Abstract
AKI is a potential complication of intravascular iodinated contrast exposure. Contrast-associated AKI, which typically manifests as small and transient decrements in kidney function that develop within several days of contrast administration, is associated with serious adverse outcomes, including progressive kidney dysfunction and death. However, a causal link between the small increases in serum creatinine that characteristically occur with contrast-associated AKI and serious adverse outcomes remains unproven. This is important given mounting evidence that clinically indicated, potentially lifesaving radiographic procedures are underutilized in patients with CKD. This has been hypothesized to be related to provider concern about precipitating contrast-associated AKI. Intravascular gadolinium-based contrast, an alternative to iodinated contrast that is administered with magnetic resonance imaging, has also been linked with potential serious adverse events, notably the development of nephrogenic systemic fibrosis in patients with severe impairment in kidney function. Patients hospitalized in the intensive care unit frequently have clinical indications for diagnostic and therapeutic procedures that involve the intravascular administration of contrast media. Accordingly, critical care providers and others treating critically ill patients should possess a sound understanding of the risk factors for and incidence of such outcomes, the ability to perform evidence-based risk-benefit assessments regarding intravascular contrast administration, and knowledge of empirical data on the prevention of these iatrogenic complications.
Keywords: critical care nephrology and acute kidney injury series, contrast media
Introduction
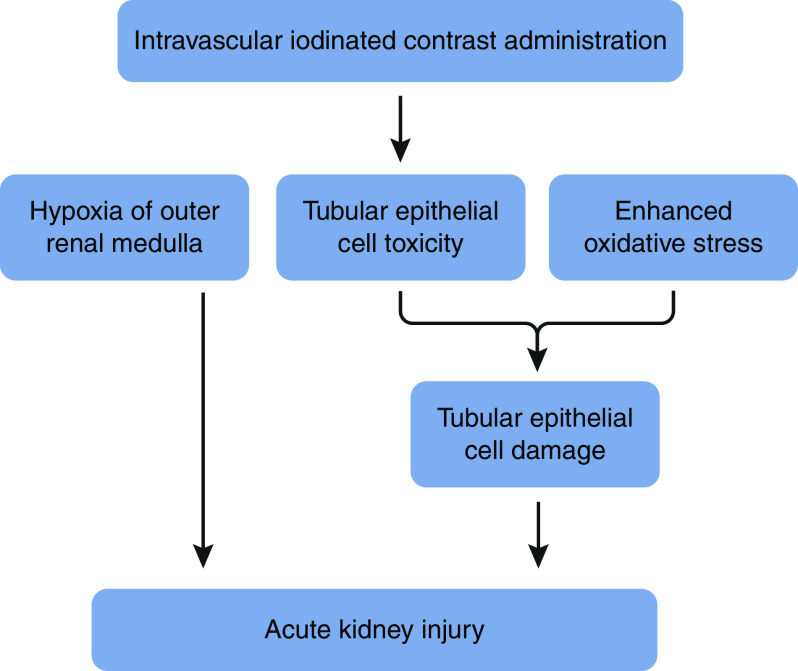
The nephrotoxic effects of intravascular iodinated contrast implicated in the pathophysiology of contrast-associated AKI (CA-AKI) include oxygen supply/demand mismatch from hemodynamic perturbations in the medulla of the kidney, a vascular territory particularly prone to hypoxia; direct toxicity to tubular epithelial cells (hypothesized to be related to DNA damage, mitochondrial dysfunction, and apoptosis); altered endothelial cell function; and generation of reactive oxygen species that contribute to oxidative stress and enhance tubular damage (1,2) (Figure 1). Postcontrast AKI has been referred to as contrast-induced AKI, although this term has fallen out of favor with the recognition that factors other than contrast likely contribute to postprocedure kidney injury. The incidence of CA-AKI varies on the basis of the burden of comorbid illness, type of procedure performed, and the threshold increase in serum creatinine used to define AKI. In patients with stage 3 or 4 CKD, Weisbord et al. (3) documented that the incident rates of CA-AKI, defined by increases in serum creatinine of ≥25%, were 13%, 9%, and 7% following nonemergent noncoronary angiography, coronary angiography, and contrast-enhanced computed tomography (CT), respectively. Among patients in the surgical intensive care unit (ICU), Valette et al. (4) reported that up to 19% of patients developed CA-AKI, with 10% of patients requiring kidney replacement therapy (KRT). In a review of various studies of patients in the ICU by Case et al. (5), the incidence of CA-AKI ranged from 12% to 19%.
Figure 1.
Proposed pathophysiologic mechanisms of kidney injury with iodinated contrast. Pathophysiology of contrast-induced AKI involves medullary hypoxia, direct tubular toxicity, and generation of reactive oxygen species.
Pathophysiology and Incidence of AKI Associated with Iodinated Contrast
Notwithstanding these seemingly alarming rates of CA-AKI, interpreting the true incidence of kidney injury attributable to iodinated contrast requires recognition that elevations in serum creatinine may occur coincidentally and independently of contrast exposure. In a cohort of 11,588 patients, many of whom had intact to near-intact kidney function and therefore low a priori risk for kidney injury from contrast, Bruce et al. (6) reported that the frequency of AKI following noncontrast CT (9%) was similar to that following CT enhanced with iso-osmolal iodixanol (10%) or low-osmolal iohexol (10%). However, because many patients were at low risk for AKI, comparisons of CA-AKI rates across groups are less informative. Multiple additional observational studies compared the incidence of AKI following CT imaging with and without intravascular contrast using propensity score analyses (7–12). A meta-analysis by McDonald et al. (8) of more than a dozen such studies found a similar risk of AKI with contrast-enhanced compared with noncontrasted procedures (relative risk, 0.79; 95% confidence interval [95% CI], 0.62 to 1.02). Several studies of patients in the ICU have observed similar findings (10,13–15). In a cohort of patients with sepsis and AKI, Goto et al. (14) compared rates of kidney injury and mortality between those who did and did not receive intravascular contrast in a propensity score–matched analysis. The rates of kidney injury and death were comparable between the groups, leading the authors to conclude that one-time contrast exposures were not associated with worsening kidney function or mortality in this critically ill patient population.
Careful inspection of these studies reveals key methodologic limitations, most importantly the fact that many patients were not at higher risk for AKI. Moreover, those who did not receive intravascular contrast consistently demonstrated a higher level of comorbidity and greater baseline level of risk for AKI. Despite efforts to adjust for differences in baseline risk factors, including with the use of propensity score matching for known confounders, the relative risks/odds ratios (ORs) for several studies revealed strong trends toward lower rates of AKI among patients who received contrast (8,16). As iodinated contrast media are not nephroprotective, this observation likely reflects residual confounding and almost certainly underscores the higher baseline risk for kidney injury among those who did not receive contrast. These studies emphasize that baseline serum creatinine fluctuations and causal factors distinct from iodinated contrast administration must be considered when assessing the risk for and incidence of CA-AKI, particularly when this condition is defined by nominal changes in serum creatinine.
Risk Factors for Contrast-Associated AKI
Risk factors for CA-AKI can be categorized as patient related or procedure related (Table 1). Preexisting CKD is the chief patient-specific risk factor with a graded and direct relationship between CKD severity and AKI risk (17). Diabetes enhances the risk in those with CKD but does not appear to increase risk in the absence of underlying impairment in kidney function (18). Absolute or effective depletion in intravascular volume may amplify the effects of contrast-induced renal vasoconstriction and increase CA-AKI risk (19,20). Similarly, nonsteroidal anti-inflammatory drugs, which inhibit vasodilatory prostaglandin synthesis, may elevate risk (21). Use of large volumes of contrast augments risk, although the precise amount of contrast above which risk rises significantly has not been definitively determined (22–25). Repeated administrations of intravascular contrast within short time frames also elevate risk. Low-osmolal contrast is less nephrotoxic than high-osmolal contrast, although there do not appear to be meaningful differences in risk between iso- and low-osmolal contrast (26). Finally, risk appears greater following intra-arterial compared with intravenous (IV) contrast exposure; however, this may reflect differences between patient populations undergoing different procedures. Additionally, cholesterol emboli are a risk factor for AKI that is unique to angiographic procedures.
Table 1.
Contrast-associated AKI risk factors
| Patient Associated | Procedure Associated |
|---|---|
| Reduced GFR, acute or chronica | High-osmolal contrast |
| Diabetes mellitusb | Large volume of contrast |
| Reduced intravascular volume | Serial contrast procedures |
| Concomitant nephrotoxic medications |
Defined as eGFR <45 ml/min per 1.73 m2 with other risk factors or <30 ml/min per 1.73 m2.
Augments risk in patients with underlying kidney function impairment.
Association of Contrast-Associated AKI with Adverse Outcomes and Implications for Clinical Care
Multiple studies have documented the association of CA-AKI with heightened short- and long-term mortality (Table 2) (23,27–29). McCullough et al. (23) reported that, among patients undergoing percutaneous coronary intervention, those with CA-AKI were more likely to experience nosocomial death than those without CA-AKI (7% versus 1%, P<0.001) (23). Solomon et al. (30) demonstrated an approximately three-fold greater risk of death, stroke, myocardial infarction, and/or kidney failure at 1 year among patients with postangiography CA-AKI compared with those without CA-AKI. CA-AKI also associates with greater health care utilization as evidenced by a study by Adolph et al. (31), which found that patients with CA-AKI were hospitalized an average of 2 days longer than those without CA-AKI. Additionally, a decision analysis by Subramanian et al. (32) documented that CA-AKI was associated with higher hospital expenses of approximately $10,000. CA-AKI is also linked with more rapid progression of underlying CKD (33–36). Goldenberg et al. (33) demonstrated that patients with transient CA-AKI experienced greater eGFR loss 2 years following angiography than patients without postangiography CA-AKI (ΔeGFR of −20±11 versus −6±16 ml/min per 1.73 m2, P=0.02).
Table 2.
Contrast-associated AKI and mortality risk
| Study Authors | No. of Patients | Contrast-Associated AKI Definition | Adjusted Odds Ratio/Hazard Ratio | 95% Confidence Interval |
|---|---|---|---|---|
| Short-term mortality | ||||
| Levy et al. (92) | 357 | ↑ Cr≥25% to ≥2.0 mg/dl | 5.5 | 2.9 to 13.2 |
| Gruberg et al. (93) | 439 | ↑ SCr>25% | 3.9 | 2.0 to 7.6 |
| Shema et al. (94) | 1111 | ↑ Cr≥50% or ↓ eGFR≥25% | 3.9 | 1.2 to 12.0 |
| McCullough et al. (23) | 1826 | ↑ Cr>25% | 6.6 | 3.3 to 12.9 |
| From et al. (28) | 3236 | ↑ Cr≥25% or ≥0.5 mg/dl | 3.4 | 2.6 to 4.4 |
| Rihal et al. (95) | 7586 | ↑ Cr>0.5 mg/dl | 10.8 | 6.9 to 17.0 |
| Bartholomew et al. (27) | 20,479 | ↑ Cr≥1.0 mg/dl | 22 | 16 to 31 |
| Weisbord et al. (3,29,67) | 27,608 | ↑ Cr=0.25–0.5 mg/dl | 1.8 | 1.4 to 2.5 |
| Longer-term mortality | ||||
| Goldenberg et al. (33) | 78 | ↑ Cr≥0.5 mg/dl or ≥25% | 2.7 | 1.7 to 4.5 |
| Solomon et al. (30) | 294 | ↑ Cr≥0.3 mg/dl | 3.2a | 1.1 to 8.7 |
| Harjai et al. (96) | 985 | ↑ Cr≥0.5 mg/dl | 2.6 | 1.5 to 4.4 |
| Roghi et al. (97) | 2860 | ↑ Cr≥0.5 mg/dl | 1.8 | 1.0 to 3.4 |
| Brown et al. (98) | 7856 | ↑ Cr≥0.5 mg/dl | 3.1 | 2.4 to 4.0 |
Cr, creatinine; SCr, serum creatinine.
Denotes the incident rate ratio of the composite outcome of death, cerebrovascular accident, myocardial infarction, or kidney failure.
Importantly, the causal nature of the associations of CA-AKI, defined by small upticks in serum creatinine, with such serious adverse outcomes remains unproven. It is plausible that transient CA-AKI represents a marker of patients more vulnerable to adverse events because of greater underlying comorbidity, more severe acute illness, and/or less kidney reserve rather than a mediator of such outcomes (37). Recognition of this is critical as past research strongly suggests that providers may overestimate the risk for CA-AKI and associated adverse outcomes, leading to an underutilization of clinically indicated and potentially lifesaving contrast-enhanced procedures in those with kidney disease. Nearly two decades ago, Chertow et al. (38) compared the use of invasive coronary care, including coronary angiography with percutaneous intervention, for the treatment of acute myocardial infarction in 15,093 patients with CKD and 42,191 without CKD. Patients with CKD were >50% less likely to undergo coronary angiography than those without CKD after adjusting for the appropriateness of such care (OR, 0.47; 95% CI, 0.40 to 0.52). Among those who underwent angiography, revascularization was performed less frequently in patients with CKD than those without CKD (55% versus 62%, P<0.001). Numerous other studies have documented that patients with CKD who are hospitalized with acute coronary syndrome, including ST segment elevation myocardial infarction, are considerably less likely to undergo invasive coronary care than those without CKD (Table 3) (38–54). Although less well studied than in the setting of atherosclerotic cardiac disease, utilization of contrast-enhanced procedures has also been studied in patients with peripheral vascular disease and CKD. In a cohort of 6227 patients with advanced lower limb ischemia, O’Hare et al. (55) found that those with eGFR values of 30–59 ml/min per 1.73 m2 were significantly less likely to undergo revascularization than patients with eGFR values >60 ml/min per 1.73 m2 (adjusted OR, 0.84; 95% CI, 0.72 to 0.96). More severe reductions in eGFR were associated with even less frequent revascularization.
Table 3.
Studies documenting the differential use of invasive treatment for acute coronary syndrome in patients with and without CKD
| Study Authors | CKD, N | Clinical Presentation | Invasive Treatment, % | |
|---|---|---|---|---|
| CKD | No CKD | |||
| Keeley et al. (45) | 1654 | ACS | 16a | 22a |
| Freeman et al. (43) | 889 | ACS | 27a | 43a |
| Keough-Ryan et al. (46) | 5549 | ACS | 24a | 31a |
| Medi et al. (48) | 778 | ACS | 37a | 46a |
| Lau et al. (47) | 1227 | ACS | 33a | 57a |
| Han et al. (44) | 6560 | NSTE-ACS | 30a | 54a |
| Rhee et al. (50) | 1540 | NSTE-ACS | 32a | 42a |
| Goldenberg et al. (33) | 13,141 | NSTE-ACS | 50 | 68 |
| Chertow et al. (38) | 15,093 | NSTEMI/STEMI | 55 | 62 |
| Charytan et al. (40,56) | 1210 | NSTEMI/STEMI | 9a | 54a |
| Shlipak et al. (52) | 47,644 | NSTEMI/STEMI | 16a | 24a |
| Saad et al. (51) | 199 | NSTEMI/STEMI | 76 | 94 |
| Fox et al. (42)b | 13,069 | NSTEMI | 45a | 72a |
| Fox et al. (42)b | 5808 | STEMI | 66a | 73a |
| Szummer et al. (53)c | 4839 | NSTEMI | 34a | 58a |
| Szummer et al. (53)c | 2569 | STEMI | 70a | 77a |
| Szummer et al. (54) | 5689 | NSTEMI | 34a | 57a |
| Medi et al. (49) | 3450 | STEMI | 46a | 62a |
| Bhatt et al. (39) | 2475 | NSTE-ACS | OR, 0.51; 95% CI, 0.46 to 0.58 | |
| Chew et al. (41) | 923 | ACS | OR, 0.35; 95% CI, 0.21 to 0.60 | |
ACS, acute coronary syndrome; NSTE-ACS, non-ST elevation acute coronary syndrome; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; OR, odds ratio; 95% CI, 95% confidence interval.
Calculated on the basis of raw data from the original manuscript.
The same study from Fox et al. (42) with two separate analyses.
The same study from Szummer et al. (53) with two separate analyses.
This practice, which has been termed “renalism,” is of significant clinical relevance given evidence demonstrating reductions in short- and long-term mortality with the use of invasive coronary care compared with conservative treatment among patients with CKD (33,42,46,56). This finding was highlighted in a meta-analysis by Shaw et al. (57) that included ten clinical trials and observational studies comprising 147,908 patients with non-ST elevation acute coronary syndrome and CKD. Compared with conservative treatment, early invasive care was associated with a nearly 50% lower mortality (relative risk, 0.53; 95% CI, 0.45 to 0.62).
Data demonstrating reduced mortality following contrast-enhanced cardiac procedures in patients with CKD must be interpreted in the context of our current understanding of the relationship between CA-AKI and serious adverse outcomes, specifically that the small elevations in serum creatinine that define CA-AKI have not been confirmed to be causally linked with mortality. Accordingly, clinically indicated contrast-enhanced procedures should typically be performed in patients at heightened risk of kidney injury with the implementation of evidence-based prevention if there are no equally diagnostic/therapeutic imaging options that do not require contrast.
Prevention of Contrast-Associated AKI
Once determined that a procedure with intravascular iodinated contrast is indicated, implementing evidence-based preventive care is important. A multitude of past clinical trials provide evidence on the efficacy of various interventions, although many were limited by small sample sizes and inadequate power to detect differences in less common but more serious patient-centered outcomes. Nonetheless, prior research has focused on four principal strategies to mitigate risk: (1) KRT to remove contrast from the circulation, (2) use of less nephrotoxic contrast media formulations, (3) prescribing medications that mechanistically counteract the nephrotoxic effects of contrast, and (4) administering IV crystalloid to mitigate the adverse hemodynamic effects and direct tubular toxicity of contrast. Prophylactic pericontrast hemodialysis has been shown to be potentially harmful and is not recommended (58). Data regarding continuous KRT are conflicting, with insufficient evidence to support this prophylactic strategy (59,60). Over several decades, the chemical formulation of iodinated contrast has evolved. Previous generation “high-osmolal” agents were associated with significantly elevated rates of CA-AKI compared with “low-osmolal” contrast (18). However, trials and meta-analyses comparing low- with iso-osmolal formulations have been conflicting (61). The American College of Cardiology/American Heart Association and the European Society of Urogenital Radiology clinical practice guidelines recommend using either low- or iso-osmolal contrast in at-risk patients (62,63).
Multiple pharmacologic agents have been evaluated for CA-AKI prevention. Some were found to be ineffective and even potentially deleterious (e.g., dopamine), whereas others have conflicting data on efficacy (64) (Table 4). Discordant findings of trials and meta-analyses that investigated N-acetylcysteine (NAC) resulted in lingering uncertainty on its potential benefits (65,66). The Prevention of Serious Adverse Events Following Angiography (PRESERVE) trial, which enrolled 4993 patients undergoing nonemergent angiography, demonstrated that compared with placebo, oral NAC was not associated with a reduction in 90-day death, need for dialysis, persistent impairment in kidney function (OR, 1.02; 95% CI, 0.78 to 1.33), or CA-AKI (9% in NAC and 9% for placebo; OR, 1.06; 95% CI, 0.87 to 1.28) (67). Hence, there is currently no role for NAC or other oral agents in CA-AKI prevention.
Table 4.
Pharmacologic agents evaluated for the prevention of contrast-associated AKI
| Ineffective | Indeterminate Effectiveness |
|---|---|
| Furosemidea | Atrial natriuretic peptide |
| Dopaminea | Theophylline/aminophylline |
| Fenoldopama | Atorvastatin/rosuvastatin |
| Calcium channel blockers | Prostaglandin analogs |
| N-acetylcysteine | Allopurinol |
| Acetazolamide |
Potentially deleterious.
The cornerstone of prevention of CA-AKI is the provision of isotonic IV crystalloid. Several past studies support the use of IV volume expansion (68,69). Recent research has focused on IV fluid composition, specifically the comparison of isotonic sodium bicarbonate with isotonic sodium chloride. An initial trial by Merten et al. (70) that enrolled 119 patients found a lower incidence of CA-AKI with IV isotonic bicarbonate than IV isotonic saline (2% versus 14%, P=0.02). This striking effect of bicarbonate spawned a proliferation of clinical trials and meta-analyses that reported conflicting results (36,71–77). To resolve the persistent clinical uncertainty, the aforementioned PRESERVE trial also randomized high-risk patients to receive IV isotonic sodium bicarbonate (n=2511) or IV isotonic sodium chloride (n=2482) prior to, during, and following angiography (67). Compared with IV sodium chloride, sodium bicarbonate was not associated with lower rates of 90-day death, need for dialysis, persistent decrement in kidney function (OR, 0.93; 95% CI, 0.72 to 1.22), or incident CA-AKI (10% for sodium bicarbonate and 8% for sodium chloride; OR, 1.16; 95% CI, 0.96 to 1.41) (64). Thus, on the basis of current evidence, IV sodium-based isotonic crystalloid with either bicarbonate or chloride as the component anion should be considered the standard of care to mitigate CA-AKI risk. However, sodium chloride is generally preferred given its lower cost, availability, and avoidance of the risk for errors in formation. A series of trials of patients undergoing angiography has investigated the RenalGuard device, which matches IV fluid with furosemide-induced urinary flow, for the prevention of CA-AKI (78,79). Although these studies demonstrated lower risk for CA-AKI and associated adverse outcomes with this treatment system, they were limited by small sample sizes and/or the use of surrogate primary outcomes. Larger trials powered to definitively determine the effect of this intervention on serious, adverse, patient-centered outcomes are needed before recommendations can be made for its use.
A Practical Evidence-Based Approach to Risk Mitigation of Contrast-Associated AKI in the Intensive Care Unit Setting
Patients in the ICU in need of iodinated contrast imaging procedures who are at heightened risk for CA-AKI should undergo such procedures if there are no comparably accurate imaging options that do not use iodinated contrast, albeit with the implementation of preventive therapy when clinically feasible. Enhanced risk is typically defined on the basis of the level of kidney function. An eGFR of <60 ml/min per 1.73 m2 is an appropriate threshold below which preventive care should be implemented, if feasible, in those in need of intra-arterial procedures. As patients undergoing contrasted CT are generally believed to be at lower risk for CA-AKI, a lower eGFR threshold is reasonable for the implementation of preventive care, although equipoise exists on whether an eGFR of <30 or <45 ml/min per 1.73 m2 is most appropriate (80). In patients with underlying diabetes or other risk factors, an eGFR<45 ml/min per 1.73 m2 is an appropriate threshold to define risk in those receiving IV contrast. A important caveat is that these recommendations assume a steady-state serum creatinine, which is necessary for the accurate calculation of eGFR (81). Clearly, a disproportionate number of patients in the ICU have fluctuating serum creatinine and/or frank AKI. Such patients should be considered at heighted risk and receive appropriate preventive care when feasible.
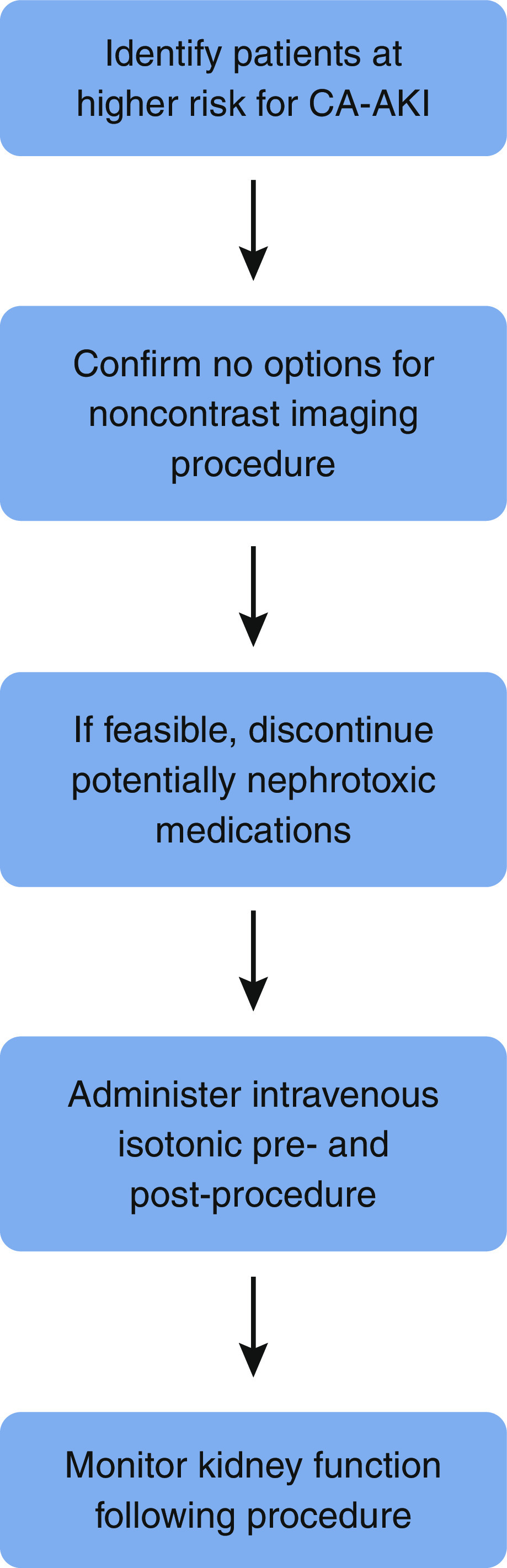
The following are evidence-based steps that are appropriate in high-risk patients undergoing contrast-enhanced procedures (Figure 2). Nonsteroidal anti-inflammatories should be discontinued prior to contrast exposure. The minimum required volume of low- or iso-osmolal contrast should be administered. If feasible, isotonic IV crystalloid should be administered prior to and following nonemergent contrast-enhanced procedures, whereas postprocedure IV isotonic crystalloid should be administered following emergent procedures even if preprocedure administration is not feasible. Short durations of IV fluid (e.g., 3 ml/kg per hour for 1 hour prior to contrast and 1.5 ml/kg per hour for 4–6 hours postcontrast) have not been shown to be inferior to more sustained volume expansion and may be more practical in the ICU setting. The Prevention of Contrast Renal Injury with Different Hydration Strategies trial showed that providing targeted IV fluid volumes to patients with elevated left ventricular end diastolic pressure undergoing coronary angiography is both safe and effective (82). Accordingly, cautious IV fluid administration should be implemented, when feasible, in patients with nondecompensated heart failure. Kidney function should be followed in the days after contrast administration to identify evidence of early kidney injury.
Figure 2.
Recommended approach to prevention of contrast-associated AKI (CA-AKI).
Gadolinium-Based Contrast and Nephrogenic Systemic Fibrosis
Until the mid-2000s, gadolinium-enhanced magnetic resonance imaging (MRI) was a preferred imaging modality in patients with advanced kidney disease. Although there is some evidence for direct gadolinium nephrotoxicity, current evidence suggests that gadolinium, when used at typical clinical doses, imparts minimal risk for significant AKI (83). However, studies have linked gadolinium-based contrast agents (GBCAs) with the development of nephrogenic systemic fibrosis (NSF), an often debilitating fibrosing disorder of skin, connective tissue, and, in certain cases, internal organs in patients with significantly impaired kidney function (84–86). Skin is the primary site of fibrosis and typically presents with thickening and induration. Systemic involvement of internal organs and the musculoskeletal system, also characterized by fibrosis, can result in organ dysfunction. The initial clinical manifestations of NSF typically present within weeks to several months following GBCA exposure, and definitive diagnosis requires skin/soft tissue biopsy and clinical/histologic correlation (87). Although improvement in symptoms may accompany AKI recovery or transplantation, there is currently no well-established effective therapeutic intervention, and the development of this condition commonly leads to significant morbidity and mortality.
Because of its toxicity in vivo, free gadolinium is complexed to a chelate/ligand (86). GBCAs are classified on the basis of the strength of their association with NSF (88). Group 1 agents are complexed to a linear ligand and have been associated with nearly all cases of NSF reported in the literature. Conversely, group 2 GBCAs, all but one of which are complexed to a macrocyclic ligand, have been independently associated with few to no cases of NSF. The group 3 agent gadoxetate disodium has not been associated with cases of NSF, although it is characterized by less frequent use than group 1 and 2 agents. Understanding the molecular differences and epidemiologic evidence regarding the differential incidence of NSF among these three chemical groups is essential for NSF prevention.
Upon recognition of the association of gadolinium-based contrast with NSF, providers became wary of performing MRIs with IV GBCAs, particularly those agents most commonly associated with NSF, in patients with severely impaired kidney function. However, available evidence demonstrates that the risk for NSF with group 2 GBCAs is exceedingly low (although not zero, particularly for repeated episodes of contrast exposure or a single high-dose exposure). Such agents have become the standard in patients with impairment in kidney function who require MRI with contrast (89). This practice is supported by a recent meta-analysis by Woolen et al. (89) that included 16 studies comprising 4931 patients with stage 4 or 5 CKD, including those dependent on dialysis, who received a group 2 GBCA. None of the patients developed NSF, with an upper bound of the two-sided 95% CI of 0.07% (89).
Prevention of Nephrogenic Systemic Fibrosis
Among high-risk patients being considered for GBCA-enhanced imaging, evaluation of alternative radiographic modalities that do not utilize GBCA but provide comparable diagnostic information is important. However, much like the recommended approach with intravascular iodinated contrast, clinically indicated GBCA-enhanced MRIs should typically be performed in at-risk patients if comparably accurate noncontrasted imaging is not an option, albeit with evidence-based preventive care. Patients at risk for NSF in whom preventive care is critical include those with kidney failure on dialysis (hemodialysis or peritoneal dialysis), CKD with an eGFR <30 ml/min per 1.73 m2, or AKI (90). The crux of preventive care in such patients involves the use of group 2 GBCAs in the lowest necessary dose. Group 1 and 3 GBCAs should not be administered to these individuals. There is no direct evidence that postexposure dialysis mitigates NSF risk. However, hemodialysis does efficiently remove gadolinium, which may attenuate NSF risk if the pathogenesis is not immediate following GBCA exposure (91). If feasible, patients with kidney failure or AKI who are already on hemodialysis should undergo the GBCA-enhanced procedure prior to a hemodialysis treatment. Data on the risk-benefit ratio of performing hemodialysis following GBCA exposure in patients on long-term peritoneal dialysis, with advanced nondialysis-dependent CKD, or with severe nondialysis-dependent AKI are lacking. Considering the risks associated with dialysis catheter placement, dialyzing these patients is generally not recommended if a group 2 GBCA is administered.
Conclusions
Imaging or procedures that utilize intravascular contrast media, iodinated or gadolinium based, are commonly indicated for diagnostic and/or therapeutic purposes in patients in the ICU with acute kidney disease and/or CKD. Although these contrast agents are associated with certain adverse outcomes that should be considered prior to proceeding with their use, the risks for these outcomes, particularly when appropriate preventive interventions are implemented, should not be overestimated. Among at-risk patients with a clinical indication for a procedure that requires intravascular iodinated contrast in whom there are no equally diagnostic/therapeutic noncontrasted imaging options, the procedure should be performed, albeit after discussing the potential risks and benefits with the patient/surrogate and with the implementation of evidence-based prevention. Such prevention comprises the use of the lowest necessary volume of iso- or low-osmolal iodinated contrast, withdrawal of concomitant nephrotoxic agents when appropriate, and intravascular volume expansion with periprocedural IV crystalloid if feasible. Among those requiring an MRI with GBCA enhancement in whom alternative imaging options with equal accuracy are not available, the lowest necessary dose of a group 2 agent should be used. Adopting a practical and evidence-based approach to the use of contrast-enhanced imaging in patients in the ICU will help ensure that such procedures provide the critical information that commonly informs therapeutic interventions and optimizes patient outcomes.
Disclosures
W. Cashion reports many individual stocks but nothing in the health care or biotechnology sectors. S.D. Weisbord reports consultancy agreements with Takeda.
Funding
W. Cashion was supported by a T32 award (T32 DK061296).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
W. Cashion and S.D. Weisbord were responsible for data curation, wrote the original draft, and reviewed and edited the manuscript.
References
- 1.Faucon AL, Bobrie G, Clément O: Nephrotoxicity of iodinated contrast media: From pathophysiology to prevention strategies. Eur J Radiol 116: 231–241, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Liu ZZ, Schmerbach K, Lu Y, Perlewitz A, Nikitina T, Cantow K, Seeliger E, Persson PB, Patzak A, Liu R, Sendeski MM: Iodinated contrast media cause direct tubular cell damage, leading to oxidative stress, low nitric oxide, and impairment of tubuloglomerular feedback. Am J Physiol Renal Physiol 306: F864–F872, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Sonel AF, Fine MJ, Palevsky PM: Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med 168: 1325–1332, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Valette X, Parienti JJ, Plaud B, Lehoux P, Samba D, Hanouz JL: Incidence, morbidity, and mortality of contrast-induced acute kidney injury in a surgical intensive care unit: A prospective cohort study. J Crit Care 27: 322.e1–322.e5, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Case J, Khan S, Khalid R, Khan A: Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract 2013: 479730, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce RJ, Djamali A, Shinki K, Michel SJ, Fine JP, Pozniak MA: Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol 192: 711–718, 2009 [DOI] [PubMed] [Google Scholar]
- 7.McDonald JS, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE: Risk of intravenous contrast material-mediated acute kidney injury: A propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology 271: 65–73, 2014 [DOI] [PubMed] [Google Scholar]
- 8.McDonald JS, McDonald RJ, Comin J, Williamson EE, Katzberg RW, Murad MH, Kallmes DF: Frequency of acute kidney injury following intravenous contrast medium administration: A systematic review and meta-analysis. Radiology 267: 119–128, 2013 [DOI] [PubMed] [Google Scholar]
- 9.McDonald JS, McDonald RJ, Lieske JC, Carter RE, Katzberg RW, Williamson EE, Kallmes DF: Risk of acute kidney injury, dialysis, and mortality in patients with chronic kidney disease after intravenous contrast material exposure [published correction appears in Mayo Clin Proc 90: 1457, 2015]. Mayo Clin Proc 90: 1046–1053, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald JS, McDonald RJ, Williamson EE, Kallmes DF, Kashani K: Post-contrast acute kidney injury in intensive care unit patients: A propensity score-adjusted study. Intensive Care Med 43: 774–784, 2017 [DOI] [PubMed] [Google Scholar]
- 11.McDonald RJ, McDonald JS, Carter RE, Hartman RP, Katzberg RW, Kallmes DF, Williamson EE: Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology 273: 714–725, 2014 [DOI] [PubMed] [Google Scholar]
- 12.McDonald RJ, McDonald JS, Newhouse JH, Davenport MS: Controversies in contrast material-induced acute kidney injury: Closing in on the truth? Radiology 277: 627–632, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Ehrmann S, Badin J, Savath L, Pajot O, Garot D, Pham T, Capdevila X, Perrotin D, Lakhal K: Acute kidney injury in the critically ill: Is iodinated contrast medium really harmful? Crit Care Med 41: 1017–1026, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Goto Y, Koyama K, Katayama S, Tonai K, Shima J, Koinuma T, Nunomiya S: Influence of contrast media on renal function and outcomes in patients with sepsis-associated acute kidney injury: A propensity-matched cohort study. Crit Care 23: 249, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams LS, Walker GR, Loewenherz JW, Gidel LT: Association of contrast and acute kidney injury in the critically ill: A propensity-matched study. Chest 157: 866–876, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm-Leen E, Montez-Rath ME, Chertow G: Estimating the risk of radiocontrast-associated nephropathy. J Am Soc Nephrol 28: 653–659, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullough PA, Adam A, Becker CR, Davidson C, Lameire N, Stacul F, Tumlin J; CIN Consensus Working Panel : Risk prediction of contrast-induced nephropathy. Am J Cardiol 98[6A]: 27K–36K, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Rudnick MR, Goldfarb S, Wexler L, Ludbrook PA, Murphy MJ, Halpern EF, Hill JA, Winniford M, Cohen MB, VanFossen DB: Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: A randomized trial. The Iohexol Cooperative Study. Kidney Int 47: 254–261, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Gomes AS, Baker JD, Martin-Paredero V, Dixon SM, Takiff H, Machleder HI, Moore WS: Acute renal dysfunction after major arteriography. AJR Am J Roentgenol 145: 1249–1253, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Taliercio CP, Vlietstra RE, Fisher LD, Burnett JC: Risks for renal dysfunction with cardiac angiography. Ann Intern Med 104: 501–504, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Ahmad SR, Kortepeter C, Brinker A, Chen M, Beitz J: Renal failure associated with the use of celecoxib and rofecoxib. Drug Saf 25: 537–544, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Marenzi G, Assanelli E, Campodonico J, Lauri G, Marana I, De Metrio M, Moltrasio M, Grazi M, Rubino M, Veglia F, Fabbiocchi F, Bartorelli AL: Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med 150: 170–177, 2009 [DOI] [PubMed] [Google Scholar]
- 23.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW: Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med 103: 368–375, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Nyman U, Almén T, Aspelin P, Hellström M, Kristiansson M, Sterner G: Contrast-medium-Induced nephropathy correlated to the ratio between dose in gram iodine and estimated GFR in ml/min. Acta Radiol 46: 830–842, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Worasuwannarak S, Pornratanarangsi S: Prediction of contrast-induced nephropathy in diabetic patients undergoing elective cardiac catheterization or PCI: Role of volume-to-creatinine clearance ratio and iodine dose-to-creatinine clearance ratio. J Med Assoc Thai 93[Suppl 1]: S29–S34, 2010 [PubMed] [Google Scholar]
- 26.Laskey W, Aspelin P, Davidson C, Rudnick M, Aubry P, Kumar S, Gietzen F, Wiemer M; DXV405 Study Group : Nephrotoxicity of iodixanol versus iopamidol in patients with chronic kidney disease and diabetes mellitus undergoing coronary angiographic procedures. Am Heart J 158: 822–828.e3, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, Grines CL, O’Neill WW: Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol 93: 1515–1519, 2004 [DOI] [PubMed] [Google Scholar]
- 28.From AM, Bartholmai BJ, Williams AW, Cha SS, McDonald FS: Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc 83: 1095–1100, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Weisbord SD, Chen H, Stone RA, Kip KE, Fine MJ, Saul MI, Palevsky PM: Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol 17: 2871–2877, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Solomon RJ, Mehran R, Natarajan MK, Doucet S, Katholi RE, Staniloae CS, Sharma SK, Labinaz M, Gelormini JL, Barrett BJ: Contrast-induced nephropathy and long-term adverse events: Cause and effect? Clin J Am Soc Nephrol 4: 1162–1169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adolph E, Holdt-Lehmann B, Chatterjee T, Paschka S, Prott A, Schneider H, Koerber T, Ince H, Steiner M, Schuff-Werner P, Nienaber CA: Renal Insufficiency Following Radiocontrast Exposure Trial (REINFORCE): A randomized comparison of sodium bicarbonate versus sodium chloride hydration for the prevention of contrast-induced nephropathy. Coron Artery Dis 19: 413–419, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Subramanian S, Tumlin J, Bapat B, Zyczynski T: Economic burden of contrast-induced nephropathy: Implications for prevention strategies. J Med Econ 10: 119–134, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg I, Chonchol M, Guetta V: Reversible acute kidney injury following contrast exposure and the risk of long-term mortality. Am J Nephrol 29: 136–144, 2009 [DOI] [PubMed] [Google Scholar]
- 34.James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, Pannu N, Manns BJ, Klarenbach SW, Hemmelgarn BR; Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators : Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 123: 409–416, 2011 [DOI] [PubMed] [Google Scholar]
- 35.James MT, Ghali WA, Tonelli M, Faris P, Knudtson ML, Pannu N, Klarenbach SW, Manns BJ, Hemmelgarn BR: Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int 78: 803–809, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Maioli M, Toso A, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, Bellandi F: Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol 52: 599–604, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Rudnick M, Feldman H: Contrast-induced nephropathy: What are the true clinical consequences? Clin J Am Soc Nephrol 3: 263–272, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Chertow GM, Normand SL, McNeil BJ: “Renalism”: Inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol 15: 2462–2468, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Bhatt DL, Roe MT, Peterson ED, Li Y, Chen AY, Harrington RA, Greenbaum AB, Berger PB, Cannon CP, Cohen DJ, Gibson CM, Saucedo JF, Kleiman NS, Hochman JS, Boden WE, Brindis RG, Peacock WF, Smith SC Jr., Pollack CV Jr., Gibler WB, Ohman EM; CRUSADE Investigators : Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: Results from the CRUSADE Quality Improvement Initiative. JAMA 292: 2096–2104, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Charytan DM, Setoguchi S, Solomon DH, Avorn J, Winkelmayer WC: Clinical presentation of myocardial infarction contributes to lower use of coronary angiography in patients with chronic kidney disease. Kidney Int 71: 938–945, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Chew DP, Amerena JV, Coverdale SG, Rankin JM, Astley CM, Soman A, Brieger DB; ACACIA investigators : Invasive management and late clinical outcomes in contemporary Australian management of acute coronary syndromes: Observations from the ACACIA registry. Med J Aust 188: 691–697, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD; Acute Coronary Treatment and Intervention Outcomes Network registry : Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: A report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 121: 357–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman RV, Mehta RH, Al Badr W, Cooper JV, Kline-Rogers E, Eagle KA: Influence of concurrent renal dysfunction on outcomes of patients with acute coronary syndromes and implications of the use of glycoprotein IIb/IIIa inhibitors. J Am Coll Cardiol 41: 718–724, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Han JH, Chandra A, Mulgund J, Roe MT, Peterson ED, Szczech LA, Patel U, Ohman EM, Lindsell CJ, Gibler WB: Chronic kidney disease in patients with non-ST-segment elevation acute coronary syndromes. Am J Med 119: 248–254, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Keeley EC, Kadakia R, Soman S, Borzak S, McCullough PA: Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. Am J Cardiol 92: 509–514, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Keough-Ryan TM, Kiberd BA, Dipchand CS, Cox JL, Rose CL, Thompson KJ, Clase CM: Outcomes of acute coronary syndrome in a large Canadian cohort: Impact of chronic renal insufficiency, cardiac interventions, and anemia. Am J Kidney Dis 46: 845–855, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Lau JK, Anastasius MO, Hyun KK, Dabin B, Coverdale S, Ferry C, Hung J, Antonis P, Chew DP, Aliprandi-Costa B, Cass A, Brieger DB: Evidence-based care in a population with chronic kidney disease and acute coronary syndrome. Findings from the Australian Cooperative National Registry of Acute Coronary Care, Guideline Adherence and Clinical Events (CONCORDANCE). Am Heart J 170: 566–72.e1, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Medi C, Chew DP, Amerena J, Coverdale S, Soman A, Astley C, Rankin J, Brieger D: An invasive management strategy is associated with improved outcomes in high-risk acute coronary syndromes in patients with chronic kidney disease. Intern Med J 41: 743–750, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Medi C, Montalescot G, Budaj A, Fox KA, López-Sendón J, FitzGerald G, Brieger DB; GRACE Investigators : Reperfusion in patients with renal dysfunction after presentation with ST-segment elevation or left bundle branch block: GRACE (Global Registry of Acute Coronary Events). JACC Cardiovasc Interv 2: 26–33, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Rhee JW, Wiviott SD, Scirica BM, Gibson CM, Murphy SA, Bonaca MP, Morrow DA, Mega JL: Clinical features, use of evidence-based therapies, and cardiovascular outcomes among patients with chronic kidney disease following non-ST-elevation acute coronary syndrome. Clin Cardiol 37: 350–356, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saad M, Karam B, Faddoul G, Douaihy YE, Yacoub H, Baydoun H, Boumitri C, Barakat I, Saifan C, El-Charabaty E, Sayegh SE: Is kidney function affecting the management of myocardial infarction? A retrospective cohort study in patients with normal kidney function, chronic kidney disease stage III-V, and ESRD. Int J Nephrol Renovasc Dis 9: 5–10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB: Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 137: 555–562, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Szummer K, Lundman P, Jacobson SH, Schön S, Lindbäck J, Stenestrand U, Wallentin L, Jernberg T; SWEDEHEART : Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation 120: 851–858, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Szummer K, Lundman P, Jacobson SH, Schön S, Lindbäck J, Stenestrand U, Wallentin L, Jernberg T; SWEDEHEART : Relation between renal function, presentation, use of therapies and in-hospital complications in acute coronary syndrome: Data from the SWEDEHEART register. J Intern Med 268: 40–49, 2010 [DOI] [PubMed] [Google Scholar]
- 55.O’Hare AM, Bertenthal D, Sidawy AN, Shlipak MG, Sen S, Chren MM: Renal insufficiency and use of revascularization among a national cohort of men with advanced lower extremity peripheral arterial disease. Clin J Am Soc Nephrol 1: 297–304, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Charytan DM, Wallentin L, Lagerqvist B, Spacek R, De Winter RJ, Stern NM, Braunwald E, Cannon CP, Choudhry NK: Early angiography in patients with chronic kidney disease: A collaborative systematic review. Clin J Am Soc Nephrol 4: 1032–1043, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaw C, Nitsch D, Lee J, Fogarty D, Sharpe CC: Impact of an early invasive strategy versus conservative strategy for unstable angina and non-ST elevation acute coronary syndrome in patients with chronic kidney disease: A systematic review. PLoS One 11: e0153478, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reinecke H, Fobker M, Wellmann J, Becke B, Fleiter J, Heitmeyer C, Breithardt G, Hense HW, Schaefer RM: A randomized controlled trial comparing hydration therapy to additional hemodialysis or N-acetylcysteine for the prevention of contrast medium-induced nephropathy: The Dialysis-versus-Diuresis (DVD) Trial. Clin Res Cardiol 96: 130–139, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Cruz DN, Perazella MA, Bellomo R, Corradi V, de Cal M, Kuang D, Ocampo C, Nalesso F, Ronco C: Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: A systematic review. Am J Kidney Dis 48: 361–371, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Marenzi G, Lauri G, Campodonico J, Marana I, Assanelli E, De Metrio M, Grazi M, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL: Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med 119: 155–162, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Heinrich MC, Häberle L, Müller V, Bautz W, Uder M: Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: Meta-analysis of randomized controlled trials. Radiology 250: 68–86, 2009 [DOI] [PubMed] [Google Scholar]
- 62.European Society of Urogenital Radiology : European Society of Urogenital Radiology home, 2021. Available at: https://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
- 63.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr., Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Lincoff AM, Philippides GJ, Zidar JP; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines : 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127: e663–e828, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC: Meta-analysis: Effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med 148: 284–294, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Gonzales DA, Norsworthy KJ, Kern SJ, Banks S, Sieving PC, Star RA, Natanson C, Danner RL: A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: Unsupervised clustering to resolve heterogeneity. BMC Med 5: 32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kshirsagar AV, Poole C, Mottl A, Shoham D, Franceschini N, Tudor G, Agrawal M, Denu-Ciocca C, Magnus Ohman E, Finn WF: N-acetylcysteine for the prevention of radiocontrast induced nephropathy: A meta-analysis of prospective controlled trials. J Am Soc Nephrol 15: 761–769, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin SS, Conner TA, Chertow GM, Bhatt DL, Shunk K, Parikh CR, McFalls EO, Brophy M, Ferguson R, Wu H, Androsenko M, Myles J, Kaufman J, Palevsky PM; PRESERVE Trial Group : Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med 378: 603–614, 2018 [DOI] [PubMed] [Google Scholar]
- 68.Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H: Prevention of contrast media-associated nephropathy: Randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med 162: 329–336, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, Goel P, Hewett J: A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract 93: C29–C34, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van Moore A, Simonton CA 3rd, Rittase RA, Norton HJ, Kennedy TP: Prevention of contrast-induced nephropathy with sodium bicarbonate: A randomized controlled trial. JAMA 291: 2328–2334, 2004 [DOI] [PubMed] [Google Scholar]
- 71.Brar SS, Hiremath S, Dangas G, Mehran R, Brar SK, Leon MB: Sodium bicarbonate for the prevention of contrast induced-acute kidney injury: A systematic review and meta-analysis. Clin J Am Soc Nephrol 4: 1584–1592, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho KM, Morgan DJ: Use of isotonic sodium bicarbonate to prevent radiocontrast nephropathy in patients with mild pre-existing renal impairment: A meta-analysis. Anaesth Intensive Care 36: 646–653, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Hoste EA, De Waele JJ, Gevaert SA, Uchino S, Kellum JA: Sodium bicarbonate for prevention of contrast-induced acute kidney injury: A systematic review and meta-analysis. Nephrol Dial Transplant 25: 747–758, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Kanbay M, Covic A, Coca SG, Turgut F, Akcay A, Parikh CR: Sodium bicarbonate for the prevention of contrast-induced nephropathy: A meta-analysis of 17 randomized trials. Int Urol Nephrol 41: 617–627, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Navaneethan SD, Singh S, Appasamy S, Wing RE, Sehgal AR: Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: A systematic review and meta-analysis. Am J Kidney Dis 53: 617–627, 2009 [DOI] [PubMed] [Google Scholar]
- 76.Ozcan EE, Guneri S, Akdeniz B, Akyildiz IZ, Senaslan O, Baris N, Aslan O, Badak O: Sodium bicarbonate, N-acetylcysteine, and saline for prevention of radiocontrast-induced nephropathy. A comparison of 3 regimens for protecting contrast-induced nephropathy in patients undergoing coronary procedures. A single-center prospective controlled trial. Am Heart J 154: 539–544, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Recio-Mayoral A, Chaparro M, Prado B, Cózar R, Méndez I, Banerjee D, Kaski JC, Cubero J, Cruz JM: The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: The RENO Study. J Am Coll Cardiol 49: 1283–1288, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Briguori C, D’Amore C, De Micco F, Signore N, Esposito G, Visconti G, Airoldi F, Signoriello G, Focaccio A: Left ventricular end-diastolic pressure versus urine flow rate-guided hydration in preventing contrast-associated acute kidney injury. JACC Cardiovasc Interv 13: 2065–2074, 2020 [DOI] [PubMed] [Google Scholar]
- 79.Mattathil S, Ghumman S, Weinerman J, Prasad A: Use of the RenalGuard system to prevent contrast-induced AKI: A meta-analysis. J Interv Cardiol 30: 480–487, 2017 [DOI] [PubMed] [Google Scholar]
- 80.Kidney Disease Improving Global Outcomes (KDIGO) : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 81.Bragadottir G, Redfors B, Ricksten SE: Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury--true GFR versus urinary creatinine clearance and estimating equations. Crit Care 17: R108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brar SS, Aharonian V, Mansukhani P, Moore N, Shen AY, Jorgensen M, Dua A, Short L, Kane K: Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: The POSEIDON randomised controlled trial. Lancet 383: 1814–1823, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Martino F, Amici G, Godi I, Baretta M, Biasi C, Carta M, Corradi V, De Cal M, Knust M, Tamayod C, Varotto A, Iannucci G, Giavarina D, Savastano S, Ronco C: Gadolinium-based contrast media exposure and the possible risk of subclinical kidney damage: A pilot study. Int Urol Nephrol 53: 1883–1889, 2021 [DOI] [PubMed] [Google Scholar]
- 84.Galan A, Cowper SE, Bucala R: Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 18: 614–617, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Idée JM, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C: Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: A review. Fundam Clin Pharmacol 20: 563–576, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Wagner B, Drel V, Gorin Y: Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol 311: F1–F11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE: Nephrogenic systemic fibrosis: Clinicopathological definition and workup recommendations. J Am Acad Dermatol 65: 1095–1106.e7, 2011 [DOI] [PubMed] [Google Scholar]
- 88.ACR Committee on Drugs and Contrast Media : ACR manual on contrast media, 2021. Available at: https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf.
- 89.Woolen SA, Shankar PR, Gagnier JJ, MacEachern MP, Singer L, Davenport MS: Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: A systematic review and meta-analysis. JAMA Intern Med 180: 223–230, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weinreb JC, Rodby RA, Yee J, Wang CL, Fine D, McDonald RJ, Perazella MA, Dillman JR, Davenport MS: Use of intravenous gadolinium-based contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology 298: 28–35, 2021 [DOI] [PubMed] [Google Scholar]
- 91.Joffe P, Thomsen HS, Meusel M: Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol 5: 491–502, 1998 [DOI] [PubMed] [Google Scholar]
- 92.Levy EM, Viscoli CM, Horwitz RI: The effect of acute renal failure on mortality. A cohort analysis. JAMA 275: 1489–1494, 1996 [PubMed] [Google Scholar]
- 93.Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, Leon MB: The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol 36: 1542–1548, 2000 [DOI] [PubMed] [Google Scholar]
- 94.Shema L, Ore L, Geron R, Kristal B: Contrast-induced nephropathy among Israeli hospitalized patients: incidence, risk factors, length of stay and mortality. Isr Med Assoc J 11: 460–464, 2009 [PubMed] [Google Scholar]
- 95.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes Jr DR: Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264, 2002 [DOI] [PubMed] [Google Scholar]
- 96.Harjai KJ, Raizada A, Shenoy C, Sattur S, Orshaw P, Yaeger K, Boura J, Aboufares A, Sporn D, Stapleton D: A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol 101: 812–819, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Roghi A, Savonitto S, Cavallini C, Arraiz G, Angoli L, Castriota F, Bernardi G, Sansa M, De Servi S, Pitscheider W, Danzi GB, Reimers B, Klugmann S, Zaninotto M, Ardissino D: Impact of acute renal failure following percutaneous coronary intervention on long-term mortality. J Cardiovasc Med (Hagerstown) 9: 375–381, 2008 [DOI] [PubMed] [Google Scholar]
- 98.Brown JR, Malenka DJ, DeVries JT, Robb JF, Jayne JE, Friedman BJ, Hettleman BD, Niles NW, Kaplan AV, Schoolwerth AC, Thompson CA: Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: Insights from the Dartmouth Dynamic Registry. Catheter Cardiovasc Interv 72: 347–354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]