Abstract
Fibrosis contributes to ~45% of deaths in western countries. In chronic liver disease, fibrosis is a major factor determining outcomes, but efficient anti-fibrotic therapies are lacking. Although platelet-derived growth factor and transforming growth factor-β constitute key fibrogenic mediators, they do not account for the well-established link between cell death and fibrosis in the liver. Here, we hypothesized that damage-associated molecular patterns (DAMPs) may link epithelial cell death to fibrogenesis in the injured liver. DAMP receptor screening identified purinergic receptor P2Y14 among several candidates as highly enriched in hepatic stellate cells (HSCs), the main fibrogenic cell type of the liver. Conversely, P2Y14 ligands uridine 5'-diphosphate (UDP)-glucose and UDP-galactose were enriched in hepatocytes and were released upon different modes of cell death. Accordingly, ligand-receptor interaction analysis that combined proteomic and single-cell RNA-sequencing data revealed P2Y14 ligands and P2Y14 receptor as a link between dying cells and HSCs, respectively. Treatment with P2Y14 ligands or coculture with dying hepatocytes promoted HSC activation in a P2Y14-dependent manner. P2Y14 ligands activated extracellular signal-regulated kinase (ERK) and Yes-associated protein (YAP) signaling in HSCs, resulting in ERK-dependent HSC activation. Global and HSC-selective P2Y14 deficiency attenuated liver fibrosis in multiple mouse models of liver injury. Functional expression of P2Y14 was confirmed in healthy and diseased human liver and human HSCs. In conclusion, P2Y14 ligands and their receptor constitute a profibrogenic DAMP pathway that directly links cell death to fibrogenesis.
One Sentence Summary:
P2Y14 and its ligands constitute a DAMP receptor system linking cell death to fibrosis in the liver.
Introduction
Chronic liver disease (CLD) is estimated to cause nearly two million deaths per year worldwide (1). Like in other organ systems (2,3), fibrosis is a main contributor to mortality in CLD (4-7) . However, there are currently no U.S. Food and Drug Administration (FDA)-approved anti-fibrotic therapies and an urgent need to fill this gap (3,8). Cell death is strongly associated with the development of liver fibrosis, but mechanisms that link cell death to fibrogenesis are only poorly understood (9). One key feature of cell death is the release of damage-associated molecular patterns (DAMPs), thereby alerting inflammatory cells and triggering sterile inflammation as well as the removal of cellular debris (10). However, the effects of DAMPs on non-inflammatory cells in wound healing and fibrosis remain largely unexplored. A recent study characterized the apoptotic metabolite secretome, with a subset of these being actively secreted, and many of them actively modulating a wide range of responses such as suppression of inflammation, cell proliferation, and wound healing (11). However, it is not known whether the DAMP concept is also applicable to wound healing and fibrogenesis. Here, we hypothesized that fibrogenic cells harbor DAMP receptors, thus endowing them with the ability to sense injury in their environment and initiate wound healing as response to cell death. To explore the contribution of DAMPs to fibrogenesis, we focused on the liver as model system for several reasons: liver fibrosis is becoming an increasing health problem (12); liver fibrosis represents the key determinant of mortality (4,13-16) in non-alcoholic fatty liver disease; cell death is strongly associated with liver fibrosis and hence considered a key driver of fibrosis progression (9,17); and the liver contains hepatic stellate cells (HSCs) as a well-defined fibrogenic cell population (18) that gives rise to the great majority of extracellular matrix-producing fibroblasts. HSCs can be isolated to high purity in a quiescent state, allowing precise testing of DAMP effects on fibrogenic cell activation. Several DAMPs have been found to regulate sterile inflammation in injured livers (for example ATP, formyl peptides, mitochondrial DNA, and high-mobility group box 1 (HMGB1) (11,19-21)) and may trigger HSC activation in vitro (21). However, there is controversy surrounding the role of HMGB1 in fibrosis (20,22,23), and insufficient understanding about their role in HSC activation in vivo and the involved receptor systems. Moreover, it has been suggested that HSCs themselves can phagocytose apoptotic bodies, resulting in increased HSC activation (24,25). In summary, receptors that endow HSCs with the ability to recognize dying hepatocytes or DAMPs from dying hepatocytes could provide much needed targets for the treatment of fibrosis in the liver and other organs. Here, we identified P2Y14 and its ligands UDP-glucose, UDP-galactose and UDP-glucuronic acid as DAMP-DAMP receptor system that allows HSCs to sense hepatocyte cell death and respond to liver injury via P2Y14-dependent fibrogenic activation. Our study reveals P2Y14 and its ligands as a fibroblast-selective DAMP-DAMP receptor system that links epithelial cell death to fibrogenesis and thus represents a potential therapeutic target.
RESULTS
P2Y14 ligands and P2Y14 are a DAMP-DAMP receptor system linking hepatocytes to HSCs
Based on previous studies suggesting a role for apoptotic cell engulfment in HSC activation (24,25), we investigated the role of TAM receptors AXL and MerTK on HSCs in the development of liver fibrosis. TAM receptors have a key role in the recognition of apoptotic cells by phagocytes via phosphatidylserine-binding molecules growth arrest-specific 6 (Gas6) or Protein S (26,27). scRNA-seq revealed Axl and MerTK mRNA expression in HSCs (Fig. S1). Despite efficient deletion by LratCre, we did not observe a significant (P>=0.05) reduction of liver fibrosis, as determined by Pircrosirius Red-positive and αSMA-positive area, in carbontetrachloride (CCl4) or choline-deficient L-amino-defined high-fat diet (CDAA) mouse liver fibrosis models with HSC-selective deletion of Axl and MerTK (Fig. S1A-D). Next, we tested the alternative hypothesis that hepatocyte DAMPs trigger fibrogenesis in HSCs. For this, we employed a contact-independent co-culture system in which HSCs were exposed to dying hepatocytes. The presence of dying hepatocytes dose-dependently promoted HSC activation, as determined by an αSMA-RFP reporter, αSMA immunoblotting, and quantitative real-time PCR (Fig. 1A-B). Based on our hypothesis that high expression of DAMP receptors may endow HSCs with the ability to directly respond to liver injury, we compared gene expression profiles between ultrapure HSCs and whole liver by bulk RNA-seq. Among the top 25 HSC-enriched receptors, we found two candidate DAMP receptors, S1PR3 and P2Y14 (Fig. 1C). Further analysis as well as confirmatory qPCR of HSC-enriched candidate DAMP receptors revealed three DAMP receptors, Adora2a (44-fold), P2ry14 (70-fold) and S1pr3 (107-fold), that were enriched to a similar degree as HSC markers Des (61-fold) and Lrat (78-fold) as well as DAMP receptors Ager (25-fold), Lpar1 (8-fold), Lpar4 (11-fold) and Tlr4 (12-fold) with moderate enrichment in HSCs (Fig. 1C, Fig. S2A). These data were additionally confirmed by scRNA-seq, revealing only minimal or no enrichment of DAMP receptors such as Adora2b, Cd24a, Clec7a, Fpr1, Lpar1, Lpar4, Nlrp3, S1pr1, Siglecg, Tlr2, and Tlr9 in HSCs in normal or fibrotic mouse liver (Fig. 1C; Fig. S2A-E). We subsequently tested the role of the most HSC-enriched receptors in HSC activation and in CCl4-induced fibrogenesis in vivo. As TLR4 and RAGE (encoded by Ager) are activated by HMGB1, a DAMP with important functions in hepatocytes (19,20), we first tested whether HMGB1 and its receptors may constitute a profibrogenic DAMP system. However, HMGB1 did not promote HSC activation (Fig. 1D). Moreover, RAGE knockout, TLR4ΔHSC and HMGB1-deleted mice did not display amelioration of CCl4-induced fibrosis (Fig. 1E-F, Fig. S3A-B, Fig. S4A), consistent with previously published studies on HMGB1 in hepatocytes in four different fibrosis models (20,22) and CCl4-induced fibrosis in mice with TLR4 deletion in HSCs (23), but contradicting one study on the role of HMGB1/RAGE (23). Activation of ADORA2A, a receptor with suggested roles in HSC activation and liver fibrosis (28,29), by agonist CGS21680 reduced HSC activation (Fig. 1D), but CCl4-induced liver fibrosis was unaltered in ADORA2-deficient mice (Fig. 1G, Fig. S4B). S1P, which activates multiple S1P receptors, promoted HSC activation (Fig. 1D), but CCl4-induced liver fibrosis was not ameliorated in S1PR3-deficient mice (Fig. 1H). As RAGE, TLR4, ADORA2A, and S1PR3 did not operate as profibrogenic DAMP receptors in HSCs or CCl4-induced fibrosis, we next investigated whether other ligand-receptor pairs could mediate the effects of dying cells on HSCs, using a method termed Proteomics-scRNA-seq Ligand-Receptor Interaction (PSLRI) that integrates proteomic data from liquid chromatography–mass spectrometry (LC/MS) with scRNA-seq data, and then used the statistical framework of CellPhoneDB (30) for the inference of ligand-receptor interactions. PSLRI showed that ligands released from dying cells (11), identified by LC/MS, interacted with receptors on different liver cells, identified by scRNA-seq. Although the LCMS datasets likely did not encompass the full spectrum of mediators released from dying cells, our analysis revealed HSCs as the cell population most strongly interacting with ligands released from dying cells, and uncovered UDP-glucose, UDP-galactose, and their receptor P2Y14 as ligand-receptor pair linking dying cells and HSCs (Fig. 2A, Fig. S5). P2Y14, the DAMP receptor candidate with the second highest enrichment in HSC in our RNA-seq analysis (Fig. S2A), is a purinergic receptor with functions in dendritic cells (DCs), neutrophils, macrophages and smooth muscle cells (31-36) but unknown DAMP receptor function. Reflecting the high expression of P2Y14 on DCs (31,32), we also observed interactions between dying cells and DCs in our PSLRI analysis (Fig. 2A, Fig. S5). scRNA-seq demonstrated strong enrichment of P2Y14 in HSCs from normal and CCl4-treated mouse liver and, as expected, also in the small cluster of DCs (Fig. 2B). Likewise, qPCR demonstrated several magnitudes higher P2ry14 mRNA expression in HSCs than in hepatocytes, Kupffer cells, cholangiocytes, and liver sinusoidal endothelial cells (Fig. 2C, Fig. S6), which was further confirmed by immunohistochemistry and RNA-scope in healthy, CCl4- and bile duct ligation (BDL)-treated mouse liver as well as by immunofluorescence in cultured HSCs showing strong or almost exclusive expression in HSCs (Fig. 2D-E, Fig. S7A-C). Other purinergic receptors P2Y1, P2Y2, P2Y4, P2Y6, P2Y10, P2Y12, P2Y13 as well as P2X2, P2X3, P2X4, P2X5, P2X6, P2X7 were not enriched in HSCs in normal or fibrotic mouse liver (Fig. S8A-E). Only one other purinergic receptor, P2X1, showed enrichment in HSCs in bulk RNA-seq but was barely detectable by scRNA-seq (Fig. S8D-E). Conversely, P2Y14 ligands UDP-glucose and UDP-galactose were strongly enriched in hepatocytes (Fig. 2F), consistent with their functions in many key metabolic pathways in hepatocytes such as glycogen, glycolipid, and glycoprotein synthesis, and glucuronidation. To further tie these P2Y14 ligands to hepatocyte death and potential crosstalk to HSCs following liver injury, we determined their release from dying hepatocytes. In TNF- and Fas-induced apoptosis, we observed a strong release of UDP-glucose, and a weaker but statistically significant increase in CCl4-induced necrosis, erastin-induced ferroptosis, and RIKP3 activation-induced necroptosis (Fig. 2G) and, to a lesser degree, of UDP-galactose (Fig. S9A-D). Together with our PSLRI, IHC, bulk RNA-seq and scRNA-seq data, these results suggest hepatocyte-enriched P2Y14 ligands and HSC-enriched P2Y14 as candidate DAMP ligand-receptor system that might link cell death to HSC activation in the injured liver.
Figure 1. Contribution of DAMPs and DAMP receptors in the promotion of HSC activation and liver fibrosis.
A-B. 2x105 primary quiescent mouse HSCs from BALB/c or αSMA-RFP transgenic mice were co-cultured with increasing amounts of dying primary mouse hepatocytes, followed by fluorescent microscopy, αSMA Western blot or qPCR for Acta2 (n=3 technical replicates). C. Gene expression in primary mouse HSCs (n=4 biological replicates) and untreated whole liver (n=3 biological replicates) from BALB/c mice was compared by RNA-seq; shown are the top 25 enriched receptor genes (left panel). Expression of P2ry14 and other candidate DAMP receptors in mouse HSCs was confirmed by qPCR (n=5 HSC, n=5 livers from the same mice, right panel, biological replicates). D. Primary mouse HSCs from BALB/c mice were treated with recombinant HMGB1, Adora2a agonist CGS 21680, or sphingosine-1-phosphate followed by Western blot for αSMA (n=1 per condition). E-H. AGER knockout (n=10 WT and n=12 KO) (E), TLR4ΔHSC (n=5 TLR4fl/fl and n=5 TLR4ΔHSC) (F), ADORA2A knockout (n=9 WT and n=10 KO) (G), and S1PR3 knockout mice (n=11 WT and n=13 KO) (H) and their respective controls were treated with six injections of CCl4 (0.5 ml/kg), followed by analysis of the Picrosirius Red area. Data are shown as means ± SEM. Scale bar 200 μm.
Figure 2. Hepatocyte-enriched P2Y14 ligands and HSC-enriched P2Y14 constitute a DAMP-DAMP receptor system that may link HSCs to cell death.
A. Determination of interactions between ligands released from dying cells and receptors on liver cell populations by PSLRI. Plot shows the number of interactions with dying cells (upper panel) and specific ligand-receptor interactions (lower panel) using scRNA-seq data from normal mouse liver. B. Uniform Manifold Approximation and Projections (UMAPs) of scRNA-seq from of normal and fibrotic mouse liver showing P2ry14-expressing cells. C. P2ry14 mRNA was determined in different primary cell populations isolated from C57BL/6J mice (HSC, hepatocytes (Hep), Kupffer cells (KC), cholangiocytes (Cho) and liver sinusoidal endothelial cells (LSEC)) by qPCR (n=3-4 biological replicates per cell population). D-E. P2Y14 expression was determined by immunohistochemistry (D) or RNAscope (E) in mice co-expressing LratCre and Cre reporter tdTomato (D) or ZsGreen1 (E). F. UDP-glucose and UDP-galactose were determined by mass-spectrometry in primary hepatocytes and non-parenchymal cells (NPC) from C57BL/6J mice (n=5 Hep, n=3 NPC, biological replicates). G. UDP-glucose was determined in the supernatants of primary hepatocytes (isolated from C57BL/6J mice) after induction of different forms of cell death (apoptosis, necrosis, ferroptosis, and necroptosis, n=3 technical replicates). Data are shown as means ± SEM. Scale bar 20 μm.
P2Y14 is a profibrogenic receptor that links hepatocyte death to HSC activation
To functionally test this hypothesis, we next treated primary quiescent murine HSCs with P2Y14 ligands and determined their activation. Consistent with our hypothesis, treatment of HSCs with P2Y14 ligands UDP-glucose, UDP-galactose, and UDP-glucuronic acid induced upregulation of αSMA protein as well as Acta2 mRNA (Fig. 3A-B). As our experiments on HSC activation and the literature show roughly similar potency of all three P2Y14 ligands, with slightly higher potency of UDP-glucose and UDP-galactose than of UDP-glucuronic at mouse P2Y14 (37), we used UDP-glucose, the prototypic and best-characterized P2Y14 ligand, for most subsequent experiments. UDP-glucose induced a statistically significant but weak upregulation of Col1a1, as well as upregulation of fibrogenic genes Lox, Col4a1, and Col8a1 (Fig. 3B, Fig. S10A). The HSC-activating effect of UDP-glucose was dose-dependent and observed in a range of 0.1 to 100 μM (Fig. 3C). To exclude that HSC activation in response to UDP-glucose may have resulted from the activation of other receptors or by contaminants, we exposed P2Y14-deficient HSCs to UDP-glucose. Whereas TGFβ-mediated αSMA induction was similar in WT and P2Y14-deficient HSCs, P2Y14 deficiency prevented UDP-glucose-induced αSMA upregulation (Fig. 3D). To prove the underlying hypothesis that P2Y14 ligands are contributors to HSC activation by dying hepatocytes and therefore represent true profibrogenic DAMPs, we compared WT and P2Y14-deficient HSC activation after co-culture with dying hepatocytes, where we observed a strong reduction of HSC activation in P2Y14-deficient HSCs (Fig. 3E-G). UDP-glucose and TGFβ or angiotensin II (AngII) exerted additive effects on HSC activation (Fig. S10B-F), suggesting that P2Y14 and TGFβ or AngII activate HSCs through distinct pathways. In vivo, we observed an increase of P2Y14 mRNA in CCl4-, BDL, and Mdr2 knockout (Mdr2ko)-induced liver fibrosis (Fig. 3H). Moreover, P2Y ligands were increased in livers from Mdr2ko mice, a model of biliary injury, unaltered in BDL livers, non-significantly decreased in liver from CCl4-treated livers, and highly decreased in acetaminophen-induced liver injury (Fig. 3I, Fig. S11A-B). Decreased UDP-glucose and UDP-galactose have been described in livers from acetaminophen-treated rats (38) and reflect the utilization of P2Y14 ligands in important metabolic and detoxification pathways such as glucuronidation (39). Last, we found that P2Y14 expression, although increased in fibrotic liver, was reduced on a per-cell level in activated HSCs from CCl4-induced chronic liver injury, as shown in scRNA-seq data (Fig. 3J), suggesting negative feedback in chronic liver injury. However, whereas UDP-glucose treatment did not affect P2ry14 abundance in quiescent HSCs, treatment with TGFβ resulted in P2ry14 upregulation (Fig. 3K). In summary, P2Y14 and its ligands fulfill several criteria to constitute a profibrogenic DAMP/DAMP receptor system, namely enrichment of DAMPs within the epithelial compartment and release from dying hepatocytes, enrichment and functional expression of DAMP receptors in fibrogenic cells, as well as promotion of fibrogenesis by DAMPs released from injured hepatocytes. Our data also indicate that this system is active in multiple types of cell death, namely apoptosis, necroptosis, and necrosis, and that it dynamically responds with negative and positive regulation of P2Y14 in HSCs and the chronically injured liver.
Figure 3. P2Y14 is a profibrogenic DAMP receptor that mediates HSC activation in response to hepatocyte death.
A-C. Primary mouse HSCs (from BALB/c mice) were treated with UDP-glucose, UDP-galactose, or UDP-glucuronic acid at 10 μM (A-B) or indicated concentrations (C), followed by analysis of αSMA protein 48h later or mRNA expression 24h later (n=1-5 technical replicates /condition). D. Primary mouse HSCs from WT or P2Y14ko mice (from C57Bl/6 mice) were treated with UDP-glucose (10 μM) or TGFβ (2.5 ng/ml) for 48h, followed by αSMA Western blot (n=3 technical replicates). E-G. Primary WT or P2Y14ko αSMA-RFPpos HSCs (BALB/c background) were co-cultured with dying hepatocytes for 48h or treated with TGFβ (2.5 ng/ml) for 48h, followed by analysis of αSMA protein (E), αSMA-RFP (F) or Acta2 mRNA (G) (n=2-3 technical replicates/condition). H-I. Expression of Desmin or P2ry14 mRNA (H) or UDP-glucose content (I) were determined in livers from different injury models (n=3-8 animals per group). J. P2ry14 in HSCs from normal and CCl4-injured liver by scRNA-seq (n=1 mouse/condition). K. P2ry14 qPCR in quiescent HSCs treated with UDP-glucose or TGFβ (n=5 technical replicates/condition). Data are shown as means ± SEM. Scale bar 50 μm.
ERK mediates profibrogenic effects downstream of P2Y14
To determine mechanisms by which P2Y14 activation triggers HSC activation, we first performed RNA-seq, comparing UDP-glucose-stimulated HSCs to untreated HSCs. Gene set enrichment analysis revealed YAP as the top activated pathway in UDP-glucose-treated HSCs (Fig. 4A, Fig. S12A). Accordingly, six of the most significant top 10 genes regulated by UDP-glucose were YAP target genes, including Ctgf, Ankrd1, Serpine1, Cyr61, Palld, and Bmp10 (Fig. 4A). This was confirmed by determination of YAP nuclear translocation and qPCR of YAP target genes in UDP-glucose-stimulated HSCs (Fig. 4B-C). With previous studies having implicated YAP in HSC activation and liver fibrosis (40,41), we carefully investigated its role in UDP-glucose-induced HSC activation. However, conditionally deleting Yap via LratCre or inhibiting YAP using SuperTDU did not affect UDP-glucose-induced HSC activation (Fig. 4D-E, Fig. S12B). As second screening approach, we employed a phospho-pathway array, which revealed strong activation of ERK, a known downstream effector of P2Y14 (42), but no major effect on other pathways (Fig. 4F). Data were confirmed by Western blot, demonstrating potent ERK activation by all three P2Y14 ligands starting at 0.1 μM and as early as 7.5 minutes after stimulation (Fig. 4G,H). UDP-glucose-induced, but not PDGF-induced, ERK phosphorylation was completely abolished in P2Y14-deficient HSCs (Fig. S13). Consistent with the enrichment of P2Y14 on HSCs, there was no ERK activation in UDP-glucose-stimulated hepatocytes, Kupffer cells, and LSECs (Fig. 4I). To determine the role of ERK in P2Y14-mediated HSC activation, we pretreated HSCs with MEK inhibitor PD98059 followed by UDP-glucose stimulation. PD98059 suppressed UDP-glucose-induced upregulation of αSMA protein and Acta2 mRNA expression (Fig. 4J-K), underpinning the known profibrogenic role of this pathway in HSCs and liver fibrosis (43). Thus, it appears that UDP-induced activation of ERK, a powerful profibrogenic pathway in HSCs (44), but not upregulation of YAP is key to its profibrogenic effect.
Figure 4. P2Y14 promotes HSC activation via ERK and YAP.
A. RNA-seq and gene set enrichment pathway analysis and a heatmap of the top 6 genes in primary BALB/c mouse HSCs treated with UDP-glucose (10 μM) for 24h (n=3 technical replicates /group). B-C. qPCR for YAP target genes after 24h (B) or immunofluorescence for YAP after 2h (C) of UDP-glucose (10 μM) treatment (n=1-3 technical replicates /condition). D. qPCR for Acta2 in YAPfl/fl or YAPΔHSC HSC, treated with 10 μM UDP-glucose (n=2-4 technical replicates /condition). E. Yap deletion in mouse HSCs from 6-times CCl4-treated mice (n=1 per condition). F. Phospho-pathway screen in mouse HSCs treated with 10 μM UDP-glucose for 15 minutes. G-I. Western blot for phospho- and total ERK in (G) mouse HSCs treated with various concentrations of UDP-glucose, UDP-galactose or UDP-glucuronic acid for 15 minutes, (H) mouse HSCs treated with 10 μM UDP-glucose for various times (n=1 per concentration or time point), or (I) primary mouse HSCs, hepatocytes, macrophages and LSECs treated with 100 μM UDP-glucose. J-K. Effect of MEK inhibitor PD98059 on UDP-glucose-induced αSMA expression after 48h (J) or Acta2 mRNA after 24h treatment (K) (n=3 technical replicates/condition). Data are shown as means ± SEM. Scale bar 20 μm.
P2Y14 promotes toxic and biliary liver fibrosis
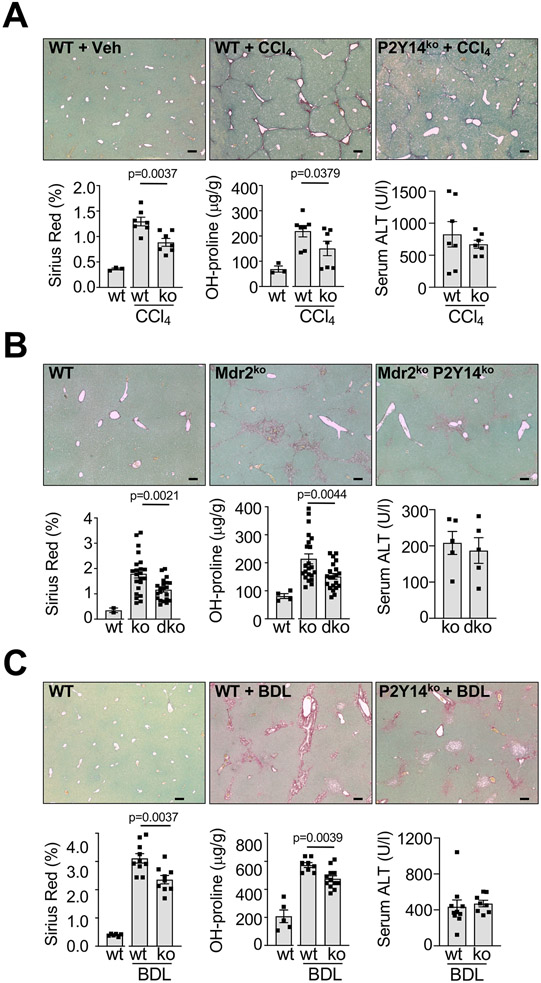
Next we sought to confirm the relevance of P2Y14 and its downstream targets for fibrogenesis in vivo. In the CCl4 fibrosis model, P2Y14-deficient mice displayed a 31.7% reduction in fibrosis, as determined by Picrosirius Red staining and confirmed by hydroxyproline measurement and αSMA immunohistochemistry (Fig. 5A, Fig. S14A). Similar reductions of Picrosirius Red were found in Mdr2ko mice (Fig. 5B, Fig. S14B), a model mimicking human cholestatic liver fibrosis caused by MDR3 mutations (45). These data were confirmed in two additional fibrosis models, induced by BDL (Fig. 5C) or 0.1% DDC diet (Fig. S14C). Because other cell populations such as DCs also expressed P2Y14 and might respond to dying cells via P2Y14, we next investigated fibrosis in bone marrow-chimeric mice that expressed P2Y14 on all cells except for bone marrow-derived cells. There was no reduction of CCl4-induced fibrosis in mice with P2Y14-deficient bone marrow (Fig. S15), thus excluding a role for P2Y14 on DC and other bone marrow-derived cell types in liver fibrogenesis. To provide positive evidence for the role of P2Y14 on HSCs in liver fibrogenesis, we generated mice with HSC-selective P2Y14 deletion (P2Y14ΔHSC) by crossing P2Y14 floxed mice with LratCre transgenic mice (18), which achieved nearly 97% reduction of P2ry14 mRNA in HSCs (Fig. S16A). Consistent with our results in global P2Y14 knockout mice, P2Y14ΔHSC mice displayed a 20% reduction in CCl4-induced fibrosis as determined by picrosirius red (P=0.0146) but an insignificant reduction in αSMA staining (P=0.1767) (Fig. S16B). A significant reduction of picrosirius red and αSMA staining was observed in P2Y14ΔHSC mice fed a CDAA diet (Fig. S16C).
Figure 5. P2Y14 promotes toxic and biliary liver fibrosis.
A-C. Liver fibrosis and injury were determined by analysis of the Picrosirius Red area, hepatic hydroxyproline content, and serum ALT in (A) WT (n=7) and P2Y14-knockout (n=7) mice treated with 6 injections of CCl4 (0.5 ml/kg), (B) in male and female Mdr2ko mice (n=22), and P2Y14/Mdr2 double knockout mice (n=21) or in (C) WT (n=10) and P2Y14-knockout (n=9) mice that underwent bile duct ligation (BDL) for 16 days. Data are shown as means ± SEM. Scale bar 200 μm.
P2Y14 is functionally expressed on human HSCs
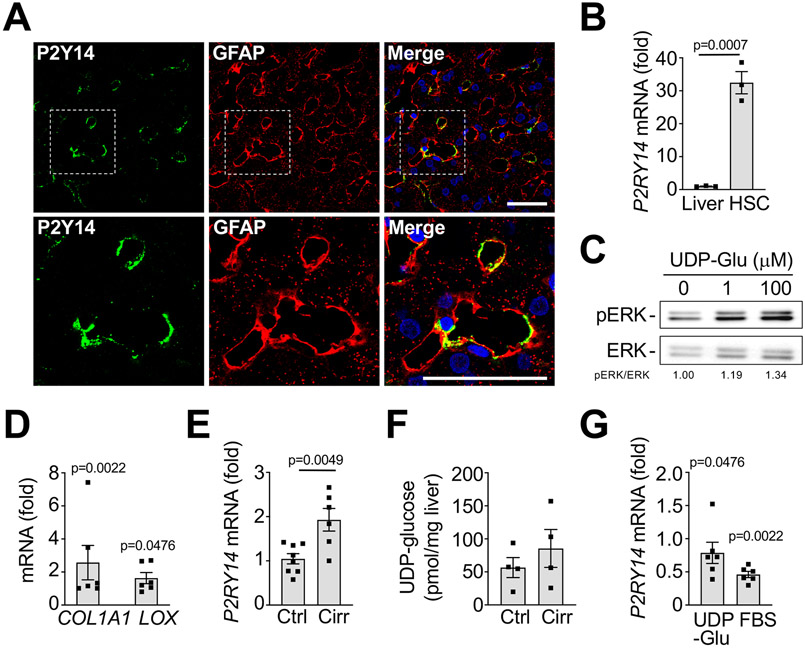
Last, we sought to confirm human relevance of this profibrogenic DAMP-DAMP receptor system. We analyzed P2Y14 expression and functions in human HSCs. Similar to our murine data, we found that P2RY14 mRNA was enriched in human HSCs and co-staining of P2Y14 with GFAP confirmed this data in human liver (Fig.6A-B, Fig. S17). Stimulation of primary human HSCs with P2Y14 ligands promoted ERK phosphorylation and incubation with UDP-glucose induced a substantial increase of COL1A1 and LOX mRNA (Fig. 6C-D), We found an increase of P2Y14 mRNA in patients with cirrhosis (Fig. 6E), whereas P2Y14 ligands UDP-glucose, UDP-galactose and UDP-glucuronic acid did not show significant differences between normal and cirrhotic livers (Fig. 6F, Fig. S18A-B), thus confirming the presence of these profibrogenic ligands in relevant settings. Furthermore, P2RY14 mRNA decreased in human HSCs activated with 10% FBS or treated with UDP-glucose (Fig. 6G), thus suggesting negative feedback regulation, which differed from our data in mouse HSCs. Together, these data confirm the presence of P2Y14 ligands in the liver; the enrichment of P2Y14 in HSCs; its contribution to fibrogenesis and dynamic regulation of P2Y14 expression in human settings. Our finding that P2Y14 is enriched in kidney and lung pericytes suggests a more universal role of P2Y14 in fibrogenesis (Fig.S19), which requires further investigation.
Figure 6. P2Y14 is enriched in human HSCs and promotes fibrogenesis.
A. Immunohistochemistry for P2Y14 and HSC marker glial fibrillary acidic protein (GFAP) in normal human liver. B. P2RY14 mRNA was determined by qPCR in human liver and primary unplated human HSCs (n=3 biological replicates each). C. Primary human HSCs were treated with 1 or 100 μM UDP-glucose for 15 minutes, followed by Western blot for phospho- and total ERK. D. Primary human HSCs were treated with 10 or 100 μM UDP-glucose for 24h, followed by qPCR for COL1A1 and LOX (n=5 biological displayed as fold induction compared to untreated control). E-F. P2RY14 mRNA was determined by qPCR, and UDP-glucose content determined by mass spectroscopy in healthy or cirrhotic human liver (n=4-8 biological replicates). G. P2RY14 mRNA was determined by qPCR in primary human HSCs treated with UDP-glucose or 10% FBS (n=5 biological replicates each, displayed as fold induction compared to untreated control). Data are shown as means ± SEM. Scale bar 50 μM.
Discussion
Our study establishes P2Y14 and its ligands as profibrogenic DAMP-DAMP receptor system in the liver and other organs, directly linking epithelial cell death to fibrogenic cell activation. The liver is an organ in which the link between epithelial cell death and fibrosis has been well established, both clinically as well as in mouse models of liver disease. However, mechanisms linking hepatocyte death to HSC activation have remained enigmatic (9,17), with the DAMP HMGB1 not playing a role in the majority of published fibrosis models (20,22,23). Moreover, key fibrogenic pathways, including TGFβ and PDGF, do not explain this link as both mediators are not known to be released upon cell death. The differential expression of P2Y14 ligand and P2Y14 in hepatocytes and HSC provide the basis for a profibrogenic DAMP-DAMP receptor system that enables sensing of hepatocyte injury by HSC, most likely in a local and gradient-dependent manner. In this system, profibrogenic DAMPs UDP-glucose and UDP-galactose are enriched in hepatocytes and released after hepatocyte death. Subsequently, P2Y14, enriched on HSCs, is activated by these ligands, promoting HSC activation and fibrogenesis. The high concentration of P2Y14 ligands in hepatocytes is consistent with their important roles in hepatocyte metabolic functions such as glycogen, glycolipid and glycoprotein synthesis, and glucuronidation, the latter representing a key step in bilirubin, bile acid and drug metabolism. Our results on the P2Y14 axis as an important DAMP system are further supported by a recent study in which both UDP-glucose and UDP-galactose were detected in the metabolite secretome of dying cells, with an active transport system enhancing their secretion (11), thus supporting the idea that release of these and other DAMPs has evolved to fine-tune responses to cell death. Consistent with the high expression of P2Y14 in HSCs and its ligands in hepatocytes, we observed a key role of this system in HSC activation in vitro, finding upregulation of fibrogenic genes Acta2, Ctgf, Serpine1 (encoding PAI-1) and Lox, and to a lesser extent, Col1a1. Accordingly, knockout of P2Y14 decreased liver fibrosis in vivo in five different injury models. The substantial but incomplete reduction of fibrosis in P2Y14-deficient mice is consistent with the presence of multiple and most likely partially redundant fibrogenic pathways and the profound role that TGFβ exerts in fibrogenesis (2,46), as well as the moderate induction of some fibrogenic pathways by P2Y14 ligands such as Col1a1 in HSCs in vitro. Moreover, we also found a reduction of P2Y14 expression upon HSC activation as negative feedback mechanisms. The concept of mechanistically distinct P2Y14- and TGFβ-mediated activation of HSCs is supported by our finding that P2Y14 ligands and TGFβ exert additive effects in HSCs.
In sum, P2Y14 is only one of several cell death-dependent and –independent pathways that contribute to liver fibrosis, consistent with the incomplete inhibition of fibrogenesis seen after P2Y14 deletion in vivo. Our results suggest that P2Y14-induced activation of ERK, a powerful profibrogenic pathway in HSCs (44), is responsible for its fibrogenic effects, whereas UDP-glucose-mediated activation of YAP did not make a major contribution to its profibrogenic effects in vitro. It is possible that the upregulation of YAP target genes by UDP-glucose was not sufficient, or that we were not able to detect a role of YAP in vitro because of the lack of other profibrogenic signals that contribute to HSC activation in vivo.
Our study contains several limitations. Although we identified P2Y14 and its ligands as link between fibrosis and cell death, it is likely that cell death promotes fibrogenesis through additional receptors activated by the many different mediators secreted from dying cells (11), or through pathways that do not involve direct interactions between dying hepatocytes and HSCs. One example is the recruitment of bone marrow-derived macrophages in response to cell death, which may promotes fibrogenesis via the release of TGFβ after efferocytosis (47). We could not find a role for TAM receptors AXL or MerTK on HSCs in fibrogenesis, consistent with our finding that macrophage-expressed MerTK regulates liver fibrosis (48), but we cannot exclude that the described phagocytosis of apoptotic bodies by HSC (24,25) occurs through other mechanisms. As P2Y14 ligand release has been described to occur in stressed cells (49), we also cannot exclude that activation of the P2Y14 profibrogenic signaling cascade in the liver can be activated by stress signals in addition to cell death, which would still consistent with its function as DAMP-DAMP receptor system. Our findings suggest that the G-protein-coupled receptor P2Y14 may be a target for anti-fibrotic therapies. P2Y14-deficient mice display no phenotype besides altered gastric emptying found in one but not another study, as well as reduced insulin secretion (34,50). However, in view of ≈20-30% reduction of liver fibrosis we observed and the downregulation of P2Y14 on HSCs in chronic liver injury, it may be necessary to combine P2Y14 inhibitors with other antifibrotic treatments or to focus on early disease stages. Last, although P2Y14 was enriched kidney and lung pericytes, we did not perform functional studies to determine the role of P2Y14 and its ligands in organs besides the liver.
Materials and Methods
Study design.
The overall objective of our study was to investigate the role of DAMP receptors in the development of liver fibrosis. We hypothesized that P2Y14 ligands are released from hepatocytes in the injured liver and activate P2Y14 on HSCs, contributing to their activation and development of liver fibrosis. After confirming activation of primary mouse HSCs by dying mouse hepatocytes, we performed screening to identify involved DAMP receptors by comparing gene expression of DAMP receptors between primary mouse HSCs from healthy untreated livers to corresponding livers by bulk RNA-seq and confirmed data by scRNA-seq and qPCR, using both C57Bl/6 and BALB/c mice. P2Y14 expression was confirmed by immunohistochemistry, immunocytofluorescence, and RNAscope. Next, we determined P2Y14 ligand expression and their release in hepatocytes by ultra-performance liquid chromatography mass spectrometry. To understand the involvement of different candidate DAMPs and DAMP receptors, we employed global and liver-specific knockout mice subjected to CCl4-induced liver injury. Mechanistic studies evaluating the role of P2Y14 were performed in primary mouse HSCs from C57Bl/6 and BALB/c mice, treating these cells with P2Y14 ligands alone or in combination with inhibitors to determine pathways mediating profibrogenic effects of P2Y14. When primary cells were isolated from knockout and wild-type mice or from multiple mice, isolations were performed on the same day by the same investigator and cells were plated, treated and harvested at the same time. The role of P2Y14 was determined in mice with global knockout of P2Y14, bone-marrow knockout of P2Y14 and HSC-selective knockout of P2Y14 in various liver fibrosis models. Each investigator conducting a specific fibrosis experiment used littermate controls (unless otherwise described) and allocated all available male mice to these experiments. For animals with genetic deletion of Mdr2, both male and female mice were used. All investigators were blinded for fibrosis and other evaluations. P2Y14 expression and functions were confirmed in human liver and primary human HSCs. Liver tissue was obtained from liver resection of patients with hepatitis C virus, alcohol-related and non-alcoholic fatty liver disease, and from normal liver tissue obtained from liver resections of colon cancer metastasis. Human studies were approved by institutional review boards (protocol Hospital Clinic of Barcelona: HCP/2015/0142 and HCB/2014/0749; protocol Hannover Medical School: #252-2008). Protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki were given and signed for all the patients. Replicates vary for each experiment and are detailed in the figure legends along with statistical tests. Additional materials and methods are available in the supplementary material.
Animal studies and mice.
All animal procedures were performed with approval by the Columbia University IACUC or review boards of Hannover Medical School animal facility (protocol number 2016/123), and were in accordance with the Guide for the Care and Use of Laboratory Animals (51). Mice were maintained on a 12-hour dark/12-hour light cycle with free access to food and water. P2Y14-deficient mice (backcrossed 8x into C57BL/6J) were obtained from the European Mouse Mutant Archive (EM:04645); S1PR3-deficient mice in C57Bl/6 background from the Mouse Mutant Regional Resource Center (B6.129S6-S1pr3tm1Rlp/Mmnc); and Adora2a-deficient mice (in mixed background) from Jax (Adora2atm1Jfc/J, Jax #010685). P2Y14-, S1PR3- and Adora2a-deficient mice were backcrossed once to the C57Bl/6 background to generate heterozygous mice which were then interbred to generate wild-type and knockout mouse lines. Mice with hepatocyte- and liver-specific deletion of HMGB1 via Alb-Cre and Mx1-Cre (backcrossed at least 5x to C57BL/6J) as well as Ager knockout mice have been described (19). P2Y14 floxed mice (backcrossed 10x into C57BL/6J) were obtained from the European Mouse Mutant Archive (EM:05368). TLR4fl/fl mice were a gift from Joel Elmquist (UT Southwestern) and YAPfl/fl mice a gift from Eric Olson (UT Southwestern); both were backcrossed at least 5x to C57BL/6J. TdTomato Cre reporter mice (Jax #007909) were obtained from Jackson laboratories. YAP1fl/fl mice have been described previously (52). P2Y14fl/fl, TLR4fl/fl and YAP1fl/fl mice were bred with LratCre transgenic mice (≥5x backcrossed into C57BL/6J) (18) for HSC-selective deletion of P2Y14, TLR4 and YAP1 in the liver. Mdr2KO mice (in FVB background) were obtained from Dr. Detlef Schuppan (University of Mainz, Germany) and interbred with P2Y14-deficient mice to generate Mdr2KO P2Y14HET in the FVB background (backcrossed 3x). P2Y14WTand P2Y14KO littermates from this breeding were used for the experiments. For isolation of αSMA-RFP HSC, αSMA-RFP mice in BALB/cJ background (53) were crossed with P2Y14-deficient mice and backcrossed to BALB/cJ at least three times. For YAP1f/f HSC isolation, mice were backcrossed twice into BALB/cJ. Primary cells were isolated from animals of both genders, always using the same gender within a specific experiment.
Induction and analysis of liver fibrosis.
Liver fibrosis was induced by the injection of six doses of CCl4 (0.5 ml/kg body weight i.p., dissolved 1:3 in corn oil; injected 2x/week) in 8-10 weeks old male mice; by ligating the common bile duct for 16 days as described (54); by genetic Mdr2 deficiency (18); or by feeding mice a diet containing 0.1% DDC for 3 weeks or L-amino acid diet with 60 kcal% fat with 0.1% methionine lacking choline (A06071302, Research Diet). To analyze liver fibrosis, Picrosirius Red staining of paraffin-embedded liver sections was performed as described (54). For quantification, at least ten pictures were taken with a 10x lens using an Olympus 71IX microscope equipped with a polarization filter. Positive area was quantified in a blinded fashion using Adobe Photoshop. Hepatic hydroxyproline content for analysis of liver fibrosis was determined as described (54).
Isolation and treatment of primary cells.
Primary mouse HSC hepatocytes, Kupffer cells, cholangiocytes, and LSECs were isolated as described (55). To determine the expression of DAMP receptors or deletion of specific genes in HSC, cells were isolated to high purity by combining gradient centrifugation and FACS sorting and never plated (55). For experiments determining HSC responses to UDP ligands, primary HSCs and other primary liver cells were plated for 5 hours in medium containing 10% FBS. Following change to medium containing 0.1% FBS, cells were treated with P2Y14 ligands. To study the crosstalk between dying hepatocytes and HSCs, primary mouse HSCs were co-cultured with primary mouse hepatocytes, that had been treated with sterile deionized water for 30 minutes to induce cell swelling and death and then were placed into transwell inserts (18). For experiments determining P2Y14 ligand release, primary mouse hepatocytes were plated in medium containing 10% FBS for 1.5 hours, followed by overnight culture in serum-free medium. Necrosis was induced by treatment with CCl4 or acetaminophen; apoptosis by treatment with ActD (0.3 μg/ml) plus recombinant mouse TNF (30 ng/ml), or ActD plus Jo2 (0.5 μg/ml); ferroptosis by treatment with erastin (10 μM). For necroptosis, male mice were intravenously injected with 2x1011 gc/mouse AAV8-TBG-mRIPK3-2XFv (56). Seven days later, primary hepatocytes were isolated, followed by treatment with 10 nM homodimerizer AP20187. The isolation of primary pulmonary and renal pericytes (57) and primary human HSCs (58) was performed as described.
Data exclusion statement.
No data points or animals were excluded from the analysis with the exception of the following. One qPCR Figure 4D and one qPCR sample in Figure S1B in Fig.4D and one qPCR sample in Figure S1B were excluded because of abnormally low 18s, defined as at least 3 CT values lower and more than 5 standard deviations outside of than the mean of all samples of the experiment. Two P2Y14 KO mice after bile duct ligation were excluded because of surgical complications, presenting with a belly full of bile fluid indicating leakage of bile into the abdomen. Six out of 20 patients were not analyzed for P2RY14 mRNA expression by qPCR because of insufficient sample quality. Last, one normal mouse liver was excluded from P2Y14 ligand measurements because of a long storage time and high discrepancy between UDP-glucose and UDP-galactose concentrations.
Statistical analysis.
All data are expressed as the mean ± SEM. Data were tested for normality prior to the use of parametric tests. For comparison of 2 groups, unpaired two-tailed t was used for normally distributed data, Mann-Whitney tests for non-normally distributed data. In experiments with more than 2 groups one-way ANOVA with post-hoc Sidak test for normally distributed data and Kruskal-Wallis with Dunn post-hoc test for not normally distributed data. Analysis was performed using GraphPad Prism version 8 for Mac. A nominal p-value of <0.05 and for GSEA, a false discovery rate (FDR) q-value <0.25 and p-value of <0.05 were considered to indicate significant enrichment.
Supplementary Material
Acknowledgements
We would like to thank Deqi Yin (Columbia University) for excellent technical assistance, Eric Olson and Joel Elmquist (both UT Southwestern) for YAP and TLR4 floxed mice.
Funding:
This study was supported by NIH grants 5R01CA200597 and 1R01DK124104 (to RFS) and 1R01DK116620 (to RFS and IT). IM was supported by a fellowship from the German Research Foundation (ME 3723/1-1, ME 3723/2-1); SA by an American Gastroenterological Association Research Scholar Award and a La Caixa Junior Leader Fellowship (847648); AF by an International Liver Cancer Association fellowship, a postdoctoral fellowship from the American Liver Foundation and a grant from the Fondation pour la Recherche Médicale; J.R.S. by a Wellcome Trust PhD fellowship (ref. 104366/Z/14/Z); N.C.H. by a Wellcome Trust Senior Research Fellowship in Clinical Science (ref. 219542/Z/19/Z) and PSB by FIS PI20/00765 and FI18/00215.
Footnotes
Competing interests: N.C.H. has received research funding from AbbVie, Pfizer, Gilead and Galecto, and has consulted or been on the advisory board for Galecto, Indalo Therapeutics, Pliant Therapeutics, GSK, Astra-Zeneca, MSD and Boehringer-Ingelheim. J.S. has received a speaker fee from Pfizer. I.A.T. has consulted for Akero, Jupiter Bioventures, Genevant, Inipharm, Novartis and Quentis and recived research grants from Takeda. C.R. is the Scientific Founder and scientific advisory board member of Surface Oncology, and scientific advisory board member of Janssen Immunology and Roche Immunology Incubator and has received research grants from Mirati Pharmaceuticals. FWRV has been on the advisory board of Chiesi and consulted for Novartis. RFS has consulted for Boehringer Ingelheim, NGM Bio, Merck and Arrowhead, and research grants from Takeda.
Data and materials availability: All data associated with this study are present in the main paper or supplementary materials. All bulk and scRNA-seq data are deposited in the Gene Omnibus database (GSE158183 and GSE172492). PSLRI package and codes are available at https://doi.org/10.5281/zenodo.6301768. Primary data are available in data file S1.
References and Notes
- 1.Asrani SK, Devarbhavi H, Eaton J, Kamath PS, Burden of liver diseases in the world. J Hepatol 70, 151–171 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Mehal WZ, Iredale J, Friedman SL, Scraping fibrosis: expressway to the core of fibrosis. Nat Med 17, 552–553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson NC, Rieder F, Wynn TA, Fibrosis: from mechanisms to medicines. Nature 587, 555–566 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F, Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 149, 389–397 e310 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lackner C, Stauber RE, Davies S, Denk H, Dienes HP, Gnemmi V, Guido M, Miquel R, Paradis V, Schirmacher P, Terracciano L, Berghold A, Pregartner G, Binder L, Douschan P, Rainer F, Sygulla S, Jager M, Rautou PE, Bumbu A, Horhat A, Rusu I, Stefanescu H, Detlefsen S, Krag A, Thiele M, Cortez-Pinto H, Moreno C, Gouw ASH, Tiniakos DG, Development and prognostic relevance of a histologic grading and staging system for alcohol-related liver disease. J Hepatol 75, 810–819 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Xu F, Moorman AC, Tong X, Gordon SC, Rupp LB, Lu M, Teshale EH, Spradling PR, Boscarino JA, Trinacty CM, Schmidt MA, Holmberg SD, I. Chronic Hepatitis Cohort Study, Holmberg SD, Teshale EH, Spradling PR, Moorman AC, Xing J, Tong X, Xu F, Gordon SC, Nerenz DR, Lu M, Lamerato L, Wang Y, Rupp LB, Akkerman N, Oja-Tebbe N, Zhang T, Li J, Sitarik A, Larkin D, Boscarino JA, Daar ZS, Curry PJ, Smith RE, Vijayadeva V, Parker JV, Schmidt MA, Donald JL, Keast EM, All-Cause Mortality and Progression Risks to Hepatic Decompensation and Hepatocellular Carcinoma in Patients Infected With Hepatitis C Virus. Clin Infect Dis 62, 289–297 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, Loomba R, Chalasani N, Kowdley K, Hameed B, Wilson LA, Yates KP, Belt P, Lazo M, Kleiner DE, Behling C, Tonascia J, N. C. R. Network, Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med 385, 1559–1569 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn TA, Ramalingam TR, Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18, 1028–1040 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luedde T, Kaplowitz N, Schwabe RF, Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 147, 765–783 e764 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaczmarek A, Vandenabeele P, Krysko DV, Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38, 209–223 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, Barron B, Walk SF, Ghesquiere B, Krupnick AS, Lorenz U, Ravichandran KS, Metabolites released from apoptotic cells act as tissue messengers. Nature 580, 130–135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris R, Harman DJ, Card TR, Aithal GP, Guha IN, Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol 2, 288–297 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R, Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 65, 1557–1565 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, Hultcrantz R, Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, Gonzalez-Fabian L, Alvarez-Quinones Sanz M, Conde-Martin AF, De Boer B, McLeod D, Hung Chan AW, Chalasani N, George J, Adams LA, Romero-Gomez M, Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 155, 443–457 e417 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z, Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology 53, 1874–1882 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Schwabe RF, Luedde T, Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol 15, 738–752 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF, Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 4, 2823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huebener P, Pradere JP, Hernandez C, Gwak GY, Caviglia JM, Mu X, Loike JD, Schwabe RF, The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest 125, 539–550 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez C, Huebener P, Pradere JP, Antoine DJ, Friedman RA, Schwabe RF, HMGB1 links chronic liver injury to progenitor responses and hepatocarcinogenesis. J Clin Invest 128, 2436–2451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An P, Wei LL, Zhao S, Sverdlov DY, Vaid KA, Miyamoto M, Kuramitsu K, Lai M, Popov YV, Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat Commun 11, 2362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khambu B, Huda N, Chen X, Antoine DJ, Li Y, Dai G, Kohler UA, Zong WX, Waguri S, Werner S, Oury TD, Dong Z, Yin XM, HMGB1 promotes ductular reaction and tumorigenesis in autophagy-deficient livers. J Clin Invest 128, 2419–2435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge X, Arriazu E, Magdaleno F, Antoine DJ, Dela Cruz R, Theise N, Nieto N, High Mobility Group Box-1 Drives Fibrosis Progression Signaling via the Receptor for Advanced Glycation End Products in Mice. Hepatology 68, 2380–2404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ, Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest 83, 655–663 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ, Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 43, 435–443 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Doran AC, Yurdagul A Jr., Tabas I, Efferocytosis in health and disease. Nat Rev Immunol 20, 254–267 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothlin CV, Hille TD, Ghosh S, Determining the effector response to cell death. Nat Rev Immunol 21, 292–304 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, Friedman SL, Cronstein BN, Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol 148, 1144–1155 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Che J, Chan ES, Cronstein BN, Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol Pharmacol 72, 1626–1636 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Efremova M, Vento-Tormo M, Teichmann SA, Vento-Tormo R, CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat Protoc 15, 1484–1506 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Skelton L, Cooper M, Murphy M, Platt A, Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol 171, 1941–1949 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Shin A, Toy T, Rothenfusser S, Robson N, Vorac J, Dauer M, Stuplich M, Endres S, Cebon J, Maraskovsky E, Schnurr M, P2Y receptor signaling regulates phenotype and IFN-alpha secretion of human plasmacytoid dendritic cells. Blood 111, 3062–3069 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Ma J, Wei K, Liu J, Tang K, Zhang H, Zhu L, Chen J, Li F, Xu P, Chen J, Liu J, Fang H, Tang L, Wang D, Zeng L, Sun W, Xie J, Liu Y, Huang B, Glycogen metabolism regulates macrophage-mediated acute inflammatory responses. Nat Commun 11, 1769 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meister J, Le Duc D, Ricken A, Burkhardt R, Thiery J, Pfannkuche H, Polte T, Grosse J, Schoneberg T, Schulz A, The G protein-coupled receptor P2Y14 influences insulin release and smooth muscle function in mice. J Biol Chem 289, 23353–23366 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scrivens M, Dickenson JM, Functional expression of the P2Y14 receptor in human neutrophils. Eur J Pharmacol 543, 166–173 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Sesma JI, Kreda SM, Steinckwich-Besancon N, Dang H, Garcia-Mata R, Harden TK, Lazarowski ER, The UDP-sugar-sensing P2Y(14) receptor promotes Rho-mediated signaling and chemotaxis in human neutrophils. Am J Physiol Cell Physiol 303, C490–498 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman K, Tsui P, Moore D, Emson PC, Vawter L, Naheed S, Lane P, Bawagan H, Herrity N, Murphy K, Sarau HM, Ames RS, Wilson S, Livi GP, Chambers JK, Cloning, pharmacology, and tissue distribution of G-protein-coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics 78, 124–128 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Hjelle JJ, Hepatic UDP-glucuronic acid regulation during acetaminophen biotransformation in rats. J Pharmacol Exp Ther 237, 750–756 (1986). [PubMed] [Google Scholar]
- 39.Andrews RS, Bond CC, Burnett J, Saunders A, Watson K, Isolation and identification of paracetamol metabolites. J Int Med Res 4, 34–39 (1976). [DOI] [PubMed] [Google Scholar]
- 40.Martin K, Pritchett J, Llewellyn J, Mullan AF, Athwal VS, Dobie R, Harvey E, Zeef L, Farrow S, Streuli C, Henderson NC, Friedman SL, Hanley NA, Piper Hanley K, PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat Commun 7, 12502 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, Hoorens A, Reynaert H, Halder G, van Grunsven LA, The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol 63, 679–688 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Fricks IP, Carter RL, Lazarowski ER, Harden TK, Gi-dependent cell signaling responses of the human P2Y14 receptor in model cell systems. J Pharmacol Exp Ther 330, 162–168 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marra F, Arrighi MC, Fazi M, Caligiuri A, Pinzani M, Romanelli RG, Efsen E, Laffi G, Gentilini P, Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor's actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology 30, 951–958 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Foglia B, Cannito S, Bocca C, Parola M, Novo E, ERK Pathway in Activated, Myofibroblast-Like, Hepatic Stellate Cells: A Critical Signaling Crossroad Sustaining Liver Fibrosis. Int J Mol Sci 20, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trauner M, Fickert P, Wagner M, MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis 27, 77–98 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Tsuchida T, Friedman SL, Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14, 397–411 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Schwabe RF, Tabas I, Pajvani UB, Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 158, 1913–1928 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai B, Dongiovanni P, Corey KE, Wang X, Shmarakov IO, Zheng Z, Kasikara C, Davra V, Meroni M, Chung RT, Rothlin CV, Schwabe RF, Blaner WS, Birge RB, Valenti L, Tabas I, Macrophage MerTK Promotes Liver Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab 31, 406–421 e407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazarowski ER, Shea DA, Boucher RC, Harden TK, Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol 63, 1190–1197 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Bassil AK, Bourdu S, Townson KA, Wheeldon A, Jarvie EM, Zebda N, Abuin A, Grau E, Livi GP, Punter L, Latcham J, Grimes AM, Hurp DP, Downham KM, Sanger GJ, Winchester WJ, Morrison AD, Moore GB, UDP-glucose modulates gastric function through P2Y14 receptor-dependent and -independent mechanisms. Am J Physiol Gastrointest Liver Physiol 296, G923–930 (2009). [DOI] [PubMed] [Google Scholar]
- 51.N. R. C. U. C. f. t. U. o. t. G. f. t. C. a. U. o. L. Animals., Guide for the Care and Use of Laboratory Animals, 8th edition. ( The National Academies Press, Washington (DC):, 2011). [Google Scholar]
- 52.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN, Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A 110, 13839–13844 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magness ST, Bataller R, Yang L, Brenner DA, A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology 40, 1151–1159 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF, TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 13, 1324–1332 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Mederacke I, Dapito DH, Affo S, Uchinami H, Schwabe RF, High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat Protoc 10, 305–315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez DA, Green DR, Generation and Use of Chimeric RIP Kinase Molecules to Study Necroptosis. Methods Mol Biol 1857, 71–83 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D, Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19, 1617–1624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coll M, El Taghdouini A, Perea L, Mannaerts I, Vila-Casadesus M, Blaya D, Rodrigo-Torres D, Affo S, Morales-Ibanez O, Graupera I, Lozano JJ, Najimi M, Sokal E, Lambrecht J, Gines P, van Grunsven LA, Sancho-Bru P, Integrative miRNA and Gene Expression Profiling Analysis of Human Quiescent Hepatic Stellate Cells. Sci Rep 5, 11549 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Love MI, Huber W, Anders S, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu Z, Eils R, Schlesner M, Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Huebener P, Gwak GY, Pradere JP, Quinzii CM, Friedman R, Lin CS, Trent CM, Mederacke I, Zhao E, Dapito DH, Lin Y, Goldberg IJ, Czaja MJ, Schwabe RF, High-mobility group box 1 is dispensable for autophagy, mitochondrial quality control, and organ function in vivo. Cell Metab 19, 539–547 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf FA, Angerer P, Theis FJ, SCANPY: large-scale single-cell gene expression data analysis. Genome Biol 19, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ilicic T, Kim JK, Kolodziejczyk AA, Bagger FO, McCarthy DJ, Marioni JC, Teichmann SA, Classification of low quality cells from single-cell RNA-seq data. Genome Biol 17, 29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lun AT, Bach K, Marioni JC, Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol 17, 75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.B. M Aparicio L, Blumberg AJ, Rabadan R, A Random Matrix Theory Approach to Denoise Single-Cell Data. Patterns, 100035 (2020. May 4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McInnes LH, J. , UMAP: uniform manifold approximation and projection for dimension reduction. arXiv:1802.03426, (2018). [Google Scholar]
- 67.Blondel VD, Guillaume JL , Lambiotte R, Lefebvre E, Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp 10008, (2008). [Google Scholar]
- 68.Rousseeuw PJ, Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. Journal of computational and applied mathematics 20, 53–65 (1987). [Google Scholar]
- 69.Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, Saadatpour A, Zhou Z, Chen H, Ye F, Huang D, Xu Y, Huang W, Jiang M, Jiang X, Mao J, Chen Y, Lu C, Xie J, Fang Q, Wang Y, Yue R, Li T, Huang H, Orkin SH, Yuan GC, Chen M, Guo G, Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 173, 1307 (2018). [DOI] [PubMed] [Google Scholar]
- 70.MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, Manuel J, Khuu N, Echeverri J, Linares I, Gupta R, Cheng ML, Liu LY, Camat D, Chung SW, Seliga RK, Shao Z, Lee E, Ogawa S, Ogawa M, Wilson MD, Fish JE, Selzner M, Ghanekar A, Grant D, Greig P, Sapisochin G, Selzner N, Winegarden N, Adeyi O, Keller G, Bader GD, McGilvray ID, Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun 9, 4383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.