Visual Abstract
Keywords: exercise, chronic kidney failure, CKD, hospitalization, death
Abstract
Background and objectives
In the EXerCise Introduction to Enhance Performance in Dialysis (EXCITE) trial, a simple, personalized 6-month walking exercise program at home during the day off of dialysis improved the functional status and the risk for hospitalization in patients with kidney failure. In this post-trial observational study, we tested whether the same intervention was associated with a lower long-term risk of death or hospitalization (combined end point) during a follow-up extended up to 36 months.
Design, setting, participants, & measurements
In total, 227 patients (exercise, n=104; control, n=123) completed the 6-month trial and entered the post-trial observational study. Data were analyzed by unadjusted and adjusted Cox regression analyses and Bayesian analysis.
Results
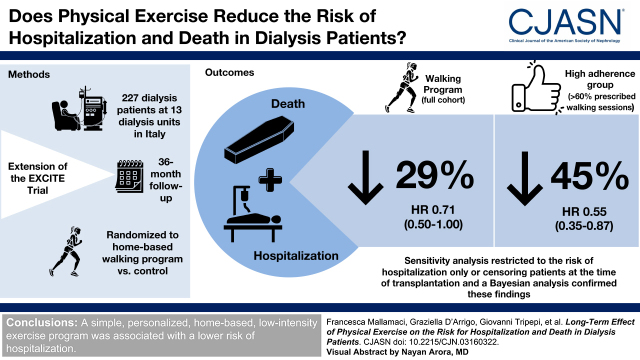
In the long-term observation (up to 36 months), 134 events were recorded (eight deaths not preceded by hospitalization and 126 hospitalizations, which were followed by death in 38 cases). The long-term risk for hospitalization or death was 29% lower (hazard ratio, 0.71; 95% confidence interval, 0.50 to 1.00), and in an analysis stratified by adherence to the walking exercise program during the 6-month trial, the subgroup with high adherence (>60% of prescribed sessions) had a 45% lower risk as compared with the control group (hazard ratio, 0.55; 95% confidence interval, 0.35 to 0.87). A Bayesian analysis showed that the posterior probability of a hazard ratio of 0.71 (95% confidence interval, 0.50 to 1.00) for the risk of the composite outcome observed in the post-trial observational study was 93% under the conservative prior and 97% under the optimistic prior. Sensitivity analyses restricted to the risk of hospitalization only or censoring patients at the time of transplantation fully confirmed these findings.
Conclusions
A simple, personalized, home-based, low-intensity exercise program was associated with a lower risk of hospitalization.
Clinical Trial registry name and registration number:
EXerCise Introduction to Enhance Performance in Dialysis (EXCITE), NCT01255969
Introduction
Physical performance is notoriously compromised in patients with kidney failure and patients maintained on long-term dialysis (1–3), and decreased physical activity has been implicated in the high risk of death (4), hospitalization (5,6), and other adverse health outcomes (7–10) in this population, including left ventricular hypertrophy (11). Over the last two decades, several clinical studies in patients with kidney failure have been performed, and two meta-analyses documented the effectiveness of various exercise programs for improving physical performance in these patients (10,12). Furthermore, another meta-analysis showed that aerobic exercise improves several hemodialysis-related symptoms, including restless legs syndrome, depression, muscle cramps, and fatigue (13). However, until now, there was no evidence that these programs, walking exercise in particular, may have a favorable effect on major clinical outcomes and on the risk for hospitalization. A trial that tested a 6-month intervention on the basis of intradialysis cycling (14) showed that this exercise may reduce health care costs during the year following the trial.
In the EXerCise Introduction to Enhance Performance in Dialysis (EXCITE) study (15), the largest randomized clinical trial on physical exercise in this population (16), we found that a 6-month simple, personalized, home-based, low-intensity training program improves physical performance in these patients. Furthermore, in a per-protocol analysis, we found that the risk of hospitalization, a secondary end point of the same trial, was 54% lower in the exercise arm than in the control arm (15).
Because the beneficial effects of physical exercise may persist for several years after the reduction or the interruption of exercise (17), we hypothesized that the 6-month training program applied in the EXCITE trial could produce benefits beyond 6 months, including a favorable trend in the risk of hospitalization. In this post-trial study, we therefore examined the long-term (36-month) effects of the walking exercise program versus standard care on the risk for hospitalization and death in patients who completed the 6-month EXCITE trial.
Materials and Methods
The EXCITE trial was a multicenter, prospective, randomized, parallel-group trial of a home-based exercise program compared with a control arm in patients on dialysis conducted between November 2009 and February 2011 at 13 nephrology units (six academic) in Italy. The detailed design of the trial, the baseline characteristics, the inclusion and the exclusion criteria, and the main results of EXCITE were published in 2017 (15). Long-term hospitalization and mortality were prespecified secondary outcome measures of the EXCITE trial.
The Exercise Intervention
The intervention in the EXCITE trial consisted of a walking exercise home program supervised by a central rehabilitation team. This intervention, reported in detail in ref. 15, is briefly described in Supplemental Material and Supplemental Table 1. Adherence to the physical exercise program was prespecified as adherence to >60% of exercise sessions (high adherence) during the first 6 months of the trial (11).
Post-Trial Observation
After the end of the 6-month trial, patients randomized to the active arm of EXCITE were advised to perform just one walking session of 10 minutes at self-selected speed without using the metronome three times per week during the dialysis interval up to the 18th month (unstructured part of the exercise program). Thereafter, up to 36 months, they were given just generic advice to maintain an active lifestyle, like the patients in the control group. A training diary was provided to each patient to register their walking sessions up to the 18th month. Total walked sessions were collected for the active group from baseline to the 18th month but not thereafter.
Outcome Measures
After the end of the trial, we continued to systematically collect follow-up information on mortality and hospitalization in all patients who completed the EXCITE trial. Death was ascertained by death certificates and by direct information obtained from the families of deceased patients who were systematically contacted (no missing contact). Causes of hospitalization were established on the basis of hospital codes that were transformed into the corresponding diagnoses reported in Table 1.
Table 1.
Causes of hospitalization in patients who completed the 6-month trial (104 in the exercise arm and 123 in the control arm) and participated in this post-trial study extending the observation up to 36 months
| Hospitalizations | Exercise Group | Control Group |
|---|---|---|
| Infection | ||
| Gastrointestinal | 5 | 10 |
| Bronchopulmonary | 1 | 3 |
| Systemic sepsis | 1 | 2 |
| Other | 2 | 3 |
| Cardiovascular | ||
| Coronary heart disease (angina and/or myocardial infarction) | 6 | 6 |
| Heart failure | 5 | 6 |
| Atrial fibrillation and other severe forms of arrythmia | 4 | 6 |
| Peripheral vascular disease | 1 | 4 |
| Cerebrovascular events | 1 | 3 |
| Vascular access–related complications | 9 | 11 |
| Unexplained syncope | 0 | 1 |
| Neurologic | ||
| Seizures | 0 | 3 |
| Severe vertigo | 0 | 1 |
| Surgical interventions (various causes) | 4 | 7 |
| Hemorrhage | 3 | 0 |
| Bone fracture | 2 | 3 |
| Other | 6 | 7 |
| Total | 50 | 76 |
The secondary analysis applied in the 6-month trial (15) focused solely on the risk of hospitalization because patients who entered the per-protocol analysis had to have completed the 6-month training program (i.e., had to survive the first 6 months). In this observational post-trial analysis in patients who completed the 6-month training program, 46 deaths occurred, 38 after the first hospitalization. In order to prevent the competing risk of death for hospitalization in this extension of the initial per-protocol analysis, we therefore adopted a composite end point combining these two outcomes.
Statistical Analyses
Data are expressed as mean and SD (normally distributed data), median and interquartile range (non-normally distributed data), or percentage frequency (binary data), and comparisons between groups were performed by t test (normally distributed data), Mann–Whitney U test (non-normally distributed data), or chi-squared test (binary data), as appropriate. We calculated Kaplan–Meier product limit estimates and compared death and hospitalization-free survival curves using the log-rank test. We calculated relative hazards according to adherence and 95% confidence intervals (95% CIs) using proportional hazards (Cox) regression. Analyses were also adjusted for risk factors that differed among the study arms with a P value =0.20 (Table 2). Missing values were <5% for all variables, except for serum cholesterol (17%). Missing values were replaced with the corresponding mean value. In survival analyses, the proportionality assumption of the effect of the allocation arm as well as of the degree of adherence to physical exercise was tested by the analysis of Schoenfeld residuals, and no violation was found. All biologically plausible effect modifications and interactions were tested, and no significant effects were found.
Table 2.
Demographic, clinical, and biochemical data of patients who completed the 6-month trial and participated in the observational study extended up to 36 months
| Characteristics | Active Arm, n=104 | Control Arm, n=123 |
|---|---|---|
| Age, yr | 63±13 | 64±14 |
| Men, N (%) | 67 (64) | 84 (68) |
| Hemodialysis/CAPD, n | 90/14 | 102/21 |
| BMI, kg/m2 | 26±4 | 27±6 |
| Smoking, N | 18 (18) | 22 (19) |
| Diabetes, N | 19 (18) | 21 (18) |
| BP systolic/diastolic, mm Hg | 132±18/72±10 | 127±18/71±12 |
| Total cholesterol, mg/dl | 164±39 | 166±39 |
| Hemoglobin, g/dl | 11±1 | 11±2 |
| Albumin, g/dl | 3.9±0.4 | 3.8±0.5 |
| Phosphate, mg/dl | 4.9±1.5 | 4.8±1.4 |
| Kt/V HD | 1.42±0.25 | 1.43±0.30 |
| Kt/V CAPD | 1.96±0.29 | 1.80±0.60 |
| Myocardial infarction, N (%) | 16 (15) | 21 (17) |
| Stroke/transient ischemic attack, % | 8 (8) | 17 (14) |
| Anginal episodes, % | 12 (11) | 16 (13) |
| Arrhythmia, % | 13 (12) | 9 (7) |
| Heart failure, % | 18 (17) | 29 (24) |
| Peripheral vascular disease, % | 7 (7) | 15 (12) |
| History of neoplasia, % | 22 (22) | 22 (18) |
| Antihypertensive therapy, % | 76 (77) | 82 (70) |
| NYHA class, % | ||
| 1 | 37 (37) | 40 (33) |
| 2 | 16 (16) | 19 (16) |
| 3–4 | 5 (5) | 12 (10) |
| Ambulation | ||
| Assisted | 4 (4) | 3 (2) |
| Independent | 100 (96) | 118 (98) |
CAPD, continuous ambulatory peritoneal dialysis; BMI, body mass index; HD, hemodialysis; NYHA, New York Heart Association class.
Bayesian Analyses
Because of random error, results reaching statistical significance in relatively small trials tend to inflate the true effect of a certain treatment (18); we performed a Bayesian analysis to better assess the uncertainty of the results of this secondary analysis under two prior distributions, in which a specific hazard ratio (HR) is given as unlikely or highly likely to occur. The HR that we used to generate the two prior distributions was the HR registered in the primary analysis of the EXCITE trial (HR [active versus control] = 0.46) (15). The posterior distributions of the HR were simulated by Monte Carlo Markov chains to describe the implications of the trial result under each prior assumption. We considered a conservative prior to represent a perspective that assumes a 54% hazard rate reduction of hospitalization/death in the active versus the control arm (i.e., a HR of 0.46) and attributes a relatively small probability (5%) to occur to such an effect. We also considered an enthusiastic prior by attributing a relatively high probability (80%) to the same effect.
Data analysis was performed by using a standard statistical package (SPSS for Windows, version 22; IBM SPSS, Chicago, IL) as well as with R 3.0.1 and the STATA statistical package (version 13).
Results
Between November 2009 and February 2011 among 714 patients, 296 in nine centers (60% of the eligible patients, 41% of the total population) were randomized to walking exercise (n=151) or usual care and normal physical activity (n=145). The original trial population and the reasons for nonparticipation and exit from the trial are detailed in the main paper reporting the main results of the EXCITE trial (15), and the CONSORT diagram of the trial is presented in Supplemental Figure 1. As described in Supplemental Figure 1, 104 patients in the active arm (hemodialysis, n=90; continuous ambulatory peritoneal dialysis [CAPD], n=14) and 123 patients in the control arm (hemodialysis, n=102; CAPD, n=21) completed the 6-month trial. The 47 patients in the exercise arm who dropped out during the 6-month trial did not differ from the 104 patients in the same group who completed the trial (Supplemental Table 2). Similarly, the 104 patients of the active arm did not differ from the 123 patients of the control group who were included in this long-term study (Table 2).
Complete information on survival and hospitalization was obtained in all patients who participated in this long-term, 36-month extended follow-up, including hospitalizations during the first 6 months.
Adherence to the Exercise Program
In total, 227 patients completed the 6-month trial: 104 in the exercise arm and 123 in the control arm. The average number of training sessions performed by patients in the exercise arm was 119±103 (range, 7–336), corresponding to 83% of the 144 prescribed sessions. Forty-six patients exceeded the number of prescribed sessions because they also did extra sessions on the dialysis days, whereas 29 performed just the minimal amount (<10%) of the prescribed sessions. The level of adherence to the exercise program was high (60% of prescribed sessions) for 55 patients and low (<60%) for 49 of them.
During the post-trial observation from 6 to 18 months, 31 patients (30% of those in the exercise arm) performed 9.6±5.2 sessions per month over the same period, whereas the remaining patients of the same arm did not perform any exercise. The 55 patients who were highly adherent during the 6-month trial performed more training sessions from the sixth to the 18th month post-trial (median, 184 sessions; interquartile range, 0–418) as compared with the 49 patients with low adherence, among whom only two patients performed a limited number of training sessions (26 and 44, respectively). A breakdown of high- and low-adherence patients is presented in Supplemental Table 3. As described in Materials and Methods, no specific recommendation for exercise was given beyond the 18th month in either group, and both patients originally allocated into the active arm and those in the control arm were given just generic advice to maintain an active lifestyle.
Observational Analysis Extended to 36 Months in Patients Who Completed the 6-Month Trial
Cumulative Incidence of the Combined End Point.
As previously reported (15), among patients who completed the 6-month trial, ten patients in the active arm and 18 in the control arm were hospitalized. In this post-trial analysis extended up to the 36th month (i.e., 30 months after the end of the trial), 50 patients in the active arm and 76 patients in the control arm were hospitalized, whereas 46 patients died, 38 of whom died after the first hospitalization. Ten patients in the active arm and 14 in the control arm underwent kidney transplantation. The cumulative incidence of the combined end point (i.e., hospitalization and death) of the extended (from the study start to the 36th month) per-protocol analysis was 29% lower (HR, 0.71; 95% CI, 0.50 to 1.00; P=0.06) in the active arm than in the control arm (Figure 1), and data adjustment for baseline variables that differed among the two study arms with a P value of 0.20, namely systolic BP, past cerebrovascular events, peripheral vascular disease, and arrhythmia (Table 2), did not modify the HR (adjusted HR, 0.69; 95% CI, 0.48 to 0.98; P=0.04).
Figure 1.
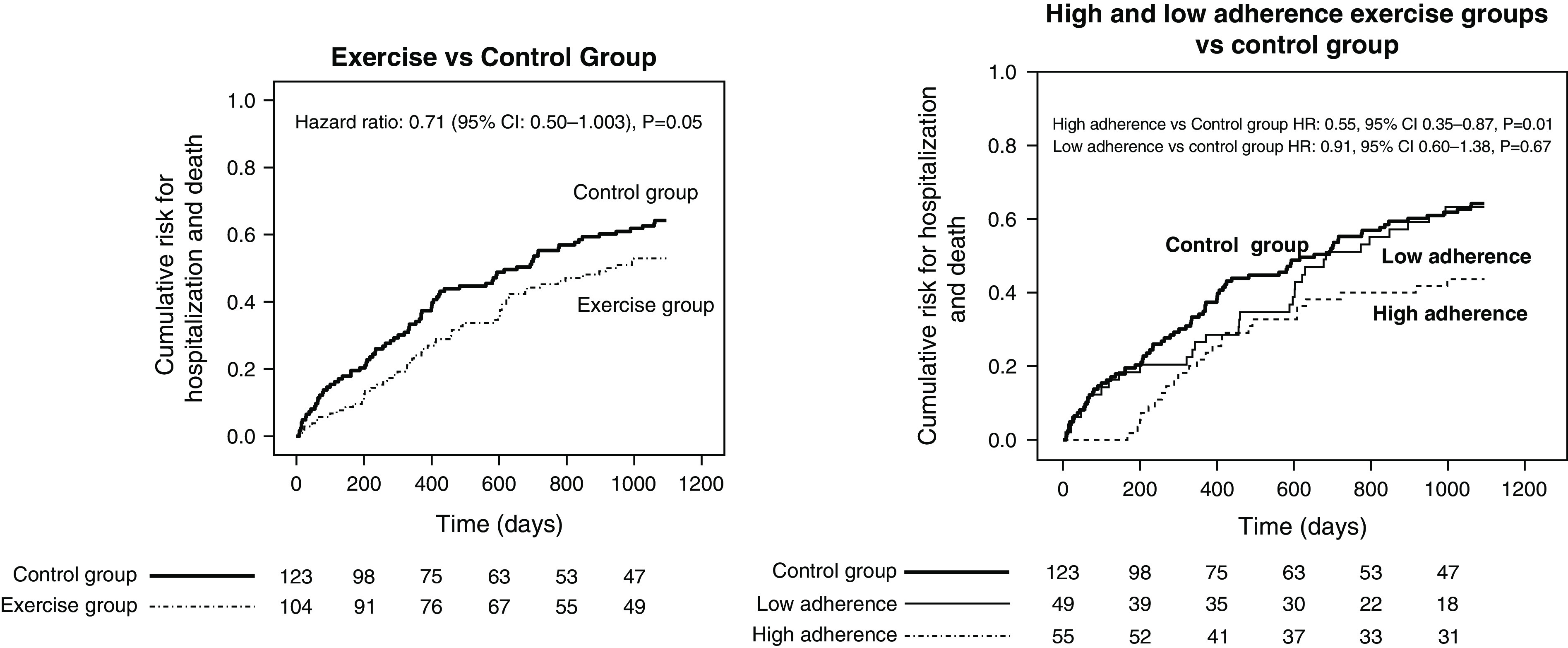
(Left panel) Cumulative incidence of hospitalization in the exercise and control arms during the EXerCise Introduction to Enhance Performance in Dialysis (EXCITE) trial (months 1–6) and the long-term post-trial observation extended up to 36 months. (Right panel) Cumulative incidence of hospitalization in the high- and low-exercise arms and in the control arms during the EXCITE trial (months 1–6) and the long-term post-trial observation (months 7–36). The graphs were derived by the reverse Kaplan–Meier option in SPSS. 95% CI, 95% confidence interval; HR, hazard ratio.
Mortality, Analyses Restricted to Hospitalization, and Causes of Hospitalization.
There was no difference in mortality among the two study arms (exercise arm: five deaths; control arm: three deaths; P=0.34), and an analysis restricted to hospitalization (Supplemental Table 4) confirmed a significant reduction in the risk for this event. The causes of hospitalization in the two groups are reported in Table 1. Hospitalizations due to infections (exercise group, n=9; control group, n=18) and cardiovascular diseases (exercise group, n=26; control group, n=37) were the main causes driving the difference among the two arms. An analysis focusing on the relationship between the adherence to the prescribed physical exercise during the 6-month trial and the long-term (up to the 36th month) risk for the combined outcome (Figure 1) showed that patients with high adherence (>60% of training sessions) had a 45% lower risk as compared with patients in the control arm (HR, 0.55; 95% CI, 0.35 to 0.87; P=0.01), whereas no difference was observed between patients with low adherence and those in the control group (HR, 0.91; 95% CI, 0.60 to 1.38; P=0.67). The risk reduction in the high-adherence arm versus the control arm was confirmed in an adjusted analysis (HR, 0.51; 95% CI, 0.31 to 0.82; P=0.005), and this was also true in an analysis restricted to the risk of hospitalization (Supplemental Table 4). Finally, an analysis censoring patients at the time of transplantation fully confirmed these relationships both in the unadjusted analysis (Figure 2) and in the adjusted analysis (exercise arm versus control arm; HR, 0.64; 95% CI, 0.44 to 0.92; P=0.02; high adherence versus control; HR, 0.48; 95% CI, 0.29 to 0.77; P=0.003).
Figure 2.
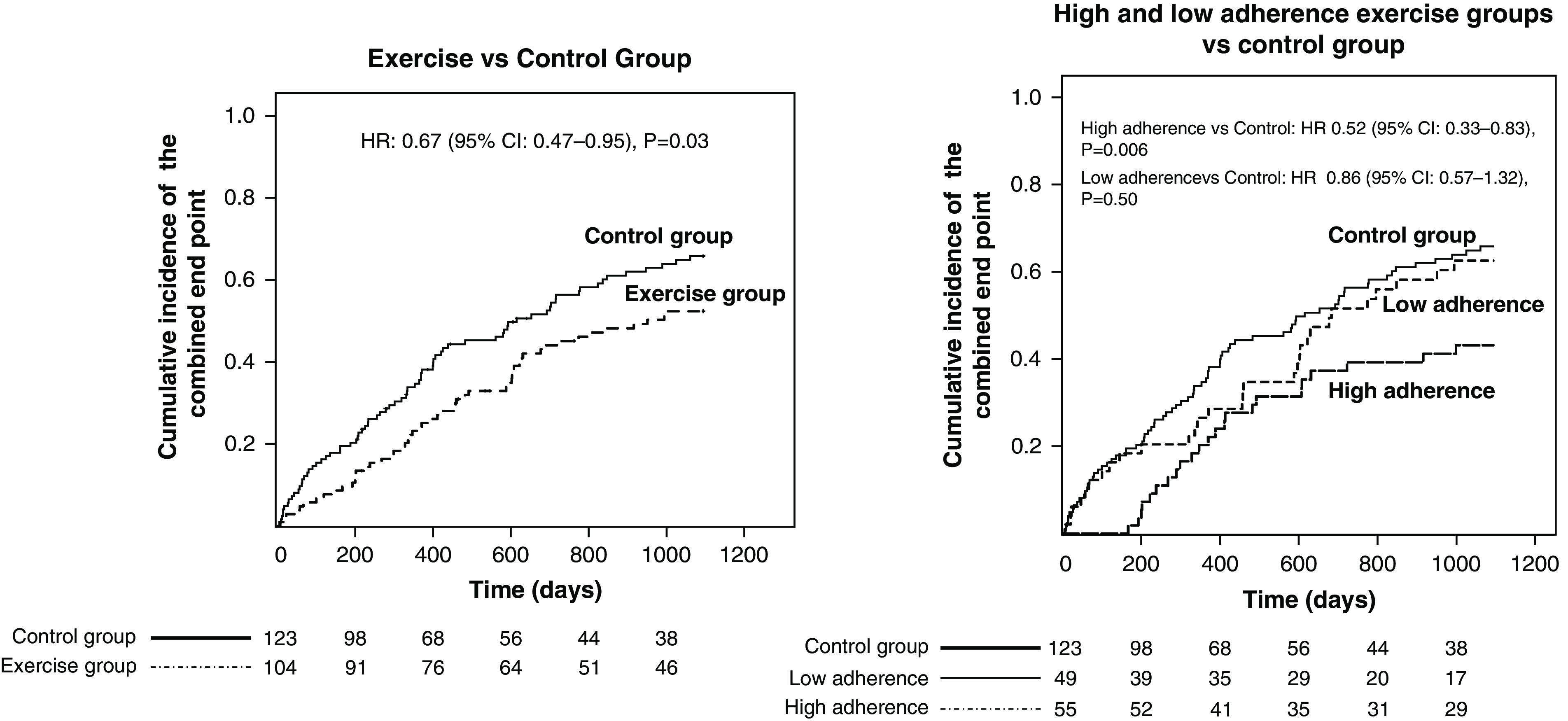
Analyses with censoring at the time of kidney transplantation. The graphs were derived by the reverse Kaplan–Meier option in SPSS.
Bayesian Analyses
The posterior probability of a HR of 0.71 for the risk of the composite outcome observed in the post-trial observational study was 93% under the conservative prior and 97% under the optimistic prior.
Discussion
The lower risk for hospitalization from a walking exercise program registered in the per-protocol analysis of the 6-month EXCITE trial was maintained up to 36 months (i.e., 30 months after the end of the same trial). Such a long-term risk reduction occurred notwithstanding that only a minority (30%) of patients in the active arm of the trial followed our recommendation to maintain just a 10-minute walking session at self-selected speed during the day off dialysis up to the 18th month and that beyond this month and up to the 36th month, no exercise intervention was applied.
The 6-month EXCITE trial tested the effect of two 10-minute sessions of walking at intensity tailored to the individual baseline performance (15); 104 of 151 patients (69%) in the active arm of the trial completed the training program and could be retested at 6 months, which is a significant proportion of the population randomized to the same arm. As previously alluded to, in a secondary per-protocol analysis, the exercise intervention significantly prolonged the hospitalization-free survival in patients in the active arm. Intention-to-treat analyses are fundamental for deriving solid recommendations for the application in clinical practice of treatments tested in randomized clinical trials. However, per-protocol analyses are needed for understanding the efficacy of treatments (19).
In this long-term post-trial observational analysis, the risk reduction of the combined end point (death or hospitalization) in patients randomized to the active arm and followed up to the 36th month was of clinical relevance (−29%). Furthermore, an analysis in the relationship to adherence to the exercise program during the 6-month trial showed that the risk reduction confined to patients who performed >60% of prescribed exercise sessions—a pre-established parameter of adherence to the study protocol—was substantial (−45%), whereas no benefit was registered in those who performed <60% of prescribed training sessions. Sensitivity analyses restricted to the risk of hospitalization only or censoring patients at the time of transplantation fully confirmed these findings.
The persistence of a beneficial effect of physical exercise beyond the actual intervention period may last several years. In the Studies Targeting Risk Reduction Interventions through Defined Exercise in middle-aged sedentary overweight or obese participants, 10 years after an 8-month exercise training intervention, cardiorespiratory fitness and metabolic parameters were better preserved in highly trained persons than in those who maintained a moderate exercise intensity or did not exercise (control) (17). In patients with cystic fibrosis, a home-based partially supervised physical conditioning program improved physical fitness, lung function, and perceived health up to 18 months after the intervention was ended (20). The long-term benefits of physical exercise programs may extend to hospitalization. Indeed, a simple inpatient intervention consisting of walking and rising from a chair, an intervention similar to that tested in the EXCITE trial, considerably decreased the risk of hospitalization-associated disability in acutely hospitalized older patients (21). A recent guideline by the UK Kidney Research Consortium suggests specific exercise targets for patients on hemodialysis (22).
In this long-term study, exercise had a beneficial effect on the risk of hospitalization for various causes, including infectious complications and cardiovascular problems. However, the number of observations in the analysis stratified by the cause of hospitalization was too small to allow for solid conclusions. Physical exercise has a favorable effect on the functioning of the immune system (23). A dose-response relationship was observed between physical exercise performed before infection and a reduction in the incidence, duration, or severity of self-reported and laboratory- or hospital-adjudicated acute upper respiratory tract infections and mortality during the 2009 influenza pandemic (24). On the other hand, a beneficial effect of physical exercise on the risk for hospitalization for cardiovascular diseases, including peripheral vascular disease (25) and heart failure, coronary heart disease (26), atrial fibrillation (27), and cerebrovascular disease (28) is well established. Larger studies are needed to confirm whether these benefits registered in the general population specifically apply to patients with kidney failure.
It is important emphasizing that our post-trial protocol contemplated the simple recommendation to maintain just one 10-minute walking session during the day off of dialysis at self-selected speed up to the 18th month and that no specific recommendation to continue physical exercise was given beyond the 18th month. This recommendation was followed by only a minority of patients (30%), whereas the majority of patients in the same arm did not perform any exercise. Beyond the 18 months, patients in the active group received just a generic recommendation to maintain an active lifestyle, which was the recommendation also given to patients in the control group. Thus, the long-term beneficial effect of the 6-month walking exercise program on the risk for hospitalization during a long-term observation extended up to 36 months is a legacy effect (i.e., a benefit that persists well beyond the temporal limit of the intervention). A legacy effect was also registered in other trials in patients on dialysis like the Frequent Hemodialysis trial, where the HR for frequent versus conventional hemodialysis 2.6 years after the end of the intervention period was 0.54 (29).
In our study, the Bayesian analyses, which considered plausible prior probabilities, helps to quantify the interpretation of the study results in the context of limited power. These analyses suggest that, although the actual long-term (36-month) benefit of the walking exercise program on the risk of hospitalization (HR, 0.71) was smaller than that registered during the 6-month trial (HR, 0.46), a clinically significant long-term benefit on the risk for hospitalization is reasonably likely. Indeed, the posterior probability of a HR of 0.71 (95% CI, 0.50 to 1.00) for the risk of the composite outcome (i.e., the HR observed in the post-trial observational study) was 93% under the conservative prior. It has to be noted that the low adherence to post-trial recommendations in the majority of patients indicates that dedicated resources are needed to maintain physical exercise programs in patients on dialysis. A study by March et al. (14) showed that a 6-month program of intradialysis cycling on health care costs during the year post-trial was cost effective.
The study has limitations. The first limitation is related to the fact that hospitalization was a secondary end point of EXCITE. This trial is the largest testing of a walking exercise program in patients with kidney failure performed so far (16). However, the power of EXCITE was calculated on the basis of the expected improvement in walking performance rather than on the risk for hospitalization. Even though the Bayesian analysis suggests that a benefit from walking exercise on the risk for hospitalization registered in the long-term analysis is very likely, observations in larger trials are needed. The dropout rate during the 6-month trial was higher in the active arm of the trial than in the control arm (47 versus 21 patients). This issue is common to most trials that test physical exercise programs (30–32). In our trial, the 47 patients who dropped out during the 6-month trial had similar demographic and clinical characteristics as compared with those who completed the 6-month trial (Supplemental Table 2), but bias or residual confounding in the benefit observed in the active group cannot be excluded. Furthermore, clinical events in this observational study were registered on the basis of hospital discharge diagnosis rather than being formally adjudicated by an external committee. The second limitation depends on the observational nature of our post-trial findings. Even though patients in the two study arms who completed the 6-month EXCITE trial were very similar in demographic and clinical characteristics, differences in unmeasured risk factors cannot be excluded. The third limitation depends on the fact that hospitalization is a surrogate end point depending on the organization of the background health system and available health resources. Therefore, findings in this post-trial long-term study in Italy should be confirmed in other countries and in other health contexts.
Overall, this study suggests that the walking exercise training applied in the EXCITE trial may reduce the long-term risk for hospitalization, particularly so in patients who had high adherence to the walking exercise program.
Disclosures
F. Mallamaci reports consultancy agreements with Fresenius; honoraria from Fresenius; and serving as a member of the editorial board of the International Journal of Nephrology, past Editor-in-Chief of Journal of Nephrology, a theme editor of Nephrology, Dialysis, and Transplantation, and a member of the editorial board of Turk Nefroloji, the Official Journal of the Turkish Society of Nephrology. C. Zoccali reports a consultancy agreement with Fresenius Medical Care, Europe; serving on the MONitoring Dialysis Outcomes (MONDO) Board; and serving as a member of the editorial boards of American Journal of Kidney Diseases, CJASN, and several internal medicine and nephrology journals. All remaining authors have nothing to disclose.
Funding
This post-trial observational study was done with the funding of the EXCITE trial, a trial supported by Italian Ministry of Health grant Bando Ricerca Finalizzata 2006 (to C. Zoccali).
Supplementary Material
Acknowledgments
Rossella Baggetta, Davide Bolignano, Silvio Bertoli, Daniele Ciurlino, Lisa Rocca-Rey, Antonio Barillà, Yuri Battaglia, Renato Rapanà, Alessandro Zuccalà, Graziella Bonanno, Pasquale Fatuzzo, Francesco Rapisarda, Stefania Rastelli, Fabrizio Fabrizi, Piergiorgio Messa, Luciano De Paola, Luigi Lombardi, Adamasco Cupisti, Giorgio Fuiano, Gaetano Lucisano, Chiara Summaria, Michele Felisatti, Enrico Pozzato, Anna Maria Malagoni, Pietro Castellino, Filippo Aucella, Samar Abd ElHafeez, Pasquale Fabio Provenzano, and Luigi Catizone all collaborated on the EXCITE trial.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
C. Zoccali conceptualized the study; C. Torino was responsible for data curation; N. Lamberti, F. Mallamaci, F. Manfredini, and C. Torino were responsible for investigation; G. D'Arrigo, G. Tripepi, and C. Zoccali were responsible for formal analysis; F. Mallamaci was responsible for validation; C. Zoccali was responsible for funding acquisition; F. Mallamaci and C. Zoccali provided supervision; F. Mallamaci and C. Zoccali wrote the original draft; and N. Lamberti, F. Mallamaci, F. Manfredini, and C. Torino reviewed and edited the manuscript.
Data Sharing Statement
The granular data of this study will be made available to interested investigators 3 months after the publication of the study. Interested investigators may contact the principal investigator and corresponding author to obtain the data. Their request should be accompanied by a clear explanation of the hypothesis they intend to test.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03160322/-/DCSupplemental.
Supplemental Material. Training program of the EXCITE trial.
Supplemental Table 1. Schematic description of the home-based training program prescribed according to the patient's functioning level.
Supplemental Table 2. Comparison of patients who exited from the 6-month trial before the sixth month (n=47) and those (n=104; completers) who completed the 6-month trial.
Supplemental Table 3. Breakdown of patients who had high adherence (>60%) to the prescribed exercise sessions during the 6-month trial and those who had low adherence (<60%).
Supplemental Table 4. Multivariable Cox regression analysis for the risk of hospitalization (N=126) and multivariable Cox regression analysis by adherence to the exercise program for the risk of hospitalization.
Supplemental Figure 1. CONSORT diagram of the EXCITE trial.
References
- 1.Painter P: Implementing exercise: What do we know? Where do we go? Adv Chronic Kidney Dis 16: 536–544, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, Kent-Braun JA: Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 57: 2564–2570, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Manfredini F, Lamberti N, Malagoni AM, Felisatti M, Zuccalà A, Torino C, Tripepi G, Catizone L, Mallamaci F, Zoccali C: The role of deconditioning in the end-stage renal disease myopathy: Physical exercise improves altered resting muscle oxygen consumption. Am J Nephrol 41: 329–336, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Martins P, Marques EA, Leal DV, Ferreira A, Wilund KR, Viana JL: Association between physical activity and mortality in end-stage kidney disease: A systematic review of observational studies. BMC Nephrol 22: 227, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stack AG, Molony DA, Rives T, Tyson J, Murthy BVR: Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis 45: 690–701, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Tentori F, Elder SJ, Thumma J, Pisoni RL, Bommer J, Fissell RB, Fukuhara S, Jadoul M, Keen ML, Saran R, Ramirez SP, Robinson BM: Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Correlates and associated outcomes. Nephrol Dial Transplant 25: 3050–3062, 2010 [DOI] [PubMed] [Google Scholar]
- 7.K/DOQI Workgroup : K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45[Suppl 3]: S1–S153, 2005 [PubMed] [Google Scholar]
- 8.Heiwe S, Jacobson SH: Exercise training in adults with CKD: A systematic review and meta-analysis. Am J Kidney Dis 64: 383–393, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Cheema BSB, Singh MA: Exercise training in patients receiving maintenance hemodialysis: A systematic review of clinical trials. Am J Nephrol 25: 352–364, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC: Effects of exercise in the whole spectrum of chronic kidney disease: A systematic review. Clin Kidney J 8: 753–765, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham-Brown MPM, March DS, Young R, Highton PJ, Young HML, Churchward DR, Dungey M, Stensel DJ, Bishop NC, Brunskill NJ, Smith AC, McCann GP, McConnachie A, Burton JO: A randomized controlled trial to investigate the effects of intra-dialytic cycling on left ventricular mass. Kidney Int 99: 1478–1486, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Clarkson MJ, Bennett PN, Fraser SF, Warmington SA: Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: A systematic review and meta-analysis. Am J Physiol Renal Physiol 316: F856–F872, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Hargrove N, El Tobgy N, Zhou O, Pinder M, Plant B, Askin N, Bieber L, Collister D, Whitlock R, Tangri N, Bohm C: Effect of aerobic exercise on dialysis-related symptoms in individuals undergoing maintenance hemodialysis: A systematic review and meta-analysis of clinical trials. Clin J Am Soc Nephrol 16: 560–574, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.March DS, Hurt AW, Grantham CE, Churchward DR, Young HML, Highton PJ, Dungey M, Bishop NC, Smith AC, Graham-Brown MPM, Cooper NJ, Burton JO: A cost-effective analysis of the CYCLE-HD randomized controlled trial. Kidney Int Rep 6: 1548–1557, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manfredini F, Mallamaci F, D’Arrigo G, Baggetta R, Bolignano D, Torino C, Lamberti N, Bertoli S, Ciurlino D, Rocca-Rey L, Barillà A, Battaglia Y, Rapanà RM, Zuccalà A, Bonanno G, Fatuzzo P, Rapisarda F, Rastelli S, Fabrizi F, Messa P, De Paola L, Lombardi L, Cupisti A, Fuiano G, Lucisano G, Summaria C, Felisatti M, Pozzato E, Malagoni AM, Castellino P, Aucella F, Abd ElHafeez S, Provenzano PF, Tripepi G, Catizone L, Zoccali C: Exercise in patients on dialysis: A multicenter, randomized clinical trial. J Am Soc Nephrol 28: 1259–1268, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarkson MJ, Bennett PN, Fraser SF, Stuart Warmington XA: Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: A systematic review and meta-analysis. Am J Physiol Renal Physiol 316: 856–872, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Johnson JL, Slentz CA, Ross LM, Huffman KM, Kraus WE: Ten-year legacy effects of three eight-month exercise training programs on cardiometabolic health parameters. Front Physiol 10: 452, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ioannidis JPA: Why most discovered true associations are inflated. Epidemiology 19: 640–648, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Tripepi G, Chesnaye NC, Dekker FW, Zoccali C, Jager KJ: Intention to treat and per protocol analysis in clinical trials. Nephrology (Carlton) 25: 513–517, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Alcaraz-Serrano V, Gimeno-Santos E, Scioscia G, Gabarrús A, Navarro A, Herrero-Cortina B, Amaro R, Fernández-Barat L, Torres A: Association between physical activity and risk of hospitalisation in bronchiectasis. Eur Respir J 55: 1902138, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Ortiz-Alonso J, Bustamante-Ara N, Valenzuela PL, Vidán-Astiz M, Rodríguez-Romo G, Mayordomo-Cava J, Javier-González M, Hidalgo-Gamarra M, Lopéz-Tatis M, Valades-Malagón MI, Santos-Lozano A, Lucia A, Serra-Rexach JA: Effect of a simple exercise program on hospitalization-associated disability in older patients: A randomized controlled trial. J Am Med Dir Assoc 21: 531–537.e1, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Baker LA, March DS, Wilkinson TJ, Billany RE, Bishop NC, Castle EM, Chilcot J, Davies MD, Graham-Brown MPM, Greenwood SA, Junglee NA, Kanavaki AM, Lightfoot CJ, Macdonald JH, Rossetti GMK, Smith AC, Burton JO: Clinical practice guideline exercise and lifestyle in chronic kidney disease. BMC Nephrol 23: 75, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieman DC, Wentz LM: The compelling link between physical activity and the body’s defense system. J Sport Health Sci 8: 201–217, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong C-M, Lai H-K, Ou C-Q, Ho S-Y, Chan K-P, Thach T-Q, Yang L, Chau Y-K, Lam T-H, Hedley AJ, Peiris JSM: Is exercise protective against influenza-associated mortality? PLoS One 3: e2108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Y, Ballew SH, Kwak L, Selvin E, Kalbaugh CA, Schrack JA, Matsushita K, Szklo M: Physical activity and subsequent risk of hospitalization with peripheral artery disease and critical limb ischemia in the ARIC Study. J Am Heart Assoc 8: e013534, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry JD, Pandey A, Gao A, Leonard D, Farzaneh-Far R, Ayers C, DeFina L, Willis B: Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail 6: 627–634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin M-N, Yang P-S, Song C, Yu HT, Kim TH, Uhm JS, Sung JH, Pak HN, Lee MH, Joung B: Physical activity and risk of atrial fibrillation: A nationwide cohort study in general population. Sci Rep 9: 13270, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CD, Folsom AR, Blair SN: Physical activity and stroke risk: A meta-analysis. Stroke 34: 2475–2481, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Chertow GM, Levin NW, Beck GJ, Daugirdas JT, Eggers PW, Kliger AS, Larive B, Rocco MV, Greene T; Frequent Hemodialysis Network (FHN) Trials Group : Long-term effects of frequent in-center hemodialysis. J Am Soc Nephrol 27: 1830–1836, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viken H, Reitlo LS, Zisko N, Nauman J, Aspvik NP, Ingebrigtsen JE, Wisløff U, Stensvold D: Predictors of dropout in exercise trials in older adults: The Generation 100 study. Med Sci Sports Exerc 51: 49–55, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Stiggelbout M, Hopman-Rock M, Tak E, Lechner L, van Mechelen W: Dropout from exercise programs for seniors: A prospective cohort study. J Aging Phys Act 13: 406–421, 2005 [PubMed] [Google Scholar]
- 32.Rossi PG, Carnaz L, Bertollo WL, Takahashi AC M: Causes of drop out from a physical exercise supervised program specific to older adults. Fisioter Mov 31: 3133, 2018 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.