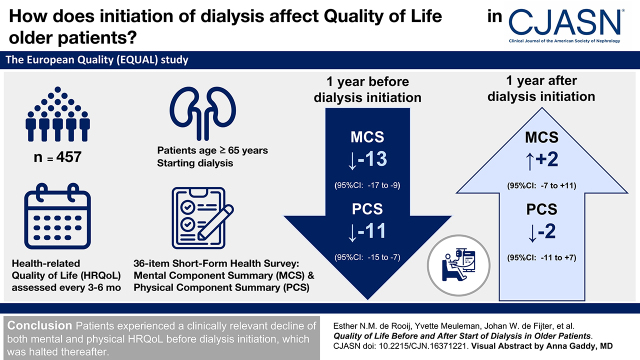
Visual Abstract
Keywords: quality of life, dialysis, end stage kidney disease, aged
Abstract
Background and objectives
In older people with kidney failure, improving health-related quality of life is often more important than solely prolonging life. However, little is known about the effect of dialysis initiation on health-related quality of life in older patients. Therefore, we investigated the evolution of health-related quality of life before and after starting dialysis in older patients with kidney failure.
Design, setting, participants, & measurements
The European Quality study is an ongoing prospective, multicenter study in patients aged ≥65 years with an incident eGFR ≤20 ml/min per 1.73 m2. Between April 2012 and December 2021, health-related quality of life was assessed every 3–6 months using the 36-item Short-Form Health Survey (SF-36), providing a mental component summary (MCS) and a physical component summary (PCS). Scores range from zero to 100, with higher scores indicating better health-related quality of life. With linear mixed models, we explored the course of health-related quality of life during the year preceding and following dialysis initiation.
Results
In total, 457 patients starting dialysis were included who filled out at least one SF-36 during follow-up. At dialysis initiation, mean ± SD age was 76±6 years, eGFR was 8±3 ml/min per 1.73 m2, 75% were men, 9% smoked, 45% had diabetes, and 46% had cardiovascular disease. Median (interquartile range) MCS was 53 (38–73), and median PCS was 39 (27–58). During the year preceding dialysis, estimated mean change in MCS was −13 (95% confidence interval, −17 to −9), and in PCS, it was −11 (95% confidence interval, −15 to −7). In the year following dialysis, estimated mean change in MCS was +2 (95% confidence interval, −7 to +11), and in PCS, it was −2 (95% confidence interval, −11 to +7). Health-related quality-of-life patterns were similar for most mental (mental health, role emotional, social functioning, vitality) and physical domains (physical functioning, bodily pain, role physical).
Conclusions
Patients experienced a clinically relevant decline of both mental and physical health-related quality of life before dialysis initiation, which stabilized thereafter. These results may help inform older patients with kidney failure who decided to start dialysis.
Introduction
Globally, the number of older (≥65 years) patients with kidney failure has increased, driven mainly by increasing prevalence of diabetes and hypertension (1). Because older patients are often ineligible for kidney transplantation, dialysis is the most common KRT. Considering the large comorbidity burden, limited functional status, and limited future perspectives in older patients with kidney failure, improving health-related quality of life (HRQoL) after the start of dialysis is deemed more important than solely the prolongation of life (2,3).
HRQoL is known to worsen considerably over time in the CKD stage 4/5 population (4). In patients on dialysis, HRQoL has also been found to be lower than in the general population (5,6). Patient-reported outcome measures, including questionnaires measuring HRQoL, are becoming more frequently incorporated into routine nephrology clinical care (7). However, to adequately interpret HRQoL outcomes for an individual patient with kidney failure, information on HRQoL levels and patterns in larger groups of patients with kidney failure is needed.
Nevertheless, there are very few studies addressing how HRQoL changes in the periods before and after dialysis initiation, and as far as we know, none are in older patients. Knowledge on the evolution of HRQoL could aid nephrologists in informing patients with kidney failure who consider starting dialysis and improve the shared decision-making process. This would especially be relevant for older patients with kidney failure considering their often limited life expectancy and treatment options. Therefore, our aim was to investigate the evolution of mental and physical HRQoL and the individual HRQoL domains in the year before and after starting dialysis in patients with kidney failure aged ≥65 years.
Materials and Methods
Study Design and Population
The European Quality (EQUAL) study on treatment in advanced CKD, starting in April 2012, is an ongoing, prospective, multicenter, follow-up study in six European countries (Germany, Italy, Poland, Sweden, The Netherlands, and the United Kingdom) as previously described in detail (8). Briefly, patients ≥65 years with advanced CKD followed in a nephrology clinic were included at an incident eGFR drop to or below 20 ml/min per 1.73 m2 in the last 6 months. Patients were excluded when the eGFR drop was the result of an acute event or when a history of KRT (i.e., dialysis initiation or kidney transplantation) was present. Identified patients who met the eligibility criteria were consecutively approached. Patients were followed every 3–6 months until kidney transplantation, death, refusal of further participation, transfer to a nonparticipating center, loss to follow-up, or end of follow-up, whichever came first. For these analyses, we included all patients who started hemodialysis or peritoneal dialysis and completed at least one HRQoL assessment during the year before or after dialysis. End of follow-up was December 2021, when the data were extracted. All patients gave written informed consent, and all local medical ethics committees or corresponding institutional review boards approved the study.
Data Collection
In the EQUAL study, patients are followed while receiving routine medical care as provided by their nephrology clinic. Data were collected every 3–6 months and entered into a web-based clinical record form that was developed for this specific purpose. Extra follow-up visits were conducted at the start of dialysis and after the eGFR dropped below 10 ml/min per 1.73 m2 for the first time. The collected data included patients’ demographics, ethnicity, primary kidney disease, comorbid conditions, physical examination, and laboratory data. All laboratory investigations and physical examinations were performed through standard protocols and procedures according to routine care at the local participating centers. In order to standardize these data, all participating centers completed a questionnaire to capture details on local laboratory methods, units of measurement, and normal ranges. Subsequently, all data were recalculated into one uniform unit of choice. The eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation (9). Primary kidney disease was classified by the treating nephrologist according to the codes of the European Renal Association (10). History of cardiovascular disease was defined as any history of cerebrovascular disease, myocardial infarction, or peripheral vascular disease.
HRQoL was assessed every 3–6 months using the 36-item Short Form Health Survey (SF-36), a 36-item questionnaire measuring HRQoL on eight domains, resulting in a mental component summary (MCS) score and a physical component summary (PCS) score. Component scores range from zero to 100, with higher scores indicating better HRQoL. The four domains of MCS are mental health, role limitations due to emotional problems, social functioning, and vitality. The four domains of PCS are physical functioning, bodily pain, role limitations due to physical problems, and general health. Following the SF-36 instructions, at least half of the items in a domain had to be completed to calculate a domain score (11). Patients could ask for the assistance of family, friends, or acquaintances in answering the questionnaires.
Statistical Analyses
For this study, baseline was defined as the date of the first dialysis treatment. Baseline characteristics are presented as mean ± SD, median (interquartile range [IQR]), or number (proportion) where appropriate. With life tables, we calculated the cumulative mortality risk during the year after dialysis initiation.
First, for our main analyses, patients were included when at least one MCS or PCS score was available in the year before or after the start of dialysis. We used linear mixed models to explore the evolution of MCS and PCS during the year preceding and the year following dialysis initiation. A random intercept and a random slope for time were used to account for repeated measurements, allowing the trajectory over time to vary between individuals. We assumed the relation between HRQoL and time to be nonlinear around the start of dialysis. Therefore, we modeled time in a three-knot restricted cubic spline function with 95% confidence intervals (95% CIs) to allow for more flexibility. The knots were chosen at the start of dialysis, 0.5 year before, and 0.5 year after. We repeated this analysis with additional knots at 1 or 3 months before and after dialysis initiation. Finally, we repeated this model with adjustments for age, sex, diabetes, and cardiovascular disease in order to correct for HRQoL data missing at random (12).
Second, we compared linear change in HRQoL during the year before and after the start of dialysis. We used three fixed variables in these linear mixed models: (1) time, (2) an indicator of whether dialysis was already started (yes or no), and (3) the interaction between time and the indicator. In this model, the interaction term estimates the difference in change in HRQoL before and after dialysis initiation.
Third, in order to identify differences in outcomes within subgroups, we studied the linear change of MCS and PCS before and after the start of dialysis after stratification at baseline for age (≥65 to <75 versus ≥75 years), sex (men versus women), smoking status (never, ex, or current smoker), history of diabetes (yes or no), history of cardiovascular disease (yes or no), Charlson comorbidity index (less than seven or greater than or equal to seven), eGFR at the start of dialysis (<10 versus ≥10 ml/min per 1.73 m2), and dialysis modality (hemodialysis versus peritoneal dialysis). Within these subgroups, we adjusted for the potential confounders age, sex, smoking, history of diabetes, history of cardiovascular disease, eGFR at dialysis initiation, and dialysis modality when appropriate.
Fourth, we assessed the linear evolution of the eight individual HRQoL domains (i.e., physical functioning, role physical, pain, general health, vitality, social function, role emotional, and mental health) in the year before and after dialysis initiation using linear mixed effect models.
Finally, we conducted three sensitivity analyses. First, we restricted our follow-up time to 6 months after dialysis initiation because patients who died in the year after did not fill out questionnaires after their death. Because these patients are more likely to have experienced worse HRQoL than patients who survived, informative dropout due to death should be considered. Second, we extended follow-up time to 3 years before and after dialysis initiation. Within this analysis, time was modeled as a four-knot restricted cubic spline with the knots chosen at the start of dialysis, 2 years before, 1 year before, and 0.5 year after. All patients with at least one MCS or PCS within this extended period were included in this analysis because we might have missed patients who only filled out an SF-36 outside the window of 1 year before dialysis initiation in our main analysis. Third, we restricted our analysis to patients who filled out questionnaires both before and after dialysis initiation. All analyses were performed using R version 4.0.3 (R Core Team, Vienna, Austria). This manuscript is in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines for reporting observational studies (13).
Results
Baseline Characteristics and Follow-Up
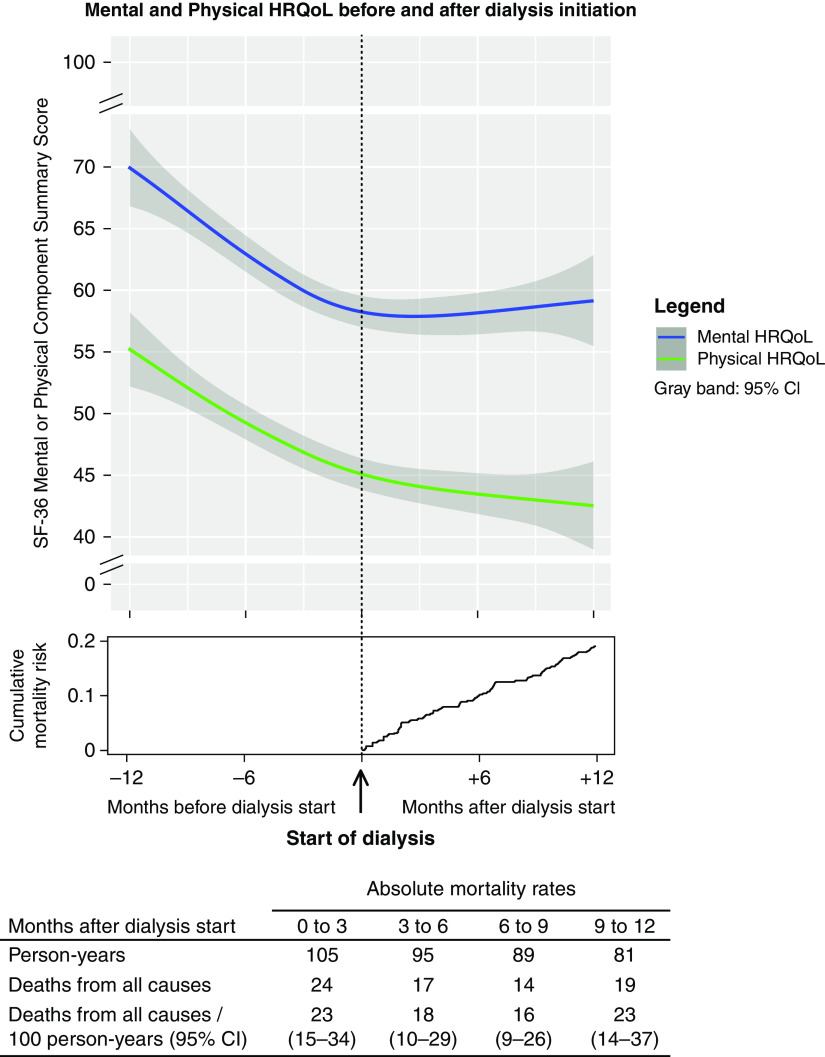
Of all EQUAL study participants who started dialysis, defined as baseline, 457 patients completed an SF-36 questionnaire during our follow-up period and were thus included in the analysis (Supplemental Figure 1). Baseline characteristics are presented in Table 1. At the start of dialysis, mean ± SD age was 76±6 years, 75% were men, 96% were White participants, 45% had diabetes, 9% were current smokers, 46% had a history of cardiovascular disease, and the mean (SD) eGFR was 8±3 ml/min per 1.73 m2. Median MCS was 53 (IQR, 38–73), and median PCS was 39 (IQR, 27–58). During 1 year after dialysis initiation, 74 patients died, of whom 24 and 41 died within 3 and 6 months of follow-up, respectively. The cumulative mortality risk at 1 year after dialysis initiation was 0.19 (Figure 1). Of the patients who died, 64% completed at least one SF-36 after dialysis initiation. Of all EQUAL study patients on dialysis, 133 (23%) were excluded for not sufficiently completing any questionnaires during our follow-up period. No relevant differences in baseline characteristics were observed between included and excluded patients (Supplemental Table 1).
Table 1.
Characteristics of 457 participants in the European Quality study on the treatment of older people with advanced CKD at the start of dialysis
| Baseline characteristics | Value |
|---|---|
| Demographics | |
| Age, yr | 76 (6) |
| Men, n (%) | 344 (75) |
| Country, n (%) | |
| Germany | 77 (17) |
| Italy | 91 (20) |
| The Netherlands | 69 (15) |
| Poland | 35 (8) |
| Sweden | 94 (21) |
| United Kingdom | 91 (20) |
| Marital status, n (%) | |
| Married | 318 (71) |
| Divorced | 27 (6) |
| Widowed | 82 (18) |
| Never married | 19 (4) |
| Education, n (%) | |
| Low | 105 (25) |
| Intermediate | 226 (54) |
| High | 90 (21) |
| Clinical characteristics | |
| Primary kidney disease, n (%) | |
| Diabetes | 111 (24) |
| Hypertension | 124 (27) |
| Systemic/glomerular/tubule-interstitial | 116 (25) |
| Other/unknown | 106 (23) |
| Dialysis modality, n (%) | |
| Hemodialysis | 315 (74) |
| Peritoneal dialysis | 110 (26) |
| Diabetes, n (%) | 199 (45) |
| Cardiovascular disease, n (%)a | 200 (46) |
| Chronic lung disease, n (%) | 53 (12) |
| Malignancy, n (%) | 95 (22) |
| Current smoking, n (%) | 40 (9) |
| BMI, kg/m2b | 27 (6) |
| Systolic BP, mm Hgb | 146 (24) |
| Diastolic BP, mm Hgb | 74 (12) |
| Blood chemistry b | |
| Creatinine, mg/dlc | 6.6 (2.3) |
| eGFR, ml/min per 1.73 m2d | 8 (3) |
| Urea nitrogen, mg/dle | 92 (42) |
| Albumin, g/dlf | 3.5 (0.6) |
| Cholesterol, mg/dlg | 159 (54) |
| HRQoL b | |
| Mental component summary score | 53 (38–73) |
| Mental health | 64 (52–80) |
| Role functioning emotional | 33 (0–100) |
| Social functioning | 63 (38–88) |
| Vitality | 40 (20–52) |
| Physical component summary score | 39 (27–58) |
| Physical functioning | 44 (20–65) |
| Bodily pain | 52 (31–100) |
| Role functioning physical | 0 (0–50) |
| General health | 40 (30–53) |
Data are expressed as number (percentage), mean (± SD), or median (interquartile range) when appropriate. BMI, body mass index; HRQoL, health-related quality of life.
Cardiovascular disease was defined as any history of a cerebral vascular accident, a myocardial infarction, or peripheral vascular disease.
Measured at the start of dialysis or within 30 days before the start of dialysis.
To convert the values for creatinine to micromoles per liter, multiply by 88.40.
eGFR was estimated on the basis of serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration formula.
To convert the values for urea nitrogen to millimoles per liter, multiply by 0.3571.
To convert the values for albumin to grams per liter, multiply by 10.
To convert the values for cholesterol to millimoles per liter, multiply by 0.02586.
Figure 1.
Evolution of mental (blue) and physical (green) health-related quality of life (HRQoL) in the year before and after the start of dialysis in 457 older patients, with a graph indicating the cumulative mortality risk and a table indicating the absolute mortality rates after dialysis initiation. 95% CI, 95% confidence interval; SF-36, 36-item Short Form Health Survey.
Questionnaires
In total, 1497 questionnaires were available during the follow-up period, with an average of 3.3 questionnaires per patient (Supplemental Figure 2). On average, questionnaires were missing in 18% and 35% of the follow-up visits in the year before or after dialysis initiation, respectively. Of all patients, 320 (70%) completed an SF-36 both before and after dialysis initiation, with a median of 135 (IQR, 90–184) days between questionnaires. Of the remaining 137 (30%) patients, 121 only filled out SF-36 questionnaires before dialysis initiation, and 16 only filled out SF-36 questionnaires after dialysis initiation. Missing questionnaires and follow-up visits are shown in Supplemental Figure 3 and Supplemental Table 2, respectively. Patients answering four or more compared with less than four SF-36 questionnaires during follow-up had a somewhat higher mean (SD) Charlson comorbidity score of 7.2±2.1 (Supplemental Table 3).
Evolution of Health-Related Quality of Life
When modeling time in a restricted cubic spline function, we observed a clear decline in HRQoL during the year before dialysis, which stabilized after dialysis initiation (Figure 1). Modeling time with knots closer to dialysis initiation or adjustments for age, sex, diabetes, and cardiovascular disease showed similar results (Supplemental Figures 4 and 5).
When assessing linear change in HRQoL during the year preceding dialysis, we observed a mean MCS decline of −13 (95% CI, −17 to −9) and a mean PCS decline of −11 (95% CI, −15 to −7) (Table 2, Supplemental Figure 6). All four individual mental HRQoL domains decreased in the year before dialysis, ranging from –9 (95% CI, –12 to –6; mental health) to –17 (95% CI, –26 to –8; role functioning emotional). The individual physical HRQoL domains of physical functioning, bodily pain, and role functioning physical decreased considerably in the year before dialysis, with scores of –11 (95% CI, –15 to –7), –9 (95% CI, –14 to –3), and –18 (95% CI, –25 to –10), respectively, whereas the change in general health was less with –2 (95% CI, –5 to +1).
Table 2.
Evolution of individual health-related quality-of-life component summary scores and individual domain scores in the year before and after the start of dialysis in 457 older patients
| Health-Related Quality of Life | Change per Year (95% Confidence Interval) | Difference in Change (95% Confidence Interval), Year after versus before the Start of Dialysis | |
|---|---|---|---|
| Year before the Start of Dialysis | Year after the Start of Dialysis | ||
| Mental component summary | −13 (–17 to –9) | +2 (–7 to +11) | +15 (+10 to +20) |
| Mental health | −9 (–12 to –6) | +3 (–5 to +11) | +12 (+7 to +17) |
| Role functioning emotional | −17 (–26 to –8) | −3 (–24 to +17) | +14 (+2 to +25) |
| Social functioning | −14 (–19 to –9) | +5 (–7 to +17) | +19 (+12 to +26) |
| Vitality | −12 (–16 to –8) | +6 (–3 to +15) | −18 (+13 to +23) |
| Physical component summary | −11 (–15 to –7) | −2 (–11 to +7) | +9 (+4 to +14) |
| Physical functioning | −11 (–15 to –7) | −1 (–11 to +10) | +10 (–4 to +13) |
| Bodily pain | −9 (–14 to –3) | −4 (–17 to +9) | +5 (+3 to +9) |
| Role functioning physical | −18 (–25 to –10) | +4 (–16 to +23) | +22 (+9 to +33) |
| General health | −2 (–5 to +1) | −1 (–9 to +6) | +1 (–4 to +5) |
In the year following dialysis, the mean MCS change was +2 (95% CI, –7 to +11), and the mean PCS change was –2 (95% CI, –11 to +7) (Table 2, Supplemental Figure 6). As for the individual mental HRQoL domains, mental health, social functioning, and vitality increased by +3 (95% CI, –5 to +11), +5 (95% CI, –7 to +17), and +6 (95% CI, –3 to +15), respectively, whereas role functioning emotional decreased by –2 (95% CI, –11 to +7). The individual physical HRQoL domains physical functioning, general health, and bodily pain decreased by –1 (95% CI, –11 to +10), –1 (95% CI, –9 to +6), and –4 (95% CI, –17 to +9), respectively, whereas role functioning physical increased by +4 (95% CI, –16 to +23) in the year after dialysis (Table 2).
Subgroup Analyses
In all subgroup analyses, MCS and PCS declined in the year before the start of dialysis and stabilized in the year after (Supplemental Table 4). Supplemental Table 5 shows median MCS and PCS at the start of dialysis for each subgroup. In patients ≥75 years compared with patients ≥65 and <75 years, adjusted MCS and PCS decreased more in the year before dialysis and increased less in the year after. At baseline, median MCS and PCS were lower in women compared with men. However, there was a faster decline of MCS and PCS in men compared with women in the year preceding dialysis, whereas this decline stabilized more in women than men after starting dialysis. Furthermore, adjusted MCS and PCS declined considerably faster before and after dialysis in current smokers when compared with former or never smokers. In patients with higher compared with lower Charlson comorbidity scores, PCS declined less in the year before the start of dialysis, whereas MCS improved less in the year after the start of dialysis.
Sensitivity Analyses
First, with follow-up restricted to 6 months after start of dialysis, MCS and PCS increased by +11 (95% CI, +3 to +19) and +6 (95% CI, –8 to +20), respectively, indicating a larger improvement after dialysis initiation than in our main analysis (Supplemental Figure 7, Supplemental Table 6). Second, by extending follow-up time from 1 to 3 years before dialysis, we included 39 additional patients and observed similar results compared with our main analysis, namely that mean MCS and PCS scores decreased by –20 (95% CI, –23 to –16) and –19 (95% CI, –23 to –15), respectively, during the 3 years before dialysis initiation (Supplemental Table 7). Especially for MCS, this decrease was fastest in the year before dialysis (Supplemental Figure 7). Third, when restricting our analysis to those who filled out questionnaires both before and after dialysis initiation (n=320), we found similar results; MCS decreased by –14 (95% CI, –18 to –10) and PCS decreased by –9 (95% CI, –14 to –5) in the year before dialysis, which stabilized in the year after with scores of +2 (95% CI, –9 to +12) and –3 (95% CI, –12 to +7), respectively.
Discussion
In this large European multicenter study of patients who started dialysis, we found clinically relevant worsening of mental and physical HRQoL of –13 and –11, respectively, during the year preceding dialysis initiation that stabilized with scores of +2 and –2, respectively, in the year after dialysis initiation.
Our results are in line with the findings of previous studies. Most studies assessing HRQoL in patients predialysis and patients on dialysis did so cross-sectionally (14–19). For example, in a cross-sectional analysis of the Dialysis Outcomes and Practice Patterns Study, 7378 prevalent patients on hemodialysis with a mean age of 59 years had much lower scores in all generic HRQoL subscales compared with their population norm values (2). Only a few studies have investigated the evolution of HRQoL in predialysis patients or patients on dialysis (4,5,20–24). The Dutch prospective PREdialysis PAtient Records-study (PREPARE) 2 cohort, including 502 patients with incident CKD stage 4/5 (nondialysis), a mean age of 65 years, and eGFR of 14 ml/min per 1.73 m2, showed that both mental and physical HRQoL decreased (by 8.9 and 7.4 points, respectively) until death or KRT, respectively (4). Furthermore, every three-point lower MCS or PCS score was associated with a hazard ratio of 1.04 (95% CI, 1.02 to 1.06) for reaching a combined poor health outcome of KRT and death (4). In 585 patients on dialysis who completed an HRQoL assessment at the start of dialysis and 12 months thereafter, HRQoL improved slightly, but this improvement was not clinically relevant (namely +1.4 and +1.4 for MCS and +0.7 and −1.0 for PCS in patients on hemodialysis and patients on peritoneal dialysis, respectively) (18). In patients on dialysis, lower mental and physical HRQoL has also been shown to be associated with hospitalization and mortality (25). The clinical relevance of changes in HRQoL depends on the type of population and intervention studied (26). In the general population with no specific intervention, clinically important differences in SF-36 scores vary from two to three points (27). In cohorts of various chronic conditions, clinically important differences range from three to five points (28). Therefore, the declines of −13 and −11 that we observed in mental and physical HRQoL, respectively, during the year preceding dialysis are substantial and thus clinically relevant for patients.
We studied the development of HRQoL before and after dialysis initiation in more detail by assessing the eight individual domains. Seven of the eight individual HRQoL domains showed a clinically relevant decline in the year before dialysis, which stabilized or improved in the year after. For example, we found a decline in the individual domain mental health of −9 points in the year before dialysis that stabilized with +3 points in the year after. This result is in line with previous studies showing that depression and depressive symptoms are highly common among patients on dialysis, with prevalence rates of 23% and 43%, respectively (29,30). In contrast, the domain general health only declined by −2 points in the year before the start of dialysis. For this individual domain, patients are asked to rate their general health, which is a broad concept compared with the other individual domains, thus leaving more room for personal interpretation (31). On a population level, this makes it more difficult to detect changes in the domain general health over time.
The steep decline of HRQoL before dialysis initiation and stabilization thereafter can be explained as follows. First, the number of uremic symptoms is known to increase considerably as kidney function declines prior to dialysis initiation (4). Assuming that dialysis treatment may alleviate uremic symptoms, this could explain the halt of the decline in mental and physical HRQoL after dialysis initiation. Second, interventions to prepare for dialysis initiation, such as vascular access creation, may also negatively affect HRQoL in the year preceding dialysis initiation (32). Third, differences in patient management before and after dialysis initiation (e.g., due to closer monitoring nearing the start of dialysis or after starting dialysis) may have influenced HRQoL. Lastly, as with any study on patient-reported outcomes, especially before and after an index event such as dialysis initiation, response shift needs to be considered. Response shift is defined as a change in the meaning of one's evaluation of a self-reported outcome, such as HRQoL, over time (26). In other words, certain experiences, such as dialysis initiation, can change someone’s frame of reference and thus their perception of HRQoL. To some extent, response shift may have contributed to the stabilization of the decrease in mental and physical HRQoL we observed after dialysis initiation.
There are several strengths to our study. First, we used a validated questionnaire to assess mental and physical HRQoL longitudinally both before and after the start of dialysis in a large cohort. This allowed us, for the first time, to describe the evolution of these important patient-reported outcomes before and after dialysis initiation in older individuals. Second, we included patients from six European countries, whereas previous studies were often restricted to a single nation. Because the perception of HRQoL can vary by country and nationality, our broad patient sample will allow for better generalizability of our results (33). However, our study also has some limitations. First, we could not include all EQUAL study patients on dialysis in this analysis because questionnaires were only available in 77%. However, there were no relevant differences in clinical characteristics at the start of dialysis between included and excluded EQUAL patients on dialysis. Second, for 18% of the follow-up visits in the year before dialysis and 35% in the year after, an SF-36 was missing. By using linear mixed effects models, we could take into account HRQoL data missing at random (e.g., a study coordinator forgot to send out an SF-36) but not data missing not at random (e.g., an SF-36 was not completed because a patient felt too sick). The latter may have resulted in an overestimation of HRQoL scores. However, an additional analysis in which we adjusted for age, sex, diabetes, and cardiovascular disease showed similar results. Third, the cumulative mortality risk at 1 year after dialysis initiation was 19% in our study. This number lies between the value of 15% established in 65- to 75-year-old European patients on dialysis and the value of 24% of European patients on dialysis >75 years old (34). After restriction of the follow-up to 6 months after starting dialysis, MCS and PCS increased slightly more. Therefore, our findings do not support the assumption that patients who died were more likely to have experienced worse HRQoL than those who remained in the study. If anything, the informative dropout due to death did not result in overestimation of the HRQoL scores that we calculated 1 year after dialysis initiation. Fourth, we did not collect data about the social support of our older population. Most likely, social support provided by, for example, families and neighbors varies between European countries and influences HRQoL. Lastly, because we only assessed patients starting dialysis, we could not investigate the evolution of HRQoL in patients not starting dialysis (e.g., those treated with conservative care or those who died before dialysis initiation). Therefore, our results are only applicable for informing patients with kidney failure who chose dialysis treatment and will survive up to dialysis initiation. As conservative care is becoming increasingly considered as an alternative to dialysis initiation in frail or older patients, assessing the effect on HRQoL of this conservative treatment would be of great value (18,22).
In conclusion, our results indicate that mental and physical HRQoL worsened considerably during the year preceding dialysis, but this decline stabilized at dialysis initiation. However, mental and physical HRQoL did not completely recover during the year after dialysis initiation. These results could help with informing older patients with kidney failure who decide to start dialysis on what to expect in the change of HRQoL.
Disclosures
F.J. Caskey reports research funding from the National Institute for Health Research and serving as treasurer, honorary secretary, and an executive committee member of the International Society of Nephrology (unpaid). F.W. Dekker reports research funding from Astellas, Chiesi, and Vifor; collaboration with the Dutch Kidney Patients Association; and collaboration with the Dutch Quality Institute for Renal Care (Nefrovisie). C. Drechsler reports research funding from Genzyme. M. Evans reports research funding from Astellas Pharma (institutional grant); payment for lectures from Astellas, AstraZeneca, Baxter Healthcare, Fresenius Medical Care, and Vifor Pharma; serving in an advisory or leadership role for Astellas, AstraZeneca, and the Vifor Pharma Advisory Board; and serving as a member of the European Renal Association (ERA) Registry Committee and a member of the steering committee of the Swedish Renal Registry. K.J. Jager reports serving on the editorial boards of African Journal of Nephrology, Journal of Renal Nutrition, Kidney International Reports, and Nephrology Dialysis Transplantation and serving on the European Renal Best Practice Committee of ERA. A.A. Pagels reports serving in an advisory or leadership role for the Swedish Renal Registry. C. Wanner reports consultancy agreements with Akebia, Bayer, Boehringer-Ingelheim, Gilead, GSK, MSD, Sanofi, Triceda, and Vifor; an Idorsia grant (to the institution) and a Sanofi grant (to the institution); honoraria from Amgen, Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, Chiesi, Eli-Lilly, FMC, Sanofi, and Takeda; serving as president of ERA; and other interests or relationships with ERA. All remaining authors have nothing to disclose.
Funding
Main funding for the EQUAL study was received from the European Renal Association and contributions from the Swedish Medical Association, the Stockholm County Council ALF Medicine and Center for Innovative Research, the Italian Society of Nephrology, the Dutch Kidney Foundation, the Young Investigators Grant in Germany, and the National Institute for Health Research in the United Kingdom.
Supplementary Material
Acknowledgments
We thank all of the patients and health professionals participating in the EQUAL study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Health Care for Older Adults with Kidney Failure,” on pages 1110–1112.
Author Contributions
F.W. Dekker, E.N.M. de Rooij, E.K. Hoogeveen, and Y. Meuleman conceptualized the study; E.N.M. de Rooij was responsible for data curation; E.N.M. de Rooij was responsible for investigation; E.N.M. de Rooij was responsible for formal analysis; F.W. Dekker, E.N.M. de Rooij, E.K. Hoogeveen, and Y. Meuleman were responsible for methodology; E.N.M. de Rooij and E.K. Hoogeveen were responsible for visualization; J.W. de Fijter, F.W. Dekker, E.K. Hoogeveen, and Y. Meuleman provided supervision; E.N.M. de Rooij wrote the original draft; and F.J. Caskey, N.C. Chesnaye, J.W. de Fijter, F.W. Dekker, E.N.M. de Rooij, C. Drechsler, M. Evans, E.K. Hoogeveen, K.J. Jager, S. Le Cessie, Y. Meuleman, A.A. Pagels, G. Porto, M. Szymczak, C. Torino, and C. Wanner reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16371221/-/DCSupplemental.
Supplemental Summary 1. Collaborator information.
Supplemental Table 1. Characteristics of 590 participants in the European Quality (EQUAL) study on the treatment of older people with advanced CKD at the start of dialysis.
Supplemental Table 2. The number (percentage) of patients who did or did not have a study visit of all included patients (n=457) within each follow-up interval.
Supplemental Table 3. Characteristics of 457 participants in the European Quality (EQUAL) study on the treatment of older people with advanced CKD at start of dialysis stratified by the number of SF-36 questionnaires completed.
Supplemental Table 4. Evolution of mental and physical HRQoL in the year before and after the start of dialysis within subgroups adjusted for potential confounders.
Supplemental Table 5. Median (IQR) HRQoL scores at the start of dialysis or within 30 days before the start of dialysis in those who filled out an SF-36 at that time.
Supplemental Table 6. Evolution of mental and physical HRQoL with restriction of follow-up to 1 year before and 0.5 year after the start of dialysis in 449 older patients.
Supplemental Table 7. Evolution of mental and physical HRQoL with extension of follow-up to 3 years before and 1 year after the start of dialysis in 496 older patients.
Supplemental Figure 1. Flow diagram indicating the selection of EQUAL study participants.
Supplemental Figure 2. Histograms indicating the number of completed SF-36 questionnaires per patient on dialysis in total (left) or during the year before or after the start of dialysis (right).
Supplemental Figure 3. Histogram indicating the number of completed and missing SF-36 questionnaires during the year before and after the start of dialysis.
Supplemental Figure 4. Evolution of mental (blue) and physical (green) HRQoL with additional knots at 3 (left) months and 1 (right) month before and after the start of dialysis in 457 older patients.
Supplemental Figure 5. Evolution of mental (blue) and physical (green) HRQoL in the year before and after the start of dialysis in 457 older patients with adjustments for age, sex, diabetes, and cardiovascular disease in order to correct for HRQoL data missing at random explained by these variables.
Supplemental Figure 6. Linear change of mental (blue) and physical (green) HRQoL in the year before and after the start of dialysis in 457 older patients, including a discontinuous change at the start of dialysis.
Supplemental Figure 7. Evolution of mental (blue) and physical (green) HRQoL with restriction of follow-up to 1 year before and 0.5 year after the start of dialysis in 449 older patients.
Supplemental Figure 8. Evolution of mental (blue) and physical (green) HRQoL with extension of follow-up to 3 years before and 1 year after the start of dialysis in 496 older patients.
References
- 1.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai CY, Floyd T, Al-Aly Z: Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 94: 567–581, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Fukuhara S, Lopes AA, Bragg-Gresham JL, Kurokawa K, Mapes DL, Akizawa T, Bommer J, Canaud BJ, Port FK, Held PJ; Worldwide Dialysis Outcomes and Practice Patterns Study : Health-related quality of life among dialysis patients on three continents: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 64: 1903–1910, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Goto NA, van Loon IN, Boereboom FTJ, Emmelot-Vonk MH, Willems HC, Bots ML, Gamadia LE, van Bommel EFH, Van de Ven PJG, Douma CE, Vincent HH, Schrama YC, Lips J, Hoogeveen EK, Siezenga MA, Abrahams AC, Verhaar MC, Hamaker ME: Association of initiation of maintenance dialysis with functional status and caregiver burden. Clin J Am Soc Nephrol 14: 1039–1047, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Goeij MC, Ocak G, Rotmans JI, Eijgenraam JW, Dekker FW, Halbesma N: Course of symptoms and health-related quality of life during specialized pre-dialysis care. PLoS One 9: e93069, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Willik EM, Hemmelder MH, Bart HAJ, van Ittersum FJ, Hoogendijk-van den Akker JM, Bos WJW, Dekker FW, Meuleman Y: Routinely measuring symptom burden and health-related quality of life in dialysis patients: First results from the Dutch registry of patient-reported outcome measures. Clin Kidney J 14: 1535–1544, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Kidney Foundation : KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis 66: 884–930, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Nair D, Wilson FP: Patient-reported outcome measures for adults with kidney disease: Current measures, ongoing initiatives, and future opportunities for incorporation into patient-centered kidney care. Am J Kidney Dis 74: 791–802, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jager KJ, Ocak G, Drechsler C, Caskey FJ, Evans M, Postorino M, Dekker FW, Wanner C: The EQUAL study: A European study in chronic kidney disease stage 4 patients. Nephrol Dial Transplant 27[Suppl 3]: iii27–iii31, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ERA Registry : (ERA) Registry Annual Report 2009, Amsterdam, The Netherlands, Academic Medical Center, Department of Medical Informatics, 2011 [Google Scholar]
- 11.Ware JE, Snow KK, Kosinski M, Gandek B: SF-36 Health Survey Manual and Interpretation Guide, Boston, New England Medical Center, The Health Institute, 1993 [Google Scholar]
- 12.Ibrahim JG, Molenberghs G: Missing data methods in longitudinal studies: A review. Test (Madr) 18: 1–43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 147: 573–577, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Fructuoso M, Castro R, Oliveira L, Prata C, Morgado T: Quality of life in chronic kidney disease. Nefrologia 31: 91–96, 2011. 10.3265/Nefrologia.pre2010.Jul.10483 [DOI] [PubMed] [Google Scholar]
- 15.Perlman RL, Finkelstein FO, Liu L, Roys E, Kiser M, Eisele G, Burrows-Hudson S, Messana JM, Levin N, Rajagopalan S, Port FK, Wolfe RA, Saran R: Quality of life in chronic kidney disease (CKD): A cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis 45: 658–666, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Kader K, Myaskovsky L, Karpov I, Shah J, Hess R, Dew MA, Unruh M: Individual quality of life in chronic kidney disease: Influence of age and dialysis modality. Clin J Am Soc Nephrol 4: 711–718, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz MC, Andrade C, Urrutia M, Draibe S, Nogueira-Martins LA, Sesso RC: Quality of life in patients with chronic kidney disease. Clinics (São Paulo) 66: 991–995, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verberne WR, Dijkers J, Kelder JC, Geers ABM, Jellema WT, Vincent HH, van Delden JJM, Bos WJW: Value-based evaluation of dialysis versus conservative care in older patients with advanced chronic kidney disease: A cohort study. BMC Nephrol 19: 205, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagels AA, Söderkvist BK, Medin C, Hylander B, Heiwe S: Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 10: 71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu AW, Fink NE, Marsh-Manzi JV, Meyer KB, Finkelstein FO, Chapman MM, Powe NR: Changes in quality of life during hemodialysis and peritoneal dialysis treatment: Generic and disease specific measures. J Am Soc Nephrol 15: 743–753, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Da Silva-Gane M, Wellsted D, Greenshields H, Norton S, Chandna SM, Farrington K: Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol 7: 2002–2009, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown MA, Collett GK, Josland EA, Foote C, Li Q, Brennan FP: CKD in elderly patients managed without dialysis: Survival, symptoms, and quality of life. Clin J Am Soc Nephrol 10: 260–268, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meuleman Y, Chilcot J, Dekker FW, Halbesma N, van Dijk S: Health-related quality of life trajectories during predialysis care and associated illness perceptions. Health Psychol 36: 1083–1091, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Chesnaye NC, Meuleman Y, de Rooij ENM, Hoogeveen EK, Dekker FW, Evans M, Pagels AA, Caskey FJ, Torino C, Porto G, Szymczak M, Drechsler C, Wanner C, Jager KJ; EQUAL Study Investigators : Health-related quality of life trajectories over time in older men and women with advanced CKD. Clin J Am Soc Nephrol 17: 205–214, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowrie EG, Curtin RB, LePain N, Schatell D: Medical outcomes study short form-36: A consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis 41: 1286–1292, 2003 [DOI] [PubMed] [Google Scholar]
- 26.van der Willik EM, Terwee CB, Bos WJW, Hemmelder MH, Jager KJ, Zoccali C, Dekker FW, Meuleman Y: Patient-reported outcome measures (PROMs): Making sense of individual PROM scores and changes in PROM scores over time. Nephrology (Carlton) 26: 391–399, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruish ME: User’s Manual for the SF-36v2 Health Survey, 3rd Ed., Lincoln, RI, QualityMetric Incorporated, 2011 [Google Scholar]
- 28.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D: Determining clinically important differences in health status measures: A general approach with illustration to the Health Utilities Index Mark II. PharmacoEconomics 15: 141–155, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Fishbane S, Strippoli GF: Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int 84: 179–191, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Schouten RW, Harmse VJ, Dekker FW, van Ballegooijen W, Siegert CEH, Honig A: Dimensions of depressive symptoms and their association with mortality, hospitalization, and quality of life in dialysis patients: A cohort study. Psychosom Med 81: 649–658, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jylhä M: What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med 69: 307–316, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Casey JR, Hanson CS, Winkelmayer WC, Craig JC, Palmer S, Strippoli GF, Tong A: Patients’ perspectives on hemodialysis vascular access: A systematic review of qualitative studies. Am J Kidney Dis 64: 937–953, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Ware JE Jr., Gandek B, Kosinski M, Aaronson NK, Apolone G, Brazier J, Bullinger M, Kaasa S, Leplège A, Prieto L, Sullivan M, Thunedborg K: The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: Results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 51: 1167–1170, 1998 [DOI] [PubMed] [Google Scholar]
- 34.ERA Registry : ERA Registry Annual Report 2019, Amsterdam, The Netherlands, Academic Medical Center, Department of Medical Informatics, 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.