Visual Abstract
Keywords: renal function decline, geriatric nephrology, glomerular filtration rate, chronic kidney disease
Abstract
Background and objectives
In older adults, data on the age-related course of GFR are scarce, which might lead to misjudgment of the clinical relevance of reduced GFR in old age.
Design, setting, participants, & measurements
To describe the course of eGFR in older adults and derive reference values in population-based individuals, we used the longitudinal design of the Berlin Initiative Study (BIS) with a repeated estimation of GFR over a median of 6.1 years of follow-up. In 2069 community-dwelling older individuals (mean inclusion age 80 years, range 70–99), GFR was estimated biennially with the BIS-2 equation, including standardized creatinine and cystatin C levels, sex, and age. We described the crude and adjusted course using a mixed-effects model and analyzed the influence of death on the GFR course applying joint models. GFR slopes were compared using GFR equations on the basis of creatinine and/or cystatin C.
Results
We observed a decreasing, thus nonlinear, eGFR decline with increasing age in a population of old adults. The estimated 1-year slope for ages 75 and 90 diminished for men from −1.67 to −0.99 and for women from −1.52 to −0.97. The modeled mean eGFR for men aged ≥79 and women ≥78 was below 60 ml/min per 1.73 m2. Multivariable adjustment attenuated slopes only minimally. Taking death into account by applying joint models did not alter the nonlinear eGFR decline. Using eGFR equations on the basis of creatinine only showed linear slope patterns in contrast to nonlinear patterns for equations including cystatin C.
Conclusions
The eGFR decline depended on sex and age and changed only marginally after multivariable adjustment but decelerated with increasing age. Equations including cystatin C demonstrated a nonlinear slope challenging the previously assumed linearity of the decline of eGFR in old age.
Introduction
The degree to which kidney function decreases in an aging kidney is still not fully understood. As observed many years ago, the decrease in kidney function starts at the age of 40 (1). Since then, data on the decline of kidney function in very old age have been scarce (2). Existing longitudinal studies in younger individuals have observed a decline in kidney function, but also preserved function in a considerable fraction of their study participants (3,4). Most of the available data either date back many years and are limited in number (3), thin out after the age of 70, or are cross-sectional in nature (5–7). In fact, the current Kidney Disease Improving Global Outcomes guidelines for definition and management of CKD (8), assessed by GFR, refer to cross-sectional data on inulin clearance from 1969 to depict the course of kidney function (7), with very few data points after the age of 80 years for men, and hardly any for women. This purely cross-sectional approach is found in most studies that demonstrate courses of GFR in older adults. Also, very few of them are community dwelling (9,10) being able to mirror a “real-world decline” in a representative population of older adults. In 2017, we published GFR changes over age strata using cross-sectional baseline data of the Berlin Initiative Study (BIS), a community-dwelling prospective cohort study of older adults (5). Interpretation of cross-sectional measurements, however, can be difficult because kidney function is modeled interindividually over different age groups, neglecting individual courses over time. Studies that possess longitudinal data with repeated eGFR to model the course in old age are rare. Besides, very few of them are able to use values of eGFR also on the basis of the endogenous biomarker cystatin C that may be more adequate in old age compared with creatinine, which is known to be confounded by sarcopenia (11).
Meanwhile, follow-up data over 8 years (median 6.1 years) of the BIS have become available with repeat biennial eGFR assessment. Thus, the primary aim of this analysis was to describe the crude and adjusted age-related course of eGFR in a population of individuals aged ≥70, and to define reference values for both sexes. Furthermore, we compared the patterns of eGFR decline in old age, calculated by five eGFR equations on the basis of different biomarkers.
Materials and Methods
Study Design
The BIS is a longitudinal population-based cohort of 2069 older adults that were recruited between November 2009 and June 2011. Since then, information has been collected biennially until October 2019, in a total of 5239 follow-up visits. A detailed description of the study design can be found elsewhere (12). The study was approved by the local ethics committee of the Charité, Berlin, Germany (EA2/009/08), and every participant gave written informed consent. BIS was registered at Deutsche Register für Klinische Studien (DRKS00017058).
Study Population
Participants were members of one of the largest German statutory health insurance (AOK Nordost Die Gesundheitskasse), all living in Berlin with ≥70 years at baseline. Exclusion criteria were age <70 and receiving RRT. Study visits included a standardized interview on comorbidities, lifestyle variables and medication, anthropometric measures such as blood pressure, pulse, body mass index (BMI), waist-to-hip ratio, and blood and urine samples. The database freeze for this analysis was December 4, 2019.
Laboratory Methods
Serum creatinine was analyzed using the isotope dilution mass spectrometry traceable enzymatic method from Roche (Crea plus; Roche Diagnostics, Mannheim, Germany) on a Roche Modular-analyzer P-Modul. The interassay coefficients of variation for serum creatinine were 2% and 3% at mean concentrations of 87.52 µmol/L (0.99 mg/dl) and 331.5 µmol/L (3.75 mg/dl), respectively.
Cystatin C was measured using the particle enhanced turbidimetric Tina-quant generation two assay on the Roche/Hitachi Cobas S system (Cobas c 501). Its interassay coefficients of variation were 2%, 3%, and 1% at mean concentrations of 1.0 mg/L, 1.7 mg/L, and 4.1 mg/L, respectively. The generation two assay by Roche is standardized to ERM-DA 471/IFCC and demonstrates excellent agreement with the Siemens particle–enhanced nephelometric N Latex assay (13). eGFR was calculated by the BIS2 equation (14), the race-free Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations CKD-Epi(crea), CKD-EPI(crea/cys) (15), CKD-EPI(cys) (16), and the European Kidney Function Consortium equation EKFC(crea) (17) (see Supplemental Appendix).
Statistical Analysis
Descriptive analysis includes means±SD or medians with the interquartile range for continuous variables, and absolute frequencies with percentages for categorical variables. The primary analysis aims to describe the course of eGFR in old age, stratified for sex, and to identify reference values for the course of kidney function decline. Patterns of eGFR course were demarcated and underlying subpopulations (age, sex) compared. Documentation ended with the death of a patient, initiation of RRT, or loss to follow-up due to other reasons (i.e., frailty, illness, and unknown reasons). Measurements can be missing due to omitted visits or failure of blood samples. Multiple imputation with ten replications was applied for missing data of available visits; if missing, eGFR was calculated on the basis of the single imputed items.
To model the overall course of eGFR, calculated repeatedly at each visit from continuous blood parameters, we used linear mixed-effects models with clustering of participants for longitudinal data separately for sex. To address the presumably decreasing, thus nonlinear, eGFR decline, age was included both as a linear and quadratic term and was centered for modeling, using random terms for intercept and age. No terms estimating individual slopes were considered, because we were aiming to model the age-dependent overall course. Although the statistical model is longitudinal in the sense that it accounts for serial correlation in repeat eGFR measurements in the same patient, the model is structured so that it primarily represents the cross-sectional relationship between mean eGFR and age between different individuals. Thus, when the term slope is used in this manuscript, it refers primarily to slope in a cross-sectional relationship between different individuals, and not to a longitudinal eGFR trajectory. Point estimates for the age-related 1-year slope were calculated as the difference between the modeled eGFR at age +0.5 and age −0.5 years.
The main research aim was to analyze the association of age and eGFR course. On the basis of current knowledge and following the confounder definition, we identified BMI, diabetes, congestive heart failure, current smoking, and diastolic and systolic BP as potential confounders all included as time-dependent variables (adjusted model). Medications were not included as they were considered parts of the causal pathway. In a sensitivity analysis, we repeated the modeling excluding patients with hospital-acquired acute kidney injuries.
To investigate the robustness of the derived GFR estimates, additional sensitivity analyses were run on patients with at least two and again with all four follow-up visits; telephone visits were not counted as regular visits because no blood samples were available. To address potential survival bias, joint models (R package jointModel, method=“piecewise-PH-aGH,” centered age was shifted to a scale >0) were calculated to assess the potential effect of the death on eGFR. Because the jointModel does only allow a mixed-model object with a simple random-effects structure, the mixed model used here is slightly deviating from those used for all other model comparisons.
Age-related reference values were calculated on the basis of all values available in 5-year intervals with downweighting for repeated measurements per interval, separately for sex. IBM SPSS Statistics (Version 25) and R (release 4.0.2) were used for analysis.
Results
Total BIS Population
The main baseline characteristics of the total BIS cohort (n=2069) by subgroups of sex are displayed in Table 1. Mean age was 80±7 years, there were slightly more females (53%), one quarter was diabetic, 79% suffered from hypertension, and one quarter was overweight (BMI ≥30). Overall mean eGFRBIS2 was 58±15 ml/min per 1.73 m2.
Table 1.
Main characteristics of the total Berlin Initiative Study population categorized by sex
| Variable | Total | Male | Female |
|---|---|---|---|
| n (%) | 2069 | 981 (47) | 1088 (53) |
| Age, yr (mean±SD), n (%) | 80±7 | 81±7 | 80±7 |
| 70–74 | 573 (28) | 251 (26) | 322 (30) |
| 75–79 | 476 (23) | 212 (22) | 264 (24) |
| 80–84 | 429 (21) | 213 (22) | 216 (20) |
| 85–89 | 385 (19) | 181 (18) | 204 (19) |
| ≥90 | 206 (10) | 124 (13) | 82 (8) |
| Smoking (ever), n (%) | 1029 (50) | 699 (71) | 330 (30) |
| Alcohol intake, n (%) | |||
| <1 per month | 914 (45) | 313 (32) | 601 (56) |
| <2 per week | 724 (35) | 358 (37) | 366 (34) |
| 6 per week or daily | 414 (20) | 299 (31) | 115 (11) |
| No comorbidities,a n (%) | 181 (9) | 62 (6) | 119 (11) |
| Hypertension,b n (%) | 1634 (79) | 777 (80) | 857 (79) |
| Diabetes mellitus,c n (%) | 539 (26) | 279 (29) | 260 (24) |
| Myocardial infarction, n (%) | 286 (14) | 199 (21) | 87 (8) |
| Stroke, n (%) | 177 (9) | 97 (10) | 80 (7) |
| Congestive heart failure,d n (%) | 591 (29) | 317 (32) | 274 (25) |
| Cancer, n (%) | 465 (23) | 249 (26) | 216 (20) |
| Anemia, n (%) | 358 (18) | 244 (26) | 114 (11) |
| Systolic BP, mm Hg (mean±SD) | 145±22 | 145±22 | 146±22 |
| Diastolic BP, mm Hg (mean±SD) | 81±13 | 81±13 | 81±13 |
| Body mass index, n (%) | |||
| <30 | 1522 (74) | 761 (78) | 761 (70) |
| ≥30 | 546 (26) | 220 (22) | 326 (30) |
| Creatinine, mg/dl, median (IQR) | 0.94 (0.79–1.15) | 1.06 (0.90–1.28) | 0.84 (0.72–1.01) |
| Cystatin C, mg/l, median (IQR) | 1.04 (0.90–1.29) | 1.08 (0.93–1.33) | 1.01 (0.88–1.23) |
| eGFR, ml/min per 1.73 m2, mean±SD | |||
| BIS2 | 58±15 | 58±16 | 58±15 |
| CKD-EpiCrea | 65±17 | 64±18 | 65±17 |
| CKD-EpiCysC | 64±21 | 64±22 | 65±20 |
| CKD-EpiCrea/CysC | 65±19 | 65±20 | 65±19 |
| eGFR <60 ml/min per 1.73 m2, n (%) | |||
| BIS2 | 1097 (53) | 520 (53) | 577 (53) |
| CKD-EpiCrea | 784 (38) | 382 (39) | 402 (37) |
| CKD-EpiCysC | 871 (42) | 427 (44) | 444 (41) |
| CKD-EpiCrea/CysC | 813 (39) | 390 (40) | 423 (39) |
| ACR, mg/g, n (%) | |||
| 30–299 | 458 (22) | 250 (26) | 208 (19) |
| ≥300 | 74 (4) | 52 (5) | 22 (2) |
Data are mean±SD, median (IQR), or absolute numbers (%). Number of missing values was <10 (<0.5%) for smoking, hypertension, diabetes, cancer, systolic and diastolic blood pressure, BMI, creatinine, cystatin C, and consequently eGFR; 12 (0.6%) for congestive heart failure, 14 (0.7%) for ACR, 17 (0.8%) for alcohol intake and Charlson Comorbidity Index, 24 (1.2%) for myocardial infarction, 25 (1.2%) for stroke, and 62 (3%) for anemia. IQR, interquartile range; BIS, Berlin Initiative Study; eGFR CKD-EPI, GFR estimated by the CKD-EPI equation incorporating creatinine (Crea), cystatin C (CysC), or both (Crea/CysC); ACR, albumin-creatinine ratio.
No comorbidities were defined as zero in the Charlson Comorbidity Index.
Hypertension was defined as prescription of antihypertensive medication.
Diabetes was defined as either HbA1c >7% and/or prescription of antidiabetic medication.
Congestive heart failure was assessed by International Classification of Diseases, 10th revision (ICD-10) coding (I50.xx, I11.0, I13.0, I13.2) as inpatient or in ≥2 different quarters as outpatient diagnosis.
Main Characteristics by Sex
In the highest age stratum, more men than women had participated in the study, and in the lowest age stratum, it was more women than men (Table 1). Male participants were more often ever smokers (71% versus 30%), and their reported daily alcohol intake was three times more frequent in men than in women (31% versus 11%). Men were leaner (BMI ≥30 kg/m2 in 22% versus 30%), but reported more frequent myocardial infarction (21% versus 8%), were more anemic (26% versus 11%), and had higher serum creatinine (1.1 versus 0.8 mg/dl) but similar cystatin C (1.1 versus 1.0 mg/l) and eGFRBIS2 values compared with women.
Repeat GFR Assessments Over Time
The BIS study collected data of 2069 elderly persons biennially with up to 8 years of follow-up (median follow-up 6.1 years). Supplemental Figure 1 presents follow-up and available eGFR measurements. The median time lag between two available visits was 24.5 months (range 11–97 months). Of 7308 visits in total, 174 eGFR values (2%) were missing. In 156 out of the 2069 participants, at least one eGFR measurement (8%) was missing at visits. For 18% one eGFR value, for 13% two, for 15% three, for 14% four, and for 40% all five eGFR values were available. Supplemental Table 1 depicts details of available eGFR values.
Until the study was completed in December 2019, 789 participants (38%) died before the intended 8-year follow-up (allowing a delay of ≤3 months, 8.25 years after the baseline visit were considered as intended end of observation), and in 19 (0.9%), RRT was initiated for ≥3 months or terminated due to death within 3 months after initiation (n=3). Supplemental Figure 1 outlines details of missing eGFR measurements or visits.
Differences of eGFR(BIS2) Slope by Age and Sex
Figure 1 demonstrates spaghetti plots of individual raw data revealing overall lower eGFR values with increasing age independent of sex. Among baseline-age strata slopes decreased from the youngest to the oldest age group (male: 2.03–1.7 ml/min per 1.73 m2; female: 1.8–1.51 ml/min per 1.73 m2). Thus, crude linear regression lines of the eGFR slopes within the age strata suggested a lower annual eGFR decline at higher ages for both sexes. In most of the participants recruited at ≥85 years, eGFR courses ranged between 30–60 ml/min per 1.73 m2.
Figure 1.
Spaghetti plots displaying individual courses of eGFRBIS2 in the total cohort (n=2069) stratified by sex and age groups at enrollment. Crude linear regression lines from mixed-effects models (linear mixed model with random intercept) within each layer are marked in red. Standardized cystatin C values were converted by the formula (−0.105 + 1.13× cystatin C). BIS, Berlin Initiative Study; CysC, cystatin C; Crea, creatinine.
eGFR(BIS2) Slope by Sex Using Linear and Quadratic Terms
In the linear mixed-effects models, age, both as a linear and quadratic term, were significantly associated with eGFR (Table 2). Men had higher eGFR values than women over the entire observed age range (Figure 2A), varying between almost 3 ml/min per 1.73 m2 at age 70 and 0.75 ml/min per 1.73 m2 at age 92. The quadratic term had a positive regression coefficient, indicating a counteracting trend to the general decline, which flattens with age. For men, the slope diminished from −1.67 at age 75 to −0.99 at age 90, for women from −1.52 to −0.97. The mixed models revealed that the estimated mean eGFR for men aged ≥79 and women ≥78 of this population-based cohort was <60 ml/min per 1.73 m2, defining decreased kidney function when applying the current Kidney Disease Improving Global Outcomes classification system. When we adjusted the models for time-dependent BMI, diabetes, congestive heart failure, smoking, and diastolic and systolic BP (Figure 2B), the slope results changed only minimally for men (−1.60 at age 75 to −0.94 at age 90) and women (−1.42 at age 75 to −1.01 at age 90).
Table 2.
Linear mixed-effects models for the five eGFR equations; pooled results of the multiple imputed data
| Value | BIS2(crea/cysC) | CKD-Epi(Crea/CysC) | CKD-Epi(CysC) | CKD-Epi(crea) | EKFC(crea) |
|---|---|---|---|---|---|
| Male | |||||
| Intercept | 53.3 (52.7 to 53.8) | 63.6 (62.8 to 64.4) | 55.9 (55.1 to 56.7) | 66.4 (65.6 to 67.1) | 55.6 (55.0 to 56.2) |
| Age, yr | −1.3 (−1.4 to −1.2) | −1.6 (−1.8 to −1.5) | −1.8 (−1.9 to −1.6) | −1.2 (−1.3 to −1.1) | −1.2 (−1.3 to −1.1) |
| Age quadratic term | 0.02 (0.01 to 0.04) | 0.02 (0.004 to 0.04) | 0.03 (0.01 to 0.05) | 0.01 (−0.01 to 0.03)a | 0.01 (−0.004 to 0.03)a |
| Female | |||||
| Intercept | 52.1 (51.7 to 52.6) | 62.8 (62.1 to 63.5) | 55.0 (54.4 to 55.7) | 65.6 (65.0 to 66.3) | 54.3 (53.8 to 54.9) |
| Age, yr | −1.2 (−1.3 to −1.2) | −1.6 (−1.7 to −1.4) | −1.7 (−1.8 to −1.6) | −1.2 (−1.3 to −1.1) | −1.2 (−1.2 to −1.1) |
| Age quadratic term | 0.02 (0.005 to 0.03) | 0.02 (−0.002 to 0.04)a | 0.03 (0.02 to 0.05) | −0.002 (−0.02 to 0.02)a | 0.002 (−0.01 to 0.02)a |
Shown are fixed parameter estimates with 95% confidence intervals in brackets. BIS2, Berliner Initiative Study; CKD-Epi, Chronic Kidney Disease Epidemiology Collaboration; Crea, creatinine; CysC, cystatin C; EKFC, European Kidney Function Consortium.
Nonsignificant factors.
Figure 2.
Course of the eGFRBIS2 for males (blue) and females (red). (A) Course in the crude mixed-effects model only including age as a linear and a quadratic term. (B) Course in the model adjusted for body mass index (BMI), diabetes, congestive heart failure, current smoking, and diastolic and systolic BP. Displayed are pooled results of the multiple imputed data with 95% confidence intervals (dashed lines). (C) Separate spaghetti plots for participants who were alive until the end of the study and those who died during the study are shown for males and females. (D) The eGFR course derived from the crude (age only) joint model incorporating death information (JM, dashed line) is compared with the course from the crude mixed-effects model ignoring death (LME, solid line). Because the function jointModel in R does only allow a mixed-model object with a simple random-effects structure, simpler random effects were modeled in the mixed-effects model (LME) in (D), causing slight deviations from (A). The association term of eGFR and death was significant in the joint model for both men (Assoct=−0.0474, P<0.001) and women (Assoct=−0.0307, P<0.001). Standardized cystatin C values were converted by the formula (−0.105 + 1.13× cystatin C).
In this high-aged cohort, 608 individuals died during the observation period (Supplemental Figure 1). When plotting the individual eGFR courses divided for participants who were alive during the entire study and those who died during the study, no obvious differences could be observed for men or women in the overall regression spline (Figure 2C). When comparing the eGFR course from the mixed-effects models ignoring the influence of death with that from the joint models taking death into account (the association of death and eGFR being significant for both sexes), the eGFR course for women changed only slightly, whereas it started somewhat steeper in the 70-year-olds, but also decelerated somewhat stronger above the age of 90 for men (Figure 2D).
In a sensitivity analysis comparing patients with fewer follow-up visits to patients with all four follow-up visits, we saw very comparable eGFR patterns (Supplemental Figure 2).
Differences of eGFR Slope by eGFR Equation
We compared eGFR values calculated with the BIS2 equation with four other eGFR-equations: the three race-free CKD-EPI equations on the basis of creatinine only, cystatin C only or the combination of both biomarkers, and the EKFC(crea) equation, all on the basis of the same mixed-effects model as described above (Figure 3, Table 2). In contrast to the three equations containing cystatin C, which all demonstrated a diminishing slope with age, the two equations on the basis of creatinine showed an almost linear decline. The CKD-Epi(cysC) equation yielded the steepest slope resulting in the lowest eGFR values above age 95.
Figure 3.

Course of eGFR. Course calculated (A) with the BIS2(cysC, crea), (B) CKD-Epi(cysC, crea), (C) CKD-Epi(cysC), (D) CKD-Epi(crea), and (E) EKFC(crea) for males (blue) and females (red). Shown are pooled results of the multiple imputed data with 95% confidence intervals (dashed lines) from the mixed-effects models separately for sex including a linear and a quadratic term for age. Standardized cystatin C values were converted by the formula (−0.105 + 1.13× cystatin C). EKFC, European Kidney Function Consortium.
Excluding patients with hospital-acquired AKI during follow-up did not change these main results (Supplemental Table 2).
Reference Values for eGFR Slope
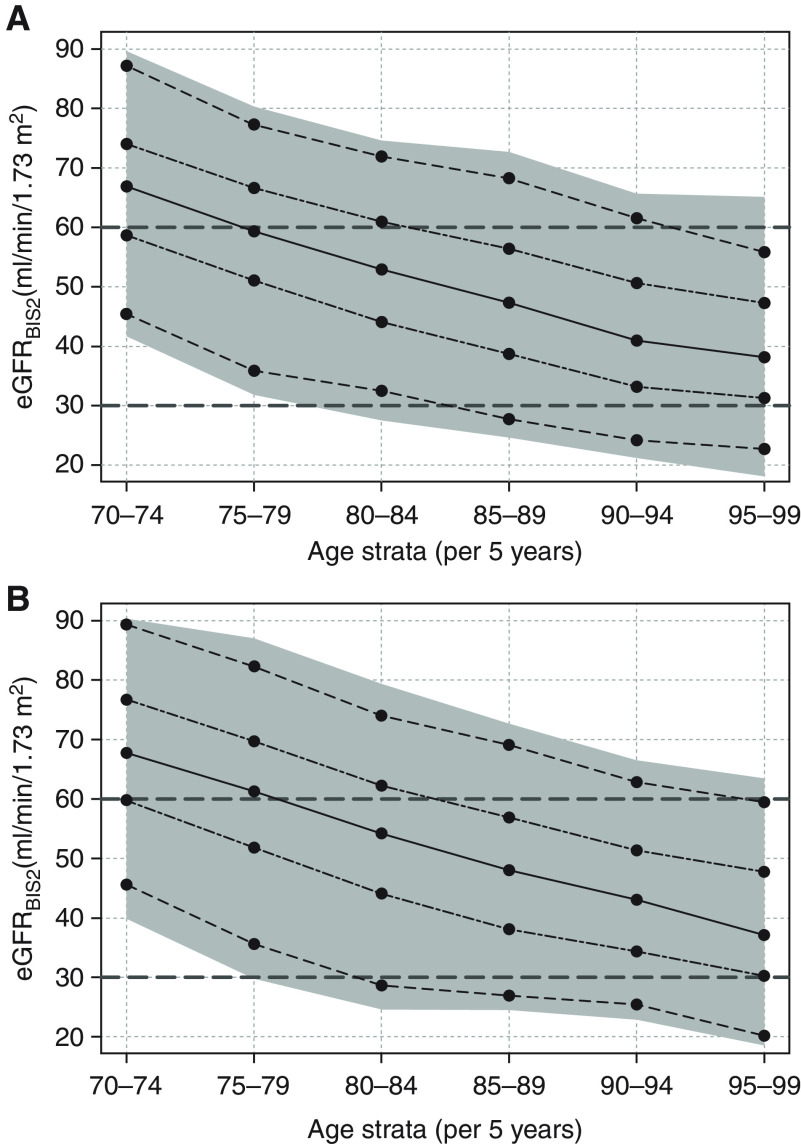
Figure 4 displays the observed median and reference values of the eGFR calculated with the BIS2(crea,cysC) by 5-year age intervals. Overall, there were no major sex differences in the slope patterns. Although still approximately 75%, the lower age group (70–74 years) presented with an eGFR >60 ml/min per 1.73 m2, and this proportion decreased to 25% among the 80–84 age group, and then further to about 5% among participants aged 90–94 years.
Figure 4.
Reference values of the eGFR. Values calculated with the BIS2 for (A) females and (B) males for 5-year age strata. To better illustrate the age-related decrease of eGFR, the cross-sectional point estimates are connected by lines. Median values are connected with a solid line, the 25th and 75th percentiles with a two-dashed line, the 5th and 95th percentiles with a dashed line, and the 95% range as gray area.
Discussion
We investigated the course of kidney function in a population-based cohort of old and very old adults, using repeat GFR estimates and observed three main findings. First, we saw a decrease of eGFRBIS2 over >6 years of follow-up, which decelerated with increasing age, exhibiting a nonlinear pattern. Despite the seemingly small coefficients, the nonlinearity reaches clinical relevance at higher ages, because the quadratic term counterbalances the linear decrease in the estimation of the eGFRBIS2 by, for example, +1.1 ml/min per 1.73 m2 at age 90 and +2.2 ml/min per 1.73 m2 at age 93. Second, using a mixed-effects model, men crossed the eGFR threshold of 60 ml/min per 1.73 m2 age ≥79, and women at ≥78 years. These slopes were only minimally attenuated after multivariable adjustment. The nonlinear pattern of eGFRBIS2 did not change after applying a joint model taking death into account. Third, the slope-pattern varied depending on the underlying eGFR equation with a nonlinear pattern if cystatin C was included, and a linear pattern if the equation was on the basis of creatinine alone.
A couple of studies have investigated kidney function decline with age, most of them on the basis of cross-sectional data only (5,6,18,19). When we analyzed cross-sectional eGFRBIS2, the GFR slope crossed the cutoff 60 ml/min per 1.73 m2 at age 77 (5), comparable with the now mixed-effects model incorporating repeat measurements, but exhibiting a different slope pattern. A recent cross-sectional study by Eriksen et al. used data from three European cohort studies (including data from the Norwegian Renal Iohexol Clearance Survey [RENIS], the Icelandic Age, Gene/Environment Susceptibility-Kidney Study [AGES-Kidney study], and the German BIS) and compared healthy and unhealthy individuals by predicting their kidney function decline on the basis of measured GFR (6). They showed that in unhealthy individuals, defined as persons with either major chronic disease or risk factors for CKD, the decline of kidney function was steeper compared with healthy individuals, suggesting that, even in healthy aging, GFR is not preserved. Compared with our data, the GFR decline was less steep, crossing 60 ml/min per 1.73 m2 at age 82 (women) and 87 (men) among the unhealthy. Potential cohort effects (a healthier Norwegian cohort and an Icelandic cohort with less diabetes) may have contributed to this overall difference of approximately 6 ml/min per 1.73 m2. Importantly, our eGFR slopes changed only minimally after adjusting for potential confounders, indicating an independent and genuine age-related kidney function decline.
Other studies were able to apply a longitudinal design (20–26), all sharing the common interest of a better understanding of the course of GFR over time. Most of these studies, however, have similar limitations because they mainly capture middle-aged instead of older adults (3,4,23,26,27), have a rather short follow-up time with few measurements (22), do not have cystatin C values (20,23,24,27), or use nonstandardized assays for creatinine or cystatin C (10). In agreement with our data, a study analyzing eGFR on the basis of cystatin C in women aged ≥75 over 10 years also demonstrated an decelerating loss of kidney function over time, with −11 ml/min per 1.73 m2 over the first 5 years, decreasing to −9 ml/min per 1.73 m2 later (25). Another recent longitudinal analysis from the European Quality Study on Treatment in Advanced CKD that looked at kidney function decline in adults ≥65 years with stage 4 and 5 CKD observed much steeper GFR slopes compared with ours (28). This difference is probably due to the high burden of comorbidities in these patients with advanced CKD, in contrast with the community-dwelling BIS participants.
Another interesting finding was that the slope pattern apparently depended on the underlying biomarker. This held true for equations with similar mathematical modeling, such as the CKD-EPI equations. It seems likely that non-GFR determinants may explain part of the difference. Muscle mass, physical activity, and protein intake are lower with increasing age, resulting in a decline of creatinine generation, leading to an overestimation of GFR. In contrast, obesity, inflammation, high-dose corticosteroids, and illnesses such as diabetes, cancer, or hyperthyroidism upregulate cystatin C (29). Especially in old age, chronic diseases and changes in muscle mass are common and may thus affect the eGFR pattern. Other studies have shown the benefit of the combination of both biomarkers for more precise GFR assessment in old age (11,14,16,30).
Despite the old age of our participants, RRT for kidney failure was initiated during follow-up in very few individuals (0.9%). The BIS is community dwelling and a representative sample of its underlying source population, the AOK insurance fund, one of the companies covering the biggest fraction of older adults in Germany. Thus, it mirrors real-world data that are in line with observations that the huge majority of older individuals die before RRT is initiated (31).
Our study has several strengths. They include its prospective design, a population-based setting, and a fairly large cohort of old and very old adults with repeat GFR estimation, on the basis of creatinine and cystatin C, over >6 years. Thus, our study fills the gap of repeat kidney function assessment in this vulnerable group of old individuals. The BIS2 equation has high validity because it was developed using the gold standard of iohexol plasma clearance in a sample of the same study population (14). In addition, creatinine and cystatin C were assessed using standardized assays.
Some limitations deserve mention. First, as expected in a prospective cohort of old individuals, we do not have the same number of eGFR estimates for all participants. Loss to follow-up can be caused by increasing frailty, immobilization, or death, and we cannot assume missing at random. We addressed the issue of survival bias (1) by restricting the analysis to subsamples with at least two or all follow-up visits and (2) by accounting for death in joint models obtaining eGFR slope patterns very similar to those of the entire cohort. These results support the notion that the participants who died during the study did not necessarily have a worse eGFR course compared with those who survived. Therefore, we feel sufficiently convinced that our eGFR models are not strongly biased to overoptimistic estimations. Second, to draw inferences for normative data from a single cohort is certainly restricted. Finally, we used eGFR and not measured GFR. The BIS2 equation, however, was developed using the iohexol plasma clearance and validated (14) and can thus be considered close to gold standard in this dataset.
In conclusion, our study shows that in older adults, the association of age and eGFR slope stayed significant after multivariable adjustment, but the eGFR decline decelerated with increasing age. This pattern remained after accounting for death. Also, the slope of eGFR decline was nonlinear when cystatin C was part of the eGFR equation, whereas it was linear when eGFR was on the basis of serum creatinine only. Overall, these slope patterns add to the notion that in very old age, kidney function decline decreases in severity as age increases.
Disclosures
D. Huscher reports receiving honoraria from 3P Clinical Research and the European Scleroderma Trials and Research Group and travel compensation from Actelion, Boehringer Ingelheim, and Shire. E. Schaeffner reports receiving research funding from Bayer, the DDnÄ Institute for Disease Management, and the ENDI foundation; receiving honoraria from Fresenius Kabi and Siemens HealthCare; and serving in an advisory or leadership role for Institut für angewandte Gesundheitsforschung, as International Editor at American Journal of Kidney Diseases (board membership), and as spokesperson for the platform for health care research at Charité-Universitätsmedizin Berlin. M. Kuhlmann reports having consultancy agreements with ICU Medical; having an ownership interest in Abbott Laboratories and BionTech; receiving research funding from Novartis; receiving honoraria from Amgen, AstraZeneca, B Braun, Bayer Vital, Baxter, Berlin Chemie, Fresenius Medical Care, Hexal, ICU Medical, Medice, and Rie-Pharm; serving on the Advisory Board for Fresenius Medical Care; and speakers bureau for Fresenius Medical Care. M. van der Giet reports having consultancy agreements with Charité Research Organization, Berlin, the Industrie Engineering und Management GmbH (IEM) Stolberg, Germany, Medtronic, Pharvaris, and Recor; having an ownership interest in Nephrolytix; receiving research funding from Bayer, the Berlin Institute of Health (BIH), IEM, and Novartis; receiving honoraria from Bayer, Berlin Chemie, Bristol Myers Squibb, Cytosorbents, IEM, Novartis, Omron, Roche, Servier, Takeda, and Vifor-Fresenius; and serving as a board member of German Hypertension Society. N. Ebert reports receiving €60,000 from Bayer Leverkusen for research cooperation on epidemiological data on kidney function in the elderly. P. Martus reports having consultancy agreements with Lilly. All remaining authors have nothing to disclose.
Funding
This work was supported by the KfH Foundation of Preventive Medicine (http://www.kfh-stiftung-praeventivmedizin.de), a nonprofit organization supporting science and research in the field of disease prevention, and the DDnÄ Institut für Disease Management (to E. Schaeffner).
Supplementary Material
Acknowledgments
The sponsors were not involved in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. The authors thank their colleagues at the 13 study sites in Berlin for providing the necessary infrastructure for the study and the health insurance fund AOK Nordost Die Gesundheitskasse for their continuous cooperation and technical support. We are indebted to the participants of the BIS for their participation and commitment.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Physiology of the Aging Kidney: We Know Where We Are Going, but We Don’t Know How…,” on pages 1107–1109.
Author Contributions
N. Ebert, D. Huscher, P. Martus, and E.S. Schaeffner conceptualized the study; N. Ebert, D. Huscher, N. Mielke, and E.S. Schaeffner were responsible for the data curation; D. Huscher was responsible for the formal analysis; E.S. Schaeffner was responsible for the funding acquisition; N. Ebert, M. Giet, D. Huscher, M. K. Kuhlmann, P. Martus, N. Mielke, E.S. Schaeffner, and A. Schneider were responsible for the investigation; D. Huscher, P. Martus, and E.S. Schaeffner were responsible for the methodology; N. Ebert, N. Mielke, and E.S. Schaeffner were responsible for the project administration; E. S. Schaeffner provided supervision; D. Huscher, N. Mielke, and E.S. Schaeffner were responsible for the visualization; D. Huscher and E.S. Schaeffner wrote the original draft; and N. Ebert, M. Giet, D. Huscher, M. K. Kuhlmann, P. Martus, N. Mielke, E.S. Schaeffner, and A. Schneider reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at https://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16631221/-/DCSupplemental.
Supplemental Appendix. List of GFR-estimating equations.
Supplemental Table 1. Availability of eGFRBIS2 values by sex and age strata.
Supplemental Table 2. Sensitivity analysis, excluding all patients with nosocomial acute kidney injury during follow-up. Linear mixed-effects models for the five eGFR equations; pooled results of the multiple imputed data.
Supplemental Figure 1. Flow chart of available eGFRBIS2 values in the BIS cohort over the entire observation.
Supplemental Figure 2. Sensitivity analysis for the course of eGFRBIS2 (1) excluding patients who were early lost to follow-up (LOF) defined as death, LOF, or only phone visit(s) after baseline or the first follow-up, and (2) restricting analysis to patients with all four follow-up visits, excluding phone visits.
References
- 1.Davies DF, Shock NW: Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 29: 496–507, 1950. 10.1172/JCI102286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delanaye P, Glassock RJ, De Broe ME: Epidemiology of chronic kidney disease: Think (at least) twice! Clin Kidney J 10: 370–374, 2017. 10.1093/ckj/sfw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Wright JT Jr, Greene T, Kusek JW, Lewis JB, Wang X, Lipkowitz MS, Norris KC, Bakris GL, Rahman M, Contreras G, Rostand SG, Kopple JD, Gabbai FB, Schulman GI, Gassman JJ, Charleston J, Agodoa LY; African American Study of Kidney Disease and Hypertension Collaborative Research Group : Long-term effects of renin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med 168: 832–839, 2008. 10.1001/archinte.168.8.832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebert N, Jakob O, Gaedeke J, van der Giet M, Kuhlmann MK, Martus P, Mielke N, Schuchardt M, Tölle M, Wenning V, Schaeffner ES: Prevalence of reduced kidney function and albuminuria in older adults: The Berlin Initiative Study. Nephrol Dial Transplant 32: 997–1005, 2017. 10.1093/ndt/gfw079 [DOI] [PubMed] [Google Scholar]
- 6.Eriksen BO, Palsson R, Ebert N, Melsom T, van der Giet M, Gudnason V, Indridason OS, Inker LA, Jenssen TG, Levey AS, Solbu MD, Tighiouart H, Schaeffner E: GFR in healthy aging: An individual participant data meta-analysis of iohexol clearance in European population-based cohorts. J Am Soc Nephrol 31: 1602–1615, 2020. 10.1681/ASN.2020020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wesson L: Physiology of the Human Kidney, New York, Grune & Stratton, 1969 [Google Scholar]
- 8.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Available at: https://kdigo.org/guidelines/ckd-evaluation-and-management/. Accessed April 14, 2021 [Google Scholar]
- 9.Delanaye P, Schaeffner E, Ebert N, Cavalier E, Mariat C, Krzesinski JM, Moranne O: Normal reference values for glomerular filtration rate: What do we really know? Nephrol Dial Transplant 27: 2664–2672, 2012. 10.1093/ndt/gfs265 [DOI] [PubMed] [Google Scholar]
- 10.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008. 10.1001/archinte.168.20.2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalrymple LS, Katz R, Rifkin DE, Siscovick D, Newman AB, Fried LF, Sarnak MJ, Odden MC, Shlipak MG: Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol 8: 2091–2099, 2013. 10.2215/CJN.02870313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaeffner ES, van der Giet M, Gaedeke J, Tölle M, Ebert N, Kuhlmann MK, Martus P: The Berlin initiative study: The methodology of exploring kidney function in the elderly by combining a longitudinal and cross-sectional approach. Eur J Epidemiol 25: 203–210, 2010. 10.1007/s10654-010-9424-x [DOI] [PubMed] [Google Scholar]
- 13.Ebert N, Delanaye P, Shlipak M, Jakob O, Martus P, Bartel J, Gaedeke J, van der Giet M, Schuchardt M, Cavalier E, Schaeffner E: Cystatin C standardization decreases assay variation and improves assessment of glomerular filtration rate. Clin Chim Acta 456: 115–121, 2016. 10.1016/j.cca.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 14.Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, Kuhlmann MK, Schuchardt M, Tölle M, Ziebig R, van der Giet M, Martus P: Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 157: 471–481, 2012. 10.7326/0003-4819-157-7-201210020-00003 [DOI] [PubMed] [Google Scholar]
- 15.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, Grams ME, Greene T, Grubb A, Gudnason V, Gutiérrez OM, Kalil R, Karger AB, Mauer M, Navis G, Nelson RG, Poggio ED, Rodby R, Rossing P, Rule AD, Selvin E, Seegmiller JC, Shlipak MG, Torres VE, Yang W, Ballew SH, Couture SJ, Powe NR, Levey AS; Chronic Kidney Disease Epidemiology Collaboration : New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 385: 1737–1749, 2021. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012. 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pottel H, Björk J, Courbebaisse M, Couzi L, Ebert N, Eriksen BO, Dalton RN, Dubourg L, Gaillard F, Garrouste C, Grubb A, Jacquemont L, Hansson M, Kamar N, Lamb EJ, Legendre C, Littmann K, Mariat C, Melsom T, Rostaing L, Rule AD, Schaeffner E, Sundin PO, Turner S, Bökenkamp A, Berg U, Åsling-Monemi K, Selistre L, Åkesson A, Larsson A, Nyman U, Delanaye P: Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate: A cross-sectional analysis of pooled data. Ann Intern Med 174: 183–191, 2021. 10.7326/M20-4366 [DOI] [PubMed] [Google Scholar]
- 18.Berg UB: Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant 21: 2577–2582, 2006. 10.1093/ndt/gfl227 [DOI] [PubMed] [Google Scholar]
- 19.Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M: Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney Int 72: 632–637, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Sesso R, Prado F, Vicioso B, Ramos LR: Prospective study of progression of kidney dysfunction in community-dwelling older adults. Nephrology (Carlton) 13: 99–103, 2008. 10.1111/j.1440-1797.2008.00919.x [DOI] [PubMed] [Google Scholar]
- 21.Muntner P: Longitudinal measurements of renal function. Semin Nephrol 29: 650–657, 2009. 10.1016/j.semnephrol.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 22.Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, Southern DA, McLaughlin K, Mortis G, Culleton BF: Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int 69: 2155–2161, 2006. 10.1038/sj.ki.5000270 [DOI] [PubMed] [Google Scholar]
- 23.Cohen E, Nardi Y, Krause I, Goldberg E, Milo G, Garty M, Krause I: A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol 27: 635–641, 2014. 10.1007/s40620-014-0077-9 [DOI] [PubMed] [Google Scholar]
- 24.Malmgren L, McGuigan FE, Berglundh S, Westman K, Christensson A, Åkesson K: Declining estimated glomerular filtration rate and its association with mortality and comorbidity over 10 years in elderly women. Nephron 130: 245–255, 2015. 10.1159/000435790 [DOI] [PubMed] [Google Scholar]
- 25.Malmgren L, McGuigan FE, Christensson A, Akesson KE: Longitudinal changes in kidney function estimated from cystatin C and its association with mortality in elderly women. Nephron 144: 290–298, 2020. 10.1159/000507256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baba M, Shimbo T, Horio M, Ando M, Yasuda Y, Komatsu Y, Masuda K, Matsuo S, Maruyama S: Longitudinal study of the decline in renal function in healthy subjects. PLoS One 10: e0129036, 2015. 10.1371/journal.pone.0129036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rijn MHC, Metzger M, Flamant M, Houillier P, Haymann JP, van den Brand JAJG, Froissart M, Stengel B: Performance of creatinine-based equations for estimating glomerular filtration rate changes over time. Nephrol Dial Transplant 35: 819–827, 2020. 10.1093/ndt/gfy278 [DOI] [PubMed] [Google Scholar]
- 28.Chesnaye NC, Dekker FW, Evans M, Caskey FJ, Torino C, Postorino M, Szymczak M, Ramspek CL, Drechsler C, Wanner C, Jager KJ: Renal function decline in older men and women with advanced chronic kidney disease-results from the EQUAL study. Nephrol Dial Transplant 36: 1656–1663, 2021. 10.1093/ndt/gfaa095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glassock RJ, Warnock DG, Delanaye P: The global burden of chronic kidney disease: Estimates, variability and pitfalls. Nat Rev Nephrol 13: 104–114, 2017. 10.1038/nrneph.2016.163 [DOI] [PubMed] [Google Scholar]
- 30.Björk J, Bäck SE, Ebert N, Evans M, Grubb A, Hansson M, Jones I, Lamb EJ, Martus P, Schaeffner E, Sjöström P, Nyman U: GFR estimation based on standardized creatinine and cystatin C: A European multicenter analysis in older adults. Clin Chem Lab Med 56: 422–435, 2018. 10.1515/cclm-2017-0563 [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011. 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.