Abstract
Aims
Pulsed field ablation (PFA) is a novel atrial fibrillation (AF) ablation modality that has demonstrated preferential tissue ablation, including no oesophageal damage, in first-in-human clinical trials. In the MANIFEST-PF survey, we investigated the ‘real world’ performance of the only approved PFA catheter, including acute effectiveness and safety—in particular, rare oesophageal effects and other unforeseen PFA-related complications.
Methods and results
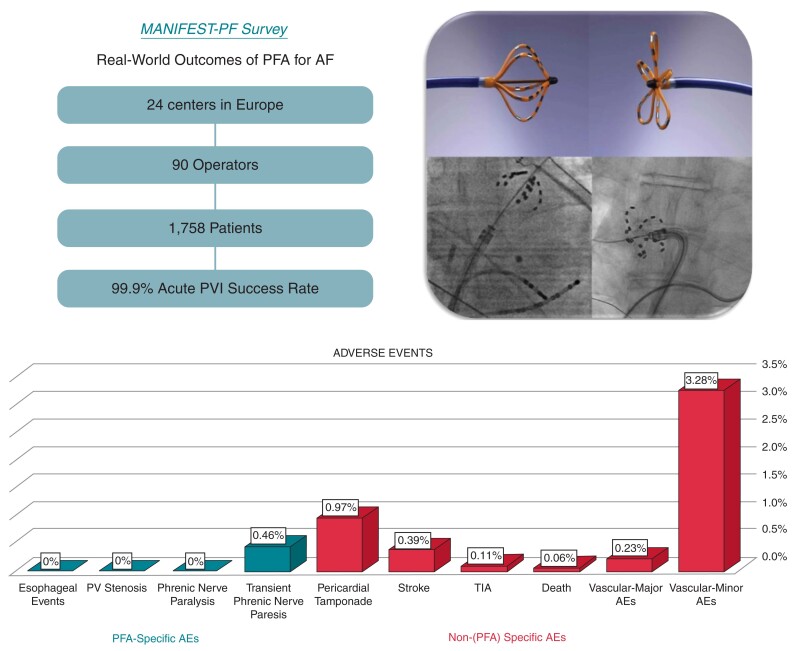
This retrospective survey included all 24 clinical centres using the pentaspline PFA catheter after regulatory approval. Institution-level data were obtained on patient characteristics, procedure parameters, acute efficacy, and adverse events. With an average of 73 patients treated per centre (range 7–291), full cohort included 1758 patients: mean age 61.6 years (range 19–92), female 34%, first-time ablation 94%, paroxysmal/persistent AF 58/35%. Most procedures employed deep sedation without intubation (82.1%), and 15.1% were discharged same day. Pulmonary vein isolation (PVI) was successful in 99.9% (range 98.9–100%). Procedure time was 65 min (38–215). There were no oesophageal complications or phrenic nerve injuries persisting past hospital discharge. Major complications (1.6%) were pericardial tamponade (0.97%) and stroke (0.4%); one stroke resulted in death (0.06%). Minor complications (3.9%) were primarily vascular (3.3%), but also included transient phrenic nerve paresis (0.46%), and TIA (0.11%). Rare complications included coronary artery spasm, haemoptysis, and dry cough persistent for 6 weeks (0.06% each).
Conclusion
In a large cohort of unselected patients, PFA was efficacious for PVI, and expressed a safety profile consistent with preferential tissue ablation. However, the frequency of ‘generic’ catheter complications (tamponade, stroke) underscores the need for improvement.
Keywords: Atrial fibrillation, Pulsed field ablation, Survey, Catheter ablation
Graphical Abstract
Graphical Abstract.
What’s new?
Investigated the real world performance of the only approved pulsed field ablation (PFA) catheter in an unselected patient population.
Largest cohort of patients undergoing PFA.
Cohort included 24 clinical centres, 90 operators, and 1758 patients
In a large cohort of unselected patients, PFA was efficacious for pulmonary vein isolation, and expressed a safety profile consistent with preferential tissue ablation.
Frequency of ‘generic’ catheter complications (tamponade, stroke) underscores the need for improvement.
Introduction
Atrial fibrillation (AF) is the most common sustained heart rhythm disorder with an increasing worldwide prevalence.1 Following seminal studies identifying the pulmonary veins (PVs) as the primary triggering sites of origin for AF, catheter-based electrical isolation of the PVs has emerged as an effective means to treat AF. Both procedural technique and catheter ablation technology—using either radiofrequency, cryothermal or laser energy—have improved over time such that AF is now the most commonly ablated arrhythmia. Indeed, clinical data support its use as first-line therapy to not only improve quality of life, but also mortality in heart failure patients, and, in concert with antiarrhythmic drugs, significantly decrease the rates of stroke and mortality in a broad spectrum of AF patients.2–7
Despite improvements in procedural outcomes, there remain safety considerations including stroke, PV stenosis, phrenic nerve palsy, pericardial tamponade and atrio-oesophageal fistula—the latter being the most dreaded complication because of an ∼50% rate of associated mortality.8 These complications highlight the weak link shared among all thermal energy-based ablation platforms: as the ablative heat (or cold) wave propagates through tissue, its destructive effect is tissue indiscriminate. Thus, collateral damage to surrounding tissue is the potential price paid for ablation transmurality.
In contrast, pulsed field ablation (PFA) is a novel ablation modality that, in pre-clinical and clinical studies, has displayed preferential tissue ablation. Pulsed field ablation involves the application of ultra-rapid (microseconds to nanoseconds) electrical pulses to generate strong electrical fields causing, among other effects, irreversible nanoscale pore formation and, ultimately, cellular death.9 Pre-clinical and clinical studies have demonstrated that by optimizing voltage amplitude, phasic waveforms and pulse sequences, one can completely avoid damage to peri-cardiac structures such as the oesophagus and phrenic nerve.10–15 While there are several PFA catheter technologies in development, the technology with the greatest amount of pre-clinical and clinical evidence is the multielectrode pentaspline PFA catheter—also the only PFA catheter with regulatory approval (CE Mark).
This pentaspline PFA catheter has been studied in both paroxysmal AF patients in the IMPULSE, PEFCAT, and PEFCAT2 trials, and persistent AF patients in the PersAFOne trial.13,16,17 On the one hand, these trials demonstrated that AF ablation was not only feasible and effective, but the theoretical safety benefits observed pre-clinically were indeed realized in clinical practice. This included the absence of: (i) oesophageal damage as evaluated by oesophagoduodenoscopy and thoracic MRI, (ii) phrenic nerve injury, or iii) PV stenosis/narrowing. On the other hand, these studies included only a modest number of patients (<150) and relatively few operators—raising the question of the pentaspline PFA catheter safety profile in a large ‘real world’ environment. Since this technology commenced limited market utilization in March 2021, we conducted the MANIFEST-PF survey of its performance in an unselected patient population in routine clinical practice. We evaluated the catheter’s acute effectiveness and safety, including rare oesophageal effects not discernable after the treatment of only ∼150 patients, and unforeseen rare PFA-related complications.
Methods
Survey overview
MANIFEST-PF is a retrospective survey of all clinical centres performing PFA after regulatory approval of the pentaspline PFA catheter (Farawave, Farapulse-Boston Scientific Inc.). The survey data form was developed by three of the authors (E.E., V.R., and M.T.) with the aim to collect comprehensive data on the methods, efficacy, and safety of the post-approval clinical use of PFA (see Supplementary material online). The survey was approved by the Ethical Committee at Homolka Hospital.
An invitation to participate in MANIFEST-PF survey was sent to all 24 centres performing PFA cases since commercialization; since invitations to participate were sent in January 2022, we included all centres that commenced cases before December 2021. All centres expressing willingness to participate were provided the comprehensive survey data form. Institution-level data were obtained on patient characteristics, procedure parameters, acute efficacy, and adverse events. Data were typically collected from each centre’s ongoing institutional level PFA database. All data forms were submitted with a shared understanding that the identity of physicians and their institutions would remain anonymous.
The pulsed field ablation procedure
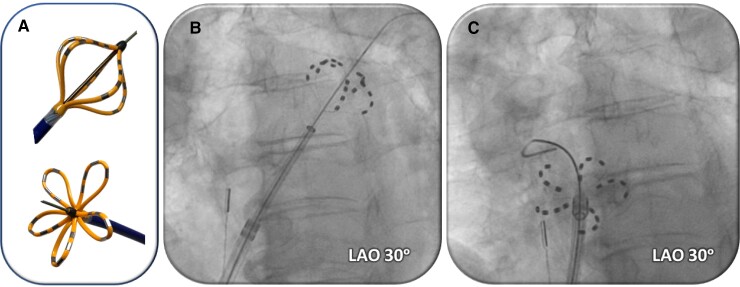
The ablation system has been previously described and, as per company protocol, physicians were trained to employ a standard protocol at all clinical sites.13,16,17 Briefly, the 12-Fr over-the-wire pentaspline PFA catheter (Farawave) is advanced through a 13-Fr steerable sheath with a transparent shaft (Faradrive) into the left atrium (LA). After positioning the recommended straight-tip 0.035 guidewire (Amplatz extra stiff straight wire; Cook Inc.) into each target PV, the PFA catheter is positioned at the ostium of each PV and a total of eight PF lesions are applied per vein: four each in ‘basket’ and ‘flower’ configurations, with rotation (halfway = 36°) between each pair of lesions (Figure 1). For posterior LA wall ablation, the catheter was placed into a flower configuration and positioned along the posterior LA to deliver overlapping sets of pulses at each location.17 The PF voltage amplitude could range between 1.8 and 2.0 kV, but 2.0 kV was typically employed. There was no oesophageal ‘management strategy’ employed during the PFA procedures—that is, unlike with thermal ablation, wherein strategies can include: reduced ablation energy application along the posterior LA wall, guidance by oesophageal temperature monitoring, mechanical oesophageal deviation, or oesophageal cooling.
Figure 1.
PFA catheter. (A) Pentaspline PFA catheter in basket (top) and flower (bottom) configurations. (B and C) Fluoroscopic images of pentaspline PFA catheter over a guidewire in the left superior (B) or right superior (C) pulmonary veins.
Survey data acquisition
The survey data form was compromised of 30 questions covering the following areas; geographical region, clinical site characteristics, baseline characteristics of PFA patients, procedural parameters, type of imaging utilized, electroanatomical mapping, additional non-PV isolation (PVI) lesion sets, and adverse events (see Supplementary material online). Major complications were defined as death, oesophageal fistula/dysmotility, PV stenosis, pericardial tamponade, stroke, phrenic nerve injury (persistent), vascular complications requiring intervention, and coronary artery spasm. If a major adverse event was identified (specifically pericardial tamponade or stroke), a root cause analysis questionnaire was sent to the clinical site (see Supplementary material online). The root cause analysis form collected information on the event details and procedure sequence at time of complication, hypothesis of most likely or clearly identified aetiology and recommendations to prevent future complications.
Data analysis and statistics
The survey data form was considered complete if at least 80% of the questions were answered. Institution-level continuous variables were reported as means with minimum and maximum values provided. Categorical variables were reported as absolute numbers and percentages. Procedural characteristics (specifically: imaging utilized, use of electroanatomical mapping, and additional non-PVI lesion sets) were reported qualitatively as never, sometimes, frequently and always. Statistical analysis was mainly descriptive with averages weighted based on the PFA volume of the clinical site as a percentage of the total cohort. A χ2 test was used to assess for a learning curve.
Results
Baseline characteristics
Clinical site characteristics
There was a 100% response rate from the 24 clinical centres with all data forms considered complete. Clinical centres were located in nine European Union countries currently performing post-approval PFA cases. As shown in Table 1, most clinical sites classified themselves as academic (70.8%), 20.8% were private and 8.3% were semi-academic. The mean number of operators/centre was 3.8 (range 2–11), with an average of 13.2 years in practice (range 5.3–22.5). The annual number of AF ablations/centre performed annually was 704 (range 300–2200). On average, the clinical sites performed their first PFA case in July 2021 (range March 2021–December 2021).
Table 1.
Clinical site characteristics
| Clinical Site Characteristics | N (24) |
|---|---|
| Practice type | |
| Academic (%) | 70.8 |
| Semi-academic (%) | 8.3 |
| Private (%) | 20.8 |
| No. of operators, mean (min–max) | 3.8 (2–11) |
| Years in practice, mean (min–max) | 13.2 (5.3–22.5) |
| Annual no. of AF ablations, mean (min–max) | 704 (300–2200) |
| No. of PFA cases in past year, mean (min–max) | 73.3 (7–291) |
| Date of first PFA case, month/year (earliest–latest) | 7/2021 (3/2021–12/2021) |
PFA, pulsed field ablation
Baseline patient characteristics
The cohort included 1758 patients who underwent PFA between March 2021 and January 2022. The mean age was 61.6 years (range 19–92) with a mean BMI of 26.7 (range 15–52) and 34.2% were female (Table 2). The mean CHA2DS2-VASc score was 2.1 (0–9) and mean LA diameter was 39 mm (range 16–73). This was the first ablation procedure for 93.5% of patients. The type of AF treated was paroxysmal (57.5%), persistent (35.2%), or long standing persistent (3.9%) with a subset of patients classified as repeat AF ablation or other LA flutters (2.9%). Atrial flutter was also treated in 1.1% of patients. The mean left ventricular ejection fraction (LVEF) was 54.7% (range 14–80), with 4.7% of patients having an LVEF < 40%. A Class I, II, III, or IV antiarrhythmic drug had failed in 65.4% of patients before PFA.
Table 2.
Baseline patient characteristics
| Baseline patient characteristics | N = 1758 patients |
|---|---|
| Demographics | |
| Age (years), mean (min–max) | 61.6 (19–92) |
| Female, % | 34.2% |
| BMI (kg/m2), mean (min–max) | 26.7 (15–52) |
| CHA2DS2-Vasc score, mean (min–max) | 2.1 (0–9) |
| Echocardiography parameters | |
| Left atrial diameter (mm), mean (min–max) | 39 (16–73) |
| LVEF (%), mean (min–max) | 54.7% (14–80) |
| Patients with LVEF < 40%, % | 4.7% |
| Past medical history | |
| Hypertension (%) | 59.1% |
| Diabetes mellitus (%) | 12.7% |
| Congestive heart failure (%) | 12.3% |
| Coronary artery disease (%) | 12.7% |
| Stroke/TIA | 6.2% |
| Medications | |
| Failed Class I or III antiarrhythmic drug (%) | 38.2% |
| Failed Class I, II, III or IV antiarrhythmic drug (%) | 65.4% |
| Vitamin K antagonist (%) | 5.1% |
| NOAC (%) | 88.3% |
| No prior oral anticoagulation (%) | 7.7% |
| Indication for ablation | |
| Paroxysmal atrial fibrillation (%) | 57.5% |
| Persistent atrial fibrillation (%) | 35.2% |
| Long standing persistent atrial fibrillation (%) | 3.9% |
| Atrial flutter (%) | 1.1% |
| Other (%) | 2.9% |
| First-ever ablation procedure (%) | 93.5% |
LVEF, left ventricular ejection fraction; NOAC, non-vitamin K antagonist oral anticoagulants.
Procedural parameters and characteristics
As shown in Table 3, most procedures used deep sedation without endotracheal intubation (82.1%), and 15.1% were discharged on the same day as the procedure. One transeptal puncture was utilized (100%), and the acute PVI success rate was 99.9% (range 98.9–100). The mean procedure time was 65 min (range 38–215), inclusive of pre- and/or post-ablation electroanatomical mapping in some patients. The fluoroscopy time was 13.7 min (range 4.5–33).
Table 3.
Procedural parameters
| Procedural parameters | Percentages (%) |
|---|---|
| General anaesthesia/intubation (%) | 17.8% |
| Deep sedation/no intubation (%) | 82.1% |
| No. of transeptal punctures, n (%) | 1 (100%) |
| PVI success rate (%), mean (min–max) | 99.9% (98.9–100) |
| Procedure time (min), mean (min–max) | 65 (38–215) |
| Fluoroscopy time (min), mean (min–max) | 13.7 (4.5–33) |
| Same day discharge (%) | 15.8% |
As shown in Table 4, pre-procedural imaging was obtained in some patients and when performed, the imaging modality was more likely to be cardiac computed tomography (CT), then transoesophageal echocardiography (TEE), and rarely magnetic resonance imaging (MRI). Intra-procedural imaging was utilized in all patients with fluoroscopy in the majority of patients, and intracardiac echocardiography (ICE) or TEE in a subset of patients. Follow-up imaging was obtained infrequently.
Table 4.
Procedural characteristics
| Procedural characteristics | Never | Sometimes | Frequently | Always |
|---|---|---|---|---|
| Pre-procedural imaging | ||||
| TEE (%) | 25 | 37.5 | 12.5 | 25 |
| CT (%) | 25 | 29.2 | 12.5 | 33.3 |
| MRI (%) | 70.8 | 25 | 4.2 | 0 |
| Intra-procedural imaging | ||||
| TEE (%) | 58.3 | 33.3 | 0 | 8.3 |
| ICE (%) | 69.6 | 8.7 | 0 | 21.7 |
| Fluoroscopy (%) | 0 | 4.3 | 4.3 | 91.3 |
| Follow-up imaging | ||||
| TEE (%) | 73.9 | 21.7 | 4.3 | 0 |
| CT (%) | 86.9 | 13.1 | 0 | 0 |
| MRI (%) | 73.9 | 26.1 | 0 | 0 |
| Electroanatomical mapping | ||||
| Paroxysmal AF (%) | 41.6 | 16.6 | 8.3 | 33.3 |
| Persistent AF (%) | 29.2 | 8.3 | 20.8 | 41.7 |
| Long standing persistent AF (%) | 37.5 | 4.2 | 8.3 | 41.7 |
| Additional lesion sets | ||||
| Roof line (%) | 50 | 25 | 12.5 | 12.5 |
| Lateral mitral isthmus line (%) | 62.6 | 33.2 | 4.2 | 0 |
| Left atrial posterior wall (%) | 25 | 45.8 | 16.7 | 12.5 |
| Anterior line (%) | 75 | 25 | 0 | 0 |
| SVC isolation (%) | 95.8 | 4.2 | 0 | 0 |
| CFAE (%) | 95.8 | 4.2 | 0 | 0 |
| LAA isolation (%) | 93.8 | 6.2 | 0 | 0 |
| Non-PV trigger (%) | 79.2 | 20.8 | 0 | 0 |
TEE, transoesophageal echocardiogram; CT, computed tomography; MRI, magnetic resonance imaging; AF, atrial fibrillation; SVC, superior vena cava; CFAE, complex fractionated atrial electrograms; LAA, left atrial appendage; Non-PV, non-pulmonary vein.
Electroanatomical mapping was performed in a subset of patients and more likely in PFA cases for persistent and long-standing persistent AF. Additional non-PVI lesion sets were performed in a subset of patients. When employed, the most common additional lesion sets, albeit not frequently, were ablation along the LA roof and LA posterior wall ‘box’ isolation, and rarely, ablation at the posterior mitral annulus.
Adverse events
Overall safety overview
As shown in Table 5, in the 1758-patient cohort, the major complication rate was 1.6%. These major complications were primarily pericardial tamponades, and to lesser extent stroke and vascular complications; mortality was rare, occurring in one patient (0.06%). The minor complication rate was 3.86%, consistent primarily of vascular complications (3.28%), but also did include two patients (0.11%) with TIAs.
Table 5.
Adverse events
| N (%) | |
|---|---|
| Major complications | 29 (1.6%) |
| Oesophageal fistula | 0 |
| Oesophageal dysmotility | 0 |
| Pulmonary vein stenosis | 0 |
| Pericardial tamponade | 17 (0.97) |
| Percutaneous treatment | 13 (0.74) |
| Surgical treatment | 4 (0.23) |
| Stroke | 7 (0.39)a |
| Phrenic nerve injury (persistent) | 0 |
| Vascular complications requiring surgery | 4 (0.23) |
| Coronary artery spasm | 1 (0.06) |
| Death | 1 (0.06)a |
| Minor complications | 68 (3.86%) |
| TIA | 2 (0.11) |
| Phrenic nerve injury (transient) | |
| Transient effect | 8 (0.46) |
| Sustained effectb | 0 |
| Vascular | |
| Hematoma | 43 (2.44) |
| Pseudoaneurysm | 4 (0.22) |
| AV fistula | 3 (0.17) |
| Other | 6 (0.34) |
| Other complications | 2 (0.11) |
One patient who sustained a stroke subsequently died.
Defined as persisting beyond hospital discharge.
Pulsed field ablation-specific adverse events
As shown in Table 5, even though no centre employed an oesophageal management strategy, there were no post-PFA oesophageal complications, including no instances of oesophageal ulcerations, atrio-oesophageal fistula or oesophageal dysmotility disorders. There were no instances of clinical PV stenosis, though it should be recognized that routine follow-up PV imaging was infrequently performed.
Persistent phrenic nerve injury did not occur in any patient. However, transient phrenic nerve paresis occurred in 0.46% (n = 8) of patients. There was near immediate recovery (within a few minutes) in three patients, and five patients had recovery by the next day. No patient had phrenic nerve injury that persisted beyond hospital discharge.
Non-pulsed field ablation-specific adverse events
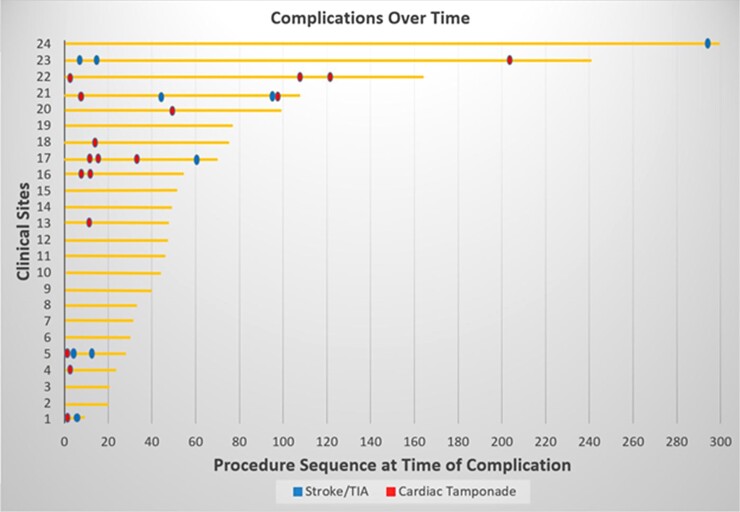
Pericardial tamponade occurred in 0.97% of patients. Most of these tamponades (0.74%) were managed with percutaneous drainage, but a substantial minority (0.23%) required surgical treatment. The other major complication, stroke, occurred in 0.39% of patients, with one stroke culminating in death (0.06%). When these complications were plotted on a timeline (Figure 2), these appeared to be a trend to fewer complications with centre experience, particularly for pericardial tamponades. That is, broken down by centre into tertiles, the majority of the tamponades (11 of 17, 65%) occurred in the first tertile, as opposed to the second and third tertiles (2 of 17, 12% and 4 of 17, 24%, respectively; P = 0.019).
Figure 2.
Complications timeline. Shown are the pericardial tamponades and strokes occurring at each centre as a sequence at the time of event. The horizontal lines represent the number of patients treated at each of the 24 centres.
Pericardial tamponade
In the root cause analysis of pericardial tamponade (Table 6), the most common cause was related to the recommended extra stiff straight guidewire used to deliver the PFA catheter. Inadvertent left atrial appendage (LAA) perforation occurred with this guidewire while attempting to engage the PVs in four patients; the involved clinical sites universally transitioned to using a J-tip wire with no subsequent cases of pericardial tamponade. The locking mechanism between the dilator and sheath was also noted to be tight enough such that unlocking the dilator could lead to sudden inadvertent forward advancement of the sheath.
Table 6.
Root cause analyses of complications
| No. of events, n (%) | |
|---|---|
| RCA for pericardial tamponade (n = 17 patients) | |
| Transeptal puncture | 2 (11.8) |
| Sheath manipulation in LA | 1 (5.9) |
| Guidewire related (straight extra stiff) | 4 (23.5) |
| Non-PFA energy (RF ablation) | 1 (5.9) |
| Coronary sinus perforation | 2 (11.8) |
| RV perforation (pacing catheter) | 3 (17.6) |
| ‘Experience-related’ | 1 (5.9) |
| No identifiable cause | 3 (17.6) |
| RCA for stroke (n = 7 patients) | |
| Catheter exchanges/sheath management | 3 (42.9) |
| Procedure duration/complexity | 2 (28.6) |
| Interrupted anticoagulation | 1 (14.3) |
| No definitive cause identified | 1 (14.3) |
Perforation also occurred unrelated to the PFA system: (i) In three patients, right ventricular perforation occurred because of a steerable decapolar catheter used for back-up ventricular pacing. (ii) The transeptal puncture was the culprit in two patients. In one patient, the left atrial anatomy was noted to be complex with a long anterior to posterior axis and an aneurysmal posterior wall, also noted during LA electroanatomical mapping as no pre-procedural imaging had been obtained. In both cases, neither TEE nor ICE imaging was employed to guide transeptal puncture, as this was not standard of care at this clinical site. (iii) In two patients, coronary sinus cannulation was difficult, resultant perforation. (iv) In one patient, pericardial tamponade occurred after a steam pop during RF ablation of the cavo-tricuspid isthmus. No identifiable cause was noted in three cases.
Stroke
In the root cause analysis, stroke was attributed to catheter exchanges and management of the 13-Fr sheath in three of seven cases; that is, despite the usage of the transparent sheath, air or thrombus was inadvertently introduced and embolized into the circulation, presumably because of insufficient aspiration and flushing with saline during catheter introductions and exchanges. Extended procedure duration and complexity were identified as a contributor to stroke in two patients. For example, one case involved complex ablation for peri-mitral atrial tachycardia with failure of RF ablation at the LA endocardium and within the coronary sinus despite use of half-normal saline and ethanol injection into the Vein of Marshall, and failure of left PVI, culminating in ‘rescue’ PFA with successful PVI. At the end of the ∼200 min procedure, the patient had right facial palsy with a small acute cerebral lesion demonstrated on CT.
In one patient, interruption of the non-vitamin K antagonist oral anticoagulant was hypothesized as possibly contributing to stroke, though this was the standard practice at this clinical site. However, this patient was also incidentally diagnosed with adenocarcinoma on a diagnostic chest CT scan which will require surgical resection. Hypercoagulability in the setting of active malignancy may have played a role.
Finally, one patient died as a result of the stroke: in this 81-year-old female with reduced LV function, during PVI with the PFA catheter, the right inferior PV was reportedly small, requiring additional time for cannulation. The patient had delayed awakening post-ablation, prompting consultation with the neurology service. The neurologist initially noted no evidence of stroke, but due to the absence of clinical improvement a CT head was obtained—this revealed a large cerebral infarction, prompting immediate successful thrombectomy. Unfortunately, the patient had poor neurological recovery and died a few days later. Autopsy revealed a normal transeptal puncture, and no unexpected cardiac damage, thrombus, or visible ablation lesions. Evaluation of the brain showed the expected large cerebral infarction.
Vascular complications
As shown in Table 5, the overall most frequent complications were of vascular aetiology. The majority of these (3.28%) was minor complications treated conservatively—most commonly haematomas (2.44%). But there also were some major vascular complications requiring surgical repair (0.23%).
Unusual adverse events
There were three unusual complications, one major and two minor, representing a rate of 0.06% each. The first was a patient presenting with coronary artery spasm during PFA along the posterior mitral isthmus region, resolving after the administration of intracoronary nitroglycerin; the details of this major complication have been published as a case report.18 Second, a 78-year-old multimorbid patient with known heart failure presented with intra-procedural haemoptysis requiring bronchoscopy and suction; the remaining course until hospital discharge was uneventful. Finally, there was one patient with a dry cough persistent for 6 weeks before spontaneously resolving.
Discussion
The MANIFEST-PF multinational survey included a cohort of 1758 consecutive unselected AF patients from all sites performing PFA with the pentaspline catheter as routine clinical practice. The major findings are as follows: (i) PFA is being employed to treat both paroxysmal and persistent AF patients, most frequently as a first-ever procedure, (ii) the acute success of PVI was good at 99.9%, (iii) there a favourable procedural workflow with >80% of cases performed without endotracheal intubation, and mean procedure time of ∼1 h, (iv) no oesophageal complications, symptomatic PV stenosis, or phrenic nerve injury persisting beyond hospital discharge—confirming the tissue preferentiality of PFA, (v) the occurrence of other non-PFA-related complications including a 1.6% rate of major complications, largely pericardial tamponade and stroke, and a 3.86% rate of minor complications, largely of vascular origin, (vi) a rare complication of coronary vasospasm was observed after PFA at the posterior mitral annulus (rate of 0.06%), and (vii) the overall procedure-related mortality was low at 0.06%.
Acute performance and workflow
Even in the hands of multiple operators, the vast majority of whom was using the pentaspline PFA catheter for the first time, there was a high acute success rate for PVI (99.9%). This is consistent with the first-in-human clinical trials with this PFA catheter, where PVI was achieved in 100%.16,17 Indeed, the overall procedure times from the MANIFEST-PF survey, 65 min (range 38–215), was similar to that observed in the first-in-human PFA trials, 96.2 ± 30.3 min; the discrepancy is most likely explained by the protocol-mandated pre-/post-PFA electroanatomical mapping and post-PVI waiting periods required in the latter trials. Similarly, the fluoroscopy times were nearly identical at 13.7 min (range 4.5–33) vs. 13.7 ± 7.8 min in the MANIFEST-PF survey and first-in-human trials, respectively.
From the perspective of procedural workflow, just as with the first-in-human trials, the majority of MANIFEST-PF survey cases was performed under deep sedation without endotracheal intubation (82.1%), all with a single transeptal puncture, and 15.1% of patients were discharged the same day as the procedure. The treated population spanned the spectrum of both chronicity of AF, with ∼40% of the cohort having non-paroxysmal AF, and comorbidities, including hypertension in ∼60%, and subsets of patients, 12.3 and 4.7% respectively, having a history of heart failure or an LVEF < 40%.
Tissue specificity of pulsed field ablation
In this ‘real world’ setting, the preferentiality of tissue susceptibility to ablation by pulsed electrical fields was evidenced by the absence of oesophageal complications, PV stenosis, and persistent phrenic nerve injury. This is consistent with prior pre-clinical and clinical studies.
Oesophagus
Oesophageal sparing was first demonstrated in porcine open-chest experiments using a different PFA system, wherein 200 Joule PF applications were delivered directly atop oesophagus: the only effects were intraepithelial vesicles in the oesophageal adventitia on Day 2 with complete normalization by Day 7.10 Using the pentaspline PFA catheter in a porcine model where the oesophagus was purposely mechanically deflected to be apposed to the inferior vena cava, we compared RF ablation to PFA; this revealed that biphasic PFA induced no chronic histopathologic oesophageal changes, while RFA resulted in varying levels of oesophageal injury including deep oesophageal ulcers, abscesses, and fistula.14
Consistent with the first-in-human PFA trials which demonstrated no oesophageal damage by both oesophagogastroduodenoscopy and MRI, the MANIFEST-PF survey identified no patients with clinical oesophageal effects—including no atrio-oesophageal fistula or dysmotility. It is striking that >1700 patients received PFA without any oesophageal ‘management’ strategy, and yet there were zero oesophageal complications—though the possibility of even rarer oesophageal damage cannot be absolutely ruled out.
Phrenic nerve injury
The risk of phrenic nerve injury with PFA has also been assessed in animal studies: (i) first with the application of a 200-Joule using a circular catheter directly within the superior vena cava, demonstrating immediate post-PFA phrenic capture in 17 of 19 animals and in all animals within 30 min,19 and (ii) with the pentaspline PFA catheter using either monophasic or biphasic waveforms.12 Similarly, there were no instances of phrenic nerve injury that persisted beyond hospital discharge. On the other hand, transient phrenic nerve paresis did occur in 0.46% of patients, all regaining normal function by the next day post-procedure. Whether this represents axonal necrosis or rather an electrical hyperpolarization is unknown—though the speed of recovery suggests the latter mechanism might be more likely.
Pulmonary vein stenosis
Beyond the pre-clinical data, in a combined analysis of four paroxysmal AF trials utilizing either PFA or RFA, PV stenosis or even narrowing was absent (0%) in the PFA cohort.20 On the other hand, after RFA, PV stenosis/narrowing was observed in 12 and 32.5% of PVs and patients, respectively.20 As compared with these first-in-human trials where PFA was performed by a small number of expert operators, there was a wide range of operator expertise with varying levels of experience (range 5.3–22.5 years) in the MANIFEST-PF cohort, but again with no instance of clinical PV stenosis. Of course, because systematic PV imaging was not performed during follow-up, we cannot rule out the possibility of asymptomatic PV stenosis.
Non-PF energy related complications
The rate of major complications was 1.6% and primarily consisted of pericardial tamponade (0.97%) and stroke (0.39%), with the only procedural mortality (0.06%) related to a stroke. While these rates are not trivial, these data should be viewed in the proper context. First, virtually all of the 90 operators included in the MANIFEST-PF survey had never used this PFA catheter beforehand, so the entire learning curve was captured within this survey—though it should be noted that these physicians were from expert centres commencing PFA. Second, for comparison, these rates are less than the rates of pericardial complications (1.52%), stroke (1.02%) and mortality (0.42%) in a large database analysis of in-hospital complications of AF ablation between 2000 and 2010 in the USA.21. Furthermore, in a contemporary analysis using the Nationwide Readmissions Database between 2010 and 2015, the early mortality following AF ablation was reportedly 0.46%.22 Indeed, the observed rate of procedure-related mortality of <1 in 1000 in the MANIFEST-PF survey compares quite favourably.
In a subset of the MANIFEST-PF cohort, 114 patients at three clinical sites, routine post-procedural brain MRI was performed to assess for asymptomatic brain embolization. As shown in Supplementary material online, Table S1, asymptomatic MRI abnormalities were identified in 20 patients (17.5%). The clinical significance of asymptomatic brain lesions remains unclear as there were no neurological abnormalities reported. Indeed, MRI-detected brain lesions after AF ablation or other catheter-based cardiovascular procedures are not uncommon, with reported rates as high as 43.2% in the multicentre prospective Mesh Ablator vs. Cryoballoon Pulmonary Vein Ablation of Symptomatic Paroxysmal AF study (MACPAF) trial.23–26
Root cause analysis
The root causes analysis revealed that most of the pericardial tamponades and strokes were attributable to catheter workflow and manipulation, independent of the energy modality. Manipulation of the extra-stiff straight-tip guidewire during pentaspline catheter positioning resulted in perforation of the LAA or PVs in four patients. Accordingly, the clinical sites evolved their practice, opting to utilize a J-tip guidewire for subsequent procedures—reporting no further tamponades. The possibility that additional operator experience will result in fewer tamponades is suggested by the timeline indicating an improvement in complication rates over time. Furthermore, a number of the tamponades were related to placement of the coronary sinus catheter into the right ventricle for back-up pacing. Interestingly, many sites have recently been switching to the administration of the vagolytic, atropine, at procedure onset, thereby obviating the need for back-up ventricular pacing.
Similarly, suboptimal transeptal sheath management was identified as a significant contributor to stroke. In order to avoid inadvertent embolization of air or thrombus, diligent saline aspiration/flushing is required with every catheter introduction or exchange, with carful inspection of the transparent sheath. The importance of this recommendation is underscored by the fact that the single mortality in this cohort was related to thromboembolism.
Rare complications
A strength of including so many patients in this survey is the ability to identify potentially rare complications. Indeed, a few such events did occur. As previously reported, one patient developed coronary vasospasm with associated ST segment elevation during mitral isthmus PFA, subsequently relieved with intracoronary nitroglycerin.18 This phenomenon of transient coronary spasm has been previously reported in pre-clinical studies.27 On the other hand, it should be recognized that coronary arterial injury may also occur with radiofrequency ablation at the mitral isthmus. In a study of 54 patients who underwent mitral isthmus ablation, 28% of the cohort developed sub-clinical angiographic changes of the circumflex artery, and a subset had significant coronary narrowing (50–84%) which resolved with intracoronary nitroglycerin.28
There was also a rare case of haemoptysis in a patient with heart failure, requiring bronchoscopy. The mechanism of this finding is unknown, but may be related not to PFA, but rather the guidewire. That is, in a prospective series of 30 patients, routine bronchoscopy was performed after PVI with the pentaspline PFA catheter. While there were no visible thermal lesions or ulcers, there were small blood clots in multiple distal segments in 12 patients (40%), without associated haemoptysis, chest discomfort or cough.29 Since the distal localization of the clots largely rules out a direct bronchial effect of PFA, the authors postulated that this was likely related to trauma from the straight-tip guidewire. On the other hand, it bears mentioning that the impact of CT-guided irreversible electroporation from within the bronchi has been evaluated in a pre-clinical study. A small amount of needle tract bleeding with mucosal injury was noted, and pathological examinations revealed necrotic vascular epithelial cells in the region of ablation, albeit with tissue normalization after 14 days.30 Whether this is clinically relevant should be the subject of future studies.
Limitations
This survey reports on the acute effects of PFA in patients with AF. The long-term clinical efficacy of the procedure cannot be judged from the present dataset. This was a retrospective survey of centre-level data; thus, the reported adverse events and outcomes were not prospectively defined. Moreover, the absence of patient-level data limits this survey’s granularity. Complications that may have occurred outside the acute treatment phase have not been systematically assessed and may have been missed. However, the high compliance rate (100%) for data acquisition, the comprehensive nature of the data acquired (majority from prospective institutional databases), and the spectrum of adverse events reported all extends credibility to the study.
Conclusion
This is the first study of the methods, acute efficacy, and safety of the post-regulatory approval use of the pentaspline PFA catheter—the first clinically approved PFA catheter to treat AF. In an unselected AF patient population in routine practice, PFA was efficacious for PVI, and expressed a safety profile consistent with preferential tissue ablation. However, the significant incidence of ‘generic’ catheter complications, particularly pericardial tamponade and stroke, underscores that there remains room for improvement.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Contributor Information
Emmanuel Ekanem, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Vivek Y Reddy, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Cardiology Department, Na Homolce Hospital, Homolka Hospital, Roentgenova 37/2, 15030 Praha 5 Prague, Czech Republic.
Boris Schmidt, MVZ CCB Frankfurt und Main-Taunus GbR, Frankfurt, Germany.
Tobias Reichlin, Inselspital—Bern University Hospital, University of Bern, Bern, Switzerland.
Kars Neven, Department of Electrophysiology, Alfried Krupp Hospital, Essen, Germany; Department of Medicine, Witten/Herdecke University, Witten, Germany.
Andreas Metzner, University Heart and Vascular Center, University of Hamburg, Hamburg, Germany.
Jim Hansen, Copenhagen University Hospital, Copenhagen, Denmark.
Yuri Blaauw, Universitair Medish Groningen, Groningen, The Netherlands.
Philippe Maury, Department of Cardiology, University Hospital Rangueil, Toulouse, France; I2MC, INSERM UMR 1297, Toulouse, France.
Thomas Arentz, Universitätsklinikum Freiburg, Breisgau, Germany.
Philipp Sommer, Clinic for Electrophysiology, Herz- und Diabeteszentrum NRW, Ruhr-University Bochum, Bad Oeynhausen, Germany.
Ante Anic, Department for Cardiovascular Diseases, University Hospital Center Split, Split, Croatia.
Frederic Anselme, Department of Cardiology, Rouen Hospital, Rouen, France.
Serge Boveda, Heart Rhythm Department, Clinique Pasteur, Toulouse, France; Universitair Ziekenhuis VUB, Brussels, Belgium.
Tom Deneke, Heart Center Bad Neustadt, Rhoen-Clinic Campus Bad Neustadt, Bad Neustadt an der Saale, Germany.
Stephan Willems, Asklepios Hospital St Georg, Hamburg, Germany.
Pepijn van der Voort, Department of Cardiology, Catharina Ziekenhuis Eindhoven, Eindhoven, The Netherlands.
Roland Tilz, Department of Rhythmology, University Heart Center, Lubeck, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany; LANS Cardio, Hamburg, Germany.
Moritoshi Funasako, Cardiology Department, Na Homolce Hospital, Homolka Hospital, Roentgenova 37/2, 15030 Praha 5 Prague, Czech Republic; Neuron Medical, Brno, Czech Republic.
Daniel Scherr, Medical University of Graz, Graz, Austria.
Reza Wakili, Department of Cardiology and Vascular Medicine, West German Heart and Vascular Center Essen, University Duisburg-Essen, Duisburg, Germany.
Daniel Steven, Universitätsklinikum Köln AöR, Köln, Germany.
Josef Kautzner, IKEM—Institute for Clinical and Experimental Medicine, Prague, Czech Republic.
Johan Vijgen, Department of Cardiology, Jessa Hospitals, Hasselt, Belgium.
Pierre Jais, IHU LIRYC, CHU Bordeaux, University of Bordeaux, Bordeaux, France.
Jan Petru, Cardiology Department, Na Homolce Hospital, Homolka Hospital, Roentgenova 37/2, 15030 Praha 5 Prague, Czech Republic.
Julian Chun, MVZ CCB Frankfurt und Main-Taunus GbR, Frankfurt, Germany.
Laurent Roten, Inselspital—Bern University Hospital, University of Bern, Bern, Switzerland.
Anna Füting, Department of Electrophysiology, Alfried Krupp Hospital, Essen, Germany; Department of Medicine, Witten/Herdecke University, Witten, Germany.
Andreas Rillig, University Heart and Vascular Center, University of Hamburg, Hamburg, Germany.
Bart A Mulder, Universitair Medish Groningen, Groningen, The Netherlands.
Arne Johannessen, Copenhagen University Hospital, Copenhagen, Denmark.
Anne Rollin, Department of Cardiology, University Hospital Rangueil, Toulouse, France.
Heiko Lehrmann, Universitätsklinikum Freiburg, Breisgau, Germany.
Christian Sohns, Clinic for Electrophysiology, Herz- und Diabeteszentrum NRW, Ruhr-University Bochum, Bad Oeynhausen, Germany.
Zrinka Jurisic, Department for Cardiovascular Diseases, University Hospital Center Split, Split, Croatia.
Arnaud Savoure, Department of Cardiology, Rouen Hospital, Rouen, France.
Stephanes Combes, Heart Rhythm Department, Clinique Pasteur, Toulouse, France; Universitair Ziekenhuis VUB, Brussels, Belgium.
Karin Nentwich, Heart Center Bad Neustadt, Rhoen-Clinic Campus Bad Neustadt, Bad Neustadt an der Saale, Germany.
Melanie Gunawardene, Asklepios Hospital St Georg, Hamburg, Germany.
Alexandre Ouss, Department of Cardiology, Catharina Ziekenhuis Eindhoven, Eindhoven, The Netherlands.
Bettina Kirstein, Department of Rhythmology, University Heart Center, Lubeck, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany; LANS Cardio, Hamburg, Germany.
Martin Manninger, Medical University of Graz, Graz, Austria.
Jan Eric Bohnen, Department of Cardiology and Vascular Medicine, West German Heart and Vascular Center Essen, University Duisburg-Essen, Duisburg, Germany.
Arian Sultan, Universitätsklinikum Köln AöR, Köln, Germany.
Petr Peichl, IKEM—Institute for Clinical and Experimental Medicine, Prague, Czech Republic.
Pieter Koopman, Department of Cardiology, Jessa Hospitals, Hasselt, Belgium.
Nicolas Derval, IHU LIRYC, CHU Bordeaux, University of Bordeaux, Bordeaux, France.
Mohit K Turagam, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Petr Neuzil, Cardiology Department, Na Homolce Hospital, Homolka Hospital, Roentgenova 37/2, 15030 Praha 5 Prague, Czech Republic.
Funding
There was no specific funding received to conduct this survey.
Data Availability
The data underlying this article cannot be shared publicly to maintain the anonymity of the participating centres.
References
- 1. Atrial fibrillation. Centers for Disease Control and Prevention. 2021 Sep 27 [accessed 2022 Jan 22]. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm.
- 2. Turagam MK, Musikantow D, Whang W, Koruth JS, Miller MA, Langan Net al. . Catheter ablation or antiarrhythmic drugs for first-line therapy of atrial fibrillation: a meta-analysis of randomized controlled trials. JAMA Cardiol 2021;6:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KHet al. . Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical Trial. JAMA 2019;321:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens Let al. . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 5. Turagam MK, Garg J, Sartori S, Dukkipati SR, Reddy VY. Catheter ablation of atrial fibrillation in patients with heart failure. Ann Intern Med 2019;171:76–7. [DOI] [PubMed] [Google Scholar]
- 6. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PAet al. . Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation 2021;143:1377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 8. Singh SM, d’Avila A, Singh SK, Stelzer P, Saad EB, Skanes Aet al. . Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm 2013;10:1591–97. [DOI] [PubMed] [Google Scholar]
- 9. Kotnik T, Rems L, Tarek M, Miklavčič D. Membrane electroporation and electropermeabilization: mechanisms and models. Annu Rev Biophys 2019;48:63–91. [DOI] [PubMed] [Google Scholar]
- 10. Neven K, van Es R, van Driel V, van Wessel H, Fidder H, Vink Aet al. . Acute and long-term effects of full-power electroporation ablation directly on the porcine esophagus. Circ Arrhythm Electrophysiol 2017;10:e004672. [DOI] [PubMed] [Google Scholar]
- 11. Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky Iet al. . Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol 2018;4:987–95. [DOI] [PubMed] [Google Scholar]
- 12. Koruth JS, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose Ret al. . Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythm Electrophysiol 2019;12:e007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet Het al. . Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 14. Koruth JS, Kuroki K, Kawamura I, Brose R, Viswanathan R, Buck EDet al. . Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol 2020;13:e008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cochet H, Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Nakashima Tet al. . Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace 2021;23:1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako Met al. . Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614–27. [DOI] [PubMed] [Google Scholar]
- 17. Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami Ket al. . Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068–80. [DOI] [PubMed] [Google Scholar]
- 18. Gunawardene MA, Schaeffer BN, Jularic M, Eickholt C, Maurer T, Akbulak RÖet al. . Coronary Spasm During Pulsed Field Ablation of the Mitral Isthmus Line. JACC Clin Electrophysiol 2021;7:1618–20. [DOI] [PubMed] [Google Scholar]
- 19. van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm 2015;12:1838–44. [DOI] [PubMed] [Google Scholar]
- 20. Kuroki K, Whang W, Eggert C, Lam J, Leavitt J, Kawamura Iet al. . Ostial dimensional changes after pulmonary vein isolation: pulsed field ablation vs radiofrequency ablation. Heart Rhythm 2020;17:1528–35. [DOI] [PubMed] [Google Scholar]
- 21. Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta Ket al. . In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013;128:2104–12. [DOI] [PubMed] [Google Scholar]
- 22. Cheng EP, Liu CF, Yeo I, Markowitz SM, Thomas G, Ip JEet al. . Risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol 2019;74:2254–64. [DOI] [PubMed] [Google Scholar]
- 23. Kim IC, Hur SH, Park NH, Jun DH, Cho YK, Nam CWet al. . Incidence and predictors of silent embolic cerebral infarction following diagnostic coronary angiography. Int J Cardiol 2011;148:179–82. [DOI] [PubMed] [Google Scholar]
- 24. Dorenbeck U, Simon B, Skowasch D, Stüsser C, Gockel A, Schild HHet al. . Cerebral embolism with interventional closure of symptomatic patent foramen ovale: an MRI-based study using diffusion-weighted imaging. Eur J Neurol 2007;14:451–4. [DOI] [PubMed] [Google Scholar]
- 25. Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber Met al. . Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010;121:870–8. [DOI] [PubMed] [Google Scholar]
- 26. Haeusler KG, Koch L, Herm J, Kopp UA, Heuschmann PU, Endres Met al. . 3 Tesla MRI-detected brain lesions after pulmonary vein isolation for atrial fibrillation: results of the MACPAF study. J Cardiovasc Electrophysiol 2013;24:14–21. [DOI] [PubMed] [Google Scholar]
- 27. Neven K, van Driel V, van Wessel H, van Es R, Doevendans PA, Wittkampf F. Epicardial linear electroporation ablation and lesion size. Heart Rhythm 2014;11:1465–70. [DOI] [PubMed] [Google Scholar]
- 28. Wong KC, Lim C, Sadarmin PP, Jones M, Qureshi N, De Bono Jet al. . High incidence of acute sub-clinical circumflex artery ‘injury’ following mitral isthmus ablation. Eur Heart J 2011;32:1881–90. [DOI] [PubMed] [Google Scholar]
- 29. Höwel D, Fueting AV, Reinsch N, Brokkaar L, Essling A, Neven K. Pulsed field ablation for atrial fibrillation is safe for the bronchial system. Clin Res Cardiol 2021. doi: 10.1007/s00392-021-01933-9. [DOI] [Google Scholar]
- 30. Sun JH, Zhu TY, Chen XH, Nie CH, Ren ZG, Zhou GHet al. . In vivo evaluation of bronchial injury of irreversible electroporation in a porcine lung ablation model by using laboratory, pathological, and CT findings. Int J Clin Exp Path 2018;11:1273–80. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly to maintain the anonymity of the participating centres.