socket contacts whereby endothelial and pericyte processes interdigitate, (2) adherent plaques, and (3) cell–cell contacts that resemble gap junctions (Carlson 1989; Armulik et al. 2005; Zhang et al. 2020). Two-way communication between endothelial cells and pericytes is important to regulate the development and function of the vasculature. During angiogenesis, as endothelial cells form capillaries, they recruit pericytes that stabilize the vessel and prevent capillary sprouting, which helps direct basement membrane formation while also promoting pericyte differentiation (Stratman et al. 2009; Liu et al. 2010; Armulik et al. 2011; Trost et al. 2016). One of the major factors in ongoing communications between endothelial and mural cells is Notch signaling. It is tantalizing to think that cell–cell interactions mediated by Notch signaling take place at this location where the plasma membrane of vascular endothelial cells and mural cells come in close proximity.
Within the context of endothelial–mural cell communication, evidence indicates that endothelial cells act primarily as signal-sending cells and VSMCs behave as signal-receiving cells (High et al. 2008). This is supported by several in vitro and in vivo studies, where coculture experiments of endothelial cells and mural cells highlighted the role of endothelial cells in promoting the activation of Notch signaling in the adjacent mural cells leading to the up-regulation of several contractile genes as well as increased expression of Jagged 1 (Fig. 4; Liu et al. 2009). Additionally, an endothelial cell–specific knockout of Jagged 1 leads to impaired VSMC differentiation in the aortic arch arteries, and postnatal endothelial knockout of Jagged 1 results in a reduction in coverage of retinal arterioles by VSMCs (High et al. 2008; Benedito et al. 2009). Intriguingly, the VSMC-specific knockout of Jagged 1 also results in improper differentiation of VSMCs indicating that Notch/Jagged 1 signaling between VSMCs is also required (Feng et al. 2010). During vascular development, Notch receptors expressed by the VSMCs bind Jagged 1 expressed by the endothelium leading to an up-regulation of the integrin αvβ3 that promotes maturation of the VSMCs and enabling adhesion of VSMCs to the endothelial basement membrane (Scheppke et al. 2012; Tian et al. 2017). In the adult vasculature, it has been shown that knockout of AKT results in a reduction in Jagged 1 expression in the endothelium and a progressive loss of VSMCs due to impaired Notch signaling (Kerr et al. 2016). Conditioned media from endothelial cells or the addition of soluble Jagged 1 are insufficient to stimulate VSMC differentiation in vitro; conversely, coculture of endothelial and VSMCs promotes differentiation (Xia et al. 2012).
Figure 4.
The role of Notch in vascular endothelial and mural cell communication. (A) Schematic of mural cell localization within branching arterioles. (B) Cross-sectional magnification of a capillary highlight pericytes (green), endothelial cells (red), and basal lamina (pink). (C) Transmission electron microscopy (TEM) images of vessels within the retinal ganglion cell layer demonstrate a peg and socket connection between the endothelial cell and pericyte. (D) Notch expressed in the mural cell (green) is activated upon ligand binding from the endothelial cell (red). (E) After a series of proteolytic events (see Fig. 1), the Notch intracellular domain (NICD) translocates to the nucleus and promotes transcription of a number of genes including PDGFRβ, smooth muscle actin (SMA), Notch 3, and Jagged 1, which promote mural cell maturation and stability. (DSL) Delta/Serrate/Lag, (RBPJK) recombination signal-binding protein for immunoglobulin κJ. (Created in BioRender.com.)
A breakdown of communication has been identified in several pathologies such as CADASIL, vascular injury, and diabetic retinopathy. Diabetic retinopathy is associated with an increased deposition of basement membrane that is believed to interfere with direct communication between endothelial and mural cells. In addition, glucose-mediated damage to the pericytes appears to lead to pericyte death and loss of pericyte coverage. Notch 1 expression has been shown to maintain the endothelium in a quiescent state (Noseda et al. 2004), whereas aberrant Notch signaling has been demonstrated to provoke vascular dysfunction (Arboleda-Velasquez et al. 2014; Kofler et al. 2015). In this context, targeting Notch 1 signaling using neutralizing antibodies may hold therapeutic potential and warrants future investigation (Miloudi et al. 2019).
The requirement for coordination of Notch signaling in the vasculature is further highlighted during vascular injury. Many signaling pathways act to repair the damage; however, if not sufficiently regulated, this process can lead to pathology such as neointimal hyperplasia, an exaggerated wound-healing process that is initiated through damage to the endothelial cells and is characterized by proliferation and migration of VSMCs to the lumen of the vessel (Wu and Zhang, 2009; Baeten and Lilly 2017). Additionally, elements of the Notch signaling pathway including Notch 1, Notch 3 and Jagged 1, Jagged 2, Hey 1, and Hey 2 are dysregulated at different phases of injury with an initial down-regulation and subsequent overexpression (Lindner et al. 2001; Gridley 2010). In vivo injury models have highlighted an important role for Notch 1 in driving migration and proliferation during neointimal hyperplasia (Li et al. 2009; Wang et al. 2018).
FUTURE DIRECTIONS
Within this article, we have summarized key components and concepts in Notch signaling during endothelial and mural cell communications in development and in the postnatal vasculature. Despite a vast and ever-growing literature regarding Notch signaling and its role in health and disease, several fundamental questions remain. The roles for Notch in the developing vasculature are largely characterized; however, pathologies such as CADASIL develop later in life and present an interesting question regarding the changing requirement for Notch signaling during aging. It is evident that Notch 3 signaling plays an important role in the differentiation of VSMCs during development, and likely during vascular remodeling processes. It remains unclear how these endothelial–VSMC interactions are affected in an adult mature VSMC.
ACKNOWLEDGMENTS
We acknowledge support by the National Institute on Aging co-funded Grants UH3 NS100121 and RF1 NS110048 to J.F.A.-V.
Footnotes
Editors: Diane R. Bielenberg and Patricia A. D'Amore
Additional Perspectives on Angiogenesis: Biology and Pathology available at www.perspectivesinmedicine.org
REFERENCES
- Akil A, Gutiérrez-García AK, Guenter R, Rose JB, Beck AW, Chen H, Ren B. 2021. Notch signaling in vascular endothelial cells, angiogenesis, and tumor progression: an update and prospective. Front Cell Dev Bio 9: 642352. 10.3389/fcell.2021.642352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabi RO, Farber G, Blobel CP. 2018. Intriguing roles for endothelial ADAM10/Notch signaling in the development of organ-specific vascular beds. Physiol Rev 98: 2025–2061. 10.1152/physrev.00029.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboleda-Velasquez JF, Zhou Z, Shin HK, Louvi A, Kim HH, Savitz SI, Liao JK, Salomone S, Ayata C, Moskowitz MA, et al. 2008. Linking Notch signaling to ischemic stroke. Proc Natl Acad Sci 105: 4856–4861. 10.1073/pnas.0709867105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboleda-Velasquez JF, Manent J, Lee JH, Tikka S, Ospina C, Vanderburg CR, Frosch MP, Rodriguez-Falcon M, Villen J, Gygi S, et al. 2011. Hypomorphic Notch 3 alleles link Notch signaling to ischemic cerebral small-vessel disease. Proc Natl Acad Sci 108: E128–E135. 10.1073/pnas.1101964108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboleda-Velasquez JF, Primo V, Graham M, James A, Manent J, D'Amore PA. 2014. Notch signaling functions in retinal pericyte survival. Invest Ophthalmol Vis Sci 55: 5191–5199. 10.1167/iovs.14-14046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. 2005. Endothelial/pericyte interactions. Circ Res 97: 512–523. 10.1161/01.RES.0000182903.16652.d7 [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Betsholtz C. 2011. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215. 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Baeten JT, Lilly B. 2015. Differential regulation of NOTCH2 and NOTCH3 contribute to their unique functions in vascular smooth muscle cells. J Biol Chem 290: 16226–16237. 10.1074/jbc.M115.655548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten JT, Lilly B. 2017. Notch signaling in vascular smooth muscle cells. Adv Pharmacol 78: 351–382. 10.1016/bs.apha.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten JT, Jackson AR, McHugh KM, Lilly B. 2015. Loss of Notch2 and Notch3 in vascular smooth muscle causes patent ductus arteriosus. Genesis 53: 738–748. 10.1002/dvg.22904 [DOI] [PubMed] [Google Scholar]
- Barrantes I, Elia AJ, Wünsch K, De Angelis MH, Mak TW, Rossant J, Conlon RA, Gossler A, de la Pompa JL. 1999. Interaction between Notch signalling and lunatic fringe during somite boundary formation in the mouse. Curr Biol 9: 470–480. 10.1016/S0960-9822(99)80212-7 [DOI] [PubMed] [Google Scholar]
- Bauer RC, Laney AO, Smith R, Gerfen J, Morrissette JJ, Woyciechowski S, Garbarini J, Loomes KM, Krantz ID, Urban Z, et al. 2010. Jagged1 (JAG1) mutations in patients with tetralogy of Fallot or pulmonic stenosis. Hum Mutat 31: 594–601. 10.1002/humu.21231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH. 2009. The Notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137: 1124–1135. 10.1016/j.cell.2009.03.025 [DOI] [PubMed] [Google Scholar]
- Blanco R, Gerhardt H. 2013. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med 3: a006569. 10.1101/cshperspect.a006569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher JM, Harrington A, Rostama B, Lindner V, Liaw L. 2013. A receptor-specific function for Notch2 in mediating vascular smooth muscle cell growth arrest through cyclin-dependent kinase inhibitor 1B. Circ Res 113: 975–985. 10.1161/CIRCRESAHA.113.301272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ. 2006. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689. 10.1038/nrm2009 [DOI] [PubMed] [Google Scholar]
- Brennan A, Kesavan A. 2017. Novel heterozygous mutations in JAG1 and NOTCH2 genes in a neonatal patient with Alagille syndrome. Case Rep Pediatr 2017: 1368189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caolo V, Molin DG, Post MJ. 2012. Notch regulation of hematopoiesis, endothelial precursor cells, and blood vessel formation: orchestrating the vasculature. Stem Cells Int 2012: 805602. 10.1155/2012/805602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EC. 1989. Fenestrated subendothelial basement membranes in human retinal capillaries. Invest Opthalmol Vis Sci 30: 1923–1932. [PubMed] [Google Scholar]
- Chen D, Schwartz MA, Simons M. 2021. Developmental perspectives on arterial fate specification. Front Cell Dev Bio 9: 691335. 10.3389/fcell.2021.691335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Álamo D, Rouault H, Schweisguth F. 2011. Mechanism and significance of cis-inhibition in Notch signalling. Curr Biol 21: R40–R47. 10.1016/j.cub.2010.10.034 [DOI] [PubMed] [Google Scholar]
- Dexter JS. 1914. The analysis of a case of continuous variation in Drosophila by a study of its linkage relations. Am Nat 48: 712–758. 10.1086/279446 [DOI] [Google Scholar]
- Diéguez-Hurtado R, Kato K, Giaimo BD, Nieminen-Kelhä M, Arf H, Ferrante F, Bartkuhn M, Zimmermann T, Bixel MG, Eilken HM, et al. 2019. Loss of the transcription factor RBPJ induces disease-promoting properties in brain pericytes. Nat Commun 10: 2817. 10.1038/s41467-019-10643-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, et al. 2004. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 18: 2730–2735. 10.1101/gad.308904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror V, Nguyen V, Walia P, Kalynyak TB, Hill JA, Johnson JD. 2007. Notch signalling suppresses apoptosis in adult human and mouse pancreatic islet cells. Diabetologia 50: 2504–2515. 10.1007/s00125-007-0835-5 [DOI] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. 2004. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18: 2474–2478. 10.1101/gad.1239004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Patterson C. 2010. Development of the endothelium: an emphasis on heterogeneity. Semin Thromb Hemost 36: 227–235. 10.1055/s-0030-1253446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eelen G, Treps L, Li X, Carmeliet P. 2020. Basic and therapeutic aspects of angiogenesis updated. Circ Res 127: 310–329. 10.1161/CIRCRESAHA.120.316851 [DOI] [PubMed] [Google Scholar]
- Ehling M, Adams S, Benedito R, Adams RH. 2013. Notch controls retinal blood vessel maturation and quiescence. Development 140: 3051–3061. 10.1242/dev.093351 [DOI] [PubMed] [Google Scholar]
- Eilken HM, Diéguez-Hurtado R, Schmidt I, Nakayama M, Jeong HW, Arf H, Adams S, Ferrara N, Adams RH. 2017. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat Commun 8: 1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldadah ZA, Hamosh A, Biery NJ, Montgomery RA, Duke M, Elkins R, Dietz HC. 2001. Familial tetralogy of Fallot caused by mutation in the jagged1 gene. Hum Mol Genet 10: 163–169. 10.1093/hmg/10.2.163 [DOI] [PubMed] [Google Scholar]
- Feng X, Krebs LT, Gridley T. 2010. Patent ductus arteriosus in mice with smooth muscle-specific Jag1 deletion. Development 137: 4191–4199. 10.1242/dev.052043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillade C, Monet-Leprêtre M, Baron-Menguy C, Joutel A. 2012. Notch signalling in smooth muscle cells during development and disease. Cardiovasc Res 95: 138–146. 10.1093/cvr/cvs019 [DOI] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. 2005. Mutations in NOTCH1 cause aortic valve disease. Nature 437: 270–274. 10.1038/nature03940 [DOI] [PubMed] [Google Scholar]
- Goldie LC, Nix MK, Hirschi KK. 2008. Embryonic vasculogenesis and hematopoietic specification. Organogenesis 4: 257–263. 10.4161/org.4.4.7416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T. 2003. Notch signaling and inherited disease syndromes. Hum Mol Genet 12: R9–R13. 10.1093/hmg/ddg052 [DOI] [PubMed] [Google Scholar]
- Gridley T. 2010. Notch signaling in the vasculature. Curr Top Dev Biol 92: 277–309. 10.1016/S0070-2153(10)92009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W. 2006. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med 10: 45–55. 10.1111/j.1582-4934.2006.tb00290.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. 1999. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055. 10.1242/dev.126.14.3047 [DOI] [PubMed] [Google Scholar]
- Henshall TL, Keller A, He L, Johansson BR, Wallgard E, Raschperger E, Mäe MA, Jin S, Betsholtz C, Lendahl U. 2015. Notch3 is necessary for blood vessel integrity in the central nervous system. Arterioscler Thromb Vasc Biol 35: 409–420. 10.1161/ATVBAHA.114.304849 [DOI] [PubMed] [Google Scholar]
- Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G. 2000. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat Cell Biol 2: 515–520. 10.1038/35019553 [DOI] [PubMed] [Google Scholar]
- High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA. 2007. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest 117: 353–363. 10.1172/JCI30070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. 2008. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci 105: 1955–1959. 10.1073/pnas.0709663105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JJ, Iruela-Arispe ML. 2007. Notch signaling in blood vessels: who is talking to whom about what? Circ Res 100: 1556–1568. 10.1161/01.RES.0000266408.42939.e4 [DOI] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cécillion M, Maréchal E, et al. 1996. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383: 707–710. 10.1038/383707a0 [DOI] [PubMed] [Google Scholar]
- Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssière C, Cruaud C, Maciazek J, Weissenbach J, Bousser MG, et al. 1997. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet 350: 1511–1515. 10.1016/S0140-6736(97)08083-5 [DOI] [PubMed] [Google Scholar]
- Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve D. 2000. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest 105: 597–605. 10.1172/JCI8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath BM, Spinner NB, Emerick KM, Chudley AE, Booth C, Piccoli DA, Krantz ID. 2004. Vascular anomalies in Alagille syndrome: a significant cause of morbidity and mortality. Circulation 109: 1354–1358. 10.1161/01.CIR.0000121361.01862.A4 [DOI] [PubMed] [Google Scholar]
- Kerr BA, West XZ, Kim YW, Zhao Y, Tischenko M, Cull RM, Phares TW, Peng XD, Bernier-Latmani J, Petrova TV, et al. 2016. Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nat Commun 7: 10960. 10.1038/ncomms10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S, Lockett TJ, Young MW. 1983. The Notch locus of Drosophila melanogaster. Cell 34: 421–433. 10.1016/0092-8674(83)90376-8 [DOI] [PubMed] [Google Scholar]
- Kofler NM, Cuervo H, Uh MK, Murtomäki A, Kitajewski J. 2015. Combined deficiency of Notch1 and Notch3 causes pericyte dysfunction, models CADASIL, and results in arteriovenous malformations. Sci Rep 5: 16449. 10.1038/srep16449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. 2000. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14: 1343–1352. 10.1101/gad.14.11.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Kirshner JR, Thurston G. 2011. Dll4-Notch signaling as a therapeutic target in tumor angiogenesis. Vasc Cell 3: 20. 10.1186/2045-824X-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC. 2004. Notch signaling: control of cell communication and cell fate. Development 131: 965–973. 10.1242/dev.01074 [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. 2001. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128: 3675–3683. 10.1242/dev.128.19.3675 [DOI] [PubMed] [Google Scholar]
- Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, et al. 1997. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 16: 243–251. 10.1038/ng0797-243 [DOI] [PubMed] [Google Scholar]
- Li K, Li Y, Wu W, Gordon WR, Chang DW, Lu M, Scoggin S, Fu T, Vien L, Histen G, et al. 2008. Modulation of Notch signaling by antibodies specific for the extracellular negative regulatory region of NOTCH3. J Biol Chem 283: 8046–8054. 10.1074/jbc.M800170200 [DOI] [PubMed] [Google Scholar]
- Li Y, Takeshita K, Liu PY, Satoh M, Oyama N, Mukai Y, Chin MT, Krebs L, Kotlikoff MI, Radtke F, et al. 2009. Smooth muscle Notch1 mediates neointimal formation after vascular injury. Circulation 119: 2686–2692. 10.1161/CIRCULATIONAHA.108.790485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. 2005. Essential role of endothelial Notch1 in angiogenesis. Circulation 111: 1826–1832. 10.1161/01.CIR.0000160870.93058.DD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbourg A, Ploom M, Elligsen D, Sörensen I, Ziegelhoeffer T, Gossler A, Drexler H, Limbourg FP. 2007. Notch ligand Delta-like 1 is essential for postnatal arteriogenesis. Circ Res 100: 363–371. 10.1161/01.RES.0000258174.77370.2c [DOI] [PubMed] [Google Scholar]
- Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. 2001. Members of the jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell–cell interaction. Am J Pathol 159: 875–883. 10.1016/S0002-9440(10)61763-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. 2003. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol 23: 14–25. 10.1128/MCB.23.1.14-25.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kennard S, Lilly B. 2009. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res 104: 466–475. 10.1161/CIRCRESAHA.108.184846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B. 2010. Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res 107: 860–870. 10.1161/CIRCRESAHA.110.218271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov I, Mikhailova N. 2018. The role of Dll4/Notch signaling in normal and pathological ocular angiogenesis: Dll4 controls blood vessel sprouting and vessel remodeling in normal and pathological conditions. J Ophthalmol 2018: 3565292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machuca-Parra AI, Bigger-Allen AA, Sanchez AV, Boutabla A, Cardona-Vélez J, Amarnani D, Saint-Geniez M, Siebel CW, Kim LA, D'Amore PA, et al. 2017. Therapeutic antibody targeting of Notch3 signaling prevents mural cell loss in CADASIL. J Exp Med 214: 2271–2282. 10.1084/jem.20161715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JJ, Iruela-Arispe ML. 2018. NOTCH regulation of the endothelial cell phenotype. Curr Opin Hematol 25: 212–218. 10.1097/MOH.0000000000000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JJ, Mosqueiro TS, Archer BJ, Jones WM, Sunshine H, Faas GC, Briot A, Aragón RL, Su T, Romay MC, et al. 2017. NOTCH1 is a mechanosensor in adult arteries. Nat Commun 8: 1620. 10.1038/s41467-017-01741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderfield LJ, High FA, Engleka KA, Liu F, Li L, Rentschler S, Epstein JA. 2012. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation 125: 314–323. 10.1161/CIRCULATIONAHA.111.047159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T. 2001. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 128: 491–502. 10.1242/dev.128.4.491 [DOI] [PubMed] [Google Scholar]
- McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. 2006. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the Notch signaling pathway. Am J Hum Genet 79: 169–173. 10.1086/505332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinney DB, Krantz ID, Bason L, Piccoli DA, Emerick KM, Spinner NB, Goldmuntz E. 2002. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation 106: 2567–2574. 10.1161/01.CIR.0000037221.45902.69 [DOI] [PubMed] [Google Scholar]
- Meester Josephina AN, Southgate L, Stittrich AB, Venselaar H, Beekmans Sander JA, den Hollander N, Bijlsma EK, Helderman-van den Enden A, Verheij JBGM, Glusman G, et al. 2015. Heterozygous loss-of-function mutations in DLL4 cause Adams–Oliver syndrome. Am J Hum Genet 97: 475–482. 10.1016/j.ajhg.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça L, Trindade A, Carvalho C, Correia J, Badenes M, Gigante J, Duarte A. 2019. Metastasis is impaired by endothelial-specific Dll4 loss-of-function through inhibition of epithelial-to-mesenchymal transition and reduction of cancer stem cells and circulating tumor cells. Clin Exp Metastasis 36: 365–380. 10.1007/s10585-019-09973-2 [DOI] [PubMed] [Google Scholar]
- Miloudi K, Oubaha M, Ménard C, Dejda A, Guber V, Cagnone G, Wilson AM, Tétreault N, Mawambo G, Binet F, et al. 2019. NOTCH1 signaling induces pathological vascular permeability in diabetic retinopathy. Proc Natl Acad Sci 116: 4538–4547. 10.1073/pnas.1814711116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr OL. 1919. Character changes caused by mutation of an entire region of a chromosome in Drosophila. Genetics 4: 275–282. 10.1093/genetics/4.3.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney DJ, Panin VM, Johnston SH, Chen J, Shao L, Wilson R, Wang Y, Stanley P, Irvine KD, Haltiwanger RS, et al. 2000. Fringe is a glycosyltransferase that modifies Notch. Nature 406: 369–375. 10.1038/35019000 [DOI] [PubMed] [Google Scholar]
- Morgan TH. 1916. Sex-linked inheritance in Drosophila. Carnegie Institution of Washington, Washington, DC. [Google Scholar]
- Morrow D, Cullen JP, Liu W, Guha S, Sweeney C, Birney YA, Collins N, Walls D, Redmond EM, Cahill PA. 2009. Sonic hedgehog induces Notch target gene expression in vascular smooth muscle cells via VEGF-A. Arterioscler Thromb Vasc Biol 29: 1112–1118. 10.1161/ATVBAHA.109.186890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem T, Bogue W, Bigit B, Cuervo H. 2020. Deficiency of Notch signaling in pericytes results in arteriovenous malformations. JCI Insight 5: e125940. 10.1172/jci.insight.125940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja S, Kapela A, Tran CH, Welsh DG, Tsoukias NM. 2013. Role of microprojections in myoendothelial feedback—a theoretical study. J Physiol 591: 2795–2812. 10.1113/jphysiol.2012.248948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito H, Iba T, Takakura N. 2020. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int Immunol 32: 295–305. 10.1093/intimm/dxaa008 [DOI] [PubMed] [Google Scholar]
- Noseda M, Chang L, McLean G, Grim JE, Clurman BE, Smith LL, Karsan A. 2004. Notch activation induces endothelial cell cycle arrest and participates in contact inhibition: role of p21Cip1 repression. Mol Cell Biol 24: 8813–8822. 10.1128/MCB.24.20.8813-8822.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. 2008. Eph-ephrin bidirectional signaling in physiology and disease. Cell 133: 38–52. 10.1016/j.cell.2008.03.011 [DOI] [PubMed] [Google Scholar]
- Polacheck WJ, Kutys ML, Yang J, Eyckmans J, Wu Y, Vasavada H, Hirschi KK, Chen CS. 2017. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 552: 258–262. 10.1038/nature24998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. 2011. Basic and therapeutic aspects of angiogenesis. Cell 146: 873–887. 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- Quillard T, Devalliere J, Chatelais M, Coulon F, Séveno C, Romagnoli M, Baillé Nion S, Charreau B. 2009. Notch2 signaling sensitizes endothelial cells to apoptosis by negatively regulating the key protective molecule survivin. PLoS ONE 4: e8244. 10.1371/journal.pone.0008244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca C, Adams RH. 2007. Regulation of vascular morphogenesis by Notch signaling. Genes Dev 21: 2511–2524. 10.1101/gad.1589207 [DOI] [PubMed] [Google Scholar]
- Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. 2009. What's where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol 36: 67–76. 10.1111/j.1440-1681.2008.05076.x [DOI] [PubMed] [Google Scholar]
- Scheppke L, Murphy EA, Zarpellon A, Hofmann JJ, Merkulova A, Shields DJ, Weis SM, Byzova TV, Ruggeri ZM, Iruela-Arispe ML, et al. 2012. Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood 119: 2149–2158. 10.1182/blood-2011-04-348706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker D, Arboleda-Velasquez JF. 2021. Notch3 signaling and aggregation as targets for the treatment of CADASIL and other NOTCH3-associated small-vessel diseases. Am J Pathol 191: 1856–1870. 10.1016/j.ajpath.2021.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisguth F. 2004. Regulation of Notch signaling activity. Curr Biol 14: R129–R138. 10.1016/j.cub.2004.01.023 [DOI] [PubMed] [Google Scholar]
- Serra H, Chivite I, Angulo-Urarte A, Soler A, Sutherland JD, Arruabarrena-Aristorena A, Ragab A, Lim R, Malumbres M, Fruttiger M, et al. 2015. PTEN mediates Notch-dependent stalk cell arrest in angiogenesis. Nat Commun 6: 7935. 10.1038/ncomms8935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawber CJ, Das I, Francisco E, Kitajewski J. 2003. Notch signaling in primary endothelial cells. Ann NY Acad Sci 995: 162–170. 10.1111/j.1749-6632.2003.tb03219.x [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. 1997. Skeletal and CNS defects in presenilin-1-deficient mice. Cell 89: 629–639. 10.1016/S0092-8674(00)80244-5 [DOI] [PubMed] [Google Scholar]
- Shen W, Huang J, Wang Y. 2021. Biological significance of NOTCH signaling strength. Front Cell Dev Bio 9: 652273. 10.3389/fcell.2021.652273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. 2007. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445: 781–784. 10.1038/nature05577 [DOI] [PubMed] [Google Scholar]
- Simpson MA, Irving MD, Asilmaz E, Gray MJ, Dafou D, Elmslie FV, Mansour S, Holder SE, Brain CE, Burton BK, et al. 2011. Mutations in NOTCH2 cause Hajdu–Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet 43: 303–305. 10.1038/ng.779 [DOI] [PubMed] [Google Scholar]
- Sjöqvist M, Andersson ER. 2019. Do as I say, Not(ch) as I do: lateral control of cell fate. Dev Biol 447: 58–70. 10.1016/j.ydbio.2017.09.032 [DOI] [PubMed] [Google Scholar]
- Solomon RA, Connolly ES Jr. 2017. Arteriovenous malformations of the brain. N Engl J Med 376: 1859-1866. 10.1056/NEJMra1607407 [DOI] [PubMed] [Google Scholar]
- Sörensen I, Adams RH, Gossler A. 2009. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113: 5680–5688. 10.1182/blood-2008-08-174508 [DOI] [PubMed] [Google Scholar]
- Southgate L, Sukalo M, Karountzos ASV, Taylor EJ, Collinson CS, Ruddy D, Snape KM, Dallapiccola B, Tolmie JL, Joss S, et al. 2015. Haploinsufficiency of the NOTCH1 receptor as a cause of Adams–Oliver syndrome with variable cardiac anomalies. Circ Cardiovasc Genet 8: 572–581. 10.1161/CIRCGENETICS.115.001086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner NB. 2000. CADASIL: Notch signaling defect or protein accumulation problem? J Clin Invest 105: 561–562. 10.1172/JCI9511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stittrich AB, Lehman A, Bodian Dale L, Ashworth J, Zong Z, Li H, Lam P, Khromykh A, Iyer RK, Vockley JG, et al. 2014. Mutations in NOTCH1 cause Adams–Oliver syndrome. Am J Hum Genet 95: 275–284. 10.1016/j.ajhg.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. 2009. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114: 5091–5101. 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez E, Bertoli MJ, Eloy JD, Shah SP. 2021. Case report and review of literature of a rare congenital disorder: Adams–Oliver syndrome. BMC Anesthesiol 21: 117. 10.1186/s12871-021-01339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Bréant C, Duarte A, Eichmann A. 2007. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci 104: 3225–3230. 10.1073/pnas.0611177104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M, Foldes G. 2018. It takes two: endothelial-perivascular cell cross-talk in vascular development and disease. Front Cardiovasc Med 5: 154. 10.3389/fcvm.2018.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, Cummins PM, Walls D, Redmond EM, Cahill PA. 2004. Notch 1 and 3 receptors modulate vascular smooth muscle cell growth, apoptosis and migration via a CBF-1/RBP-Jk dependent pathway. FASEB J 18: 1421–1423. 10.1096/fj.04-1700fje [DOI] [PubMed] [Google Scholar]
- Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. 1994. Notch1 is essential for postimplantation development in mice. Genes Dev 8: 707–719. 10.1101/gad.8.6.707 [DOI] [PubMed] [Google Scholar]
- Takeshita K, Satoh M, Ii M, Silver M, Limbourg FP, Mukai Y, Rikitake Y, Radtke F, Gridley T, Losordo DW, et al. 2007. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res 100: 70–78. 10.1161/01.RES.0000254788.47304.6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian DY, Jin XR, Zeng X, Wang Y. 2017. Notch signaling in endothelial cells: is it the therapeutic target for vascular neointimal hyperplasia? Int J Mol Sci 18: 1615. 10.3390/ijms18081615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost A, Lange S, Schroedl F, Bruckner D, Motloch KA, Bogner B, Kaser-EIchberger A, Strohmaier C, Runge C, AIgner L, et al. 2016. Brain and retinal pericytes: origin, function and role. Front Cell Neurosci 10: 20. 10.3389/fncel.2016.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadkar P, Kraman M, Despres D, Ma G, Lozier J, McCright B. 2008. Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle cells. Dev Dyn 237: 1144–1152. 10.1002/dvdy.21502 [DOI] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. 2001. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108: 161–164. 10.1016/S0925-4773(01)00469-5 [DOI] [PubMed] [Google Scholar]
- Vokes SA, Krieg PA. 2002. Endoderm is required for vascular endothelial tube formation, but not for angioblast specification. Development 129: 775–785. 10.1242/dev.129.3.775 [DOI] [PubMed] [Google Scholar]
- Wang W, Campos AH, Prince CZ, Mou Y, Pollman MJ. 2002. Coordinate Notch3-hairy-related transcription factor pathway regulation in response to arterial injury: mediator role of platelet-derived growth factor and ERK. J Biol Chem 277: 23165–23171. 10.1074/jbc.M201409200 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao N, Kennard S, Lilly B. 2012. Notch2 and Notch3 function together to regulate vascular smooth muscle development. PLoS ONE 7: e37365. 10.1371/journal.pone.0037365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pan L, Moens CB, Appel B. 2014. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 141: 307–317. 10.1242/dev.096107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang Z, Tian D, Zeng X, Liu Y, Fu Q, Liang A, Zhang Y, Gao Q, Cheng J, et al. 2018. Integrin β3 mediates the endothelial-to-mesenchymal transition via the Notch pathway. Cell Physiol Biochem 49: 985–997. 10.1159/000493229 [DOI] [PubMed] [Google Scholar]
- Warthen DM, Moore EC, Kamath BM, Morrissette JJ, Sanchez-Lara PA, Piccoli DA, Krantz ID, Spinner NB. 2006. Jagged1 (JAG1) mutations in Alagille syndrome: increasing the mutation detection rate. Hum Mutat 27: 436–443. 10.1002/humu.20310 [DOI] [PubMed] [Google Scholar]
- Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S. 1985. Nucleotide sequence from the neurogenic locus Notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell 43: 567–581. 10.1016/0092-8674(85)90229-6 [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang C. 2009. Neointimal hyperplasia, vein graft remodeling, and long-term patency. Am J Physiol Heart Circ Physiol 297: H1194–H1195. 10.1152/ajpheart.00703.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Iwata F, Grass JA, Osborne CS, Elnitski L, Fraser P, Ohneda O, Yamamoto M, Bresnick EH. 2005. Molecular determinants of NOTCH4 transcription in vascular endothelium. Mol Cell Biol 25: 1458–1474. 10.1128/MCB.25.4.1458-1474.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wang S, Oliveira DV, Del Gaudio F, Vanlandewijck M, Lebouvier T, Betsholtz C, Zhao J, Jin SB, Lendahl U, et al. 2021. The infantile myofibromatosis NOTCH3 L1519P mutation leads to hyperactivated ligand-independent Notch signaling and increased PDGFRB expression. Dis Model Mech 14: dmm046300. 10.1242/dmm.046300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Bhattacharyya A, Roszell EE, Sandig M, Mequanint K. 2012. The role of endothelial cell-bound Jagged1 in Notch3-induced human coronary artery smooth muscle cell differentiation. Biomaterials 33: 2462–2472. 10.1016/j.biomaterials.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Yang SS, Yu DY, Du YT, Wang L, Gu L, Zhang YY, Xiao M. 2020. Inhibition of Delta-like Ligand 4 enhances the radiosensitivity and inhibits migration in cervical cancer via the reversion of epithelial–mesenchymal transition. Cancer Cell Int 20: 344. 10.1186/s12935-020-01434-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. 2005. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435: 98–104. 10.1038/nature03511 [DOI] [PubMed] [Google Scholar]
- Zhang N, Gridley T. 1998. Defects in somite formation in lunatic fringe-deficient mice. Nature 394: 374–377. 10.1038/28625 [DOI] [PubMed] [Google Scholar]
- Zhang P, Yan X, Chen Y, Yang Z, Han H. 2014. Notch signaling in blood vessels: from morphogenesis to homeostasis. Sci China Life Sci 57: 774–780. 10.1007/s11427-014-4716-0 [DOI] [PubMed] [Google Scholar]
- Zhang ZS, Zhou HN, He SS, Xue MY, Li T, Liu LM. 2020. Research advances in pericyte function and their roles in diseases. Chin J Traumatol 23: 89–95. 10.1016/j.cjtee.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]