Abstract
Background
Many species of the genus Caragana have been used as wind prevention and sand fixation plants. They are also important traditional Chinese medicine, and ethnic medicine resource plant. Thus, chloroplast genomes (cp-genome) of some of these important species must be studied.
Methods
In this study, we analyzed the chloroplast genomes of C. jubata, C. erinacea, C. opulens, and C. bicolor, including their structure, repeat sequences, mutation sites, and phylogeny.
Results
The size of the chloroplast genomes was between 127,862 and 132,780 bp, and such genomes contained 112 genes (30 tRNA, 4 rRNA, and 78 protein-coding genes), 43 of which were photosynthesis-related genes. The total guanine + cytosine (G+C) content of four Caragana species was between 34.49% and 35.15%. The four Caragana species all lacked inverted repeats and can be classified as inverted repeat-lacking clade (IRLC). Of the anticipated genes of the four chloroplast genomes, introns were discovered in 17 genes, most of which were inserted by one intron. A total of 50 interspersed repeated sequences (IRSs) were found among them, 58, 29, 61, and 74 simple sequences repeats were found in C. jubata, C. bicolor, C. opulens, and C. erinacea, respectively. Analyses of sequence divergence showed that some intergenic regions (between trnK-UUU and rbcl; trnF-GAA and ndhJ; trnL-CAA and trnT-UGU; rpoB and trnC-GCA; petA and psbL; psbE and pebL; and sequences of rpoC, ycf1, and ycf2) exhibited a high degree of variations. A phylogenetic tree of eight Caragana species and another 10 legume species was reconstructed using full sequences of the chloroplast genome.
Conclusions
(1) Chloroplast genomes can be used for the identification and classification of Caragana species. (2) The four Caragana species have highly similar cpDNA G+C content. (3) IRS analysis of the chloroplast genomes showed that these four species, similar to the chloroplast genome of most legumes, lost IRLC regions. (4) Comparative cp-genomic analysis suggested that the cp genome structure of the Caragana genus was well conserved in highly variable regions, which can be used to exploit markers for the identification of Caragana species and further phylogenetic study. (5) Results of phylogenetic analyses were in accordance with the current taxonomic status of Caragana. The phylogenetic relationship of Caragana species was partially consistent with elevation and geographical distribution.
1. Introduction
About 100 Caragana species exist worldwide. Among the species in the arid and semi-arid regions of Asia and Europe, 66 species (32 endemic) can be found in China [1]. The genus Caragana is a deciduous shrub with a wide range of adaptability and strong stress tolerance. Most of the Caragana species are distributed in higher elevations and relatively harsh environments (barren, drought, heat, and cold); they are known to prevent wind and fixate sand [2–5]. Moreover, previous studies have shown that many plants have pharmacological, antibacterial, and antioxidant activities and anti-tumor, anti-HIV, and other effects [2, 5, 6]. All four species in this study have been documented in traditional Chinese ethnic medicine. Among them, C. jubata is an important Tibetan drug that can be used to treat alpine erythrocytosis and hypertension; it possesses hepatoprotective and antiviral activities[7–11].
Phylogenetic relationships in the genus Caragana remain obscure, and also have some problems in the identification of medicinal species. Only 10 cp-genome of the Caragana genus have been reported and low amount of data are available for analysis [12–14].
Chloroplasts are the posterity of ancient microbacillary endosymbionts. They are the usual organelles of green plants, which play an indispensable role in photosynthesis [15]. Ordinarily, the descendibility of the chloroplast genome is maternal in angiosperms [16]. The chloroplast genome is relatively stable in structure, and it contains a large single-copy region, small single-copy region, and two inverse repeat (IR) regions. Inverted repeat-lacking clade (IRLC) has been reported in legumes [17–20]: four Caragana species have been reported with IRLC [11–13]. Therefore, the Caragana genus can represent a lineage with extensive IRLC. However, knowledge of the pattern, origin, and evolution of plastomic IRLC within Caragana is presently limited by the scarcity of plastomic sequences. In addition, the chloroplast genomic model can be used to study molecular identification, phylogeny, species conservation, and evolution [21, 22].
In the present study, the four species of the genus Caragana from Ganzi Tibetan Autonomous Prefecture of Sichuan Province and Qinghai Province, China, were identified on the basis of the chloroplast genome. The structural characteristics, population genetics, phylogenetic relationships, and phylogenetic trees were documented.
2. Materials and methods
2.1. DNA sequencing, assembly, and validation of the chloroplast genome
The leaves of four plants were collected from Ganzi Tibetan Autonomous Prefecture of Sichuan Province and Qinghai Province, China: C. jubata location: E 97°11′18″, N 32°37′19″, altitude: 4372 m; C. erinacea location: E 98°18′13″, N 33°3′34″, altitude: 3974 m; C. opulens location: E 101°5′42″, N 31°0′8″, altitude: 2932 m; and C. bicolor location: E 100°40′26″, N 31°24′25″, altitude: 3199 m. The whole genomic DNA of Caragana species was extracted by using E.Z.N.A® Plant DNA kit [23]. The library was started by reagent (TruSeq™ Nano DNA Sample Prep Kit, Illumina) at 1 μg of DNA, and the DNA was interrupted to 300–500 bp by Covaris M220 ultrasound. Libraries were enriched, and eight cycles were amplified by polymerase chain reaction (PCR). Quantification was performed using TBS380 (Picogreen). Bridge PCR amplification was performed on cBot Truseq PE Cluster Kit v3-cBot-HS to generate clusters. Sequencing was conducted with 150 bp pair-end reads on the Illumina NovaSeq platform (Illumina, San Diego, CA, USA).
The cp reads were used to assemble sequences by spades, abyss, and soapdenovo. All of the contigs were aligned to the reference cp genome of C. korshinskii with MUMmer. Finally, the assembly results were inhole repaired with GapCloser-1.12 (OMEGA) [24].
2.2. Gene annotation and sequence analyses
Sequences were annotated by Plann [25] using the chloroplast genome of C. korshinskii from NCBI and some manual corrections. BLAST and Apollo [26] were used to check the start and stop codons and intron/exon boundaries with the cp genome of C. korshinskii as the reference sequence. The complete chloroplast genome sequence data reported in this paper have been deposited in the Genome Warehouse in National Genomics Data Center (NGDC https://ngdc.cncb.ac.cn/, accession numbers: GWHBJYO00000000, GWHBJYN00000000, GWHBJYM00000000, and GWHBJYL00000000). The structural features of the chloroplast genome were drawn by Organellar Genome DRAW [27] (http://ogdraw.mpimp-golm.mpg.de/). Protein-coding gene sequences were extracted by Geneious.
2.3. Comparison of chloroplast genomes
The chloroplast genomes of Caragana species were completed by mVISTA [28] (Shuffle-LAGAN mode) using the genome of C. korshinskii as the reference. The detecting and testing of forward, palindromic, and tandem repeats were performed using Tandem Repeats Finder [26] and REPuter [27]. In addition, the detection of simple sequence repeats (SSRs) was executed using Misa.pl [29]. The search parameters of mononucleotides, dinucleotides, trinucleotides, tetranucleotides, pentanucleotides, and hexanucleotide were set to ≥10, ≥8, ≥4, and ≥3 repeat units.
2.4. Phylogenetic analyses
Phylogenetic trees were constructed using plastid genomes of 18 species, in which Ranitomeya imitator was an outgroup. The sequences were aligned by Mafft. An unrooted phylogenetic tree with 1000 bootstrap replicates was inferred using the neighbor-joining (NJ) approach with MEGA X [30].
3. Results
3.1. DNA features of the chloroplast genome of four Caragana species
The size of chloroplast genome was between 127,862 and 132,780 bp, which is small because of the loss of the IR region. C. opulens (132780 bp) had the largest chloroplast genome, whereas C. jubata had the smallest (127862 bp). The mean value of the total guanine + cytosine (G+C) content of four Caragana species was 34.72%. Four Caragana species had a chloroplast genome with a similar structure, and all of them loss the IR region. After annotation, the whole chloroplast genome sequence of the four Caragana species was submitted to NGDC: the accession numbers are listed in Table 1.
Table 1. Summary of complete chloroplast genomes for four Caragana species.
| Scample name | Total length | GC Content (%) | Depth | accession number |
|---|---|---|---|---|
| C. jubata | 127862 | 34.49 | 749 | GWHBJYN00000000 |
| C. bicolor | 131318 | 34.54 | 979 | GWHBJYL00000000 |
| C. erinacea | 130968 | 35.15 | 451 | GWHBJYM00000000 |
| C. opulens | 132780 | 34.70 | 992 | GWHBJYO00000000 |
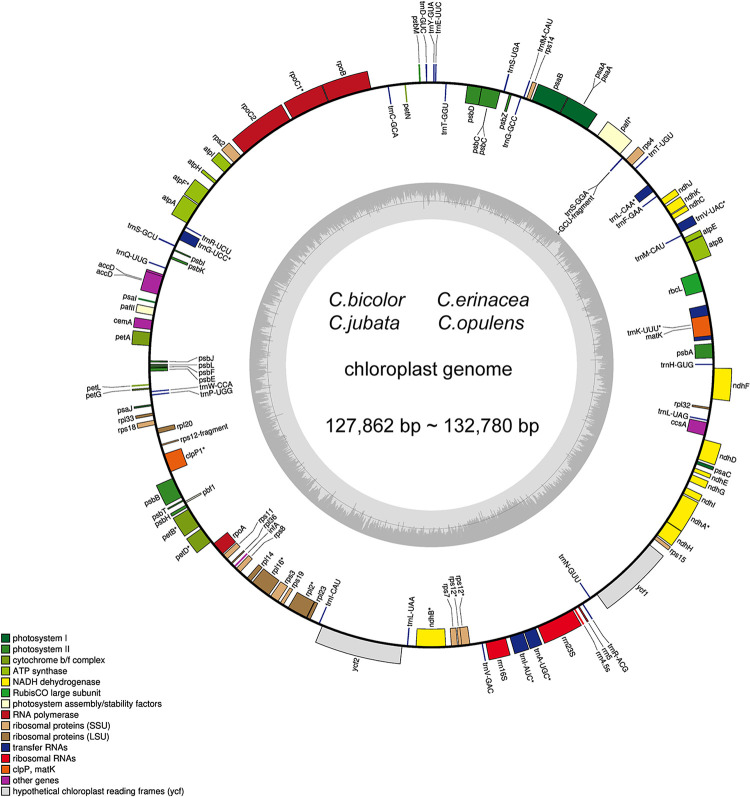
The gene maps of C. jubata, C. bicolor, C. erinacea, and C. opulens were drawn by OGDraw [24] (Fig 1) based on annotation results. A total of 112 genes were found in the chloroplast genome of C. jubata, C. bicolor, C. erinacea, and C. opulens: 30 tRNA, 4rRNA, and 78 protein-coding genes. Among the 112 genes, 43 were photosynthesis-related genes (Table 2). Most genes could be divided crudely into three groups: “photosynthesis-related,” “self-replication-related,”, and “other” groups (Table 2) [31].
Fig 1. Gene map of the chloroplast genome of four Caragana species.
Genes within the circle are transcribed clockwise, and those outside are transcribed counterclockwise. Genes belonging to different functional groups are color coded. The dark gray in the inner circle corresponds to the DNA G+C content, whereas the light-gray corresponds to the A+T content.
Table 2. Genes in the chloroplast genome of four Caragana species.
| Category | Group of genes | Name of genes |
|---|---|---|
| Self-replication | Large subunit of ribosomal proteins | Rp114, 16, 2, 20, 22, 23, 32, 33, 36 |
| Small subunit of ribosomal proteins | rps2, 3, 4, 7, 8, 11, 12, 14, 15, 18, 19 | |
| DNA-dependent RNA polymerase | rpoA, B, C1, C2 | |
| rRNA genes | rrn16Sa, rrn23Sa, rrn4.5Sa, rrn5Sa | |
| tRNA genes | trnA-UGC, trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC, trnG-UCC, trnH-GUG, trnI-AUC, trnI-CAU, trnK-UUU, trnL-CAA, trnL-UAA, trnL-UAG, trnM-CAU, trnN-GUU, trnP-UGG, trnQ-UUG, trnR-ACG, trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC, trnV-UAC, trnW-CCA, trnY-GUA | |
| Photosynthesis | Photosystem I | psaA, B, C, I, J |
| Photosystem II | psbA, B, C, D, E, F, H, I, J, K, L, M, T, Z, | |
| NADH oxidoreductase | ndhA*, B*,a, C, D, E, F, G, H, I, J, K | |
| Cytochrome b6/f complex | petA, B*, D*, G, L, N | |
| ATP synthase | atpA, B, E, F*, H, I | |
| Rubisco | rbcL | |
| Other genes | Maturase | matK |
| Protease | clpP * | |
| Envelope membrane protein | cemA | |
| Subunit acetyl-CoA-carboxylase | accD | |
| c-Type cytochrome synthesis gene | ccsA | |
| Conserved open-reading frames | Ycf1, 2 | |
| protein synthesis initiation factor | infA |
Of the anticipated genes of the chloroplast genomes of C. jubata, C. bicolor, C. erinacea, and C. opulens, introns were discovered in 17 genes: six tRNA(trnV-UAC, trnL-CAA, trnG-UCC, trnA-UGC, trnK-UUU, and trnI-AUC) genes and 11 protein-encoding genes (rpl2, rps12, rpoC1, rpl16, ndhA, ndhB, aptF, clpP, petB, pafI, and petD; Table 3). Most of the 17 intron-containing genes were inserted by one intron except for pafI, which was inserted by two introns. In the four cp-genomes, the longest intron all were trnH-UUU, which in length (2481, 2494, 2494, and 2485 bp), and contained the whole matK.
Table 3. The genes having introns in the chloroplast genome of four Caragana species and the length of the exons and introns.
| Species | Gene | Exon I (bp) | Intron I (bp) | Exon II (bp) | Intron II (bp) | Exon III (bp) |
|---|---|---|---|---|---|---|
| C. jubata | trnL-CAA | 37 | 350 | 50 | ||
| trnV-UAC | 39 | 574 | 37 | |||
| trnA-UGC | 38 | 812 | 35 | |||
| trnG-UCC | 21 | 682 | 51 | |||
| trnI-AUC | 37 | 953 | 35 | |||
| trnK-UUU | 37 | 2481 | 29 | |||
| rps12* | 26 | 232 | 591 | |||
| rpl16 | 399 | 1105 | 9 | |||
| rpl2 | 434 | 689 | 394 | |||
| rpoC1 | 430 | 790 | 1625 | |||
| ndhA | 553 | 1163 | 539 | |||
| ndhB | 762 | 685 | 777 | |||
| aptF | 145 | 702 | 410 | |||
| petB | 6 | 819 | 642 | |||
| clpP | 231 | 786 | 292 | |||
| petD | 8 | 717 | 475 | |||
| pafI | 124 | 711 | 230 | 886 | 153 | |
| C. erinacea | trnL-CAA | 37 | 555 | 50 | ||
| trnV-UAC | 39 | 572 | 37 | |||
| trnA-UGC | 38 | 808 | 35 | |||
| trnG-UCC | 21 | 679 | 51 | |||
| trnI-AUC | 37 | 956 | 35 | |||
| trnK-UUU | 37 | 2494 | 29 | |||
| rps12* | 26 | 232 | 588 | |||
| rpl16 | 399 | 945 | 9 | |||
| rpl2 | 434 | 689 | 388 | |||
| rpoC1 | 430 | 785 | 1625 | |||
| ndhA | 551 | 1194 | 541 | |||
| ndhB | 723 | 680 | 762 | |||
| petB | 6 | 822 | 642 | |||
| clpP | 363 | 843 | 231 | |||
| petD | 8 | 729 | 475 | |||
| aptF | 145 | 697 | 410 | |||
| pafI | 124 | 714 | 230 | 891 | 153 | |
| C. bicolor | trnV-UAC | 39 | 572 | 37 | ||
| trnL-CAA | 37 | 555 | 50 | |||
| trnK-UUU | 37 | 2494 | 29 | |||
| trnI-AUC | 37 | 956 | 35 | |||
| trnA-UGC | 38 | 808 | 35 | |||
| rps12 | 26 | 232 | 588 | |||
| rpoC1 | 430 | 785 | 1625 | |||
| rpl2 | 390 | 686 | 435 | |||
| rpl16 | 399 | 945 | 9 | |||
| petD | 8 | 729 | 475 | |||
| petB | 6 | 822 | 642 | |||
| pafI | 126 | 714 | 226 | 981 | 155 | |
| ndhB | 723 | 680 | 762 | |||
| ndhA | 551 | 1194 | 541 | |||
| clpP1 | 363 | 843 | 231 | |||
| atpF | 167 | 673 | 412 | |||
| C. opulens | trnV-UAC | 39 | 574 | 37 | ||
| trnL-CAA | 37 | 534 | 50 | |||
| trnK-UUU | 37 | 2485 | 29 | |||
| trnI-AUC | 37 | 953 | 35 | |||
| trnG-UCC | 21 | 682 | 51 | |||
| trnA-UGC | 38 | 810 | 35 | |||
| rps12 | 26 | 232 | 600 | |||
| rpoC1 | 430 | 789 | 1625 | |||
| rpl2 | 396 | 692 | 435 | |||
| rpl16 | 399 | 1101 | 9 | |||
| petD | 8 | 720 | 475 | |||
| petB | 6 | 826 | 642 | |||
| pafI | 126 | 702 | 226 | 873 | 155 | |
| ndhB | 723 | 685 | 762 | |||
| ndhA | 551 | 1169 | 541 | |||
| clpP1 | 363 | 1628 | 223 | |||
| atpF | 167 | 679 | 412 |
3.2 Analyses of long repetitive sequences and SSRs
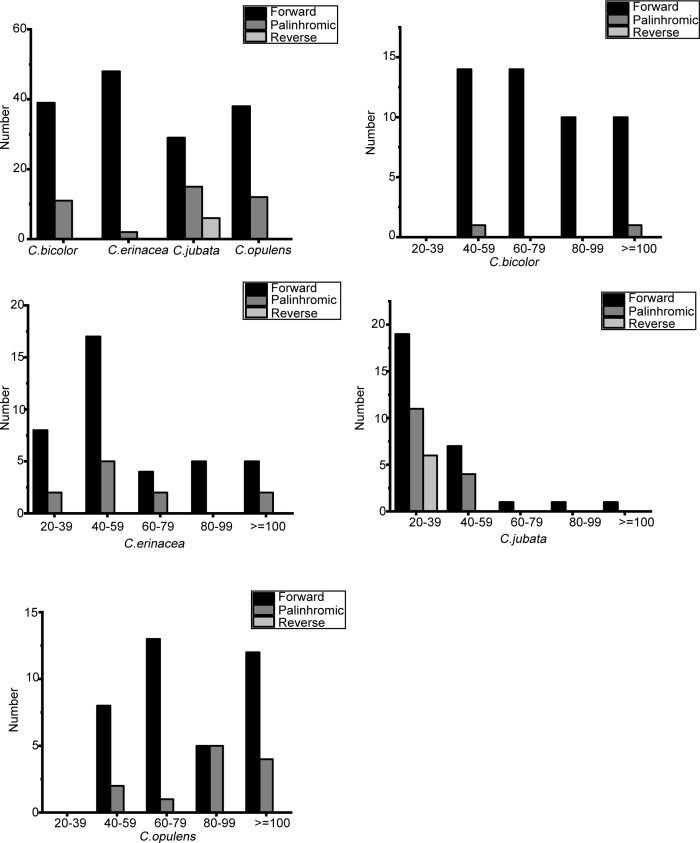
For C. jubata, C. bicolor, C. erinacea, and C. opulens, interspersed repeated sequences (IRSs) were evaluated in the chloroplast genome with a repeat-unit length of ≥ 20 bp. These sequences comprised only forward reverse and palindromic repeats, yet they lacked complementary repeats that are common in other species. Among them, a total of 50 IRSs were found. Among all types of IRS, the sequence lengths in the range of 20–39 bp occurred most frequently in C. jubata and 40–59 bp occurred most frequently in C. erinacea. Those in the range of 60–79 bp and ≥ 100 bp occurred most frequently in C. opulens. IRS analyses of chloroplast genomes are shown in Fig 2.
Fig 2. Long repetitive sequences in chloroplast genomes of four Caragana species.
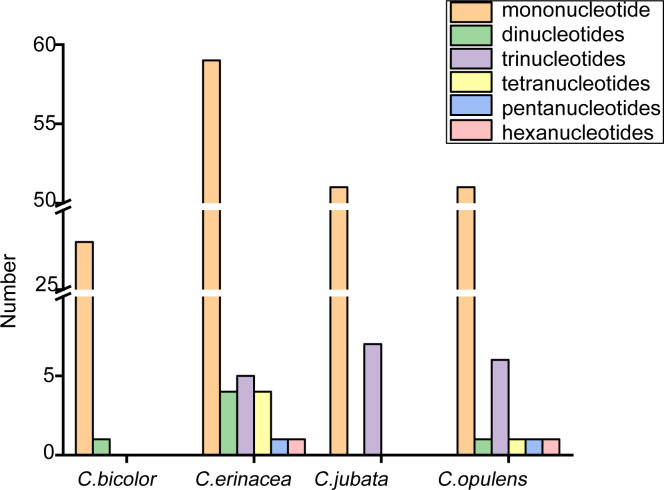
The key mutational mechanism generating SSR polymorphism is as follows: SSRS tended to undergo slipped-strand mispairing [32]. However, SSRs in chloroplast genomes are often used as genetic markers in evolutionary and population genetic studies because of their variability at the intra-specific level [33, 34]. We found 58 SSRs in C. jubata, 29 SSRs in C. bicolor, 61 SSRs in C. opulens, and 74 SSRs in C. erinacea (Fig 3).
Fig 3. SSR distribution in chloroplast genomes of four Caragana species.
3.3. Comparative genomic analysis
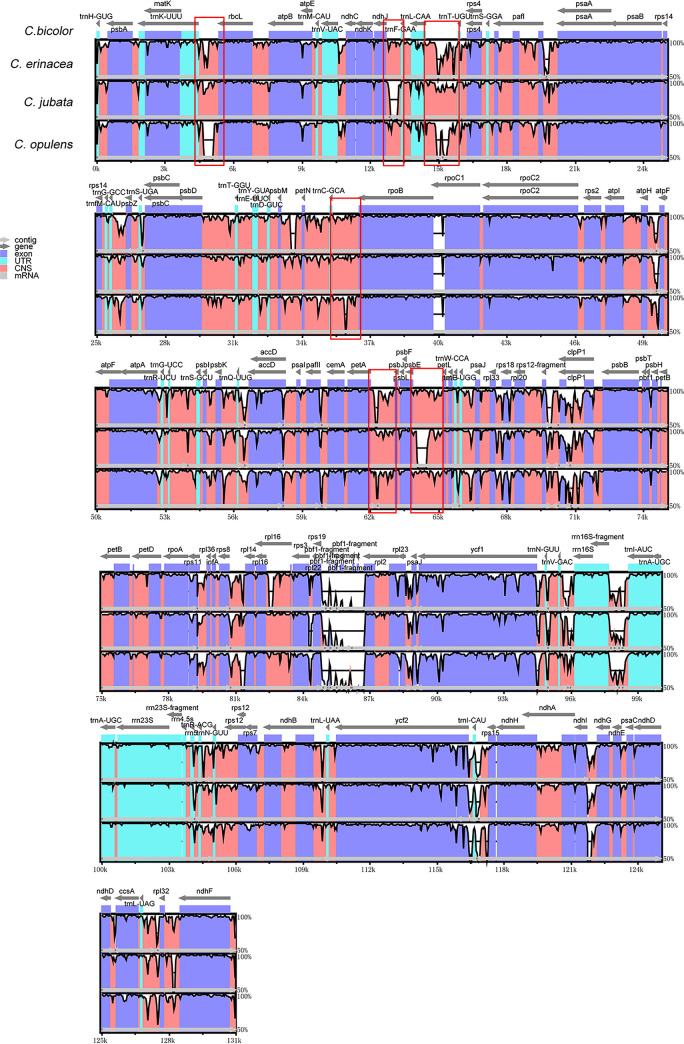
In elucidating the differences in genomic sequences of C. jubata, C. erinacea, C. opulens, and C. bicolor, we used mVISTA to detect sequence variations using the sequence in C. bicolor as a reference (Fig 4). The four genomic sequences are highly similar. However, in some intergenic spacer (IGS) regions and partial sequences, significant differences are found, such as the IGS between trnK-UUU and rbcl; trnF-GAA and ndhJ; trnL-CAA and trnT-UGU; rpoB and trnC-GCA; petA and psbL; psbE and pebL; and sequences of the rpoC, ycf1, and ycf2. The noncoding regions have different degrees of divergence, whereas the protein coding regions are highly conserved. This finding indicated that the IGS of the Caragana genus evolved rapidly. Highly variable regions can be used to exploit markers for identification and further phylogenetic study.
Fig 4. Comparative analyses of genomic differences in chloroplasts of four Caragana species.
Gray arrows and thick black lines above the alignment indicate gene orientation. Purple bars represent exons; blue bars denote untranslated regions (UTRs); pink bars represent non-coding sequences (CNS), and gray bars denote mRNA. The y-axis represents percentage identity.
3.4 Phylogenetic analyses
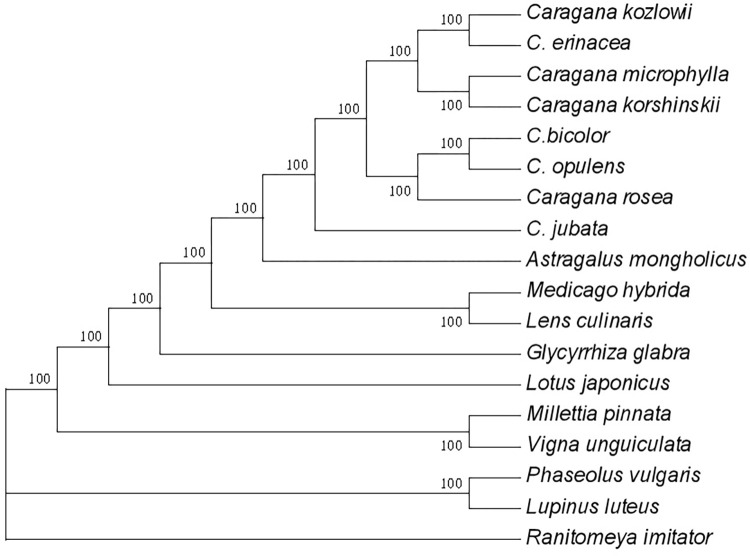
In determining the phylogenetic position of Caragana species, 18 complete chloroplast genome sequences of the Fabaceae family were constructed using the NJ tree (Fig 5). The other 14 species belong to Lens, Medicago, Caragana, Astragalus, Glycyrrhiza, Lotus, Millettia, Vigna, Phaseolus, Lupinus, and Mimosa.
Fig 5. NJ tree based on chloroplast genomes of the Fabaceae family.
The results showed that eight species from Caragana were relatives and categorized together. The following pairs showed a closer relationship: C. kozlowii and C. erinacea, C. microphylla and C. korshinskii, and C. opulens and C. bicolor. The genera Astragalus and Caragana were classified into the Subtrib. Astragalinae: C. jubata belongs to Ser. Jubatae; C. bicolor belongs to Ser. Occidentales; C. erinacea belongs to Ser. Spinosae, and C. opulens belongs to Ser. Grandiflorae Pojark. [1]. This result also fitted the clustering results based on ITS2 sequences [35].
4. Conclusions and discussion
The chloroplast genomes of 10 species of the genus Caragana have been published in the National Center for Biotechnology Information; chloroplast genomes were between 127,103 and 133,122 bp in size, and they contained 110–111 genes (30–31 tRNA, 4 rRNA, and 76 protein-coding genes). Multiple species of Caragana have been reported to loss the IR region, such as C. rosea, C. microphylla, and C. intermedia[12–14]. The chloroplast genomes of C. jubata, C. erinacea, C. opulens, and C. bicolor showed high similarity with regard to gene deletion, genome size, gene sequences, gene classes, and distribution of repeat sequences, and the lacked IRLC. An important indicator of species affinity is the content of DNA G + C [36], and the four Caragana species in this study have highly similar cpDNA G+C content.
IRS analyses of chloroplast genomes show that the four species lacked complementary repeats. Comparative cp-genomic analysis suggested that the cp genome structure of Caragana was well conserved. Highly variable regions are primarily distributed in non-coding and partial coding regions, which can be used to exploit markers for identification and further phylogenetic study.
Intron and/or gene losses in chloroplast genomes have been reported in considerable literature [37–39]. Introns can play an important role in the regulation of gene expression in a temporal and tissue-specific manner [39–41]. Regulatory mechanisms of introns in some plants and animals have been reported [42–44]. However, the relationship between intron deletion and gene expression in Caragana by transcriptome has not been published. Therefore, further research into the role of introns in Caragana species is necessary.
Advances in phylogenetic analysis can reveal the evolution of chloroplast genomes, including nucleotide substitutions and structural changes [45, 46]. Our results of phylogenetic analysis were consistent with the status of the major taxa within the genus Caragana [1]. Species from the genus Caragana were monophyletic, and C. jubata, C. erinacea, C. opulens, and C. bicolor could be differentiated from other Caragana species. The current study demonstrated that chloroplast genomes can be used for the identification and classification of Caragana species. In addition, the phylogenetic relationship of Caragana species is related to elevation and geographical distribution (GD). For example, C. kozlowii (altitude: 3100–4300 m; GD: Qinghai, E Xizang) and C. erinacea (altitude: 2000–4000 m, GD: Qinghai, Xizang, Gansu, Ningxia, W Sichuan, NW Yunnan) are distributed at altitudes of 3100–4000 levels in Qinghai and Tibet. In addition, C. microphylla (altitude: 1800–3800 m, GD: Nei Mongol, Jilin, Liaoning) and C. korshinskii (altitude: 900–2400 m, GD: Nei Mongol, Gansu, Ningxia, Qinghai, W Shanxi) are distributed at altitudes of 1800–2400 levels in Nei Mongo. The phylogenetic relationship of Caragana species partially consistent with elevation and GD. This finding may provide a reference for further study on the relationship between distribution and evolution of Caragana species.
Caragana species have a large altitude span and wide GD, showing strong environmental adaptability[1–5]. Our results can provide valuable information for genetic transformation, the development of population genetic surveys, and evolutionary studies. Plastids contain a range of genes associated with photosynthesis, and photosystem II is a key component of high temperature, drought stress, and many other stresses [47, 48]. However, the strong environmental adaptability mechanism of the genus Caragana remains unclear because of the lack of research and data[3, 12]. Our results can provide data for further investigation of the discovery of adaptability and strong adversity resistance genes of Caragana species. Our research data complement the database of herbgenomics [49, 50].
Data Availability
The datasets generated during the current study are available in The Genome Warehouse in National Genomics Data Center (NGDC, https://ngdc.cncb.ac.cn/)[1][2] (accession numbers: GWHBJYO00000000, GWHBJYN00000000, GWHBJYM00000000, and GWHBJYL00000000).
Funding Statement
This work was supported by the Major Project of "National key research and development plan project projects", under Grant #2019YFC1712302, 2019YFC1712305. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wu Z. Flora of China. Beijing, China: Science Press; 2010. 528–45 p. [Google Scholar]
- 2.Meng Q, Niu Y, Niu X, Roubin RH, Hanrahan JR. Ethnobotany, phytochemistry and pharmacology of the genus Caragana used in traditional Chinese medicine. Journal of Ethnopharmacology. 2009;124(3). doi: 10.1016/j.jep.2009.04.048 [DOI] [PubMed] [Google Scholar]
- 3.Kang H-M, Chen K, Bai J, Wang G. Antioxidative system’s responses in the leaves of six Caragana species during drought stress and recovery. Acta Physiologiae Plantarum. 2012;34(6). [Google Scholar]
- 4.Xiao C-W, Sun OJ, Zhou G-S, Zhao J-Z, Wu G. Interactive effects of elevated CO2 and drought stress on leaf water potential and growth in Caragana intermedia. Trees Structure and Function. 2005;19(6). [Google Scholar]
- 5.Khan S, Nazir M, Raiz N, Saleem M, Zengin G, Fazal G, et al. Phytochemical profiling, in vitro biological properties and in silico studies on Caragana ambigua stocks (Fabaceae): A comprehensive approach. Industrial Crops & Products. 2019;131. [Google Scholar]
- 6.Luo HF, Zhang LP, Hu CQ. ChemInform Abstract: Five Novel Oligostilbenes from the Roots of Caragana sinica. ChemInform. 2001;32(37). [Google Scholar]
- 7.Kakorin PA, Tereshkina OI, Ramenskaya GV. Potential Biological Activity and Chemical Composition of Caragana Jubata (Pall.) Poir. (Review). Pharmaceutical Chemistry Journal. 2018;52(6). [Google Scholar]
- 8.A KP, V BI, O TY, V RG, A DT, G KV. [Hepatoprotective activity of aqueous extract from Caragana jubata (Pall.) Poir. shoots in the model of acute hepatitis induced by acetaminophen in rats]. Biomeditsinskaia khimiia. 2018;64(3). [DOI] [PubMed] [Google Scholar]
- 9.KP A., PI B., RE D., ÉK I., RG V., PL A., et al. Biologically Active Compounds in Aqueous Extracts of Caragana jubata (Pall.) Poir. Pharmaceutical Chemistry Journal. 2018;51(11). [Google Scholar]
- 10.Liaquat A, Saima K, Mamona N, Naheed R, Saima N, Gokhan Z, et al. Chemical profiling, in vitro biological activities and Pearson correlation between phenolic contents and antioxidant activities of Caragana brachyantha Rech.f. South African Journal of Botany. 2021;140. [Google Scholar]
- 11.Kamila Z, Gihwan L, Aizhamal B, Bari SA, Ho KJ, Yoon KJ, et al. Inhibitory mechanism of O-methylated quercetins, highly potent β-secretase inhibitors isolated from Caragana balchaschensis (Kom.) Pojark. Journal of Ethnopharmacology. 2021;272. [DOI] [PubMed] [Google Scholar]
- 12.Jiang M, Chen H, He S, Wang L, Chen AJ, Liu C. Sequencing, Characterization, and Comparative Analyses of the Plastome of Caragana rosea var. rosea. International Journal of Molecular Sciences. 2018;19(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z-l, Ma L-y, Yao H-b, Yang X, Luo J-h, Gong X, et al. Complete chloroplast genome of Caragana intermedia (Fabaceae), an endangered shrub endemic to china. Conservation Genetics Resources. 2016;8(4). [Google Scholar]
- 14.Liu B-b, Duan N, Zhang H-l, Liu S, Shi J-w, Chai B-f. Characterization of the whole chloroplast genome of Caragana microphylla Lam (Fabaceae). Conservation Genetics Resources. 2016;8(4). [Google Scholar]
- 15.Rehder A. Bibliography of Cultivated Trees and Shrubs Hardy in the Cooler Temperate Regions of the Northern Hemisphere. Arnold Arboretum of Harv. 1949. [Google Scholar]
- 16.O BJ, M RA, C ZP. Chloroplasts extend stromules independently and in response to internal redox signals. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(32). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanjun L, Dapeng N, Yujun W, Junjie S, Xincun W, Dan Y, et al. Intraspecific and heteroplasmic variations, gene losses and inversions in the chloroplast genome of Astragalus membranaceus. Scientific reports. 2016;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D PJ, F TW. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell. 1982;29(2). [DOI] [PubMed] [Google Scholar]
- 19.Jamal S, Erika S, Nicholas E, Jin Z, A BN, Muhammed M, et al. Evolutionary and biotechnology implications of plastid genome variation in the inverted-repeat-lacking clade of legumes. Plant biotechnology journal. 2014;12(6). [DOI] [PubMed] [Google Scholar]
- 20.Domingos C, P dQL, Toby PR, C dLH, Emile F, F WM, et al. Revisiting the phylogeny of papilionoid legumes: New insights from comprehensively sampled early-branching lineages. American journal of botany. 2012;99(12). [DOI] [PubMed] [Google Scholar]
- 21.Yingchun H, Quan Z, Guangyuan R, Sodmergen. Occurrence of plastids in the sperm cells of Caprifoliaceae: biparental plastid inheritance in angiosperms is unilaterally derived from maternal inheritance. Plant & cell physiology. 2008;49(6). [DOI] [PubMed] [Google Scholar]
- 22.Daniell H, Kumar S, Dufourmantel N. Breakthrough in chloroplast genetic engineering of agronomically important crops. Trends in Biotechnology. 2005;23(5). doi: 10.1016/j.tibtech.2005.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jun-Bo Y, De-Zhu L, Hong-Tao L. Highly effective sequencing whole chloroplast genomes of angiosperms by nine novel universal primer pairs. Molecular ecology resources. 2014;14(5). [DOI] [PubMed] [Google Scholar]
- 24.D AR, J TJ, Ibai I-A, Ferdinand M, Carlos G-M, Elisa dlC-M, et al. A single three-dimensional chromatin compartment in amphioxus indicates a stepwise evolution of vertebrate Hox bimodal regulation. Nature genetics. 2016;48(3). [DOI] [PubMed] [Google Scholar]
- 25.M LT, R ES. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic acids research. 1997;25(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A FK, Lior P, Alexander P, M RE, Inna D. VISTA: computational tools for comparative genomics. Nucleic acids research. 2004;32(Web Server issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.G B. Tandem repeats finder: a program to analyze DNA sequences. Nucleic acids research. 1999;27(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marc L, Oliver D, Ralph B. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Current genetics. 2007;52(5–6). [DOI] [PubMed] [Google Scholar]
- 29.S K, V CJ, E O, C S, J S, R G. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic acids research. 2001;29(22). doi: 10.1093/nar/29.22.4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebastian B, Thomas T, Thomas M, Uwe S, Martin M. MISA-web: a web server for microsatellite prediction. Bioinformatics (Oxford, England). 2017;33(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seung-Bum L, Charalambos K, Robert J, Jessica H, Luke T, Christopher T, et al. The complete chloroplast genome sequence of Gossypium hirsutum: organization and phylogenetic relationships to other angiosperms. BMC Genomics. 2006;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.T C, M H, C G, R J. A generic intron increases gene expression in transgenic mice. Molecular and cellular biology. 1991;11(6). doi: 10.1128/mcb.11.6.3070-3074.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sajjad A, Latif KA, Aaqil KM, Muhammad W, Sang-Mo K, Byung-Wook Y, et al. Chloroplast genomes of Arabidopsis halleri ssp. gemmifera and Arabidopsis lyrata ssp. petraea: Structures and comparative analysis. Scientific reports. 2017;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wenpan D, Chao X, Tao C, Kui L, Shiliang Z. Sequencing angiosperm plastid genomes made easy: a complete set of universal primers and a case study on the phylogeny of saxifragales. Genome biology and evolution. 2013;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao D, Zhang Y, Fu S. Molecular identification of Tibetan medicinal plants based on ITS2 sequence. Chinese Traditional and Herbal Drugs. 2019;50(12):2967–75. [Google Scholar]
- 36.Koichiro T, Daniel P, Nicholas P, Glen S, Masatoshi N, Sudhir K. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution. 2011;28(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Y, Liying C, Claude d, Bernard M, Jijun T. Gene rearrangement analysis and ancestral order inference from chloroplast genomes with inverted repeat. BMC Genomics. 2008;9(Suppl+1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Downie SR, Llanas E, Downie DSK. Multiple Independent Losses of the rpoC1 Intron in Angiosperm Chloroplast DNA’s. Systematic Botany. 1996;21(2). [Google Scholar]
- 39.BR G. Alternative splicing: increasing diversity in the proteomic world. Trends Genet 2001. 2 p. [DOI] [PubMed] [Google Scholar]
- 40.Jiang X, Yang C, Baosheng L, Shuiming X, Qinggang Y, Rui B, et al. Panax ginseng genome examination for ginsenoside biosynthesis. GigaScience. 2017;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hir HL, Nott A, Moore MJ. How introns influence and enhance eukaryotic gene expression. Trends in Biochemical Sciences. 2003;28(4). doi: 10.1016/S0968-0004(03)00052-5 [DOI] [PubMed] [Google Scholar]
- 42.Deng-Ke N, Yu-Fei Y. Why eukaryotic cells use introns to enhance gene expression: splicing reduces transcription-associated mutagenesis by inhibiting topoisomerase I cutting activity. Biology direct. 2011;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.J C, M F, V W. Introns increase gene expression in cultured maize cells. Genes & development. 1987;1(10). [DOI] [PubMed] [Google Scholar]
- 44.Shahram E, Dinah A, Ian K, B RA. The effects of a stimulating intron on the expression of heterologous genes in Arabidopsis thaliana. Plant biotechnology journal. 2013;11(5). [DOI] [PubMed] [Google Scholar]
- 45.Saski C, Lee S-B, Daniell H, Wood TC, Tomkins J, Kim H-G, et al. Complete Chloroplast Genome Sequence of Glycine max and Comparative Analyses with other Legume Genomes. Plant Molecular Biology. 2005;59(2). [DOI] [PubMed] [Google Scholar]
- 46.C HR, Matthew FH, L BJ, K JR. Extensive rearrangements in the chloroplast genome of Trachelium caeruleum are associated with repeats and tRNA genes. Journal of molecular evolution. 2008;66(4). [DOI] [PubMed] [Google Scholar]
- 47.Mulo P, Sakurai I, Aro EM. Strategies for psbA gene expression in cyanobacteria, green algae and higher plants: from transcription to PSII repair. Biochimica et biophysica acta. 2012;1817(1):247–57. Epub 2011/05/14. doi: 10.1016/j.bbabio.2011.04.011 . [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto Y, Aminaka R, Yoshioka M, Khatoon M, Komayama K, Takenaka D, et al. Quality control of photosystem II: impact of light and heat stresses. Photosynthesis research. 2008;98(1–3):589–608. Epub 2008/10/22. doi: 10.1007/s11120-008-9372-4 . [DOI] [PubMed] [Google Scholar]
- 49.Xianmei Y, Baosheng L, Shuai G, Conglian L, Jin P, Jiang X, et al. The chloroplasts genomic analyses of Rosa laevigata, R. rugosa and R. canina. Chinese medicine. 2020;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shu-Dong Z, Jian-Jun J, Si-Yun C, W CM, E SD, Hong-Tao L, et al. Diversification of Rosaceae since the Late Cretaceous based on plastid phylogenomics. The New phytologist. 2017;214(3). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available in The Genome Warehouse in National Genomics Data Center (NGDC, https://ngdc.cncb.ac.cn/)[1][2] (accession numbers: GWHBJYO00000000, GWHBJYN00000000, GWHBJYM00000000, and GWHBJYL00000000).