Abstract
The Drosophila connectome project aims to map the synaptic connectivity of entire larval and adult fly neural networks, which is essential for understanding nervous system development and function. So far, the project has produced an impressive amount of electron microscopy data that has facilitated reconstructions of specific synapses, including many in the larval locomotor circuit. While this breakthrough represents a technical tour de force, the data remain underutilized, partly because of a lack of functional validation of reconstructions. Attempts to validate connectivity posited by the connectome project, have mostly relied on behavioral assays and/or GFP reconstitution across synaptic partners (GRASP) or GCaMP imaging. While these techniques are useful, they have limited spatial or temporal resolution. Electrophysiological assays of synaptic connectivity overcome these limitations. Here, we combine patch-clamp recordings with optogenetic stimulation in male and female larvae, to test synaptic connectivity proposed by connectome reconstructions. Specifically, we use multiple driver lines to confirm that several connections between premotor interneurons and the anterior corner cell motoneuron are, as the connectome project suggests, monosynaptic. In contrast, our results also show that conclusions based on GRASP imaging may provide false-positive results regarding connectivity between cells. We also present a novel imaging tool, based on the same technology as our electrophysiology, as a favorable alternative to GRASP imaging. Finally, of eight Gal4 lines tested, five are reliably expressed in the premotor interneurons they are targeted to. Thus, our work highlights the need to confirm functional synaptic connectivity, driver line specificity, and use of appropriate genetic tools to support connectome projects.
SIGNIFICANCE STATEMENT The Drosophila connectome project aims to provide a complete description of connectivity between neurons in an organism that presents experimental advantages over other models. It has reconstructed hundreds of thousands of synaptic connections of the fly larva by manual identification of anatomic landmarks present in serial section transmission electron microscopy (ssTEM) volumes of the larval CNS. We use a highly reliable electrophysiological approach to verify these connections, providing useful insight into the accuracy of work based on ssTEM. We also present a novel imaging tool for validating excitatory monosynaptic connections between cells and show that several genetic driver lines designed to target neurons of the larval connectome exhibit nonspecific and/or unreliable expression.
Keywords: connectome, Drosophila, electrophysiology, genetics, optogenetics, synapse
Introduction
Invertebrate models are often used for experiments on neural circuits, because they offer several advantages over mammals (Hunter et al., 2021). One is the large number of “identified neurons” in invertebrates, which occupy reliable anatomic positions across preparations. This is important for work that combines cell-specific genetic manipulation with electrophysiology in Drosophila (Baines and Bate, 1998; Baines et al., 1999, 2001, 2002; Choi et al., 2004; Worrell and Levine, 2008; Ryglewski et al., 2012; Srinivasan et al., 2012a, b; Kadas et al., 2017). Indeed, identified neurons are amenable to cell type-specific genetic manipulation via “driver lines,” such as split-GAL4 lines (Kohsaka et al., 2014; Fushiki et al., 2016; Hasegawa et al., 2016; Schneider-Mizell et al., 2016; Kohsaka et al., 2019). It is therefore significant that, recently, the entire Drosophila first-instar larval nervous system was imaged using serial section transmission electron microscopy (ssTEM; Ohyama et al., 2015; Schneider-Mizell et al., 2016; Gerhard et al., 2017; Larderet et al., 2017; Saumweber et al., 2018; Zarin et al., 2019) and annotated into a CATMAID (Collaborative Annotation Toolkit for Massive Amounts of Image Data; Saalfeld et al., 2009) dataset. This dataset is being used by the Drosophila connectome project to identify all neurons in the larval CNS and to characterize the connections between them. The project has already reconstructed the majority of the CNS, and a large number of putative neuronal connections has been published (Kohsaka et al., 2014; Heckscher et al., 2015; Itakura et al., 2015; Fushiki et al., 2016; Hasegawa et al., 2016; Schneider-Mizell et al., 2016; Yoshikawa et al., 2016; Zwart et al., 2016; Burgos et al., 2018; Carreira-Rosario et al., 2018; Zarin et al., 2019). Researchers are making use of this information and cell type-associated transgenic expression lines to investigate the physiology and function of neurons and neural networks (Giachello et al., 2019; Ackerman et al., 2021; Giachello et al., 2021). The accuracy of this and future work is, therefore, predicated on the accuracy of manual reconstructions conducted as part of the connectome project, and the expression patterns of related driver lines. Both may be subject to error and require validation.
Most attempts to validate connections posited by ssTEM reconstruction have focused on behavioral analysis and/or functional imaging using GFP reconstitution across synaptic partners (GRASP) or GCaMP, which can suggest that activity is coordinated across neurons (Hasegawa et al., 2016; Kohsaka et al., 2019; Zarin et al., 2019). However, the value of using these techniques to validate synaptic connectivity is limited by a lack of spatial or temporal resolution that makes it difficult to determine whether cells are monosynaptically or polysynaptically connected. Given this limitation, a combination of optogenetics, Ca2+ imaging, and pharmacology has been used to determine monosynaptic connectivity (Sales et al., 2019). However, results generated by this approach may be complicated by variability in responses recorded from the postsynaptic neuron. In contrast, electrophysiology provides unambiguous evidence for monosynaptic connections between cells, and is regarded as the “gold standard” for doing so. It is perhaps surprising then, that few publications have used electrophysiology to functionally validate a connection posited in the connectome (Fushiki et al., 2016). This may be because of the difficulty of the technique and the corresponding paucity of researchers able to use it.
In this study, we used a whole-cell patch-clamp electrophysiology-based assay for connectivity to validate connections posed by connectome project reconstructions. Specifically, we screened Gal4 driver lines that were reported to target expression of Gal4 to five identified premotor interneurons (PMINs): cholinergic A27h, A18a (also called CLI2), and A18b3 (also called CLI1), plus GABAergic A23a and A31k. These interneurons were proposed to monosynaptically connect to the anterior corner cell [aCC (also called MN1-lb)] motoneuron (Hoang and Chiba, 2001); however, this connectivity had not been functionally validated before the present work. We adapted a protocol called “TERPS” (Zhang and Gaudry, 2016), which uses genetically targeted expression of a tetrodotoxin (TTX)-insensitive bacterial cation channel, voltage-gated sodium channel from Bacillus halodurans (NaChBac; Ren et al., 2001), to test whether neurons are monosynaptically connected (Zhang and Gaudry, 2018; Suzuki et al., 2020) and showed that four of the five premotor interneurons are connected to aCC. We also highlighted the limitations of GRASP, which has been shown previously, and in the present research, to infer a direct connection between A18b3 and aCC (Hasegawa et al., 2016). This contrasts with the more accurate results we generated using TERPS, which demonstrate that this is not the case. Finally, we show that not all Gal4 lines express as suggested by name, and so, highlight the importance of careful characterization of expression before lines are used to infer cell or network function.
Materials and Methods
Experimental design
Drosophila rearing and stocks.
All Drosophila stocks were kept on standard corn meal medium, at 25°C. The following lines provided the optogenetic and other transgenic tools necessary to manipulate and record the connectivity of neurons: ChR; NaChBac (w*; 20xUAS-T159C-ChR2; UAS-NaChBac-EGFP/TM6CSb, Tb), which was created by crossing y1,w*; PBac{20xUAS-ChR2.T195C-HA}VK00018; Dr1/TM6CSb, Tb (stock #52258, Bloomington Drosophila Stock Center (BDSC), Bloomington, IN) and y1,w*; P{UAS-NaChBac-EGFP}1/TM3Sb (stock #9467, BDSC); and w*; UAS-H134R-ChR2; (gift from Stefan Pulver; University of ST. Andrews, Scotland, U.K.) and w*; P(y[+t7.7] w*; P{20xUAS-Chronos-mVenus}attP40 (stock #77115, BDSC). These lines were also combined with one expressing an RCaMP transgene (described in the subsection Molecular biology) to produce the imaging tool for assessing monosynaptic connectivity between neurons, as follows: w[*]; DvGlut-T2A-QF2, P{y[+t7.7], 5xQUAS IVS syn21 RCaMP1b P2A nls-GFP P10 in su(Hw)attP5, 20xUAS-ChR2.T159C-HA}VK00018/CyO, Dfd-GMR-YFP; UAS-NaChBac-EGFP}1 in 85A, 87D/TM6bSb, and Dfd-GMR-YFP.
Driver lines targeting Gal4 expression to interneurons were as follows: w1118; +; R36G02-Gal4 (“A27h-Gal4”; stock #49939, BDSC), which expresses in the premotor interneuron A27h, as well as in three other neurons (Fushiki et al., 2016): w1118; +; 47E12-Gal4 (CLIs-Gal4; stock #50317, BDSC), which expresses in A18b3 (also called CLI1) and A18a (also called CLI2), plus a range of other interneurons and some sensory neurons (Hasegawa et al., 2016); w−; +; R47E12-Gal4; cha3.3-Gal80 (“CLI1/2-Gal4”), which exploits Gal80 to facilitate more specific expression to A18b3 and A18a, than 47E12-Gal4 does alone (Hasegawa et al., 2016); w−; tsh-Gal80; R47E12-Gal4; cha3.3-Gal80 (“CLI1-Gal4”), which is specific for A18b3 (Hasegawa et al., 2016); w−; +; R15B07-Gal4 (“CLI2-Gal4”), which is specific for A18a (Hasegawa et al., 2016); and w−; +; GAD1-T2A-Gal4 expresses in all GABAergic neurons (Diao et al., 2015). We used three driver lines reported to express in the GABAergic premotor interneuron A23a: R78F07-Gal4 (Zarin et al., 2019), R78F07-AD; R49C08-DBD split Gal4, and R41G07-AD; R78F07-DBD split Gal4 (also called SS04495-Gal4; Kohsaka et al., 2019). We also used three driver lines reported to express in the GABAergic premotor interneuron A31k: R87H09-Gal4 (Zarin et al., 2019), R20A03-AD; R87H09-DBD split Gal4 (A. Zarin), and R20A03-AD; R93B07-DBD split Gal4 (also called SS04399-Gal4; Kohsaka et al., 2019). For cyan GRASP experiments, we used the following lines: w-; UAS-CD4::spCer1–10, LexAop-CD4::spGFP11/CyO, Dfd-GMR-YFP; RN2-FLP (hopA), tub-FRT-stop-FRT-LexA::VP16, 13xLexAop2-myr::YPet (attP2)/TM6b, Sb, Dfd-GMR-YFP, and line y1 w*; P{w[+mC]=UAS-CD4-tdTom}7M1 (stock #35841, BDSC).
Molecular biology.
To generate a cyan fluorescent version of GRASP, four mutations (Y66W, S72A, H148D, and N149I) were introduced into the 1-10 fragment of spGFP. The mutated sequence was codon optimized and synthesized by Integrated DNA technologies (IDT). PAT2-SP::Cerulean1-10 and CD2 coding sequences, as well the pJFRC161 vector backbone, were PCR amplified, all with overlapping ends, then ligated via Gibson Assembly. Flies were transformed by BestGene.
The Cerulean1-10 coding sequence was as follows: ATGTCCAAGGGCGAGGAGCTGTTCACCGGCGTGGTGCCCATCCTGGTGGAGCTGGACGGCGACGTGAACGGCCACAAGTTCTCCGTGCGCGGCGAGGGCGAGGGCGACGCCACCATCGGCAAGCTGACCCTGAAGTTCATCTGCACCACCGGCAAGCTGCCCGTGCCCTGGCCCACCCTGGTGACCACCCTGACCTGGGGCGTGCAGTGCTTCGCCCGCTACCCCGACCACATGAAGCGCCACGACTTCTTCAAGTCCGCCATGCCCGAGGGCTACGTGCAGGAGCGCACCATCTCCTTCAAGGACGACGGCAAGTACAAGACCCGCGCCGTGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGCATCGAGCTGAAGGGCACCGACTTCAAGGAGGACGGCAACATCCTGGGCCACAAGCTGGAGTACAACTTCAACTCCGACATCGTGTACATCACCGCCGACAAGCAGAAGAACGGCATCAAGGCCAACTTCACCGTGCGCCACAACGTGGAGGACGGCTCCGTGCAGCTGGCCGACCACTACCAGCAGAACACCCCCATCGGCGACGGCCCCGTGCTGCTGCCCGACAACCACTACCTGTCCACCCAGACCGTGCTGTCCAAGGACCCCAACGAGAAGGGCACC.
The primers used were as follows: Cerulean1-10 forward, GAAACGACTAACCCTAATTCTTATCCTTTACTTCAGGCGGCCGCGGCTCGAGCAGATATCGACAAGTTTGTAC; Cerulean1-10 reverse, CTCTGCAGTCAGTTGTGGTGCCCTTCTCGTTG; CD2 forward, CACCACAACTGACTGCAGAGACAGTGGGAC; CD2 reverse, CCAATTATGTCACACCACAGAAGTAAGGTTCCTTCACAAAGATCCTCTAGACACCACTTTGTACAAGAAAGCTG; pJFRC161 forward, TCTAGAGGATCTTTGTGAAGGAAC; pJFRC161 reverse, CTCGAGCCGCGGCCGCCTGAAGTAAAG.
To generate the 5xQUAS-IVS-syn21-RCaMP1b-P2A-nls::GFP-P10 transgene necessary for the RCaMP imaging tool for assessing monosynaptic connectivity between neurons, we used a commercial gene synthesis (GenScript) for the insert and upstream activation sequence, followed by restriction enzyme-based cloning into the pJFRC7 backbone (Pfeiffer et al., 2010), whose 20xUAS sequences were replaced with 5xQUAS (Potter et al., 2010). Within the bicistronic insert, the red-shifted calcium indicator with nuclear export sequence (NES-jRCaMP1b; Dana et al., 2016) is separated from EGFP with a nuclear localization sequence by the self-cleaving peptide sequence P2A (Kim et al., 2011; Daniels et al., 2014). Three translational enhancers (IVS, Syn21, and p10) were added to 5′ or 3′ untranslated regions (Pfeiffer et al., 2012). A fly stock was then generated by inserting this construct into the su(Hw)attP5 landing site using phiC31 site-specific recombination (Groth et al., 2004).
Gal4 expression and GRASP imaging.
Newly hatched male and female larvae containing one copy of the GAL4-producing transgenes and 10xUAS-IVS-myr::GFP transgene reporter inserted in attP2 (Pfeiffer et al., 2010) were kept on apple juice agar plates with yeast paste food, at 25°C until the third-instar stage of development (48 h after larval hatching). Each larva was dissected in extracellular saline to isolate the CNS, using a hypodermic syringe needle (30 gauge; BD Microlance) as a scalpel. The CNS was then transferred onto poly-l-lysine-coated (Sigma-Aldrich) cover glass and fixed with 4% paraformaldehyde (Agar Scientific) in saline for 15 min, at room temperature. Following the standard procedures of washes in PBS containing 0.3% Triton X-100 and 0.25% (w/v) bovine serum albumin (Sigma-Aldrich), Gal4-directed expression of membrane-targeted GFP was visualized in the longitudinal Fasciclin II-positive axon tracts (Landgraf et al., 2003) via chicken anti-GFP (1:5000; catalog #ab13970, Abcam), visualized with donkey anti-chicken-CF488A (1:1000; catalog #20166, Biotium) and mouse anti-Fasciclin II (1:20; stock #1D4, DSHB), visualized with goat anti-mouse-StarRed (1:2000; Abberior). Stained nerve cords were cleared in 70% glycerol, then mounted in EverBrite medium (Biotium and Cambridge BioScience) and sandwiched under a second cover glass, with thin aluminum foil strips used as spacers. Imaging was performed with one of the following two confocal point scanning microscopes: Leica model SP5 with a 63×/1.2 numerical aperture (NA) glycerol immersion objective; or an Olympus model FV3000 with a 60×/1.3 NA silicone oil-immersion objective.
For GRASP imaging, male and female larvae were dissected in extracellular saline (135 mm NaCl, 5 mm KCl, 4 mm MgCl2 · 6H2O, 2 mm CaCl2 · 2H2O, 5 mm TES (triethylsilane), and 36 mm sucrose, at pH 7.15) to isolate the ventral nerve cord (VNC) and brain lobes. This preparation was placed on a poly-l-lysine (Sigma-Aldrich)-coated cover glass, dorsal side up, and imaged immediately using a custom-built spinning disk confocal field scanning system consisting of the following: a field scanner (model CSU-22, Yokagawa), mounted on a fixed-stage upright microscope frame (model BX51-WI, Olympus), equipped with a single objective piezo focusing device (Physik Instruments), a 60×/1.2 NA water-immersion objective (Olympus), an external filter wheel (Sutter Instrument), and a programmable XY stage (Prior). Images were acquired at an effective voxel size of 0.217 × 0.217 × 0.3 µm using a back-thinned electron-multiplying CCD camera (model Evolve, Photometrics), operated via MetaMorph software (Molecular Devices).
Electrophysiology.
Electrophysiological recordings were performed as previously described (Baines et al., 1990; Marley and Baines, 2011), in mostly third (L3) or occasionally first (L1)-instar larvae (as relevant to Results). In brief, the protocol for assessing monosynaptic connectivity between neurons was as follows: male and female larvae were dissected as for the GRASP imaging signal, and the isolated CNS preparation was glued to a Sylgard-coated cover slip on a slide. Before recording, interneuron Gal4 and GFP-tagged reporter line expression [e.g., “CLI2” (Gal4 x UAS-T159C-ChR2; UAS-NaChBac-EGFP)] was checked by momentarily exposing preparations to blue light (470 nm LED; bandwidth, 25 nm; irradiance, 15.62 mW · cm−2; OptoLED, Cairn Instruments), while viewing them under an microscope (model BX51WI, Olympus). Once expression was confirmed, aCC motoneurons present in the VNC segments that expressed the interneuron Gal4 and reporter, were identified using bright-field microscopy. Protease (Sigma-Aldrich) was applied to those segments to remove overlaying glia, to facilitate access to aCC for patching. The 2 μm TTX was pipetted directly into the extracellular saline and given ∼60 s to diffuse across the preparation, unless the experiment was a TTX-free control. Other drugs [10 μm picrotoxin (PTX), 1 mm gabazine, or 167–200 μm mecamylamine] were used to abolish responses observed in the presence of TTX, and so, were pipetted into the extracellular saline following a first and before a second optogenetic stimulation protocol (see below).
Whole-cell voltage-clamp or current-clamp recordings were made from A27h or aCC, using thick-walled borosilicate glass pipettes (GC100F-10, Harvard Apparatus) that were fire polished to resistances of 10–15 MΩ (for L3 aCC) or 15–20 MΩ (L1 aCC or L3 A27h) and filled with intracellular saline (140 mm K+-d-gluconate, 2 mm MgCl2 · 6H2O, 2 mm EGTA, 5 mm KCl, and 20 mm HEPES, at pH 7.4).KCl, CaCl2, MgCl2, and sucrose used to make extracellular and/or intracellular saline were from Thermo Fisher Scientific. All other chemicals were from Sigma-Aldrich. Recordings were made using a Multiclamp 700B amplifier controlled by pCLAMP (version 10.4), via a analog-to-digital converter (model Digidata 1440A, Molecular Devices). Traces were sampled at 20 kHz and filtered online at 10 kHz. Once the “whole-cell” conformation was achieved, input resistance was measured, and only cells with an input resistance ≥0.5 GΩ and membrane resistance (Vm) less than of equal to –40 mV were used for experiments.
Voltage-clamp recordings were performed at −60 mV for excitatory interneurons and −40 mV for inhibitory interneurons (A27h, A18a, A18b3, A23a, and A31k, respectively) to promote and standardize driving force to ensure reliable inputs to aCC. Inputs were elicited by optogenetic stimulation of the interneuron, and the amplitude of input was calculated as the change in current (in picoamperes; normalized for cell capacitance and determined by integration of the area under a capacity transient generated by a −60 to −90 mV step protocol) from baseline to peak, following stimulation. Excitation of channelrhodopsin (ChR) was achieved using a 470 nm LED connected to the microscope. Light output was controlled by Clampex (version 10.4) and was pulsed onto the preparation for 1 s per one sweep. Specifically, the Clampex stimulation protocol was five sweeps (repetitions) of 1 s LED off, 1 s LED on, 1 s off, for each preparation.
Current-clamp recordings were made by injecting current (∼10 pA) sufficient to evoke action potentials (APs) in aCC, at a frequency of ∼3–8 Hz. Cells were then subjected to the same optogenetic stimulation used for voltage-clamp experiments (1 s LED off, 1 s on, 1 s off) to assess the impact of interneuron input on aCC firing frequency. Frequency was calculated as the number of action potentials occurring during the 1 s before, during, and after optogenetic stimulation.
RCaMP imaging.
Male and female larvae were dissected and mounted as for electrophysiology, and 2 μm TTX was added to the saline droplet and left to incubate for 5 min. The preparation was positioned under a 40× water-immersion lens on a microscope (model BX51WI, Olympus), and Gal4-UAS expression [e.g., A27h (Gal4 driving UAS-NaChBac-EGFP)] was confirmed as it was for electrophysiology (momentary exposure to 470 nm light). Imaging was recorded through excitation filters #378827 and #348474, plus a #378710 barrier filter (Chroma Technology) by a digital camera (model ORCA-Flash4.0, Hamamatsu), at a frame rate of 10 Hz in Winflour version 4.1.5. Light at 470 nm was used to stimulate ChR and depolarize premotor interneurons expressing Gal4, while light at 590 nm was used to visualize RCaMP activity in aCC. Activity was imaged for 60–430 s, with the 590 nm LED switched off between stimulations for longer recordings, to prevent bleaching fluorophores. After imaging, ROIs were drawn around aCC soma present in segments in which premotor interneuron Gal4 was expressed. The fluorescence changes observed in these ROIs were normalized to an ROI positioned in a dark area of the images, quantified in arbitrary units, then exported to ClampFit (version 10) to generate data traces.
Drugs.
TTX was from Alomone Labs. PTX, gabazine (SR95531), and mecamylamine were from Sigma-Aldrich.
Statistical analysis
Electrophysiology data were imported into Excel (Microsoft), and statistical tests were performed in GraphPad Prism (version 7; GraphPad Software). Statistical tests were not applied to measurements of synaptic inputs or RCaMP imaging, because of the descriptive nature of these data. Conversely, repeated-measures one-way ANOVA with Bonferroni's post hoc multiple-comparisons tests were applied to firing plots, to quantify premotor drive to aCC. p Values <0.05 were considered significant, and levels of significance were represented as follows: *p < 0.05 and **p < 0.01. Comparisons that did not reach significance are not marked. Figures were edited to improve presentation in Illustrator CS3 (Adobe).
Results
Validating the NaChBac tool and confirming A27h is monosynaptically connected to aCC
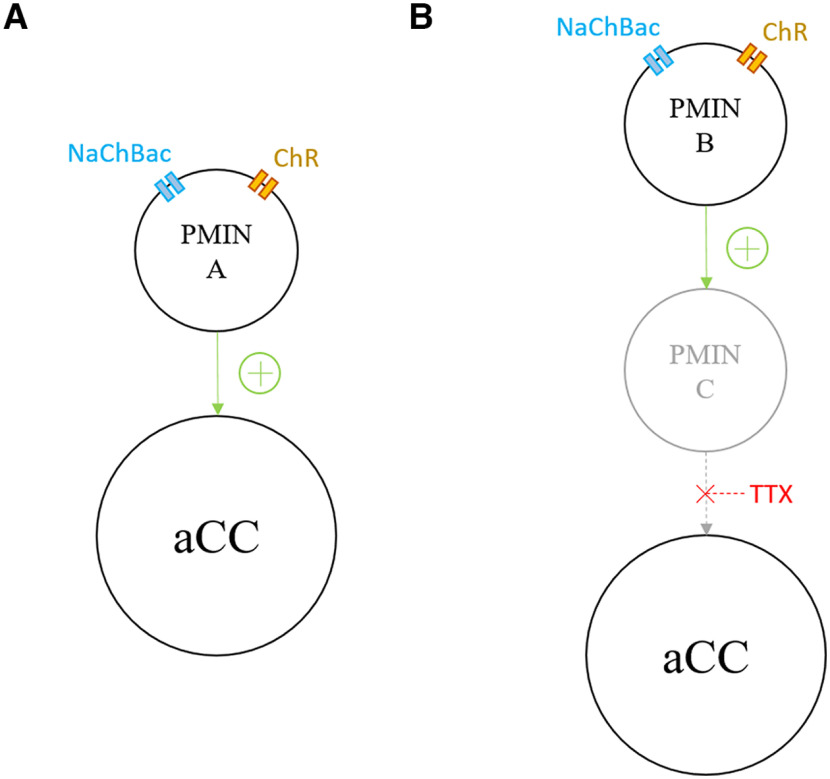
We adapted TERPS (Zhang and Gaudry, 2016), which exploits the insensitivity of the NaChBac to TTX (Ren et al., 2001), to demonstrate that NaChBac-expressing neurons are monosynaptically connected to postsynaptic cells (Zhang and Gaudry, 2016, 2018; Suzuki et al., 2020). We generated a transgenic stock containing both UAS-NaChBac and the T159C variant of UAS-ChR (Berndt et al., 2011) to allow them to be expressed simultaneously. Crossing this stock to interneuron-specific Gal4 lines and recording optogenetically induced synaptic drive in the postsynaptic aCC motoneuron, in the presence of TTX, therefore demonstrates monosynaptic connectivity between cells (Fig. 1).
Figure 1.
TERPS tool mechanism. A, B, UAS-ChR and UAS-NaChBac (NaChBac) expression are driven by a Gal4 line, in PMIN A and B). A, Light stimulation of ChR in PMIN A increases its open probability so that an inward, nonspecific cation current depolarizes the cell. This depolarization gates the voltage-sensitive NaChBac channel, which carries a strongly depolarizing sodium current. This induces synaptic transmission to neighboring aCC. B, PMIN B expresses ChR and NaChBac; however, it is not monosynaptically connected to aCC: it is polysynaptically connected to the motoneuron via PMIN C. TTX blocks the Nav channels in PMIN C, so that there is no synaptic transmission between it and aCC, following optogenetic stimulation of PMIN B.
We validated our tool by crossing it to R36G02-Gal4 (Fushiki et al., 2016), which is expressed in a number of INs, including A27h (Fig. 2A). Before the current work, A27h was the only IN that possessed a connection to aCC predicted by network reconstruction, which had been verified by electrophysiology (Fushiki et al., 2016). Whole-cell patch-clamp recordings from A27h expressing ChR and NaChBac, demonstrated the functional properties of the latter. As expected, optogenetic stimulation of A27h induced a large and slowly inactivating depolarization that is characteristic of NaChBac conductance, in the presence of 2 μm TTX (Fig. 3A, left, arrowhead). Importantly, evoked action potential firing in A27h (Fig. 3A, right, arrow) is absent in the presence of TTX (Fig. 3B). Depolarization of A27h via injection of constant current (1 pA steps/0.5 s) showed that NaChBac activates at −65 ± 12 mV (Fig. 3B). The rate of inactivation of NaChBac increases steeply as a function of voltage (Ren et al., 2001), so we determined NaChBac steady-state inactivation by measuring the peak amplitude of the current activated by ChR (470 nm, 1 s; Fig. 3C). This showed that NaChBac activity is severely reduced at prepulse membrane potentials more positive than −40 mV (Fig. 3D), which is consistent with NaChBac expressed in CHO-K1 cells (Ren et al., 2001). This severe reduction in activity at greater than –40 mV should be considered when planning and interpreting experiments using this tool (see Discussion).
Figure 2.
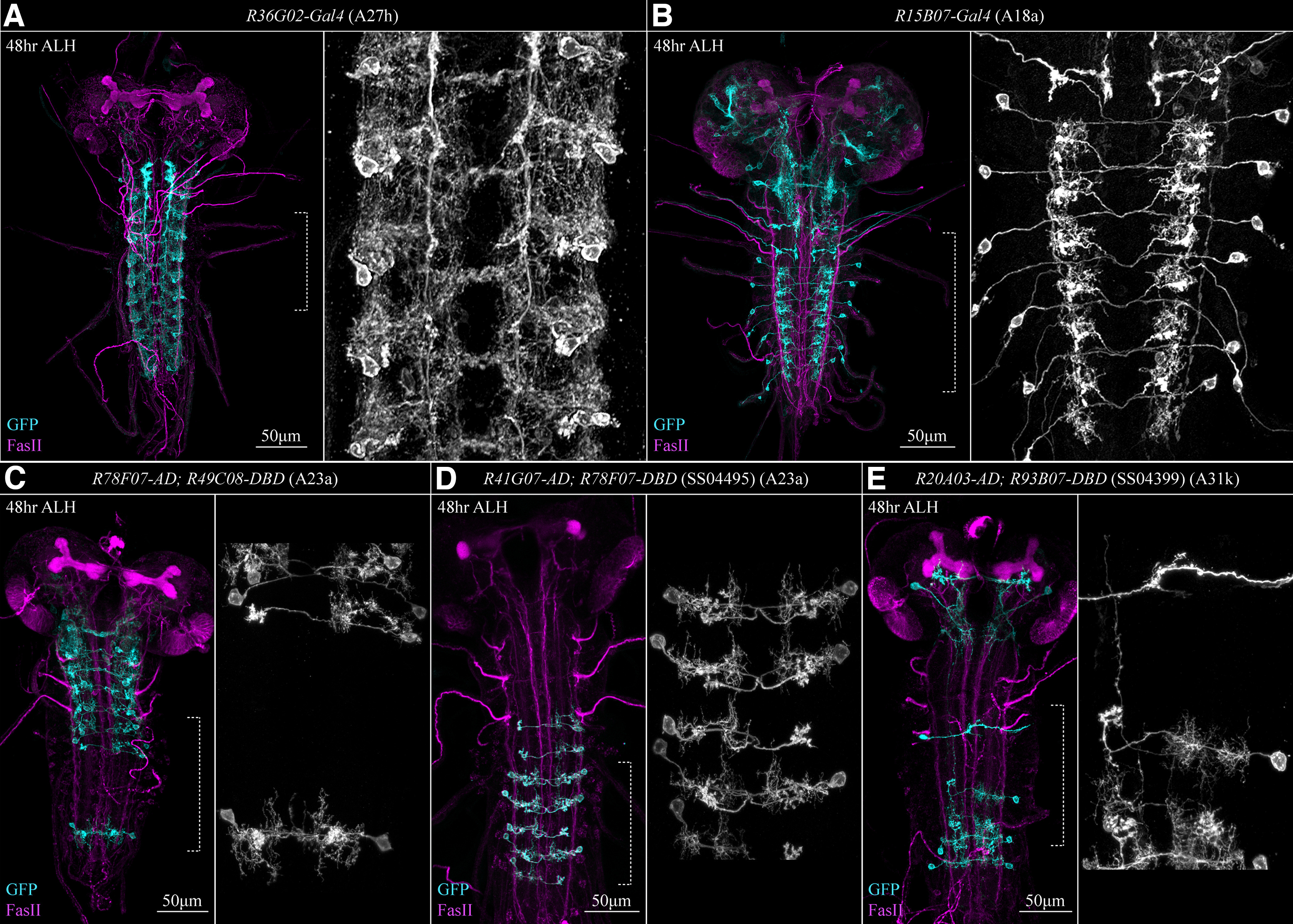
Gal4 line expression. A, R36G02-Gal4 (A27h-Gal4) is expressed in several cell types, including A27h. B, 15B07-Gal4 (CLI2-Gal4) is specific to A18a (CLI2) interneurons. C, R78F07-AD; R49C08-DBD is predominantly expressed in thoracic segments of the VNC and appears to be specific to A23a. D, R41G07-AD; R78F07-DBD, also called SS04495-Gal4, is expressed in abdominal segments of the VNC and also appears to be specific to A23a. Note that in contrast to these images, our electrophysiology and pharmacology suggested that neither R78F07-AD; R49C08-DBD nor SS04495-Gal4 was specific to A23a. E, R20A03-AD; R93B07-DBD, also called SS04399-Gal4, is strongly expressed in only the most posterior abdominal segments of the VNC and is specific to A31k interneurons. All expression patterns were imaged 48 h AEL. Left-hand panels show merged images, while right-hand panels show an enlarged GFP channel section of the left panel (indicated by dotted square bracket) that highlights individual neurons.
Figure 3.
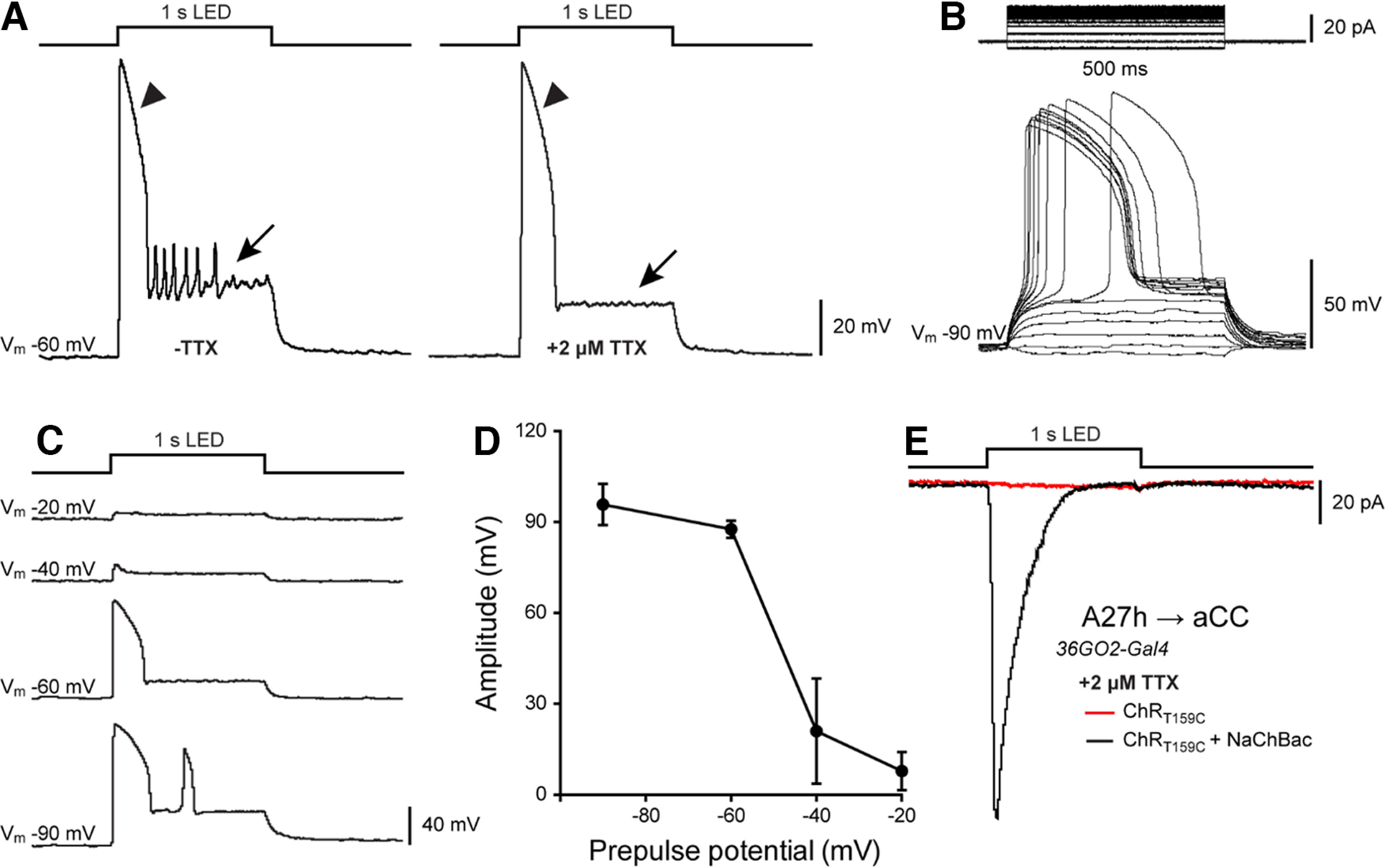
ChR; NaChBac is a powerful tool to verify monosynaptic connectivity. A, A representative current-clamp recording from the A27h IN overexpressing both ChR and NaChBac. Optogenetic stimulation (λ470 nm, 1 s) induced the activation of NaChBac, which persists in the presence of 2 μm TTX (arrowheads). Conversely, APs produced by the activation of endogenous voltage-gated sodium channels were blocked after TTX application (arrows). B, Voltage dependence of NaChBac activation recorded from A27h in current clamp. A27h depolarization was elicited by injecting constant current steps (1 pA steps/0.5 s, Vm = −90mV) in the presence of TTX. C, D, Voltage-dependent inactivation of NaChBac. Peak amplitude was recorded and measured from A27h held at different prepulse voltages (from −90 to −20 mV) during optogenetic stimulation (470 nm, 1 s). NaChBac activation is reduced at Vm more positive than −40 mV. Note that there is a second activation (peak) of NaChBaC at −90 mV. D, Averaged data ± SEM (n = 3) for traces shown in C. E, Sample recording of synaptic drive to aCC, recorded in voltage-clamp, following optogenetic activation of A27h (470 nm, 1 s). In the presence of TTX, coexpression and activation of both ChR and NaChBac in A27h produced a clear synaptic input in aCC (inward current, black trace), thus confirming the existence of a monosynaptic connection between these two neurons. As a control, TTX successfully blocked aCC inputs when only ChR, but not NaChBac, was expressed in A27h (red trace). These results validate that A27h is monosynaptically connected to aCC and validate the use of ChR; NaChBac to identify other monosynaptically connected neuron pairs.
A18a but not A18b3, is monosynaptically connected to aCC
The A18a and A18b3 interneurons (originally named CLI2 and CLI1, respectively) were first identified by their rhythmic activity, which correlates with locomotion (Hasegawa et al., 2016). We tested four different Gal4 lines that have been reported to be expressed in A18a and A18b3, in the trunk of the nerve cord: R47E12-Gal4 (also called “CLIs-Gal4”) targets A18a and A18b3, some uncharacterized interneurons, plus some sensory neurons; R47E12-Gal4; cha3.3-Gal80 (also called “CLI1/2-Gal4”) is only expressed in A18a and A18b3 (Hasegawa et al., 2016); and tsh-Gal80; R47E12-Gal4, cha3.3-Gal80 (also called “CLI1-Gal4”) is specific for A18b3 (Hasegawa et al., 2016) and R15B07-Gal4 (also called CLI2-Gal4), which is specific for A18a (Fig. 2B). Perhaps unsurprisingly, the most broadly expressed line, R47E12-Gal4 (also called CLIs-Gal4), provided the largest synaptic drive to aCC in the absence of TTX. This drive is notably reduced, but not fully blocked, by TTX [−9.55 ± 3.19 pA/pF (n = 4) to −3.57 ± 0.92 pA/pF; n = 6; Fig. 4A–C). The fact that stimulation of R47E12-Gal4-expressing neurons is more excitatory before the application of TTX than after, suggests that additional neurons (in addition to A18a and A18b3) express Gal4 and are directly or indirectly connected to aCC.
Figure 4.
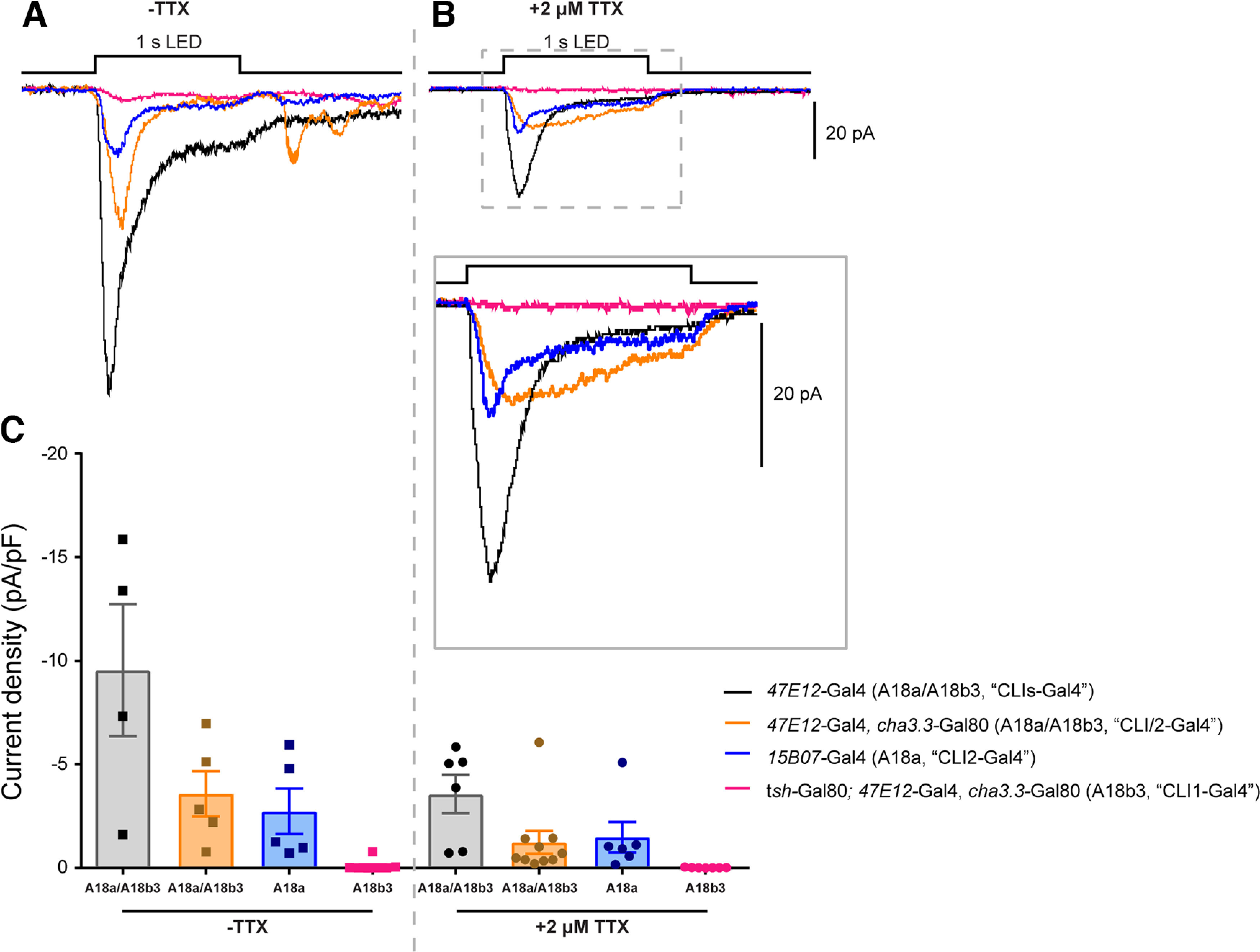
A18a, but not A18b3, is monosynaptically connected to the aCC motoneuron. A, B, Excitatory synaptic inputs to aCC recorded in the absence (A) and presence (B and inset) of TTX. Four different Gal4 lines were used to target ChR and NaChBac expression to the CLI interneurons A18a and A18b3. C, Quantification of the aCC synaptic drive revealed that A18a, but not A18b3, is monosynaptically connected to aCC. The TTX-induced reduction, but not elimination, of synaptic current amplitudes in some Gal4 expression lines [e.g., R47E12-Gal4 (CLIs-Gal4)] suggests the presence of additional Gal4-expressing interneurons not directly connected the aCC motoneuron, but indirectly contributing to its overall excitation following optogenetic stimulation in the absence of TTX.
Our results using R47E12-Gal4; cha3.3-Gal80 (CLI/2-Gal4) and R15B07-Gal4 (CLI2-Gal4) demonstrated a decrease in synaptic drive in the presence versus the absence of TTX. Currents recorded using R47E12-Gal4; cha3.3-Gal80 decreased from −3.59 ± 1.10 pA/pF (n = 5) to −1.25 ± 0.55 pA/pF (n = 10), while those recorded using R15B07-Gal4 dropped from −2.74 ± 1.09 pA/pF (n = 5) to −1.49 ± 0.73 pA/pF (n = 6). Interestingly, we recorded similar current amplitudes following depolarization of R47E12-Gal4; cha3.3-Gal80- and R15B07-Gal4-expressing neurons, which suggests that A18a (CLI2) provides the majority of the excitatory presynaptic input to aCC. This was confirmed by the fact that optogenetically stimulating A18b3 (CLI1) using tsh-Gal80; R47E12-Gal4, cha3.3-Gal80 did not provide synaptic drive to aCC, in either the absence or presence of TTX [−0.09 ± 0.09 pA/pF (n = 8) to −0.01 ± 0.01 pA/pF (n = 9)]. Thus, we conclude that A18a, but not A18b3, is monosynaptically connected to aCC. This is in agreement with the description in the study by Zarin et al. (2019) and suggests that R15B07-Gal4 is the most accurate and reliable Gal4 line to use for testing CLI input to aCC.
aCC receives GABAergic inputs
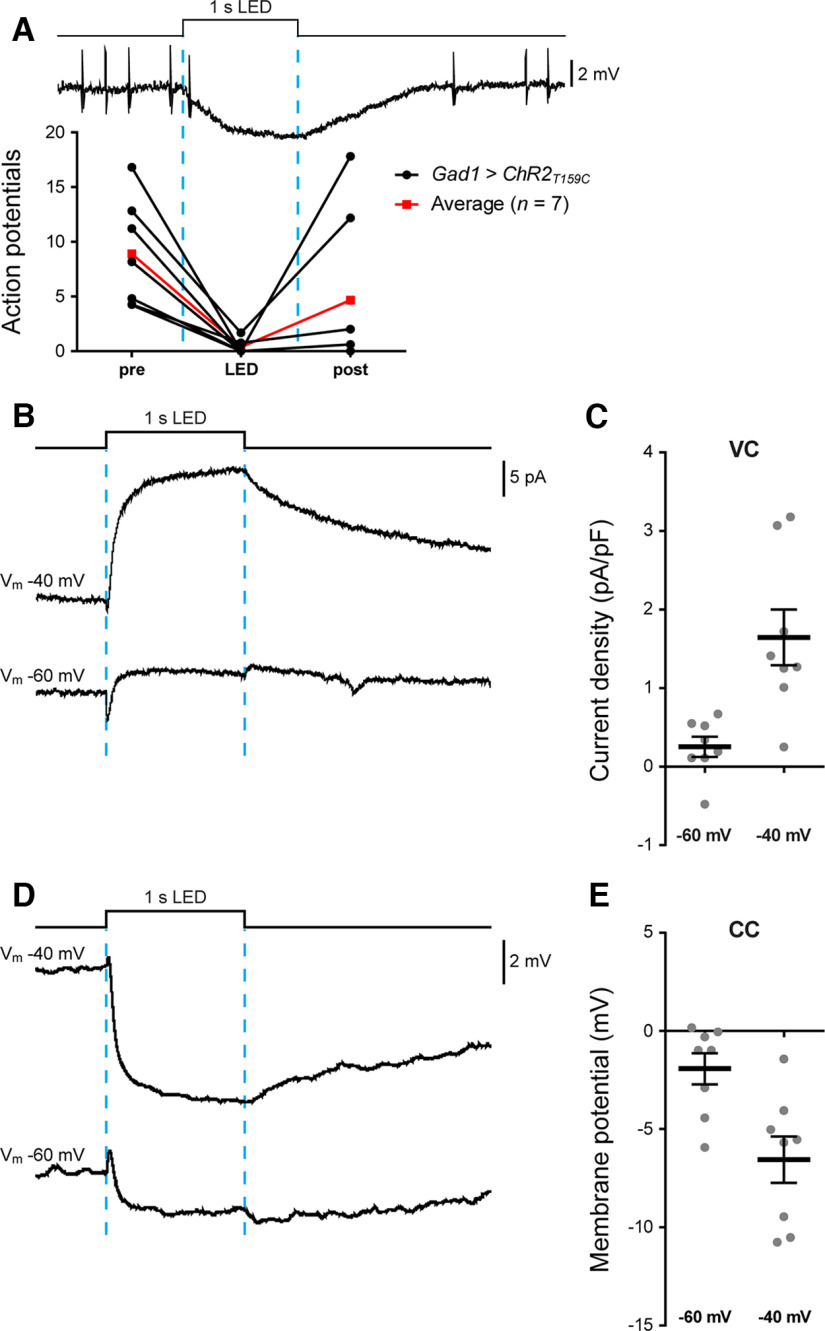
The connectome identifies inhibitory neurons making direct synaptic connections to motoneurons, including aCC (Kohsaka et al., 2019). In contrast, however, to the single cholinergic connection (A27h to aCC) that was established before our work was conducted (Fushiki et al., 2016), none of the proposed GABAergic inputs had been validated by electrophysiology. We therefore began by attempting to demonstrate GABAergic input to aCC, by expressing NaChBac in all GABAergic neurons (GAD1-T2A-Gal4). Widespread expression of NaChBac driven by GAD1-T2A-Gal4 was lethal, so we expressed ChR in GABAergic neurons instead, and recorded the synaptic input to aCC. aCC is not tonically active, so we injected current to evoke AP firing, before optogenetic stimulation. Activation of GABAergic neurons produced a significant decrease in AP firing in aCC (F(2,18) = 8.391, p = 0.0137, repeated-measures one-way ANOVA) for the duration of the light pulse (8.89 ± 1.85-0.35 ± 0.24, n = 7, p = 0.0103, Bonferroni's post hoc test; Fig. 5A). Firing resumed shortly after the cessation of light stimulation (4.26 ± 2.75 vs 8.89 ± 1.85, n = 7, p = 0.1166, Bonferroni's post hoc test). As further evidence for inhibitory drive, voltage-clamp traces showed increasing synaptic current density as aCC was depolarized away from the chloride reversal potential. A current of +0.25 ± 0.13 pA/pF (n = 8) at −60 mV increased, as expected for Cl– ions, to +1.65 ± 0.36 pA/pF (n = 8) in the same cells at −40 mV (Fig. 5B,C). Similarly, current-clamp recordings clearly exhibited an increasing hyperpolarizing drive to aCC of −1.93 ± 0.80 and −6.56 ± 1.18 mV, at −60 or −40 mV, respectively (Fig. 5D,E).
Figure 5.
aCC motoneurons receive inputs from GABAergic interneurons. A, Sample trace and quantification of APs evoked by injecting a suprathreshold depolarizing current into aCC. Optogenetic activation of all GABAergic interneurons (GAD1-T2A-Gal4>UAS- T159C-ChR; 470 nm, 1 s) almost completely inhibited AP firing in aCC (n = 7; black lines), clearly showing that aCC receives inhibitory inputs. Average values are shown in red. B, C, Sample trace and quantification of the inhibitory drive to aCC recorded in voltage-clamp mode. The same cells were recorded at Vm of −40 and −60 mV. As expected, we observed a large outward current (at −40 mV) that attenuated at more negative potentials (−60 mV) close to the chloride reversal potential (approximately –70 mV). D, E, Sample trace and quantification of the inhibitory drive to aCC recorded in current-clamp mode showing a clear hyperpolarization of aCC and similar attenuation at –60 mV compared with –40 mV.
A23a is monosynaptically connected to aCC
A23a was first described as a GABAergic interneuron presynaptic to the aCC motoneuron, which is active during both forward and backward peristaltic waves (Kohsaka et al., 2019). We tested three Gal4 lines reported to be expressed in A23a, as follows: R78F07-Gal4 (Zarin et al., 2019); R78F07-AD; R49C08-DBD split Gal4 (A. Zarin); and R41G07-AD 78F07-DBD split Gal4, also called SS04495-Gal4 (Kohsaka et al., 2019).
We found that optogenetic activation of R78F07-Gal4-expressing neurons usually caused a decrease in AP firing (F(2,15) = 5.005, p = 0.0216, repeated-measures one-way ANOVA), consistent with an inhibitory input to aCC (4.70 ± 1.07 vs 1.32 ± 0.61 APs, n = 6, p = 0.0406, Bonferroni's post hoc test; post-LED = 5.02 ± 1.01 APs; Fig. 6A). R78F07-Gal4, however, exhibits an expression pattern that is more diverse than expected; it targets some interneurons that are not A23a (Ackerman et al., 2021). This lack of specificity is reflected in the heterogeneity of responses recorded from aCC. In the absence of TTX, inhibitory inputs prevailed (input average: −1.73 ± 1.02 mV at −40 mV, n = 7; Fig. 6B), while recordings performed in the presence of TTX revealed an additional excitatory component (five of eight cells). This suggests that the inhibitory input includes a contribution from neurons polysynaptically connected to aCC, and that this GAL4 is expressed in monosynaptically connected excitatory premotor interneurons (input average: −0.46 ± 0.75 mV at −40mV, n = 10; Fig. 6B). This is illustrated in Figure 6C, where the inhibitory component becomes excitatory after TTX application. In summary, our results suggest that R78F07-Gal4 is not selective, or reliable, for activation of the A23a inhibitory premotor interneuron.
Figure 6.
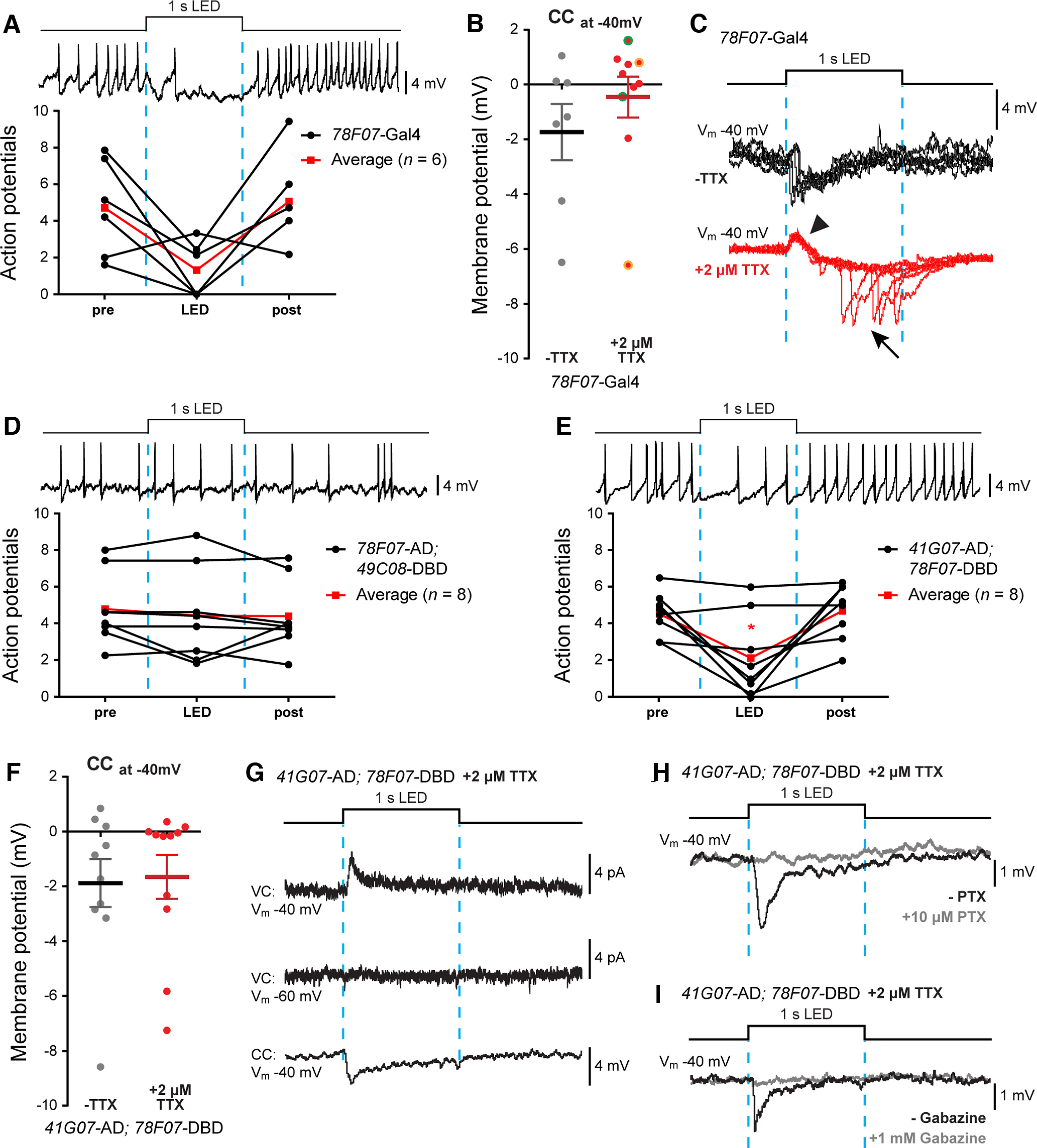
A23a and aCC are monosynaptically connected. A, Optogenetic activation of R78F07-Gal4 driving ChR; NaChBac reduces AP firing in aCC (elicited by the injection of constant current). On average, we observed an inhibitory effect (n = 6; black lines). Average values are shown in red. B, Quantification of the synaptic inputs recorded from aCC (Vm, –40 mV) following optogenetic activation of R78F07-Gal4 driving ChR; NaChBac before and after 2 μm TTX application. In the presence of TTX, we observed a heterogeneous range of inputs with excitation prevailing over inhibition. Some recordings (two of eight cells) showed a biphasic connection where both the excitatory and inhibitory components were observed in the same cell (values are highlighted with a different color, +TTX group). C, Raw electrophysiological sweeps from an example of a biphasic connection obtained with optogenetic activation of R78F07-Gal4. The same cell was recorded five times during optogenetic stimulation before (black traces) and after (red traces) TTX exposure. While the inhibitory component seems to prevail before applying TTX, the isolation of NaChBac-overexpressing neurons (+TTX) resulted in a reliable excitatory component (arrowhead) followed by a delayed erratic inhibitory component (arrow). D, Optogenetic activation of R78F07-AD; R49C08-DBD split Gal4 did not affect aCC firing (n = 8; black lines). Average values are shown in red. E, Optogenetic activation of R41G07-AD; R78F07-DBD split Gal4 significantly reduced aCC firing (n = 8; black lines). Average values are shown in red. F, Quantification of the synaptic drive to aCC (Vm, −40mV) following optogenetic activation of R41G07-AD; R78F07-DBD split Gal4 in the absence, or presence, of 2 μm TTX. The prevalence of inhibitory inputs suggests a better specificity for this line in targeting A23a compared with previous tested lines. G, Sample traces showing the optogenetic activation of R41G07-AD; R78F07-DBD split Gal4, driving ChR; NaChBac. aCC were recorded both in voltage clamp (both at −60 and −40 mV) and in current clamp (at −40 mV) in the presence of TTX. H, I, Sample traces confirming that the A23a → aCC synapse is GABAergic. Cells were recorded, as previously described, before (black trace) and after (gray trace) bath application of 10 μm picrotoxin (H) or 1 mm gabazine (I), two blockers of the Drosophila GABAA receptor. In both cases, aCC inputs were abolished.
Next, we tested two split-Gal4 lines designed for specific expression in A23a. The first split Gal4 tested, R78F07-AD; R49C08-DBD, was predominantly expressed in thoracic segments of the VNC. As expected, this expression appeared to be specific to A23a (Fig. 2C). Optogenetic activation of R78F07-AD; R49C08-DBD often reduced evoked action potential firing in aCC. In some experiments, however, R78F07-AD; R49C08-DBD-expressing cells excited the aCC. Indeed, it did so consistently, so the mean effect of stimulation on aCC AP firing was not significant (F(2,21) = 0.8322, p = 0.4005, repeated-measures one-way ANOVA; 4.78 ± 0.69 vs 4.42 ± 0.90 APs, n = 8, p > 0.99, Bonferroni's post hoc test; post-LED = 4.39 ± 0.68 APs; Fig. 6D). This suggests that R78F07-AD; R49C08-DBD was expressed in A23a, but also in excitatory (presumed cholinergic) INs that synapse with aCC. We tested and supported this suggestion by showing R78F07-AD; R49C08-DBD input was always inhibitory when experiments were conducted in the presence of 200 µm mecamylamine.
The second A23a-split line we used was SS04495-Gal4: R41G07-AD; R78F07-DBD (Kohsaka et al., 2019). It was predominantly expressed in abdominal segments of the VNC; however, the degree of expression in specific segments was variable (Fig. 2D). Moreover, the expression pattern includes at least one neuron that is not A23a. Optogenetic activation of cells expressing SS04495-Gal4 led to a significant reduction in evoked AP firing in aCC (F(2,21) = 9.662, p = 0.0141, repeated-measures one-way ANOVA; from 4.50 ± 0.42 to 2.16 ± 0.80 APs, n = 8, p > 0.0415, Bonferroni's post hoc test; post-LED = 4.70 ± 0.54 APs; Fig. 6E). The same stimulation produced a mostly reliable hyperpolarizing effect on aCC that was unaffected by the application of TTX [−1.88 ± 0.87 mV (n = 10) vs −1.66 ± 0.80 mV at −40 mV, n = 11, –TTX vs +TTX, p = 0.8502, t test, t(19) = 0.1915; Fig. 6F). Only one of the traces shown here, and two of six recorded from crosses to UAS-Chronos-mVenus, demonstrated an excitatory effect of SS04495-Gal4-expressing cells on aCC (which was blocked by 200 μm mecamylamine; data not shown). SS04495-Gal4 is therefore more reliably associated with the expected effect of the GABAergic interneuron (A23a) on aCC than the other lines we tested.
Finally, to support our proposal that SS04495-Gal4 was the most reliable of those currently available for the expression in A23a, we confirmed that the inhibition we observed was because of the movement of Cl– ions. Currents recorded from cells held at −40 mV were larger than from those held at −60 mV (Fig. 6G), and the inhibitory effect of the A23a-to-aCC connection was blocked by PTX (10 μm; Fig. 6H) and gabazine (1 mm; Fig. 6I).
A31k is monosynaptically connected to aCC
A31k was identified as a GABAergic IN connected to aCC (Schneider-Mizell et al., 2016) and later shown to inhibit motor activity (Clark et al., 2018; Zarin et al., 2019) downstream of “canon” neurons (Hiramoto et al., 2021). We tested the following three different drivers to verify A31k-to-aCC connectivity: R87H09-Gal4 (Zarin et al., 2019); R20A03-AD; R87H09-DBD split Gal4 (A. Zarin); and R20A03-AD; R93B07-DBD split Gal4 (also called “SS04399-Gal4”; Kohsaka et al., 2019).
Evoked AP firing in aCC was not changed by optogenetic activation of A31k in the absence or presence of TTX, using either R87H09-Gal4 (F(2,12) = 0.5102, p = 0.5559, repeated-measures one-way ANOVA; 6.73 ± 0.81 vs 6.68 ± 0.90 APs, pre-LED vs LED, respectively; n = 7, p > 0.99, repeated-measures one-way ANOVA followed by Bonferroni's post hoc test; post-LED = 6.46 ± 0.90 APs; data not shown) or R20A03-AD; R87H09-DBD split Gal4 (F(2,6) = 1.733, p = 0.3134, repeated-measures one-way ANOVA; 3.20 ± 0.56 vs 3.53 ± 0.43 APs, pre-LED vs LED, respectively; n = 3, p > 0.99, Bonferroni's post hoc test; post-LED = 3.73 ± 0.50 APs; Fig. 7A). Similarly, voltage-clamp recordings provided no indication of input from Gal4-expressing neurons to aCC in both driver lines (data not shown). Given that our recordings were conducted in third-instar animals and the connectome was generated from a first-instar larva, we repeated our experiments at L1. Our results mirrored those observed at the L3 stage, with no change in evoked AP firing recorded in aCC following optogenetic stimulation of R20A03-AD; R87H09-DBD split Gal4-expressing neurons (F(2,12) = 0.5404, p = 0.5928, repeated-measures one-way ANOVA; from 5.99 ± 0.97 to 5.98 ± 1.14; n = 5, p > 0.99, Bonferroni's post hoc test; post-LED = 6.41 ± 0.83 APs; Fig. 7B). Thus, the lack of aCC response to R87H09-Gal4 and R20A03-AD; R87H09-DBD split Gal4-expressing cell stimulation is probably not because of changes in connectivity occurring during development. It more likely reflects inaccurate and/or unreliable expression.
Figure 7.
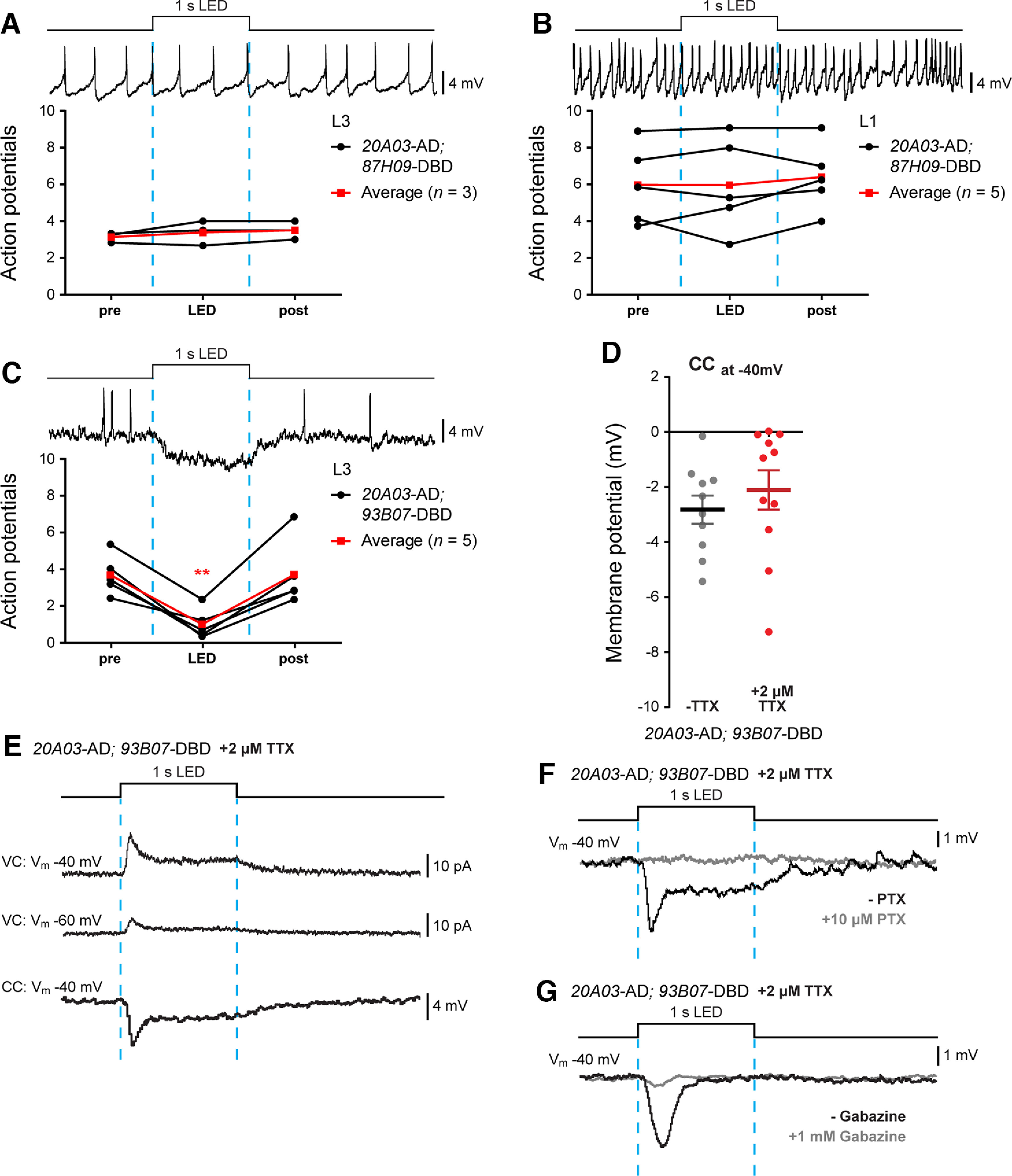
A31k and aCC are monosynaptically connected. A, B, Optogenetic activation of R20A03-AD; R87H09-DBD split Gal4 did not produce detectable changes in aCC firing (evoked by current injection) recorded at both L3 (n = 3, black lines; A) and L1 (n = 5, black lines; B). Average values are shown in red. C, Optogenetic activation of R20A03-AD; R93B07-DBD split Gal4 significantly reduced aCC firing (n = 5; black lines). Average values are shown in red. D, Quantification of the synaptic drive to aCC (Vm, −40 mV) following optogenetic activation of R20A03-AD; R93B07-DBD split Gal4 in the absence or presence of 2 μm TTX. E, Sample traces showing the optogenetic activation of R20A03-AD; R93B07-DBD split Gal4. aCC neurons were recorded both in voltage (Vm, −60 and −40 mV) and in current-clamp (−40 mV) configurations, in the presence of TTX. F, G, Sample traces confirming the GABAergic connection between A31k and aCC. Cells were recorded, as previously described, before (black trace) and after (gray trace) the bath application of 10 μm picrotoxin (F) or 1 mm gabazine (G). In both cases, aCC inputs were abolished.
In contrast to results generated using R87H09-Gal4 and R20A03-AD; R87H09-DBD, optogenetic activation of neurons targeted by the split-Gal4 driver line SS04399-Gal4 (R20A03-AD; R93B07-DBD) produced a clear inhibition of evoked AP firing in aCC (F(2,12) = 20.22, p = 0.0011, repeated-measures one-way ANOVA; from 3.66 ± 0.49 to 0.99 ± 0.37 APs; n = 5, p = 0.0088, Bonferroni's post hoc test; post-LED = 3.68 ± 0.81 APs; Fig. 7C). Current-clamp recordings confirmed the presence of a hyperpolarizing drive with no significant reduction after the application of TTX [−2.82 ± 0.51 mV (n = 10) vs −2.11 ± 0.72 mV at −40 mV (n = 11), –TTX vs +TTX, p = 0.4347, t test, t(19) = 0.798; Fig. 7D]. Similar to A23a, optogenetic activation of A31k produces an outward current in aCC clamped at −40 mV, which is reduced at −60 mV (Fig. 7E). Bath application of PTX (Fig. 7F) or gabazine (Fig. 7G) blocked this inhibition, which is consistent with it being carried by Cl– ions. Note that SS04399-Gal4 is predominantly expressed in the two most posterior abdominal segments of the VNC, at 48 h after egg laying (AEL; Fig. 2E, expression pattern). In summary, our results indicate that A31k makes a robust GABAergic, monosynaptic connection with aCC. The premotor neuron can be accurately and reliably targeted in posterior abdominal segments by the split Gal4 line SS04399-Gal4: R20A03-AD; R93B07-DBD (Kohsaka et al., 2019).
TERPS is more accurate than GRASP and can be adapted for imaging excitatory monosynaptic connections between neurons
GRASP and calcium imaging represent the primary techniques by which connectivity proposed by reconstruction of neural networks, had been verified before the present study. This type of validation may, however, be limited by the spatial and temporal resolution of these methods. We demonstrated this limitation by direct comparison of GRASP imaging to our electrophysiology results for A18a (also called CLI2) and A18b3 (also called CLI1). Prior research used GRASP to infer “direct” connections between both A18a and A18b3 and aCC (Hasegawa et al., 2016), and using a similar system we also found GRASP-positive cell–cell contacts (Fig. 8). In contrast, the more accurate, functional connectivity assessment we made using TERPS showed that while A18a is connected to aCC, A18b3 is not (Fig. 4C). Thus, GRASP should be used to indicate close apposition between cells, rather than synaptic connectivity (Couton et al., 2015).
Figure 8.
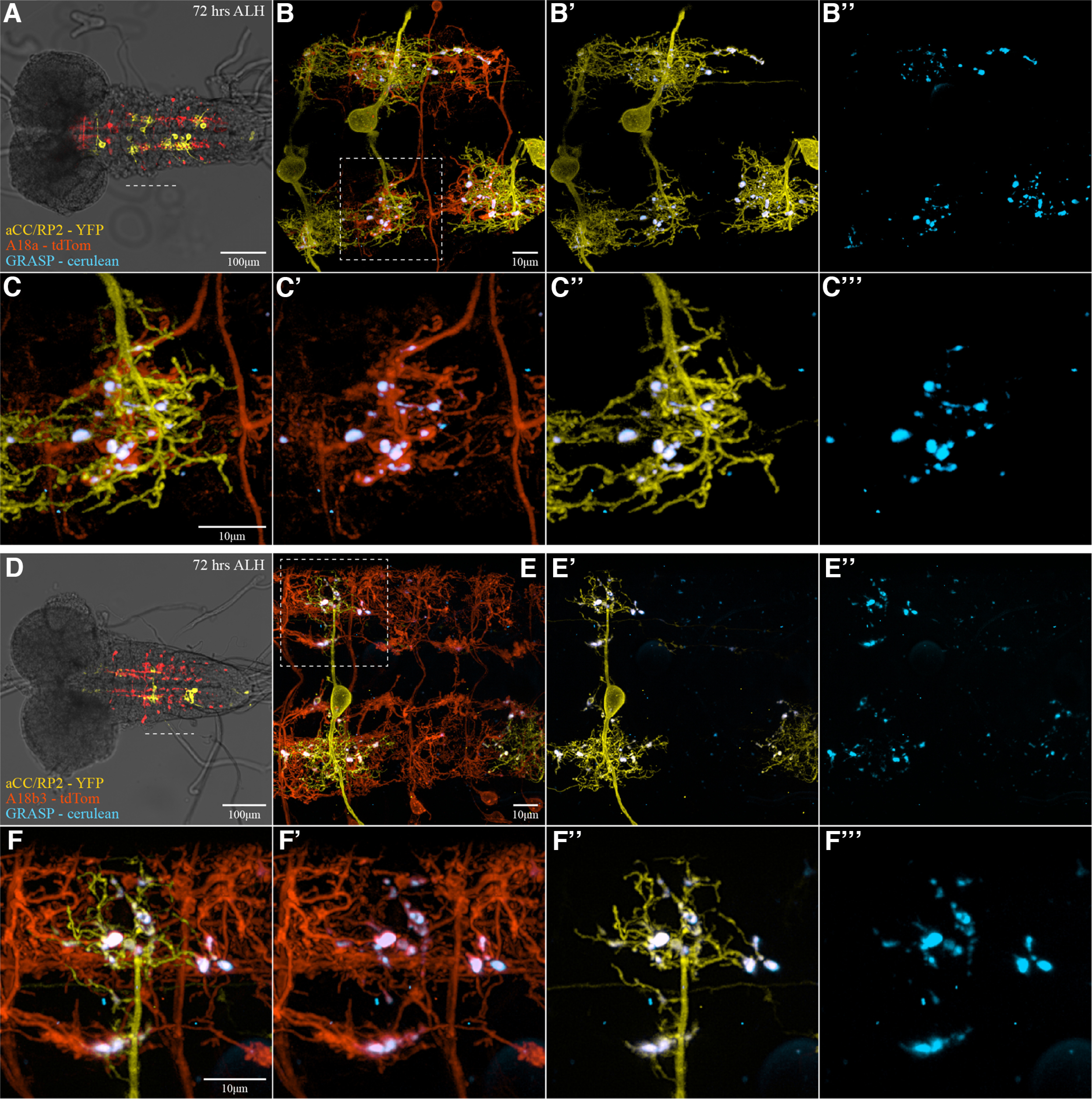
GRASP is not specific to direct synaptic partners. A–F, Three-color fluorescence imaging of nerve cords 72 h after larval hatching performed to examine the cerulean-GRASP signal between aCC and RP2 motoneurons with two different premotor interneurons: A18a (also called CLI2) and A18b3 (also called CLI1). A and D show overlays of fluorescence with bright-field illumination taken with a 10× objective, providing an overview of the whole nerve cord, with the dashed white line indicating the region imaged with 60× objective, shown to the right (B, E). The dashed white boxes in B and E (aCC dendritic regions) outline regions magnified in C and F. A–C, 15B07-Gal4 drives the expression of UAS-CD4::tdTomato in A18a interneurons (red). D–F, tsh-Gal80; R47E12-Gal4, Cha3.3-Gal80 targets the expression of UAS-CD4::tdTomato to A18b3 interneurons (red). A–F, Subsets of aCC were stochastically labeled with LexAop2-myr::YPet (yellow) via Flippase (Dhawan et al., 2021), which is expressed in those neurons. Cell–cell contacts are visualized using a cyan version of GRASP, UAS-CD4::spCerulean1-10, expressed by the respective interneurons, while the complementary LexAop-CD4::spGFP11 is expressed in subsets of aCC. At points of sufficient proximity between cell pairs, cerulean fluorescent protein is reconstituted (cyan). Robust cerulean fluorescence is detected using both A18a and A18b3 Gal4 drivers, at contact sites with aCC.
Electrophysiology is difficult to perform and requires expensive, specialist equipment. Similarly, our research is one of very few to deploy TERPS to assess monosynaptic connectivity between neurons in Drosophila (Zhang and Gaudry, 2018; Suzuki et al., 2020). This difficulty and the expense of electrophysiology, combined with the novel nature of our adaptation of TERPS, inspired us to design a tool that can be used more easily to conduct similar work. Specifically, we created a stock that facilitates targeted expression of UAS-ChR and UAS-NaChBac in neurons for testing monosynaptic input to glutamatergic neurons (including all motoneurons). In addition to ChR and NaChBac, the line expresses RCaMP1b in motoneurons (genotype: w[*]; DvGlut-T2A-QF2, P{y[+t7.7], 5xQUAS IVS syn21 RCaMP1b P2A nls-GFP P10 in su(Hw)attP5, 20xUAS-ChR2.T159C-HA}VK00018/CyO, Dfd-GMR-YFP; UAS-NaChBac-EGFP}1 in 85A and 87D/TM6bSb, and Dfd-GMR-YFP). It can, therefore, be crossed to interneuron-specific Gal4 lines to establish monosynaptic connectivity between those (interneurons) and motoneurons by functional calcium imaging.
We validated the tool by crossing it with R36G02-Gal4 (A27h) and by imaging calcium activity in aCC following optogenetic activation of this premotor interneuron. Stimulation of A27h, in the presence of TTX, elicited a robust change in calcium levels in aCC (n = 5; Fig. 9A, left). As expected, the application of mecamylamine (which inhibits all cholinergic synaptic transmission) abolished this response (Fig. 9A, right), leaving only a smaller light-induced artifact produced by the blue LED used to excite ChR (Fig. 9C, arrowhead in CS wild-type controls). Thus, our results validate the imaging tool by confirming those generated by electrophysiology. We also tested the potential to use the tool to establish monosynaptic connectivity between inhibitory premotor interneurons and aCC, using A31k-specific SS04399-Gal4 (Kohsaka et al., 2019; Fig. 9B). We were unable to validate the tool for this application, as aCC must be active before input from inhibitory neurons for a noticeable change in calcium levels to be observed.
Figure 9.
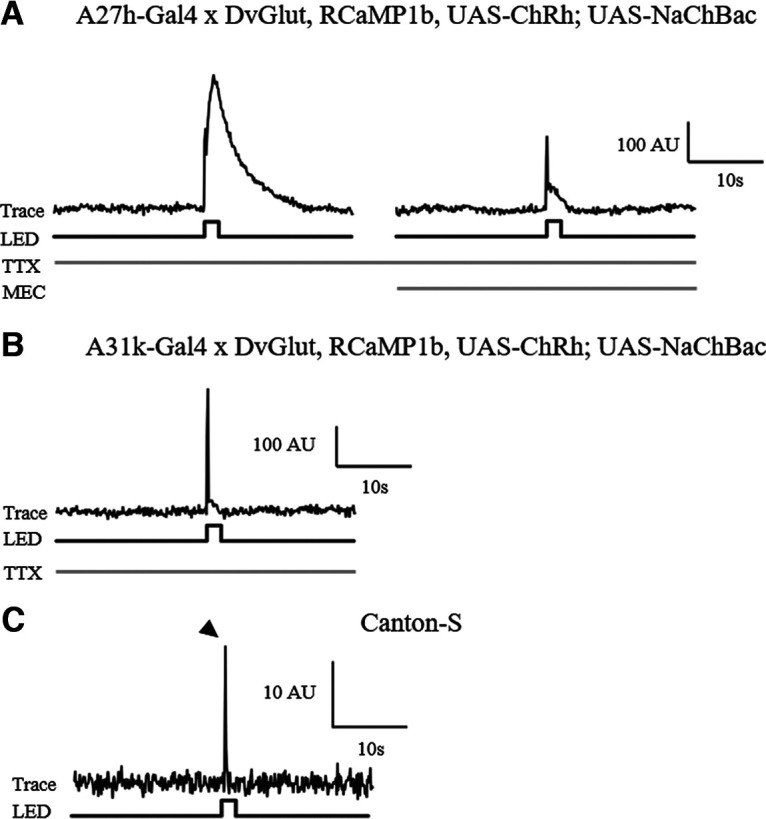
A TERPS and RCaMP imaging tool can identify excitatory monosynaptic connections between cells. A, Representative trace showing that optogenetic activation of A27h expressing NaChBac, in the presence of 2 μm TTX, induces a clear change in calcium levels (indicated by a change in RCaMP fluorescence) in aCC (∼200 A.U. above baseline, n = 5). The addition of the cholinergic blocker mecamylamine (200 μm) to the same preparation abolishes this response (right-hand panel). All that remains is a smaller light-induced artifact because of the activation of the blue LED. B, Representative trace for optogenetic activation of A31k-expressing NaChBac, in the presence of 2 μm TTX, shows that the activation of this premotor interneuron does alter calcium levels in aCC (no change in arbitrary units above baseline, in addition to the peak explained in C; n = 5). C, Representative trace for flashing Canton-S wild-type larvae, showing that it is light from the blue LED that produces the recording artifact (arrowhead; n = 1).
Discussion
In this study, we used electrophysiology to validate monosynaptic connectivity of four identified premotor interneurons with the aCC motoneuron in Drosophila larvae (Fig. 10). Two are cholinergic and form part of the excitatory input to motoneurons, and two are GABAergic and inhibitory (Baines et al., 1999; Rohrbough and Broadie, 2002; Kohsaka et al., 2014; Itakura et al., 2015; Fushiki et al., 2016; Zwart et al., 2016; Kohsaka et al., 2019; Zarin et al., 2019). Together, they form part of a central pattern generator composed of interneurons and motoneurons, which regulates locomotion in Drosophila larvae. This locomotor circuit is central to much of the work directed toward establishing the fly connectome, so it is significant that our combination of electrophysiology with TTX-resistant NaChBac (Zhang and Gaudry, 2018) supports certain connectome data (Zarin et al., 2019). Importantly, our data also highlight the potential for inaccuracy when connectivity is not validated by electrophysiology (e.g., in the case of A18b3). This confirms that electrophysiology represents the “gold standard” test for functional connectivity between neurons and poses that it should be used to check all new connections proposed by reconstructions. It may, however, be difficult to do so. Testing every synapse associated with each of the ∼10,000 neurons present in the larval CNS (Heimbeck et al., 1999) represents an immense amount of work. It may be more practical to use electrophysiology or the imaging tool we presented in this research intermittently to confirm the principles by which connections are proposed.
Figure 10.
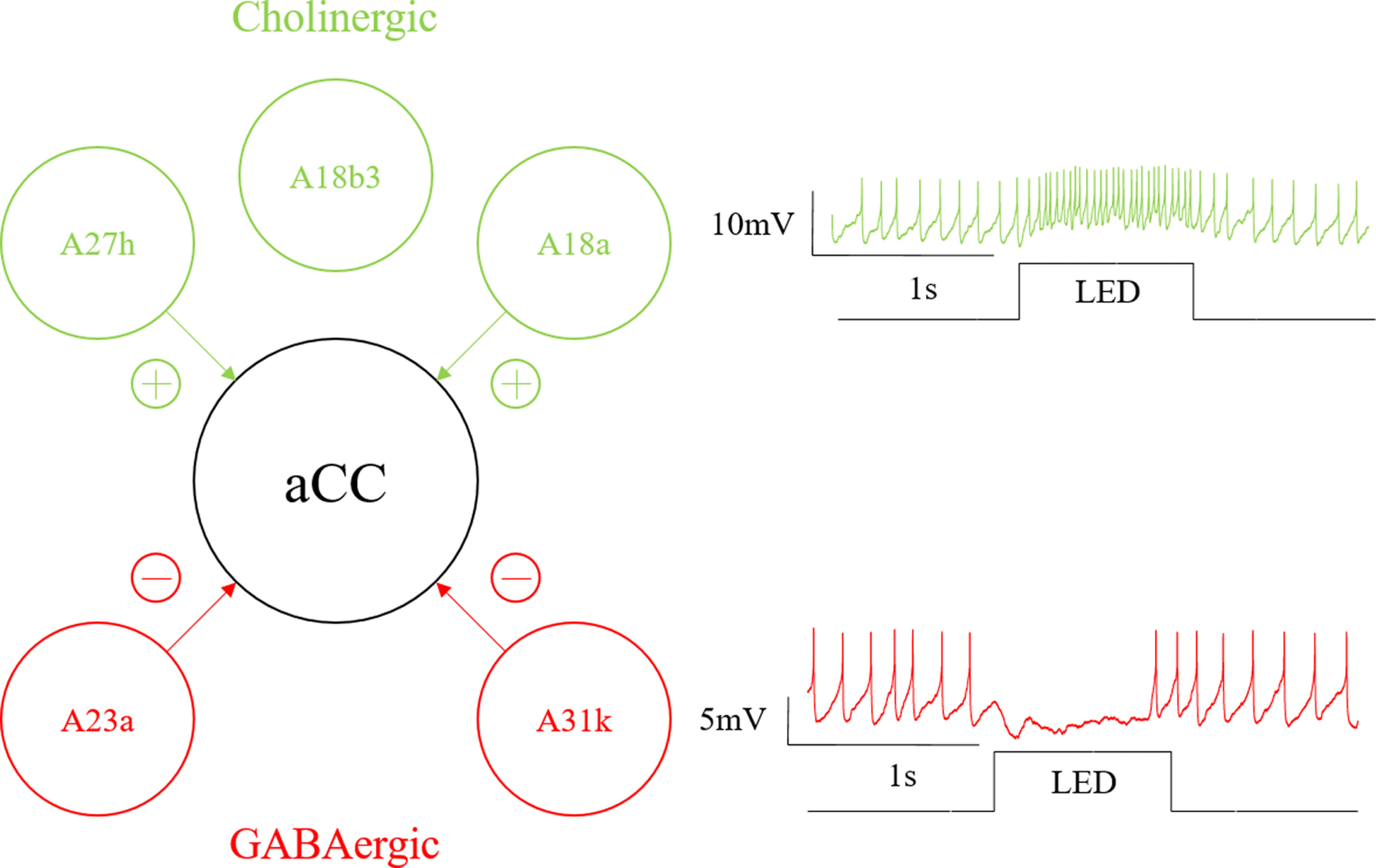
aCC-associated circuit connectivity. Schematic showing relationship between the cholinergic (A27h, A18b3, and A18a) and GABAergic (A23a and A31k) premotor interneurons we tested for monosynaptic connectivity to the motoneuron aCC. Arrows depict synapses with connectivity verified by electrophysiology. Traces to the right of the schematic are representative examples of the effect of cholinergic and GABAergic monosynaptic inputs on evoked action potentials aCC, taken from recordings from A27h and A31k stimulation, respectively.
Given that the NaChBac tool presented here may be used to provide further insights into the accuracy of connections predicted by the CATMAID dataset, it is important to briefly revisit the main caveat to using the tool. That is, if a presynaptic neuron has a resting membrane potential at or above −40 mV, the NaChBac ion channel will be inactivated (Ren et al., 2001). Consequently, stimulating a presynaptic cell and recording a lack of response from a supposed postsynaptic partner (in the presence of TTX, which blocks endogenous NaV channels) may be misinterpreted to mean that two cells are not monosynaptically connected, when, in fact, they might be. Given that most electrophysiologists do not include cells resting at less than –40 mV in their research (more depolarized cells tend to fire spontaneous action potentials that reflect damage), this should not impact the vast majority of potential applications for this tool. However, in conditions where a cell membrane potential is unknown (e.g., when using the imaging tool we presented), the persistence of synaptic drive in the presence of TTX is strong evidence for monosynaptic connectivity, but its absence is not definitive evidence against it.
The kinetics of NaChBac are radically different from those of the endogenous Na+ channel Paralytic, so there is little to be gained from analyzing the biophysics of the synaptic drive when NaChBac is expressed (Baines and Bate, 1998; Baines et al., 2001; Ren et al., 2001). Another, more general, issue with the ectopic expression of an ion channel (particularly one of bacterial origin) in a neuron, is that its presence may be sufficient to alter development and/or physiology (Zhang and Gaudry, 2018). Indeed, we show here that the expression of NaChBac in all GABAergic neurons is embryonic lethal. This technique is also specific to the detection of synaptic couplings that involve ionotropic receptors, which cause significant change to the postsynaptic membrane potential (and/or Ca2+ influx) and neurons that spike (i.e., excludes graded neurons). The tool cannot be applied to reliably detect synapses that rely on metabotropic receptors, which alter second-messenger signaling, unless ionic movements across the neuronal membrane form part of the activated downstream signaling pathway.
GRASP has been used to validate synaptic connectivity (Hasegawa et al., 2016; Sales et al., 2019); however, we demonstrate that it is limited by poor spatial resolution (Fig. 8). We therefore present an alternative, image-based tool that may be used to establish excitatory monosynaptic connections between neurons if access to electrophysiology is limited. This tool cannot identify inhibitory synapses as it is presented; however, it is conceivable that it could be adapted to do so. For example, combining the imaging tool with a high K+ saline (or similar) that first excites postsynaptic cells, may facilitate observation of a reduction of activity because of inhibitory inputs.
In addition to identifying the most accurate and reliable Gal4s for A27h, CLI2, A23a, and A31k [R36G02-Gal4 (A27h-Gal4); R15B07-Gal4 (CLI2-Gal4); SS04495-Gal4 (“A23a-Gal4”); and SS04399-Gal4 (“A31k-Gal4”)], we observed several that demonstrated a considerable lack of specificity and variability in the expression of different Gal4 lines, despite them targeting the same premotor interneurons (Table 1). For some (e.g., R78F07-Gal4), the issue was clearly the degree of specificity. Indeed, even the most accurate line for targeting Gal4 expression to A23a (SS04495-Gal4) must be used in conjunction with mecamylamine, to block nonspecific expression from providing excitatory inputs to postsynaptic cells. For others (e.g., R87H09-Gal4, R20A03-AD, and R87H09-DBD), the explanation for the discrepancies we observed is less apparent, but may be explained by differing strengths of Gal4 activity. It is our recommendation, therefore, that future work using relatively “new” Gal4 lines must take time to carefully characterize their expression pattern and confirm the proposed monosynaptic connectivity using one of the tools presented in this study.
Table 1.
Summary of Gal4 line results
| Driver line | Other names | Target interneurons | Connected to aCC | Reference |
|---|---|---|---|---|
| R36G02-Gal4 | A27 h-GAL4 | A27h | ✓ | Fushiki et al. (2016) |
| R15B07-Gal4 | CLI2-GAL4 | A18a | ✓ | A. Zarin |
| R47E12 -Gal4 | CLIs-GAL4 | A18a/A18b3 | ✓ | Hasegawa et al. (2016) |
| R47E12 -Gal4; cha3.3-Gal80 | CLI-GAL4 | A18a/A18b3 | ✓ | Hasegawa et al. (2016) |
| tsh-Gal80; R47E12-Gal4, cha3.3-Gal80 | CLI1-GAL4 | A18b3 | x | Hasegawa et al. (2016) |
| R78F07 -Gal4 | A23a | ✓ | Zarin et al. (2019) | |
| SS04495-Gal4 | R41G07-AD; R78F07-DBD | A23a | ✓ | Kohsaka et al. (2019) |
| No label | R78F07-AD; R49C08-DBD | A23a | ✓ | A. Zarin |
| SS04399-Gal4 | R20A03-AD; R93B07-DBD | A31k | ✓ | Kohsaka et al. (2019) |
| A31K | x | Zarin et al. (2019) | ||
| R87H09 -Gal4 | R20A03-AD; R87H09-DBD | A31K | x | A. Zarin |
In the Driver line column, bold type indicates confidence in the monosynaptic connection to aCC; underlining indicates either no connection or a possible connection to aCC which is conflated by lack of specificity of driver.
In summary, our results validate that the premotor interneurons A27h, A18a, A23a, and A31k are monosynaptically connected to the aCC motoneuron, and thus confirm that the reconstructions detailed in the study by Zarin et al. (2019) are accurate. We demonstrate that electrophysiology deserves its reputation as the current gold standard of validation for functional connectivity between neurons (by contrasting it with GRASP) and, moreover, provide an imaging-based tool that others may use to verify excitatory monosynaptic connections. We identify specific GAL4 lines that are reliably and accurately expressed in A27h, A18a, A23a, and A31k, and so enable further study of these interneurons and their connections. This research, therefore, supports the ongoing effort to establish accurate connectomes in Drosophila larvae and other animals, and the development of tools for validating interneuronal connections.
Footnotes
This work was supported by funding from the Biotechnology and Biological Sciences Research Council to R.A.B. (Grant BB/N/014561/1) and to M.L. (Grant BB/R016666/1) and by a Joint Wellcome Trust investigator award to R.A.B. and M.L. (Grant 217099/Z/19/Z). S.C. was supported by a Dorothy Hodgkin Fellowship from the Royal Society to S.C. (Grant DH120072), and benefited from core support from the MRC (Grant MC-U105188491). Work on this project benefited from the Manchester Fly Facility, established through funds from the University and the Wellcome Trust (Grant 087742/Z/08/Z); and from the Imaging Facility, Department of Zoology, University of Cambridge, which was supported by Dr. Matthew Wayland and funds from Wellcome Trust Equipment Grant WT079204 with contributions by the Sir Isaac Newton Trust in Cambridge, including Research Grant 18.07ii(c). We thank Chris Doe for generous provision of GAL4 lines and for advice in conducting this study, and Jan Felix Evers for designing a cyan fluorescent version of GRASP (GFP Reconstitution across Synaptic Partners).
The authors declare no competing financial interests.
References
- Ackerman SD, Perez-Catalan NA, Freeman MR, Doe CQ (2021) Astrocytes close a motor circuit critical period. Nature 592:414–420. 10.1038/s41586-021-03441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Bate M (1998) Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci 18:4673–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Lange AB, Downer RG (1990) Proctolin in the innervation of the locust mandibular closer muscle modulates contractions through the elevation of inositol trisphosphate. J Comp Neurol 297:479–486. [DOI] [PubMed] [Google Scholar]
- Baines RA, Robinson SG, Fujioka M, Jaynes JB, Bate M (1999) Postsynaptic expression of tetanus toxin light chain blocks synaptogenesis in Drosophila. Curr Biol 9:1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M (2001) Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci 21:1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Seugnet L, Thompson A, Salvaterra PM, Bate M (2002) Regulation of synaptic connectivity: levels of Fasciclin II influence synaptic growth in the Drosophila CNS. J Neurosci 22:6587–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Schoenenberger P, Mattis J, Tye KM, Deisseroth K, Hegemann P, Oertner TG (2011) High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc Natl Acad Sci U|S|A 108:7595–7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos A, Honjo K, Ohyama T, Qian CS, Shin GJE, Gohl DM, Silies M, Tracey WD, Zlatic M, Cardona A, Grueber WB (2018) Nociceptive interneurons control modular motor pathways to promote escape behavior in Drosophila. Elife 7:e26016. 10.7554/eLife.26016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira-Rosario A, Zarin AA, Clark MQ, Manning L, Fetter RD, Cardona A, Doe CQ (2018) MDN brain descending neurons coordinately activate backward and inhibit forward locomotion. Elife 7:e38554. 10.7554/eLife.38554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JC, Park D, Griffith LC (2004) Electrophysiological and morphological characterization of identified motor neurons in the Drosophila third instar larva central nervous system. J Neurophysiol 91:2353–2365. [DOI] [PubMed] [Google Scholar]
- Clark MQ, Zarin AA, Carreira-Rosario A, Doe CQ (2018) Neural circuits driving larval locomotion in Drosophila. Neural Dev 13: 10.1186/s13064-018-0103-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couton L, Mauss AS, Yunusov T, Diegelmann S, Evers JF, Landgraf M (2015) Development of connectivity in a motoneuronal network in Drosophila larvae. Curr Biol 25:568–576. 10.1016/j.cub.2014.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman Jp, Tsegaye G, Holt GT, Hu A, Walpita D, Patel R, Macklin JJ, Bargmann CI, Ahrens MB, Schreiter ER, Jayaraman V, Looger LL, Svoboda K, Kim DS (2016) Sensitive red protein calcium indicators for imaging neural activity. Elife 5:e12727. 10.7554/eLife.12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Rossano AJ, Macleod GT, Ganetzky B (2014) Expression of multiple transgenes from a single construct using viral 2A peptides in Drosophila. PLoS One 9:e100637. 10.1371/journal.pone.0100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan S, Myers P, Bailey DMD, Ostrovsky Ad, Evers JF, Landgraf M (2021) Reactive oxygen species mediate activity-regulated dendritic plasticity through NADPH oxidase and aquaporin regulation. Front Cell Neurosci 15:641802. 10.3389/fncel.2021.641802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao FQ, Ironfield H, Luan HJ, Diao FC, Shropshire WC, Ewer J, Marr E, Potter CJ, Landgraf M, White BH (2015) Plug-and-play genetic access to Drosophila cell types using exchangeable exon cassettes. Cell Rep 10:1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushiki A, Zwart MF, Kohsaka H, Fetter RD, Cardona A, Nose A (2016) A circuit mechanism for the propagation of waves of muscle contraction in Drosophila. Elife 5:e13253. 10.7554/eLife.13253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard S, Andrade I, Fetter RD, Cardona A, Schneider-Mizell CM (2017) Conserved neural circuit structure across Drosophila larval development revealed by comparative connectomics. Elife 6:e29089. 10.7554/eLife.29089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachello CNG, Fan YN, Landgraf M, Baines RA (2019) Activity manipulation of an excitatory interneuron, during an embryonic critical period, alters network tuning of the Drosophila larval locomotor circuit. bioRxiv 780221. doi: 10.1101/780221. [DOI] [Google Scholar]
- Giachello CNG, Fan YN, Landgraf M, Baines RA (2021) Nitric oxide mediates activity-dependent change to synaptic excitation during a critical period in Drosophila. Sci Rep 11:20286. 10.1038/s41598-021-99868-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth AC, Fish M, Nusse R, Calos MP (2004) Construction of transgenic Drosophila by using the site-specific integrase from phage phi C31. Genetics 166:1775–1782. 10.1093/genetics/166.4.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa E, Truman JW, Nose A (2016) Identification of excitatory premotor interneurons which regulate local muscle contraction during Drosophila larval locomotion. Sci Rep 6:30806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckscher ES, Zarin AA, Faumont S, Clark MQ, Manning L, Fushiki A, Schneider-Mizell CM, Fetter RD, Truman JW, Zwart MF, Landgraf M, Cardona A, Lockery SR, Doe CQ (2015) Even-Skipped(+) interneurons are core components of a sensorimotor circuit that maintains left-right symmetric muscle contraction amplitude. Neuron 88:314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbeck G, Bugnon V, Gendre N, Haberlin C, Stocker RF (1999) Smell and taste perception in Drosophila melanogaster larva: toxin expression studies in chemosensory neurons. J Neurosci 19:6599–6609. 10.1523/JNEUROSCI.19-15-06599.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto A, Jonaitis J, Niki S, Kohsaka H, Fetter RD, Cardona A, Pulver SR, Nose A (2021) Regulation of coordinated muscular relaxation in Drosophila larvae by a pattern-regulating intersegmental circuit. Nat Commun 12:2943. 10.1038/s41467-021-23273-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang B, Chiba A (2001) Single-cell analysis of Drosophila larval neuromuscular synapses. Dev Biol 229:55–70. [DOI] [PubMed] [Google Scholar]
- Hunter I, Coulson B, Zarin AA, Baines RA (2021) The Drosophila larval locomotor circuit provides a model to understand neural circuit development and function. Front Neural Circuits 15:684969. 10.3389/fncir.2021.684969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura Y, Kohsaka H, Ohyama T, Zlatic M, Pulver SR, Nose A (2015) Identification of inhibitory premotor interneurons activated at a late phase in a motor cycle during Drosophila larval locomotion. PLoS One 10:e0136660. 10.1371/journal.pone.0136660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadas D, Klein A, Krick N, Worrell JW, Ryglewski S, Duch C (2017) Dendritic and axonal L-type calcium channels cooperate to enhance motoneuron firing output during Drosophila larval locomotion. J Neurosci 37:10971–10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, Kim MK, Shin BA, Choi SY (2011) High cleavage efficiency of a 2A peptide derived from porcine Teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6:e18556. 10.1371/journal.pone.0018556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka H, Takasu E, Morimoto T, Nose A (2014) A group of segmental premotor interneurons regulates the speed of axial locomotion in Drosophila larvae. Curr Biol 24:2632–2642. [DOI] [PubMed] [Google Scholar]
- Kohsaka H, Zwart MF, Fushiki A, Fetter RD, Truman JW, Cardona A, Nose A (2019) Regulation of forward and backward locomotion through intersegmental feedback circuits in Drosophila larvae. Nat Commun 10:2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M, Sanchez-Soriano N, Technau GM, Urban J, Prokop A (2003) Charting the Drosophila neuropile: a strategy for the standardised characterisation of genetically amenable neurites. Dev Biol 260:207–225. [DOI] [PubMed] [Google Scholar]
- Larderet I, Fritsch PM, Gendre N, Neagu-Maier GL, Fetter RD, Schneider-Mizell CM, Truman JW, Zlatic M, Cardona A, Sprecher SG (2017) Organization of the Drosophila larval visual circuit. Elife 6:e28387. 10.7554/eLife.28387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley R, Baines RA (2011) Dissection of first- and second-instar Drosophila larvae for electrophysiological recording from neurons: the flat (or fillet) preparation. Cold Spring Harb Protoc 2011:pdb.prot065649. 10.1101/pdb.prot065649 [DOI] [PubMed] [Google Scholar]
- Ohyama T, Schneider-Mizell CM, Fetter RD, Aleman JV, Franconville R, Rivera-Alba M, Mensh BD, Branson KM, Simpson JH, Truman JW, Cardona A, Zlatic M (2015) A multilevel multimodal circuit enhances action selection in Drosophila. Nature 520:633–639. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TTB, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM (2010) Refinement of tools for targeted gene expression in drosophila. Genetics 186:735–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Truman JW, Rubin GM (2012) Using translational enhancers to increase transgene expression in Drosophila. Proc Natl Acad Sci U|S|A 109:6626–6631. 10.1073/pnas.1204520109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Tasic B, Russler EV, Liang L, Luo LQ (2010) The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell 141:536–548. 10.1016/j.cell.2010.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Navarro B, Xu H, Yue L, Shi Q, Clapham DE (2001) A prokaryotic voltage-gated sodium channel. Science 294:2372–2375. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K (2002) Electrophysiological analysis of synaptic transmission in central neurons of Drosophila larvae. J Neurophysiol 88:847–860. [DOI] [PubMed] [Google Scholar]
- Ryglewski S, Lance K, Levine RB, Duch C (2012) Ca(v)2 channels mediate low and high voltage-activated calcium currents in Drosophila motoneurons. J Physiol 590:809–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalfeld S, Cardona A, Hartenstein V, Tomancak P (2009) CATMAID: collaborative annotation toolkit for massive amounts of image data. Bioinformatics 25:1984–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales EC, Heckman EL, Warren TL, Doe CQ (2019) Regulation of subcellular dendritic synapse specificity by axon guidance cues. Elife 8:e43478. 10.7554/eLife.43478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumweber T, Rohwedder A, Schleyer M, Eichler K, Chen YC, Aso Y, Cardona A, Eschbach C, Kobler O, Voigt A, Durairaja A, Mancini N, Zlatic M, Truman JW, Thum AS, Gerber B (2018) Functional architecture of reward learning in mushroom body extrinsic neurons of larval Drosophila. Nat Commun 9:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Mizell CM, Gerhard S, Longair M, Kazimiers T, Li F, Zwart MF, Champion A, Midgley FM, Fetter RD, Saalfeld S, Cardona A (2016) Quantitative neuroanatomy for connectomics in Drosophila. Elife 5:e12059. 10.7554/eLife.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Lance K, Levine RB (2012a) Segmental differences in firing properties and potassium currents in Drosophila larval motoneurons. J Neurophysiol 107:1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Lance K, Levine RB (2012b) Contribution of EAG to excitability and potassium currents in Drosophila larval motoneurons. J Neurophysiol 107:2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Schenk JE, Tan H, Gaudry Q (2020) A population of interneurons signals changes in the basal concentration of serotonin and mediates gain control in the Drosophila antennal lobe. Curr Biol 30:1110–1118.e4. 10.1016/j.cub.2020.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell JW, Levine RB (2008) Characterization of voltage-dependent Ca2+ currents in identified Drosophila motoneurons in situ. J Neurophysiol 100:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S, Long H, Thomas JB (2016) A subset of interneurons required for Drosophila larval locomotion. Mol Cell Neurosci 70:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarin AA, Mark B, Cardona A, Litwin-Kumar A, Doe CQ (2019) A multilayer circuit architecture for the generation of distinct locomotor behaviors in Drosophila. Elife 8:e51781. 10.7554/eLife.51781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XN, Gaudry Q (2016) Functional integration of a serotonergic neuron in the Drosophila antennal lobe. Elife 5:e16836. 10.7554/eLife.16836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gaudry Q (2018) Examining monosynaptic connections in Drosophila using tetrodotoxin resistant sodium channels. J Vis Exp (132):57052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart MF, Pulver SR, Truman JW, Fushiki A, Fetter RD, Cardona A, Landgraf M (2016) Selective inhibition mediates the sequential recruitment of motor pools. Neuron 91:615–628. 10.1016/j.neuron.2016.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]