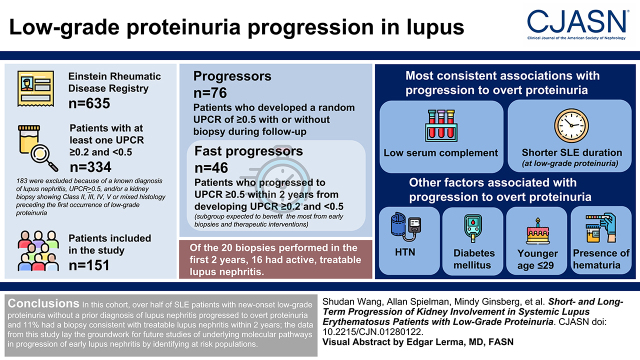
Visual Abstract
Keywords: proteinuria, systemic lupus erythematosus
Abstract
Background and objectives
Lupus nephritis remains a common cause of morbidity and mortality in systemic lupus erythematosus (SLE). Current guidelines recommend performing a kidney biopsy at a urine protein-creatinine ratio of ≥0.5 g/g. However, cross-sectional studies reported a high prevalence of active histologic lupus nephritis lesions, and even chronic irreversible scarring, in patients with low-grade proteinuria. This study was initiated to assess disease progression in patients with SLE and low-grade proteinuria to identify risk factors for progression to overt proteinuria suggestive of clinical lupus nephritis.
Design, setting, participants, & measurements
Patients with SLE who had an incident urinary protein-creatinine ratio of ≥0.2 and <0.5 g/g without known lupus nephritis were identified from the Einstein Rheumatic Disease Registry. Patients who developed a random urinary protein-creatinine ratio of ≥0.5 g/g with or without biopsy during the follow-up period were defined as “progressors.” Patients who progressed to a urinary protein-creatinine ratio of ≥0.5 g/g within 2 years of developing a urinary protein-creatinine ratio of ≥0.2 and <0.5 g/g were defined as “fast progressors,” a subgroup expected to benefit most from early biopsies and therapeutic interventions.
Results
Among 151 eligible patients with SLE and low-grade proteinuria at study entry, 76 (50%) progressed to a urinary protein-creatinine ratio of ≥0.5 g/g, of which 44 underwent a clinically indicated biopsy. The median (interquartile range) time from a urinary protein-creatinine ratio of ≥0.2 and <0.5 g/g to progression was 1.2 (0.3–3.0) years. Of the 20 biopsies performed in the first 2 years, 16 specimens showed active, treatable lupus nephritis. Low complement and shorter SLE duration at low-grade proteinuria onset were associated with progression to overt proteinuria across different analyses. Other associated factors included hypertension, diabetes mellitus, younger age, and the presence of hematuria.
Conclusions
In this longitudinal cohort of patients with SLE and low-grade proteinuria at study entry, over half progressed to a urinary protein-creatinine ratio of ≥0.5 g/g in a short time period.
Introduction
Lupus nephritis remains a common cause of significant morbidity and mortality in systemic lupus erythematosus (SLE). Nearly 60% of adults with SLE develop clinically apparent lupus nephritis in their lifetime (1). Lupus nephritis carries the highest standard mortality ratio in SLE (with rates almost four-fold higher than those without kidney involvement) (2). Although encouraging results from recent phase 3 studies supported approval of belimumab and voclosporin for lupus nephritis (3,4), >50% of patients did not meet the primary end points in either trial. Indeed, early diagnosis and treatments are key to reduce kidney inflammation more effectively, which often leads to cumulative and irreversible kidney damage. This is especially relevant as molecular advances on the basis of new approaches, such as single cell transcriptomics, are anticipated to provide novel targets for intervention (5).
While elevated proteinuria with or without active urine sediment signals kidney inflammation, percutaneous kidney biopsy is the gold standard for diagnosis and prognostication of lupus nephritis. The 2012 American College of Rheumatology guidelines recommend a kidney biopsy in patients with proteinuria of ≥1.0 g/d and/or proteinuria ≥0.5 g/d accompanied by hematuria or cellular casts (6). Both the 2019 European League Against Rheumatism/European Renal Association–European Dialysis and Transplant Association and the 2021 Kidney Disease Improving Global Outcomes guidelines recommend performing a biopsy for proteinuria ≥0.5 g/d or active urine sediment (7,8).
However, several cross-sectional studies reported a high prevalence of active histologic lupus nephritis lesions in patients with low-grade proteinuria of <0.5 g/d (9–12). This subset of patients with SLE and low-grade proteinuria may have early active lupus nephritis that, if untreated, will quickly progress to overt kidney disease. Many of these patients already have chronic, irreversible scarring by the time of their first biopsy (13). Yet, other patients with SLE and low-grade proteinuria never progress to clinically significant kidney disease. Therefore, it is important to identify patients with SLE and low-grade proteinuria who may develop clinically apparent lupus nephritis. Early biopsy should be considered in these patients to initiate early treatment and to minimize progression to chronic kidney damage/kidney failure.
Accordingly, this study was initiated to assess disease progression in patients with SLE and low-grade proteinuria and to identify characteristics associated with progression to increasing proteinuria. This was approached by leveraging a longitudinal cohort of patients with SLE followed at an urban tertiary care center.
Materials and Methods
Study Population
Eligible patients were initially identified from the Einstein Rheumatic Disease Registry, a longitudinal registry that includes 635 prevalent and incident adult and pediatric patients with SLE who met the American College of Rheumatology criteria or the Systemic Lupus International Collaborating Clinics classification criteria (14,15). The median (interquartile range; IQR) age of the cohort participants at SLE diagnosis is 29 (21–39) years old; 80% are women, 40% self-identify as Black, and 49% self-identify as Hispanic/Latino according to the 2015 National Institutes of Health (NIH) race and ethnicity categories and definitions (16). Participants provide a written informed consent for their data to be used in future studies. The data are stored in a REDCap database interfaced with the Montefiore Hospital Electronic Data Warehouse (which contains laboratory and pathology data from 1998 to present) to allow for direct download of the laboratory and pathology data.
This project was approved by the Montefiore Einstein Institutional Review Board and adherent to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies.
Study Sample and Data
Patients with SLE who had at least one value of random urinary protein-creatinine ratio (UPCR) of ≥0.2 and <0.5 g/g were defined as having a low-grade proteinuria (UPCR ≥0.2 and <0.5 g/g), similar to the definitions used in prior studies (9,12,17). The index study date was defined as the first known date of low-grade proteinuria. Patients who previously had a UPCR of ≥0.5 g/g, a known diagnosis of lupus nephritis, and/or a documented kidney biopsy specimen showing class II, III, IV, and V lupus nephritis or mixed histology preceding the first occurrence of low-grade proteinuria were excluded.
Histopathology data were extracted from clinical biopsy reports; all kidney biopsy specimens at our center were reviewed by nephropathologists with expertise in lupus nephritis in accordance with the 1995 revised World Health Organization criteria (18) before 2004, the 2003 International Society of Nephrology (ISN)/Renal Pathology Society (RPS) criteria (19) between 2004 and 2019, and revised ISN/RPS criteria (20) after 2019.
Although biopsy reports, laboratory data, and demographics were available from 1998 onward, medical notes (including medications and comorbidities) were only accessible after 2008. Medical records and pathology reports were reviewed by a rheumatologist (A.B.) who was blinded to the outcome. Patients who developed a random UPCR of ≥0.5 g/g with or without biopsy during the follow-up period were defined as “progressors.” Twenty-four-hour urine collection was not used to identify progressors in this study because UPCR is the uniform screening test performed at our institution for all patients with SLE. Patients who remained at a UPCR of <0.5 g/g at all time points were defined as “nonprogressors.” Patients who progressed to a UPCR of ≥0.5 g/g within 2 years of developing a UPCR of ≥0.2 and <0.5 g/g were defined as “fast progressors,” a subgroup that would be expected to benefit the most from early biopsies and therapeutic interventions. Those who progressed after 2 years were defined as “slow progressors.”
Statistical Analyses
On the basis of the laboratory cutoff at our center, high anti–double-stranded DNA (anti-dsDNA) antibody levels were defined as >70 U, low serum C3 was defined as <80 mg/dl, and low serum C4 was defined as <20 mg/dl. Low C3 and/or C4 were combined as “low complement.” GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration 2021 equation (21) and analyzed as ≤90 versus >90 ml/min.
The type and mechanisms of missing data were evaluated, including whether data was missing at random. A multiple imputation approach was used to impute for missing data and compare the results obtained from the data with imputation to those obtained from complete data as a primary approach. Complete case was used as a secondary approach.
The primary comparisons were performed between progressors and nonprogressors. Additional comparisons were performed between fast progressors and slow progressors/nonprogressors.
In the time-dependent analysis, the primary outcome was defined as time to a UPCR of ≥0.5 g/g (with or without biopsy). Nonprogressors were censored at the time of the last documented visit, on or before September 30, 2021. In the additional time-dependent comparisons of fast progressors, slow progressors, and nonprogressors, time to the outcome was defined as time from a UPCR of <0.5 g/g to the development of a UPCR of ≥0.5 g/g with or without biopsy within 2 years from a UPCR of <0.5 g/g. Slow progressors were censored at 2 years and nonprogressors were censored at the time of last documented visit or at the end of 2 years (whichever occurred earlier).
Cox regression analysis was used to estimate hazard ratios and to adjust for potential confounders, including age at index date, sex, and race (Black versus other). Additional variables were considered on the basis of the bivariate comparisons if the P value was <0.20.
To place our finding in a clinical context, sensitivity, specificity, and negative and positive predictive values were calculated for the variables associated with progression to overt proteinuria. For the purposes of these calculations, continuous variables were dichotomized at the median. Sensitivity analyses were performed as follows: using clinically indicated biopsy as the outcome, excluding slow progressors from the study sample (i.e., comparing fast progressors with nonprogressors), defining fast progressors as patients who developed a UPCR of ≥0.5 g/g within 1 year instead of 2 years from the low-grade proteinuria onset, and limiting the sample to patients with baseline UPCR of ≥0.25 and <0.5 g/g using a more narrow definition of low-grade proteinuria as described in prior studies (9,12). We also analyzed a subset of patients with complete medication and comorbidity data whose index date was on or after January 2008.
To study why some patients progressed without developing hypocomplementemia, we conducted two additional comparisons: (1) progressors with normal versus low complement and (2) progressors with normal complement versus nonprogressors.
Results
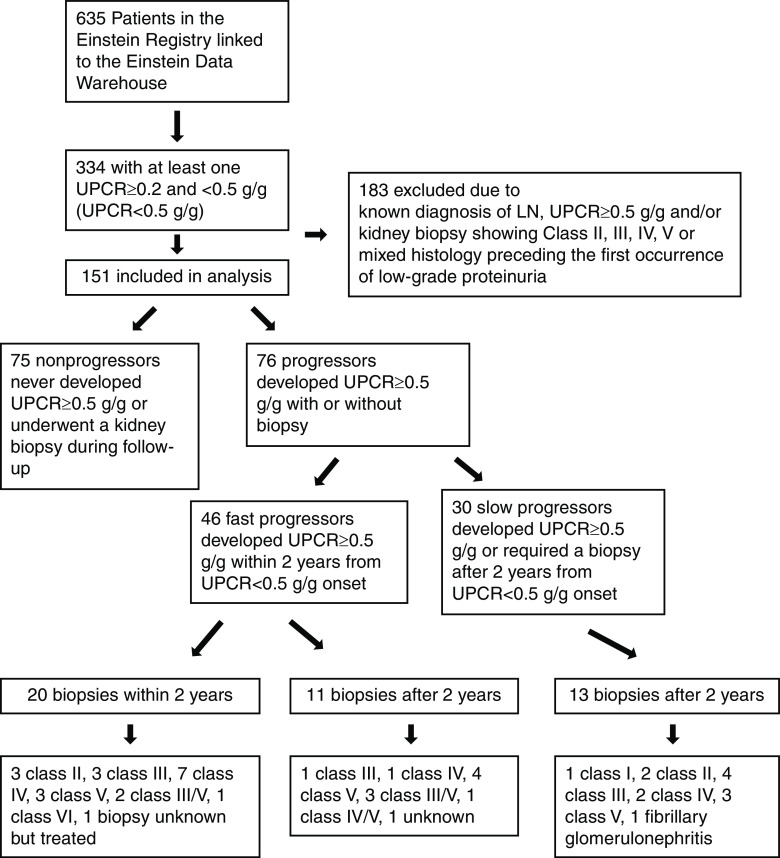
Of the 635 patients with SLE enrolled in the Einstein Registry and linked with the laboratory data in the Montefiore Hospital Electronic Data Warehouse, 334 had at least one documented random UPCR of ≥0.2 and <0.5 g/g between 1999 and 2020 (Figure 1). Of these, 183 were excluded because of a known diagnosis of lupus nephritis, UPCR of ≥0.5 g/g, and/or a kidney biopsy showing class II, III, IV, and V lupus nephritis or mixed histology preceding the first occurrence of low-grade proteinuria. Therefore, a total of 151 patients were included in the main analyses. The median (IQR) follow-up time from low-grade proteinuria to last follow-up visit in the Montefiore Hospital Electronic Data Warehouse was 8.2 (4.3–12.0) years (Table 1). The median (IQR) age was 33 (24–46) years, 11% were men, 39% were Black, and 56% were Hispanic/Latino.
Figure 1.
Study flow chart. From 635 SLE patients enrolled in the Einstein Registry, 151 SLE patients with at least one documented random UPCR of ≥0.2 and <0.5 g/g were included after applying the exclusion criteria. Seventy-six were progressors who developed a UPCR of ≥0.5 with or without biopsy during follow-up. Among these progressors, 46 were fast progressors who developed the outcome within 2 years from UPCR <0.5 g/g onset. Of the 20 biopsies performed in the first 2 years, 16 had active, treatable lupus nephritis. LN, lupus nephritis; UPCR, urinary protein-creatinine ratio.
Table 1.
Characteristics of participants in the Einstein Rheumatic Disease Registry at the time of first urinary protein-creatinine ratio between 0.2 and <0.5 g/g
| Characteristic | Total (n=151) | Outcome Urinary Protein-Creatinine Ratio ≥0.5 g/g (with or without Biopsy) | Outcome Urinary Protein-Creatinine Ratio ≥0.5 g/g (with or without Biopsy) within 2 Years from the Index Date | ||
|---|---|---|---|---|---|
| Progressors (n=76) | Nonprogressors (n=75) | Fast Progressors (n=46) | Slow Progressors/ Nonprogressors (n=105) | ||
| Demographics | |||||
| Age at index date (yr), median (IQR) | 33 (24–46) | 31 (21–43)a | 36 (28–50)a | 29 (21–41) | 34 (27–47) |
| Men, n (%) | 16 (11) | 9 (12) | 7 (9) | 6 (13) | 10 (10) |
| Black, n (%) | 59 (39) | 32 (42) | 27 (36) | 18 (39) | 41 (39) |
| Hispanic/Latino, n (%) | 85 (56) | 42 (55) | 43 (57) | 27 (59) | 58 (55) |
| SLE diagnosis to low-grade proteinuria onset (yr), median (IQR) | 3 (0–10) | 0 (0–2)a | 6 (1–12)a | 1 (0–4)a | 6 (1–12)a |
| Comorbidities b | |||||
| History of hypertension, n (%) | 20 (17) | 14 (25)a | 6 (10)a | 7 (21) | 13 (16) |
| History of diabetes, n (%) | 10 (9) | 7 (13) | 3 (5) | 6 (17)a | 4 (5)a |
| Medications b | |||||
| Hydroxychloroquine, n (%) | 78 (67) | 35 (63) | 43 (70) | 20 (58) | 59 (69) |
| Azathioprine, n (%) | 20 (17) | 8 (14) | 12 (20) | 2 (6)a | 18 (22)a |
| Mycophenolate mofetil, n (%) | 5 (5) | 4 (7) | 1 (2) | 3 (9) | 2 (2) |
| Other immunosuppressives, n (%)c | 11 (10) | 7 (13) | 4 (7) | 4 (12) | 7 (8) |
| ACEI/ARB, n (%) | 5 (4) | 3 (5) | 2 (3) | 1 (3) | 4 (5) |
| Corticosteroids, n (%) | 72 (62) | 38 (68) | 34 (56) | 21 (62) | 51 (61) |
| Corticosteroid dose (mg of prednisone equivalent), median (IQR) | 5 (0–20) | 5 (0–20) | 5 (0–18) | 5 (0–20) | 5 (0–20) |
| Laboratory and urine | |||||
| WBC (k/μl), median (IQR) | 6.2 (4.4–7.6) | 6.1 (4.5–7.2) | 6.2 (4.0–8.3) | 6.4 (4.3–7.4) | 6.2 (4.4–7.7) |
| Absolute neutrophil count (k/μl), median (IQR) | 4.1 (2.6–5.5) | 4.3 (2.4–6.0) | 4.0 (2.8–5.3) | 4.0 (2.6–5.3) | 4.2 (2.6–5.5) |
| Absolute lymphocyte count (k/μl), median (IQR) | 1.2 (0.9–1.7) | 1.2 (0.9–1.7) | 1.2 (0.85–1.7) | 1.3 (0.9–1.8) | 1.2 (0.8–1.6) |
| Platelet count (k/μl), median (IQR) | 257 (197–333) | 257 (191–322) | 266 (200–335) | 251 (197–333) | 265 (205–322) |
| GFR on the basis of CKD-EPI 2021 ≤90 ml/min, n (%) | 33 (22) | 17 (22) | 16 (21) | 12 (26) | 21 (20) |
| Serum albumin (g/dl), median (IQR) | 3.8 (3.4–4.2) | 3.8 (3.4–4.2) | 3.1 (2.8–3.4) | 3.7 (3.3–3.7) | 3.9 (3.4–4.2) |
| Low complement (C3 and/or C4), n (%) | 91 (66) | 56 (82)a | 35 (51)a | 37 (86)a | 54 (57)a |
| Missing 14 (9%) patients | |||||
| dsDNA positive, n (%) | 76 (56) | 41 (61) | 35 (51) | 24 (62) | 52 (54) |
| Missing 15 (10%) patients | |||||
| UPCR at index date (g/g), median (IQR) | 0.27 (0.23–0.37) | 0.29 (0.23–0.38) | 0.26 (0.22–0.32) | 0.29 (0.24–0.39) | 0.26 (0.22–0.35) |
| Urine RBC >5 HPF, n (%) | 55 (36) | 34 (45)a | 21 (28)a | 19 (41) | 36 (34) |
IQR, interquartile range; ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; WBC, white blood cells; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; dsDNA, double-stranded DNA; UPCR, urinary protein-creatinine ratio; RBC, red blood cells; HPF, high-powered field.
Statistically significant differences (P<0.05) between groups.
Medication and comorbidity data available for only 117 patients: 56 progressors and 61 nonprogressors.
Cyclophosphamide, belimumab, rituximab, methotrexate, leflunomide, and intravenous immunoglobulin.
Among the 151 patients with SLE who had a UPCR of ≥0.2 and <0.5 g/g, 76 (50%) progressed to a UPCR of ≥0.5 g/g (with or without kidney biopsy). The median (IQR) time from a UPCR of ≥0.2 and <0.5 g/g to progression was 1.2 (0.3–3.0) years. A total of 75 patients with SLE were nonprogressors who never developed a UPCR of ≥0.5 g/g or underwent a kidney biopsy during follow-up. Among the 76 progressors, 46 (61%) progressed to a UPCR of ≥0.5 g/g within the first 2 years of follow-up, and 30 (39%) progressed after 2 years.
Forty-four (58%) progressors underwent a kidney biopsy during the follow-up period (five had chart documentation only and 39 had available biopsy reports); 20 biopsies were performed in the first 2 years of follow-up and 24 biopsies were performed after 2 years. In addition to lupus nephritis, patients presented with the following concurrent findings: fibrillary GN (n=1), sickle cell nephropathy (n=1), and atherosclerosis/hypertensive nephropathy (n=6). Thirteen biopsy specimens showed full-house immunofluorescence (defined as the presence of IgG, IgM, IgA, C1q, and C3 deposits). Additional histologic findings included cellular/fibrocellular crescents (n=10), necrosis (n=4), interstitial inflammation (n=3), endocapillary proliferation (n=5), glomerulosclerosis (n=15), and interstitial fibrosis/tubular atrophy (n=7). Twenty biopsy specimens obtained in the first 2 years of follow-up included three showing class II lupus nephritis, three with class III, seven with class IV, three with class V, two with class III/V, and one with class VI lupus nephritis; one biopsy was performed at an outside hospital and was not available (although this patient was started on immunosuppression on the basis of the biopsy result). Progressors who underwent a biopsy were younger compared with progressors who did not undergo a biopsy: median (IQR) age of 25 (20–39) and 36 (28–50) years old, respectively (P=0.007).
Comparison between Progressors and Nonprogressors
Compared with nonprogressors, progressors to a UPCR of ≥0.5 g/g with or without biopsy were younger than nonprogressors, with a median age (IQR) 31 (21–43) versus 36 (28–50) years old (P=0.004; Table 1). The median (IQR) duration of SLE at the time of low-grade proteinuria onset was shorter among progressors than nonprogressors: 0 (0–2) years versus 6 (1–12) years, respectively (P=0.01). There were no differences with respect to sex or race and ethnicity between the two groups. A higher proportion of progressors than nonprogressors had low complement (C3 and/or C4): 82% versus 51% (P<0.001). In addition, 45% of progressors had evidence of hematuria (more than five red blood cells per high-powered field), as compared with 28% of nonprogressors (P=0.03). There were no statistically significant differences in random UPCR at index date, GFR, anti-dsDNA antibody levels, or serum albumin.
In the Cox model (Table 2, model 1), low complement was associated with a higher risk of progression to a UPCR of ≥0.5 g/g after adjusting for age, sex, race, and hematuria (hazard ratio, 2.6; 95% confidence interval, 1.4 to 4.8; P=0.003). Similar results were obtained using complete case analyses (Supplemental Table 1, model 1).
Table 2.
Clinical characteristics associated with progression from low-grade proteinuria to urinary protein-creatinine ratio of ≥0.5 g/g
|
Characteristics Associated with Outcomes |
N (%) Events | Unadjusted Hazard Ratio (95% Confidence Interval) | P Value | Adjusted Hazard Ratio (95% Confidence Interval)a | P Value |
|---|---|---|---|---|---|
| Model 1 (outcome: progression to UPCR ≥0.5 g/g; n=151) | |||||
| Age at index date, per 10 yr older | 76 (50) | 0.79 (0.66 to 0.94) | 0.009 | 0.96 (0.78 to 1.2) | 0.70 |
| Duration of SLE at index date, per 1 yr longer | 76 (50) | 0.95 (0.91 to 0.98) | 0.005 | 0.96 (0.92 to 1.0) | 0.06 |
| Low C3 and/or C4 | 76 (50) | <0.001 | 0.003 | ||
| No | 1 (reference) | 1 (reference) | |||
| Yes | 2.9 (1.6 to 5.2) | 2.6 (1.4 to 4.8) | |||
| Model 2 (outcome: progression to UPCR ≥0.5 g/g within 2 years; n=151) | |||||
| Age at index date, per 10 yr older | 46 (30) | 0.82 (0.65 to 1.0) | 0.07 | 1.0 (0.79 to 1.2) | 0.93 |
| Duration of SLE at index date, per 1 yr longer | 46 (30) | 0.91 (0.86 to 0.97) | 0.003 | 0.93 (0.87 to 0.99) | 0.02 |
| Low C3 and/or C4 | 46 (30) | 0.006 | 0.02 | ||
| No | 1 (reference) | 1 (reference) | |||
| Yes | 3.6 (1.4 to 8.9) | 2.9 (1.1 to 7.8) | |||
A multiple imputation approach was used to impute for missing data.
All models are adjusted for race, sex, and urine red blood cell count of more than five per high-powered field.
In the subset of 117 patients with available medication and comorbidity data, there was no association between the baseline medications and progression to a UPCR of ≥0.5 g/g. The proportion of patients with baseline hypertension was higher among progressors than nonprogressors (Table 1). In the Cox model (Supplemental Table 2, model 1), younger age at index date, low complement, and baseline hypertension were associated with the higher risk of the outcome.
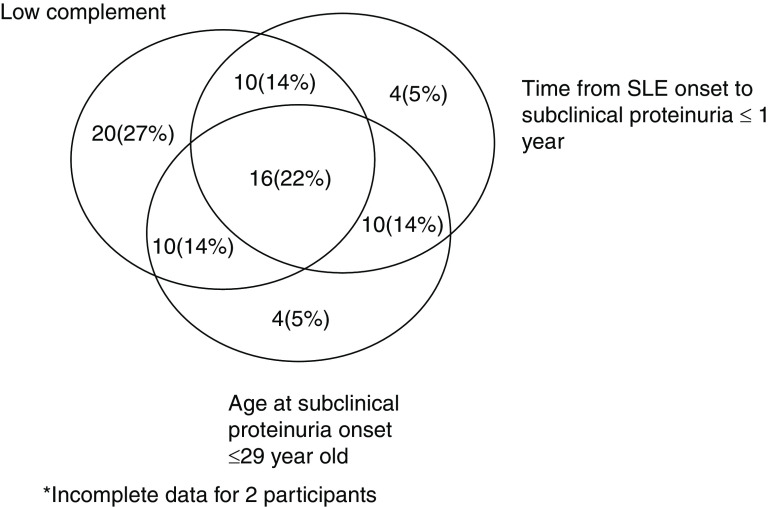
Sensitivity, specificity, and negative and positive predictive values for progression to overt proteinuria (UPCR ≥0.5 g/g) for low complement, age <29 years old, and SLE duration at biopsy of <1 year are provided in Table 3. The presence of low complement had the highest sensitivity (82%) and negative predictive value (94%) among the three variables. A Venn diagram (Figure 2) demonstrates the relationship among these variables.
Table 3.
Positive and negative predictive values for progression to overt proteinuria with or without biopsy during follow-up
| Factors Associated with Progression to Overt Proteinuria | Sensitivity, % | Specificity, % | Positive Predictive Value, % | Negative Predictive Value, % |
|---|---|---|---|---|
| Low complement | 82 | 49 | 62 | 94 |
| Age ≤29 yr | 53 | 28 | 43 | 38 |
| SLE duration at biopsy ≤1 yr | 53 | 32 | 44 | 40 |
Figure 2.
Venn diagram of the relationship between the main predictors of UPCR ≥0.5 g/g (with or without biopsy), n=74.
Comparison between Fast Progressors and Slow Progressors/Nonprogressors
Compared with slow progressors/nonprogressors, fast progressors included a higher proportion of patients with low complement and shorter SLE duration in the bivariate comparisons (Table 1). Both variables were associated with a higher risk of fast progression in the Cox regression models (Table 2, model 2; Supplemental Table 1, model 2).
In the subset of patients with data on medications and comorbidities, fast progressors had higher rates of diabetes mellitus and lower frequency of azathioprine use (Table 1). In the Cox model, shorter duration of SLE at index date, younger age, and low complement were associated with progression to a UPCR of ≥0.5 g/g within 2 years of follow-up (Supplemental Table 2, model 2). When fast progressors were compared with nonprogressors (Supplemental Table 2, model 3), low complement and shorter duration of SLE at index date were associated with progression. The overall results of the additional analyses with clinically indicated kidney biopsy as outcome and other sensitivity analyses were similar to the main analyses (Supplemental Table 2, models 4–5, Supplemental Tables 3 and 4).
Compared with progressors with normal complement, progressors with low complement had shorter duration from SLE diagnosis to low-grade proteinuria onset and included a higher proportion of patients with elevated anti-dsDNA antibody levels, history of diabetes, and use of immunosuppressives. Compared with nonprogressors, progressors with normal complement included a higher proportion of patients with diabetes and mycophenolate mofetil use. However, these comparisons are difficult to interpret given multiple potential confounders and small numbers of patients in each subgroup (Supplemental Table 5).
Discussion
In this longitudinal cohort of 151 patients with SLE and low-grade proteinuria at study entry, over half progressed to a UPCR of ≥0.5 g/g in a short period of 1.2 years. Low complement and shorter duration of SLE at the onset of the low-grade proteinuria were associated with progression to the outcome across various analyses. To date, this is the only longitudinal study evaluating the progression of SLE from low-grade proteinuria to clinically relevant proteinuria and/or kidney biopsy.
The current approach to lupus nephritis follows a proteinuria-centric paradigm. Evaluation of patients with SLE for lupus nephritis is triggered by detecting proteinuria above a certain threshold, most commonly a UPCR of 0.5–1.0 g/g. Although some patients with lupus nephritis will also have glomerular hematuria, hematuria alone does not generally result in a diagnostic kidney biopsy because hematuria is multifactorial, especially in young women. Challenging this conventional approach, pooled data from several studies demonstrate that about half of patients with SLE who have normal kidney function and low-grade proteinuria (UPCR ≥0.2 and <0.5 g/g), with or without glomerular hematuria, will have significant immune-mediated kidney injury (class III, IV, or V) on biopsy (9,10,22–25). This study confirms that over half of patients with SLE and low-grade proteinuria progress to overt proteinuria, supporting the role for early biopsy to diagnose lupus nephritis, even in the absence of hematuria. Importantly, 16 of 20 (80%) patients who initially had low-grade proteinuria had treatable lupus nephritis by biopsy by the time of progression, suggesting lupus nephritis can be diagnosed and treated earlier. The presence of low complement had the highest sensitivity and specificity with regard to progression to overt proteinuria, underscoring the need to monitor complement level closely in the presence of low-grade proteinuria to identify patients in the early stages of lupus nephritis.
The logical extension in considering the clinical implications of low-grade proteinuria would be to obtain a kidney biopsy at these lower thresholds for these patients at higher risk. Therefore, invoking the safety of kidney biopsy is relevant: recent published data from the Accelerating Medicines Partnership (AMP) showed 34 of 475 (7%) patients with SLE, who were followed prospectively for safety, experienced a related adverse event from a kidney biopsy, with 18 (4%) events deemed serious enough to require hospitalization (26). Comparatively, despite additional research tissue being obtained, patients in AMP had fewer adverse events than reported in the historical cohorts and meta-analyses of patients undergoing clinically indicated biopsies (27,28).
This study has several limitations related to its retrospective design. First, the study may have been underpowered to detect significance for some of the variables included in the analyses. Second, biopsies were not performed or delayed for some patients with clinically relevant proteinuria for a variety of reasons, including differences in the biopsy threshold guidelines over the years; patient preferences; socioeconomic barriers to care/insurance; high risk for complications, mainly due to antiphospholipid syndrome/anticoagulation; and severe multiorgan disease requiring rapid escalation of immunosuppression for nonkidney manifestations. In addition, NIH activity and chronicity scores were not consistently available in all kidney biopsy reports, some dating back to the late 1990s/early 2000s. Therefore, we could not determine if delays in biopsies resulted in higher chronicity. Lastly, data on active urinary sediment were not routinely available at our center. Random spot UPCR measurements may not be as accurate as a first morning urine or 24-hour collections and can introduce some heterogeneity. However, the results were consistent using two different definitions of low-grade proteinuria.
This study was not designed to assess whether medications, including hydroxychloroquine, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers may delay proteinuria onset in patients with SLE. The retrospective design also precluded us from studying whether early biopsies or treatments of low-grade proteinuria result in lower rates of progression to CKD, kidney failure, or death. These critical questions will need to be addressed using randomized trials or large-scale pharmacoepidemiologic studies to minimize biases inherent in retrospective studies. Hydroxychloroquine blood levels and treatment adherence measures were not available for the majority of patients in this study and would be important to account for in future studies.
Using real-world longitudinal data from a large ethnically and racially diverse SLE population from an urban tertiary care center is a major strength. The conclusions were robust across multiple sensitivity analyses, including different definitions of low-grade proteinuria range.
Importantly, the data from this study lay the groundwork for future studies of underlying molecular pathways in progression of early lupus nephritis by identifying at-risk populations. Early lupus nephritis diagnosis and effective intervention reduce adverse, long-term kidney complications. The ability to prevent injury should result in less overall exposure to toxic medications and better preservation of kidney mass. There is currently a dearth of reliable clinical markers to predict which patients with SLE who have low-grade proteinuria should be biopsied. Understanding the molecular mechanisms activated in the kidney at the earliest stage in high-risk populations will be critical for developing biomarkers of early disease.
Disclosures
J. Buyon reports having consultancy agreements with Boomcom, Equillium, GlaxoSmithKline, Janssen, L and M Healthcare Communications, Merck Sharp Dohme, and UpToDate. M. Petri reports having consultancy agreements with Alexion, Amgen, AnaptysBio, Argenx, AstraZeneca, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline, IQVIA, Janssen, Kira Pharmaceuticals, MedShr, and Sanofi; receiving research funding from AstraZeneca, Eli Lilly, Exagen, GlaxoSmithKline, Janssen, and Thermo Fisher; and serving on a speakers bureau for Aurinia and Focus MedEd. B.H. Rovin reports having consultancy agreements with, and receiving honoraria from, Alexion, AstraZeneca, Aurinia, Biocryst, Biogen, BMS, Calliditas, Chemocentryx, Corrona, EMD Serono/Merck, Exagen, Galapagos, Genentech, Horizon, Human Genome Sciences (GlaxoSmithKline), Idorsia, Janssen, MedImmune, Morphosys, Novartis, Omeros, Otsuka, Resonance, Retrophin, RILITE Foundation, Roche, and Vistera; serving in an advisory or leadership role for American Society of Nephrology (ASN) Kidney Week, CureGN, Kidney Disease Improving Global Outcomes, Kidney International, Kidney International Reports, Lupus Foundation of America, Nephrology Dialysis and Transplantation, and UpToDate; working with ASN (mostly educational courses), ISN, LFA, and the National Kidney Foundation; and receiving research funding from Biogen. A. Spielman reports having consultancy agreements with Shelter Island Risk. All remaining authors have nothing to disclose.
Funding
This research was supported by the National Center for Advancing Translational Science Einstein – Montefiore CTSA grant number KL2 TR002558 to S. Wang, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases grants K23 AR068441 to A. Broder and 1UC2AR081039-01 to J. Buyon, M. Petri, B.H. Rovin, and A. Broder.
Supplementary Material
Acknowledgment
All individuals with direct involvement of this study are listed as authors.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
A. Broder was responsible for project administration; A. Broder, J. Buyon, M. Petri, and B.H. Rovin conceptualized the study and provided supervision; A. Broder, J. Buyon, M. Petri, B.H. Rovin, and S. Wang reviewed and edited the manuscript and were responsible for funding acquisition and investigation; A. Broder, J. Buyon, and S. Wang wrote the original draft; A. Broder, M. Ginsberg, and A. Spielman were responsible for data curation, formal analysis, and methodology; A. Broder and S. Wang were responsible for validation and visualization; A. Spielman was responsible for software; and all authors were responsible for resources.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01280122/-/DCSupplemental.
Supplemental Table 1. Clinical characteristics associated with progression from low-grade proteinuria to urine protein-creatinine ratio (UPCR) ≥0.5 g/g using case complete analyses.
Supplemental Table 2. Cox proportional hazard models for the sensitivity analyses.
Supplemental Table 3. Baseline comparisons between progressors and nonprogressors; fast progressors and slow/nonprogressors with kidney biopsy as the outcome.
Supplemental Table 4. Cox proportional hazard models for progressors versus nonprogressors and fast progressors versus slow/nonprogressors with clinically indicated kidney biopsy as the outcome.
Supplemental Table 5. Comparison of progressors with normal versus low complement; progressors with normal complement versus nonprogressors.
References
- 1.Izmirly PM, Wan I, Sahl S, Buyon JP, Belmont HM, Salmon JE, Askanase A, Bathon JM, Geraldino-Pardilla L, Ali Y, Ginzler EM, Putterman C, Gordon C, Helmick CG, Parton H: The incidence and prevalence of systemic lupus erythematosus in New York county (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis Rheumatol 69: 2006–2017, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mok CC, Kwok RC, Yip PS: Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 65: 2154–2160, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, Amoura Z, Yu X, Mok CC, Santiago MB, Saxena A, Green Y, Ji B, Kleoudis C, Burriss SW, Barnett C, Roth DA: Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med 383: 1117–1128, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, Gibson K, Kaplan J, Lisk L, Navarra S, Parikh SV, Randhawa S, Solomons N, Huizinga RB: Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 397: 2070–2080, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Liossis SN, Staveri C: What’s new in the treatment of systemic lupus erythematosus. Front Med (Lausanne) 8: 655100, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, Ramsey-Goldman R, Singh K, Khalighi M, Choi SI, Gogia M, Kafaja S, Kamgar M, Lau C, Martin WJ, Parikh S, Peng J, Rastogi A, Chen W, Grossman JM; American College of Rheumatology : American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 64: 797–808, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group : KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-Glomerular-Diseases-Guideline-2021-English.pdf. Accessed July 7, 2022 [DOI] [PubMed] [Google Scholar]
- 8.Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, Boletis J, Frangou E, Houssiau FA, Hollis J, Karras A, Marchiori F, Marks SD, Moroni G, Mosca M, Parodis I, Praga M, Schneider M, Smolen JS, Tesar V, Trachana M, van Vollenhoven RF, Voskuyl AE, Teng YKO, van Leew B, Bertsias G, Jayne D, Boumpas DT: 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 79: 713–723, 2020 [DOI] [PubMed] [Google Scholar]
- 9.De Rosa M, Rocha AS, De Rosa G, Dubinsky D, Almaani SJ, Rovin BH: Low-grade proteinuria does not exclude significant kidney injury in lupus nephritis. Kidney Int Rep 5: 1066–1068, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zabaleta-Lanz ME, Muñoz LE, Tapanes FJ, Vargas-Arenas RE, Daboin I, Barrios Y, Pinto JA, Bianco NE: Further description of early clinically silent lupus nephritis. Lupus 15: 845–851, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Malvar A, Pirruccio P, Alberton V, Lococo B, Recalde C, Fazini B, Nagaraja H, Indrakanti D, Rovin BH: Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant 32: 1338–1344, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christopher-Stine L, Siedner M, Lin J, Haas M, Parekh H, Petri M, Fine DM: Renal biopsy in lupus patients with low levels of proteinuria. J Rheumatol 34: 332–335, 2007 [PubMed] [Google Scholar]
- 13.Wakiguchi H, Takei S, Kubota T, Miyazono A, Kawano Y: Treatable renal disease in children with silent lupus nephritis detected by baseline biopsy: Association with serum C3 levels. Clin Rheumatol 36: 433–437, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr, Magder LS: Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64: 2677–2686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health : Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes, 2015. Available at: https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html. Accessed July 7, 2022
- 17.Hanly JG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, Bae SC, Bernatsky S, Clarke AE, Wallace DJ, Merrill JT, Isenberg DA, Rahman A, Ginzler EM, Petri M, Bruce IN, Dooley MA, Fortin P, Gladman DD, Sanchez-Guerrero J, Steinsson K, Ramsey-Goldman R, Khamashta MA, Aranow C, Alarcón GS, Fessler BJ, Manzi S, Nived O, Sturfelt GK, Zoma AA, van Vollenhoven RF, Ramos-Casals M, Ruiz-Irastorza G, Lim SS, Kalunian KC, Inanc M, Kamen DL, Peschken CA, Jacobsen S, Askanase A, Theriault C, Farewell V: A longitudinal analysis of outcomes of lupus nephritis in an international inception cohort using a multistate model approach. Arthritis Rheumatol 68: 1932–1944, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Churg JBJ, Glassock RJ: Renal Disease: Classification and Atlas of Glomerular Diseases, 2nd Ed., New York, Igaky-Shoin, 1995 [Google Scholar]
- 19.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M; International Society of Nephrology Working Group on the Classification of Lupus Nephritis; Renal Pathology Society Working Group on the Classification of Lupus Nephritis : The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65: 521–530, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D’Agati VD, Ferrario F, Haas M, Jennette JC, Joh K, Nast CC, Noël LH, Rijnink EC, Roberts ISD, Seshan SV, Sethi S, Fogo AB: Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 93: 789–796, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, Grams ME, Greene T, Grubb A, Gudnason V, Gutiérrez OM, Kalil R, Karger AB, Mauer M, Navis G, Nelson RG, Poggio ED, Rodby R, Rossing P, Rule AD, Selvin E, Seegmiller JC, Shlipak MG, Torres VE, Yang W, Ballew SH, Couture SJ, Powe NR, Levey AS; Chronic Kidney Disease Epidemiology Collaboration : New creatinine- and cystatin c-based equations to estimate GFR without race. N Engl J Med 385: 1737–1749, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavragani CP, Fragoulis GE, Somarakis G, Drosos A, Tzioufas AG, Moutsopoulos HM: Clinical and laboratory predictors of distinct histopathogical features of lupus nephritis. Medicine (Baltimore) 94: e829, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakasugi D, Gono T, Kawaguchi Y, Hara M, Koseki Y, Katsumata Y, Hanaoka M, Yamanaka H: Frequency of class III and IV nephritis in systemic lupus erythematosus without clinical renal involvement: An analysis of predictive measures. J Rheumatol 39: 79–85, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Chedid A, Rossi GM, Peyronel F, Menez S, Atta MG, Bagnasco SM, Arend LJ, Rosenberg AZ, Fine DM: Low-level proteinuria in systemic lupus erythematosus. Kidney Int Rep 5: 2333–2340, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birmingham DJ, Merchant M, Waikar SS, Nagaraja H, Klein JB, Rovin BH: Biomarkers of lupus nephritis histology and flare: Deciphering the relevant amidst the noise. Nephrol Dial Transplant 32: i71–i79, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deonaraine KK, Carlucci PM, Fava A, Li J, Wofsy D, James JA, Putterman C, Diamond B, Davidson A, Fine DM, Monroy- Trujillo J, Atta MG, Haag K, Rao DA, Apruzzese W, Belmont HM, Izmirly PM, Wu M, Connery S, Payan-Schober F, Furie RA, Berthier CC, Dall’Era M, Cho K, Kamen DL, Kalunian K, Anolik J, Ishimori M, Weisman MH, Petri MA, Buyon JP; Accelerating Medicines Partnership RA/SLE network : Safety of procuring research tissue during a clinically indicated kidney biopsy from patients with lupus: Data from the Accelerating Medicines Partnership RA/SLE Network. Lupus Sci Med 8: e000522, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen TK, Estrella MM, Fine DM: Predictors of kidney biopsy complication among patients with systemic lupus erythematosus. Lupus 21: 848–854, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poggio ED, McClelland RL, Blank KN, Hansen S, Bansal S, Bomback AS, Canetta PA, Khairallah P, Kiryluk K, Lecker SH, McMahon GM, Palevsky PM, Parikh S, Rosas SE, Tuttle K, Vazquez MA, Vijayan A, Rovin BH; Kidney Precision Medicine Project : Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol 15: 1595–1602, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.