Cell walls are an important structural component of prokaryotic organisms and essential for many aspects of their life. Particularly, the diverse structures of the outermost boundary layers strongly reflect adaptations of organisms to specific ecological and environmental conditions (6).
Over the past 3 decades of research, it has become apparent that one of the most common surface structures on archaea and bacteria are monomolecular crystalline arrays of proteinaceous subunits termed surface layers or S-layers (125, 131, 132). Since S-layer-carrying organisms are ubiquitous in the biosphere and because S-layers represent one of the most abundant cellular proteins, it is now obvious that these metabolically expensive products must provide the organisms with an advantage of selection in very different habitats (133). This minireview provides a brief survey of the current state of our knowledge about S-layers with a particular focus on molecular biological and genetic aspects. Other recent reviews (5, 7, 127, 133, 135) are recommended for a more detailed introduction to and treatises on this subject.
OCCURRENCE, STRUCTURE, AND ASSEMBLY
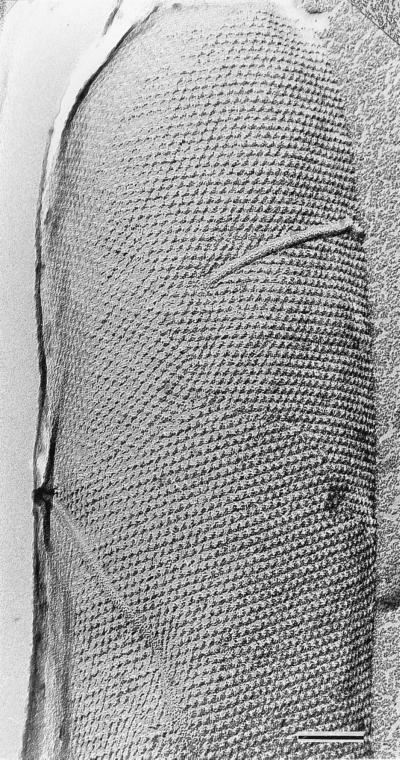
S-layers have now been identified in hundreds of different species belonging to all major phylogenetic groups of bacteria, and they represent a feature common to almost all archaea (for a recent compilation, see reference 133). This widespread occurrence on prokaryotic organisms has not always been appreciated, since S-layers are often lost during prolonged cultivation under laboratory conditions. Consequently, fresh isolates should be examined by electron microscopic techniques as soon as possible, preferably by freeze-etching of pellets of unwashed cells (Fig. 1) in complete medium (64, 125). For high-resolution studies on the mass distribution of S-layer lattices, negatively stained preparations of isolated or recrystallized S-layers have been used (for reviews, see references 4, 5, 50, and 130). More recently, high-resolution images of S-layers were also obtained by applying underwater atomic-force microscopy (38, 135).
FIG. 1.
Electron micrograph of a freeze-etched preparation showing a whole cell with a hexagonally ordered S-layer lattice. Bar, 100 nm.
The S-layer lattices can have oblique (p1, p2) square (p4), or hexagonal (p3, p6) symmetry. The data now available show that hexagonal symmetry is predominant among archaea (for a compilation, see reference 133). Depending on the lattice type, one morphological unit consists of one, two, four, three, or six identical (glyco)protein subunits, respectively, and they exhibit center-to-center spacings of approximately 2.5 to 35 nm. Most S-layers are 5 to 25 nm thick, and they reveal a rather smooth outer surface and a more corrugated inner surface. Among S-layer lattices of archaea, pillar-like extensions on the inner surface can be observed (80, 99, 100, 103). Since S-layers are monomolecular assemblies of identical subunits, they exhibit pores identical in size and morphology. In numerous S-layer lattices, two or even more distinct classes of pores (generally in the 2- to 8-nm range) could be identified, occupying up to 70% of the surface area. Since S-layers are found in gram-positive and gram-negative bacteria and archaea, they can be associated with quite different supramolecular cell envelope structures. In gram-positive bacteria and archaea, the S-layer subunits are linked to the peptidoglycan-containing layer or to the pseudomurein. In gram-negative bacteria, attachment involves components of the outer membrane (e.g., lipopolysaccharides [LPS]). In archaea lacking a rigid wall layer, S-layers are the only wall component, being closely associated with the plasma membrane (38, 59).
S-layers represent unique model systems for studying the dynamic process of assembly of a supramolecular structure during cell growth and cell division. Different methods have been developed for the detachment of S-layers and for their disintegration into protomeric units (5, 62, 90, 133). Most S-layer proteins can be solubilized with high concentrations of agents that break hydrogen bonds (e.g., guanidine hydrochloride). Particularly S-layer proteins from gram-negative bacteria may be disintegrated by applying metal-chelating agents or cation substitution. These data have shown that the individual subunits of S-layers interact with each other and with the supporting cell envelope components through noncovalent forces. Isolated S-layer subunits frequently maintain the ability to recrystallize into regular arrays in suspension or on surfaces (including that cell envelope component they were originally associated with) upon removal of the agent used for their isolation (5, 105, 115, 126, 133). Up to now, the most detailed in vivo and in vitro assembly studies were performed with S-layers from members of the family Bacillaceae (115, 129, 133). It was shown that differences in the surface net charge and in the hydrophobicity of the inner and outer surfaces, binding domains specific to components of the supporting rigid envelope layer and defined intersubunit binding properties, are responsible for the proper orientation and incorporation of the S-layer subunits on cell surfaces. From a more general point of view, it is now evident that S-layers are dynamic closed surface crystals with the intrinsic ability to continuously assume a structure of low free energy during cell growth and division. In most bacteria and archaea, the rate of synthesis of S-layer subunits appears to be strictly controlled, as at different growth rates only small amounts are detectable in the medium. Analysis of the lattice faults in S-layers of lobed archaea possessing hexagonal arrays as the exclusive wall component provided strong evidence that complementary pairs of pentagons and heptagons play an important role as sites for the incorporation of new subunits in the formation and maintenance of the cell structure and in the fission process. The latter was assumed to be dependent on the ratio of the increase in protoplast volume to the increase in actual S-layer surface area during cell growth (106).
S-LAYER PROTEINS AND GENES
Chemical analyses of isolated S-layers showed that they are mostly composed of a single protein or glycoprotein species with apparent relative molecular weights of 40,000 to 200,000. Only the S-layers of a few organisms, such as that of Clostridium difficile (140) and Bacillus anthracis (39, 84, 85), consist of two types of S-layer subunits which together form a defined lattice type but do not cross-react with polyclonal antibodies. S-layer protein EA1 of B. anthracis was proposed to establish the main lattice, whereas the second S-layer protein, Sap, is most probably only incorporated into this template. Two superimposed S-layer lattices consisting of different types of S-layer subunits were identified in the cell envelopes of Brevibacillus brevis (formerly Bacillus brevis; 154) and Aquaspirillum serpens (58).
Amino acid analyses revealed that S-layer proteins of organisms from different phylogenetic branches are rather similar in overall composition (5, 115, 128, 133). Typically, S-layer proteins possess a high content of acidic and hydrophobic amino acids. Lysine is the predominant basic amino acid, while the arginine, histidine, and methionine content is generally low and cysteine was only detected in a few S-layer proteins (86, 115). According to sequence analyses, the isoelectric points (pIs) of most S-layer proteins are in the weakly acidic pH range. An exception was found for the S-layer proteins of Methanothermus fervidus (17) and for different lactobacilli (11), which possess pIs of 8.4 and 9 to 11, respectively. According to circular-dichroism measurements, approximately 20% of the amino acids are organized as α helices and about 40% occur as β sheets. Aperiodic foldings and β-turn content may vary between 5 and 45%. Secondary-structure predictions based on protein sequence data (see Table 1) revealed that most α-helical segments are arranged in the N-terminal part of the proteins. For the remaining part of the sequences, mainly short β-strands connected by loops and turns have been predicted.
TABLE 1.
Survey of S-layer proteins whose amino acid sequences are known
| Species | Strain | Gene | No. of amino acids, including N-terminal leader peptide/ N-terminal leader peptide | Lattice typea | GenBank accession no. | Reference |
|---|---|---|---|---|---|---|
| Aeromonas hydrophila | TF7 | ahs | 467/19 | S | L37348 | 141 |
| Aeromonas salmonicida | A450 | vapA | 502/21 | S | M64655 | 23 |
| Bacillus anthracis | Sterne derivative substrain 9131 | sap | 814/29 | Oc | Z36946 | 39 |
| eag | 862/29 | O | X99724 | 85 | ||
| Brevibacillus brevis (Bacillus brevis) | 47 | owp | 1,004/24 | M14238 | 144 | |
| mwp | 1,053/23 | H | M19115 | 145 | ||
| Brevibacillus brevis (Bacillus brevis) | HPD31 | HWP | 1,087/23 | H | D90050 | 33 |
| Bacillus licheniformis | HM105 | olpA | 874/29 | U38842 | 156 | |
| Bacillus sphaericus | P1 | sequence 8 | 1,252/30 | S | A45814 | 26 |
| Bacillus sphaericus | 2362 | gene 125 | 1,176/30 | S | M28361 | 14 |
| gene 80 | 745 (silent) | 14 | ||||
| Bacillus stearothermophilus | PV72/p6 | sbsA | 1,228/30 | H | X71092 | 67 |
| PV72/p2 | sbsB | 920/31 | O | X98095 | 65 | |
| Bacillus stearothermophilus | ATCC 12980 | sbsC | 1,099/30 | O | AF055578 | 53 |
| Bacillus thuringiensis | ctc | 842/29 | AJ012290 | |||
| Campylobacter fetus subsp. fetus | sapA | 933/none | H, Sb | J05577 | 10 | |
| Campylobacter fetus subsp. fetus | 23B | sapA1 | 920/none | H, Sb | L15800 | 146 |
| Campylobacter fetus subsp. fetus | 82-40LP3 | sapA2 | 1,109/none | H, Sb | S76860 | 31 |
| Campylobacter fetus subsp. fetus | 84-91 | sapB | 936/none | U25133 | 31 | |
| CIP 5396T | sapB2 | 1,112/none | AF048699 | |||
| Campylobacter rectus | 314 | crs | 1,361/none | AF010143 | 149 | |
| Caulobacter crescentus | CB15 | rsaA | 1,026/none | H | M84760 | 44 |
| Clostridium thermocellum | NCIMB 10682 | slpA | 1,036/26 | O | U79117 | 72 |
| Corynebacterium glutamicum | ATCC 17965 | csp2 | 510/30 | H | X69103 | 102 |
| Deinococcus radiodurans | HPI gene | 1,036/31 | H | M17895 | 98 | |
| Halobacterium halobium | csg | 852/34 | H | J02767 | 69 | |
| Haloferax volcanii | 828/34 | H | M62816 | 139 | ||
| Lactobacillus acidophilus | ATCC 4356 | slpA | 444/24 | O | X89375 | 12 |
| slpB | 456 (silent) | X89376 | 12 | |||
| Lactobacillus brevis | ATCC 8287 | 465/30 | O | Z14250 | 147 | |
| Lactobacillus crispatus | JCM 5810 | cbsA | 440/30 | O | AF001313 | |
| Lactobacillus helveticus | CNRZ 892 | slpH1 | 440/30 | O | X91199 | 18 |
| Lactobacillus helveticus | CNRZ 1269 | slpH2 | 440/30 | O | X92752 | 92 |
| Methanococcus voltae | sla | 565/12 | H | M59200 | 60 | |
| Methanosarcina mazei | S-6 | slgB | 652/31 | H | X77929 | 155 |
| Methanothermus fervidus | DSM 2088 | slgA | 593/22 | H | X58297 | 17 |
| Methanothermus sociabilis | DSM 3496 | slgA | 593/22 | H | X58296 | 17 |
| Rickettsia prowazekii | Brein 1 | spaP | 1,612/32 | M37647 | 20 | |
| Rickettsia rickettsii | R | p120 | 1,645/32 | X16353 | 45 | |
| Rickettsia typhii | Wilmington | slpT | 1,645/32 | L04661 | 48 | |
| Serratia marcescens | Isolate 8000 | slaA | 1,004/none | AB007125 | 56 | |
| Staphylothermus marinus | F1 | 1,524/putative | US7967 | 99 | ||
| Thermoanaerobacter kivui (Acetogenium kivui) | DSM 2030 | slp | 762/26 | H | M31069 | 101 |
| Thermus thermophilus | HB8 | slpA | 917/27 | H, S | X57333 | 40 |
H, hexagonal; S, square; O, oblique.
In Campylobacter fetus subsp. fetus, the lattice type was found to be dependent on the molecular weights of the S-layer subunits (H, 97,000; S, 127,000 and 149,000).
Presumably.
S-layer proteins of many archaea and those of gram-positive bacteria can possess covalently bound carbohydrate chains (for reviews, see references 70, 86, 87, and 89). Typically, the glycan chains are composed of 20 to 50 identical repeating units containing neutral hexoses, pentoses, heptoses, 6-deoxyhexoses, and amino sugars. The glycan chains of S-layer proteins from gram-positive bacteria are more similar to the LPS occurring in the outer membranes of gram-negative bacteria than to eukaryotic glycan structures (118). Core oligosaccharides of S-layer glycoproteins consist of two to four sugar residues attaching the carbohydrate chains mostly via O-glycosidic linkages (galactose–tyrosine, glucose–tyrosine, N-acetylgalactosamine–serine, N-acetyl galactosamine–threonine) to the protein moiety (86, 87, 153). However, in a few examples, N-glycosidic linkages (asparagine–rhamnose) have been observed (86, 87). S-layer glycoproteins of gram-positive bacteria possess two to four glycosylation sites, whereas up to 25 glycosylation sites have been determined for those of archaea in which N-glycosidic linkages predominate (70). The presence of sulfate residues in the glycan chains of the extremely halophilic bacterium Halobacterium halobium (82) endows the S-layer lattice with a strong net negative charge. Since only neutral glycan chains were identified for the S-layer protein of the moderate halophile Haloferax volcanii (81), it was suggested that the negative charges are required for stabilizing of the S-layer lattice (138). A different type of posttranslational modification than glycosylation was reported for the S-layer protein of Aeromonas hydrophila. Phosphorylation of tyrosine residues decreases the pI of this S-layer protein from 6.7 to 4.6 (141).
During the last decade, numerous S-layer genes from organisms having quite different taxonomical positions have been cloned and sequenced (for reviews, see references 66 and 135). A survey of all of the sequenced S-layer genes is given in Table 1. Although it was proposed for several years that sequence identities among S-layer proteins are extremely rare or do not even exist, it is now evident that high sequence identities are limited to the N-terminal region, which is responsible for binding of the S-layer subunits to the underlying cell envelope layer (135). On the other hand, only low sequence identities (∼20%) were found for the middle and C-terminal parts, comprising those domains that are involved in the self-assembly process and that are exposed inside the pores and on the S-layer surface. Despite these low sequence identities, conserved four- to six-amino-acid sequences were observed at relatively constant distances over the whole middle and the corresponding C-terminal parts of S-layer proteins from different Bacillus spp. (SbsC, Sap, CTC, OlpA, and SbsB; Table 1), most probably assembling into oblique lattices (53). Considering the highly competitive situation of closely related organisms in their natural habitats, it is obvious that the S-layer surface has to contribute to diversification rather than to conservation. With respect to this, the importance of S-layer variation leading to the expression of alternative S-layer genes under different stress factors such as those imposed by the immune system of a host in response to an S-layered pathogen or drastic changes in the growth and environmental conditions for nonpathogens is conceivable (29, 112).
For S-layered bacteria with a generation time of 20 min, approximately 500 S-layer subunits have to be synthesized per second, translocated through the cell wall, and incorporated into the existing S-layer lattice (128). To keep the bacterial surface completely covered with a closed protein lattice, promoters preceding S-layer genes must be very strong (66). Actually, the promoter of the S-layer gene of Lactobacillus acidophilus is twice as efficient as that preceding the gene encoding lactate dehydrogenase (12, 13, 104), which is considered to be one of the strongest promoters in bacteria. Two promoters were identified for the S-layer gene of L. brevis ATCC 8287 (55), A. salmonicida (24), and B. stearothermophilus ATCC 12980 (53), which are most probably used during different growth stages. The P2 promoter preceding the S-layer gene sbsC in B. stearothermophilus ATCC 12980 seems to be 2.4 times more frequently used in exponentially growing cells than the P1 promoter (53). The existence of three tandemly arranged promoters has been reported for the cwp operon of B. brevis 47 (1), an organism which concomitantly produces two different types of S-layer proteins that assemble sequentially into superimposed S-layer lattices. The promoter of the slpA gene encoding the S-layer protein of L. brevis (147) was recently exploited for heterologous expression of proteins and was recognized in Lactococcus lactis, L. plantarum, and L. gasseri (54).
With the exception of the S-layer proteins of the gram-negative bacteria Caulobacter sp. and Campylobacter sp., all of those sequenced to date are produced with an N-terminal secretion signal peptide which is cleaved off after translocation through the plasma membrane. Linker peptide insertions at the C-terminal end of the S-layer protein of Caulobacter crescentus (Table 1) indicated the existence of a type I secretion signal (8, 9). A similar observation was reported for the S-layer protein of Campylobacter fetus, which also reveals potential secretion signals at the C-terminal part (142). In Serratia marcescens, the slaA gene is located in the upstream region of the lip exporter genes and most probably encodes an S-layer protein (56). The putative S-layer protein is strictly recognized by the lip system, which belongs to the ATP-binding cassette exporter and which is responsible for signal peptide-independent secretion of a lipase and a metalloprotease. On the other hand, the S-layer proteins VapA and AhsA of the gram-negative bacteria A. salmonicida and A. hydrophila are produced with either a 21- or a 19-amino-acid-long signal peptide (23, 24, 141). A conserved gene (abcA) which is located immediately downstream of the vapA gene and affects its expression in Escherichia coli was identified. The corresponding AbcA protein possesses P-loop-containing residues at the N terminus, suggesting that this protein has ATP-binding activity and belongs to the ABC family of transport proteins (25). Further, it could be demonstrated that AbcA is a bifunctional protein in which the N-terminal part regulates the synthesis of the O-polysaccharide side chains of the LPS, functioning as a binding site for the VapA protein. The C-terminal leucine zipper increases expression of the vapA gene and can thus be considered as a transcriptional activator of the P2 promoter (25, 95, 96).
The existence of a translational autoregulation system was reported for the S-layer protein SlpA of Thermus thermophilus HB8 (41). Three genes which specifically repressed the expression of the S-layer protein promoter were cloned in E. coli. Sequence comparison revealed that one of these genes encodes a protein (Rep54) which corresponds to the C-terminal fragment of the S-layer protein and could bind to the 5′-untranslated region of the S-layer protein mRNA.
ATTACHMENT OF S-LAYER PROTEINS TO THE UNDERLYING CELL ENVELOPE LAYER
In gram-positive bacteria, the rigid cell envelope layer is composed of peptidoglycan and accessory (secondary) cell wall polymers which may be teichoic acids, teichuronic acids, lipoteichoic acids, or lipoglycans (2, 93). The polymer chains are either covalently linked to the peptidoglycan backbone via phosphodiester bonds or tethered to a lipid anchor moiety (2). Most of the biological functions discussed for secondary cell wall polymers, such as binding of cations, keeping of the peptidoglycan sacculus in an expanded state by charge repulsion, binding of protons to create an acidic cell wall during bacterial growth and division, or provision of a physical barrier to prevent diffusion of nutrients and metabolites, were seen in context with their net negative charge, and only for B. subtilis were the teichoic acids reported to serve as a binding site for a cell wall autolysin (49).
By sequence comparison, S-layer-homologous (SLH) motifs (74) have been identified at the N-terminal part of many S-layer proteins (14, 26, 33, 39, 40, 51, 65, 71, 72, 85, 97, 144) and at the C-terminal end of cell-associated exoenzymes (71, 78, 79) and other exoproteins (71, 73) of gram-positive bacteria. According to their origin (S-layer proteins, cell-associated exoproteins, porins), SLH motifs have been divided into three main groups whose specific properties have recently been reviewed by Engelhardt and Peters (38). Typically, S-layer proteins and cell-associated exoenzymes possess three repeats of SLH motifs each consisting of 50 to 60 amino acids. Due to their wide distribution among gram-positive bacteria, SLH motifs were suggested to anchor cell-associated exoproteins permanently or transiently to the cell surface (74). In contrast to the original assumption that peptidoglycan functions as a binding site (74), it is now evident that secondary cell wall polymers serve as anchoring structures for many S-layer proteins, cell-associated exoenzymes, and exoproteins (15, 22, 51, 72, 73, 83, 107).
First studies regarding the importance of secondary cell wall polymers for anchoring of S-layer proteins to the rigid cell wall layer (107) were carried out with the S-layer protein SbsB (65) from B. stearothermophilus PV72/p2, an oxygen-induced variant strain of B. stearothermophilus PV72/p6 (112). The S-layer protein SbsB carries three typical SLH motifs at the N-terminal part. Affinity studies using the whole S-layer protein (107) and an N-terminally truncated form (Δ3 SLH motifs; amino acids 208 to 920; Moll, unpublished data), as well as native peptidoglycan-containing sacculi and those extracted with hydrofluoric acid, leading to pure peptidoglycan, revealed that the N-terminal part recognizes a secondary cell wall polymer composed mainly of N-acetylglucosamine and N-acetylmannosamine and not the peptidoglycan as a binding site (107). However, affinity studies with proteolytic cleavage fragments indicated the coexistence of a binding region for peptidoglycan and the secondary cell wall polymer on the N-terminal part of this S-layer protein (111). The isolated secondary cell wall polymer had unique effects on the S-layer protein SbsB (110). Firstly, the presence of the secondary cell wall polymer inhibited the in vitro self-assembly of the guanidine hydrochloride-extracted S-layer protein and kept the S-layer protein in a water-soluble state. Under these conditions, the S-layer protein showed a significantly enhanced tendency to recrystallize into closed monolayers on those solid supports to which the subunits bind with their outer surface. Secondly, the polymer chains protected the S-layer protein against proteolytic attack and specifically masked an N-terminal endoproteinase Glu-C cleavage site. Preliminary studies indicate that a highly specific lectin-type recognition mechanism (110) exists between the S-layer protein and this type of secondary cell wall polymer.
More recently, the complete structure of the secondary cell wall polymer functioning as a binding site for the SLH motifs of the S-layer protein from B. sphaericus CCM 2177 was provided by nuclear magnetic resonance analysis (51). This secondary cell wall polymer is a teichuronic acid and is composed of disaccharide repeating units having the structure →3)-[4,6-O-(1-carboxyethylidene)]∼0.5-β-d-ManpNAc-(1→4)-β-d-GlcpNAc-(1→. In contrast to the SLH motifs of most S-layer proteins, which reveal a positive net charge, those of B. sphaericus strains (14, 26) are net negatively charged, which explains why bivalent cations are required for binding of the S-layer subunits to the rigid cell envelope layer (51).
Experimental evidence that the SLH motifs recognize a cell envelope component that could be extracted from peptidoglycan-containing sacculi with hydrofluoric acid was further provided for the S-layer proteins Sap and EA1 of B. anthracis (22, 85) and SlpA of C. thermocellum (22, 72) and the S-layer protein and cell wall-associated xylanase of Thermoanaerobacterium thermosulfurigenes EM1 (15). In affinity studies in which peptidoglycan-containing sacculi were used as a binding matrix, the Kd values for the polypeptides comprising either the SLH motifs of EA1 and Sap were 1.8 × 10−7 and 1.0 × 10−7 M, respectively (22). Similar values were determined for the SLH motifs and peptidoglycan-containing sacculi of C. thermocellum (22). In all cases, pure peptidoglycan was unable to bind the SLH motifs (22).
In contrast to most S-layer proteins of gram-positive bacteria, those of B. stearothermophilus wild-type strains (53, 65) and Lactobacillus (11, 12, 18, 92, 147) do not possess SLH motifs. Nevertheless, the N-terminal part of the S-layer proteins of B. stearothermophilus wild-type strains is highly conserved and recognizes a net negatively charged secondary cell wall polymer as the binding site (35, 53). This type of secondary cell wall polymer is composed of tetrasaccharide repeating units that contain glucose, N-acetylglucosamine, and 2,3-diacetamido-2,3-dideoxymannuronic acid in a molar ratio of 1 to 1 to 2 (117). Sequence analysis of the S-layer proteins from B. stearothermophilus PV72/p6 and ATCC 12980 (Table 1) revealed an accumulation of arginine, lysine, and tyrosine at the N-terminal part. Because of a high density of positively charged amino acids, the N terminus possesses a positive net charge, which is in contrast to that of the whole S-layer proteins (53). Thus, bivalent cations were not required for binding of the S-layer subunits to the rigid cell wall layer (35), indicating the existence of direct electrostatic interactions between the N-terminal region and the secondary cell wall polymer. The accumulation of tyrosine in the N-terminal part is especially interesting, since in carbohydrate-binding proteins such as lectins, tyrosine interacts with N-acetylated sugars via hydrogen bonds (108, 152). The production of truncated forms of SbsC confirmed that the N-terminal part is not involved in the self-assembly process (35, 53) and seems to fold independently of the remainder of the protein sequence. According to secondary-structure prediction, about 70% of the N-terminal amino acids are organized as α helices.
As described above, the S-layer proteins of Lactobacillus do not possess SLH motifs (11, 12, 18, 92, 147). In earlier studies, Masuda and Kawata (77) reported that these S-layer proteins recognized a neutral polysaccharide but not teichoic acids or the peptidoglycan as a binding site. To conclude, the S-layer proteins from most gram-positive bacteria can be considered cell surface-located carbohydrate-binding proteins with the ability to self-assemble into monomolecular crystalline arrays (110). For a better understanding of the importance of secondary cell wall polymers for S-layer protein folding, prevention of self-assembly within the peptidoglycan-containing layer (16), and anchoring of the S-layer subunits to the cell surface, more detailed studies are required. According to the suggestion that S-layers may be considered cell surface-located carbohydrate-binding proteins, the N-terminal region of the S-layer protein of the gram-negative bacterium C. crescentus recognizes distinct oligosaccharides of the LPS as binding sites (9, 148). Two serotypes that depend on the type of LPS present in the outer membrane are distinguished in C. fetus, a pathogen for humans and ungulates (29). In type A cells, the S-layer protein SapA and its homologs are bound via a conserved N-terminal region of 184 amino acids to the type A LPS. The S-layer protein SapB and its homologs possess a different N-terminal region and recognize cells with type B LPS as a binding site (31, 32).
The example of Corynebacterium glutamicum ATCC 17965 demonstrated that in gram-positive bacteria, interactions other than that of the S-layer protein–polysaccharide type may exist. The S-layer protein of C. glutamicum ATCC 17965 has a hydrophobic C-terminal end which binds to a hydrophobic cell wall layer possessing a high content of mycolic acids (21). In archaea missing a rigid cell wall layer, the S-layer subunits closely interact with the plasma membrane. The S-layer protein of H. halobium shows a hydrophobic stretch of 21 amino acids on its C-terminal part which integrates into the plasma membrane (69). A similar observation was reported for the S-layer protein of Staphylothermus marinus, which carries an extremely stable protease on its 70-nm-long stalk (80, 99).
S-LAYER-DEFICIENT PHENOTYPES AND S-LAYER VARIATION
The importance of insertion sequence (IS) elements for the generation of S-layer-deficient phenotypes has been demonstrated for at least three different organisms. In general terms, IS elements are small translocating DNA segments which occur on the host chromosome or on plasmids with the potential to move within and between bacterial genomes (76). Because of the existence of IS target sequences, transposition does not occur randomly. The integration of IS elements can either repress or activate genes located downstream. Variants of A. salmonicida, a fish pathogen, with S-layer-deficient phenotypes were generated by insertion of two different IS elements into different sites of the vapA gene and its promoter region (47). The bacteria with S-layer-deficient phenotypes were isolated after a temperature upshift from 20 to 30°C. While ISAS1 was unique among reported IS elements, ISAS2 showed strong similarities to transposases encoded by the IS30 family (76). However, both IS elements were found to be restricted to A. salmonicida, where they occurred in a low copy number. The insertion of IS elements was linked to the attenuation or complete loss of virulence (47). Temperature-dependent transposition was also described for IS4712 (120), an IS element which inhibits the expression of the S-layer gene sbsA (67) in B. stearothermophilus PV72/p6, leading to the S-layer-deficient phenotype strain PV72/T5 (112). Integration of IS4712 into the upstream region of the sbsA gene was induced by cultivating the organism at 67°C instead of 57°C. An S-layer-deficient phenotype variant of B. stearothermophilus ATCC 12980 was isolated from agar plates which were stored for at least 2 years at 4°C. PCR analysis revealed that the coding region of the sbsC gene was interrupted by a new type of IS element designated ISBst12, which currently cannot be attributed to any of the known IS families. Southern hybridization showed that ISBst12 was present in multiple copies in the S-layer-carrying strain, as well as in that with the S-layer-deficient phenotype (34). Interestingly, ISBst12 was also detected in B. stearothermophilus PV72/p6, its oxygen-induced strain variant PV72/p2, and the strain with the S-layer-deficient phenotype (PV72/T5). These findings are in agreement with the observation that organisms carrying one type of IS element tend to possess other types since multiple ISs can be delivered by a single vector (76).
S-layer variation leads to the expression of different types of S-layer genes or to the recombination of partial coding sequences. S-layer variation was studied in detail for C. fetus, an important pathogen for humans and ungulates (29), but was also observed for nonpathogens such as B. stearothermophilus (112, 121). In the case of pathogens, S-layer variation can be considered a kind of antigenic variation responding to the lytic activity of the immune system of the host cells. Both type A and B cells of C. fetus can produce multiple S-layer proteins with a single form predominating (31, 32). The apparent molecular weights of SapA and its homologs lie between 97,000 and 149,000, and depending on the molecular size, the S-layer subunits assemble into lattices with either hexagonal or square symmetry (42). Eight or nine S-layer gene cassettes are present in C. fetus wild-type cells and are tightly clustered on the genome in a region of less than 93 kb. Several studies confirmed that antigenic variation is due to the recombination of partial coding sequences (30, 31). In addition to promotor inversion between two oppositely oriented gene cassettes, one of the S-layer gene cassettes bracketing the invertible element with a nonflanking, previously silent gene cassette is exchanged. Thus, C. fetus uses a single promoter strictly by a single DNA inversion event at frequencies independent of DNA fragment size, permitting expression of different S-layer gene cassettes (29, 30). Although the S-layer protein SapB and its homologs possess an N-terminal region different from that of SapA, noncoding regions 300 bp upstream and 1,000 bp downstream of the sapA and sapB open reading frames, including promotor and transcriptional terminator sequences, were essentially identical for both types of cells (31).
Environmental factors inducing change in S-layer gene expression in nonpathogens were studied for B. stearothermophilus (112, 121). Under oxygen-limited growth conditions, the sbsA gene (67) encoding the S-layer protein SbsA, which assembles into a hexagonally ordered lattice type, was stably expressed during continuous cultivation of B. stearothermophilus PV72/p6 (112). After a change to non-oxygen-limited growth conditions (oxygen stress), expression of the second S-layer gene sbsB (65) was induced. The S-layer protein SbsB assembles into an oblique lattice and shows only 25% identity to SbsA. Change in S-layer gene expression was highly coordinated with the synthesis of a different type of secondary cell wall polymer (112). Interestingly, the S-layer protein SbsA recognized both types of secondary cell wall polymers as binding sites, guaranteeing complete coverage of the cell surface during the oxygen-induced switch. On the other hand, the S-layer protein SbsB showed only affinity for binding of the newly synthesized secondary cell wall polymer. Preliminary studies indicate that the sbsB gene is generated from partial coding sequences, while the sbsA gene is disrupted during the switch (121).
FUNCTIONAL ASPECTS
Although considerable knowledge has accumulated on the structure, assembly, biochemistry, and genetics of S-layers, relatively little information is available about their specific functions (5, 109, 127). Considering that S-layer-carrying organisms are ubiquitous in the biosphere, the supramolecular concept of a closed, isoporous, crystalline surface layer could have the potential to fulfill a broad spectrum of functions. In functional terms, S-layers must not be seen as isolated components since they are generally only part of more complex envelope structures.
Because S-layer lattices possess pores identical in size and morphology in the 2- to 8-nm range, they work as precise molecular sieves, providing sharp cutoff levels for the bacterial cells (113). The largest pores in the 5- to 8-nm range were found in S-layer lattices of those archaea lacking a rigid cell wall layer in which these crystalline arrays fulfill a shape-determining or shape-maintaining function (3, 4, 50, 80, 99). As a general feature, the S-layer subunits of archaea lacking a rigid cell wall layer possess long, hydrophobic protrusions which integrate into the plasma membrane. Thereby, a kind of periplasmic space is formed between the S-layer and the plasma membrane where secreted macromolecules involved in nutrient degradation, nutrient transport, and folding and export of proteins could be stored (46, 103). According to the proposed function, the S-layer glycoprotein of S. marinus is held at a distance of as much as 70 nm from the plasma membrane by membrane-anchored stalks. Furthermore, two copies of a hyperthermostable protease showing significant similarity to subtilisin are attached near the middle of each stalk. This enzyme is resistant to heat inactivation to 95°C in the free form and to 125°C in the stalk-bound form (80, 99).
S-layers from Bacillaceae were found to function as adhesion sites for cell-associated exoenzymes. The high-molecular-weight exoamylase from two B. stearothermophilus wild-type strains were bound to the S-layer surface in a density that did not disturb diffusion of nutrients or metabolites through the S-layer lattice (36, 37). S-layer-associated exoenzymes were also described for T. thermohydrosulfurigenes (71, 78, 79).
Regarding protective functions, S-layers from gram-negative bacteria such as A. salmonicida, C. fetus, A. serpens, and C. crescentus were found to protect the cells from attack by bacterial parasites such as Bdellovibrio bacteriovorus, but they could not shield them from other predators like protozoa (63). A quite interesting type of protective function was reported for the S-layer lattice of Synechococcus GL-24 (122, 123), a cyanobacterium capable of growing in lakes with exceptionally high calcium and sulfate ion concentrations. The hexagonally ordered S-layer lattice functions as a template for fine-grain mineralization and is continuously shed from the cell surface to prevent clogging of further cell envelope layers.
S-layers can contribute to virulence when they are present as a structural component of the cell envelope of pathogens. The A-layer of A. salmonicida endows this organism with high or intermediate resistance to the bactericidal activity of complement in immune and nonimmune sera. Furthermore, the A-layer plays an important role in uptake of porphyrins and shows unique immunoglobulin- and extracellular matrix protein-binding capabilities (28, 43). A similar observation was reported for the S-layer from B. cereus isolated from periodontal infections (61). Only the S-layer-carrying cells adhered to matrix proteins and were resistant to polymorphonuclear leukocytes in the absence of opsonins. The serum resistance of C. fetus was found to be due to the inability of complement component C3b to bind to the S-layer surface. Thus, effective opsonization occurred only after the addition of specific antibodies (29). In Rickettsia prowazekii and R. typhii, causing either epidemic or endemic typhus, the S-layer protein is responsible for humoral and cell-mediated immunity (19). In L. acidophilus, the S-layer is responsible for the adhesion of the bacterial cells to the intestinal epithelium (119).
APPLICATION POTENTIAL
During the last 10 years, studies on the structure, morphogenesis, genetics, and function of S-layers revealed that these isoporous monomolecular arrays have a considerable application potential in biotechnology, molecular nanotechnology, and biomimetics (for reviews, see references 105, 116, 134, and 137). Many applications depend on the ability of isolated S-layer proteins to assemble into regular arrays in suspension or on suitable surfaces (e.g., silicon wafers, metals, and polymers) or interfaces (e.g., lipid films and liposomes) upon removal of the disrupting agent used for their isolation.
Since S-layer lattices are assemblies of identical (glyco)protein species, these crystalline arrays exhibit repetitive physicochemical properties down to the subnanometer scale and possess pores identical in size and morphology. S-layers from various Bacillaceae were shown to be suitable for the production of isoporous ultrafiltration membranes with well-defined molecular weight cutoffs (113, 114, 134, 136). The S-layer lattice and the pore areas of S-layers contain functional groups (carboxylic acid, amine, and hydroxyl groups) which are aligned in well-defined positions and orientations. Accordingly, highly reproducible chemical modifications can be applied for optimization of molecular sieving properties and nonspecific adsorption (antifouling) characteristics (150, 151). The repetitive features of S-layers have led to their use as immobilization matrices for binding of monolayers of functional molecules (e.g., enzymes, antibodies, and immunogens) in a geometrically well-defined way. This application potential has been exploited for the production of bioanalytical sensors, immunoassays, affinity microparticles, and affinity membranes (116, 137).
Another line of development is directed toward the use of S-layer self-assembly products in suspension as a combined carrier-adjuvant system against infection with pathogenic bacteria in the immunotherapy of cancers and in antiallergic immunotherapy (52, 91, 135). On the other hand, S-layers of pathogenic organisms were identified to be essential for virulence. Whole-cell preparations or partially purified cell products are currently used as attenuated vaccines against fish pathogens (57, 143). Another broad application for S-layers is based on the ability of subunits to recrystallize into coherent lattices on functional lipid membranes (105, 135), including liposomes (68, 75, 94). These S-layer-stabilized lipid membranes mimic the supramolecular structure of those archaeal envelopes which possess S-layers as exclusive wall components or virus envelopes. They can be used in diagnostics, as vaccines, for drug targeting or delivery, and for gene therapy (115, 116, 135, 137). Alternative or complementary to existing S-layer technologies, genetic approaches are used to incorporate functional domains (proteins and glycans) in S-layer subunits while maintaining their ability to self-assemble into regular arrays (137).
Finally, S-layer lattices recrystallized on solid supports (e.g., silicon wafers) can be patterned by microlithographic procedures as required for lab-on-a-chip technologies (105, 135). The observation that S-layers of cyanobacteria may induce precipitation of minerals (122, 123) led to the concept of their use as templates for the formation of regular arrays of metal clusters as required in molecular electronics and nonlinear optics (27, 105, 124).
CONCLUSION
It is now evident that S-layers are the most common cell surface components of prokaryotic organisms. In comparison with the considerable accumulation of data concerning the structure, chemistry, assembly, and genetics of S-layer proteins, relatively little is known about their evolutionary relationship; their biosynthesis, including glycosylation; their transfer through intermediate envelope layers to sites of lattice extension; and their functional potential. Since S-layers of gram-positive and gram-negative bacteria can be lost in the course of subculturing in the laboratory, elucidation of their functional significance for a particular organism requires ecological approaches mimicking complex natural habitats and potential hazards. Although structurally diverse S-layers with barely any sequence identities in the constituent subunits are observed even among strains of the same species, these proteins must have domains with common structural and functional significance. In this context, it is important to remember that some organisms possess multiple S-layer genes and can even assemble different superimposed S-layers on their surface. This diversity and differentiation potential is further stressed by the observation that in S-layer proteins of strains of the same species can be either glycosylated or not (87, 88).
Finally, since S-layers can be considered supramolecular structures with the intrinsic ability to assume closed structures with low free energy during cell growth, it is tempting to speculate that S-layers, like membranes, could have fulfilled barrier and supporting functions as required by self-reproducing systems during the early periods of biological evolution (132).
ACKNOWLEDGMENTS
This work was supported in part by the Austrian Science Foundation (project 12938) and by the Ministry of Research and Transportation.
We thank Dieter Moll for critical reading of the manuscript.
REFERENCES
- 1.Adachi T, Yamagata H, Tsukagoshi N, Udaka S. Multiple and tandemly arranged promotors of the cell wall protein operon in Bacillus brevis 47. J Bacteriol. 1989;171:1010–1016. doi: 10.1128/jb.171.2.1010-1016.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald A R, Hancock I C, Harwood C R. Cell wall structure, synthesis, and turnover. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C.: American Society for Microbiology; 1993. pp. 381–410. [Google Scholar]
- 3.Baumeister W, Lembcke G. Structural features of archaebacterial cell envelopes. J Bioenerg Biomembr. 1992;24:567–575. doi: 10.1007/BF00762349. [DOI] [PubMed] [Google Scholar]
- 4.Baumeister W, Lembcke G, Dürr R, Phipps B. Electron crystallography of bacterial surface proteins. In: Freyer J R, Dorset D L, editors. Electron crystallography of organic molecules. Dordrecht, The Netherlands: Kluwer; 1990. pp. 283–296. [Google Scholar]
- 5.Beveridge T J. Bacterial S-layers. Curr Opin Struct Biol. 1994;4:204–212. [Google Scholar]
- 6.Beveridge T J, Graham L L. Surface layers of bacteria. Microbiol Rev. 1991;55:684–705. doi: 10.1128/mr.55.4.684-705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beveridge T J, Koval S F. Advances in paracrystalline surface layers. New York, N.Y: Plenum Press; 1993. [Google Scholar]
- 8.Bingle W H, Nomellini J F, Smit J. Cell-surface display of a Pseudomonas aeruginosa strain K pilin peptide within the paracrystalline S-layer of Caulobacter crescentus. Mol Microbiol. 1997;26:277–288. doi: 10.1046/j.1365-2958.1997.5711932.x. [DOI] [PubMed] [Google Scholar]
- 9.Bingle W H, Nomellini J F, Smit J. Linker mutagenesis of the Caulobacter crescentus S-layer protein: toward a definition of an N-terminal anchoring region and a C-terminal secretion signal and the potential for heterologous protein secretion. J Bacteriol. 1997;179:601–611. doi: 10.1128/jb.179.3.601-611.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaser M, Gotschlich E C. Surface array protein of Campylobacter fetus cloning and gene structure. J Biol Chem. 1990;265:14529–14535. [PubMed] [Google Scholar]
- 11.Boot H, Pouwels P. Expression, secretion and antigenic variation of bacterial S-layer proteins. Mol Microbiol. 1996;21:1117–1123. doi: 10.1046/j.1365-2958.1996.711442.x. [DOI] [PubMed] [Google Scholar]
- 12.Boot H J, Kolen C P A M, Pouwels P H. Identification, cloning, and nucleotide sequence of a silent S-layer protein gene of Lactobacillus acidophilus ATCC 4356 which has extensive similarity with the S-layer protein gene of this species. J Bacteriol. 1995;177:7222–7230. doi: 10.1128/jb.177.24.7222-7230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boot H J, Kolen C P A M, Andreadaki F J, Leer R J, Pouwels P H. The Lactobacillus acidophilus S-layer protein gene expression site comprises two consensus promoter sequences, one of which directs transcription of stable mRNA. J Bacteriol. 1996;178:5388–5394. doi: 10.1128/jb.178.18.5388-5394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowditch R D, Baumann P, Yousten A A. Cloning and sequencing of the gene encoding a 125-kilodalton surface-layer protein from Bacillus sphaericus 2362 and of a related cryptic gene. J Bacteriol. 1989;171:4178–4188. doi: 10.1128/jb.171.8.4178-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brechtel E, Bahl H. In Thermoanaerobacterium thermosulfurigenes EM1 S-layer homology domains do not attach to peptidoglycan. J Bacteriol. 1999;181:5017–5023. doi: 10.1128/jb.181.16.5017-5023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breitwieser A, Gruber K, Sleytr U B. Evidence for an S-layer protein pool in the peptidoglycan of Bacillus stearothermophilus. J Bacteriol. 1992;174:8008–8015. doi: 10.1128/jb.174.24.8008-8015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bröckel G, Behr M, Fabry S, Hensel R, Kaudewitz H, Biendl E, König H. Analysis and nucleotide sequence of the genes encoding the surface-layer glycoprotein of the hyperthermophilic methanogens Methanothermus fervidus and Methanothermus sociabilis. Eur J Biochem. 1991;199:147–152. doi: 10.1111/j.1432-1033.1991.tb16102.x. [DOI] [PubMed] [Google Scholar]
- 18.Callegari L, Riboli B, Sanders W, Sandro Coconelli P, Kok J, Venema G, Morelli L. The S-layer gene of Lactobacillus helveticus CNRZ 892: cloning, sequencing and heterologous expression. Microbiology. 1998;144:719–726. doi: 10.1099/00221287-144-3-719. [DOI] [PubMed] [Google Scholar]
- 19.Carl M, Dasch G A. The importance of the crystalline surface layer protein of rickettsiae in T-cell immunity. J Autoimmun. 1989;2:81–91. doi: 10.1016/0896-8411(89)90119-4. [DOI] [PubMed] [Google Scholar]
- 20.Carl M, Dobson M E, Ching W M, Dasch G A. Characterization of the gene encoding the protective paracrystalline-surface-layer protein of Rickettsia prowazekii: presence of a truncated identical homolog in Rickettsia typhii. Proc Natl Acad Sci USA. 1990;87:8237–8241. doi: 10.1073/pnas.87.21.8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chami M, Bayan N, Peyret J L, Gulik-Krzywicki T, Leblon G, Schechter E. The S-layer protein of Corynebacterium glutamicum is anchored to the cell wall by its C-terminal hydrophobic domain. Mol Microbiol. 1997;23:483–492. doi: 10.1046/j.1365-2958.1997.d01-1868.x. [DOI] [PubMed] [Google Scholar]
- 22.Chauvaux S, Matuschek M, Beguin P. Distinct affinity of binding sites for S-layer homologous domains in Clostridium thermocellum and Bacillus anthracis. J Bacteriol. 1999;181:2455–2458. doi: 10.1128/jb.181.8.2455-2458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu S, Cavaignac S, Feutrier J, Phipps B M, Kostrzynska M, Kay W W, Trust T J. Structure of the tetragonal surface virulence array protein and gene of Aeromonas salmonicida. J Biol Chem. 1991;266:15258–15265. [PubMed] [Google Scholar]
- 24.Chu S, Gustafson C E, Feutrier J, Cavaignac S, Trust T J. Transcriptional analysis of the Aeromonas salmonicida S-layer protein gene vapA. J Bacteriol. 1993;175:7968–7975. doi: 10.1128/jb.175.24.7968-7975.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu S, Trust T J. An Aeromonas salmonicida gene which influences A-protein expression in Escherichia coli encodes a protein containing an ATP-binding cassette and maps beside the surface array protein gene. J Bacteriol. 1993;175:3105–3114. doi: 10.1128/jb.175.10.3105-3114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deblaere, R. 20 July 1995. Expression of surface layer proteins. Patent WO 9529371-A.
- 27.Dieluweit S, Pum D, Sleytr U B. Formation of a gold superlattice on an S-layer with square lattice symmetry. Supramol Sci. 1998;5:15–19. [Google Scholar]
- 28.Doig P, Emödy L, Trust T J. Binding of laminin and fibronection by the trypsin-resistant major structural domain of the crystalline virulence surface array protein of Aeromonas salmonicida. J Biol Chem. 1992;267:43–49. [PubMed] [Google Scholar]
- 29.Dworkin J, Blaser M J. Molecular mechanisms of Campylobacter fetus surface layer protein expression. Mol Microbiol. 1997;26:433–440. doi: 10.1046/j.1365-2958.1997.6151958.x. [DOI] [PubMed] [Google Scholar]
- 30.Dworkin J, Shedd O L, Blaser M J. Nested DNA inversion of Campylobacter fetus S-layer genes is recA dependent. J Bacteriol. 1997;179:7523–7529. doi: 10.1128/jb.179.23.7523-7529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dworkin J, Tummuru M K, Blaser M J. Segmental conservation of sapA sequences in type B Campylobacter fetus cells. J Biol Chem. 1995;270:15093–15101. doi: 10.1074/jbc.270.25.15093. [DOI] [PubMed] [Google Scholar]
- 32.Dworkin J, Tummuru M K R, Blaser M J. A lipopolysaccharide-binding domain of the Campylobacter fetus S-layer protein resides within the conserved N terminus of a family of silent and divergent homologs. J Bacteriol. 1995;177:1734–1741. doi: 10.1128/jb.177.7.1734-1741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebisu S, Tsuboi A, Takagi H, Naruse Y, Yamagata H, Tsukagoshi N, Udaka S. Conserved structures of cell wall protein genes among protein-producing Bacillus brevis strains. J Bacteriol. 1990;172:1312–1320. doi: 10.1128/jb.172.3.1312-1320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egelseer, E. M., R. Idris, M. Jarosch, T. Danhorn, U. B. Sleytr, and M. Sára. Characterization of ISBst12, a novel type of insertion sequence element causing loss of S-layer gene expression in Bacillus stearothermophilus ATCC 12980. Submitted for publication. [DOI] [PubMed]
- 35.Egelseer E M, Leitner K, Jarosch M, Hotzy C, Zayni S, Sleytr U B, Sára M. The S-layer proteins of two Bacillus stearothermophilus wild-type strains are bound via their N-terminal region to a secondary cell wall polymer of identical chemical composition. J Bacteriol. 1998;180:1488–1495. doi: 10.1128/jb.180.6.1488-1495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egelseer E M, Schocher I, Sára M, Sleytr U B. The S-layer fom Bacillus stearothermophilus DSM 2358 functions as an adhesion site for a high-molecular-weight amylase. J Bacteriol. 1995;177:1444–1451. doi: 10.1128/jb.177.6.1444-1451.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egelseer E M, Schocher I, Sleytr U B, Sára M. Evidence that an N-terminal S-layer protein fragment triggers the release of a cell-associated high-molecular-weight amylase from Bacillus stearothermophilus ATCC 12980. J Bacteriol. 1996;178:5602–5609. doi: 10.1128/jb.178.19.5602-5609.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelhardt H, Peters J. Structural research on surface layers: a focus on stability, surface layer homology domains, and surface layer-cell wall interactions. J Struct Biol. 1998;124:276–302. doi: 10.1006/jsbi.1998.4070. [DOI] [PubMed] [Google Scholar]
- 39.Etienne-Toumelin I, Sirard J-C, Duflot E, Mock M, Fouet A. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J Bacteriol. 1995;177:614–620. doi: 10.1128/jb.177.3.614-620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faraldo M M, de Pedro M A, Berenguer J. Sequence of the S-layer gene of Thermus thermophilus HB8 and functionality of its promoter in Escherichia coli. J Bacteriol. 1992;174:7458–7462. doi: 10.1128/jb.174.22.7458-7462.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Herrero L A, Olabarria G, Berenguer J. Surface proteins and a novel transcription factor regulate the expression of the S-layer gene in Thermus thermophilus HB8. Mol Microbiol. 1997;24:61–72. doi: 10.1046/j.1365-2958.1997.3191683.x. [DOI] [PubMed] [Google Scholar]
- 42.Fujimoto S, Takade A, Amako K, Blaser M J. Correlation between molecular size of the surface arrray protein and morphology and antigenicity of the Campylobacter fetus S-layer protein. Infect Immun. 1991;59:2017–2022. doi: 10.1128/iai.59.6.2017-2022.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garduno R, Phipps B M, Kay W W. Physical and functional S-layer reconsitution in Aeromonas salmonicida. J Bacteriol. 1995;177:2684–2694. doi: 10.1128/jb.177.10.2684-2694.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilchrist A, Fisher J A, Smit J. Nucleotide sequence analysis of the gene encoding the Caulobacter cresentus paracrystalline surface layer protein. J Bacteriol. 1992;170:4706–4713. doi: 10.1139/m92-033. [DOI] [PubMed] [Google Scholar]
- 45.Gilmore R D, Joste N, McDonald G A. Cloning, expression and sequence analysis of the gene encoding the 120 kD-surface exposed protein in Rickettsia rickettsii. Mol Microbiol. 1989;3:1579–1586. doi: 10.1111/j.1365-2958.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 46.Graham L, Nanninga N, Beveridge T J. Periplasmic space and the concept of periplasm. Trends Biochem Sci. 1991;16:328–329. doi: 10.1016/0968-0004(91)90135-i. [DOI] [PubMed] [Google Scholar]
- 47.Gustafson C E, Chu S, Trust T J. Mutagenesis of the paracrystalline surface protein array of Aeromonas salmonicida by endogeneous insertion elements. J Mol Biol. 1994;237:452–463. doi: 10.1006/jmbi.1994.1247. [DOI] [PubMed] [Google Scholar]
- 48.Hahn M J, Kim K K, Kim I. Cloning and sequence analysis of the gene encoding the crystalline surface protein of Rickettsia typhii. Gene. 1993;133:129–133. doi: 10.1016/0378-1119(93)90237-w. [DOI] [PubMed] [Google Scholar]
- 49.Herbold D R, Glaser L. Interaction of N-acetylmuramic acid L-alanine amidase with cell wall polymers. J Biol Chem. 1975;250:1676–1680. [PubMed] [Google Scholar]
- 50.Hovmöller S, Sjögren A, Wang D N. The structure of crystalline bacterial surface layers. Prog Biophys Mol Biol. 1988;51:131–163. doi: 10.1016/0079-6107(88)90012-0. [DOI] [PubMed] [Google Scholar]
- 51.Ilk N, Kosma P, Puchberger M, Egelseer E M, Mayer H F, Sleytr U B, Sára M. Structural and functional analyses of the secondary cell wall polymer of Bacillus sphaericus CCM 2177 serving as an S-layer-specific anchor. J Bacteriol. 1999;181:7643–7646. doi: 10.1128/jb.181.24.7643-7646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jahn-Schmid B, Messner P, Unger F M, Sleytr U B, Scheiner O, Kraft D. Toward selective elicitation of TH1-controlled vaccination responses: vaccine applications of bacterial surface layer proteins. J Biotechnol. 1996;44:225–231. doi: 10.1016/0168-1656(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 53.Jarosch, M., E. M. Egelseer, D. Mattanovich, U. B. Sleytr, and M. Sára. The S-layer gene sbsC of Bacillus stearothermophilus ATCC 12980: molecular characterization and heterologous expression in Escherichia coli. Microbiology, in press. [DOI] [PubMed]
- 54.Kahala M, Palva A. The expression signals of the Lactobacillus brevis slpA gene direct efficient heterologous protein production in lactic acid bacteria. Appl Microbiol Biotechnol. 1999;51:71–78. doi: 10.1007/s002530051365. [DOI] [PubMed] [Google Scholar]
- 55.Kahala M, Savijoki K, Palva A. In vivo expression of the Lactobacillus brevis S-layer gene. J Bacteriol. 1997;179:284–286. doi: 10.1128/jb.179.1.284-286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawai E, Akatsuka H, Idei A, Shibatani T, Omori K. Serratia marcescens S-layer protein is secreted extracellularly via an ATP-binding cassette exporter, the Lip system. Mol Microbiol. 1998;27:941–952. doi: 10.1046/j.1365-2958.1998.00739.x. [DOI] [PubMed] [Google Scholar]
- 57.Kay W W, Trust T J. Form and functions of the regular surface array (S-layer) of Aeromonas salmonicida. Experientia. 1991;47:412–414. [PubMed] [Google Scholar]
- 58.Kist M, Murray R G E. Components of the regular surface array of Aquaspirillum serpens MW5 and their assembly in vitro. J Bacteriol. 1984;157:599–606. doi: 10.1128/jb.157.2.599-606.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.König H. Archaebacterial cell envelopes. Can J Microbiol. 1988;34:395–406. [Google Scholar]
- 60.Konisky J, Lynn D, Hoppert M, Mayer F, Haney P. Identification of the Methanococcus voltae S-layer structural gene. J Bacteriol. 1994;176:1790–1792. doi: 10.1128/jb.176.6.1790-1792.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotiranta A, Lounatmaa K, Kari K, Kerusuo E, Haapasalo M. Function of the S-layer of some Gram-positive bacteria in phagocytosis. FEMS Microbiol Rev. 1997;20:110–114. [Google Scholar]
- 62.Koval S F. Paracrystalline protein surface arrays on bacteria. Can J Microbiol. 1988;34:407–414. [Google Scholar]
- 63.Koval S F. The effect of S-layers and cell surface hydrophobicity on prey selection by bacterivorous protozoa. FEMS Microbiol Rev. 1997;20:138–142. [Google Scholar]
- 64.Koval S F, Murray R G E. The superficial protein arrays on bacteria. Microbiol Sci. 1986;3:357–362. [PubMed] [Google Scholar]
- 65.Kuen B, Koch A, Asenbauer E, Sára M, Lubitz W. Molecular characterization of the second S-layer gene sbsB of Bacillus stearothermophilus PV72 expressed by oxidative stress. J Bacteriol. 1997;179:1664–1670. doi: 10.1128/jb.179.5.1664-1670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuen B, Lubitz W. Analysis of S-layer proteins and genes. In: Sleytr U B, Messner P, Pum D, Sára M, editors. Crystalline bacterial cell surface proteins. Austin, Tex: Academic Press, Landes Company; 1996. pp. 77–102. [Google Scholar]
- 67.Kuen B, Sleytr U B, Lubitz W. Sequence analysis of the sbsA gene encoding the 130 kDa surface layer protein of Bacillus stearothermophilus PV72. Gene. 1994;145:115–120. doi: 10.1016/0378-1119(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 68.Küpcü S, Sára M, Sleytr U B. Liposomes coated with crystalline bacterial cell surface protein (S-layer) as immobilization structures for macromolecules. Biochim Biophys Acta. 1995;1235:263–269. doi: 10.1016/0005-2736(95)80013-6. [DOI] [PubMed] [Google Scholar]
- 69.Lechner J, Sumper M. The primary structure of a prokaryotic glycoprotein. Cloning and sequencing of the cell surface gene of halobacteria. J Biol Chem. 1987;262:9724–9729. [PubMed] [Google Scholar]
- 70.Lechner J, Wieland F. Structure and biosynthesis of procaryotic glycoproteins. Annu Rev Biochem. 1989;58:173–194. doi: 10.1146/annurev.bi.58.070189.001133. [DOI] [PubMed] [Google Scholar]
- 71.Leibovitz E, Lemaire M, Miras I, Salamitou S, Beguin P, Ohayon H, Gounon P, Matuschek M, Sahm K, Bahl H. Occurrence and function of a common domain in S-layer and other exocellular proteins. FEMS Microbiol Rev. 1997;20:127–133. [Google Scholar]
- 72.Lemaire M, Miras I, Gounon P, Beguin P. Identification of a region responsible for binding to the cell wall within the S-layer protein of Clostridium thermocellum. Microbiology. 1998;144:211–217. doi: 10.1099/00221287-144-1-211. [DOI] [PubMed] [Google Scholar]
- 73.Lemaire M, Ohayon H, Gounon P, Fujino T, Beguin P. OlpB, a new outer layer protein of Clostridium thermocellum and binding of its S-layer-like domain to components of the cell envelope. J Bacteriol. 1995;177:2451–2459. doi: 10.1128/jb.177.9.2451-2459.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lupas A, Engelhardt H, Peters J, Santarius U, Volker S, Baumeister W. Domain structure of the Acetogenium kivui surface layer revealed by electron crystallography and sequence analysis. J Bacteriol. 1994;176:1224–1233. doi: 10.1128/jb.176.5.1224-1233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mader C, Küpcü S, Sára M, Sleytr U B. Stabilizing effect of an S-layer on liposomes towards thermal or mechanical stress. Biochim Biophys Acta. 1999;1418:106–116. doi: 10.1016/s0005-2736(99)00030-9. [DOI] [PubMed] [Google Scholar]
- 76.Mahillon J, Chandler M. Insertion sequences. Microbiol Mol Biol Rev. 1998;62:725–774. doi: 10.1128/mmbr.62.3.725-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masuda K, Kawata T. Reassembly of a regularly arranged protein in the cell wall of Lactobacillus buchneri and its reattachment to cell walls: chemical modification studies. Microbiol Immunol. 1985;29:927–938. [PubMed] [Google Scholar]
- 78.Matuschek M, Burchhardt G, Sahm K, Bahl H. Pullulanase of Thermoanaerobacterium thermohydrosulfurigenes EM1 (Clostridium thermosulfurogenes): molecular analysis of the gene, composite structure of the enzyme, and a common model for its attachment to the cell surface. J Bacteriol. 1994;176:3295–3302. doi: 10.1128/jb.176.11.3295-3302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matuschek M, Sahm K, Zibat H, Bahl H. Characterization of genes from Thermoanaerobacterium thermosulfurigenes EM1 that encode two glycosyl hydrolases with conserved S-layer-like domain. Mol Gen Genet. 1996;252:493–496. doi: 10.1007/BF02173016. [DOI] [PubMed] [Google Scholar]
- 80.Mayr J, Lupas A, Kellermann J, Eckerskorn C, Baumeister W, Peters J. A hyperthermostable protease of the subtilisin family bound to the surface layer of the archaeon Staphylothermus marinus. Curr Biol. 1996;6:739–749. doi: 10.1016/s0960-9822(09)00455-2. [DOI] [PubMed] [Google Scholar]
- 81.Mengele R, Sumper M. Drastic differences in glycosilation of related S-layer glycoproteins from moderate and extreme halophiles. J Biol Chem. 1992;267:8182–8185. [PubMed] [Google Scholar]
- 82.Mescher M F, Strominger J L. Purification and characterization of a prokaryotic glycoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976;251:2005–2014. [PubMed] [Google Scholar]
- 83.Mesnage S, Tosi-Couture E, Fouet A. Production and cell surface anchoring of functional fusion proteins between the SLH motifs of the Bacillus anthracis S-layer proteins and the Bacillus subtilis levansucrase. Mol Microbiol. 1999;31:927–936. doi: 10.1046/j.1365-2958.1999.01232.x. [DOI] [PubMed] [Google Scholar]
- 84.Mesnage S, Tosi-Couture E, Gounon P, Mock M, Fouet A. The capsule and S-layer: two independent and yet compatible macromolecular structures in Bacillus anthracis. J Bacteriol. 1998;180:52–58. doi: 10.1128/jb.180.1.52-58.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mesnage S, Tosi-Couture E, Mock M, Gounon P, Fouet A. Molecular characterization of the Bacillus anthracis main S-layer component: evidence that it is the major cell-associated antigen. Mol Microbiol. 1997;23:1147–1155. doi: 10.1046/j.1365-2958.1997.2941659.x. [DOI] [PubMed] [Google Scholar]
- 86.Messner P. Chemical composition and biosynthesis of S-layers. In: Sleytr U B, Messner P, Pum D, Sára M, editors. Crystalline bacterial cell surface layer proteins (S-layers). Austin, Tex: Academic Press, Landes Company; 1996. pp. 35–76. [Google Scholar]
- 87.Messner P. Bacterial glycoproteins. Glycoconj J. 1997;14:3–11. doi: 10.1023/a:1018551228663. [DOI] [PubMed] [Google Scholar]
- 88.Messner P, Hollaus F, Sleytr U B. Paracrystalline cell wall surface layers of different Bacillus stearothermophilus strains. Int J Syst Bacteriol. 1984;34:202–210. [Google Scholar]
- 89.Messner P, Sleytr U B. Bacterial surface layer glycoproteins. Glycobiology. 1991;1:545–555. doi: 10.1093/glycob/1.6.545. [DOI] [PubMed] [Google Scholar]
- 90.Messner P, Sleytr U B. Crystalline bacterial cell surface layers. Adv Microbiol Physiol. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- 91.Messner P, Unger F M, Sleytr U B. Vaccine development based on S-layer technology. In: Sleytr U B, Messner P, Pum D, Sára M, editors. Crystalline bacterial cell surface layer proteins (S-layers). Austin, Tex: Landes Company, Academic Press; 1996. pp. 161–173. [Google Scholar]
- 92.Morelli L, Callegari M-L. Surface layer of Lactobacillus helveticus CNRZ 892. FEMS Microbiol Lett. 1997;20:118–121. doi: 10.1111/j.1574-6968.2000.tb09243.x. [DOI] [PubMed] [Google Scholar]
- 93.Navarre W W, Schneewind O. Surface protein of gram-positive bacteria and mechanisms of their targeting to the cell envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nomellini J F, Küpcü S, Sleytr U B, Smit J. Factors controlling in vitro recrystallization of the Caulobacter crescentus paracrystalline S-layer. J Bacteriol. 1997;179:6349–6354. doi: 10.1128/jb.179.20.6349-6354.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noonan B, Trust T J. Molecular analysis of an A-protein secretion mutant of Aeromonas salmonicida reveals a surface layer-specific secretion pathway. J Mol Biol. 1995;248:316–327. doi: 10.1016/s0022-2836(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 96.Noonan B, Trust T J. The leucine zipper of Aeromonas salmonicida AbcA is required for the transcriptional activation of the P2 promoter of the surface-layer structural gene, vapA, in Escherichia coli. Mol Microbiol. 1995;17:379–386. doi: 10.1111/j.1365-2958.1995.mmi_17020379.x. [DOI] [PubMed] [Google Scholar]
- 97.Olabarria G, Carrascosa J L, de Pedro M A, Berenguer J. A conserved motif in S-layer proteins is involved in peptidoglycan binding in Thermus thermophilus. J Bacteriol. 1996;178:4765–4772. doi: 10.1128/jb.178.16.4765-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peters J, Baumeister W. Molecular cloning, expression, and characterization of the gene for the surface (HPI)-layer protein of Deinococcus radiodurans in Escherichia coli. J Bacteriol. 1986;167:1048–1054. doi: 10.1128/jb.167.3.1048-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peters J, Nitsch M, Kühlmorgen B, Golbik R, Lupas A, Kellermann J, Engelhardt H, Pfander J P, Müller S, Goldie K, Engel A, Stetter K O, Baumeister W. Tetrabrachion: a filamentous archaebacterial surface protein assembly of unusual structure and extreme stability. J Mol Biol. 1995;245:385–401. doi: 10.1006/jmbi.1994.0032. [DOI] [PubMed] [Google Scholar]
- 100.Peters J, Baumeister W, Lupas A. Hyperthermostable surface layer protein tetrabrachion from the arachaebacterium Staphlyothermus marinus: evidence for the presence of a right-handed coiled coil derived from primary structure. J Mol Biol. 1996;257:1031–1041. doi: 10.1006/jmbi.1996.0221. [DOI] [PubMed] [Google Scholar]
- 101.Peters J, Peters M, Lottspeich F, Baumeister W. S-layer protein gene of Acetogenium kivui: cloning and expression in Escherichia coli and determination of the nucleotide sequence. J Bacteriol. 1989;171:6307–6315. doi: 10.1128/jb.171.11.6307-6315.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peyret J L, Bayan N, Joliff G, Gulik-Krzywicki T, Mathieu L, Shechter E, Leblon G. Characterization of the cspB gene encoding PS2, an ordered surface layer protein in Corynebacterium glutamicum. Mol Microbiol. 1993;9:97–109. doi: 10.1111/j.1365-2958.1993.tb01672.x. [DOI] [PubMed] [Google Scholar]
- 103.Phipps B, Huber R, Baumeister W. The cell envelope of the hyperthermophilic archaebacterium Pyrobaculum organothrophum consists of two regularly arrayed protein layers: three-dimensional structure of the outer layer. Mol Microbiol. 1991;5:523–528. doi: 10.1111/j.1365-2958.1991.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 104.Pouwels P, Kolen C P A M, Boot H J. S-layer protein genes in Lactobacillus. FEMS Microbiol Rev. 1997;20:78–82. [Google Scholar]
- 105.Pum D, Sleytr U B. The application of bacterial S-layers in molecular nanotechnology. Trends Biotechnol. 1999;17:8–12. [Google Scholar]
- 106.Pum D, Messner P, Sleytr U B. Role of the S-layer in morphogenesis and cell division of the archaebacterium Methanocorpusculum sinense. J Bacteriol. 1991;173:6865–6873. doi: 10.1128/jb.173.21.6865-6873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ries W, Hotzy C, Schocher I, Sleytr U B, Sára M. Evidence that a secondary cell wall polymer recognizes the N-terminal part of the S-layer protein from Bacillus stearothermophilus PV72/p2. J Bacteriol. 1997;179:3892–3898. doi: 10.1128/jb.179.12.3892-3898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rini J M. Lectin structure. Annu Rev Biophys Biomol Struct. 1995;24:551–577. doi: 10.1146/annurev.bb.24.060195.003003. [DOI] [PubMed] [Google Scholar]
- 109.Sára M, Egelseer E M. Functional aspects of S-layers. In: Sleytr U B, Messner P, Pum D, Sára M, editors. Crystalline bacterial cell surface layer proteins (S-layers). Austin, Tex: Landes Company, Academic Press; 1996. pp. 103–131. [Google Scholar]
- 110.Sára M, Dekitsch C, Mayer H F, Egelseer E M, Sleytr U B. Influence of the secondary cell wall polymer on the reassembly, recrystallization and stability properties of the S-layer protein from Bacillus stearothermophilus PV72/p2. J Bacteriol. 1998;180:4146–4153. doi: 10.1128/jb.180.16.4146-4153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sára M, Egelseer E M, Dekitsch C, Sleytr U B. Identification of two binding domains, one for peptidoglycan and another for a secondary cell wall polymer, on the N-terminal part of the S-layer protein SbsB from Bacillus stearothermophilus PV72/p2. J Bacteriol. 1998;180:6780–6783. doi: 10.1128/jb.180.24.6780-6783.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sára M, Kuen B, Mayer H F, Mandl F, Schuster K C, Sleytr U B. Dynamics in oxygen-induced changes in S-layer protein synthesis from Bacillus stearothermophilus PV72 and the S-layer deficient variant T5 in continuous culture and studies on the cell wall composition. J Bacteriol. 1996;178:2108–2117. doi: 10.1128/jb.178.7.2108-2117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sára M, Sleytr U B. Molecular-sieving through S-layers of Bacillus stearothermophilus strains. J Bacteriol. 1987;169:4092–4098. doi: 10.1128/jb.169.9.4092-4098.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sára M, Sleytr U B. Production and characteristics of ultrafiltration membranes with uniform pores from two-dimensional arrays of proteins. J Membr Sci. 1987;33:27–49. [Google Scholar]
- 115.Sára M, Sleytr U B. Crystalline bacterial cell surface layers (S-layers): from cell structure to biomimetics. Prog Biophys Mol Biol. 1996;65:83–111. doi: 10.1016/s0079-6107(96)00007-7. [DOI] [PubMed] [Google Scholar]
- 116.Sára M, Sleytr U B. Biotechnology and biomimetic with crystalline bacterial cell surface layers (S-layers) Micron. 1996;27:141–156. doi: 10.1016/0968-4328(96)80628-7. [DOI] [PubMed] [Google Scholar]
- 117.Schäffer C, Kählig H, Christian R, Schulz G, Zayni S, Messner P. The diacetamidodideoxymannuronic acid-containing glycan chain of Bacillus stearothermophilus NRS 2004/3a represents the secondary cell wall polymer of wild-type B. stearothermophilus strains. Microbiology. 1999;145:1575–1583. doi: 10.1099/13500872-145-7-1575. [DOI] [PubMed] [Google Scholar]
- 118.Schäffer C, Wugeditsch T, Neuninger C, Messner P. Are S-layer glycoproteins and lipopolysaccharides related? Microb Drug Resist. 1996;2:17–23. doi: 10.1089/mdr.1996.2.17. [DOI] [PubMed] [Google Scholar]
- 119.Schneitz C, Nuotio L, Lounatmaa K. Adhesion of Lactobacillus acidophilus to avian intestinal epithelial cells mediated by the crystalline bacterial cell surface layer (S-layer) J Appl Bacteriol. 1992;74:290–299. doi: 10.1111/j.1365-2672.1993.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 120.Scholz H. Genetische Analyse der S-layer Protein Variation in Bacillus stearothermophilus PV72. Ph.D. thesis. Vienna, Austria: University of Vienna; 1998. [Google Scholar]
- 121.Scholz H, Kuen B, Lubitz W, Sára M. S-layer variation in Bacillus stearothermophilus. FEMS Microbiol Rev. 1997;20:69–78. [Google Scholar]
- 122.Schultze-Lam S, Beveridge T J. Nucleation of celestite and strontianite in a cyanobacterial S-layer. Appl Environ Microbiol. 1994;60:447–453. doi: 10.1128/aem.60.2.447-453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schultze-Lam S, Harauz G, Beveridge T J. Participation of a cyanobacterial S layer in fine-grain mineral formation. J Bacteriol. 1992;174:7971–7981. doi: 10.1128/jb.174.24.7971-7981.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shenton W, Pum D, Sleytr U B, Mann S. Synthesis of cadmium sulphide super-lattices using self-assembled bacterial S-layers. Nature. 1997;389:585–587. [Google Scholar]
- 125.Sleytr U B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly and function. Int Rev Cytol. 1978;53:1–64. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- 126.Sleytr U B. Heterologous reattachment of regular arrays of glycoproteins on bacterial cell surface. Nature. 1975;257:400–402. doi: 10.1038/257400a0. [DOI] [PubMed] [Google Scholar]
- 127.Sleytr U B, Beveridge T J. Bacterial S-layers. Trends Microbiol. 1999;7:253–260. doi: 10.1016/s0966-842x(99)01513-9. [DOI] [PubMed] [Google Scholar]
- 128.Sleytr U B, Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- 129.Sleytr U B, Messner P. Self-assembly of crystalline bacterial cell surface layers (S-layers) In: Plattner H, editor. Electron microscopy of subcellular dynamics. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 13–31. [Google Scholar]
- 130.Sleytr U B, Messner P, Pum D. Analysis of crystalline bacterial surface layers by freeze-etching, metal shadowing, negative staining and ultrathin sectioning. Methods Microbiol. 1988;20:29–60. [Google Scholar]
- 131.Sleytr U B, Messner P, Pum D, Sára M. Crystalline bacterial cell surface layers. Berlin, Germany: Springer-Verlag; 1988. [Google Scholar]
- 132.Sleytr U B, Messner P, Pum D, Sára M. Crystalline bacterial cell surface layers. Mol Microbiol. 1993;10:911–916. doi: 10.1111/j.1365-2958.1993.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 133.Sleytr U B, Messner P, Pum D, Sára M. Crystalline bacterial cell surface proteins. Austin, Tex: Landes Company, Academic Press; 1996. [Google Scholar]
- 134.Sleytr U B, Messner P, Pum D, Sára M. Immobilised macromolecules: application potentials. London, England: Springer-Verlag; 1993. [Google Scholar]
- 135.Sleytr U B, Messner P, Pum D, Sára M. Crystalline bacterial cell surface layers (S-layers): from supramolecular cell structure to biomimetics and nanotechnology. Angew Chem Int Ed. 1999;38:1034–1054. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1034::AID-ANIE1034>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 136.Sleytr, U. B., and M. Sára. 21 June 1988. Structure with membranes having continuous pores. U.S. patent 4,752,395.
- 137.Sleytr U B, Sára M. Bacterial and archaeal S-layer proteins: structure-function relationships and their biotechnological applications. Trends Biotechnol. 1997;15:20–26. doi: 10.1016/S0167-7799(96)10063-9. [DOI] [PubMed] [Google Scholar]
- 138.Sumper M. S-layer glycoproteins from moderately and extremely halophilic archaeobacteria. In: Beveridge T J, Koval S F, editors. Advances in paracrystalline bacterial surface layers. New York, N.Y: Plenum Press; 1993. pp. 109–118. [Google Scholar]
- 139.Sumper M, Berg E, Mengele R, Strobel I. Primary structure and glycosylation of the S-layer protein of Haloferax volcanii. J Bacteriol. 1990;172:7111–7118. doi: 10.1128/jb.172.12.7111-7118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takeoka A, Takumi K, Kawata T. Purification and characterization of S-layer proteins from Clostridium difficile GAI 0714. J Gen Microbiol. 1991;137:261–267. doi: 10.1099/00221287-137-2-261. [DOI] [PubMed] [Google Scholar]
- 141.Thomas S R, Trust T J. Tyrosine phosphorylation of the tetragonal paracrystalline array of Aeromonas hydrophila: molecular cloning and high-level expression of the S-layer protein gene. J Mol Biol. 1995;245:568–581. doi: 10.1006/jmbi.1994.0047. [DOI] [PubMed] [Google Scholar]
- 142.Thompson S A, Shedd O L, Ray K C, Beins M H, Jorgensen J P, Blaser M J. Campylobacter fetus surface layer proteins are transported by a type I secretion system. J Bacteriol. 1998;180:6450–6458. doi: 10.1128/jb.180.24.6450-6458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thornton J C, Garduno R A, Newman S G, Kay W W. Surface-disorganized, attenuated mutants of Aeromonas salmonicida as furunculosis live vaccines. Microb Pathog. 1991;11:85–99. doi: 10.1016/0882-4010(91)90002-r. [DOI] [PubMed] [Google Scholar]
- 144.Tsuboi A, Uchihi R, Tabata R, Takahashi Y, Hashiba H, Sasaki T, Yamagata H, Tsukagoshi N, Udaka S. Characterization of the genes coding for two major cell wall proteins from protein-producing Bacillus brevis 47: complete nucleotide sequence of the outer wall protein gene. J Bacteriol. 1986;168:365–373. doi: 10.1128/jb.168.1.365-373.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsuboi A, Tsukagoshi N, Udaka S. Characterization of the genes for the hexagonally arranged surface layer proteins in protein-producing Bacillus brevis 47: complete nucleotide sequence of the middle wall protein gene. J Bacteriol. 1988;177:614–620. doi: 10.1128/jb.170.2.935-945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tummuru M, Blaser M J. Rearrangement of sapA homologs with conserved and variable regions in Campylobacter fetus. Proc Natl Acad Sci USA. 1993;90:7265–7269. doi: 10.1073/pnas.90.15.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vidgren G, Palva I, Pakkanen R, Lounatmaa K, Palva A. S-layer protein gene of Lactobacillus brevis: cloning by polymerase chain reaction and determination of nucleotide sequence. J Bacteriol. 1992;174:7419–7427. doi: 10.1128/jb.174.22.7419-7427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Walker S G, Nedra Karunaratne D, Ravenscroft N, Smit J. Characterization of mutants of Caulobacter crescentus defective in surface attachment of the paracrystalline surface layer. J Bacteriol. 1994;176:6312–6323. doi: 10.1128/jb.176.20.6312-6323.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang B, Kraig E, Kolodrubetz D. A new member of the S-layer protein family: characterization of the crs gene from Campylobacter rectus. Infect Immun. 1998;66:1521–1526. doi: 10.1128/iai.66.4.1521-1526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weigert S, Sára M. Surface modification of an ultrafiltration membrane with crystalline structure and studies on interactions with selected protein molecules. J Membr Sci. 1995;106:147–159. [Google Scholar]
- 151.Weigert S, Sára M. Ultrafiltration membranes from crystalline bacterial cell surface layers as model systems for studying the influence of surface properties on protein adsorption. J Membr Sci. 1996;121:185–196. [Google Scholar]
- 152.Weis W I. Cell-surface carbohydrate recognition by animal and viral lectins. Curr Opin Struct Biol. 1997;7:624–630. doi: 10.1016/s0959-440x(97)80070-x. [DOI] [PubMed] [Google Scholar]
- 153.Wugedtisch T, Zacchara N E, Puchberger M, Kosma P, Gooley A A, Messner P. Structural heterogeneity in the core oligosaccharide of the S-layer glycoprotein from Aneurinibacillus thermoaerophilus DSM 1055. Glycobiology. 1999;9:787–795. doi: 10.1093/glycob/9.8.787. [DOI] [PubMed] [Google Scholar]
- 154.Yamada H, Tsukagoshi N, Udaka S. Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium Bacillus brevis 47. J Bacteriol. 1981;148:322–332. doi: 10.1128/jb.148.1.322-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yao R, Macario A J, Conway de Macario E. An archaeal S-layer gene homolog with repetitive units. Biochim Biophys Acta. 1994;1219:607–700. doi: 10.1016/0167-4781(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 156.Zhu X, Mc Veigh R R, Malathi P, Ghosh B K. The complete nucleotide sequence of the Bacillus licheniformis NM105 S-layer encoding gene. Gene. 1996;173:189–194. doi: 10.1016/0378-1119(96)00233-8. [DOI] [PubMed] [Google Scholar]